Biconometric Connections in Dental Implants: A Pilot Mechanical Study
Abstract
1. Introduction
2. Materials and Methods
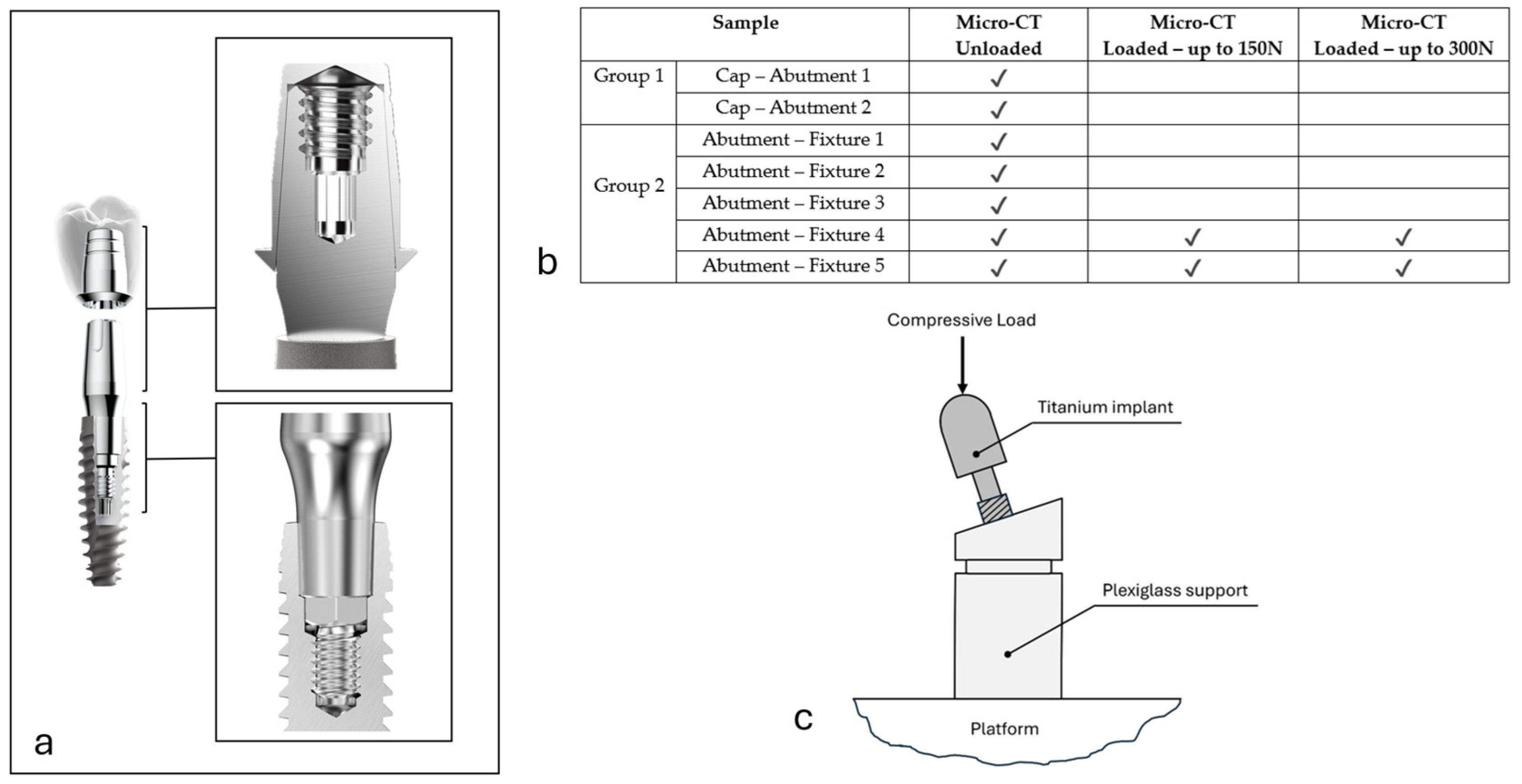
2.1. Experiment Workflow
2.2. Implant Manufacturing
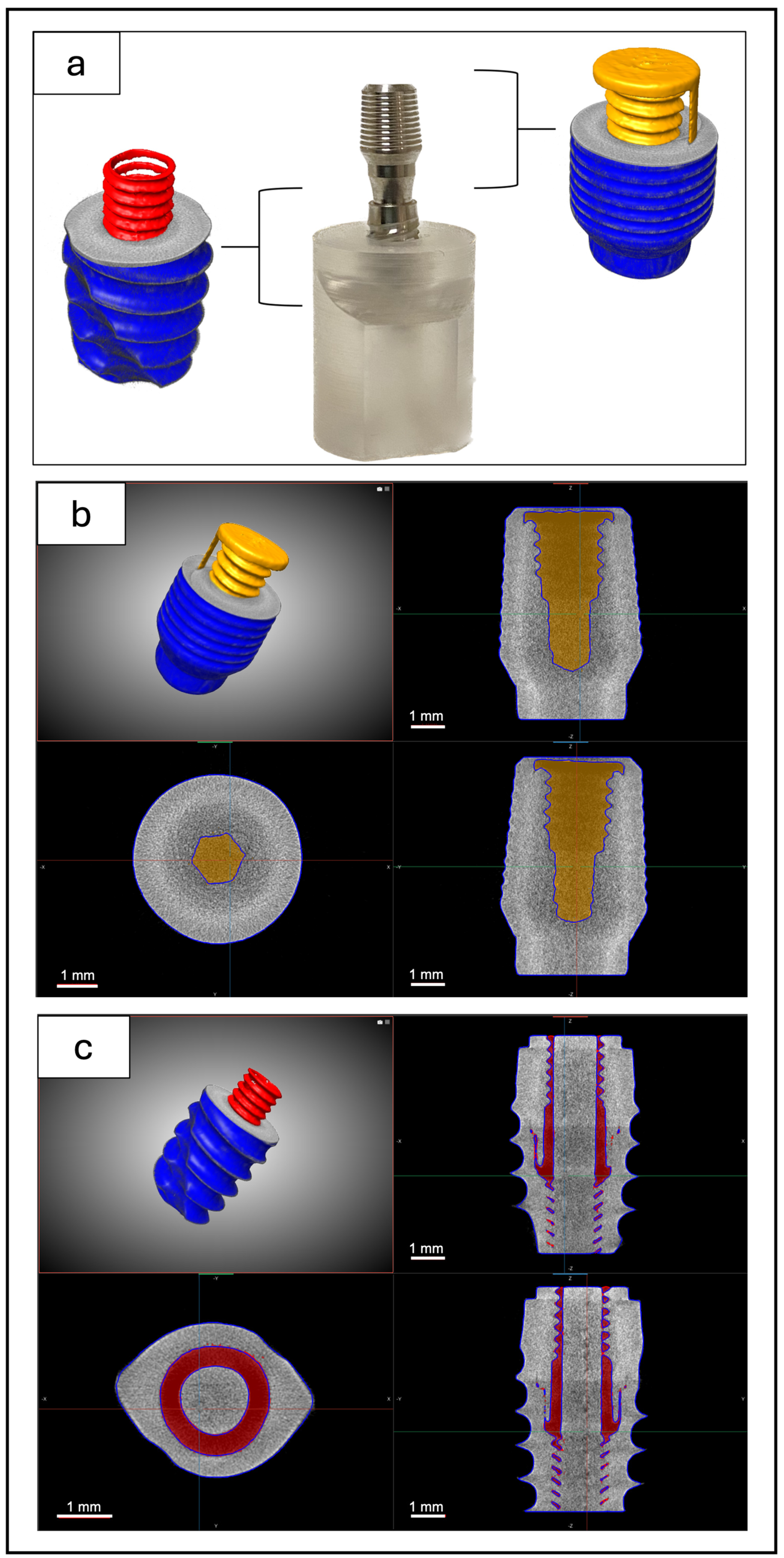
2.3. X-Ray Micro-CT Analysis
2.4. Image Segmentation, Thickness Map, and Image Registration
2.5. Mechanical Compression Tests
3. Results
3.1. Micro-CT Analysis
3.2. Mechanical Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Micro-CT | Micro-computed tomography |
References
- Fernandes, G.V.d.O.; Martins, B.G.d.S.; Fraile, J.F. Revisiting Peri-Implant Diseases in Order to Rethink the Future of Compromised Dental Implants: Considerations, Perspectives, Treatment, and Prognosis. Dent. Med. Probl. 2024, 61, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, L.; Montevecchi, M.; Ostanello, M.; Checchi, V. Recognition and Treatment of Peri-Implant Mucositis: Do We Have the Right Perception? A Structured Review. Dent. Med. Probl. 2021, 58, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, J.; Takeichi, T.; Sol Gaviria, A.; Peter, L.; Katsoulis, K. Misfit of Implant Prostheses and Its Impact on Clinical Outcomes. Definition, Assessment and a Systematic Review of the Literature. Eur. J. Oral Implantol. 2017, 10 (Suppl. S1), 121–138. [Google Scholar]
- Abarno, S.; Gehrke, A.; Dedavid, B.; Gehrke, S. Stress Distribution around Dental Implants, Generated by Six Different Ceramic Materials for Unitary Restoration: An Experimental Photoelastic Study. Dent. Med. Probl. 2021, 58, 453–461. [Google Scholar] [CrossRef]
- Biju, D.; Arumugam, P.; Kannan, S.; Kumar Yadalam, P.; Ronsivalle, V.; Cicciù, M.; Minervini, G. Development, Characterization, and Biocompatibility and Corrosion Analyses of a Silver-Decorated Graphene Oxide and Chitosan Surface Coating for Titanium Dental Implants: A Preliminary Report. Dent. Med. Probl. 2024, 61, 627–632. [Google Scholar] [CrossRef]
- Krebs, M.; Schmenger, K.; Neumann, K.; Weigl, P.; Moser, W.; Nentwig, G. Long-Term Evaluation of ANKYLOS® Dental Implants, Part I: 20-Year Life Table Analysis of a Longitudinal Study of More Than 12,500 Implants. Clin. Implant. Dent. Relat. Res. 2015, 17, e275–e286. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Peri-implant Health. J. Periodontol. 2018, 89, S230–S236. [Google Scholar] [CrossRef]
- Arai, Y.; Takashima, M.; Matsuzaki, N.; Takada, S. Marginal Bone Loss in Dental Implants: A Literature Review of Risk Factors and Treatment Strategies for Prevention. J. Prosthodont. Res. 2024, 69, 12–20. [Google Scholar] [CrossRef]
- Broggini, N.; McManus, L.M.; Hermann, J.S.; Medina, R.; Schenk, R.K.; Buser, D.; Cochran, D.L. Peri-Implant Inflammation Defined by the Implant-Abutment Interface. J. Dent. Res. 2006, 85, 473–478. [Google Scholar] [CrossRef]
- Piattelli, A.; Vrespa, G.; Petrone, G.; Iezzi, G.; Annibali, S.; Scarano, A. Role of the Microgap Between Implant and Abutment: A Retrospective Histologic Evaluation in Monkeys. J. Periodontol. 2003, 74, 346–352. [Google Scholar] [CrossRef]
- Molinero-Mourelle, P.; Cascos-Sanchez, R.; Yilmaz, B.; Lam, W.Y.H.; Pow, E.H.N.; Del Río Highsmith, J.; Gómez-Polo, M. Effect of Fabrication Technique on the Microgap of CAD/CAM Cobalt–Chrome and Zirconia Abutments on a Conical Connection Implant: An In Vitro Study. Materials 2021, 14, 2348. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Fenton, A. Screw- Versus Cement-Retained Implant Prostheses: A Systematic Review of Prosthodontic Maintenance and Complications. Int. J. Prosthodont. 2015, 28, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Polo, M.; Ortega, R.; Gómez-Polo, C.; Celemin, A.; Del Rio Highsmith, J. Factors Affecting the Decision to Use Cemented or Screw-Retained Fixed Implant-Supported Prostheses: A Critical Review. Int. J. Prosthodont. 2018, 31, 43–54. [Google Scholar] [CrossRef]
- Degidi, M.; Nardi, D.; Sighinolfi, G.; Degidi, D. The Conometric Concept for the Definitive Rehabilitation of a Single Posterior Implant by Using a Conical Indexed Abutment: A Technique. J. Prosthet. Dent. 2020, 123, 576–579. [Google Scholar] [CrossRef]
- Degidi, M.; Nardi, D.; Sighinolfi, G.; Degidi, D.; Piattelli, A. The Conometric Concept: Definitive Rehabilitation of the Single Posterior Implant Using the Acuris Abutment: A Preliminary Report. Clin. Oral Implants Res. 2020, 31, 223. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Nogueira-Filho, G.; Tenenbaum, H.C.; Lai, J.Y.; Brito, C.; Döring, H.; Nonhoff, J. Performance of Conical Abutment (Morse Taper) Connection Implants: A Systematic Review. J. Biomed. Mater. Res. A 2014, 102, 552–574. [Google Scholar] [CrossRef]
- Arshad, M.; Mahgoli, H.; Payaminia, L. Effect of Repeated Screw Joint Closing and Opening Cycles and Cyclic Loading on Abutment Screw Removal Torque and Screw Thread Morphology: Scanning Electron Microscopy Evaluation. Int. J. Oral Maxillofac. Implants 2018, 33, 31–40. [Google Scholar] [CrossRef]
- Vinhas, A.S.; Aroso, C.; Salazar, F.; López-Jarana, P.; Ríos-Santos, J.V.; Herrero-Climent, M. Review of the Mechanical Behavior of Different Implant–Abutment Connections. Int. J. Environ. Res. Public Health 2020, 17, 8685. [Google Scholar] [CrossRef]
- Rack, A.; Rack, T.; Stiller, M.; Riesemeier, H.; Zabler, S.; Nelson, K. In Vitro Synchrotron-Based Radiography of Micro-Gap Formation at the Implant-Abutment Interface of Two-Piece Dental Implants. J. Synchrotron Radiat. 2010, 17, 289–294. [Google Scholar] [CrossRef]
- Zipprich, H.; Weigl, P.; Ratka, C.; Lange, B.; Lauer, H.C. The Micromechanical Behavior of Implant-Abutment Connections under a Dynamic Load Protocol. Clin. Implant. Dent. Relat. Res. 2018, 20, 814–823. [Google Scholar] [CrossRef]
- Hermann, J.S.; Schoolfield, J.D.; Schenk, R.K.; Buser, D.; Cochran, D.L. Influence of the Size of the Microgap on Crestal Bone Changes Around Titanium Implants. A Histometric Evaluation of Unloaded Non-Submerged Implants in the Canine Mandible. J. Periodontol. 2001, 72, 1372–1383. [Google Scholar] [CrossRef]
- Ricomini Filho, A.P.; Fernandes, F.S.d.F.; Straioto, F.G.; Silva, W.J.d.; Del Bel Cury, A.A. Preload Loss and Bacterial Penetration on Different Implant-Abutment Connection Systems. Braz. Dent. J. 2010, 21, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Piermatti, J.; Yousef, H.; Luke, A.; Mahevich, R.; Weiner, S. An In Vitro Analysis of Implant Screw Torque Loss With External Hex and Internal Connection Implant Systems. Implant. Dent. 2006, 15, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, K.; Ayukawa, Y.; Matsuzaki, T.; Kihara, M.; Koyano, K. The Influence of Implant–Abutment Connection on the Screw Loosening and Microleakage. Int. J. Implant. Dent. 2018, 4, 11. [Google Scholar] [CrossRef]
- Tripodi, D.; Vantaggiato, G.; Scarano, A.; Perrotti, V.; Piattelli, A.; Iezzi, G.; D’Ercole, S. An In Vitro Investigation Concerning the Bacterial Leakage at Implants With Internal Hexagon and Morse Taper Implant-Abutment Connections. Implant. Dent. 2012, 21, 335–339. [Google Scholar] [CrossRef]
- Kowalski, J.; Puszkarz, A.K.; Radwanski, M.; Sokolowski, J.; Cichomski, M.; Bourgi, R.; Hardan, L.; Sauro, S.; Lukomska-Szymanska, M. Micro-CT Evaluation of Microgaps at Implant–Abutment Connection. Materials 2023, 16, 4491. [Google Scholar] [CrossRef]
- Keklikoglou, K.; Arvanitidis, C.; Chatzigeorgiou, G.; Chatzinikolaou, E.; Karagiannidis, E.; Koletsa, T.; Magoulas, A.; Makris, K.; Mavrothalassitis, G.; Papanagnou, E.-D.; et al. Micro-CT for Biological and Biomedical Studies: A Comparison of Imaging Techniques. J. Imaging 2021, 7, 172. [Google Scholar] [CrossRef]
- Sacker, T.N.; Trentin, M.S.; dos Santos, T.M.P.; Lopes, R.T.; Rivaldo, E.G. In Vitro Microtomography Evaluation of the Implant-Abutment Interface—Gap Microtomography Evaluation/ Avaliação Da Microtomografia in Vitro Da Interface Implante-Pilar—Avaliação Da Microtomografia de Gap. Braz. J. Dev. 2021, 7, 57552–57565. [Google Scholar] [CrossRef]
- Radwanski, M.; Leski, M.; Puszkarz, A.K.; Sokolowski, J.; Hardan, L.; Bourgi, R.; Sauro, S.; Lukomska-Szymanska, M. A Micro-CT Analysis of Initial and Long-Term Pores Volume and Porosity of Bioactive Endodontic Sealers. Biomedicines 2022, 10, 2403. [Google Scholar] [CrossRef]
- Riberti, N.; D’Amico, E.; Pierfelice, T.V.; Furlani, M.; Giuliani, A.; Piattelli, A.; Iezzi, G.; Comuzzi, L. Case Report of a Dental Implant with Conometric Abutment–Prosthetic Cap Connection: Advanced High-Resolution Imaging and Peri-Implant Connective Tissue Performance. Clin. Pract. 2024, 14, 556–569. [Google Scholar] [CrossRef]
- Ceddia, M.; Comuzzi, L.; Di Pietro, N.; Romasco, T.; Specchiulli, A.; Piattelli, A.; Trentadue, B. Finite Element Analysis (FEA) for the Evaluation of Retention in a Conometric Connection for Implant and Prosthesis. Osteology 2023, 3, 140–156. [Google Scholar] [CrossRef]
- Makovetsky, R.; Piche, N.; Marsh, M. Dragonfly as a Platform for Easy Image-Based Deep Learning Applications. Microsc. Microanal. 2018, 24, 532–533. [Google Scholar] [CrossRef]
- Lupi, S.M.; Todaro, C.; De Martis, D.; Blasi, P.; Rodriguez y Baena, R.; Storelli, S. The Conometric Connection for the Implant-Supported Fixed Prosthesis: A Narrative Review. Prosthesis 2022, 4, 458–467. [Google Scholar] [CrossRef]
- Degidi, M.; Nardi, D.; Sighinolfi, G.; Piattelli, A. The Conometric Concept: Definitive Fixed Lithium Disilicate Restorations Supported by Conical Abutments. J. Prosthodont. 2018, 27, 605–610. [Google Scholar] [CrossRef]
- Lupi, S.M.; De Martis, D.; Todaro, C.; Isola, G.; Beretta, M.; Rodriguez y Baena, R. Conometric Connection for Implant-Supported Crowns: A Prospective Clinical Cohort Study. J. Clin. Med. 2023, 12, 7647. [Google Scholar] [CrossRef]
- Sharma, V.; Paliwal, J.; Bhansali, S.; Meena, K.; Gupta, N.; Dadarwal, A. Evaluation of Clinical and Radiographic Outcome of Friction Fit Conical Abutment System in Implant-Supported Dental Prostheses: An in Vivo Study. J. Indian Prosthodont. Soc. 2022, 22, 29. [Google Scholar] [CrossRef]
- Bressan, E.; Venezze, A.; Magaz, V.; Lops, D.; Ghensi, P. Fixed Conometric Retention with CAD/CAM Conic Coupling Abutments and Prefabricated Syncone Caps: A Case Series. Int. J. Periodontics Restor. Dent. 2018, 38, 277–280. [Google Scholar] [CrossRef]
- Bressan, E.; Sbricoli, L.; Guazzo, R.; Bambace, M.; Lops, D.; Tomasi, C. Five-Year Prospective Study on Conometric Retention for Complete Fixed Prostheses. Int. J. Oral Implantol. 2019, 12, 105–113. [Google Scholar]
- Gehrke, P.; Burg, S.; Peters, U.; Beikler, T.; Fischer, C.; Rupp, F.; Schweizer, E.; Weigl, P.; Sader, R.; Smeets, R.; et al. Bacterial Translocation and Microgap Formation at a Novel Conical Indexed Implant Abutment System for Single Crowns. Clin. Oral Investig. 2022, 26, 1375–1389. [Google Scholar] [CrossRef]
- Gehrke, P.; Hartjen, P.; Smeets, R.; Gosau, M.; Peters, U.; Beikler, T.; Fischer, C.; Stolzer, C.; Geis-Gerstorfer, J.; Weigl, P.; et al. Marginal Adaptation and Microbial Leakage at Conometric Prosthetic Connections for Implant-Supported Single Crowns: An In Vitro Investigation. Int. J. Mol. Sci. 2021, 22, 881. [Google Scholar] [CrossRef]
- Murmura, G.; Di Iorio, D.; Cicchetti, A.R.; Sinjari, B.; Caputi, S. In Vitro Analysis of Resistance to Cyclic Load and Preload Distribution of Two Implant/Abutment Screwed Connections. J. Oral Implantol. 2013, 39, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Cashman, P.M.; Schneider, R.L.; Schneider, G.B.; Stanford, C.M.; Clancy, J.M.; Qian, F. In Vitro Analysis of Post-Fatigue Reverse-Torque Values at the Dental Abutment/Implant Interface for a Unitarian Abutment Design. J. Prosthodont. 2011, 20, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Rocha Bernardes, S.; Chiarello de Mattos, M.d.G.; Hobkirk, J.; Faria Ribeiro, R. Loss of Preload in Screwed Implant Joints as a Function of Time and Tightening/Untightening Sequences. Int. J. Oral Maxillofac. Implant. 2014, 29, 89–96. [Google Scholar] [CrossRef]
- Shin, H.-M.; Huh, J.-B.; Yun, M.-J.; Jeon, Y.-C.; Chang, B.M.; Jeong, C.-M. Influence of the Implant-Abutment Connection Design and Diameter on the Screw Joint Stability. J. Adv. Prosthodont. 2014, 6, 126–132. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; León-Cano, A.; Monje, A.; Ortega-Oller, I.; O′Valle, F.; Catena, A. Abutment Height Influences the Effect of Platform Switching on Peri-implant Marginal Bone Loss. Clin. Oral Implant. Res. 2016, 27, 167–173. [Google Scholar] [CrossRef]
- Laleman, I.; Lambert, F.; Gahlert, M.; Bacevic, M.; Woelfler, H.; Roehling, S. The Effect of Different Abutment Materials on Peri-implant Tissues—A Systematic Review and Meta-analysis. Clin. Oral Implant. Res. 2023, 34, 125–142. [Google Scholar] [CrossRef]
- Deporter, D. Dental Implant Design and Optimal Treatment Outcomes. Int. J. Periodontics Restor. Dent. 2009, 29, 625–633. [Google Scholar]
- Caricasulo, R.; Malchiodi, L.; Ghensi, P.; Fantozzi, G.; Cucchi, A. The Influence of Implant-abutment Connection to Peri-implant Bone Loss: A Systematic Review and Meta-analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 653–664. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Du, W.; Zhou, X.-D.; Yu, H.-Y. Review of Research on the Mechanical Properties of the Human Tooth. Int. J. Oral Sci. 2014, 6, 61–69. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riberti, N.; Furlani, M.; Tognoli, E.; Piattelli, A.; Comuzzi, L.; Giuliani, A.; Gatto, A. Biconometric Connections in Dental Implants: A Pilot Mechanical Study. Materials 2025, 18, 1415. https://doi.org/10.3390/ma18071415
Riberti N, Furlani M, Tognoli E, Piattelli A, Comuzzi L, Giuliani A, Gatto A. Biconometric Connections in Dental Implants: A Pilot Mechanical Study. Materials. 2025; 18(7):1415. https://doi.org/10.3390/ma18071415
Chicago/Turabian StyleRiberti, Nicole, Michele Furlani, Emanuele Tognoli, Adriano Piattelli, Luca Comuzzi, Alessandra Giuliani, and Andrea Gatto. 2025. "Biconometric Connections in Dental Implants: A Pilot Mechanical Study" Materials 18, no. 7: 1415. https://doi.org/10.3390/ma18071415
APA StyleRiberti, N., Furlani, M., Tognoli, E., Piattelli, A., Comuzzi, L., Giuliani, A., & Gatto, A. (2025). Biconometric Connections in Dental Implants: A Pilot Mechanical Study. Materials, 18(7), 1415. https://doi.org/10.3390/ma18071415












