Overview of Recent Developments in Composite Epoxy Resin in Organic Coating on Steel (2020–2024)
Abstract
1. Introduction
2. Epoxy Coatings Modified by Metals and Their Compounds
2.1. Modification of Zinc-Rich Epoxy Coating
2.2. Titanium-Modified Epoxy Coatings
2.3. Silicon-Modified Epoxy Coatings
2.4. Epoxy Coatings Modified by Other Metal Compounds
3. Epoxy Coatings Modified by Organic Compounds
3.1. Epoxy Coatings Modified by N Organic Compounds
3.2. Epoxy Coatings Modified by Natural Organic Compounds
3.3. Organic Coatings Modified by Other Organic Compounds
4. Organometallic-Compound-Modified Epoxy Coatings
4.1. Epoxy Coatings Modified by Metal–Organic Frameworkds
4.2. Epoxy Coatings Modified by Other Organometallic Compounds
5. Carbon-Material-Modified Epoxy Coatings
5.1. Graphene-Modified Epoxy Coatings
5.2. Epoxy Coatings Modified by Other Carbon Materials
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, G.; Bi, Z.; Wang, S.; Liu, J.; Xing, X.; Li, Z.; Wang, B. A comprehensive review on smart anti-corrosive coatings. Prog. Org. Coat. 2020, 148, 105821. [Google Scholar] [CrossRef]
- Kausar, A. Performance of corrosion protective epoxy blend-based nanocomposite coatings: A review. Polym. Plast. Technol. Mater. 2020, 59, 658–673. [Google Scholar] [CrossRef]
- Xavier, J.R. Superior barrier, hydrophobic, and mechanical properties of the epoxy nanocomposite containing mixed metal oxides. J. Adhes. Sci. Technol. 2023, 37, 1394–1418. [Google Scholar] [CrossRef]
- Kumari, S.; Saini, A.; Dhayal, V. Metal oxide based epoxy coatings for corrosion protection of steel. Mater. Today Proc. 2021, 43, 3105–3109. [Google Scholar] [CrossRef]
- Yap, S.W.; Johari, N.; Mazlan, S.A.; Ahmad, S.N.A.S.; Arifin, R.; Hassan, N.A.; Johari, M.A.F. Superhydrophobic zinc oxide/epoxy coating prepared by a one-step approach for corrosion protection of carbon steel. J. Mater. Res. Technol. 2023, 25, 5751–5766. [Google Scholar] [CrossRef]
- Guo, S.-Y.; Luo, H.-H.; Tan, Z.; Chen, J.-Z.; Zhang, L.; Ren, J. Impermeability and interfacial bonding strength of TiO2-graphene modified epoxy resin coated OPC concrete. Prog. Org. Coat. 2021, 151, 106029. [Google Scholar] [CrossRef]
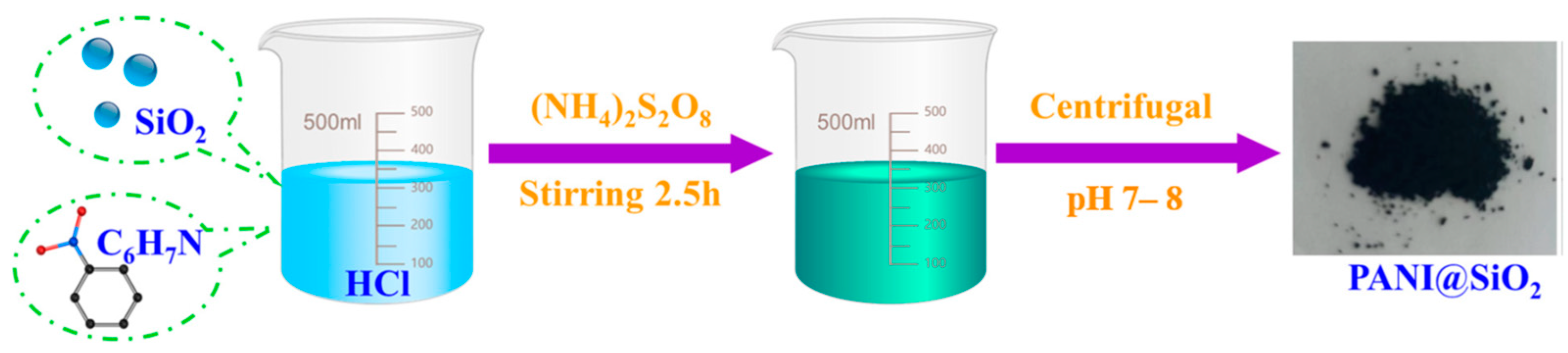
- Lei, Y.; Qiu, Z.; Tan, N.; Du, H.; Li, D.; Liu, J.; Liu, T.; Zhang, W.; Chang, X. Polyaniline/CeO2 nanocomposites as corrosion inhibitors for improving the corrosive performance of epoxy coating on carbon steel in 3.5% NaCl solution. Prog. Org. Coat. 2020, 139, 105430. [Google Scholar] [CrossRef]
- Xavier, J.R. Electrochemical, mechanical and adhesive properties of surface modified NiO-epoxy nanocomposite coatings on mild steel. Mater. Sci. Eng. B 2020, 260, 114639. [Google Scholar]
- Feng, Z.; Wan, R.; Chen, S.; Tang, X.; Ju, H.; Li, Y.; Song, G.-L. In-situ repair of marine coatings by a Fe3O4 nanoparticle-modified epoxy resin under seawater. Chem. Eng. J. 2022, 430, 132827. [Google Scholar] [CrossRef]
- Pandis, P.K.; Papaioannou, S.; Siaperas, V.; Terzopoulos, A.; Stathopoulos, V.N. Evaluation of Zn- and Fe-rich organic coatings for corrosion protection and condensation performance on waste heat recovery surfaces. Int. J. Thermofluids 2020, 3–4, 100025. [Google Scholar] [CrossRef]
- Qi, C.; Dam-Johansen, K.; Weinell, C.E.; Bi, H.; Wu, H. Enhanced anticorrosion performance of zinc rich epoxy coatings modified with stainless steel flakes. Prog. Org. Coat. 2022, 163, 106616. [Google Scholar] [CrossRef]
- Xing, C.; Wang, W.; Qu, S.; Tang, Y.; Zhao, X.; Zuo, Y. Degradation of zinc-rich epoxy coating in 3.5% NaCl solution and evolution of its EIS parameters. J. Coat. Technol. Res. 2021, 18, 843–860. [Google Scholar] [CrossRef]
- Qi, C.; Weinell, C.E.; Dam-Johansen, K.; Wu, H. Assessment of anticorrosion performance of zinc-rich epoxy coatings added with zinc fibers for corrosion protection of steel. ACS Omega 2023, 8, 1912–1922. [Google Scholar] [CrossRef]
- Mamudu, U.; Omeiza, L.A.; Hussin, M.R.; Subramanian, Y.; Azad, A.K.; Alnarabiji, M.S.; Ebenso, E.E.; Lim, R.C. Recycled eggshell waste in zinc-rich epoxy coating for corrosion protection of mild steel in a controlled elevated temperature saline environment. Prog. Org. Coat. 2024, 186, 108025. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Ghaderi, S.; Sadeghi, M.; Gorji, B.; Ahmadijokani, F.; Ramazani, S.A.A.; Mahdavian, M.; Arjmand, M. Enhanced active/barrier corrosion protective properties of epoxy coatings containing eco-friendly green inorganic/organic hybrid pigments based on zinc cations/Ferula Asafoetida leaves. J. Mol. Liq. 2021, 323, 114584. [Google Scholar] [CrossRef]
- Amanian, S.; Naderi, R.; Mahdavian, M. The role of an in-situ grown Zn-Al layered double hydroxide conversion coating in the protective properties of epoxy coating on galvanized steel. J. Electrochem. Soc. 2022, 169, 031511. [Google Scholar] [CrossRef]
- Rahmani, M.H.; Naderi, R.; Mahdavian, M. Pulse-reverse electrodeposition of a conversion coating based on zinc cation and 3-nitrobenzoic acid on carbon steel to enhance adhesion and protective function of epoxy coating. Prog. Org. Coat. 2022, 172, 107124. [Google Scholar]
- Kabaoglu, E.; Karabork, F.; Kayan, D.B.; Akdemir, A. Improvement of anti-corrosion performance (surface and near the cut edge) and mechanical properties of epoxy coatings modified with nano, micro and hybrid ZnO particles. J. Compos. Mater. 2023, 57, 451–463. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Ly, L.T.N.; Nguyen, T.X.; Dao, H.P.; Nguyen, S.A.; Tran, T.H.; Vu, T.Q.; Ngo, Q.T.C.; Nguyen, T.N.; Thai, H. The effect of organotitanate-modified zinc oxide nanoparticles on some characteristics and anticorrosion protection properties of epoxy coating. J. Coat. Technol. Res. 2024, 22, 255–268. [Google Scholar] [CrossRef]
- Jia, Z.; Hong, R. Anticorrosive and photocatalytic properties research of epoxy-silica organic–inorganic coating. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126647. [Google Scholar]
- Dagdag, O.; Guo, L.; Safi, Z.; Verma, C.; Ebenso, E.E.; Wazzan, N.; Masroor, S.; Haldhar, R.; Jodeh, S.; El Gouri, M. Epoxy resin and TiO2 composite as anticorrosive material for carbon steel in 3% NaCl medium: Experimental and computational studies. J. Mol. Liq. 2020, 317, 114249. [Google Scholar] [CrossRef]
- Fadl, A.M.; Abdou, M.I.; Hamza, M.A.; Sadeek, S.A. Corrosion-inhibiting, self-healing, mechanical-resistant, chemically and UV stable PDMAS/TiO2 epoxy hybrid nanocomposite coating for steel petroleum tanker trucks. Prog. Org. Coat. 2020, 146, 105715. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Chen, F.; He, X.; Ma, Y.; Zhang, L.; Sheng, X.; Chen, Y.; Shchukina, E.; Shchukin, D. Reinforced anticorrosion performance of waterborne epoxy coating with eco-friendly L-cysteine modified Ti3C2Tx MXene nanosheets. Prog. Org. Coat. 2021, 161, 106478. [Google Scholar] [CrossRef]
- Yan, H.; Cai, M.; Wang, J.; Zhang, L.; Li, H.; Li, W.; Fan, X.; Zhu, M. Insight into anticorrosion/antiwear behavior of inorganic-organic multilayer protection system composed of nitriding layer and epoxy coating with Ti3C2Tx MXene. Appl. Surf. Sci. 2021, 536, 147974. [Google Scholar] [CrossRef]
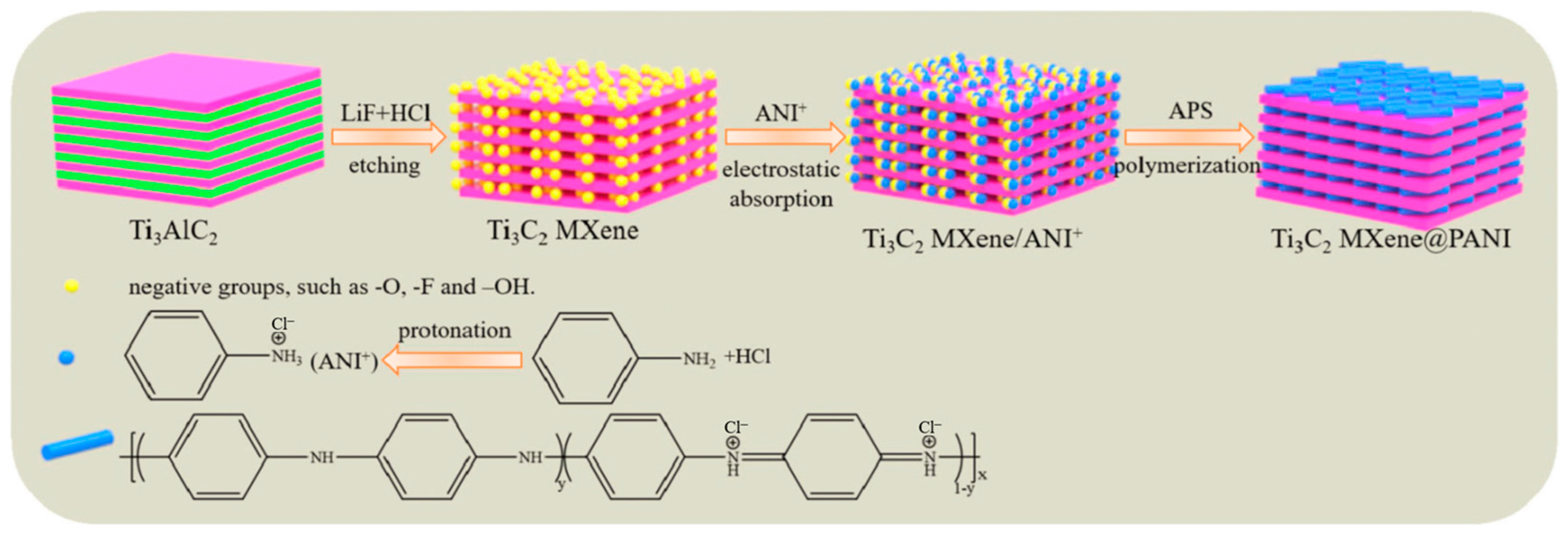
- Li, C.; Xu, J.; Xu, Q.; Xue, G.; Yu, H.; Wang, X.; Lu, J.; Cui, G.; Gu, G. Synthesis of Ti3C2 MXene@PANI composites for excellent anticorrosion performance of waterborne epoxy coating. Prog. Org. Coat. 2022, 165, 106673. [Google Scholar] [CrossRef]
- Kim, B.G.; Choi, H.-H.; Park, H.-Y.; Kwon, M.S.; Byeun, Y.-K.; Kang, S.; Jung, Y.-G.; Son, J.-H.; Yang, S. Preparation and characterization of organic–inorganic hybrid coatings for improving the insulation properties of electrical steel. J. Coat. Technol. Res. 2023, 20, 1383–1393. [Google Scholar] [CrossRef]
- Mastalska-Popławska, J.; Kadac, K.; Izak, P.; Gierej, M.; Stempkowska, A.; Góral, Z. The influence of ceramic additives on intumescence and thermal activity of epoxy coatings for steel. J. Appl. Polym. Sci. 2021, 138, 49914. [Google Scholar] [CrossRef]
- He, X.; Cui, C.; Chen, Y.; Zhang, L.; Sheng, X.; Xie, D. MXene and polymer collision: Sparking the future of high-performance multifunctional coatings. Adv. Funct. Mater. 2024, 34, 2409675. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, C.; Zhang, Z.; Li, L.; Fan, X.; Zhu, M. Hierarchical design of microcapsules-based epoxy resin coating enhanced with Ti3C2Tx for improving thermal, tribological, anti-corrosive performance. Carbon 2024, 228, 119379. [Google Scholar] [CrossRef]
- Pessoa, M.O.; Freitas, B.R.; de Oliveira Braga, J.; Santos, S.L.B.S.; da Silva, B.P.; Capelossi, V.R.; Cotting, F. Anticorrosion and adhesion performance of a monolayer and double layer silane-epoxy coating systems applied on carbon steel. Surf. Coat. Technol. 2024, 485, 130909. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, M.; Pei, X.; Liu, S.; Luo, S.; Yan, M.; Shao, R.; Sun, Y.; Xu, W.; Xu, Z. Improving corrosion resistance of epoxy coating by optimizing the stress distribution and dispersion of SiO2 filler. Prog. Org. Coat. 2023, 179, 107522. [Google Scholar]
- Fernández-Álvarez, M.; Velasco, F.; de la Fuente, D.; Bautista, A. Powder organic coatings functionalized with calcium ion-exchanged silica corrosion inhibitors. Surf. Coat. Technol. 2024, 477, 130377. [Google Scholar]
- Atta, A.M.; Ezzat, A.O.; El-Saeed, A.M.; Tawfeek, A.M.; Sabeela, N.I. Self-healing of chemically bonded hybrid silica/epoxy for steel coating. Prog. Org. Coat. 2020, 141, 105549. [Google Scholar]
- Li, X.; Liu, Z.; Hong, P.; Chen, L.; Liu, X. Synthesis of organic and inorganic hybrid nanoparticles as multifunctional photoinitiator and its application in UV-curable epoxy acrylate-based coating systems. Prog. Org. Coat. 2020, 141, 105565. [Google Scholar]
- Ma, I.W.; Sh, A.; Bashir, S.; Kumar, S.S.; Ramesh, K.; Ramesh, S. Development of active barrier effect of hybrid chitosan/silica composite epoxy-based coating on mild steel surface. Surf. Interfaces 2021, 25, 101250. [Google Scholar]
- Yang, W.; Feng, W.; Liao, Z.; Yang, Y.; Miao, G.; Yu, B.; Pei, X. Protection of mild steel with molecular engineered epoxy nanocomposite coatings containing corrosion inhibitor functionalized nanoparticles. Surf. Coat. Technol. 2021, 406, 126639. [Google Scholar]
- Geng, Y.; Liu, Y.; Liu, A.; Li, S.; Zhang, H. Improved interfacial interactions and corrosion resistance of epoxy coated reinforcement by pre-electrodeposited silane layer. Prog. Org. Coat. 2022, 173, 107171. [Google Scholar]
- Zhu, H.; Liu, J.; Lu, X.; Wang, D.; Geng, T.; Feng, L.; Liang, D.; Ma, X.; Hu, Z. Wettability and anticorrosion behavior of organic-inorganic hybrid superhydrophobic epoxy coatings containing triazine corrosion inhibitor loaded in mesoporous molecular sieve. J. Taiwan Inst. Chem. Eng. 2022, 141, 104604. [Google Scholar]
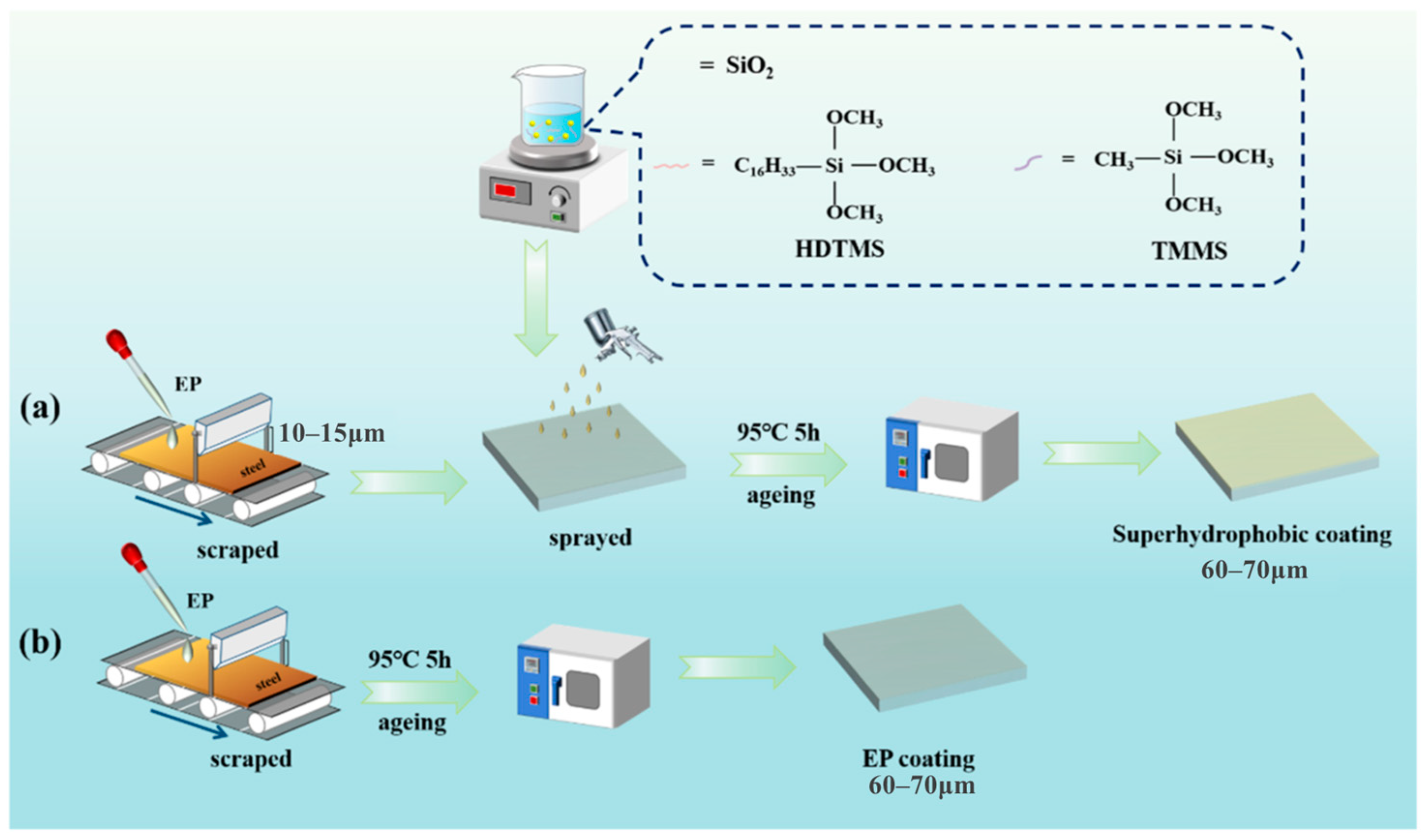
- Zeng, Q.; Kang, L.; Fan, J.; Song, L.; Wan, S.; Liao, B.; Guo, X. Durable superhydrophobic silica/epoxy resin coating for the enhanced corrosion protection of steel substrates in high salt and H2S environments. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130137. [Google Scholar]
- El-Lateef, H.M.A.; Khalaf, M.M. Fabrication and characterization of alumina-silica/poly(o-toluidine) nanocomposites as novel anticorrosive epoxy coatings films on carbon steel. Microchem. J. 2020, 158, 105129. [Google Scholar]
- Rêgo, G.C.; Bandeira, R.M.; van Drunen, J.; Tremiliosi-Filho, G.; Casteletti, L.C. Multi-layer organic-inorganic hybrid anticorrosive coatings for protection of medium carbon steel. Mater. Chem. Phys. 2023, 303, 127841. [Google Scholar] [CrossRef]
- Zin, I.M.; DatskoKhlopyk, B.M.O.P.; Sobodosh, N.Y.; Korniy, S.A. Effect of zeolite-phosphate anti-corrosion pigment on protective properties of epoxy coating on carbon steel. Mater. Sci. 2024, 59, 658–665. [Google Scholar] [CrossRef]
- Xiong, H.; Qi, F.; Zhao, N.; Yuan, H.; Wan, P.; Liao, B.; Ouyang, X. Effect of organically modified sepiolite as inorganic nanofiller on the anti-corrosion resistance of epoxy coating. Mater. Lett. 2020, 260, 126941. [Google Scholar] [CrossRef]
- Nikafshar, S.; McCracken, J.; Dunne, K.; Nejad, M. Improving UV-Stability of epoxy coating using encapsulated halloysite nanotubes with organic UV-Stabilizers and lignin. Prog. Org. Coat. 2021, 151, 105843. [Google Scholar] [CrossRef]
- Xavier, J.R.; Raja Beryl, J.; Ravisankar, N. A study on the influence of silanized clay on the barrier, hydrophobic and mechanical properties of epoxy coated steel in natural seawater. J. Adhes. 2023, 99, 1889–1915. [Google Scholar] [CrossRef]
- Atta, A.M.; Ezzat, A.O.; El-Saeed, A.M.; Wahby, M.H.; Abdallah, M.M.S. Superhydrophobic organic and inorganic clay nanocomposites for epoxy steel coatings. Prog. Org. Coat. 2020, 140, 105502. [Google Scholar] [CrossRef]
- Beryl, J.R.; Xavier, J.R. Electrochemical and mechanical studies of epoxy coatings containing eco-Friendly nanocomposite consisting of silane functionalized clay-epoxy on mild steel. J. Bio- Tribo-Corros. 2020, 6, 126. [Google Scholar] [CrossRef]
- Jing, Y.; Meng, F.; Wang, F.; Liu, L. Design of an anticorrosion/bactericidal dual functional organic coating based on the slippery liquid-infused porous surface. Appl. Surf. Sci. 2023, 639, 158214. [Google Scholar]
- Fan, W.; Wang, H.; Wang, C.; Liu, Z.; Zhu, Y.; Li, K. Epoxy coating capable of providing multi-component passive film for long-term anti-corrosion of steel. Appl. Surf. Sci. 2020, 521, 146417. [Google Scholar] [CrossRef]
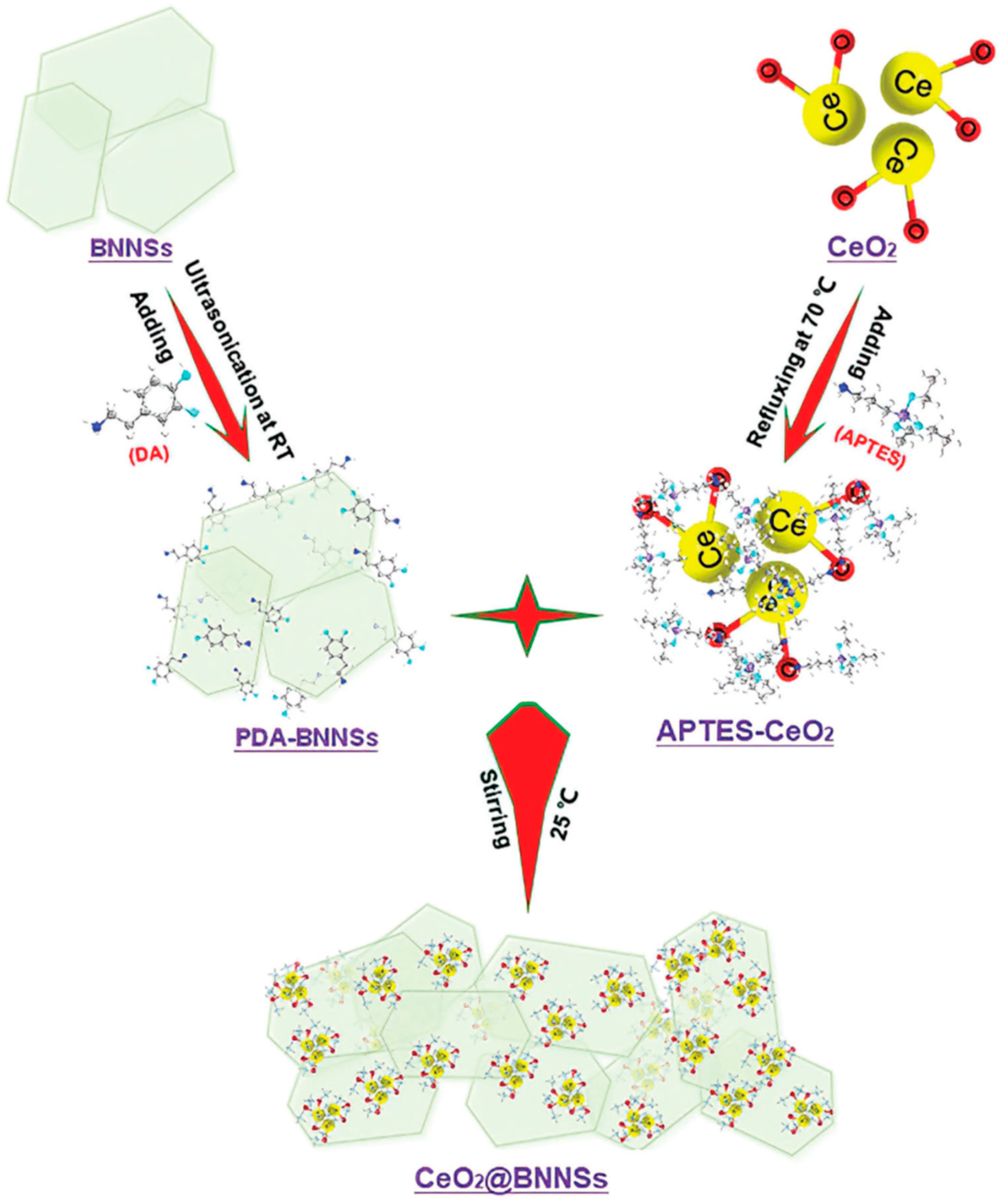
- Duan, H.; Ji, J.; Cao, C.; Bai, W.; Song, Z.; Xue, Y.; Tang, C. Enhanced anti-corrosion performance of carbon steels via CeO2@BNNSs/epoxy resin composite coatings. Macromol. Chem. Phys. 2023, 224, 2300006. [Google Scholar] [CrossRef]
- Wan, P.; Zhao, N.; Qi, F.; Zhang, B.; Xiong, H.; Yuan, H.; Liao, B.; Ouyang, X. Synthesis of PDA-BN@f-Al2O3 hybrid for nanocomposite epoxy coating with superior corrosion protective properties. Prog. Org. Coat. 2020, 146, 105713. [Google Scholar]
- Saber, D.; Alghtani, A.H.; Ahmed, E.M.; Felemban, B.F.; Ali, H.T.; Megahed, M.; El-Aziz, K.A. Enhancement of barrier and mechanical performance of steel coated with epoxy filled with micron and nano alumina fillers. Mater. Res. 2022, 25, e20210413. [Google Scholar]
- Feng, Z.; Huang, J.; Guo, H.; Zhang, X.; Li, Y.; Fang, B.; Li, Y.; Song, G.L.; Liu, J. A magnetic “Band-Aid” incorporated with Fe3O4 NPs modified epoxy binder for in-situ repair of organic coating under seawater. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132317. [Google Scholar]
- Peng, G.; Qiao, Q.; Huang, K.; Wu, J.; Wang, Y.; Fu, X.; Zhang, Z.; Fang, T.; Zhang, B.; Huang, Y.; et al. Ni-Fe-MoO42− LDHs/epoxy resin varnish: A composite coating on carbon steel for long-time and active corrosion protection. Prog. Org. Coat. 2020, 140, 105514. [Google Scholar]
- Xavier, J.R. Investigation on the anticorrosion, adhesion and mechanical performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-MoO3 on mild steel. J. Adhes. Sci. Technol. 2020, 34, 115–134. [Google Scholar]
- Asemabadi, Z.; Behzadnasab, M.; Mohammadloo, H.E.; Ramezanpour, J.; Zargarian, S. Investigating the role of silane treatment in enhancing the anti-corrosion properties of MoS2 particle-embedded epoxy coatings on steel. Surf. Coat. Technol. 2024, 477, 130348. [Google Scholar]
- Zhou, S.; Yan, J.; Yan, H.; Zhang, Y.; Huang, J.; Zhao, G.; Liu, Y. ZrO2-anchored rGO nanohybrid for simultaneously enhancing the wear resistance and anticorrosion performance of multifunctional epoxy coatings. Prog. Org. Coat. 2022, 166, 106795. [Google Scholar]
- Xavier, J.R. Improvement in Corrosion Resistance and Mechanical properties of epoxy coatings on steel with the addition of thiadiazole treated ZrC. J. Mater. Eng. Perform. 2023, 32, 3980–3994. [Google Scholar]
- Bahremand, F.; Shahrabi, T.; Ramezanzadeh, B. Epoxy coating anti-corrosion properties enhancement via the steel surface treatment by nanostructured samarium oxide-poly-dopamine film. J. Hazard. Mater. 2021, 403, 123722. [Google Scholar]
- Dagdag, O.; El Harfi, A.; Safi, Z.; Guo, L.; Verma, C.; Ebenso, E.E.; Wazzan, N.; El Gouri, M. Fabrication on designing of a macromolecular epoxy resin as anti-corrosive coating material for electrocatalytically deposited cadmium on 15CDV6 steel in 3% NaCl solution. J. Mater. Res. Technol. 2020, 9, 5549–5563. [Google Scholar]
- Sun, M.; Ma, Z.; Li, A.; Zhu, G.; Zhang, Y. Anticorrosive performance of polyaniline/waterborne epoxy/poly(methylhydrosiloxane) composite coatings. Prog. Org. Coat. 2020, 139, 105462. [Google Scholar]
- Hao, Y.; Sun, W.; Jiang, L.; Cui, J.; Zhang, Y.; Song, L.; Zhang, Y. Self-healing effect of epoxy coating containing mesoporous polyaniline hollow spheres loaded with benzotriazole. Prog. Org. Coat. 2021, 159, 106445. [Google Scholar]
- Hu, C.; Li, T.; Yin, H.; Hu, L.; Tang, J.; Ren, K. Preparation and corrosion protection of three different acids doped polyaniline/epoxy resin composite coatings on carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126069. [Google Scholar]
- Yao, Y.; Sun, H.; Zhang, Y.; Yin, Z. Corrosion protection of epoxy coatings containing 2-hydroxyphosphonocarboxylic acid doped polyaniline nanofibers. Prog. Org. Coat. 2020, 139, 105470. [Google Scholar]
- Liu, T.; Wei, J.; Ma, L.; Liu, S.; Zhang, D.; Zhao, H. Effect of polyaniline-based plate on the anticorrosion performance of epoxy coating. Prog. Org. Coat. 2021, 151, 106109. [Google Scholar]
- Diraki, A.; Omanovic, S. Smart PANI/epoxy anti-corrosive coating for protection of carbon steel in sea water. Prog. Org. Coat. 2022, 168, 106835. [Google Scholar]
- Sang, Y.; Liu, Q.; Wang, S.; Dong, S.; Fan, Y.; Zhao, X.; Li, S. Synthetic polyaniline-boron nitride-aqueous epoxy resin composite coating for improving the corrosion resistance of hot-dip galvanized steel plates. Appl. Surf. Sci. 2022, 592, 153229. [Google Scholar]
- Liu, T.; Zhao, Y.; Deng, Y.; Ge, H. Preparation of fully epoxy resin microcapsules and their application in self-healing epoxy anti-corrosion coatings. Prog. Org. Coat. 2024, 188, 108247. [Google Scholar]
- Bi, Y.; Li, J.; Zhuang, J.; Zhang, P.; Yang, J.; Liao, B.; Wang, Q.; Li, W. Unraveling the detrimental effect of polymeric microcapsules on the corrosion performance of epoxy resin coatings. Prog. Org. Coat. 2024, 194, 108620. [Google Scholar]
- Dagdag, O.; Hsissou, R.; El Harfi, A.; Berisha, A.; Safi, Z.; Verma, C.; Ebenso, E.E.; Touhami, M.E.; El Gouri, M. Fabrication of polymer based epoxy resin as effective anti-corrosive coating for steel: Computational modeling reinforced experimental studies. Surf. Interfaces 2020, 18, 100454. [Google Scholar]
- Liu, T.; Liu, Y.; Qu, D.; Chen, S.; Chen, W.; Han, L.; Qiu, Z.; Zhu, L.; Chen, M. Smart self-healing coating based on the covalent organic frameworks (COF LZU-1) for corrosion protection of steel. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133246. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, H.; Li, J.; Zhang, D.; Zheng, W.; Wang, L. POSS-tetraaniline based giant molecule: Synthesis, self-assembly, and active corrosion protection of epoxy-based organic coatings. Corros. Sci. 2020, 168, 108555. [Google Scholar] [CrossRef]
- Atta, A.M.; Azzam, E.M.S.; Alenezi, K.M.; El Moll, H.; Mechi, L.; El-Sofany, W.I. New epoxy and hardener system based on an imidazolium ionic liquid as an anticorrosive coating for steel in the marine environment. ACS Omega 2023, 8, 16315–16326. [Google Scholar] [CrossRef]
- Xia, Y.; Tong, L.; Feng, X.; Zhang, S.; Xiang, H.; He, Y.; Liu, X. An investigation on the mechanical and corrosion protection properties of poly(arylene ether nitrile) reinforced epoxy coating. Prog. Org. Coat. 2024, 192, 108463. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Echihi, S.; Benzidia, B.; Cherrouf, S.; Haldhar, R.; Alvi, P.A.; Kaya, S.; Serdaroğlu, G.; Zarrouk, A. Performance of curing epoxy resin as potential anticorrosive coating for carbon steel in 3.5% NaCl medium: Combining experimental and computational approaches. Chem. Phys. Lett. 2021, 783, 139081. [Google Scholar] [CrossRef]
- Attaei, M.; Calado, L.M.; Taryba, M.G.; Morozov, Y.; Shakoor, R.A.; Kahraman, R.; Marques, A.C.; Montemor, M.F. Autonomous self-healing in epoxy coatings provided by high efficiency isophorone diisocyanate (IPDI) microcapsules for protection of carbon steel. Prog. Org. Coat. 2020, 139, 105445. [Google Scholar] [CrossRef]
- Attaei, M.; Calado, L.M.; Morozov, Y.; Taryba, M.G.; Shakoor, R.A.; Kahraman, R.; Marques, A.C.; Montemor, M.F. Smart epoxy coating modified with isophorone diisocyanate microcapsules and cerium organophosphate for multilevel corrosion protection of carbon steel. Prog. Org. Coat. 2020, 147, 105864. [Google Scholar] [CrossRef]
- Robert, T.; Eschig, S.; Sangermano, M.; Ocepek, M. Biobased aromatic building blocks for coating applications. Curr. Opin. Green Sustain. Chem. 2024, 49, 100962. [Google Scholar] [CrossRef]
- Sreehari, H.; Sethulekshmi, A.S.; Saritha, A. Bio epoxy coatings: An emergent green anticorrosive coating for the future. Macromol. Mater. Eng. 2022, 307, 2200004. [Google Scholar] [CrossRef]
- Ammar, S.; Iling, A.W.M.; Ramesh, K.; Ramesh, S. Development of fully organic coating system modified with epoxidized soybean oil with superior corrosion protection performance. Prog. Org. Coat. 2020, 140, 105523. [Google Scholar] [CrossRef]
- Li, J.; Shi, H.; Liu, F.; Han, E.-H. Self-healing epoxy coating based on tung oil-containing microcapsules for corrosion protection. Prog. Org. Coat. 2021, 156, 106236. [Google Scholar]
- Veedu, K.K.; Mohan, S.; Somappa, S.B.; Gopalan, N.K. Eco-friendly anticorrosive epoxy coating from Ixora leaf extract: A promising solution for steel protection in marine environment. J. Clean. Prod. 2022, 340, 130750. [Google Scholar] [CrossRef]
- Veedu, K.K.; Banyangala, M.; Kalarikkal, T.P.; Somappa, S.B.; Gopalan, N.K. Green approach in anticorrosive coating for steel protection by Gliricidia sepium leaf extract and silica hybrid. J. Mol. Liq. 2023, 369, 120967. [Google Scholar]
- Qi, C.; Li, Z.; Bi, H.; Dam-Johansen, K. Towards sustainable steel corrosion protection: Expanding the applicability of natural hydrolyzable tannin in epoxy coatings via metal complexation. Electrochim. Acta 2024, 497, 144546. [Google Scholar] [CrossRef]
- Teijido, R.; Monteserin, C.; Blanco, M.; Odriozola, J.L.L.; Olabarria, M.I.M.; Vilas-Vilela, J.L.; Lanceros-Méndez, S.; Zhang, Q.; Ruiz-Rubio, L. Exploring anti-corrosion properties and rheological behaviour of tannic acid and epoxidized soybean oil-based fully bio-based epoxy thermoset resins. Prog. Org. Coat. 2024, 196, 108719. [Google Scholar]
- Azani, N.F.S.M.; Hussin, M.H. Comparison of cellulose nanocrystal (CNC) filler on chemical, mechanical, and corrosion properties of epoxy-Zn protective coatings for mild steel in 3.5% NaCl solution. Cellulose 2021, 28, 6523–6543. [Google Scholar]
- Diógenes, O.B.F.; de Oliveira, D.R.; da Silva, L.R.R.; Linhares, B.G.; Mazzetto, S.E.; Lomonaco, D.; Araujo, W.S. Acetylated lignin as a biocomponent for epoxy coating—Anticorrosive performance analysis by accelerated corrosion tests. Surf. Coat. Technol. 2023, 474, 130116. [Google Scholar]
- Nunes, M.S.; Bandeira, R.M.; Figueiredo, F.C.; Junior, J.R.D.S.; de Matos, J.M.E. Corrosion protection of stainless steel by a new and low-cost organic coating obtained from cashew nutshell liquid. J. Appl. Polym. Sci. 2022, 140, e53420. [Google Scholar]
- da Silva, L.R.R.; Avelino, F.; Diogenes, O.B.F.; Sales, V.D.O.F.; da Silva, K.T.; Araujo, W.S.; Mazzetto, S.E.; Lomonaco, D. Development of BPA-free anticorrosive epoxy coatings from agroindustrial waste. Prog. Org. Coat. 2020, 139, 105449. [Google Scholar] [CrossRef]
- Abbout, S.; Hsissou, R.; Chebabe, D.; Erramli, H.; Hajjaji, N. Investigation of the anti-corrosion properties of Galactomannan as additive in epoxy coatings for carbon steel: Rheological and electrochemical study. Inorg. Chem. Commun. 2021, 134, 108971. [Google Scholar]
- Xiao, X.; Ye, Z.; Meng, G.; Gu, L. Mussel-inspired preparation of superhydrophobic mica nanosheets for long-term anticorrosion and self-healing performance of epoxy coatings. Prog. Org. Coat. 2023, 178, 107456. [Google Scholar]
- Balakrishnan, A.; Govindaraj, S.; Dhaipule, N.G.K.; Thirumalaisamy, N.; Anne, R.S.; Sublime, N.; Philip, J. Enhancing microbiologically influenced corrosion protection of carbon steels with silanized epoxy-biocide hybrid coatings. Environ. Sci. Pollut. Res. 2024, 31, 13302–13326. [Google Scholar]
- Zhou, Y.; Chen, G.; Yan, S.; Ni, C.; Yu, L.; Li, X. Epoxy composite coating with excellent anticorrosion and self-healing properties based on acrylate copolymers. Prog. Org. Coat. 2022, 172, 107098. [Google Scholar] [CrossRef]
- Chen, P.; Wang, G.; Li, J.; Zhang, M.; Qiao, X. Preparation of textured epoxy resin coatings for excellent hydrophobicity and corrosion resistance. Prog. Org. Coat. 2023, 175, 107312. [Google Scholar]
- Mallakpour, S.; Nikkhoo, E.; Hussain, C.M. Application of MOF materials as drug delivery systems for cancer therapy and dermal treatment. Coord. Chem. Rev. 2022, 451, 214262. [Google Scholar]
- Ma, S.; Han, W.; Han, W.; Dong, F.; Tang, Z. Recent advances and future perspectives in MOF-derived single-atom catalysts and their application: A review. J. Mater. Chem. A 2023, 11, 3315–3363. [Google Scholar]
- Liu, K.G.; Bigdeli, F.; Panjehpour, A.; Jhung, S.H.; Al Lawati, H.A.J.; Morsali, A. Potential applications of MOF composites as selective membranes for separation of gases. Coord. Chem. Rev. 2023, 496, 215413. [Google Scholar]
- Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. Development of metal-organic framework (MOF) decorated graphene oxide nanoplatforms for anti-corrosion epoxy coatings. Carbon 2020, 161, 231–251. [Google Scholar]
- Zhang, Y.; Tian, H.; Sui, X.; Wang, X.; Zhou, F.; Zhang, X. The Improved antiwear and anticorrosion properties of epoxy resin with metal–organic framework ZIF-8 containing lubrication oil. Langmuir 2022, 38, 10649–10661. [Google Scholar]
- He, Z.; Lin, H.; Zhang, X.; Chen, Y.; Bai, W.; Lin, Y.; Jian, R.; Xu, Y. Self-healing epoxy composite coating based on polypyrrole@MOF nanoparticles for the long-efficiency corrosion protection on steels. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130601. [Google Scholar]
- Wan, S.; Chen, H.; Ma, X.; Chen, L.; Lei, K.; Liao, B.; Dong, Z.; Guo, X. Anticorrosive reinforcement of waterborne epoxy coating on Q235 steel using NZ/BNNS nanocomposites. Prog. Org. Coat. 2021, 159, 106410. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; SaadatAbadi, A.R. ZIF-67 metal-organic framework decorated g-C3N4 nanosheets with inbuilt organic-inorganic corrosion inhibitor for self-healing epoxy anti-corrosion coating. Surf. Coat. Technol. 2024, 492, 131218. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Abadchi, M.R.; Mirzaee, M.; Tabar, F.A.; Ramezanzadeh, B. Recent advances and future perspectives for carbon nanostructures reinforced organic coating for anti-corrosion application. Surf. Interfaces 2021, 23, 100994. [Google Scholar]
- Hsia, H.-H.; Chen, Y.-L.; Tai, Y.-T.; Tian, H.-K.; Kung, C.-W.; Liu, W.R. Two-dimensional metal–organic frameworks/epoxy composite coatings with superior O2/H2O resistance for anticorrosion applications. ACS Appl. Mater. Interfaces 2024, 16, 41421–41434. [Google Scholar] [CrossRef] [PubMed]
- Alipanah, N.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G. MIL-88A (Fe) filler with duplicate corrosion inhibitive/barrier effect for epoxy coatings: Electrochemical, molecular simulation, and cathodic delamination studies. J. Ind. Eng. Chem. 2021, 97, 200–215. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Chen, Y.; Tian, X.; Liu, L.; Wang, Y.; Guo, R.; Yan, H. Dual-inhibitor composite BTA/PPy/MIL-88(Fe) for active anticorrosion of epoxy resin coatings. J. Ind. Eng. Chem. 2023, 119, 660–673. [Google Scholar]
- Keshmiri, N.; Najmi, P.; Ramezanzadeh, M.; Ramezanzadeh, B. Designing an eco-friendly lanthanide-based metal organic framework (MOF) assembled graphene-oxide with superior active anti-corrosion performance in epoxy composite. J. Clean. Prod. 2021, 319, 128732. [Google Scholar]
- Wei, R.; Liu, Z.; Wei, W.; Wang, S.; Lv, Y.-J.; Han, G.-C. Anticorrosion performance of hydrophobic acid-modified-MOFs/epoxy coatings. Colloid Interface Sci. Commun. 2022, 46, 100580. [Google Scholar]
- Li, K.; Yu, Z.; Tang, J.; Guo, S.; Peng, B.; Yu, P.; Lin, B. +Improving the corrosion protection properties of epoxy resin coating by using HKUST-1-BTA@e-Mica two-dimensional nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134869. [Google Scholar] [CrossRef]
- Fadl, A.M.; Sadeek, S.A.; Magdy, L.; Abdou, M.I.; El-Shiwiniy, W.H. Multi-functional epoxy composite coating incorporating mixed Cu(II) and Zr(IV) complexes of metformin and 2,2\-bipyridine as intensive network cross-linkers exhibiting anti-corrosion, self-healing and chemical-resistance performances for steel petroleum platforms. Arab. J. Chem. 2021, 14, 103367. [Google Scholar]
- Butt, A.M.; Wang, Y.; Ma, H.; Li, H. The preparation of cerium nitrate and attapulgite based superhydrophobic epoxy coatings for the corrosion protection of Q355 mild steel surface. Surf. Coat. Technol. 2023, 473, 129977. [Google Scholar]
- Chopra, I.; Ola, S.K.; Gopalakrishnan, S.; Dhayal, V. Tailoring epoxy coating with acetoxime derivative of zinc for advanced anticorrosive performance on mild steel: Experimental and computational insights. J. Mol. Model. 2023, 29, 300. [Google Scholar]
- Peng, Y.; Hughes, A.E.; Mardel, J.I.; Deacon, G.B.; Junk, P.C.; Catubig, R.A.; Forsyth, M.; Hinton, B.R.W.; Somers, A.E. Dual function of rare earth carboxylate compounds on the barrier properties and active corrosion inhibition of epoxy coatings on mild steel. Prog. Org. Coat. 2023, 185, 107870. [Google Scholar]
- Zhang, Q.; Shen, M.; Liu, X.; Fu, H.; Yang, W.; Zhao, J.; Wang, J.; Du, Y.; Ma, C. Construction and utilization of rare earth complexes as efficient corrosion inhibitor in epoxy coating. Prog. Org. Coat. 2024, 186, 108024. [Google Scholar]
- Ren, P.; Li, J.; Wang, L.; Guo, H.; Lei, B.; Feng, Z.; Meng, G. Organo-cerium as a quick repair agent for coating damage on carbon steel. J. Mater. Eng. Perform. 2023, 32, 9755–9764. [Google Scholar]
- Madhusudhana, A.M.; Mohana, K.N.S.; Hegde, M.B.; Nayak, S.R.; Rajitha, K.; Swamy, N.K. Functionalized graphene oxide-epoxy phenolic novolac nanocomposite: An efficient anticorrosion coating on mild steel in saline medium. Adv. Compos. Hybrid Mater. 2020, 3, 141–155. [Google Scholar]
- Zhang, C.; Li, W.; Liu, C.; Zhang, C.; Cao, L.; Kong, D.; Wang, W.; Chen, S. Effect of covalent organic framework modified graphene oxide on anticorrosion and self-healing properties of epoxy resin coatings. J. Colloid Interface Sci. 2022, 608, 1025–1039. [Google Scholar]
- Mourya, P.; Goswami, R.N.; Saini, R.; Ray, A.; Khatri, O.P. Epoxy coating reinforced with graphene-PANI nanocomposites for enhancement of corrosion-resistance performance of mild steel in saline water. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133500. [Google Scholar]
- Yan, H.; Zhang, L.; Li, H.; Fan, X.; Zhu, M. Towards high-performance additive of Ti3C2/graphene hybrid with a novel wrapping structure in epoxy coating. Carbon 2020, 157, 217–233. [Google Scholar]
- Zhang, J.; Kong, G.; Li, S.; Le, Y.; Che, C.; Zhang, S.; Lai, D.; Liao, X. Graphene-reinforced epoxy powder coating to achieve high performance wear and corrosion resistance. J. Mater. Res. Technol. 2022, 20, 4148–4160. [Google Scholar]
- Ye, Y.; Chen, H.; Zou, Y.; Ye, Y.; Zhao, H. Corrosion protective mechanism of smart graphene-based self-healing coating on carbon steel. Corros. Sci. 2020, 174, 108825. [Google Scholar]
- Xavier, J.R. Novel multilayer structural epoxy composite coating containing graphene oxide and silanized chromium carbide for the protection of steel structures. J. Coat. Technol. Res. 2022, 19, 1713–1730. [Google Scholar]
- Dhongde, N.R.; Baranwal, P.K.; Rajaraman, P.V. Functionalization of graphene oxide with an ionic liquid (1-butyl-3-methylimidazolium acetate): Preparation of epoxy-based coating on carbon steel for anticorrosive applications. J. Appl. Polym. Sci. 2023, 140, e54026. [Google Scholar]
- Vinodhini, S.P.; Xavier, J.R. Evaluation of corrosion protection performance and mechanical properties of epoxy-triazole/graphene oxide nanocomposite coatings on mild steel. J. Mater. Sci. 2021, 56, 7094–7110. [Google Scholar]
- Zhou, X.; Huang, H.; Zhu, R.; Chen, R.; Sheng, X.; Xie, D.; Mei, Y. Green modification of graphene oxide with phytic acid and its application in anticorrosive water-borne epoxy coatings. Prog. Org. Coat. 2020, 143, 105601. [Google Scholar]
- Zhu, Q.; Li, E.; Liu, X.; Song, W.; Li, Y.; Wang, X.; Liu, C. Epoxy coating with in-situ synthesis of polypyrrole functionalized graphene oxide for enhanced anticorrosive performance. Prog. Org. Coat. 2020, 140, 105488. [Google Scholar]
- Xiang, Q.; Qiang, Y.; Guo, L. Designing a novel GO@AAP reinforced epoxy coating for achieving the long-term corrosion protection of steel substrate. Prog. Org. Coat. 2023, 174, 107293. [Google Scholar]
- Li, X.; Chen, X.; Chen, J.; Huo, D.; Gao, X.; Dong, J.; Yin, Y.; Liu, J.; Nan, D. Development of an epoxy resin-based anticorrosive coating enhanced by monolayer amino-modified graphene oxide. Prog. Org. Coat. 2024, 194, 108624. [Google Scholar]
- Diraki, A.; Omanovic, S. Anticorrosive properties of the double-layer PANI-(graphene oxide)/epoxy coating in protecting carbon steel in saltwater. J. Coat. Technol. Res. 2023, 20, 995–1006. [Google Scholar]
- Kooshksara, M.M.; Mohammadi, S. Investigation of the in-situ solvothermal reduction of multi-layered Graphene oxide in epoxy coating by acetonitrile on improving the hydrophobicity and corrosion resistance. Prog. Org. Coat. 2021, 159, 106432. [Google Scholar]
- Xiong, L.; Yu, M.; Li, Y.; Kong, X.; Li, S.; Liu, J. Modified salicylaldehyde@ZIF-8/graphene oxide for enhancing epoxy coating corrosion protection property on AA2024-T3. Prog. Org. Coat. 2020, 142, 105562. [Google Scholar]
- Chen, C.; He, Y.; Xiao, G.; Zhong, F.; Xia, Y.; Wu, Y. Graphic C3N4-assisted dispersion of graphene to improve the corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2020, 139, 105448. [Google Scholar]
- Xavier, J.R.; Bhaskar, R.; Subramanian, S. Multifunctional graphitic carbon nitride/manganese dioxide/epoxy nanocomposite coating on steel for enhanced anticorrosion, flame retardant, mechanical, and hydrophobic properties. J. Ind. Eng. Chem. 2024, 134, 514–536. [Google Scholar]
- Souto, L.F.C.; Soares, B.G. Polyaniline/carbon nanotube hybrids modified with ionic liquids as anticorrosive additive in epoxy coatings. Prog. Org. Coat. 2020, 143, 105598. [Google Scholar]
- Nayak, S.R.; Mohana, K.N.S.; Hegde, M.B.; Rajitha, K.; Madhusudhana, A.M.; Naik, S.R. Functionalized multi-walled carbon nanotube/polyindole incorporated epoxy: An effective anti-corrosion coating material for mild steel. J. Alloys Compd. 2021, 856, 158057. [Google Scholar]
- Wan, S.; Chen, H.; Cai, G.; Liao, B.; Guo, X. Functionalization of h-BN by the exfoliation and modification of carbon dots for enhancing corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2022, 165, 106757. [Google Scholar]

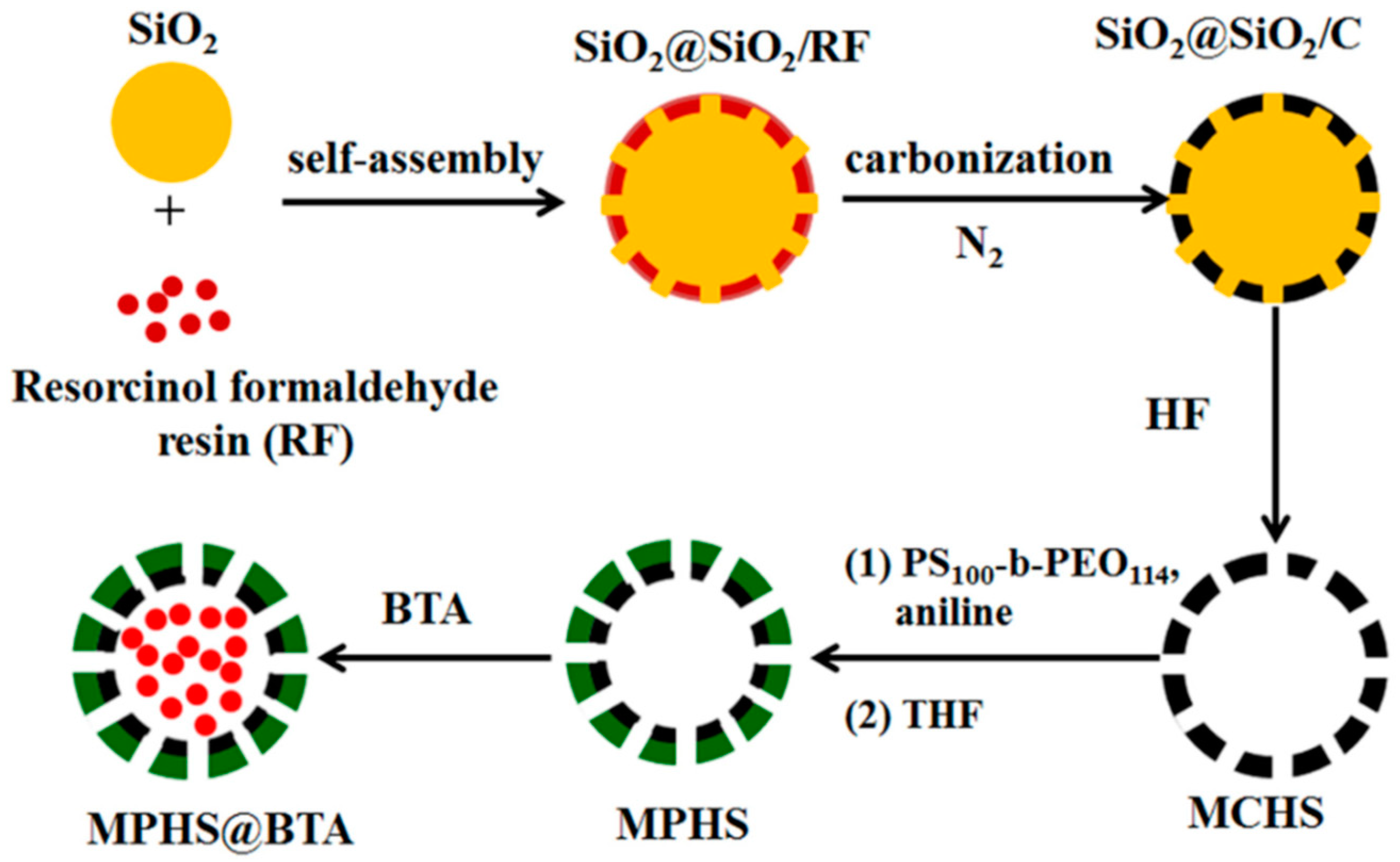
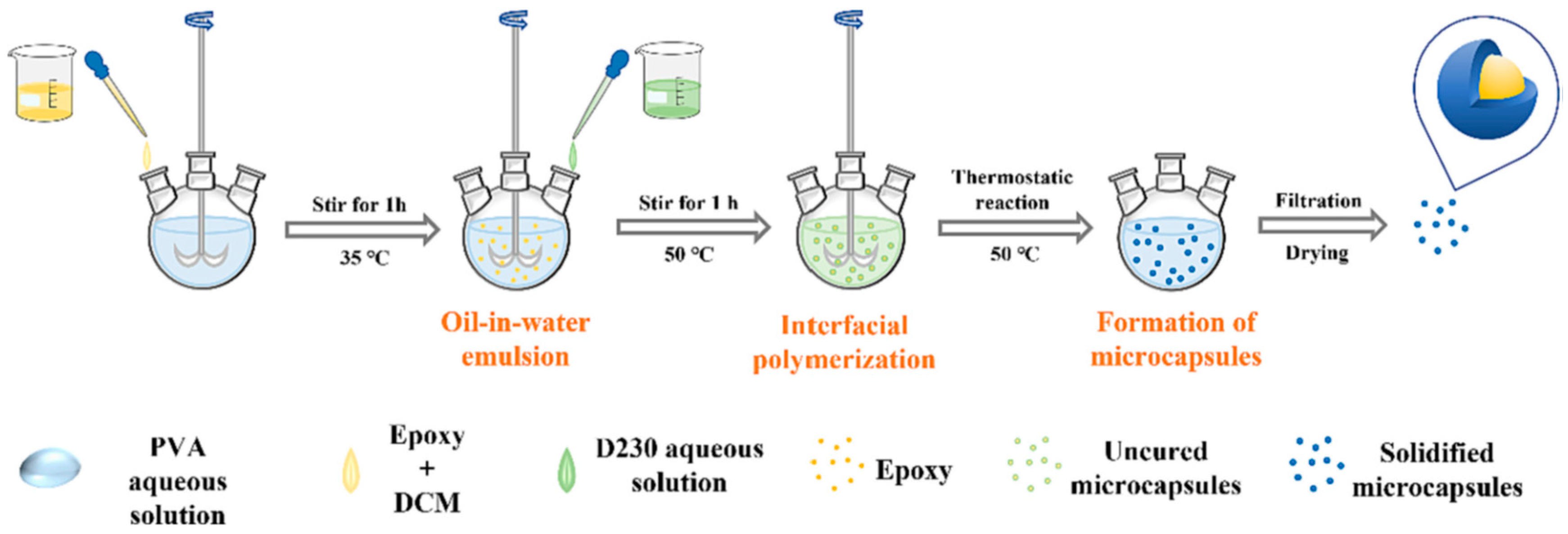
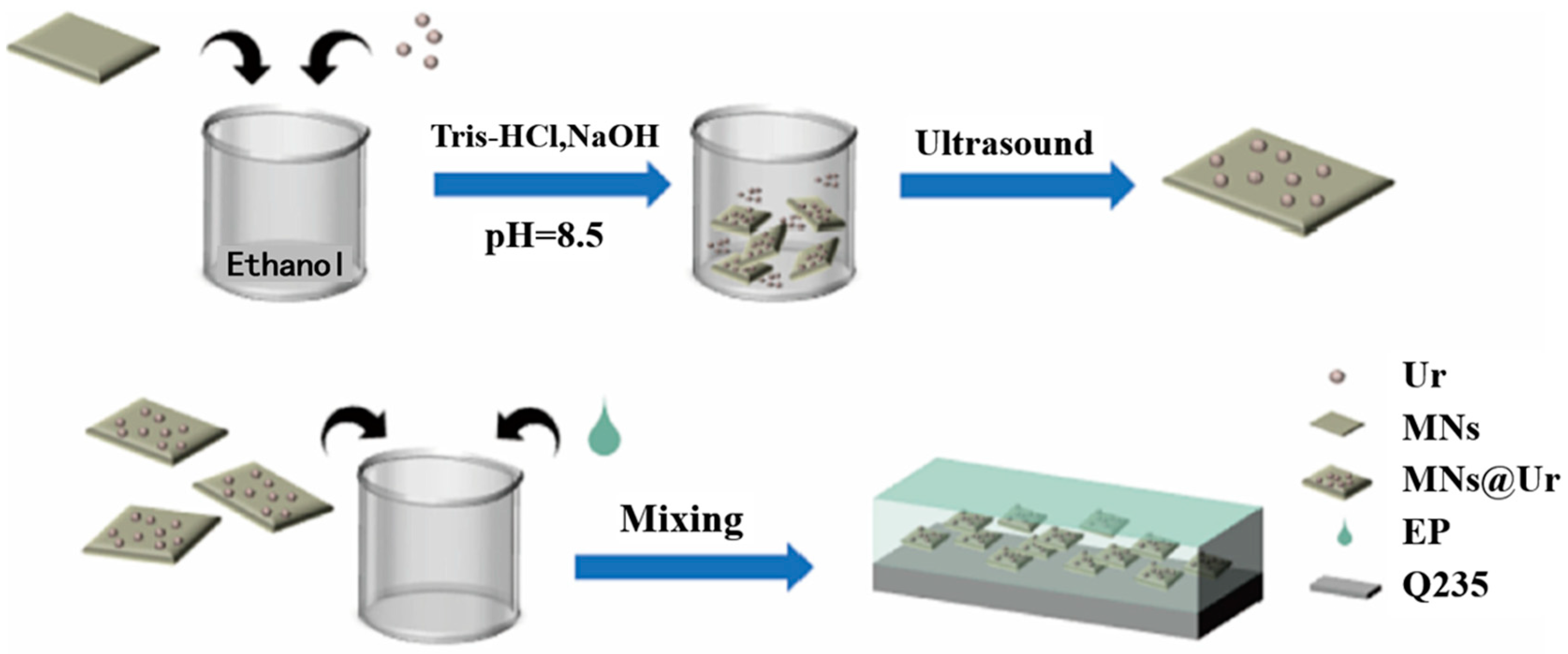
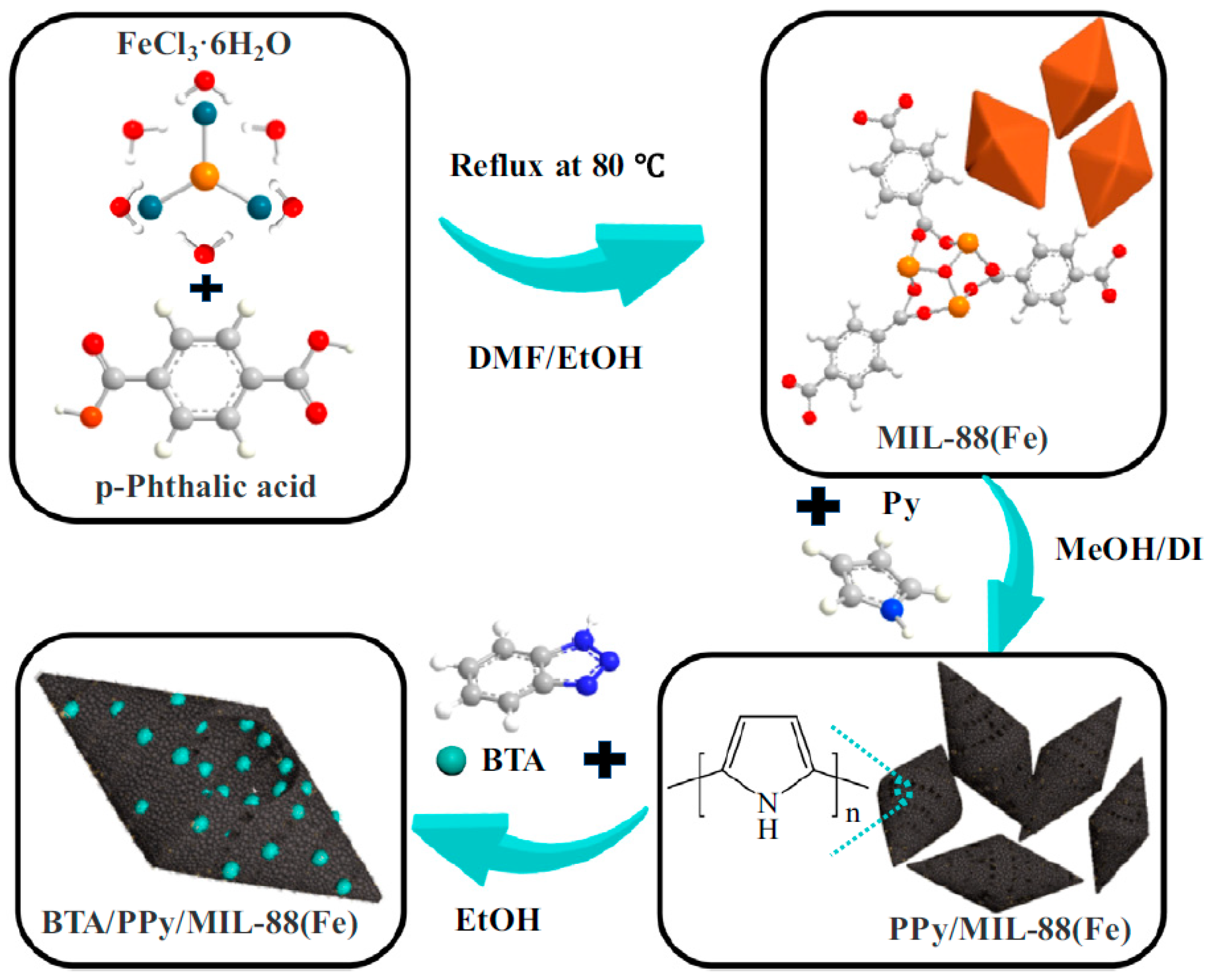
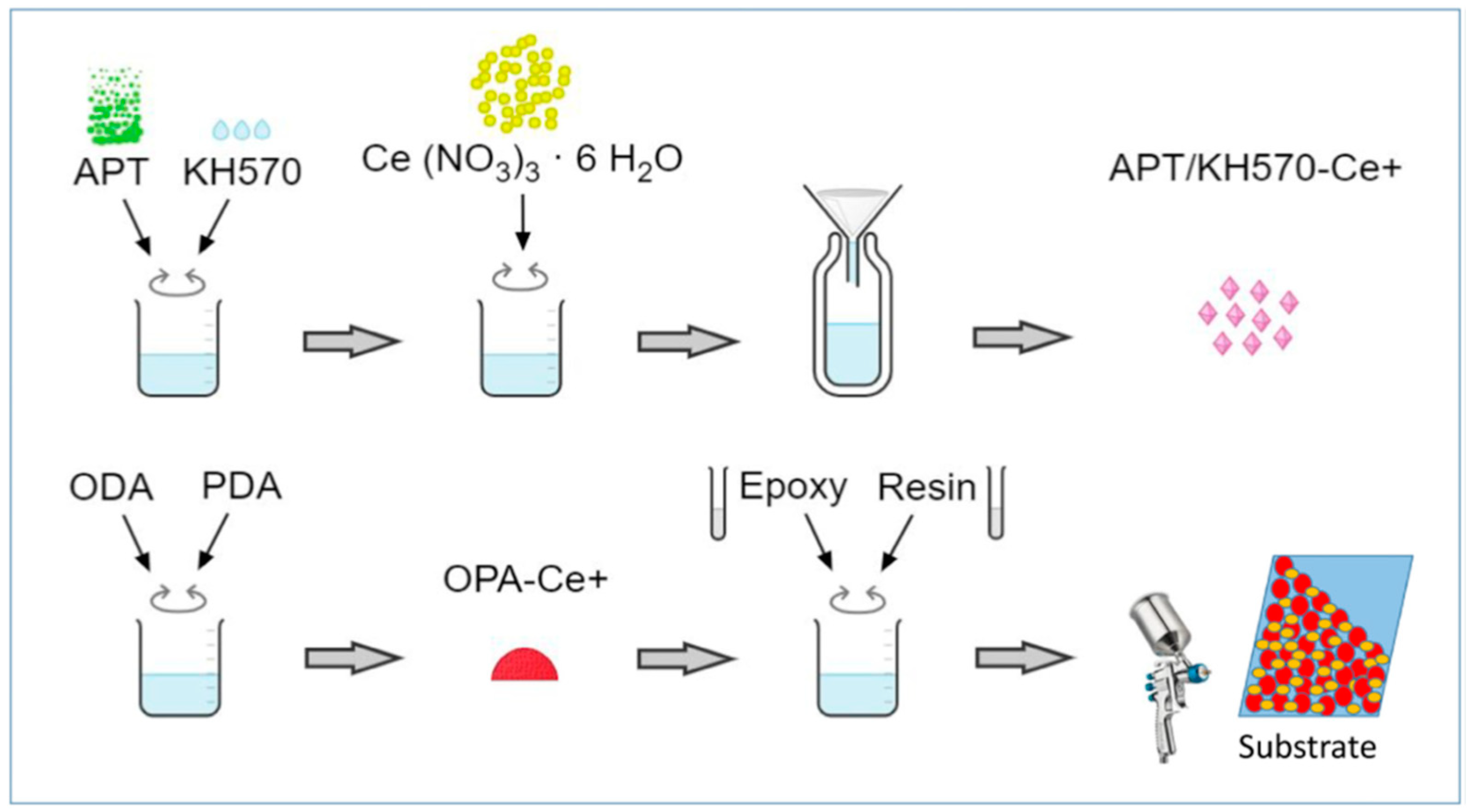
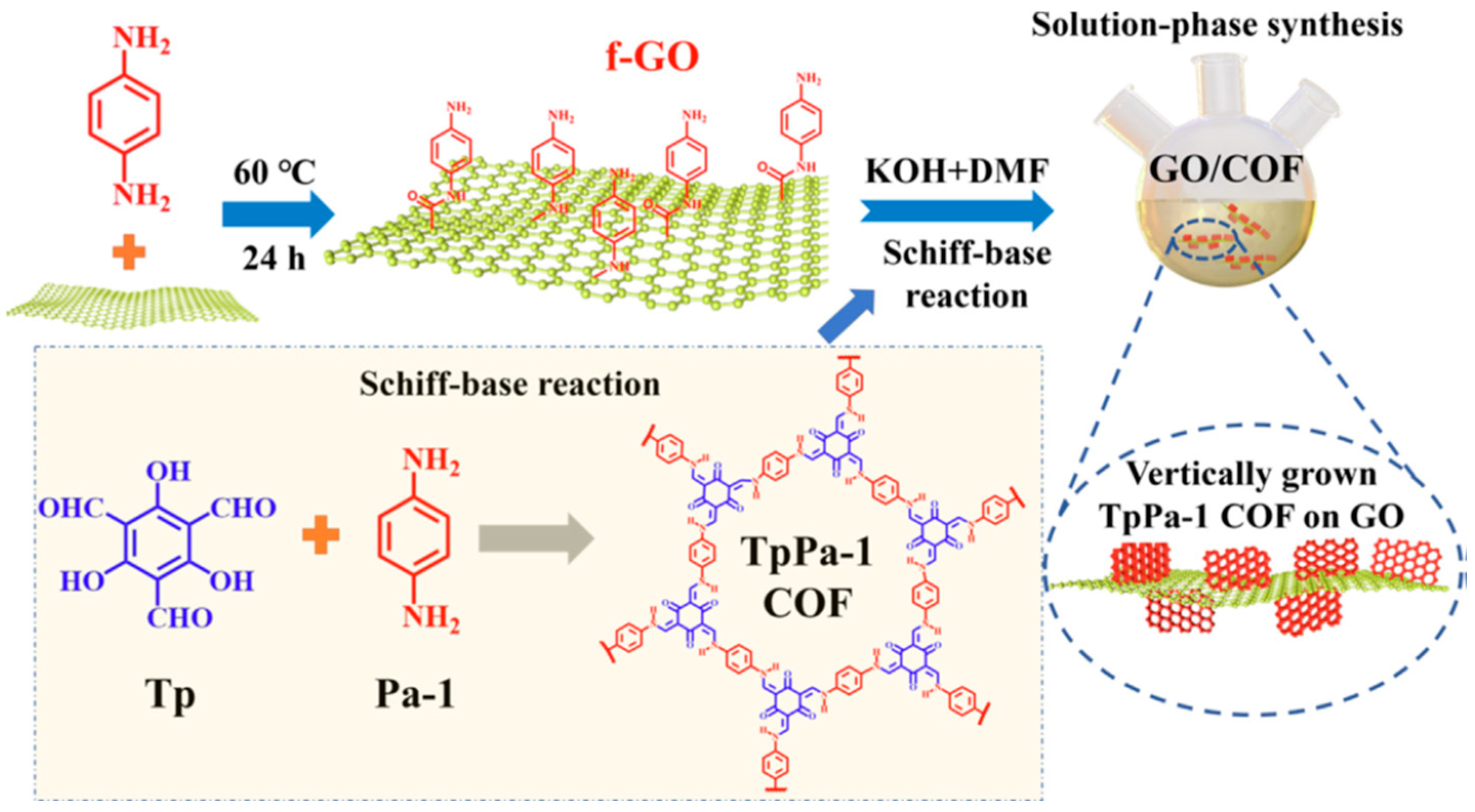

| Corrosion Protection Method | Benefits | Limitations |
|---|---|---|
| Epoxy coatings modified by metals and their compounds |
|
|
| Zinc-rich epoxy coatings |
|
|
| Titanium-modified epoxy coatings |
|
|
| Silicon-modified epoxy coatings |
|
|
| Epoxy coatings modified by other metal compounds |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Yang, K.; Wu, J.; Wu, M.; Li, Y. Overview of Recent Developments in Composite Epoxy Resin in Organic Coating on Steel (2020–2024). Materials 2025, 18, 1531. https://doi.org/10.3390/ma18071531
Hao J, Yang K, Wu J, Wu M, Li Y. Overview of Recent Developments in Composite Epoxy Resin in Organic Coating on Steel (2020–2024). Materials. 2025; 18(7):1531. https://doi.org/10.3390/ma18071531
Chicago/Turabian StyleHao, Jianghua, Kun Yang, Jiaye Wu, Mingzhu Wu, and Ying Li. 2025. "Overview of Recent Developments in Composite Epoxy Resin in Organic Coating on Steel (2020–2024)" Materials 18, no. 7: 1531. https://doi.org/10.3390/ma18071531
APA StyleHao, J., Yang, K., Wu, J., Wu, M., & Li, Y. (2025). Overview of Recent Developments in Composite Epoxy Resin in Organic Coating on Steel (2020–2024). Materials, 18(7), 1531. https://doi.org/10.3390/ma18071531







