Synthesis of Sandwich-Structured Zeolite Molecular Sieves and Their Adsorption Performance for Volatile Hydrocarbons
Abstract
1. Introduction
- (1)
- High Pressure Drop and Mass Transfer Resistance:
- (2)
- Bed Instability and Safety Hazards:
- (3)
- High Energy Consumption for Regeneration:
2. Materials and Methods
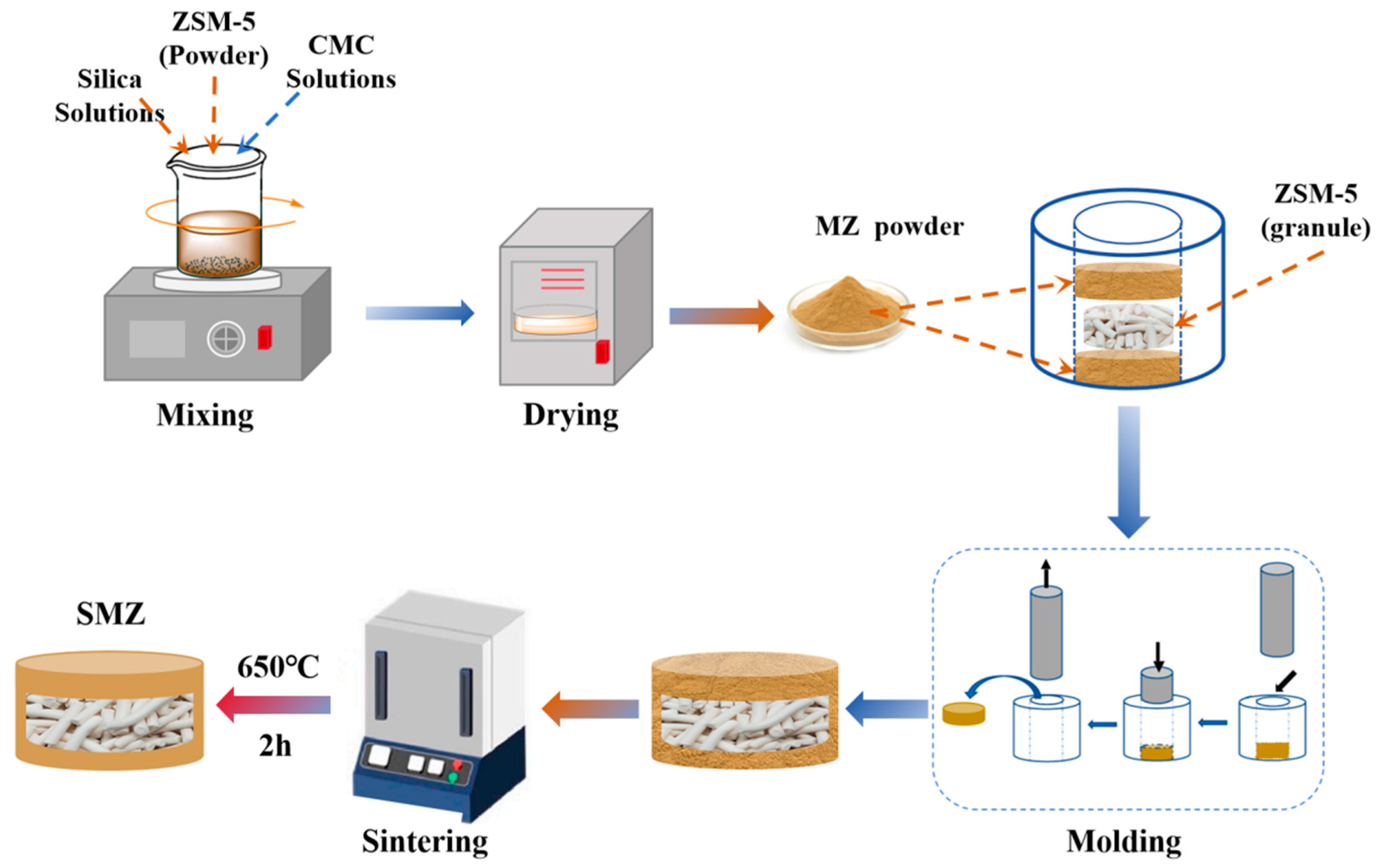
2.1. Fabrication of Sandwich-Structured Monolith
2.2. Material Characterization Methods
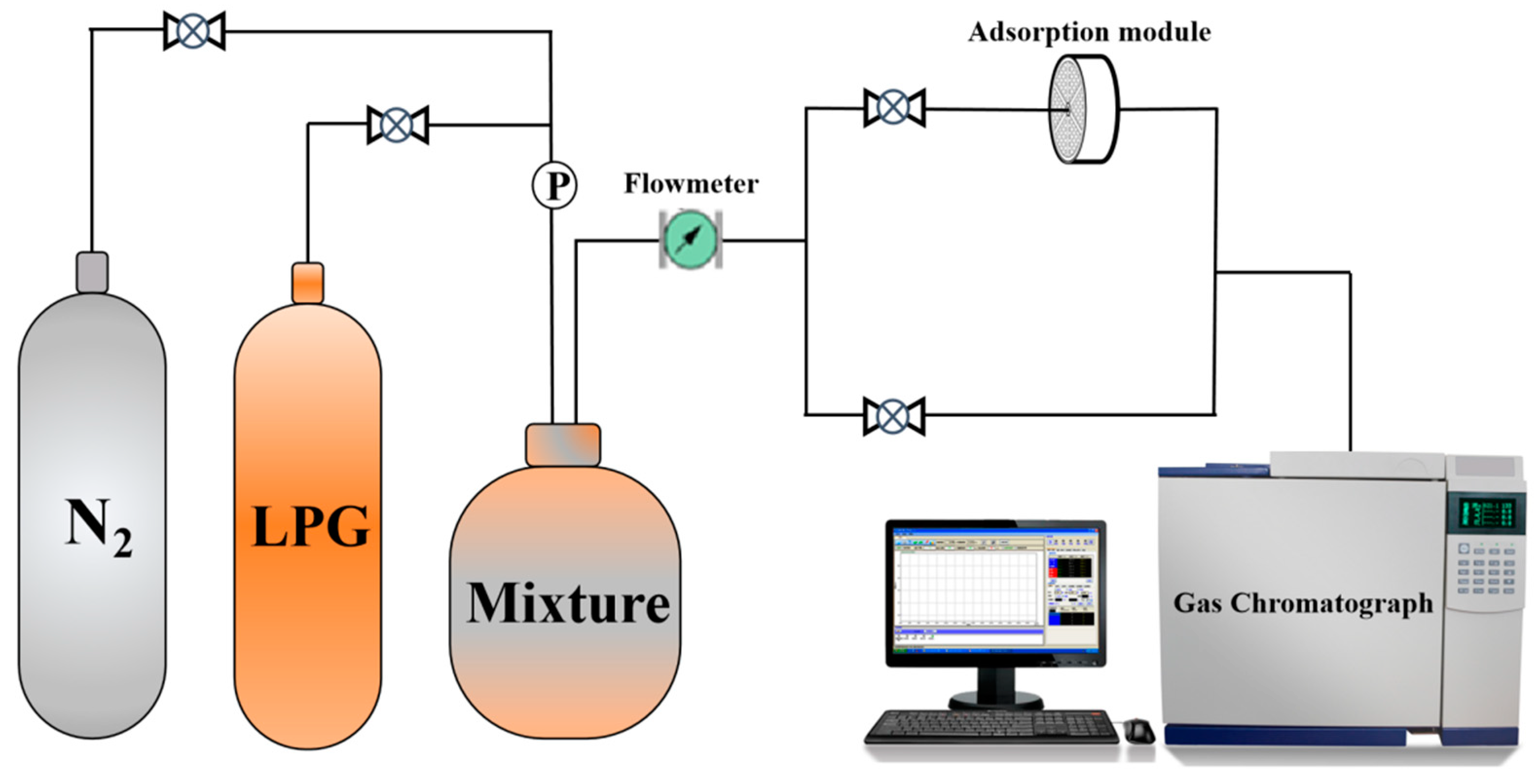
2.3. Adsorption Performance Evaluation
3. Results and Discussion
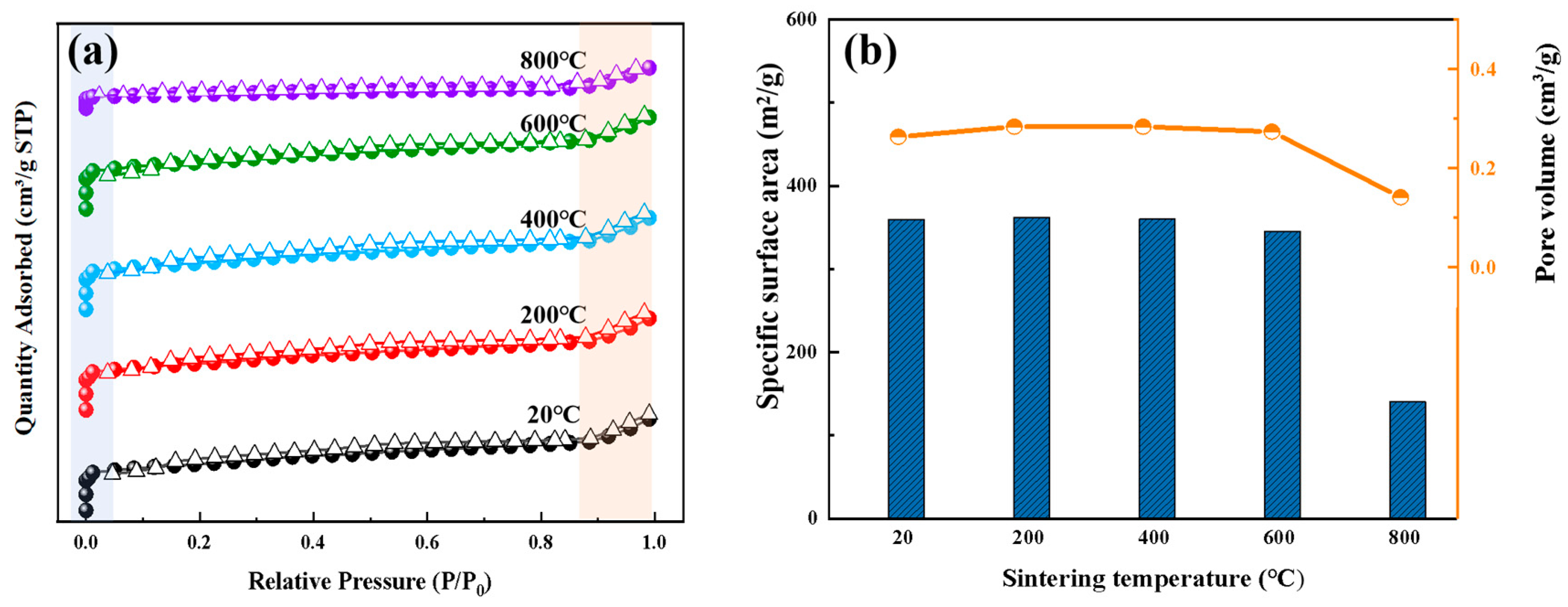
3.1. Thermal Stability and Pore Evolution of ZSM-5
3.2. Mechanical Strength and Stability of SMZ
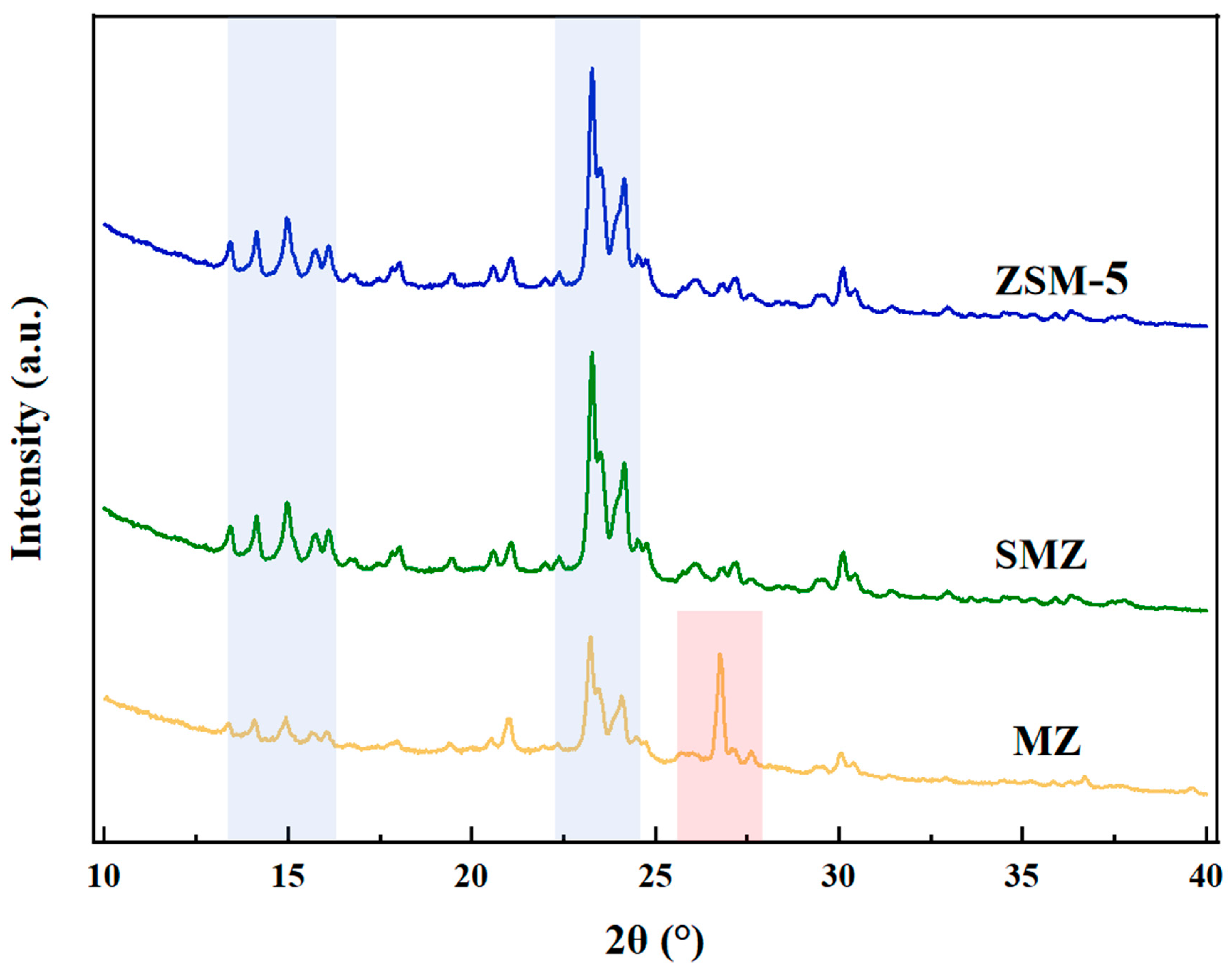
3.3. Characteristics of SMZ
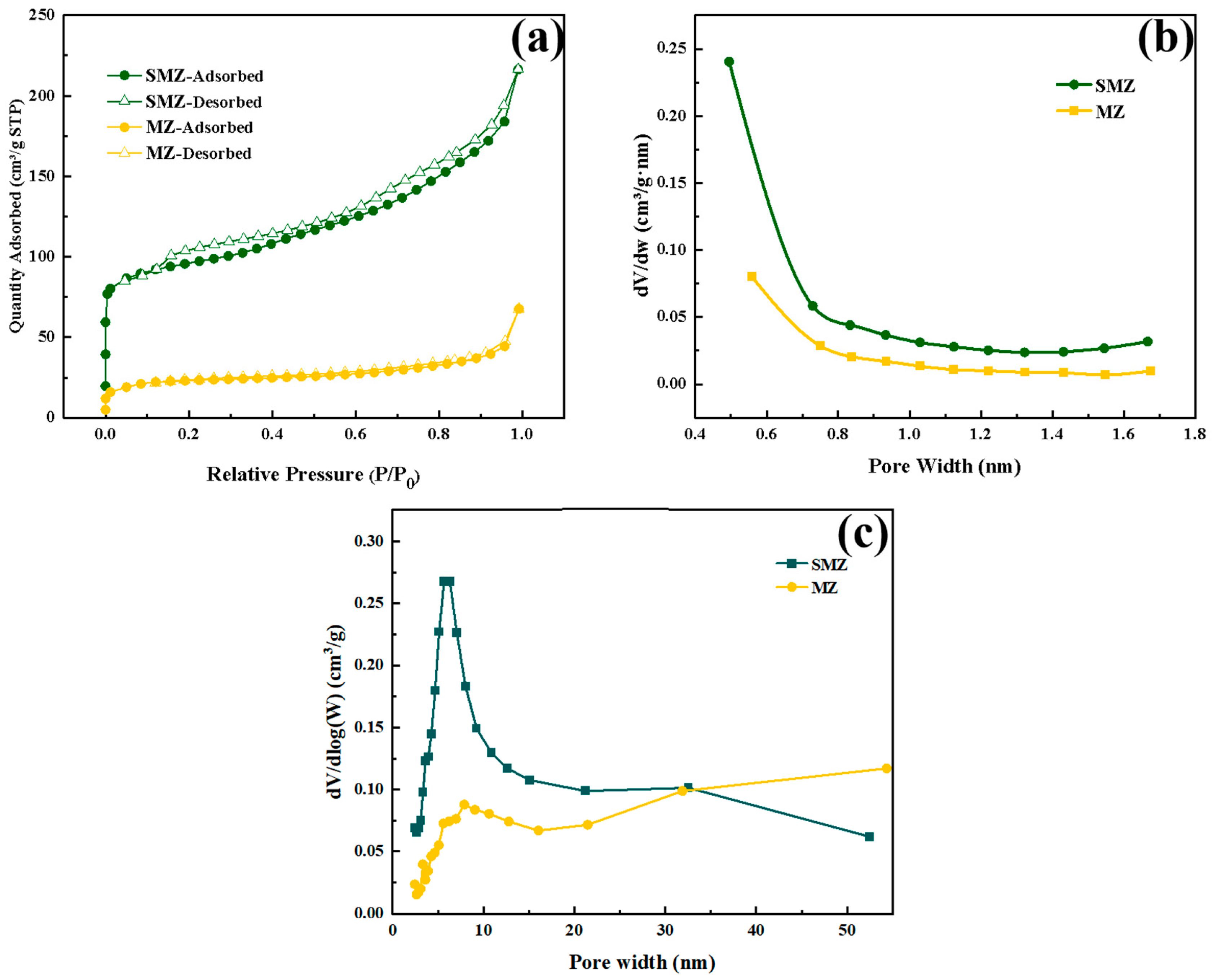
3.4. Specific Surface Area and Pore Structure Analysis
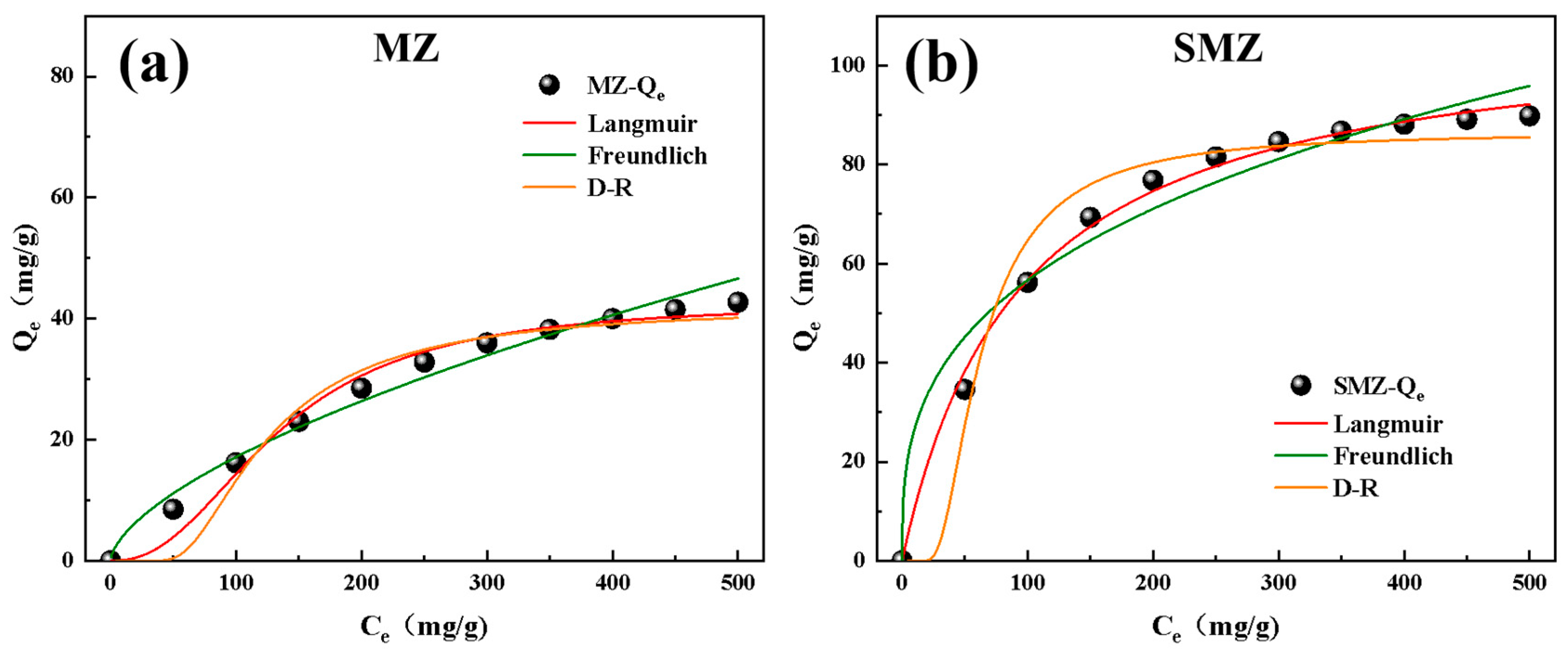
3.5. Dynamic Adsorption and Isotherm Modeling
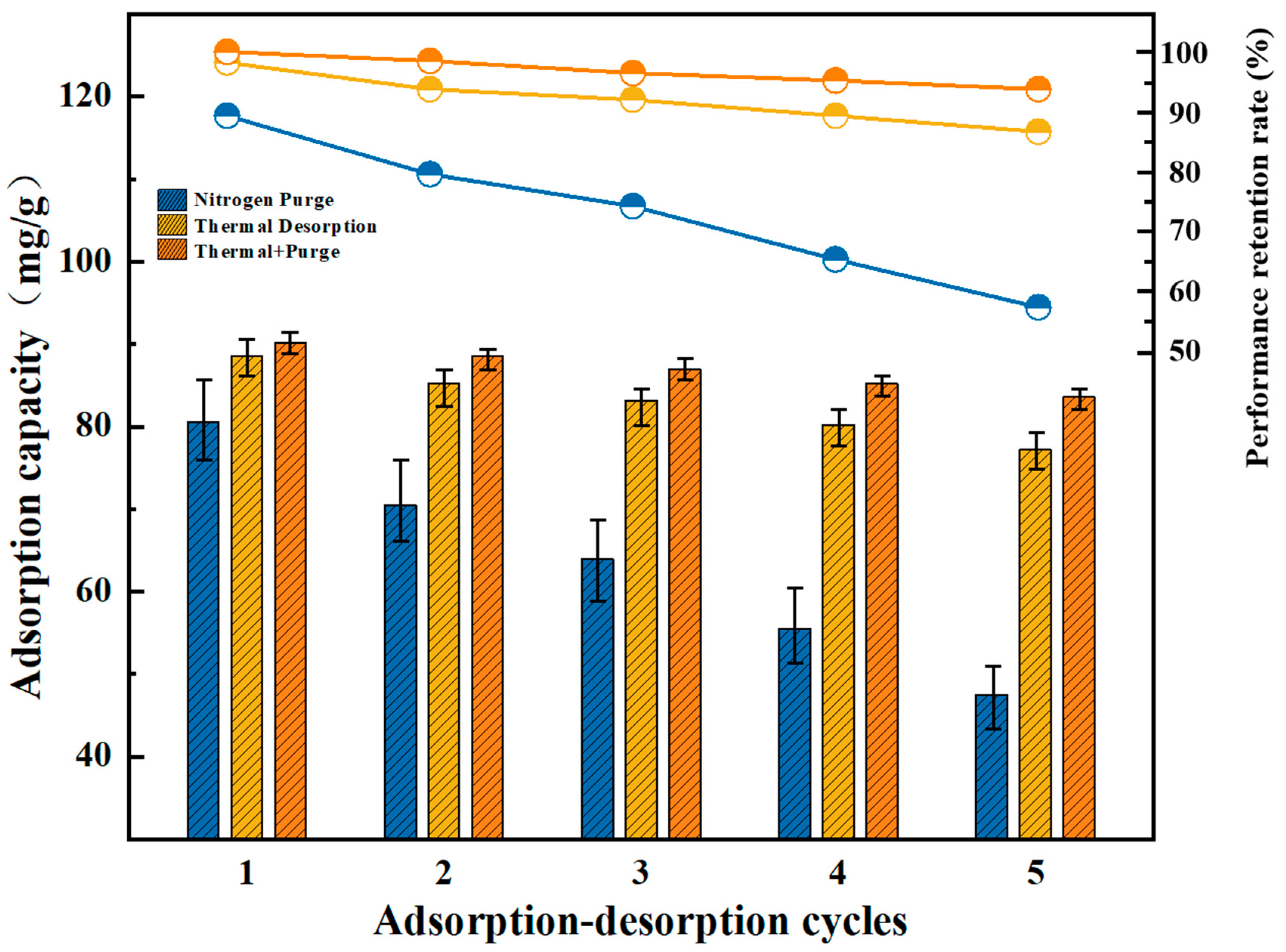
3.6. Desorption and Regeneration Performance
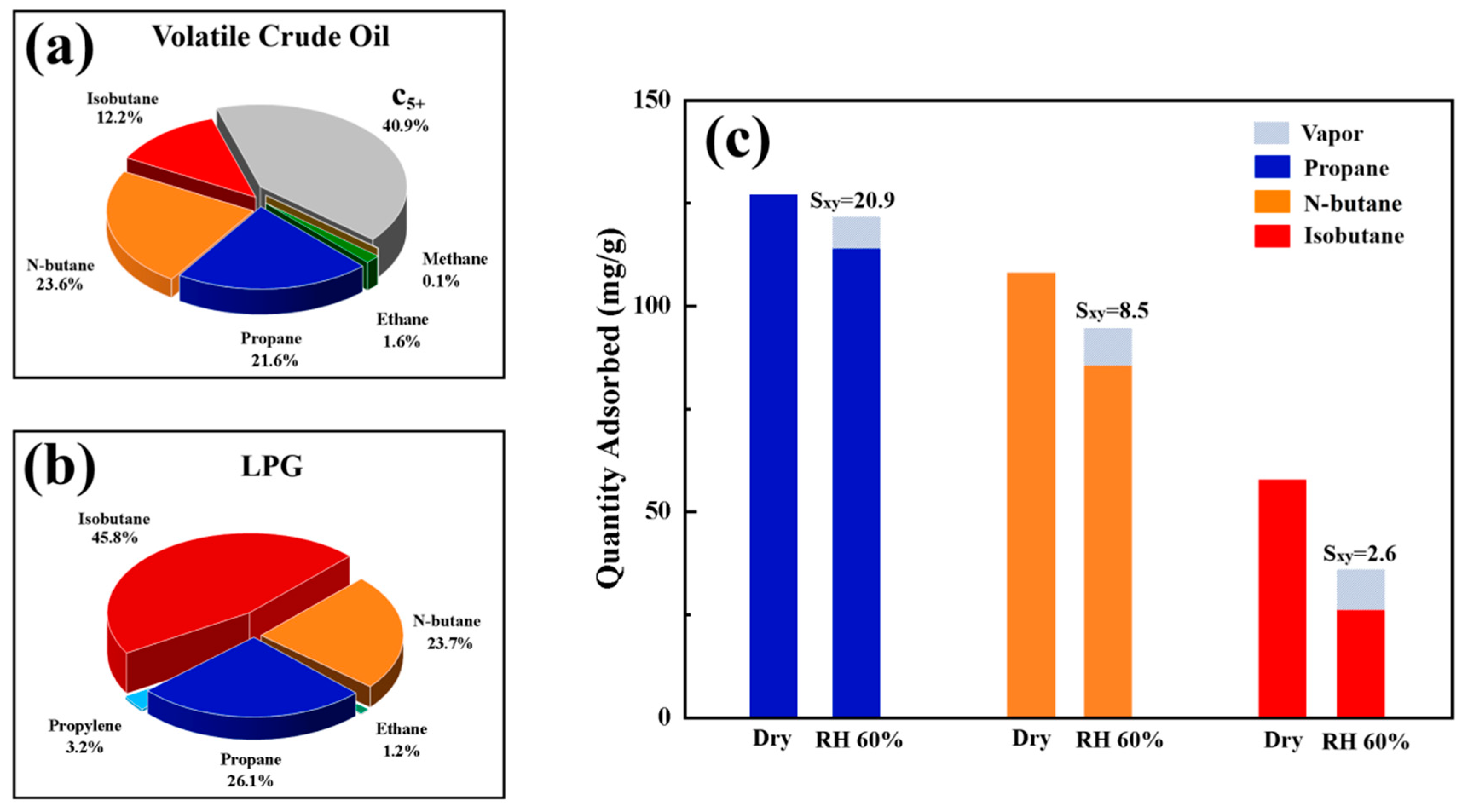
3.7. Adsorption Performance of Volatile Components in Crude Oil
4. Conclusions
- (1)
- Adsorption Efficiency: SMZ exhibited superior adsorption capacities for propane (127.6 mg/g) and n-butane (118.2 mg/g), with a 106% improvement over conventional monolithic zeolites. Adsorption isotherm models (Langmuir, Freundlich, D-R) validated its microporous dominance and homogeneous surface interactions.
- (2)
- Regeneration Stability: Combined thermal desorption (250 °C) and nitrogen purging achieved >95% capacity retention over five cycles, ensuring energy-efficient regeneration for industrial cyclic operations.
- (3)
- Mechanical Durability: SMZ sintered at 600 °C showcased high compressive strength (12.7 MPa) and vibration resistance, with minimal mass loss (<2.2%) under simulated pressure-swing adsorption (PSA) conditions, guaranteeing long-term structural robustness.
- (4)
- Pore Engineering: The hierarchical pore architecture (0.495 nm micropores + 2–10 nm mesopores) optimized mass transfer, enabling rapid VOC capture even under 60% humidity, while binder-free fabrication eliminated pore-blocking risks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z.P. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic Combustion of VOCs on Non-Noble Metal Catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Zheng, B.; Tong, D.; Lei, Y.; Liu, F.; Hong, C.P.; Kang, S.C.; Yan, L.; Zhang, Y.X.; et al. Persistent Growth of Anthropogenic Non-Methane Volatile Organic Compound (NMVOC) Emissions in China during 1990–2017: Drivers, Speciation and Ozone Formation Potential. Atmos. Chem. Phys. 2019, 19, 8897–8913. [Google Scholar] [CrossRef]
- Zheng, C.H.; Shen, J.L.; Zhang, Y.X.; Huang, W.W.; Zhu, X.B.; Wu, X.C.; Chen, L.H.; Gao, X.; Cen, K.F. Quantitative Assessment of Industrial VOC Emissions in China: Historical Trend, Spatial Distribution, Uncertainties, and Projection. Atmos. Environ. 2017, 150, 116–125. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Adebayo, B.; Gelles, T. Abatement of Gaseous Volatile Organic Compounds: A Process Perspective. Catal. Today 2020, 350, 100–119. [Google Scholar] [CrossRef]
- Gelles, T.; Krishnamurthy, A.; Adebayo, B. Abatement of Gaseous Volatile Organic Compounds: A Material Perspective. Catal. Today 2020, 350, 3–18. [Google Scholar] [CrossRef]
- Long, C.; Liu, P.; Li, Y.; Li, A.M.; Zhang, Q.X. Characterization of Hydrophobic Hyper-Cross-Linked Polymer as an Adsorbent for Removal of Chlorinated Volatile Organic Compounds. Environ. Sci. Technol. 2011, 45, 4506–4512. [Google Scholar] [CrossRef]
- Li, R.N.; Chong, S.J.; Altaf, N.; Gao, Y.S.; Louis, B.; Wang, Q. Synthesis of ZSM-5/Siliceous Zeolite Composites for Improvement of Hydrophobic Adsorption of Volatile Organic Compounds. Front. Chem. 2019, 7, 620. [Google Scholar] [CrossRef]
- Chun, J.; Kang, S.; Park, N.; Park, E.J.; Jin, X.; Kim, K.D.; Seo, H.O.; Lee, S.M.; Kim, H.J.; Kwon, W.H.; et al. Metal-Organic Framework@Microporous Organic Network: Hydrophobic Adsorbents with a Crystalline Inner Porosity. J. Am. Chem. Soc. 2014, 136, 6786–6789. [Google Scholar] [CrossRef]
- Kim, K.J.; Ahn, H.G. The Effect of Pore Structure of Zeolite on the Adsorption of VOCs and Their Desorption Properties by Microwave Heating. Microporous Mesoporous Mater. 2012, 152, 78–83. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Al-Muhtaseb, A.H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Patdhanagul, N.; Srithanratana, T.; Rangsriwatananon, K.; Hengrasmee, S. Ethylene adsorption on cationic surfactant modified zeolite NaY. Microporous Mesoporous Mater. 2010, 131, 97–102. [Google Scholar] [CrossRef]
- Liu, S.; Peng, Y.; Chen, J.J.; Shi, W.B.; Yan, T.; Li, B.; Zhang, Y.N.; Li, J.H. Engineering surface functional groups on mesoporous silica: Towards a humidity-resistant hydrophobic adsorbent. J. Mater. Chem. A 2018, 6, 13769–13777. [Google Scholar] [CrossRef]
- Sarker, A.I.; Aroonwilas, A.; Veawab, A. Equilibrium and Kinetic Behaviour of CO2 Adsorption onto Zeolites, Carbon Molecular Sieve and Activated Carbons. Energy Procedia 2017, 114, 2450–2459. [Google Scholar] [CrossRef]
- Girimonte, R.; Formisani, B.; Testa, F. Adsorption of CO2 on a confined fluidized bed of pelletized 13X zeolite. Powder Technol. 2017, 311, 9–17. [Google Scholar] [CrossRef]
- Valverde, J.M.; Quintanilla, M.A.S. Attrition of Ca based CO2 adsorbents by a high velocity gas jet. AIChE J. 2013, 59, 1096–1107. [Google Scholar] [CrossRef]
- Lu, X.; Ren, T.; Cao, P.; Wang, Z.; Liu, L.; He, J.; Chen, X.; May, E.F.; Li, G.K. Construction of high performance binder-free zeolite monolith. Chem. Eng. J. 2022, 447, 137558. [Google Scholar] [CrossRef]
- Vasiliev, P.O.; Shen, Z.J.; Hodgkins, R.P.; Bergstrom, L. Meso/macroporous, mechanically stable silica monoliths of complex shape by controlled fusion of mesoporous spherical particles. Chem. Mater. 2006, 18, 4933–4938. [Google Scholar] [CrossRef]
- Thakkar, H.; Lawson, S.; Rownaghi, A.A. Development of 3D-printed polymer-zeolite composite monoliths for gas separation. Chem. Eng. J. 2018, 348, 109–116. [Google Scholar] [CrossRef]
- Li, Y.Y.; Perera, S.P.; Crittenden, B.D.; Bridgwater, J. The effect of the binder on the manufacture of a 5A zeolite monolith. Powder Technol. 2001, 116, 85–96. [Google Scholar] [CrossRef]
- Aranzabal, A.; Iturbe, D.; Romero-Saez, M.P.; Gonzalez-Marcos, M.P.; Gonzalez-Velasco, J.R.; Gonzalez-Marcos, J.A. Optimization of process parameters on the extrusion of honeycomb shaped monolith of H-ZSM-5 zeolite. Chem. Eng. J. 2010, 162, 415–423. [Google Scholar] [CrossRef]
- Whiting, G.T.; Chung, S.H.; Stosic, D.; Chowdhury, A.D.; van der Wal, L.I.; Fu, D.; Zecevic, J.; Travert, A.; Houben, K.; Baldus, M.; et al. Multiscale mechanistic insights of shaped catalyst body formulations and their impact on catalytic properties. ACS Catal. 2019, 9, 4792–4803. [Google Scholar] [CrossRef]
- Raganati, F.; Alfe, M.; Gargiulo, V.; Chirone, R.; Ammendola, P. Isotherms and thermodynamics of CO2 adsorption on a novel carbon-magnetite composite sorbent. Chem. Eng. Res. Des. 2018, 134, 540–552. [Google Scholar] [CrossRef]
- Tang, X.; Ripepi, N.; Stadie, N.P.; Yu, L.; Hall, M.R. A dual-site Langmuir equation for accurate estimation of high pressure deep shale gas resources. Fuel 2016, 185, 10–17. [Google Scholar] [CrossRef]
- Wang, Z.; Goyal, N.; Liu, L.; Tsang, D.C.W.; Shang, J.; Liu, W.; Li, G. N–doped porous carbon derived from polypyrrole for CO2 capture from humid flue gases. Chem. Eng. J. 2020, 396, 125376. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Zhang, H.; Yan, J.; Wu, Y.; Wu, D.; Chen, H.; Ma, X. Effect of hollow structure on the acetone adsorption property of tungsten-substituted MFI zeolite. CIESC J. 2022, 73, 1194–1206. [Google Scholar] [CrossRef]
- Chafik, T.; Harti, S.; Cifredo, G.; Gatica, J.M.; Vidal, H. Easy extrusion of honeycomb-shaped monoliths using Moroccan natural clays and investigation of their dynamic adsorptive behavior towards VOCs. J. Hazard. Mater. 2009, 170, 87–95. [Google Scholar] [CrossRef]
- Rioland, G.; Nouali, H.; Daou, T.J.; Faye, D.; Patarin, J. Adsorption of volatile organic compounds in composite zeolites pellets for space decontamination. Adsorption 2017, 23, 395–403. [Google Scholar] [CrossRef]
- Chouat, N.; Maziz, A.; Bensafi, B. Hierarchized ZSM-5 Zeolite Synthesis using N-Methyldiethanolamine as a Novel Desilicate Agent: Impact on Methanol Adsorption and Resistance Improvement to Coke Poisoning. J. Inorg. Organomet. Polym. 2024, 34, 5154–5164. [Google Scholar] [CrossRef]
- Yue, M.B.; Sun, L.B.; Zhuang, T.T.; Dong, X.; Chun, Y.; Zhu, J.H. Directly transforming as-synthesized MCM-41 to mesoporous MFI zeolite. J. Mater. Chem. 2008, 18, 2044–2050. [Google Scholar] [CrossRef]
- Wang, C.; Guo, H.; Leng, S.Z.; Yu, J.; Feng, K.; Cao, L.; Huang, J. Regulation of hydrophilicity/hydrophobicity of aluminosilicate zeolites: A review. Crit. Rev. Solid. State Mater. Sci. 2021, 46, 330–348. [Google Scholar] [CrossRef]
- Chen, C.; Son, W.J.; You, K.S.; Ahn, J.W.; Ahn, W.S. Carbon Dioxide Capture Using Amine-Impregnated HMS Having Textural Mesoporosity. Chem. Eng. J. 2010, 161, 46–52. [Google Scholar] [CrossRef]
- Khoramzadeh, E.; Mofarahi, M.; Lee, C.H. Equilibrium Adsorption Study of CO2 and N2 on Synthesized Zeolites 13X, 4A, 5A, and Beta. J. Chem. Eng. Data 2019, 64, 5648–5664. [Google Scholar] [CrossRef]
- Genli, N.; Kutluay, S.; Baytar, O.; Sahin, Ö. Preparation and Characterization of Activated Carbon from Hydrochar by Hydrothermal Carbonization of Chickpea Stem: An Application in Methylene Blue Removal by RSM Optimization. Int. J. Phytoremediation 2022, 24, 88–100. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Liu, Y.S.; Li, Z.Y.; Yang, X. Performance of mesoporous silicas (MCM-41 and SBA-15) and carbon (CMK-3) in the removal of gas-phase naphthalene: Adsorption capacity, rate and regenerability. RSC Adv. 2016, 6, 21193–21203. [Google Scholar] [CrossRef]
- Akinpelu, A.A.; Ali, M.E.; Johan, M.R.; Saidur, R.; Chowdhury, Z.Z.; Shemsi, A.M.; Saleh, T.A. Effect of the Oxidation Process on the Molecular Interaction of Polyaromatic Hydrocarbons (PAH) with Carbon Nanotubes: Adsorption Kinetic and Isotherm Study. J. Mol. Liq. 2019, 289, 111107. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Mofarahi, M. Pure and Binary Adsorption Equilibria of Methane and Nitrogen on Zeolite 5A. J. Chem. Eng. Data 2014, 59, 626–639. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Equilibrium Isotherms Studies for Light Hydrocarbons Adsorption on 4A Molecular Sieve Zeolite. J. Pet. Sci. Eng. 2013, 108, 316–320. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Lu, H.F.; Jiang, B.; Zhu, Q.L.; Zhou, Y.; Huang, H.F.; Chen, Y.F. Adsorption and Electrothermal Desorption of Volatile Organic Compounds on Activated Carbon Fiber. Chin. J. Environ. Sci. 2016, 36, 1981–1987. [Google Scholar]
- Jia, H.X.; Huang, W.; Ren, C.M.; Su, L.X.; Pei, P. Study on the Regeneration Mechanism of Silica Gel for Ammonia Adsorption; Guangzhou Chemical Industry: Guangzhou, China, 2023; Volume 51, pp. 32–35. ISSN 1004-1014. [Google Scholar] [CrossRef]
- Huang, H.F.; Chen, H.Y.; Chen, H.; Lu, H.F. Adsorption and Desorption Properties of Polymeric Resin for n-Hexane. J. Zhejiang Univ. Technol. 2015, 43, 532–536. [Google Scholar]
- Li, Y.; Zhang, H.X.; Yan, K.L.; Wang, Q.; Zou, B.; Jiang, S.X. Adsorption of Volatile Organic Compounds (VOCs) by MOF Materials. Guangzhou Chem. Ind. 2016, 44, 3. [Google Scholar]
- Luo, Y. Simulation Experimental Study on Propane Concentration Detection in Crude Oil Volatiles Using Laser Gas Analyzer. Contemp. Chem. Ind. 2016, 45, 2036–2038. [Google Scholar]
- Xu, J.N.; Li, Q.; Qin, Y.C.; Song, L.J. Molecular Simulation of Adsorption Behavior of Butane Isomers on MFI Zeolite. Pet. Refin. Chem. Eng. 2022, 53, 46–53. [Google Scholar]
- Huang, H.F.; Rong, W.J.; Gu, Y.Y.; Chang, R.Q.; Lu, H.F. Adsorption and desorption of VOCs on the ZSM-5 zeolite. Acta Sci. Circumstantiae 2014, 34, 3144–3151. [Google Scholar]
- Yue, X.; Wang, S.; Gao, J.; Wang, S.; Ding, W. Effects of mesopore size on ethyl acetate adsorption–desorption behaviors over hierarchical ZSM-5/MCM-41 molecular sieves. Sep. Purif. Technol. 2024, 336, 126228. [Google Scholar] [CrossRef]
- Chen, G.; Liu, W.; Li, Q.; Wang, Y.; Sun, L.; Zhao, J.; Dong, J.; Xu, S.; Yu, X.; Wang, M.; et al. Adsorption mechanism and quantum chemical calculation of six aliphatic VOCs on industrial ZSM-5 zeolites. J. Solid State Chem. 2025, 344, 125181. [Google Scholar] [CrossRef]






| Component | Content (%) | |
|---|---|---|
| ZSM-5 | SMZ | |
| SiO2 | 98.5687 | 98.6912 |
| Na2O | 0.4465 | 0.3865 |
| Al2O3 | 0.2924 | 0.2655 |
| Fe2O3 | 0.1842 | 0.1632 |
| P2O5 | 0.1355 | 0.1165 |
| K2O | 0.1326 | 0.1218 |
| CaO | 0.0438 | 0.0324 |
| MgO | 0.0230 | 0.0152 |
| Other | 0.1733 | 0.2077 |
| Sample | SBET (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Average Pore Size (nm) | Micropore Size (nm) |
|---|---|---|---|---|---|
| MZ | 106.25 | 0.12 | 0.05 | 5.27 | 0.553 |
| SMZ | 349.51 | 0.37 | 0.26 | 4.19 | 0.495 |
| Theoretical Model | Parameter | MZ | SMZ |
|---|---|---|---|
| Langmuir model | qe/(mg·g−1) | 42.7 | 109.2 |
| KL/(L·mg−1) | 0.073 | 0.24 | |
| R2 | 0.93 | 0.98 | |
| Freundlich model | KF/(L·mg−1) | 18.5 | 52.3 |
| n | 1.8 | 2.2 | |
| R2 | 0.96 | 0.93 | |
| D-R model | qe/(mg·g−1) | 42.1 | 88.4 |
| β/(mol2·kJ−2) | 6.2 × 10−7 | 2.5 × 10−7 | |
| E/(kJ/mol) | 7.7 | 11.8 | |
| R2 | 0.95 | 0.97 |
| Materials | Adsorbate | qe/(mg·g−1) | References |
|---|---|---|---|
| Activated biochar | Phenol | 106.2 | [38] |
| ZSM-5 | propane | 101.6 | [39] |
| 4A molecular sieve | Ethane | 78.3 | [40] |
| SMZ | LPG | 109.2 | This study |
| Materials | Adsorbate | Desorption Method | Recovery Rate | References |
|---|---|---|---|---|
| Activated Carbon | Benzene | Electro-thermal | 79% (4 times) | [41] |
| Silica Gel | Ammonia | Vacuum + purging | 81% (3 times) | [42] |
| Polymer Resin | n-Hexane | Thermal | 32% (4 times) | [43] |
| MOF | n-Hexane | Radiation | 83% (3 times) | [44] |
| SMZ | LPG | Thermal + Purging | 96% (5 times) | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Qi, W.; Nie, L.; Wang, B. Synthesis of Sandwich-Structured Zeolite Molecular Sieves and Their Adsorption Performance for Volatile Hydrocarbons. Materials 2025, 18, 1758. https://doi.org/10.3390/ma18081758
Liu T, Qi W, Nie L, Wang B. Synthesis of Sandwich-Structured Zeolite Molecular Sieves and Their Adsorption Performance for Volatile Hydrocarbons. Materials. 2025; 18(8):1758. https://doi.org/10.3390/ma18081758
Chicago/Turabian StyleLiu, Tongyuan, Wenxing Qi, Lihong Nie, and Beifu Wang. 2025. "Synthesis of Sandwich-Structured Zeolite Molecular Sieves and Their Adsorption Performance for Volatile Hydrocarbons" Materials 18, no. 8: 1758. https://doi.org/10.3390/ma18081758
APA StyleLiu, T., Qi, W., Nie, L., & Wang, B. (2025). Synthesis of Sandwich-Structured Zeolite Molecular Sieves and Their Adsorption Performance for Volatile Hydrocarbons. Materials, 18(8), 1758. https://doi.org/10.3390/ma18081758





