Study of Properties of Novel Geopolymers Prepared with Slate Stone Cutting Sludge and Activated with Olive Stone Bottom Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Properties
2.2. Mixture Design and Methodology
3. Results and Discussions
3.1. Physical Characterization of Geopolymer
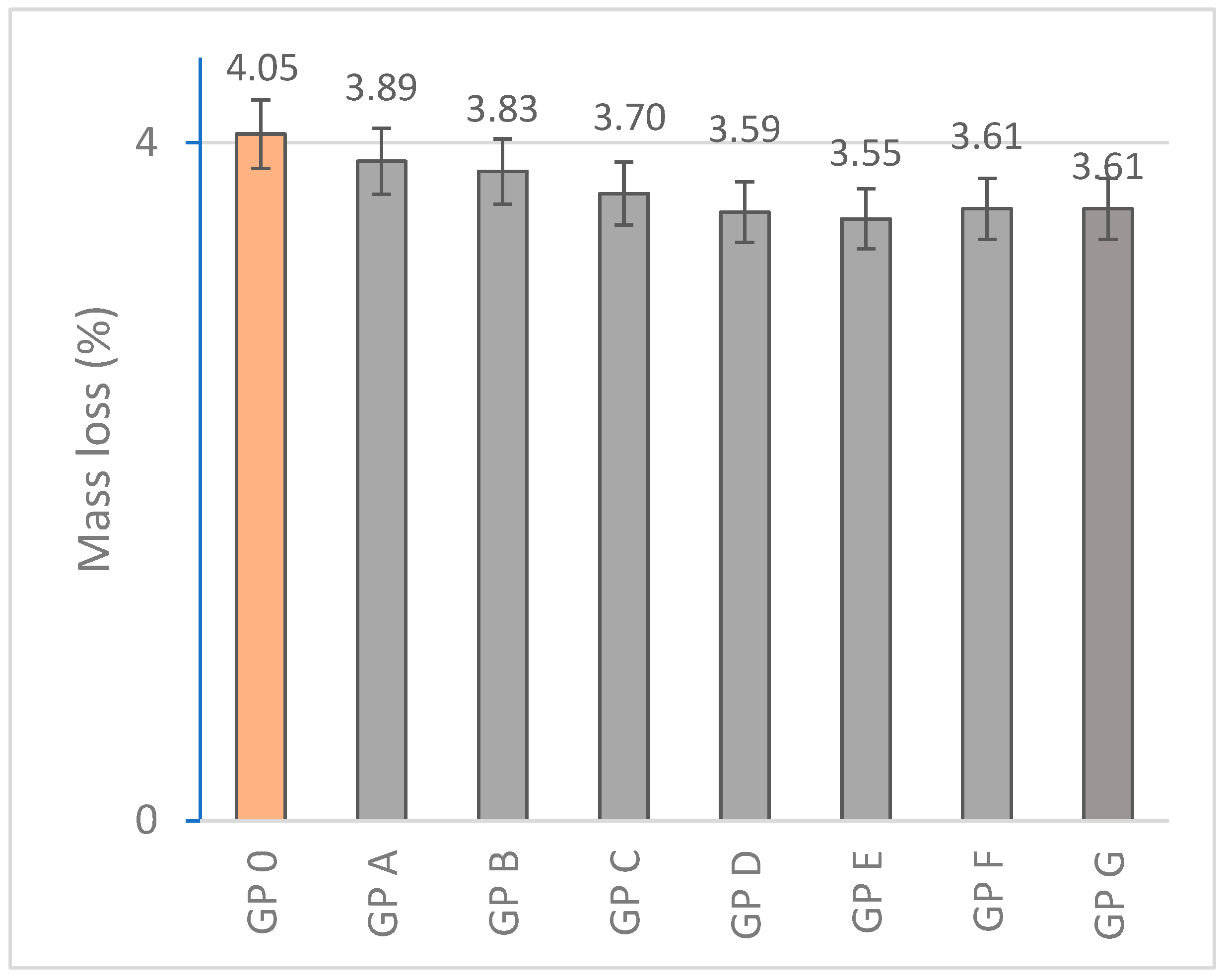
3.1.1. Determination of Mass Loss
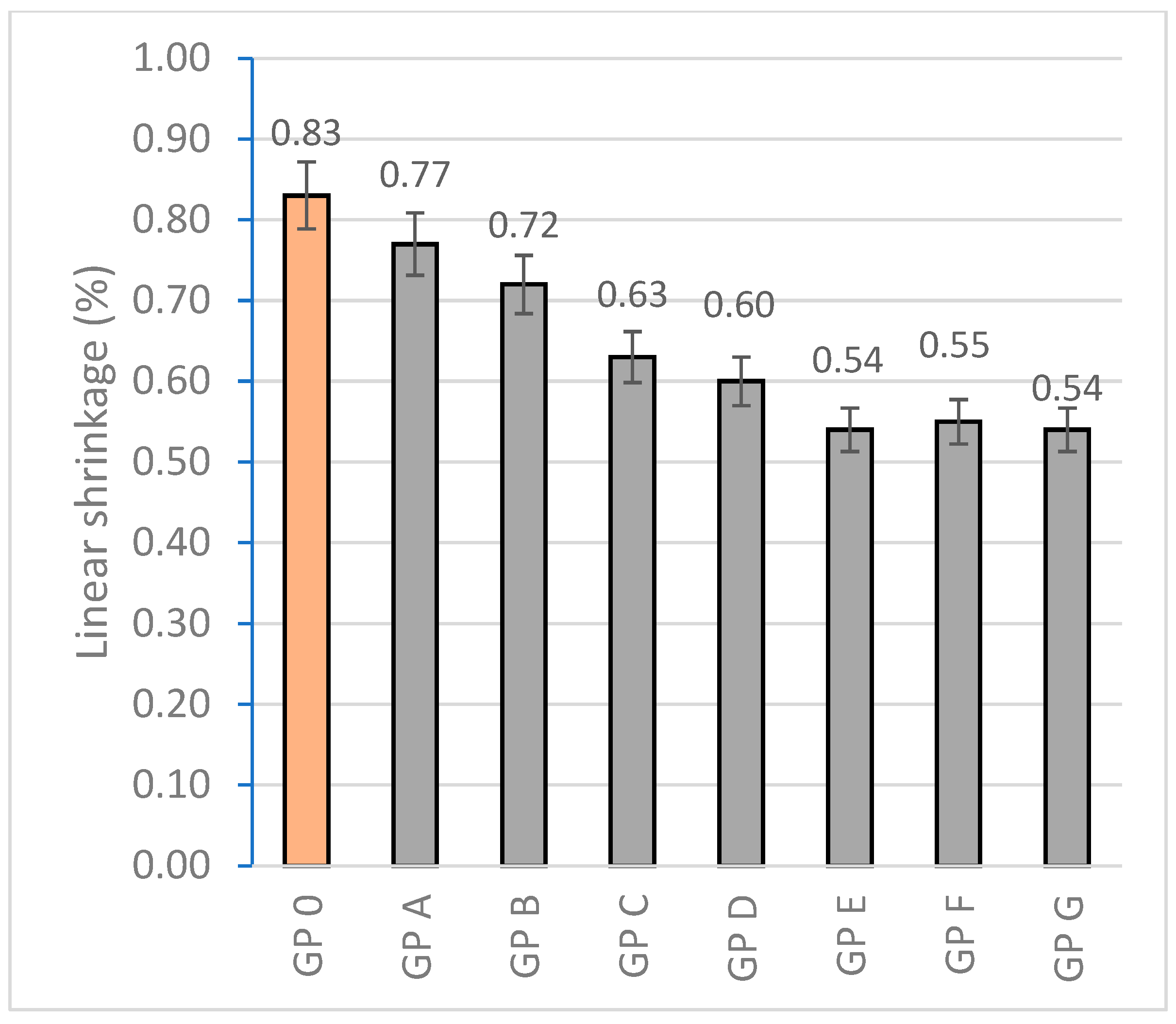
3.1.2. Determination of Linear Shrinkage
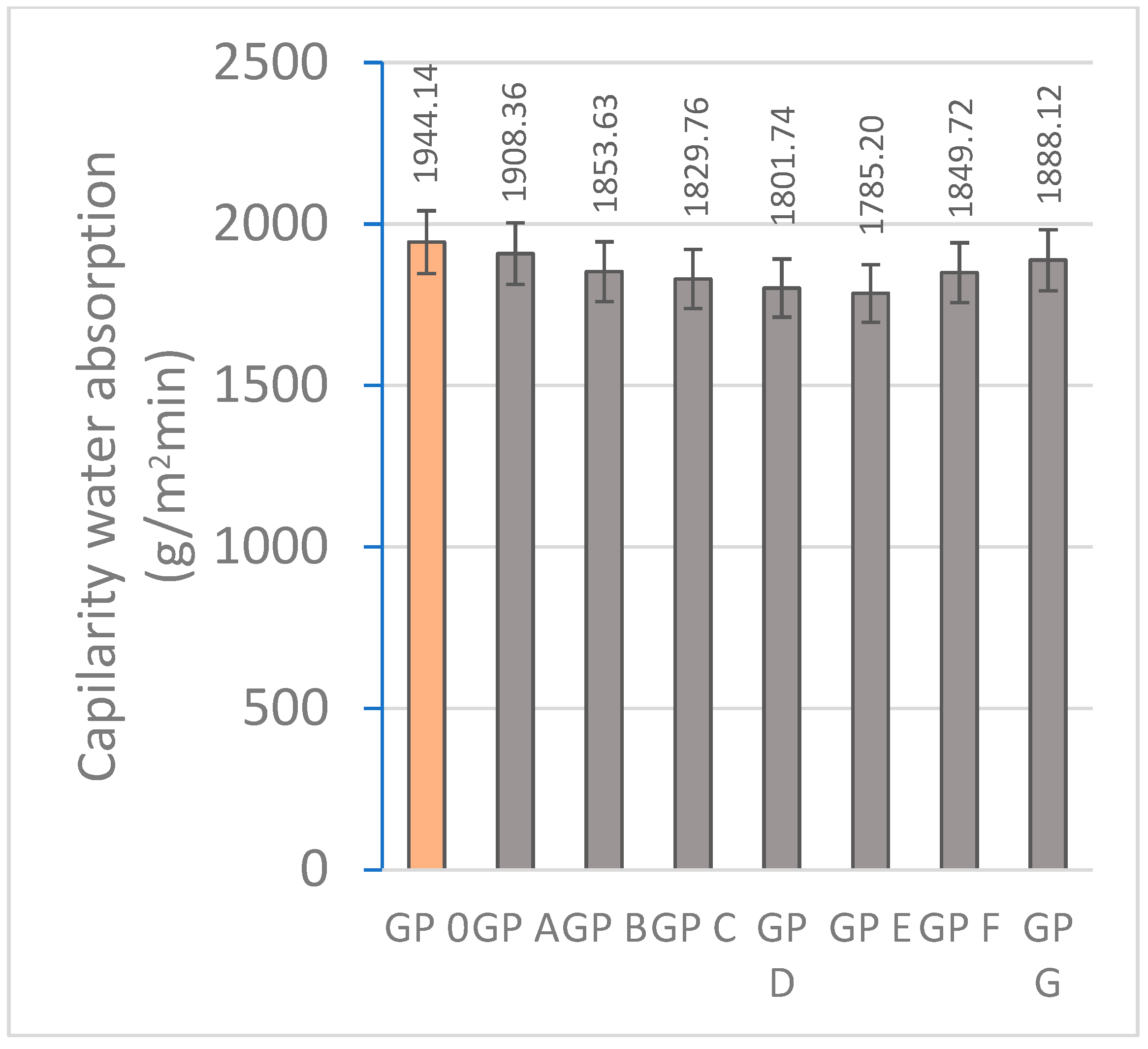
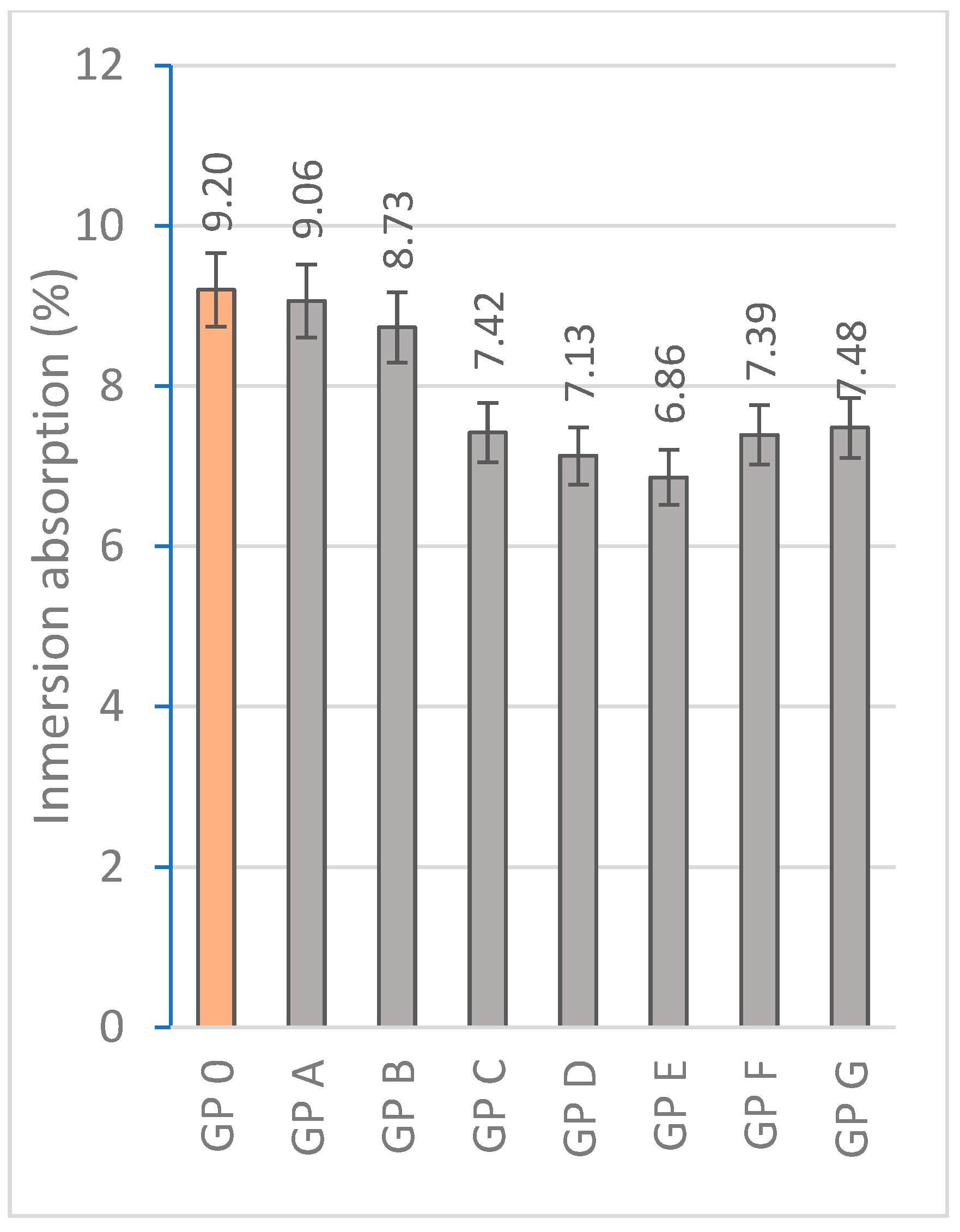
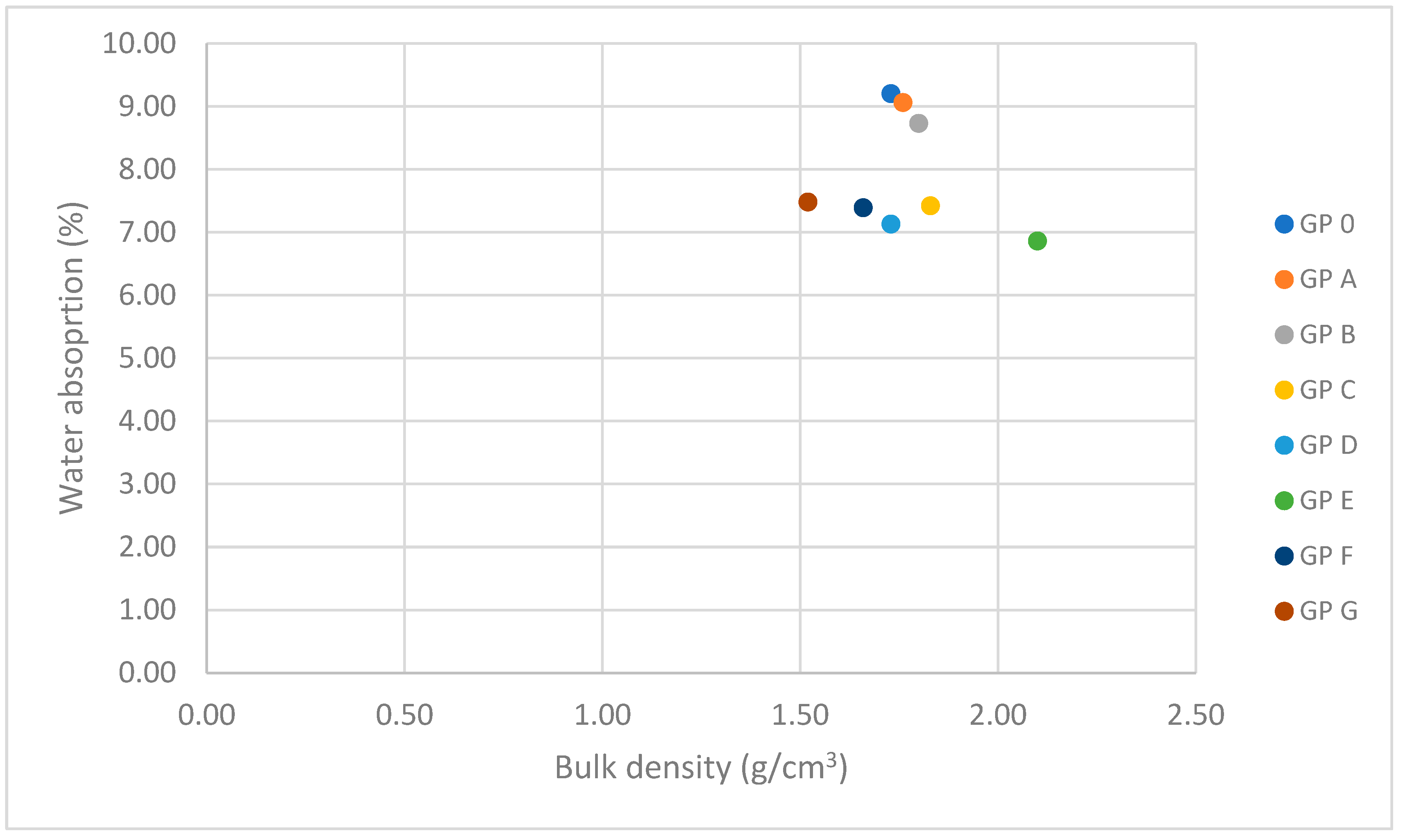
3.1.3. Determination of Water Absorption by Capillary Action and Immersion
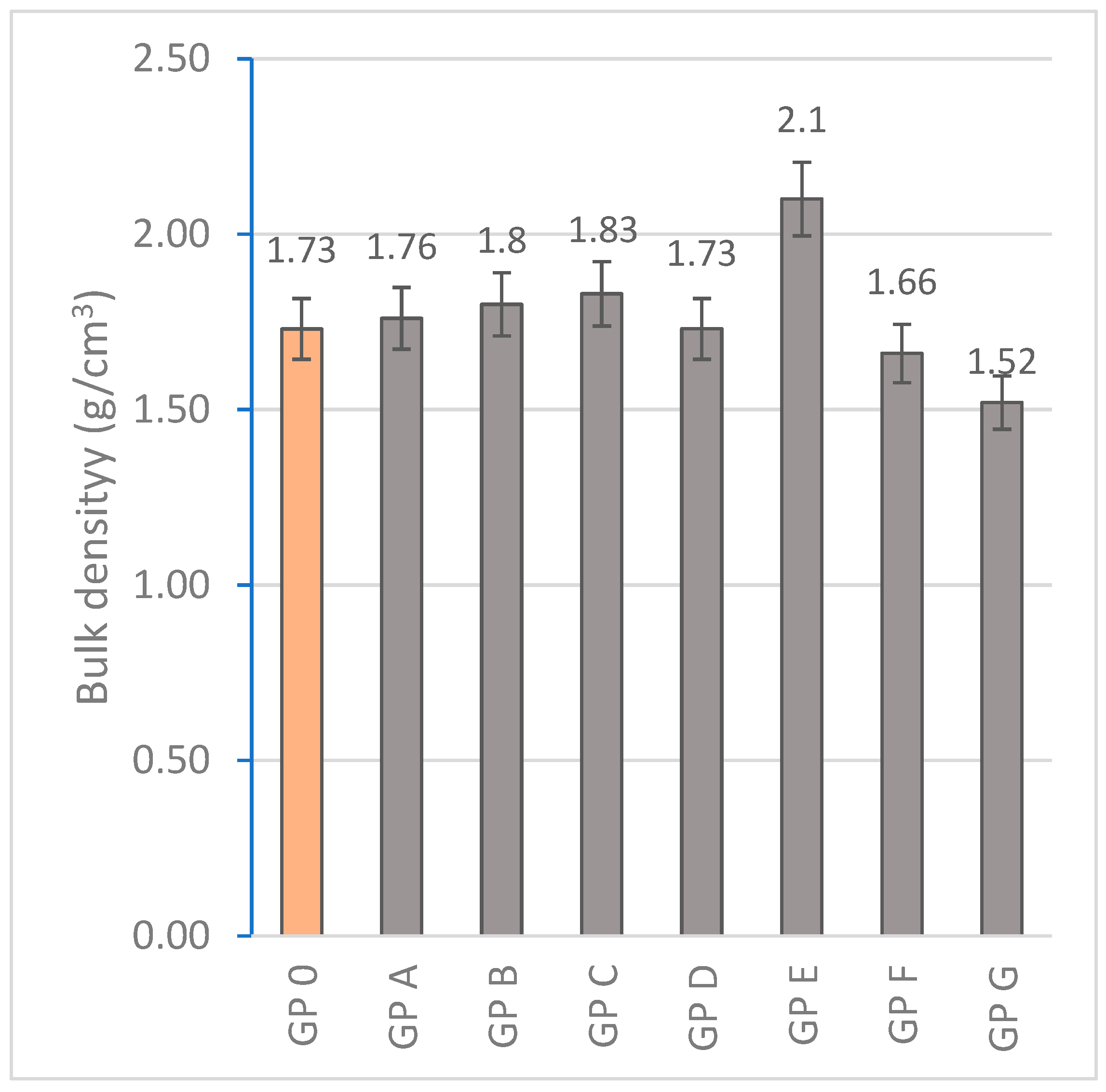
3.1.4. Determination of Bulk Density
3.1.5. Determination of Porosity
3.1.6. Determination of Thermal Conductivity
3.1.7. Evaluation of Freeze–Thaw Cycles
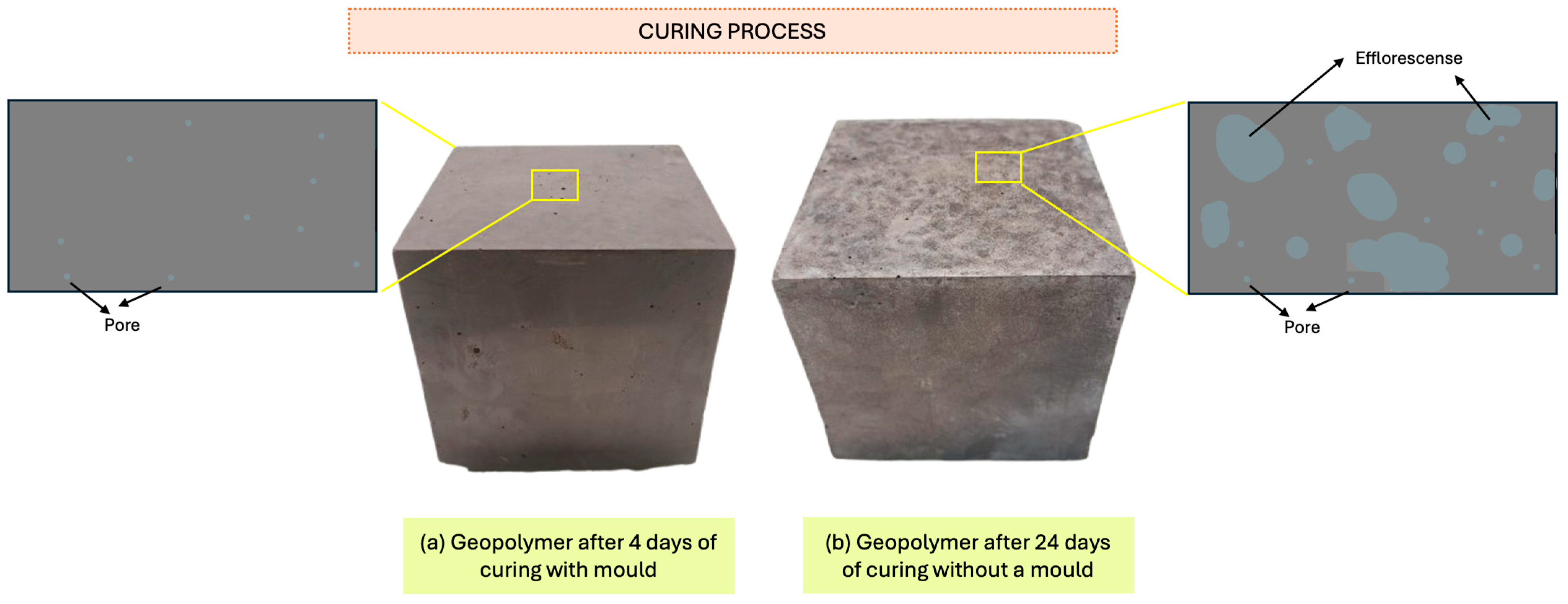
3.1.8. Efflorescence Evaluation
3.2. Chemical Characterization of Geopolymers
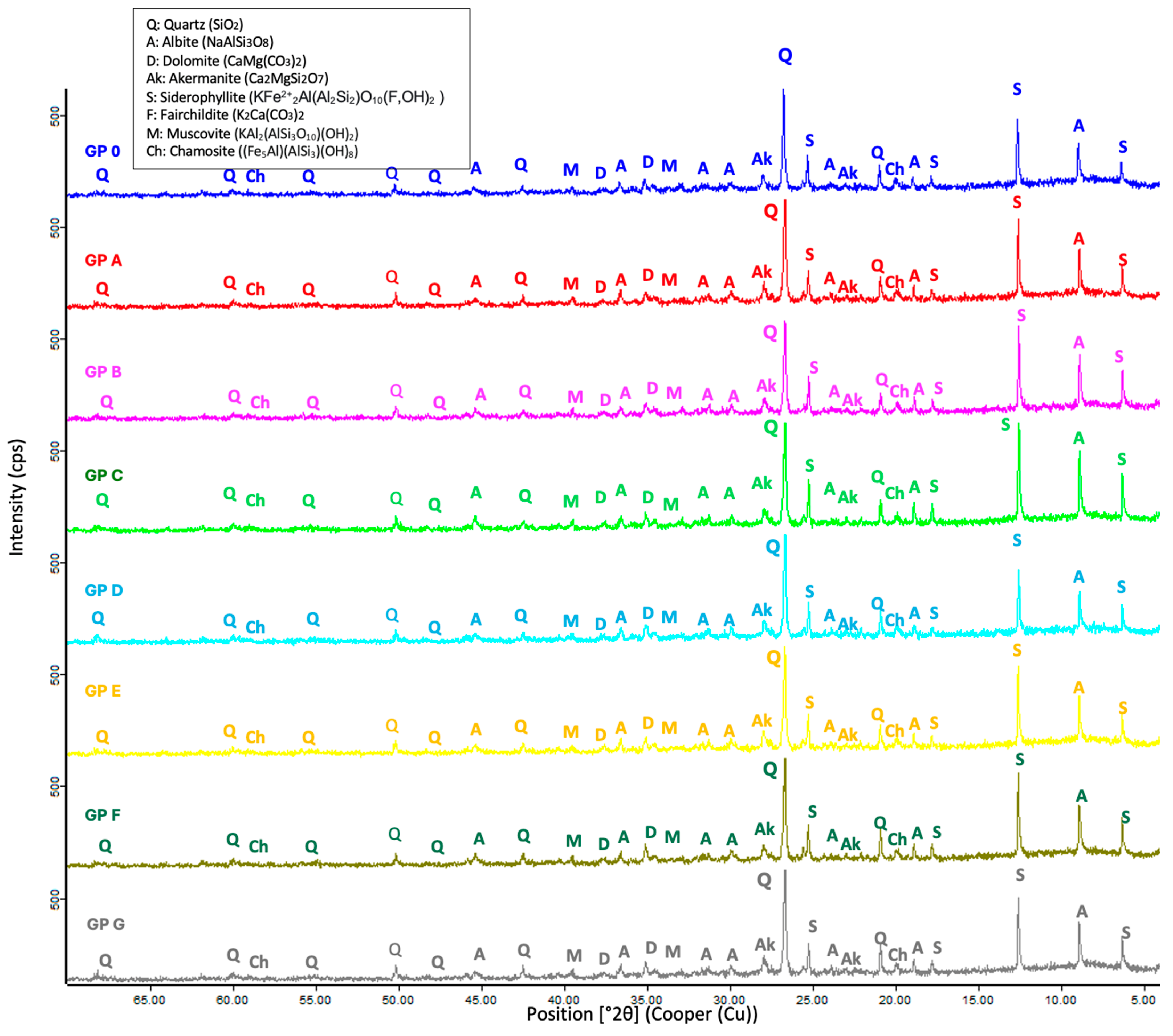
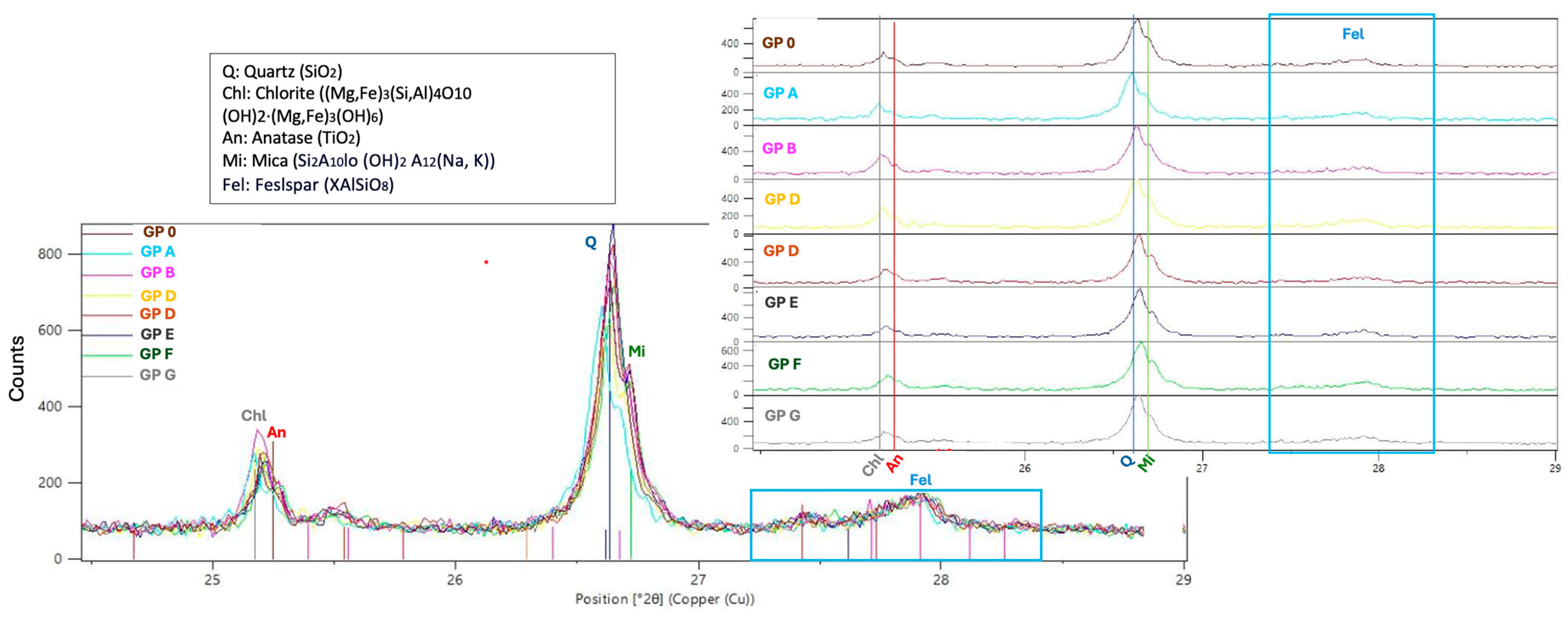
3.2.1. XRD Analysis
3.2.2. FTIR Analysis
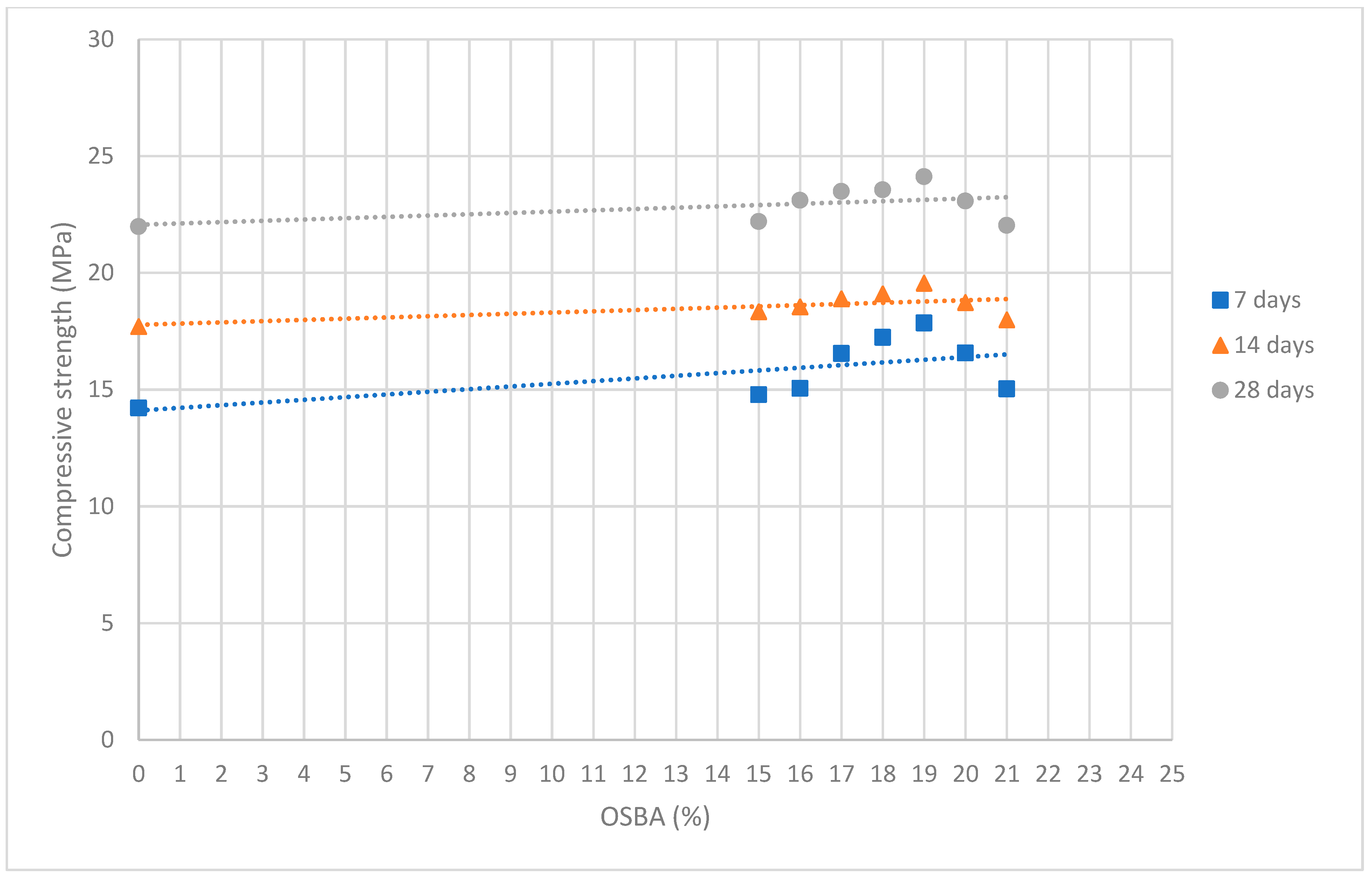
3.3. Mechanical Characterization of Geopolymers
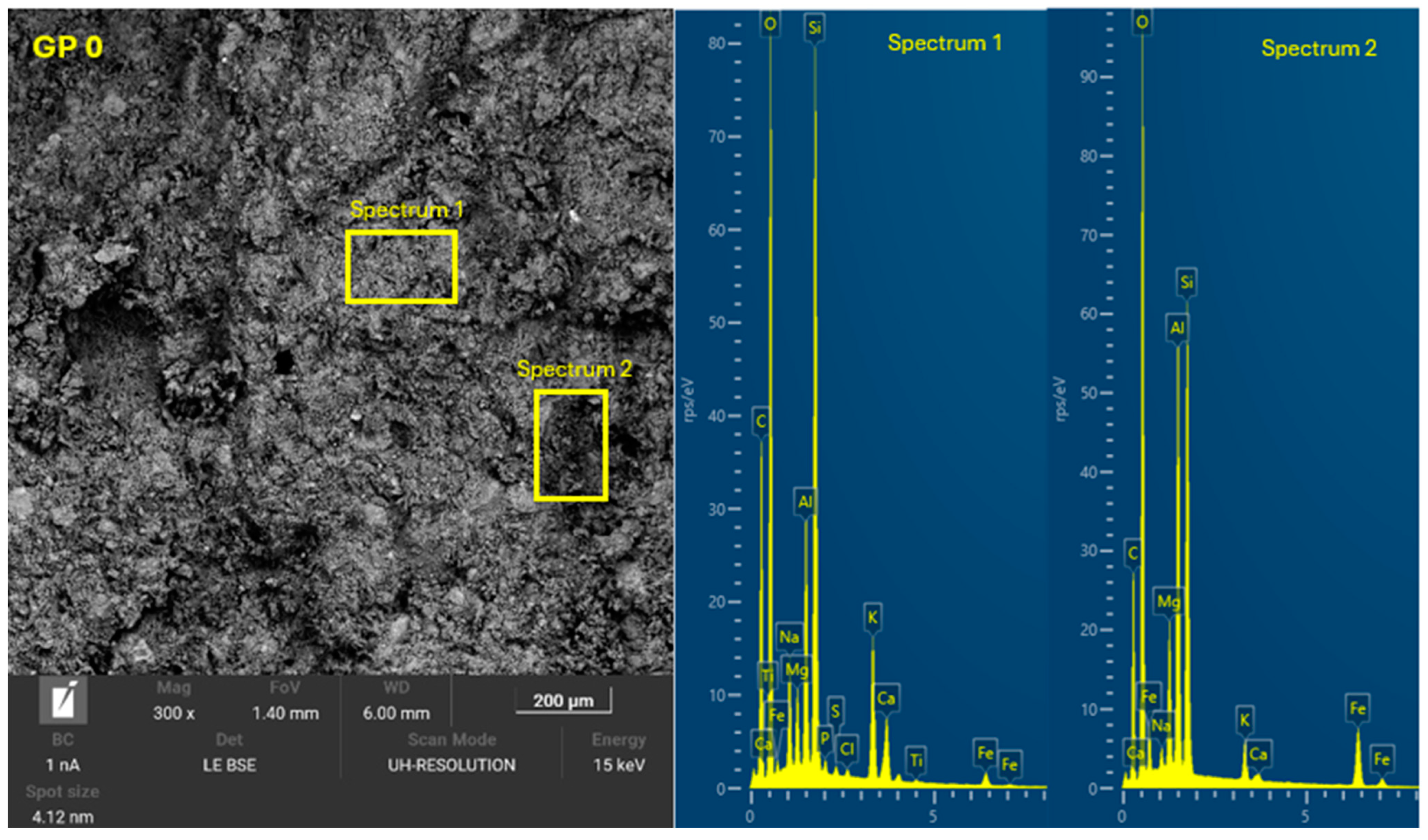
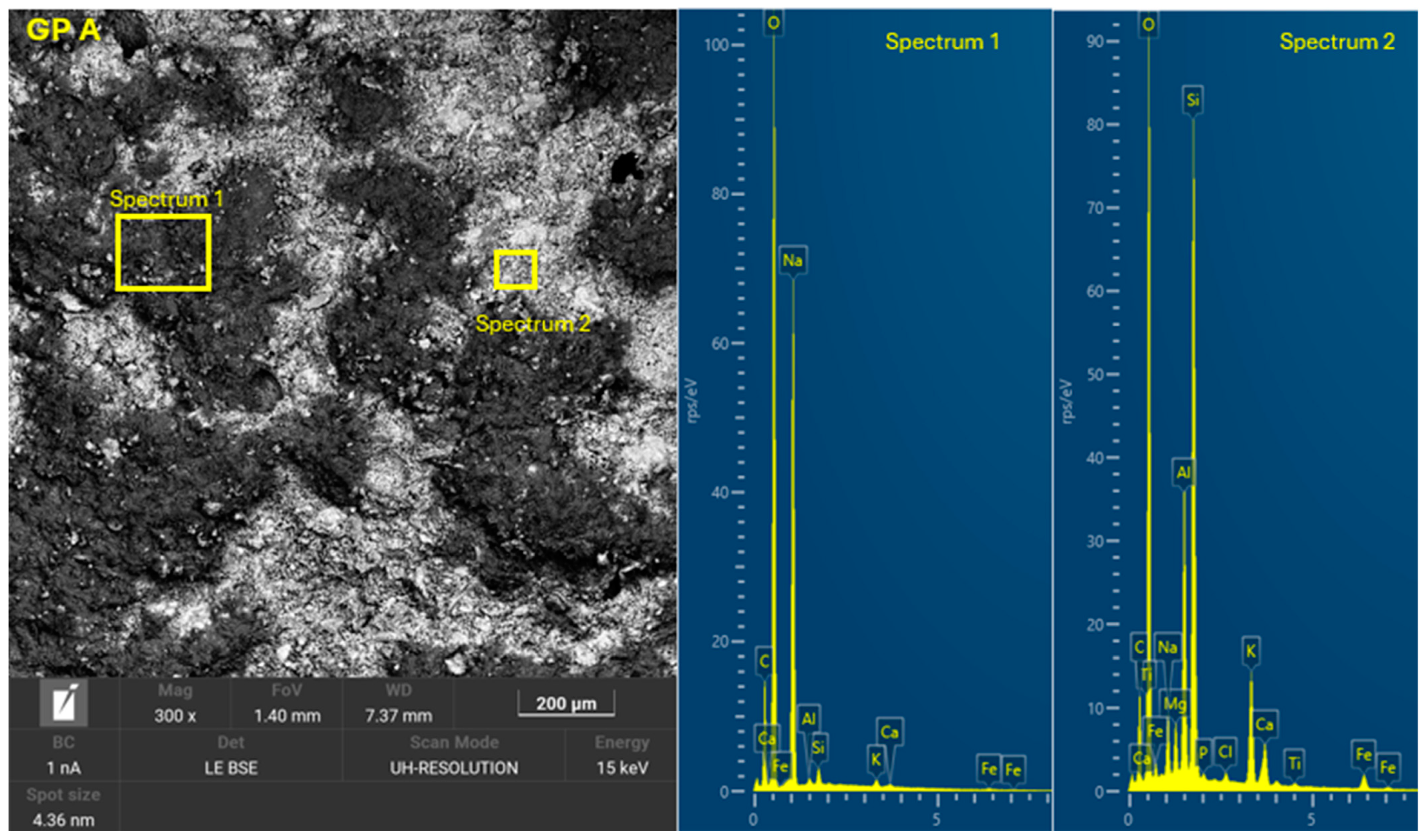
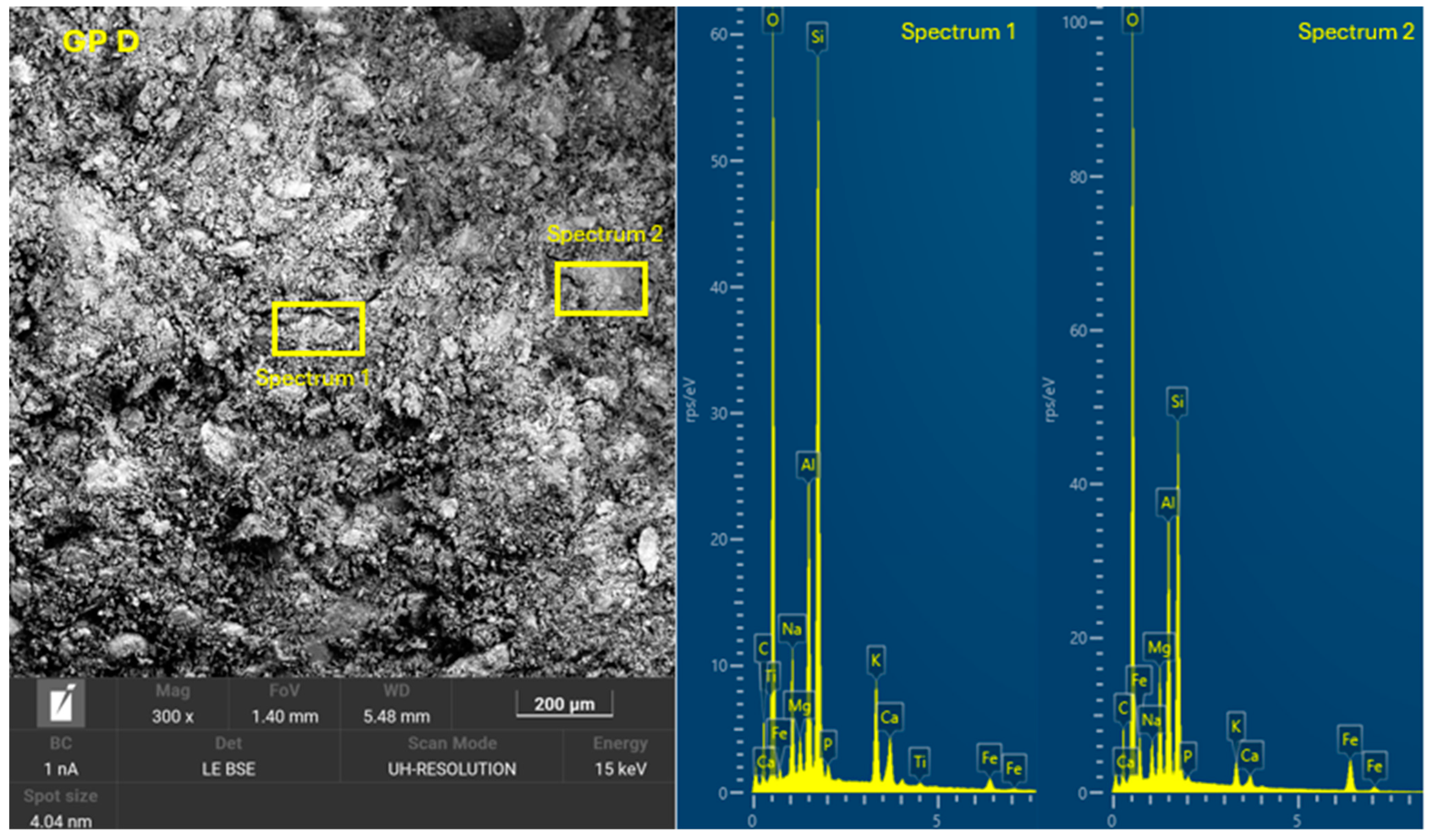
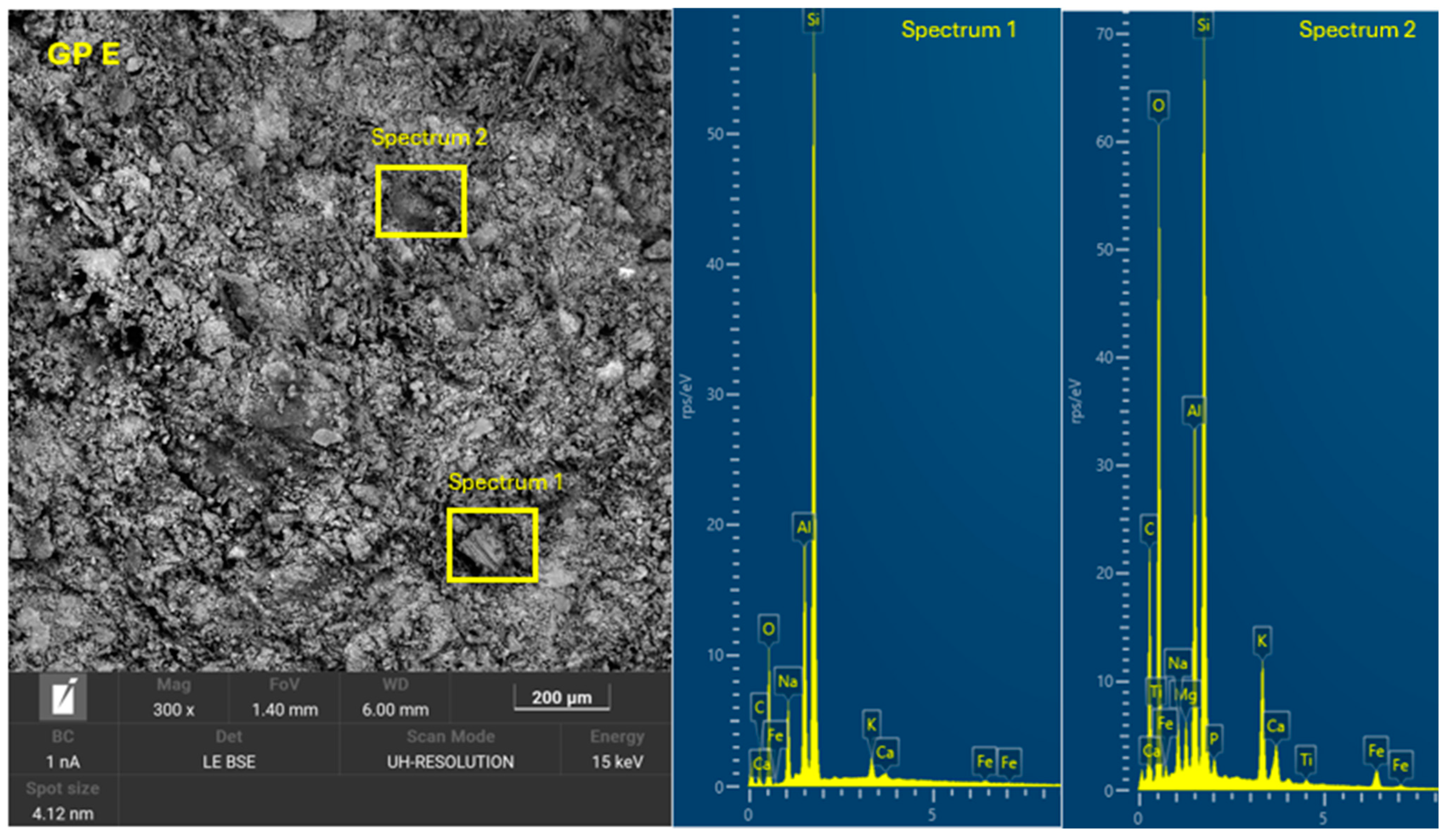
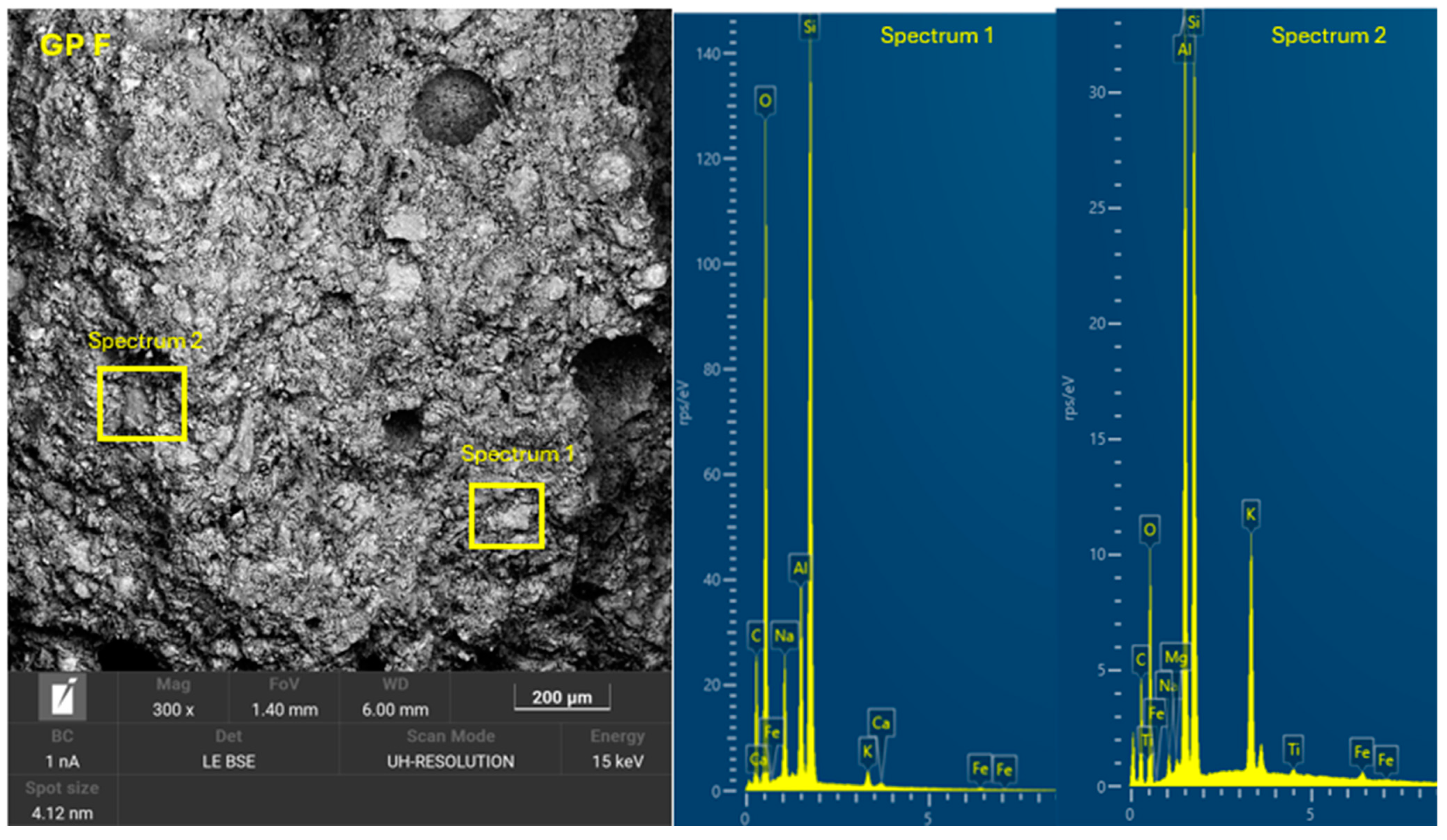
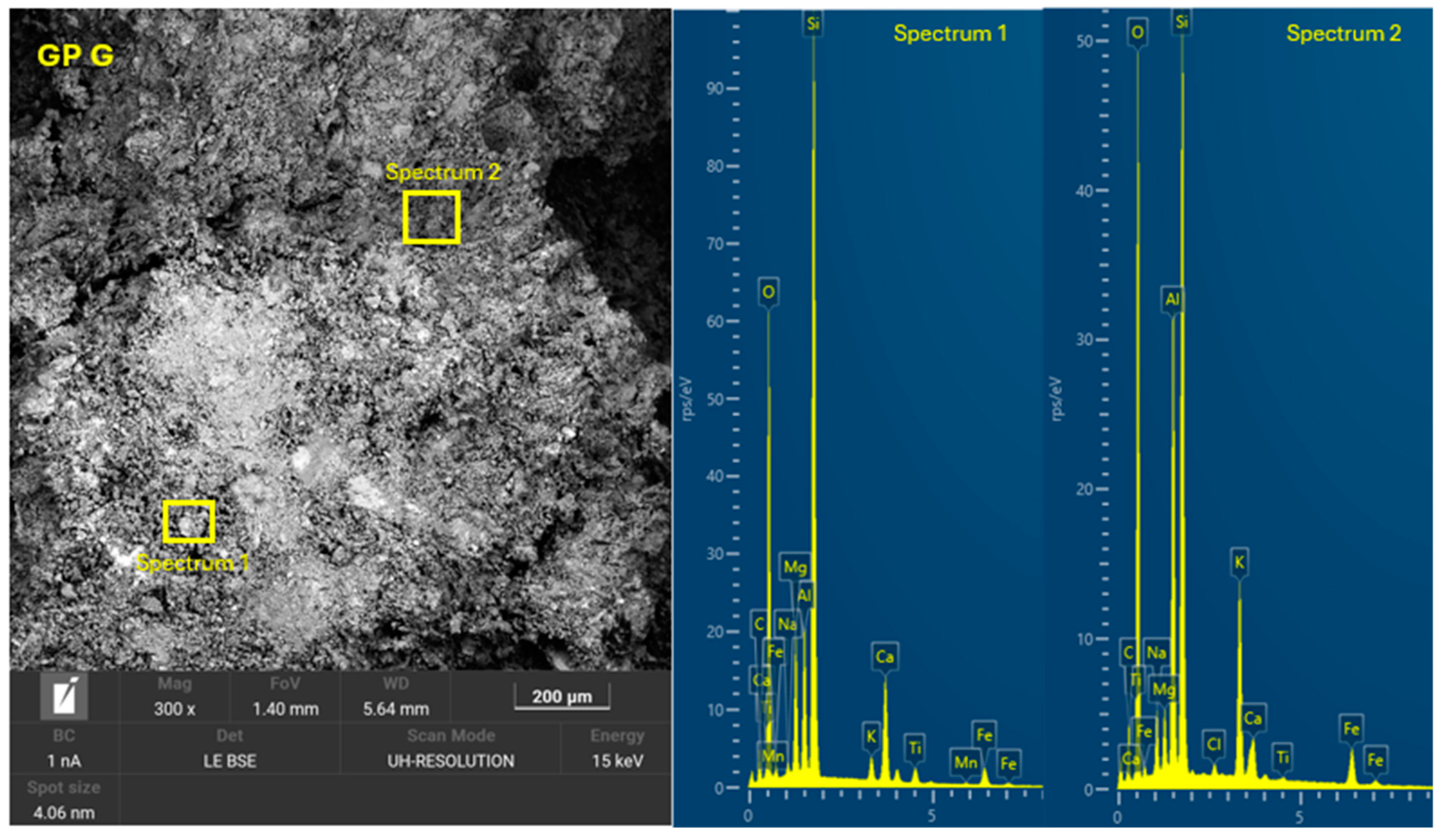
3.4. Microscopic Characterization of Geopolymers
4. Conclusions
- Physical characterization showed that GP E performed best with a Na2SiO3/OSBA ratio of 1.0 which favors the formation of (C, N)-A-S-H gels and forms dense matrices with reduced porosity.
- The XRD analysis of the mortars indicates that the geopolymers are composed of crystalline phases with quartz inclusions, suggesting the incomplete dissolution of the precursors. The presence of albite plays a key role in the formation of the geopolymer gel, influencing the structure and cohesion of the matrix, which may affect both the reactivity of the system and its mechanical properties.
- The compressive strength results demonstrate the feasibility of using OSBA in the activation of SSCS- and CH-based geopolymers. GP E showed an increase in compressive strength value and reached 24.12 MPa at 28 days of curing, 15.16% higher than GP 0 influenced by the reactivity of the geopolymer gel.
- SEM-EDX showed the formation of C-A-S-H and N-A-S-H phases present in the geopolymer gel forming an interconnected network. The resulting gel composition is characterized by balanced Si/Al and Na/Si ratios.
- The manufacturing process of geopolymers would be scalable due to the fact that high curing temperatures are not required and that the main materials come from the by-products of other industries that are obtained at low cost.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.H.; Wang, H.; Zhong, W.L.; Fan, L.F. Development of a high-strength lightweight geopolymer concrete for structural and thermal insulation applications. Case Stud. Constr. Mater. 2024, 21, e03949. [Google Scholar] [CrossRef]
- Nasreddine, H.; Salem, T.; Djerbi, A.; Dujardin, N.; Gautron, L. Enhanced thermal insulation behavior of metakaolin-based geopolymer reinforced by miscanthus fibers. Appl. Clay Sci. 2024, 258, 107496. [Google Scholar] [CrossRef]
- IEA. IEA—International Energy Agency—IEA. (s. f.). Available online: https://www.iea.org/data-and-statistics (accessed on 19 December 2024).
- IEA. Energy Efficiency 2024—Analysis—IEA. (2024, 1 Noviembre). Available online: https://www.iea.org/reports/energy-efficiency-2024 (accessed on 19 December 2024).
- Contreras-Aguilar, J.A.; Gijón-Rivera, M.; Rivera-Solorio, C.I.; Noh-Pat, F. Thermo-economic assessment of a double-layer phase-change material in building roofs in a semi-arid climate. Therm. Sci. Eng. Prog. 2025, 57, 103093. [Google Scholar] [CrossRef]
- Flores Nicolás, M.; Chávez, M.M.; Vlasova, M.; Pi Puig, T. Low-temperature sintering of ceramic bricks from clay, waste glass and sand. Boletín De La Soc. Española De Cerámica Y Vidr. 2024, 63, 377–388. [Google Scholar] [CrossRef]
- Jayaweera, J.M.N.; Narayana, M.; Adikary, S.U. Modeling and simulation of ceramic tiles linear shrinkage variation during the sintering process. Forces Mech. 2024, 15, 100274. [Google Scholar] [CrossRef]
- Belfiore, C.M.; Parisi, S.; Menta, S.; Mazzoleni, P. Use of volcanic ash and chamotte as substitute temper in the production of ceramic tiles. Appl. Clay Sci. 2024, 262, 107603. [Google Scholar] [CrossRef]
- Bintarto, R.; Purnowidodo, A.; Darmadi, D.B.; Widodo, T.D. The effect of composite thickness as thermal insulation roof coating on room temperature reduction. Salud Cienc. Y Tecnologia 2022, 2, 100543. [Google Scholar] [CrossRef]
- Siriboon, J.; Magaraphan, R. Devulcanization and functionalization of ground tire rubber for the novel metal sheet roof with strong sound absorber and thermal insulation. Clean. Eng. Technol. 2025, 24, 100865. [Google Scholar] [CrossRef]
- Yin, Y.; Song, Y.; Chen, W.; Yan, Y.; Wang, X.; Hu, J.; Zhao, B.; Ren, S. Thermal environment analysis of enclosed dome with double-layered PTFE fabric roof integrated with aerogel-glass wool insulation mats: On-site test and numerical simulation. Energy Build. 2022, 254, 111621. [Google Scholar] [CrossRef]
- Arumugam, C.; Shaik, S.; Roy, A.; Kontoleon, K.J.; Cuce, E.; Shaik, A.H.; Chakraborty, S.; Alwetaishi, M.; Cuce, P.M.; Gupta, M. Analysis of the benefits of adopting roof sandwich panels integrated with PCM versus PUR to mitigate energy costs and carbon dioxide emissions. J. Energy Storage 2024, 77, 109947. [Google Scholar] [CrossRef]
- Mostafa, S.A.; Agwa, I.S.; Elboshy, B.; Zeyad, A.M.; Seddik Hassan, A.M. The effect of lightweight geopolymer concrete containing air agent on building envelope performance and internal thermal comfort. Case Stud. Constr. Mater. 2024, 20, e03365. [Google Scholar] [CrossRef]
- Narattha, C.; Wattanasiriwech, S.; Wattanasiriwech, D. Sustainable, multifunctional fly ash geopolymer composite with rice husk aggregates for improved acoustic, hygric, and thermal performance. Constr. Build. Mater. 2024, 445, 137743. [Google Scholar] [CrossRef]
- de Masi, R.F.; Ruggiero, S.; Russo, A.; Vanoli, G.P. Geopolymer-based insulated wall incorporating construction and demolition waste: Experimental assessment of thermo-physical behavior. Energy Build. 2024, 322, 114743. [Google Scholar] [CrossRef]
- El hammouti, A.; Channouf, S.; Charai, M.; Horma, O.; Miri, H.; Mezrhab, A.; Karkri, M.; Tankari, M.A. Resource deposit, characterization and energy saving potential of olive pomace as a promising aggregate for energy efficient earth bricks in eastern Morocco. Constr. Build. Mater. 2023, 393, 131989. [Google Scholar] [CrossRef]
- el Hammouti, A.; Charai, M.; Mezrhab, A.; Karkri, M. Fired and unfired clay bricks incorporating olive pomace: A comparative study. Mater. Today Proc. 2022, 60, 359–364. [Google Scholar] [CrossRef]
- Khobragade, K. Impacr of minning activity on enrinment: An overview. Int. J. Sci. Res. Publ. 2020, 20, 10191. [Google Scholar] [CrossRef]
- Youssef, N.; Rabenantoandro, A.Z.; Dakhli, Z. Reuse of waste bricks: A new generation of geopolymer bricks. SN Appl. Sci. 2019, 1, 1252. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahmed, I.; Basit, A.; Umar, M.; Liaqat, N.; Khan, M.N.A. Use of slate in making lightweight concrete. IOP Conf. Ser. Mater. Sci. Eng. 2018, 414, 012007. [Google Scholar] [CrossRef]
- Astariani, N.K.; Salain, I.M.A.K.; Sutarja, I.N.; Widiarsa, I.B.R. Setting time of geopolymer binder based on Umeanyar slate stone powder. IOP Conf. Ser. Earth Environ. Sci. 2021, 871, 012002. [Google Scholar] [CrossRef]
- Mousa, A. Utilization of coal bottom ash from thermal power plants as a cement replacement for building: A promising sustainable practice. J. Build. Eng. 2023, 74, 106885. [Google Scholar] [CrossRef]
- Park, J.; Wang, J.J.; Seo, D. Agronomic and environmental performance of bottom ash discharged from biomass-based thermal power plant. Sustain. Chem. Pharm. 2023, 33, 101097. [Google Scholar] [CrossRef]
- Nieves, L.J.J.; Elyseu, F.; Goulart, S.; De Souza Pereira, M.; Valvassori, E.Z.; Bernardin, A.M. Use of fly and bottom ashes from a thermoelectrical plant in the synthesis of geopolymers: Evaluation of reaction efficiency. Energy Geosci. 2020, 2, 167–173. [Google Scholar] [CrossRef]
- Lan, T.; Meng, Y.; Ju, T.; Chen, Z.; Du, Y.; Deng, Y.; Song, M.; Han, S.; Jiang, J. Synthesis and application of geopolymers from municipal waste incineration fly ash (MSWI FA) as raw ingredient—A review. Resour. Conserv. Recycl. 2022, 182, 106308. [Google Scholar] [CrossRef]
- Łach, M.; Mierzwiński, D.; Korniejenko, K.; Mikuła, J.; Hebda, M. Geopolymers as a material suitable for immobilization of fly ash from municipal waste incineration plants. J. Air Waste Manag. Assoc. 2018, 68, 1190–1197. [Google Scholar] [CrossRef]
- Alhawat, M.; Yildirim, G.; Ashour, A.; Ozcelikci, E.; Aldemir, A.; Sahmaran, M. A study on the influencing parameters in developing construction and demolition waste-based geopolymer concretes and their sustainability assessment. Constr. Build. Mater. 2024, 426, 136143. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Cao, Z.; Blom, J.; Vuye, C.; Gu, F. Feasibility of using building-related construction and demolition waste-derived geopolymer for subgrade soil stabilization. J. Clean. Prod. 2024, 450, 142001. [Google Scholar] [CrossRef]
- Fatimah, I.; Citradewi, P.W.; Iqbal, R.M.; Ghazali, S.A.I.S.M.; Yahya, A.; Purwiandono, G. Geopolymer from tin mining tailings waste using Salacca leaves ash as activator for dyes and peat water adsorption. S. Afr. J. Chem. Eng. 2023, 43, 257–265. [Google Scholar] [CrossRef]
- Chkala, H.; Ighir, S.; Ettahiri, W.; Taleb, M.; Chigr, M.; el Mansouri, N.E. Feasibility of incorporating leaf date palm fibers in geopolymer composites made from mining waste. Constr. Build. Mater. 2024, 428, 136188. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Q.; Liu, Y.; Xue, T.; Yu, Q.; Chen, S. Influence of new organic alkali activators on microstructure and strength of fly ash geopolymer. Ceram. Int. 2022, 48, 12442–12449. [Google Scholar] [CrossRef]
- Santos, A.; Andrejkovičová, S.; Perná, I.; Almeida, F.; Rocha, F. Mechanical and thermal properties of geopolymers derived from metakaolin with iron mine waste. Appl. Clay Sci. 2024, 258, 107452. [Google Scholar] [CrossRef]
- Yanti, E.D.; Mubarok, L.; Erlangga, B.D.; Widyaningsih, E.; Jakah; Pratiwi, I.; Rinovian, A.; Nugroho, T.; Herbudiman, B. Utilization of various ceramic waste as fine aggregate replacement into fly ash-based geopolymer. Mater. Lett. 2024, 357, 135651. [Google Scholar] [CrossRef]
- Onaizi, A.M.; Tang, W.; Amran, M.; Liu, Y.; Sajjad, U.; Alhassan, M. Towards increased adoption of furnace bottom ash as sustainable building materials: Characterization, standardization, and applications. J. Build. Eng. 2024, 82, 108274. [Google Scholar] [CrossRef]
- Ullah, A.; Kassim, A.; Rashid, A.S.A.; Huang, Y.; Mohd Yunus, N.Z.; Zhu, C.; Khan, I.; Apandi, N.M. Strength development of bottom ash based geopolymer and their application in columns to improve soft soil underneath embankment: Achieving sustainability in ground improvement. Transp. Geotech. 2025, 50, 101463. [Google Scholar] [CrossRef]
- Bian, Z.; Lu, J.X.; Huang, Y.; Xuan, D.; Ou, G.; Sun Poon, C. Recycling of waste glass in lightweight geopolymer using incineration bottom ash as a foaming agent: Towards energy conservation. Constr. Build. Mater. 2023, 400, 132632. [Google Scholar] [CrossRef]
- Informe. Redes de Calor con Biomasa (2022). AVEBIOM. (Consulted 14th November). Available online: https://www.avebiom.org/proyectos/informes (accessed on 21 December 2024).
- Font, A.; Soriano, L.; de Moraes Pinheiro, S.M.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. Design and properties of 100% waste-based ternary alkali-activated mortars: Blast furnace slag, olive-stone biomass ash and rice husk ash. J. Clean. Prod. 2020, 243, 118568. [Google Scholar] [CrossRef]
- de Moraes Pinheiro, S.M.; Font, A.; Soriano, L.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. Olive-stone biomass ash (OBA): An alternative alkaline source for the blast furnace slag activation. Constr. Build. Mater. 2018, 178, 327–338. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Tayeh, B.A.; Abu Aisheh, Y.I.; Salih, M.N.A. Utilising olive-stone biomass ash and examining its effect on green concrete: A review paper. J. Mater. Res. Technol. 2023, 24, 7091–7107. [Google Scholar] [CrossRef]
- Los Santos-Ortega, J.; Fraile-García, E.; Ferreiro-Cabello, J. Environmental assessment of the use of ground olive stones in mortars. Reduction of CO2 emissions and production of sustainable mortars for buildings. Environ. Impact Assess. Rev. 2025, 110, 107709. [Google Scholar] [CrossRef]
- Petlitckaia, S.; Gharzouni, A.; Hyvernaud, E.; Texier-Mandoki, N.; Bourbon, X.; Rossignol, S. Influence of the nature and amount of carbonate additions on the thermal behaviour of geopolymers: A model for prediction of shrinkage. Constr. Build. Mater. 2021, 296, 123752. [Google Scholar] [CrossRef]
- Mao, L.; Zhou, H.; Peng, M.; Hu, L.; Zhang, W. Effects of waste glass particle size on improving the property and environmental safety of fired brick containing electroplating sludge. Constr. Build. Mater. 2020, 257, 119583. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Silveira, N.C.G.; Martins, M.L.F.; da Silva Bezerra, A.C.; da Silva Araújo, F.G. Ecological geopolymer produced with a ternary system of red mud, glass waste, and Portland cement. Clean. Eng. Technol. 2022, 6, 100379. [Google Scholar] [CrossRef]
- Pu, S.; Xu, B.; Duan, W.; Yao, H.; Wu, Z.; Mei, G.; Cai, G. A green phosphate-based geopolymer adsorbent or binder with high specific surface area for environmental applications. Constr. Build. Mater. 2023, 408, 133738. [Google Scholar] [CrossRef]
- Zheng, P.; Tan, X.; Du, Z.; Liu, X.; Zhou, Y.; Shen, K.; Guan, B.; Chen, W. Microscopic damage and deterioration of carbonaceous slate in cold region subjected to freeze-thaw cycles. J. Rock Mech. Geotech. Eng. 2025, 441, 137471. [Google Scholar] [CrossRef]
- Santos, F.D.; da Conceição LRv Ceron, A.; de Castro, H.F. Chamotte clay as potential low cost adsorbent to be used in the palm kernel biodiesel purification. Appl. Clay Sci. 2017, 149, 41–50. [Google Scholar] [CrossRef]
- Dehghani, A.; Aslani, F.; Ghaebi Panah, N. Effects of initial SiO2/Al2O3 molar ratio and slag on fly ash-based ambient cured geopolymer properties. Constr. Build. Mater. 2021, 293, 123527. [Google Scholar] [CrossRef]
- Lei, Z.; Pavia, S. Geopolymer based on biomass ash from agricultural residues. Constr. Build. Mater. 2024, 441, 137471. [Google Scholar] [CrossRef]
- Bernabeu, J.P.; Monzó, J.; Borrachero, M.V.; Soriano, L.; Tashima, M.M. Activadores alternativos para cementos de activación alcalina. Rev. Alconpat 2022, 12, 16–31. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, Z.; Zhang, J.; Kang, Z.; Pu, S.; Wan, Y. Utilization of OPC and FA to enhance reclaimed lime-fly ash macadam based geopolymers cured at ambient temperature. Constr. Build. Mater. 2021, 303, 124378. [Google Scholar] [CrossRef]
- Ju, S.; Bae, S.; Jung, J.; Park, S.; Pyo, S. Use of coal bottom ash for the production of sodium silicate solution in metakaolin-based geopolymers concerning environmental load reduction. Constr. Build. Mater. 2023, 391, 131846. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Z.; Huang, F.; Ni, C.; Wu, J.; Zheng, B. Utilizing municipal solid waste incineration bottom ash and volcanic tuff to produce geopolymer materials. Constr. Build. Mater. 2024, 425, 136015. [Google Scholar] [CrossRef]
- Capasso, Y.; Leer, S.; Flora, A.; Ferona, C.; Cioffi, D.; Liguori, B. Reuse of mining waste as aggregates in fly ash-based geopolymers. J. Clean. Prod. 2019, 220, 108568. [Google Scholar] [CrossRef]
- della Rocca, D.G.; de Noni Júnior, A.; Rodríguez-Aguado, E.; Peralta, R.A.; Rodríguez-Castellón, E.; Puma, G.L.; Moreira, R.F.P.M. Mechanistic insights on the catalytic ozonation of trimethoprim in aqueous phase using geopolymer catalysts produced from mining waste. J. Environ. Chem. Eng. 2023, 11, 111163. [Google Scholar] [CrossRef]
- Gier Della Rocca, D.; Santos e Sousa, F.A.; Domingos Ardisson, J.; Peralta, R.A.; Rodríguez-Castellón, E.; Peralta Muniz Moreira, R.d.F. Magnetic mining waste based-geopolymers applied to catalytic reactions with ozone. Heliyon 2023, 9, e17097. [Google Scholar] [CrossRef]
- Kang, X.; Gan, Y.; Chen, R.; Zhang, C. Sustainable eco-friendly bricks from slate tailings through geopolymerization: Synthesis and characterization analysis. Constr. Build. Mater. 2021, 278, 122337. [Google Scholar] [CrossRef]
- Bouchukhi, A.; Amar, M.; Arroug, L.; Mahdi, A.; Haddaji, Y. Characterizing nano-indentation and microstructural properties of mine tailings-based geopolymers. Case Stud. Constr. Mater. 2024, 21, e03899. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, C.; Zhang, Z.; Jiang, S.; Ma, Z. Development of sustainable geopolymer with excavation soil powder as precursor: Cementitious properties and thermal-activated modification. J. Build. Eng. 2024, 91, 109745. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, C.; Lu, X.; Huang, B.; Chen, Z.; Lian, C. Effect of silica fume on the efflorescence, strength, and micro-properties of one-part geopolymer incorporating sewage sludge ash. Constr. Build. Mater. 2024, 436, 136840. [Google Scholar] [CrossRef]
- Gado, R.A.; Hebda, M.; Łach, M.; Mikuła, J. Alkali Activation of Waste Clay Bricks: Influence of The Silica Modulus, SiO2/Na2O, H2O/Na2O Molar Ratio, and Liquid/Solid Ratio. Materials 2020, 13, 383. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, R.; Jiang, X.; Li, W.; Zhu, X.; Huang, B. Effect of particle size and curing temperature on mechanical and microstructural properties of waste glass-slag-based and waste glass-fly ash-based geopolymers. J. Clean. Prod. 2020, 273, 122970. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Jin, C. Use of slaked lime and Portland cement to improve the resistance of MSWI bottom ash-GBFS geopolymer concrete against carbonation. Constr. Build. Mater. 2018, 166, 290–300. [Google Scholar] [CrossRef]
- Le Ping, K.K.; Cheah, C.B.; Liew, J.J.; Siddique, R.; Tangchirapat, W.; Johari, M.A.B.M. Coal bottom ash as constituent binder and aggregate replacement in cementitious and geopolymer composites: A review. J. Build. Eng. 2022, 52, 104369. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolímero: Química y Aplicaciones, 5th ed.; Institut Géopolymère: Saint-Quentin, Francia, 2020; ISBN 978-2-9544531-1-8. [Google Scholar]
- UNE-EN 772-16:2011; Methods of Test for Masonry Units—Part 16: Determination of Dimensions. AENOR: Madrid, Spain, 2011. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0047875 (accessed on 8 January 2025).
- UNE-EN 772-11:2011; Methods of Test for Masonry Units—Part 11: Determination of Water Absorption of Aggregate Concrete, Autoclaved Aerated Concrete, Manufactured Stone and Natural Stone Masonry Units Due to Capillary Action and the Initial Rate of Water Absorption of Clay Masonry Units. AENOR: Madrid, Spain, 2011. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0047874 (accessed on 8 January 2025).
- UNE-EN 772-21:2011; Methods of Test for Masonry Units—Part 21: Determination of Water Absorption of Clay and Calcium Silicate Masonry Units by Cold Water Absorption. AENOR: Madrid, Spain, 2011. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0047877 (accessed on 8 January 2025).
- UNE-EN 772-7:1999; Methods of Test for Masonry Units-Part 7: Determination of Water Absorption of Clay Masonry Damp Proof Course Units by Boiling in Water. AENOR: Madrid, Spain, 1999. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0009121 (accessed on 8 January 2025).
- UNE-EN 772-4:1999; Methods of Test for Masonry Units-Part 4: Determination of Real and Bulk Density and of Totral and Open Porosity por Natural Stone Masonry Units. AENOR: Madrid, Spain, 1999. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0009120 (accessed on 8 January 2025).
- UNE-EN 15304:2011; Determination of the Freeze-Thaw Resistance of Autoclaved Aerated Concrete. AENOR: Madrid, Spain, 2011. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0048353 (accessed on 8 January 2025).
- UNE-EN 12667:2002; Thermal Performance of Building Materials and Products. Determination of Thermal Resistance by Means of Guarded Hot Plate and Heat Flow Meter Methods. Prod. High Medium Thermal Resistance. AENOR: Madrid, Spain, 2002. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0027459 (accessed on 8 January 2025).
- UNE-EN 772-1:2011+A1:2016; Methods of Test for Masonry Units—Part 1: Determination of Compressive Strength. AENOR: Madrid, Spain, 2016. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=norma-une-en-772-1-2011-a1-2016-n0056681 (accessed on 8 January 2025).
- Ma, H.; Zhu, H.; Wu, C.; Fan, J.; Yang, S.; Hang, Z. Effect of shrinkage reducing admixture on drying shrinkage and durability of alkali-activated coal gangue-slag material. Constr. Build. Mater. 2021, 270, 121372. [Google Scholar] [CrossRef]
- el Abd, A.; Taman, M.; Behiry, R.N.; El-Naggar, M.R.; Eissa, M.; Hassan, A.M.A.; Bar, W.A.; Mongy, T.; Osman, M.; Hassan, A.; et al. Neutron imaging of moisture transport, water absorption characteristics and strength properties for fly ash/slag blended geopolymer mortars: Effect of drying temperature. Constr. Build. Mater. 2024, 449, 138436. [Google Scholar] [CrossRef]
- Wu, T.; Wu, J.; Zheng, C.; Wang, J. Evaluation of freeze-thaw resistance of geopolymer concrete incorporating GFRP waste powder. J. Build. Eng. 2024, 90, 109465. [Google Scholar] [CrossRef]
- Shee-Ween, O.; Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.A.B.; Li Ngee, H.; Chan, L.W.L.; Wan-En, O.; Jaya, N.A.; Yong-Sing, N. Cold-pressed fly ash geopolymers: Effect of formulation on mechanical and morphological characteristics. J. Mater. Res. Technol. 2021, 15, 3028–3046. [Google Scholar] [CrossRef]
- Mahfoud, E.; Ndiaye, K.; Maherzi, W.; Aggoun, S.; Benzerzour, M.; Abriak, N.E. Mechanical properties and shrinkage performance of one-part-geopolymer based on fly ash and micronized dredged sediments. Dev. Built Environ. 2023, 16, 100253. [Google Scholar] [CrossRef]
- Dinh, H.L.; Liu, J.; Doh, J.H.; Ong, D.E.L. Influence of Si/Al molar ratio and ca content on the performance of fly ash-based geopolymer incorporating waste glass and GGBFS. Constr. Build. Mater. 2024, 411, 134741. [Google Scholar] [CrossRef]
- Zhan, X.; Tang, L.; Yue, Z.; Lu, H.; Wang, J. Enhanced mechanical properties and mechanism of iron ore tailings-based geopolymers modified by municipal solid waste incineration fly ash. J. Build. Eng. 2024, 98, 111456. [Google Scholar] [CrossRef]
- Liang, D.; Tao, L.; Wang, F.; Lv, G. Synthesis of geopolymers using municipal solid waste incineration fly ash and construction and demolition waste: Mechanical and thermal activation. J. Environ. Chem. Eng. 2023, 11, 111249. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Refaat, O.A.; Xue, S.; Liew, K.M.; Dai, J.G. A comparative study of wet-mix vs. dry-mix to prepare geopolymer artificial aggregates utilizing municipal solid waste incineration bottom ash (IBA) via crushing technique. J. Build. Eng. 2024, 96, 110538. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Development and characterization of binary recycled ceramic tile and brick wastes-based geopolymers at ambient and high temperatures. Constr. Build. Mater. 2021, 301, 124138. [Google Scholar] [CrossRef]
- Ye, T.; Xiao, J.; Duan, Z.; Li, S. Geopolymers made of recycled brick and concrete powder—A critical review. Constr. Build. Mater. 2022, 330, 127232. [Google Scholar] [CrossRef]
- Hanumananaik, M.; Subramaniam, K.V.L. Shrinkage in low-calcium fly ash geopolymers for precast applications: Reaction product content and pore structure under drying conditions. J. Build. Eng. 2023, 78, 107583. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Z.; Sun, H.; Huo, W.; Zhang, J.; Wan, Y.; Zhang, C. Durability of waste concrete powder-based geopolymer reclaimed concrete under carbonization and freeze–thaw cycles. Constr. Build. Mater. 2023, 403, 133155. [Google Scholar] [CrossRef]
- Zhang, H.; Sarker, P.K.; Xiao, L.; Ai, J.; He, B.; Ren, Q.; Zhu, X.; Zhang, Y. Durability of low-carbon geopolymer mortar: Different responses to cryogenic attack caused by water content and freeze-thaw mediums. Cem. Concr. Compos. 2023, 139, 105065. [Google Scholar] [CrossRef]
- Min, Y.; Wu, J.; Li, B.; Zhang, M.; Zhang, J. Experimental study of freeze–thaw resistance of a one-part geopolymer paste. Case Stud. Constr. Mater. 2022, 17, e01269. [Google Scholar] [CrossRef]
- Huang, M.; Bao, S.; Zhang, Y.; Zhou, Z.; Jiao, X. Efflorescence behavior and mechanism of burnt coal cinder-based geopolymers under different alkali activators. Constr. Build. Mater. 2024, 455, 139057. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; Carvalho, J.M.F.; Ribeiro, J.C.L.; Albuini-Oliveira, N.M.; Andrade, I.K.R. Evaluation of eco-efficient geopolymer using chamotte and waste glass-based alkaline solutions. Case Stud. Constr. Mater. 2022, 16, e00847. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Durón-Sifuentes, M.; Díaz-Guillén, J.A.; Escalante-García, J.I. Effect of waste glass incorporation on the properties of geopolymers formulated with low purity metakaolin. Cem. Concr. Compos. 2020, 107, 103492. [Google Scholar] [CrossRef]
- Ghanim, H.A.A.E.; Alengaram, U.J.; Bunnori, N.M.; Ibrahim, M.S.I. Innovative in-house sodium silicate derived from coal bottom ash and its impact on geopolymer mortar. J. Build. Eng. 2025, 99, 111428. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Gado, R.A.; Feltin, N.; Ducourtieux, S.; Devoille, L. Recycling and utilization assessment of waste fired clay bricks (Grog) with granulated blast-furnace slag for geopolymer production. Process Saf. Environ. Prot. 2016, 103, 237–251. [Google Scholar] [CrossRef]
- Rashad, A.M.; Mohamed, R.A.E.; Zeedan, S.R.; El-Gamal, A.A. Basalt powder as a promising candidate material for improving the properties of fly ash geopolymer cement. Constr. Build. Mater. 2024, 435, 136805. [Google Scholar] [CrossRef]
- Kim, B.; Kang, J.; Shin, Y.; Yeo, T.M.; Heo, J.; Um, W. Effect of Si/Al molar ratio and curing temperatures on the immobilization of radioactive borate waste in metakaolin-based geopolymer waste form. J. Hazard. Mater. 2023, 458, 131884. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.H.; Dinh, H.L.; Ong, D.E.L.; Zi, G.; You, I. Effect of Si/Al molar ratio on the strength behavior of geopolymer derived from various industrial waste: A current state of the art review. Constr. Build. Mater. 2022, 329, 127134. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.H.; Ong, D.E.L.; Wang, S.; Yang, Y.; Dinh, H.L.; Zi, G. Correlation between dissolubilities of Si, Al, and Fe from aluminosilicate precursor and strength of fly ash-based geopolymer. Constr. Build. Mater. 2023, 393, 132107. [Google Scholar] [CrossRef]
- Zhang, S.; Keulen, A.; Arbi, K.; Ye, G. Waste glass as partial mineral precursor in alkali-activated slag/fly ash system. Cem. Concr. Res. 2017, 102, 29–40. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Villacorte-Tabelin, M. Synthesis and characterization of coal fly ash and palm oil fuel ash modified artisanal and small-scale gold mine (ASGM) tailings based geopolymer using sugar mill lime sludge as Ca-based activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef]
- Oyun-Erdene, G.; Temuujin, J. Effect of Mechanical Activation of Fluidized Bed Fly Ash on Geopolymer Properties. Solid State Phenom. 2019, 288, 51–58. [Google Scholar] [CrossRef]
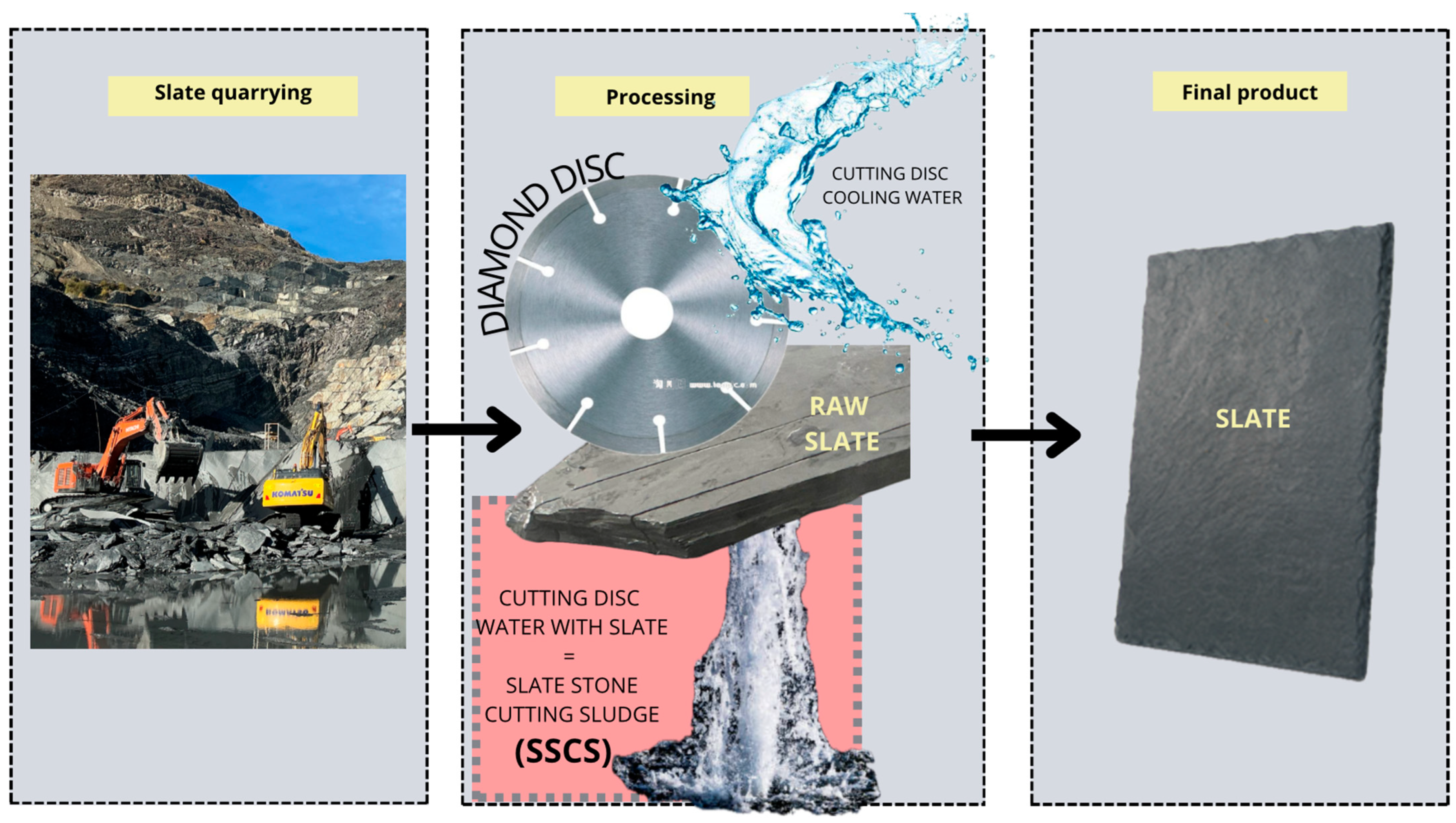

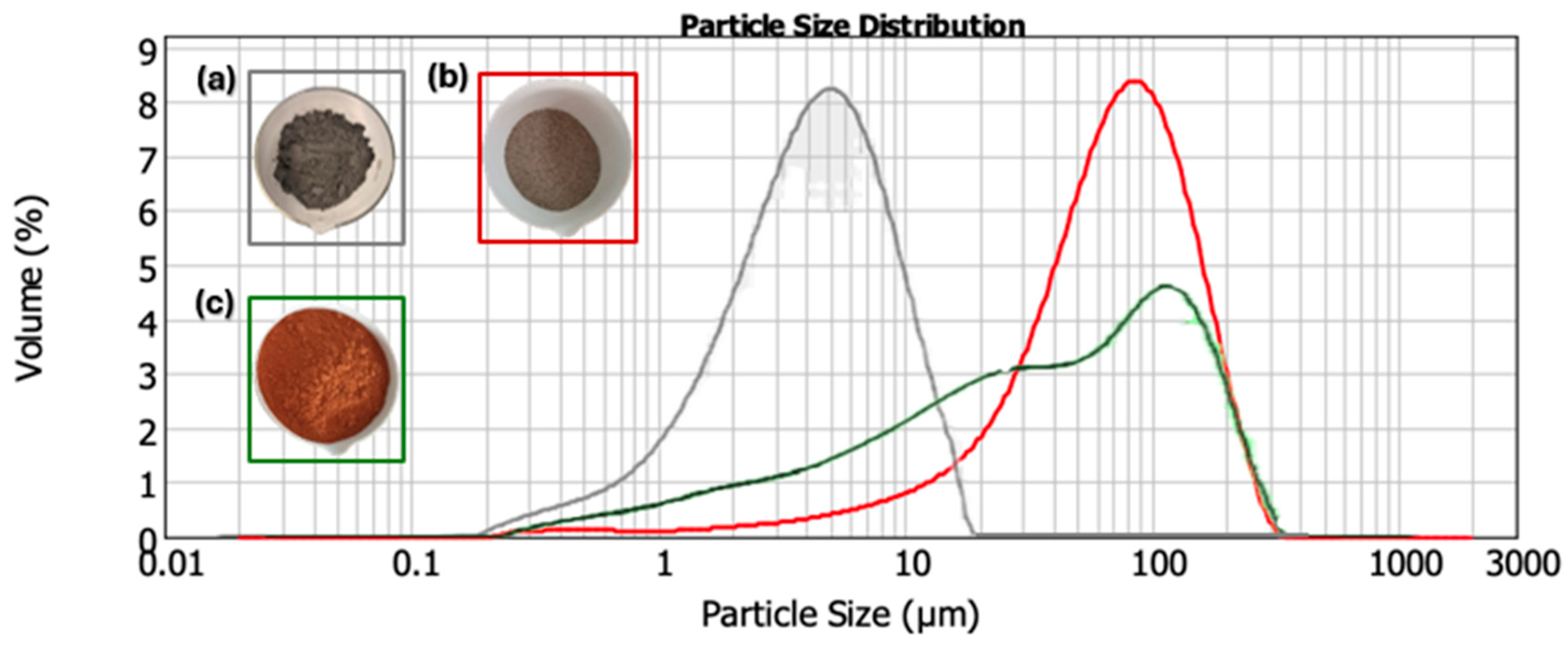

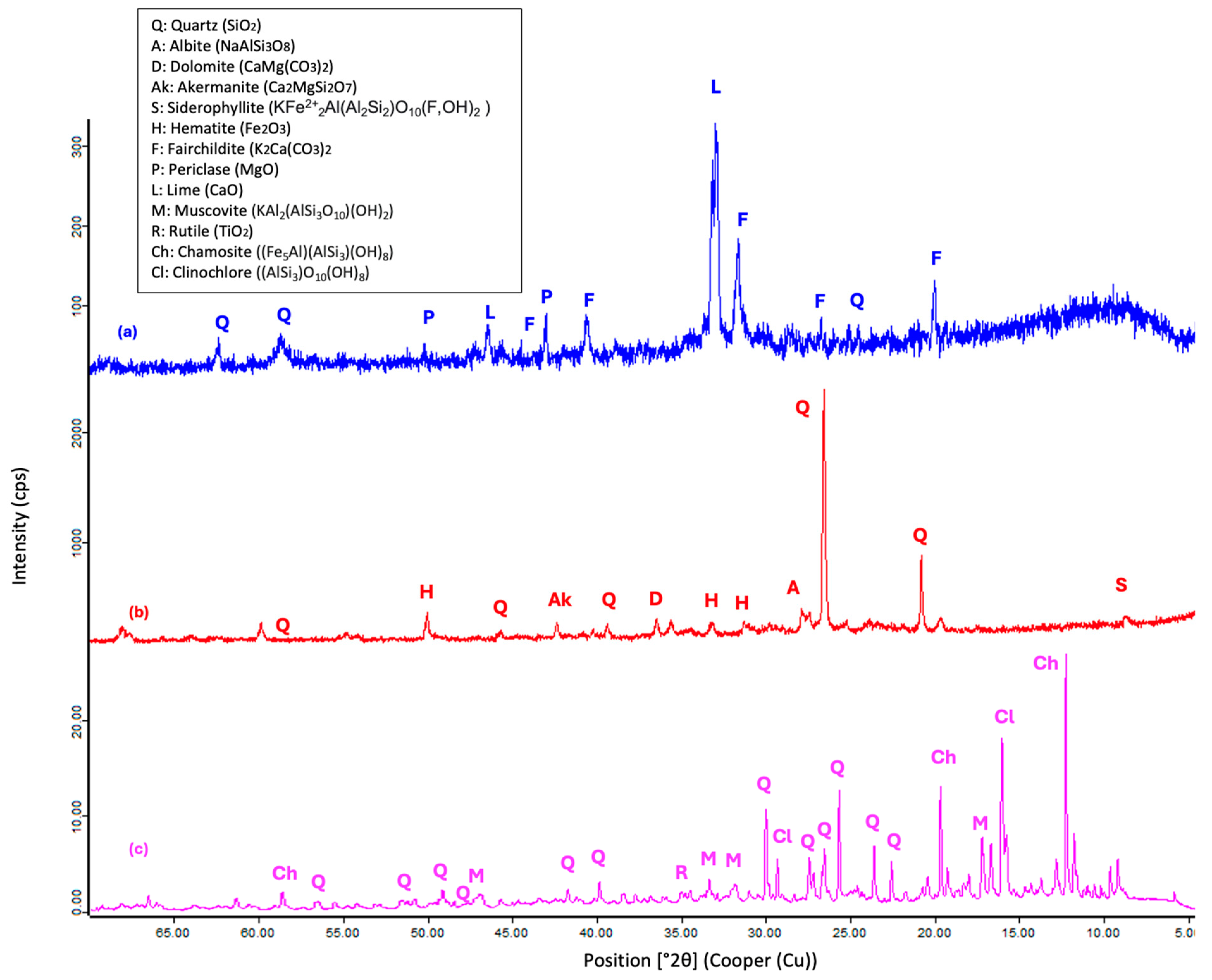
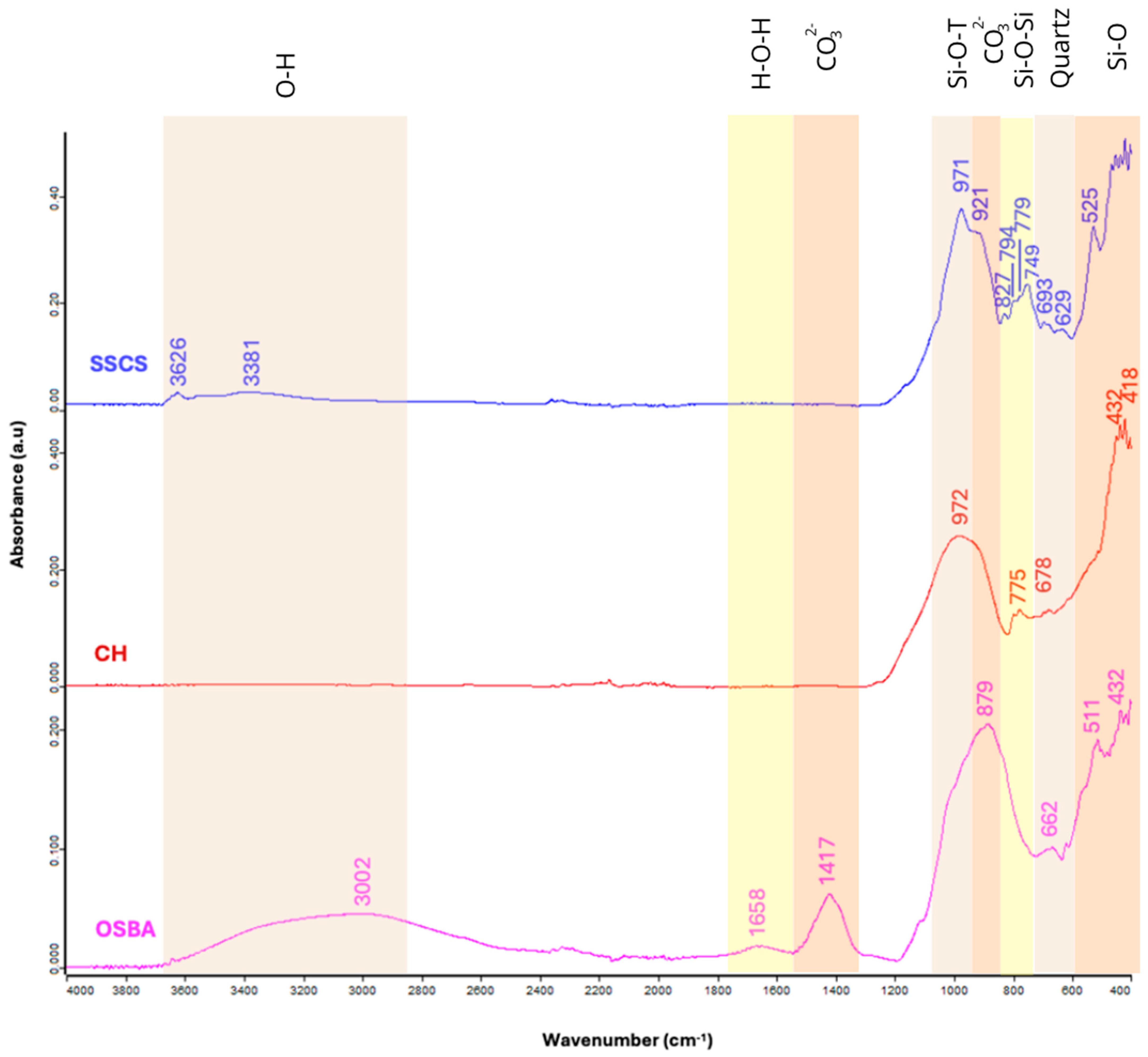
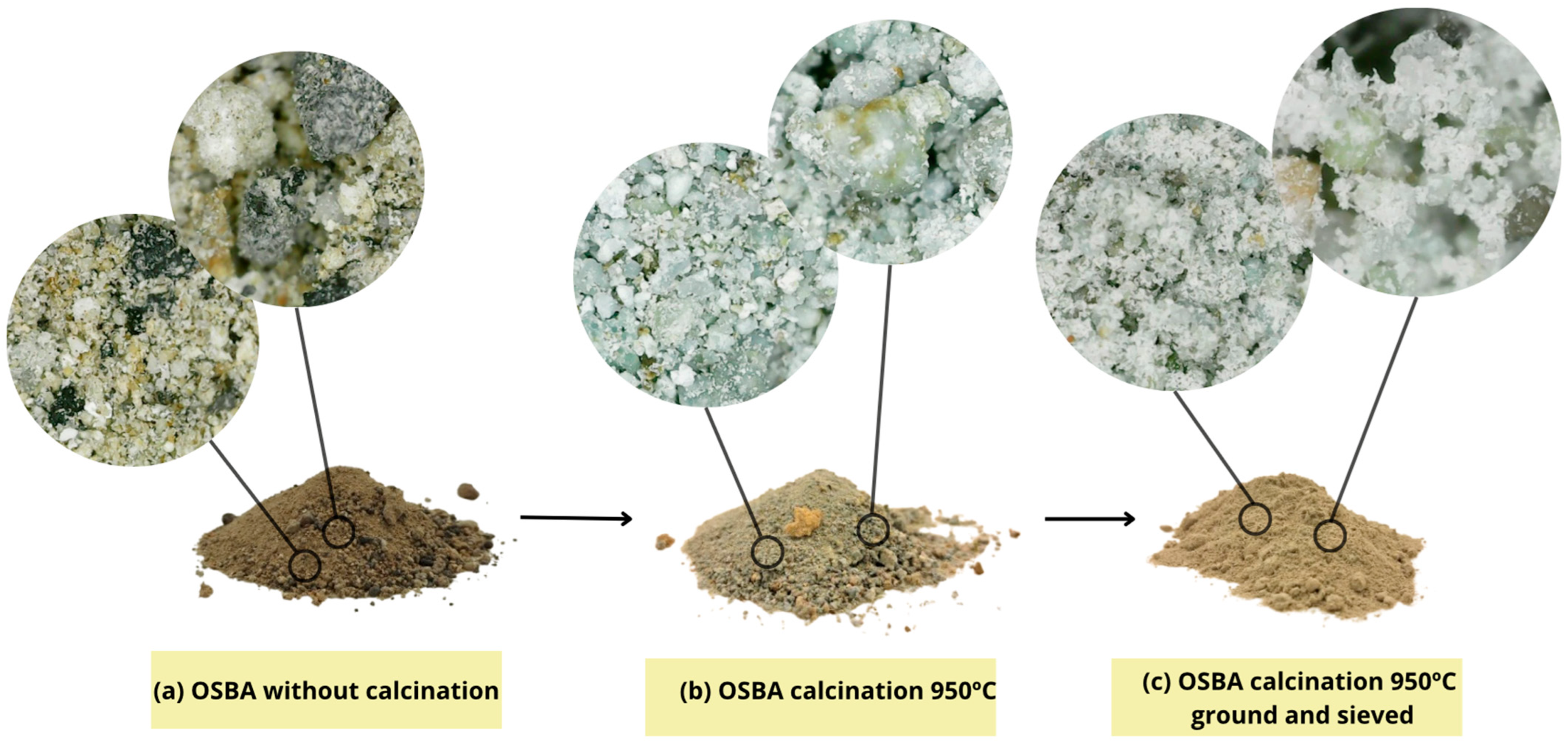








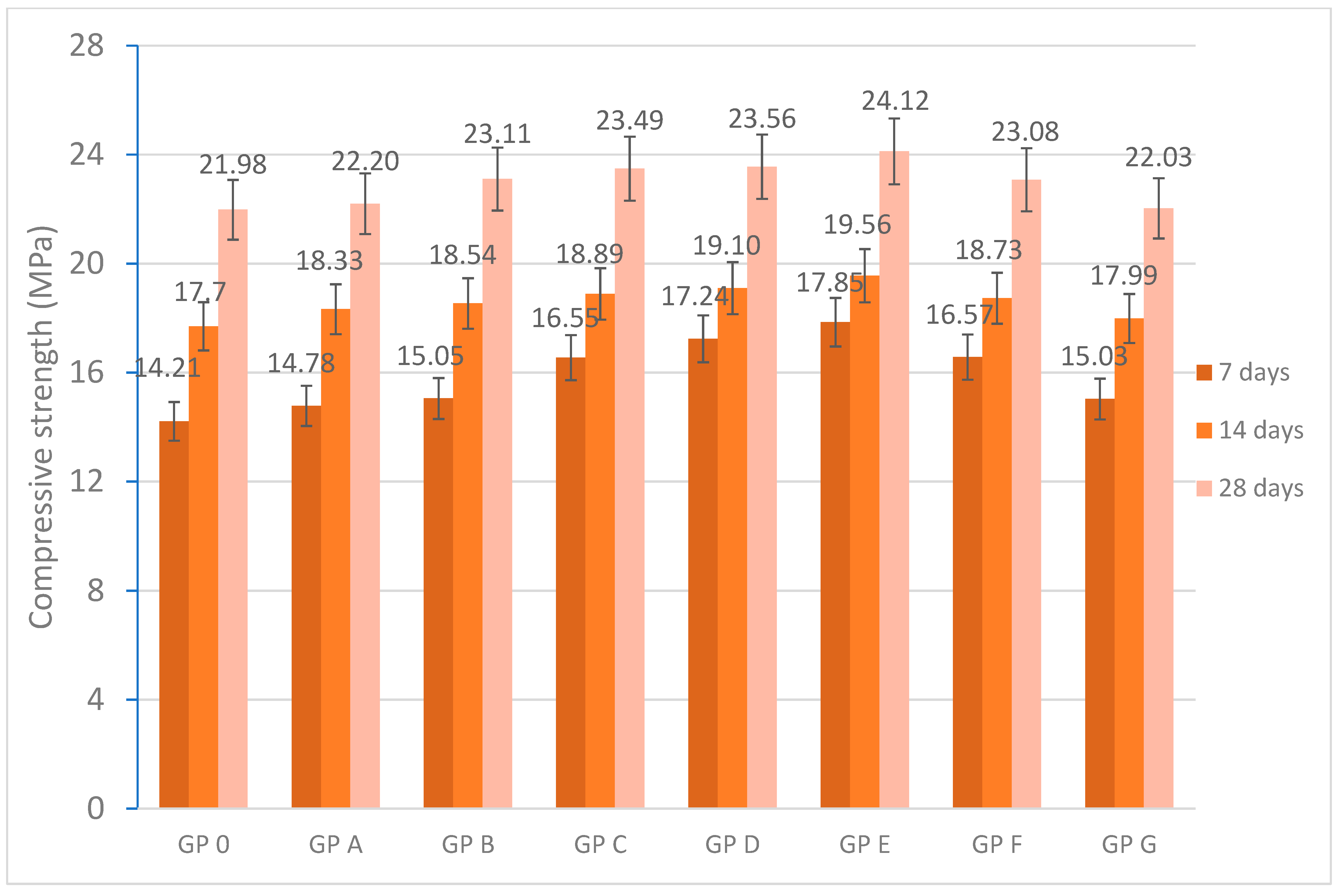


| Raw Material | SSCS | OSBA | CH |
|---|---|---|---|
| C (%) | 0.580 | 3.870 | 0.424 |
| N (%) | 0.030 | 0.011 | 0.001 |
| H (%) | 0.380 | 3.035 | 0.001 |
| Raw Materials | Density (Kg/m3) |
|---|---|
| SSCS | 2.49 ± 0.15 |
| OSBA | 2.74 ± 0.19 |
| CH | 2.53 ± 0.21 |
| Raw Material | SSCS | OSBA | CH |
|---|---|---|---|
| K2O | 4.54 | 27.48 | 5.12 |
| CaO | 0.462 | 34.22 | 7.22 |
| Al2O3 | 21.97 | 0.779 | 15.41 |
| SiO2 | 50.34 | 4.12 | 58.98 |
| P2O5 | 0.263 | 1.85 | 0.155 |
| MgO | 2.50 | 3.82 | 2.40 |
| Fe2O3 | 10.38 | 0.94 | 7.84 |
| Cl | - | 0.092 | 0.028 |
| Na2O | 1.37 | 0.761 | 0.367 |
| SO3 | 0.501 | 0.361 | 0.863 |
| TiO2 | 1.22 | 0.060 | 0.882 |
| MnO | 0.099 | 0.125 | 0.109 |
| V2O5 | 0.035 | - | 0.026 |
| CuO | 0.027 | 0.055 | 0.011 |
| Cr2O3 | 0.021 | 0.048 | 0.017 |
| ZnO | 0.016 | - | 0.046 |
| NiO | - | 0.041 | - |
| Function Group | Wavenumber Range (cm−1) | FTIR Peaks (cm−1) | References | ||
|---|---|---|---|---|---|
| Raw Materials | SSCS | OSBA | CH | ||
| Stretching vibration O-H | 3626–3002 | 3326, 3381 | 3002 | - | [53] |
| Bending vibration H-O-H | 1648 | - | 1648 | - | [53,54] |
| Asymmetric stretching vibration CO | 1417 | - | 1417 | - | [55] |
| Asymmetric stretching vibration Si-O-T | 972–921 | 971, 921 | - | 972 | [56,57] |
| CO bonds vibrations in carbonate groups | 879–827 | 827 | 879 | - | [58,59] |
| Bending symmetric stretching Si-O-Si | 794–749 | 794, 779, 749 | - | 775 | [58,59,60] |
| Bending vibration in quartz | 693–629 | 693, 629 | 662 | 678 | [60] |
| Bending vibration Si-O | 525–418 | 525 | 511, 432 | 432, 418 | [61,62] |
| Specimen | SSCS (%) | CH (%) | OSBA (%) | Na2SiO3 (%) | Wd (%) | NaOH 12M (%) | Na2SiO3/OSBA | NaOH/OSBA | Liquid/Binder Ratio | pH Alkaline Solution |
|---|---|---|---|---|---|---|---|---|---|---|
| GP 0 | 35.0 | 0.0 | 0.0 | 37.0 | 11.0 | 17 | - | 1.32 | 1.86 | 12.14 |
| GP A | 25.0 | 15.0 | 15.0 | 34.0 | 11.0 | 0 | 1.31 | - | 1.50 | 13.41 |
| GP B | 25.0 | 15.0 | 16.0 | 32.0 | 12.0 | 0 | 1.22 | - | 1.50 | 13.28 |
| GP C | 25.0 | 15.0 | 17.0 | 30.0 | 13.0 | 0 | 1.14 | - | 1.50 | 13.19 |
| GP D | 25.0 | 15.0 | 18.0 | 28.0 | 14.0 | 0 | 1.07 | - | 1.50 | 13.01 |
| GP E | 25.0 | 15.0 | 19.0 | 26.0 | 15.0 | 0 | 1.00 | - | 1.50 | 12.92 |
| GP F | 25.0 | 15.0 | 20.0 | 24.0 | 16.0 | 0 | 0.94 | - | 1.50 | 12.87 |
| GP G | 25.0 | 15.0 | 21.0 | 22.0 | 17.0 | 0 | 0.88 | - | 1.50 | 12.75 |
| Specimen | Si/Al | Ca/Si | K/Si | Na/Si |
|---|---|---|---|---|
| GP 0 | 4.65 | 0.01 | 0.49 | 0.07 |
| GP A | 4.92 | 0.21 | 1.00 | 0.19 |
| GP B | 4.79 | 0.26 | 1.04 | 0.21 |
| GP C | 4.66 | 0.27 | 1.08 | 0.22 |
| GP D | 4.54 | 0.31 | 1.13 | 0.23 |
| GP E | 4.41 | 0.34 | 1.18 | 0.25 |
| GP F | 4.29 | 0.37 | 1.24 | 0.26 |
| GP G | 4.16 | 0.40 | 1.31 | 0.28 |
| Parameter | Standard | Equipment |
|---|---|---|
| Weight loss | - | RB-30KG Cobos (Balanzas Cobos, Hospitalet de Llobregat, Spain) |
| Linear shrinkage | UNE-EN 772-16 [68] | Digital gauge |
| Capillary water absorption | UNE-EN 772-11 [69] | Stopwatch and balance RB-30KG Cobos |
| Cold water absorption | UNE-EN 772-21 [70] | Thermostatic bath Bunsen and balance RB-30KG Cobos (Bunsen, Madrid, Spain) |
| Boiling water absorption | UNE-EN 772-7 [71] | Thermostatic bath Bunsen and balance RB-30KG Cobos |
| Bulk density and open porosity | UNE-EN 772-4 [72] | Hydrostatic balance |
| Freeze–thaw resistance | UNE-EN 15304 [73] | Freezer |
| Thermal conductivity | UNE-EN 12667:2002 [74] | HFM 446 Lambda Eco-Line Netzsch (Netzsch, High Franconia, Germany) |
| Compressive strength | UNE-EN 772-1:2001+A1:2016 [75] | Shimadzu AG-300 KNX (Shimadzu, Korneuburg, Austria) |
| DRX | - | Pioner S4 Explorer Bruker (Bruker AXS GmbH, Karlsruhe, Germany) |
| FTIR | - | FT-IR Vertex 70 Bruker (Bruker AXS GmbH, Karlsruhe, Germany) |
| SEM-EDX | - | Microscope Carl Zeiss Merlin (Zeiss GmbH, Jena, Germany) |
| Specimen | Mass Loss (%) | Linear Shrinkage (%) | Capillarity Water Absorption (g/m2min) | Immersion Absorption (%) | Bulk Density (g/cm3) | Porosity (%) | Thermal Conductivity (W/mK) |
|---|---|---|---|---|---|---|---|
| GP 0 | 4.05 ± 0.41 | 0.83 ± 0.10 | 1944.14 ± 68 | 9.20 ± 0.18 | 1.73 ± 0.15 | 25.48 ± 2.06 | 0.698 ± 0.015 |
| GP A | 3.89 ± 0.35 | 0.77 ± 0.14 | 1908.36 ± 71 | 9.06 ± 0.15 | 1.76 ± 0.08 | 23.70 ± 4.38 | 0.456 ± 0.087 |
| GP B | 3.83 ± 0.46 | 0.72 ± 0.11 | 1852.63 ± 48 | 8.73 ± 0.20 | 1.80 ± 0.21 | 22.68 ± 3.80 | 0.497 ± 0.041 |
| GP C | 3.70 ± 0.37 | 0.62 ± 0.09 | 1801.74 ± 56 | 7.42 ± 0.13 | 1.83 ± 0.23 | 20.97 ± 1.44 | 0.584 ± 0.093 |
| GP D | 3.59 ± 0.29 | 0.60 ± 0.12 | 1829.76 ± 32 | 7.13 ± 0.09 | 2.10 ± 0.19 | 19.54 ± 2.31 | 0.612 ± 0.105 |
| GP E | 3.55 ± 0.30 | 0.54 ± 0.13 | 1785.20 ± 41 | 6.86 ± 0.22 | 1.73 ± 0.17 | 19.88 ± 2.47 | 0.688 ± 0.056 |
| GP F | 3.61 ± 0.45 | 0.55 ± 0.09 | 1849.72 ± 44 | 7.39 ± 0.15 | 1.66 ± 0.21 | 20.43 ± 2.27 | 0.654 ± 0.09 |
| GP G | 3.61 ± 0.32 | 0.54 ± 0.11 | 1888.12 ± 28 | 7.48 ± 0.14 | 1.52 ± 0.15 | 22.49 ± 3.71 | 0.620 ± 0.110 |
| Function Group | Wavenumber Range (cm−1) | FTIR Peaks (cm−1) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw Materials | GP 0 | GP A | GP B | GP C | GP D | GP E | GP F | GP G | ||
| Stretching vibration O-H | 3352–3174 | 3285 | 3352 | 3351 | 3335 | 3335 | 3235 | 3177 | 3174 | [59,92,93,94] |
| Bending vibration H-O-H | 1651–1650 | 1650 | 1651 | 1650 | 1650 | 1650 | 1651 | 1650 | 1650 | [54,58] |
| Asymmetric stretching vibration CO | 1482–1378 | 1482 | 1457 | 1417 | 1416 | 1410 | 1380 | 1378 | 1396 | [92,94] |
| Asymmetric stretching vibration Si-O-T | 975–973 | 974 | 975 | 973 | 973 | 974 | 975 | 974 | 973 | [54,91,93] |
| Bending symmetric stretching Si-O-Si | 774–754 | 754 | 774 | 773 | 773 | 775 | 774 | 773 | 773 | [54] |
| Bending vibration in quartz | 527–526 | 525 | 527 | 526 | 525 | 525 | 526 | 526 | 526 | [92] |
| Bending vibration Si-O | 418–401 | 401 | 418 | 418 | 418 | 418 | 418 | 418 | 418 | [58,94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picazo Camilo, E.; Valenzuela Expósito, J.J.; Carrillo Beltrán, R.; Perea Toledo, G.E.; Corpas Iglesias, F.A. Study of Properties of Novel Geopolymers Prepared with Slate Stone Cutting Sludge and Activated with Olive Stone Bottom Ash. Materials 2025, 18, 1774. https://doi.org/10.3390/ma18081774
Picazo Camilo E, Valenzuela Expósito JJ, Carrillo Beltrán R, Perea Toledo GE, Corpas Iglesias FA. Study of Properties of Novel Geopolymers Prepared with Slate Stone Cutting Sludge and Activated with Olive Stone Bottom Ash. Materials. 2025; 18(8):1774. https://doi.org/10.3390/ma18081774
Chicago/Turabian StylePicazo Camilo, Elena, Juan José Valenzuela Expósito, Raúl Carrillo Beltrán, Griselda Elisabeth Perea Toledo, and Francisco Antonio Corpas Iglesias. 2025. "Study of Properties of Novel Geopolymers Prepared with Slate Stone Cutting Sludge and Activated with Olive Stone Bottom Ash" Materials 18, no. 8: 1774. https://doi.org/10.3390/ma18081774
APA StylePicazo Camilo, E., Valenzuela Expósito, J. J., Carrillo Beltrán, R., Perea Toledo, G. E., & Corpas Iglesias, F. A. (2025). Study of Properties of Novel Geopolymers Prepared with Slate Stone Cutting Sludge and Activated with Olive Stone Bottom Ash. Materials, 18(8), 1774. https://doi.org/10.3390/ma18081774








