Anisotropic Conductivity and Mechanical Strength Enhancements in Gel Polymer Electrolyte Films by Hot Pressing
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Film Preparation
2.3. Characterization
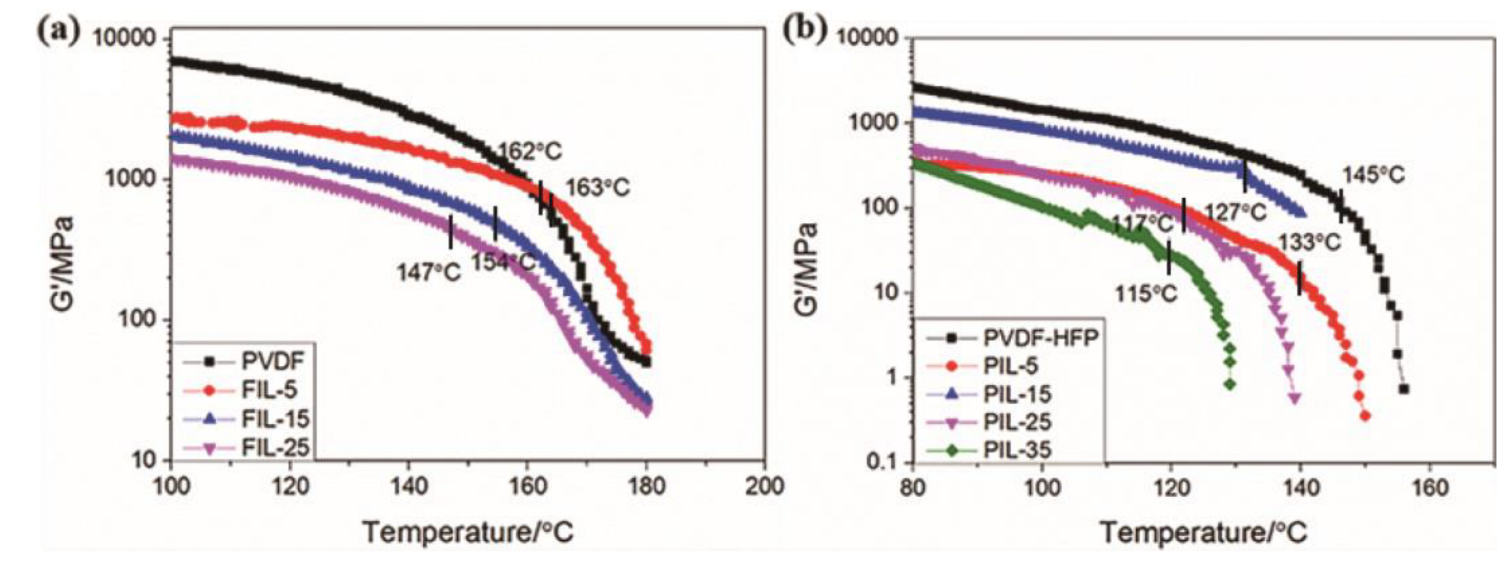
2.3.1. Dynamic Mechanical Thermal Analysis
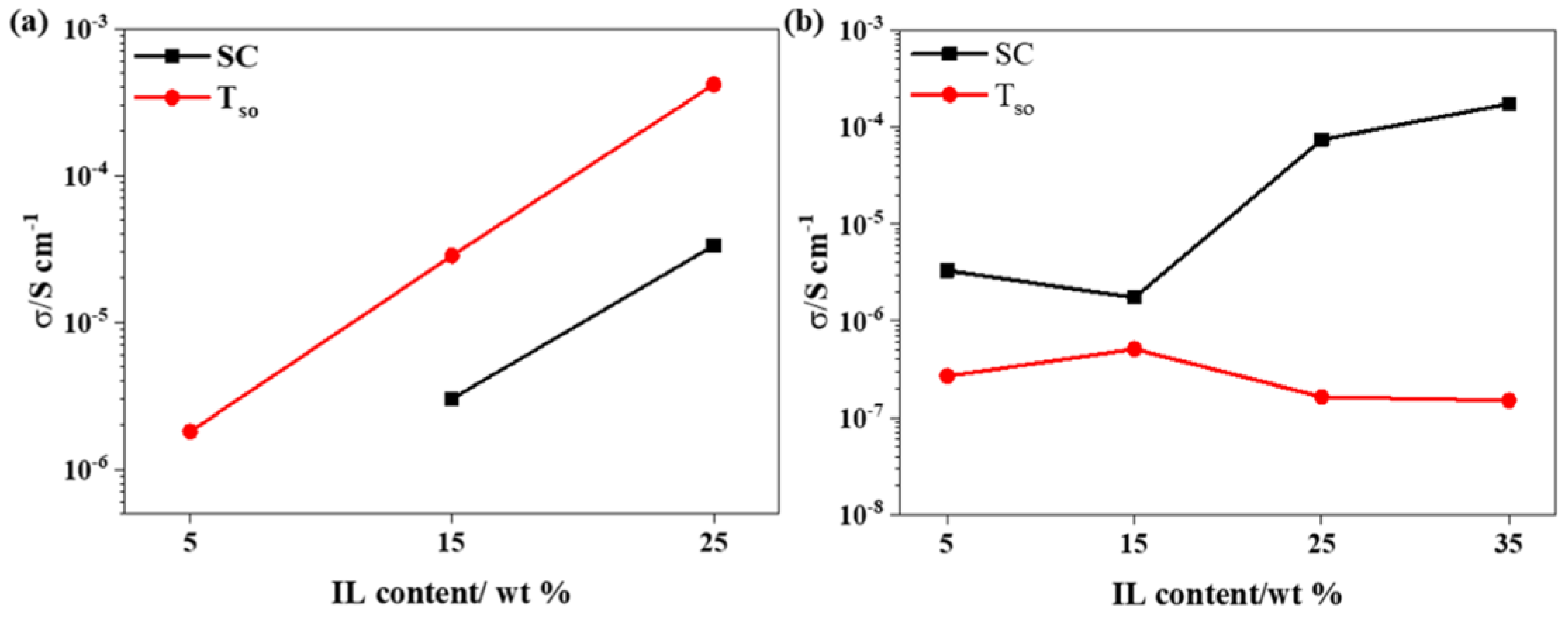
2.3.2. Ionic Conductivity
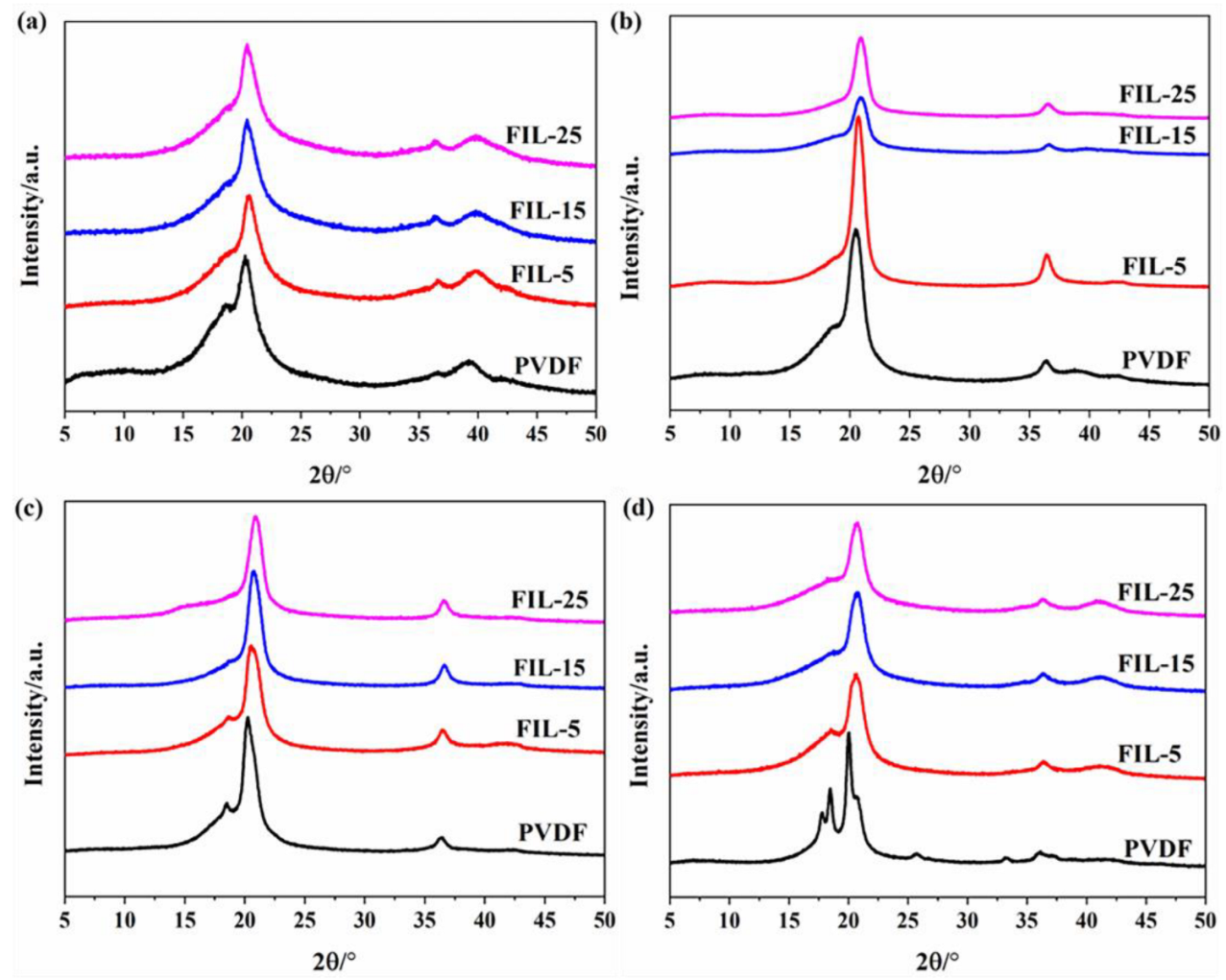
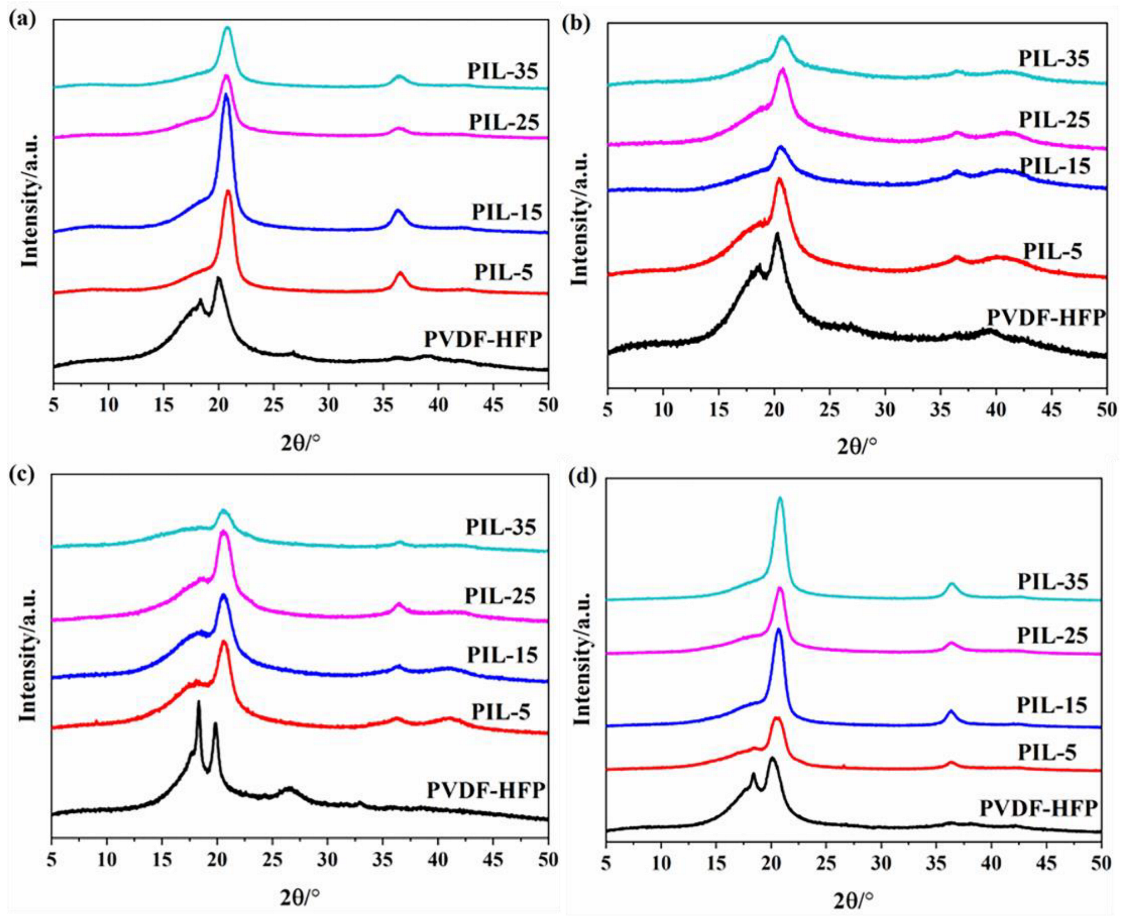
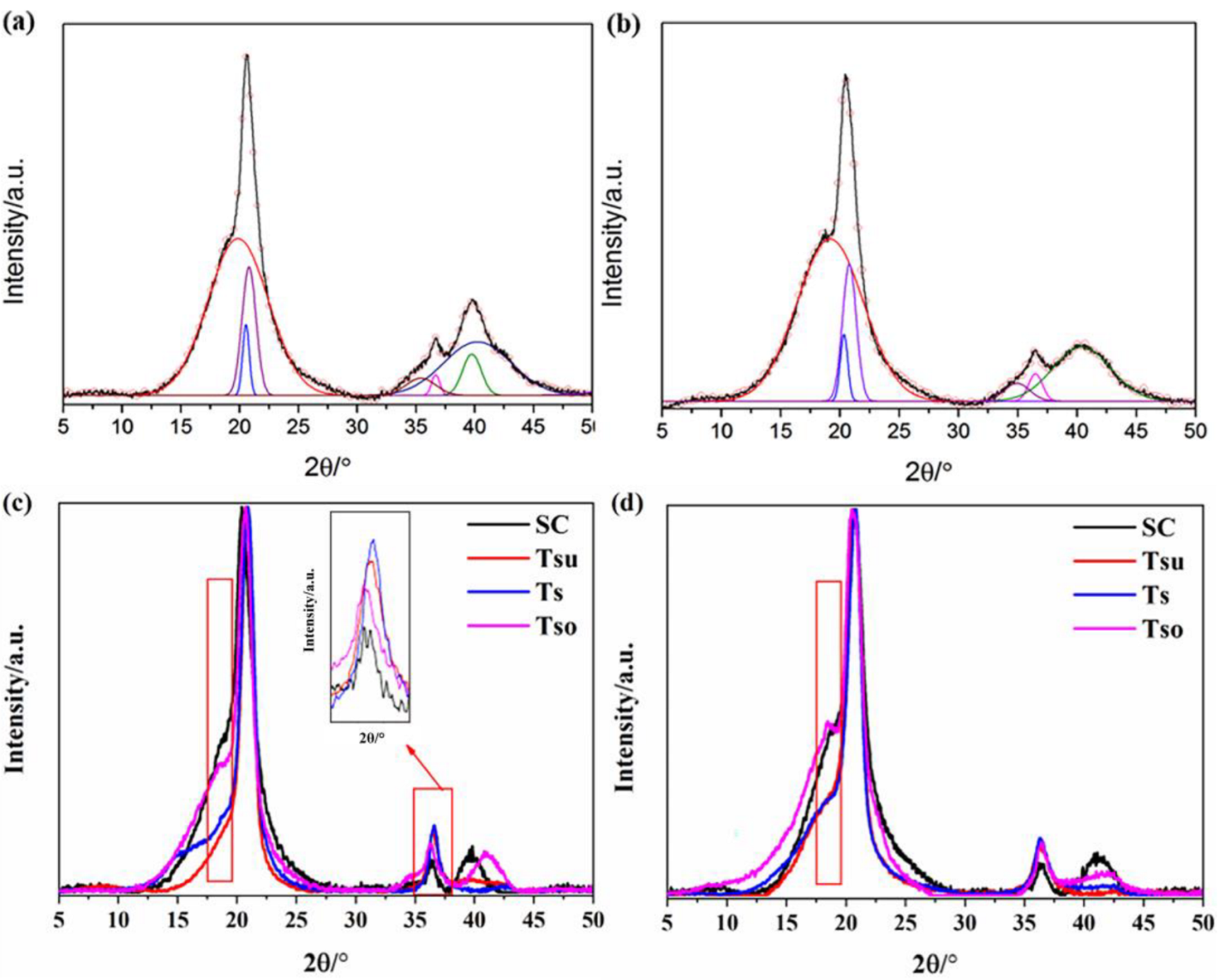
2.3.3. X-Ray Diffraction
2.3.4. Differential Scanning Calorimetry (DSC)
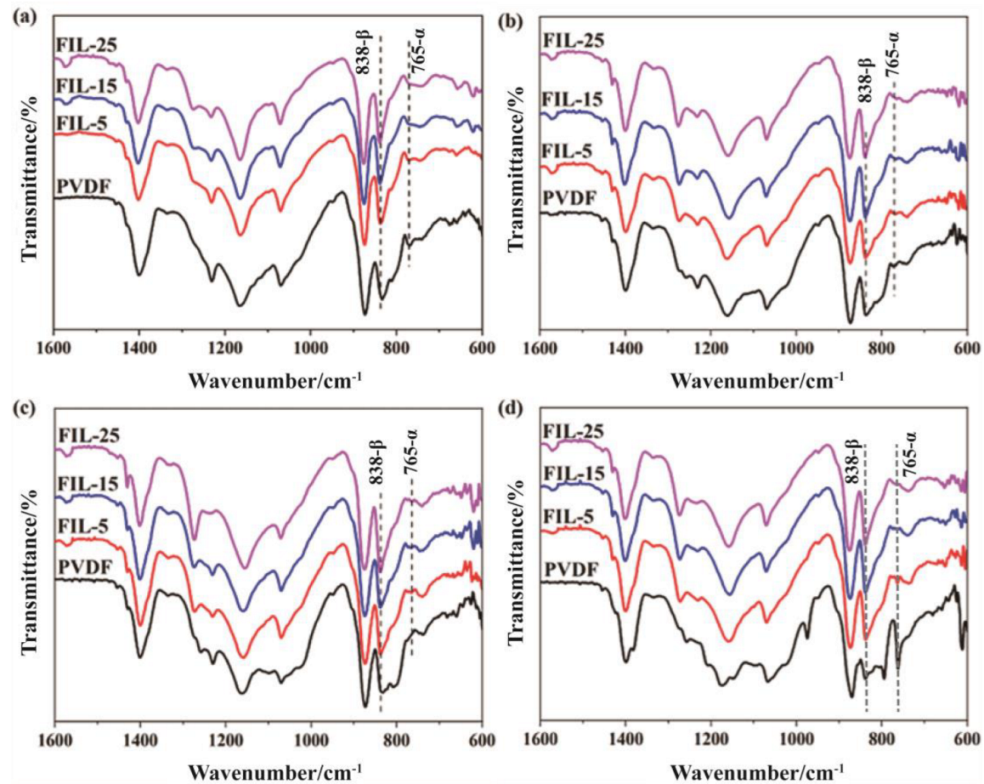
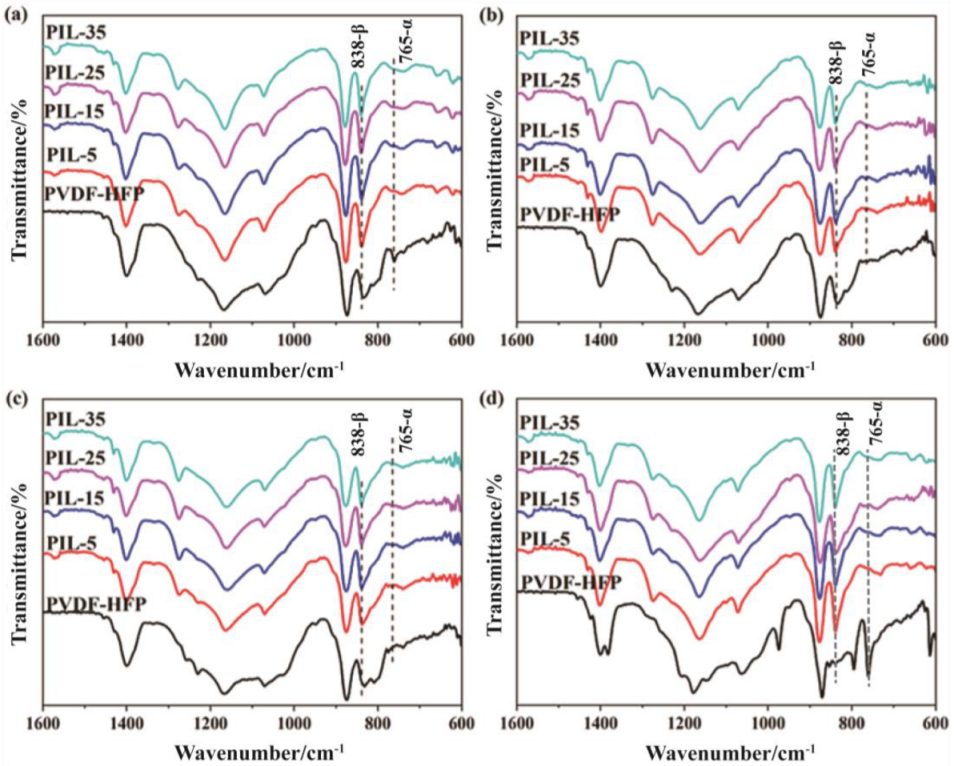
2.3.5. Fourier Transform Infrared Spectroscopy (FTIR)
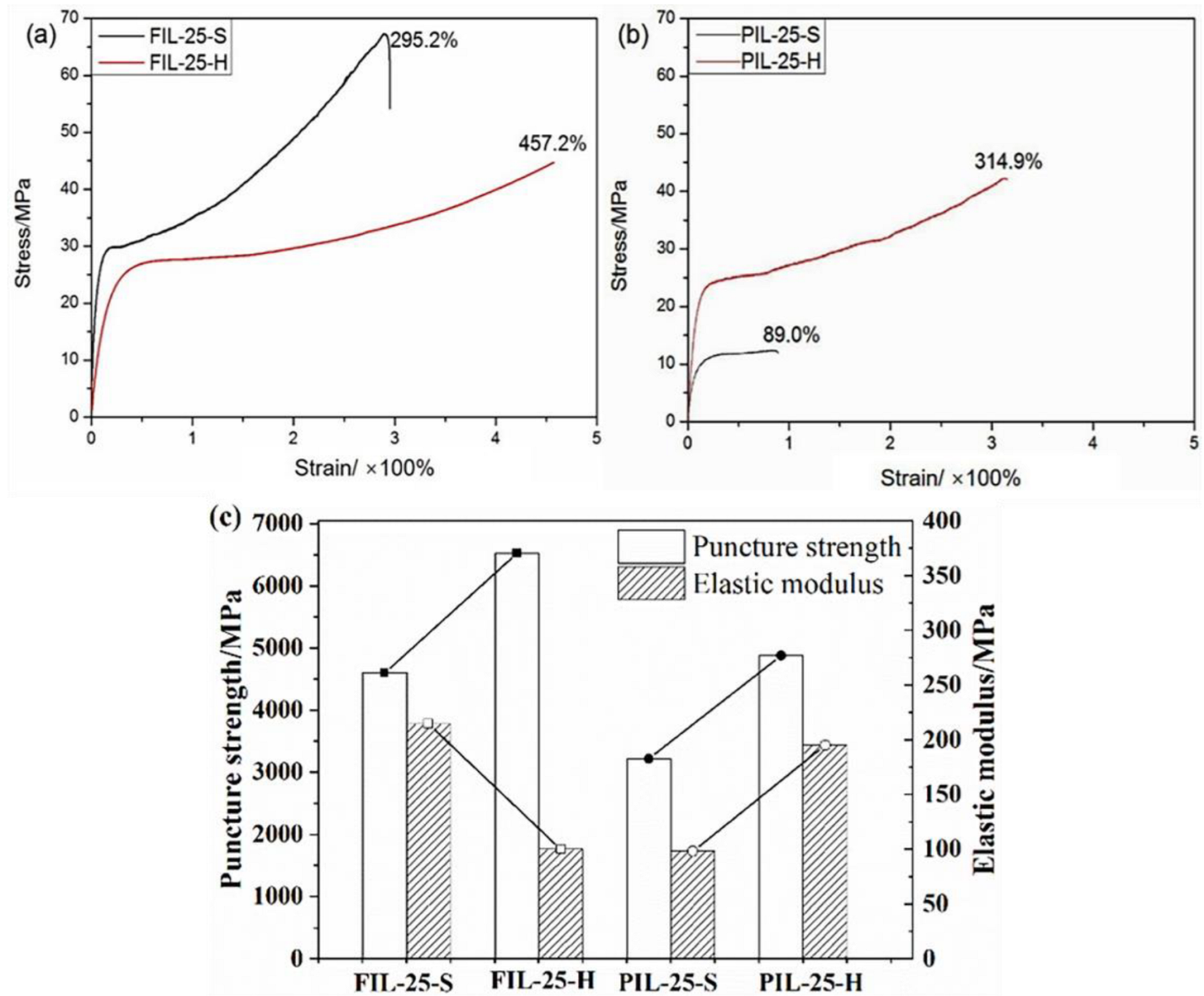
2.3.6. Mechanical Properties
3. Results and Discussion
3.1. Ionic Conductivities of the GPEs
3.2. Crystallinity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Zhu, M.; Kang, P.; Zhu, T.; Yuan, H.; Lan, J.; Yang, X.; Sui, G. Self-Enhancing Gel Polymer Electrolyte by In Situ Construction for Enabling Safe Lithium Metal Battery. Adv. Sci. 2022, 9, 2103663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, G.; Li, X.; Zhang, S.; Chen, R.; Wang, X.; Gao, Y.; Wang, Z.; Chen, L. Polymer electrolytes based on interactions between solvent-Li+ complex and solvent-modified polymer. Energy Storage Mater. 2022, 51, 443–452. [Google Scholar] [CrossRef]
- Ma, C.; Cui, W.; Liu, X.; Ding, Y.; Wang, Y. In situ preparation of gel polymer electrolyte for lithium batteries: Progress and perspectives. InfoMat 2022, 4, e12232. [Google Scholar] [CrossRef]
- Xu, P.; Chen, H.; Zhou, X.; Xiang, H. Gel polymer electrolyte based on PVDF-HFP matrix composited with rGO-PEG-NH2 for high-performance lithium ion battery. J. Membr. Sci. 2021, 617, 118660. [Google Scholar] [CrossRef]
- Liang, S.; Yan, W.; Wu, X.; Zhang, Y.; Zhu, Y.; Wang, H.; Wu, Y. Gel polymer electrolytes for lithium ion batteries: Fabrication, characterization and performance. Solid State Ion. 2018, 318, 2–18. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, P.; Shi, C.; Chen, L.; Dai, J.; Zhao, J. The functional separator coated with core-shell structured silica-poly (methyl methacrylate) sub-microspheres for lithium-ion batteries. J. Membr. Sci. 2015, 474, 148–155. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, J.; Du, Z.; Jia, X.; Qu, Y.; Yu, F.; Du, J.; Chen, Y. High-strength and flexible cellulose/PEG based gel polymer electrolyte with high performance for lithium ion batteries. J. Membr. Sci. 2020, 593, 117428. [Google Scholar] [CrossRef]
- Fasciani, C.; Panero, S.; Hassoun, J.; Scrosati, B. Novel configuration of poly(vinylidenedifluoride)-based gel polymer electrolyte for application in lithium-ion batteries. J. Power Sources 2015, 294, 180–186. [Google Scholar] [CrossRef]
- Li, X.; Qian, K.; He, Y.-B.; Liu, C.; An, D.; Li, Y.; Zhou, D.; Lin, Z.; Li, B.; Yang, Q.-H.; et al. A dual-functional gel-polymer electrolyte for lithium ion batteries with superior rate and safety performances. J. Mater. Chem. A 2017, 5, 18888–18895. [Google Scholar] [CrossRef]
- Zhou, D.; He, Y.-B.; Cai, Q.; Qin, X.; Li, B.; Du, H.; Yang, Q.-H.; Kang, F. Investigation of cyano resin-based gel polymer electrolyte: In situ gelation mechanism and electrode-electrolyte interfacial fabrication in lithium-ion battery. J. Mater. Chem. A 2014, 2, 20059–20066. [Google Scholar] [CrossRef]
- Celik, M.; Kizilaslan, A.; Can, M.; Cetinkaya, T.; Akbulut, H. Electrochemical investigation of PVDF: HFP gel polymer electrolytes for quasi-solid-state Li-O2 batteries: Effect of lithium salt type and concentration. Electrochim. Acta 2021, 371, 137824. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, F.; Zhou, Z.; Li, J.; Tang, Y. A Flexible Dual-Ion Battery Based on PVDF-HFP-Modified Gel Polymer Electrolyte with Excellent Cycling Performance and Superior Rate Capability. Adv. Energy Mater. 2018, 8, 1801219. [Google Scholar] [CrossRef]
- Ferrari, S.; Quartarone, E.; Mustarelli, P.; Magistris, A.; Fagnoni, M.; Protti, S.; Gerbaldi, C.; Spinella, A. Lithium ion conducting PVdF-HFP composite gel electrolytes based on N-methoxyethyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)-imide ionic liquid. J. Power Sources 2010, 195, 559–566. [Google Scholar] [CrossRef]
- Le, H.T.T.; Duc Tung, N.; Kalubarme, R.S.; Cao, G.; Park, C.-N.; Park, C.-J. Composite Gel Polymer Electrolyte Based on Poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) with Modified Aluminum Doped Lithium Lanthanum Titanate (A-LLTO) for High-Performance Lithium Rechargeable Batteries. ACS Appl. Mater. Interfaces 2016, 8, 20710–20719. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, S.; Shi, Y.; Yang, Y.; Wu, Y. A trilayer poly(vinylidene fluoride)/polyborate/poly(vinylidene fluoride) gel polymer electrolyte with good performance for lithium ion batteries. J. Mater. Chem. A 2013, 1, 7790–7797. [Google Scholar] [CrossRef]
- Castillo, J.; Santiago, A.; Judez, X.; Garbayo, I.; Coca Clemente, J.A.; Carmen Morant-Minana, M.; Villaverde, A.; Antonio Gonzalez-Marcos, J.; Zhang, H.; Armand, M.; et al. Safe, Flexible, and High-Performing Gel-Polymer Electrolyte for Rechargeable Lithium Metal Batteries. Chem. Mater. 2021, 33, 8812–8821. [Google Scholar] [CrossRef]
- Qian, J.; Jin, B.; Li, Y.; Zhan, X.; Hou, Y.; Zhang, Q. Research progress on gel polymer electrolytes for lithium-sulfur batteries. J. Energy Chem. 2021, 56, 420–437. [Google Scholar]
- Chen, X.; Yi, L.; Zou, C.; Liu, J.; Yu, J.; Zang, Z.; Tao, X.; Luo, Z.; Guo, X.; Chen, G.; et al. High-Performance Gel Polymer Electrolyte with Self-Healing Capability for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 5267–5276. [Google Scholar] [CrossRef]
- Gou, J.; Liu, W.; Tang, A.; Xie, H. A phosphorylated nanocellulose/hydroxypropyl methylcellulose composite matrix: A biodegradable, flame-retardant and self-standing gel polymer electrolyte towards eco-friendly and high safety lithium ion batteries. Eur. Polym. J. 2021, 158, 110703. [Google Scholar] [CrossRef]
- Ma, F.; Liu, Y.; Huang, T.; Du, X.; Lu, Q.; Kid, K. Facile in situ polymerization synthesis of poly(ionic liquid)-based polymer electrolyte for high-performance solid-state batteries. Energy Convers. Manag. X 2024, 22, 100570. [Google Scholar] [CrossRef]
- Yi, L.; Zou, C.; Chen, X.; Liu, J.; Cao, S.; Tao, X.; Zang, Z.; Liu, L.; Chang, B.; Shen, Y.; et al. One-Step Synthesis of PVDF-HFP/PMMA-ZrO2 Gel Polymer Electrolyte to Boost the Performance of a Lithium Metal Battery. ACS Appl. Energy Mater. 2022, 5, 7317–7327. [Google Scholar] [CrossRef]
- Song, S.; Wang, J.; Tang, J.; Muchakayala, R.; Ma, R. Preparation, properties, and Li-ion battery application of EC + PC-modified PVdF-HFP gel polymer electrolyte films. Ionics 2017, 23, 3365–3375. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, J.; Chen, S.; Zhang, J.; Wang, X. Controlled crystallization of poly(vinylidene fluoride) chains from mixed solvents composed of its good solvent and nonsolvent. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 575–581. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, S.; Chen, S.; Zhang, J.; Wang, X. Crystallization behavior and hydrophilicity of poly(vinylidene fluoride)/poly(methyl methacrylate)/poly(vinyl pyrrolidone) ternary blends. Polym. Int. 2012, 61, 477–484. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Chen, S.; Wang, X.; Wang, J. Effect of PPEGMA content on the structure and hydrophilicity of PVDF/PPEGMA blends prepared by in situ polymerization. Colloid Polym. Sci. 2013, 291, 1573–1580. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, J.; Chen, S.; Zhang, J.; Wang, X. Hydrophilic modification of poly(vinylidene fluoride) (PVDF) by in situ polymerization of methyl methacrylate (MMA) monomer. Colloid Polym. Sci. 2010, 288, 1327–1332. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, S.; Chen, S.; Zhang, J.; Wang, X. Properties and crystallization behavior of poly (vinylidene fluoride) (PVDF)/thermoplastic polyurethane elastomer (TPU) blends. Desalination Water Treat. 2011, 34, 184–189. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, J.; Chen, S.; Wang, X. β-Phase of poly(vinylidene fluoride) formation in poly(vinylidene fluoride)/poly(methyl methacrylate) blend from solutions. Appl. Surf. Sci. 2008, 254, 5635–5642. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, W.; Cheng, H.; Chen, S.; Wang, L. Effect of ionic liquid on crystallization kinetics and crystal form transition of poly(vinylidene fluoride) blends. J. Therm. Anal. Calorim. 2018, 132, 1153–1165. [Google Scholar] [CrossRef]
- ISO 527; Plastics—Determination of Tensile Properties—Part 1: General Principles. ISO: Geneva, Switzerland, 2019.
- Tang, J.; Muchakayala, R.; Song, S.; Wang, M.; Kumar, K.N. Effect of EMIMBF4 ionic liquid addition on the structure and ionic conductivity of LiBF4-complexed PVdF-HFP polymer electrolyte films. Polym. Test. 2016, 50, 247–254. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, Q.; Wang, L.; Xu, Z.; Li, Y.; Huang, S. Li7La3Zr2O12 Ceramic Nanofiber-Incorporated Solid Polymer Electrolytes for Flexible Lithium Batteries. ACS Appl. Energy Mater. 2020, 3, 5238–5246. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Kim, D. Crystallinity, morphology, mechanical properties and conductivity study of in situ formed PVdF/LiClO4/TiO2 nanocomposite polymer electrolytes. Electrochim. Acta 2007, 52, 3181–3189. [Google Scholar] [CrossRef]
- Zhai, W.; Zhu, H.-j.; Wang, L.; Liu, X.-m.; Yang, H. Study of PVDF-HFP/PMMA blended micro-porous gel polymer electrolyte incorporating ionic liquid [BMIM]BF4 for Lithium ion batteries. Electrochim. Acta 2014, 133, 623–630. [Google Scholar] [CrossRef]
- Zhang, Y.; Ben Jar, P.Y.; Xue, S.; Li, L. Quantification of strain-induced damage in semi-crystalline polymers: A review. J. Mater. Sci. 2018, 54, 62–82. [Google Scholar]
- Pereira, N.; Gonçalves, S.; Barbosa, J.C.; Gonçalves, R.; Tubio, C.R.; Vilas-Vilela, J.L.; Costa, C.M.; Lanceros-Mendez, S. High dielectric constant poly(vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) for capacitive pressure and bending sensors. Polymer 2021, 214, 123349. [Google Scholar] [CrossRef]
- Räntzsch, V.; Haas, M.; Özen, M.B.; Ratzsch, K.-F.; Riazi, K.; Kauffmann-Weiss, S.; Palacios, J.K.; Müller, A.J.; Vittorias, I.; Gisela, G.; et al. Polymer crystallinity and crystallization kinetics via benchtop 1H NMR relaxometry: Revisited method, data analysis, and experiments on common polymers. Polymer 2018, 145, 162–173. [Google Scholar] [CrossRef]
- Cha, B.J.; Yang, J.M. Preparation of poly(vinylidene fluoride) hollow fiber membranes for microfiltration using modified TIPS process. J. Membr. Sci. 2007, 291, 191–198. [Google Scholar] [CrossRef]
- Tseng, J.-K.; Yin, K.; Zhang, Z.; Mackey, M.; Baer, E.; Zhu, L. Morphological effects on dielectric properties of poly(vinylidene fluoride-co-hexafluoropropylene) blends and multilayer films. Polymer 2019, 172, 221–230. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Farooqui, U.R.; Hamid, N.A. Effect of graphene oxide (GO) on Poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) polymer electrolyte membrane. Polymer 2018, 142, 330–336. [Google Scholar] [CrossRef]
- Mohamed, H.F.M.; Hassanien, M.H.M.; Gomaa, A.G.R.; Aboud, A.A.A.; Ragab, S.A.M.; El-Gamal, A.-R.A.A.; Saeed, W.R.M. The electric field effect on the nanostructure, transport, mechanical, and thermal properties of polymer electrolyte membrane. J. Polym. Res. 2021, 28, 216. [Google Scholar] [CrossRef]
- Correia, D.M.; Costa, C.M.; Rodríguez-Hernández, J.C.; Tort Ausina, I.; Biosca, L.T.; Torregrosa Cabanilles, C.; Meseguer-Dueñas, J.M.; Lanceros-Méndez, S.; Gomez Ribelles, J.L. Effect of Ionic Liquid Content on the Crystallization Kinetics and Morphology of Semicrystalline Poly(vinylidene Fluoride)/Ionic Liquid Blends. Cryst. Growth Des. 2020, 20, 4967–4979. [Google Scholar] [CrossRef]
- Dong, L.; Li, R.; Xiong, C.; Quan, H. Effect of heat treatment on the electrical properties of lead zirconate titanate/poly (vinylidene fluoride) composites. Polym. Int. 2010, 59, 756–758. [Google Scholar] [CrossRef]
- Kelly, G.M.; Elman, J.F.; Jiang, Z.; Strzalka, J.; Albert, J.N.L. Thermal transitions in semi-crystalline polymer thin films studied via spectral reflectance. Polymer 2018, 143, 336–342. [Google Scholar] [CrossRef]
- Jamalpour, S.; Ghahramani, M.; Ghaffarian, S.R.; Javanbakht, M. The effect of poly(hydroxyl ethyl methacrylate) on the performance of PVDF/P(MMA-co-HEMA) hybrid gel polymer electrolytes for lithium ion battery application. Polymer 2020, 195, 122427. [Google Scholar] [CrossRef]
- Mandal, A.; Nandi, A.K. Ionic liquid integrated multiwalled carbon nanotube in a poly(vinylidene fluoride) matrix: Formation of a piezoelectric beta-polymorph with significant reinforcement and conductivity improvement. ACS Appl. Mater. Interfaces 2013, 5, 747–760. [Google Scholar] [CrossRef]
- Huang, P.; Xu, S.; Zhong, W.; Fu, H.; Luo, Y.; Xiao, Z.; Zhang, M. Carbon quantum dots inducing formation of β phase in PVDF-HFP to improve the piezoelectric performance. Sens. Actuators A Phys. 2021, 330, 112880. [Google Scholar] [CrossRef]
| Polymer/wt% | [HMIM]Cl/wt% | Abbreviation | |
|---|---|---|---|
| PVDF | 95 | 5 | FIL-5 |
| 85 | 15 | FIL-15 | |
| 75 | 25 | FIL-25 | |
| PVDF-HFP | 95 | 5 | PIL-5 |
| 85 | 15 | PIL-15 | |
| 75 | 25 | PIL-25 | |
| 65 | 35 | PIL-35 |
| Sample | σSC */S·cm−1 | σTsu */S·cm−1 | σTs */S·cm−1 | σTso */S·cm−1 |
|---|---|---|---|---|
| FIL-5 | - | 3.39 × 10−7 | 3.83 × 10−7 | 1.81 × 10−6 |
| FIL-15 | 3.02 × 10−6 | 1.85 × 10−5 | 6.75 × 10−6 | 2.85 × 10−5 |
| FIL-25 | 3.34 × 10−5 | 7.49 × 10−5 | 9.39 × 10−5 | 4.17 × 10−4 |
| PIL-5 | 3.30 × 10−6 | 3.91 × 10−8 | 1.73 × 10−7 | 2.69 × 10−7 |
| PIL-15 | 1.74 × 10−6 | 1.52 × 10−5 | 1.49 × 10−7 | 5.12 × 10−7 |
| PIL-25 | 7.39 × 10−5 | 4.21 × 10−6 | 6.07 × 10−7 | 1.64 × 10−7 |
| PIL-35 | 1.73 × 10−4 | 1.02 × 10−4 | 2.06 × 10−8 | 1.51 × 10−7 |
| Sample | σPS/MPa | σt/MPa | εb/% | E/MPa |
|---|---|---|---|---|
| FIL-25-S | 4604.6 | 67.25 | 295.2 | 214.5 |
| FIL-25-H | 6527.3 | 46.83 | 457.2 | 100.1 |
| PIL-25-S | 3220.4 | 12.45 | 89.0 | 98.1 |
| PIL-25-H | 4880.3 | 42.33 | 314.9 | 195.2 |
| Sample | XCsc/% | XCTsu/% | XCTs/% | XCTso/% |
|---|---|---|---|---|
| PVDF | 32.2 | 27.8 | 33.4 | 37.8 |
| FIL-5 | 19.1 | 48.7 | 43.8 | 39.8 |
| FIL-15 | 19.1 | 33.9 | 45.2 | 37.3 |
| FIL-25 | 18.4 | 42.0 | 35.0 | 32.0 |
| PVDF-HFP | 27.1 | 30.0 | 25.3 | 25.4 |
| PIL-5 | 16.1 | 42.8 | 28.3 | 28.6 |
| PIL-15 | 15.0 | 41.6 | 38.6 | 28.3 |
| PIL-25 | 19.3 | 32.1 | 36.3 | 28.4 |
| PIL-35 | 14.2 | 34.2 | 49.3 | 17.1 |
| Sample | FCSC(β)/% | FCTsu(β)/% | FCTs(β)/% | FCTso(β)/% |
|---|---|---|---|---|
| PVDF | 78.0 | 91.1 | 98.8 | 32.3 |
| FIL-5 | 90.3 | 98.6 | 97.8 | 94.8 |
| FIL-15 | 92.5 | 98.7 | 98.4 | 98.8 |
| FIL-25 | 93.6 | 98.7 | 98.4 | 99.1 |
| PVDF-HFP | 71.5 | 99.0 | 92.9 | 3.1 |
| PIL-5 | 96.4 | 98.0 | 97.8 | 99.3 |
| PIL-15 | 96.5 | 98.3 | 98.8 | 98.8 |
| PIL-25 | 96.3 | 98.6 | 98.4 | 98.6 |
| PIL-35 | 96.8 | 98.0 | 97.9 | 97.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Zhang, H.; Chen, S. Anisotropic Conductivity and Mechanical Strength Enhancements in Gel Polymer Electrolyte Films by Hot Pressing. Materials 2025, 18, 1751. https://doi.org/10.3390/ma18081751
Fang Z, Zhang H, Chen S. Anisotropic Conductivity and Mechanical Strength Enhancements in Gel Polymer Electrolyte Films by Hot Pressing. Materials. 2025; 18(8):1751. https://doi.org/10.3390/ma18081751
Chicago/Turabian StyleFang, Zhifan, Hao Zhang, and Shuangjun Chen. 2025. "Anisotropic Conductivity and Mechanical Strength Enhancements in Gel Polymer Electrolyte Films by Hot Pressing" Materials 18, no. 8: 1751. https://doi.org/10.3390/ma18081751
APA StyleFang, Z., Zhang, H., & Chen, S. (2025). Anisotropic Conductivity and Mechanical Strength Enhancements in Gel Polymer Electrolyte Films by Hot Pressing. Materials, 18(8), 1751. https://doi.org/10.3390/ma18081751






