Preparation, Properties, and Interaction Mechanism of High-Ratio DCLR-Modified Asphalt
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Asphalt
2.1.2. DCLR and BRA
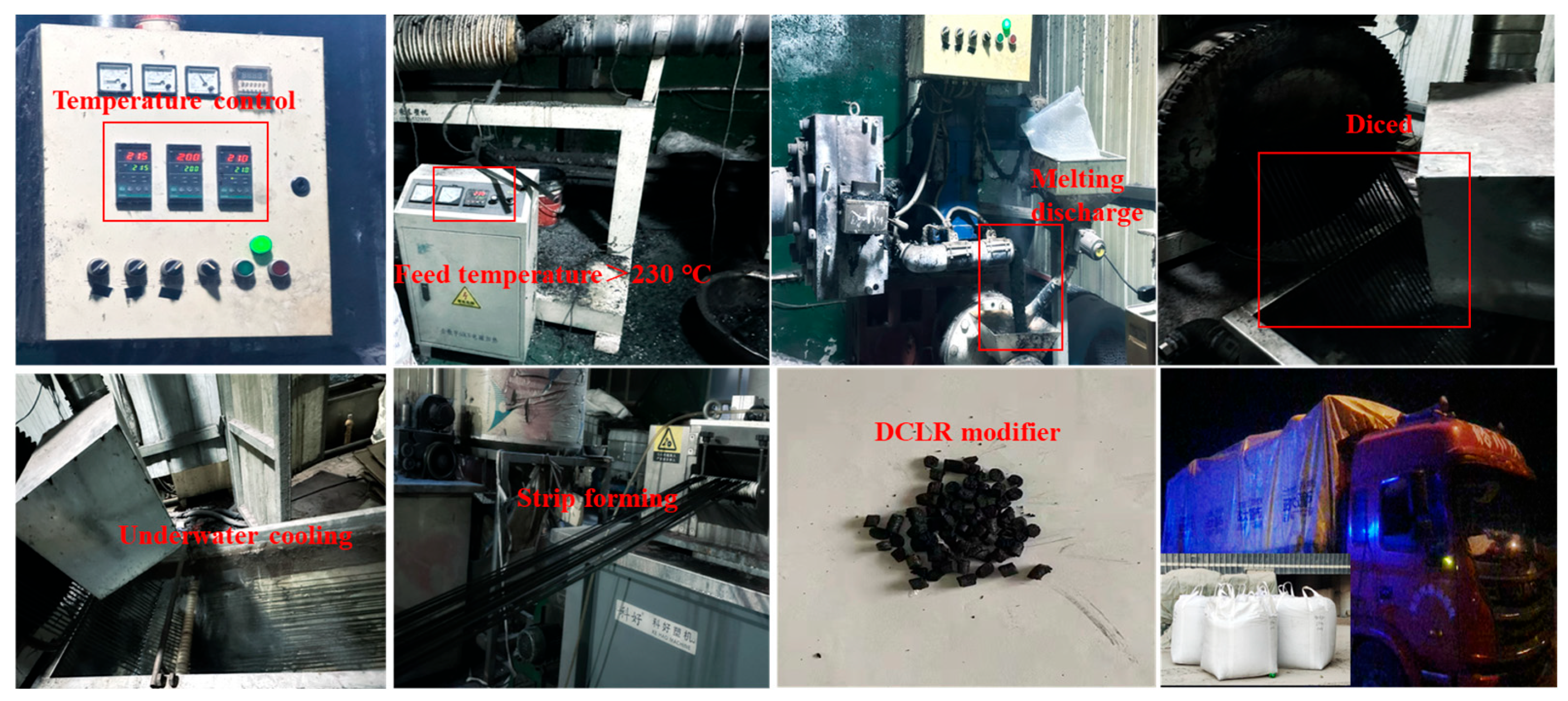
2.2. The Preparation of Modified Asphalt
2.3. Asphalt Performance Test
2.4. Composition and Structural Performance Testing
2.4.1. Determination of Four Components of Asphalt
2.4.2. Organic Element Analysis Testing
2.4.3. Fourier Transformation Infrared Spectroscopy (FTIR) Test
2.4.4. Gel Permeation Chromatography (GPC) Test
2.5. Microscopic Performance Testing
2.5.1. Thermogravimetric Infrared Spectroscopy Combined Method (Tg-FTIR)
2.5.2. X-Ray Diffraction Test (XRD)
2.5.3. Scanning Electron Microscopy (SEM) Test
2.5.4. Brunauer–Emmett–Teller (BET) Testing Method
2.6. Research Flowchart
3. Results and Discussion
3.1. The Composition and Structure of DCLR
3.1.1. FTIR Spectrum Comparative Analysis of DCLR
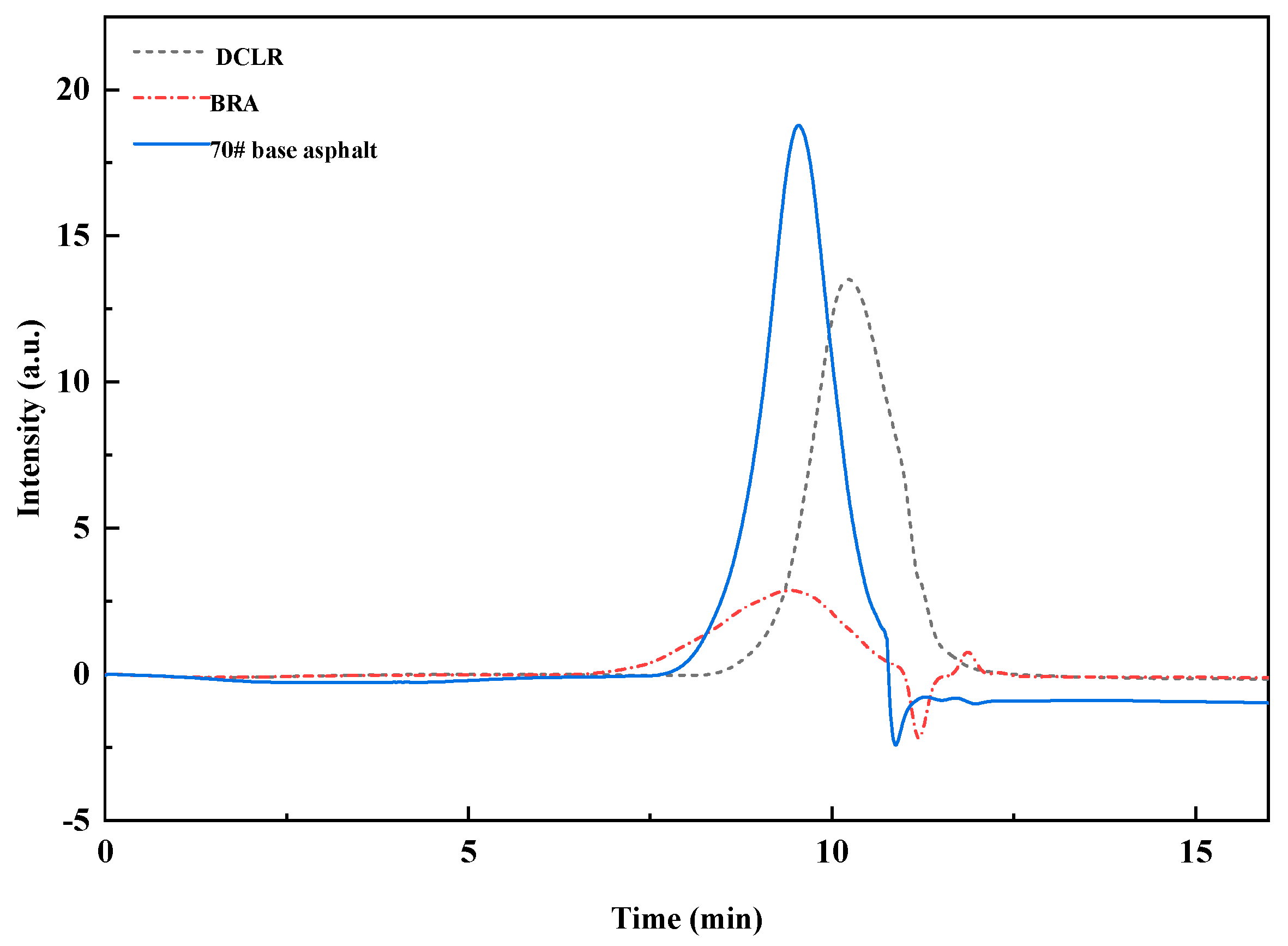
3.1.2. Relative Molecular Weight Distribution of DCLR
3.2. Research on Multiscale Microscopic Characteristics of DCLR
3.2.1. Comparative Analysis of Microscopic Morphology of DCLR
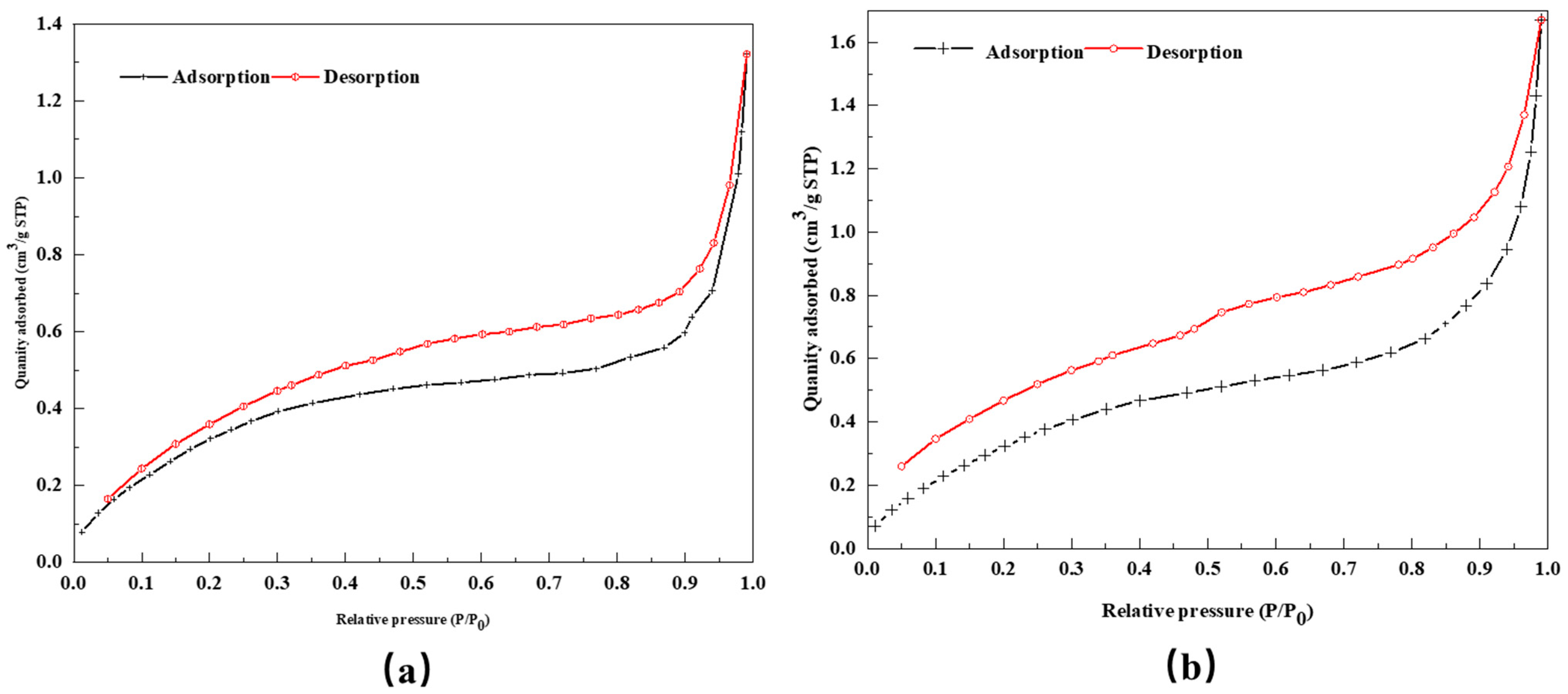
3.2.2. Study on the Pore Structure and Pore Size Distribution of DCLR
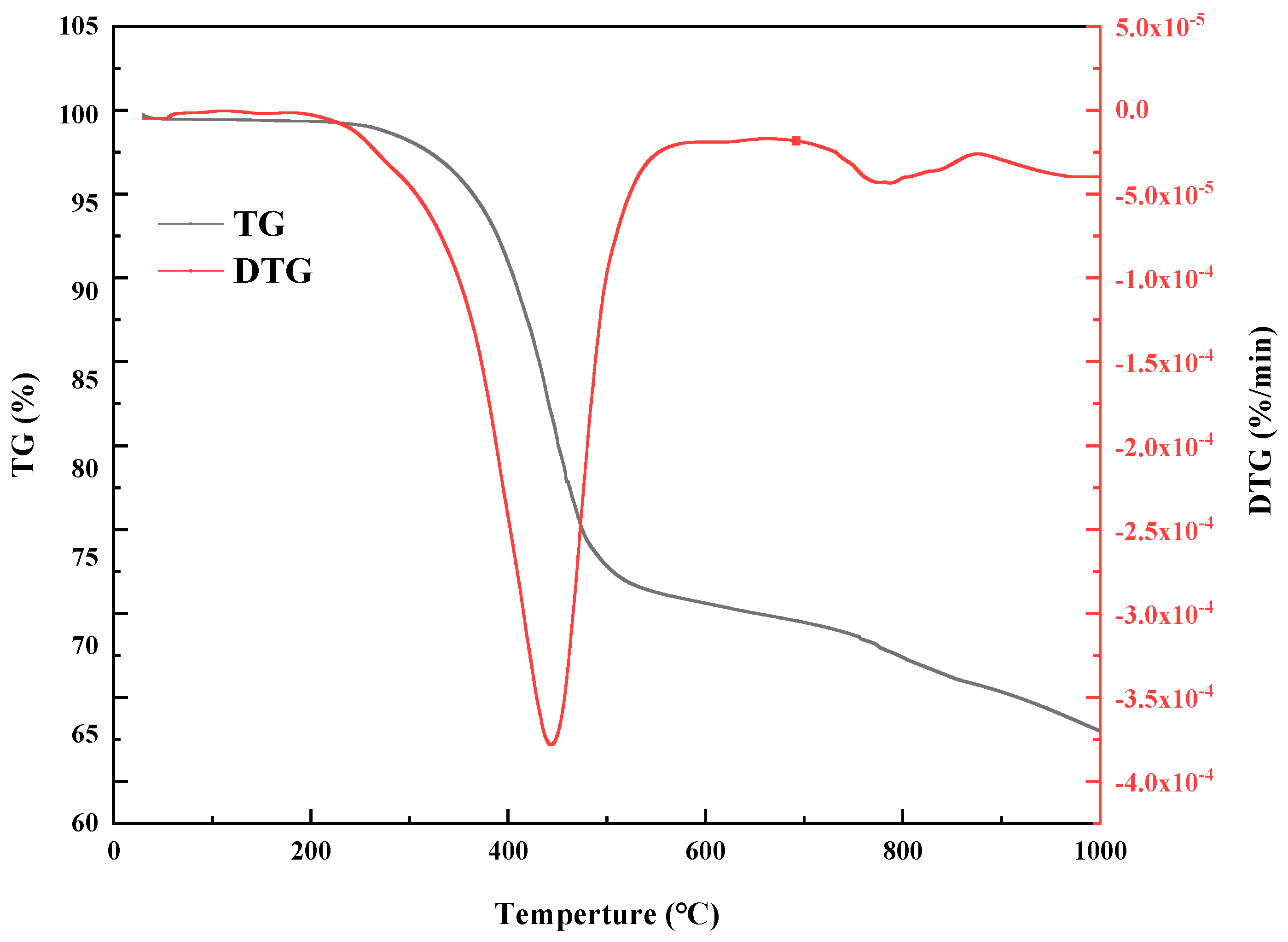
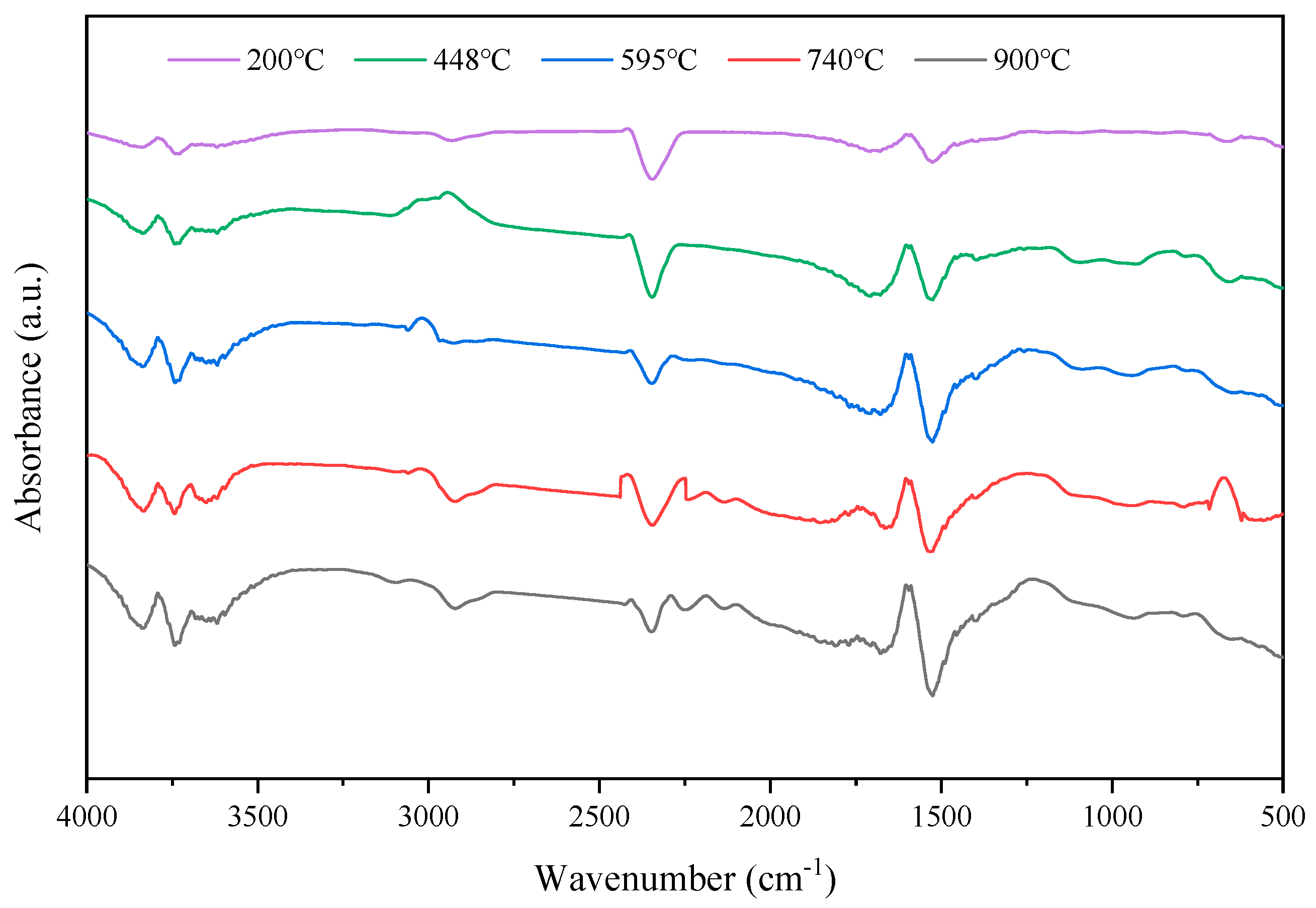
3.2.3. Tg-FTIR Analysis of DCLR
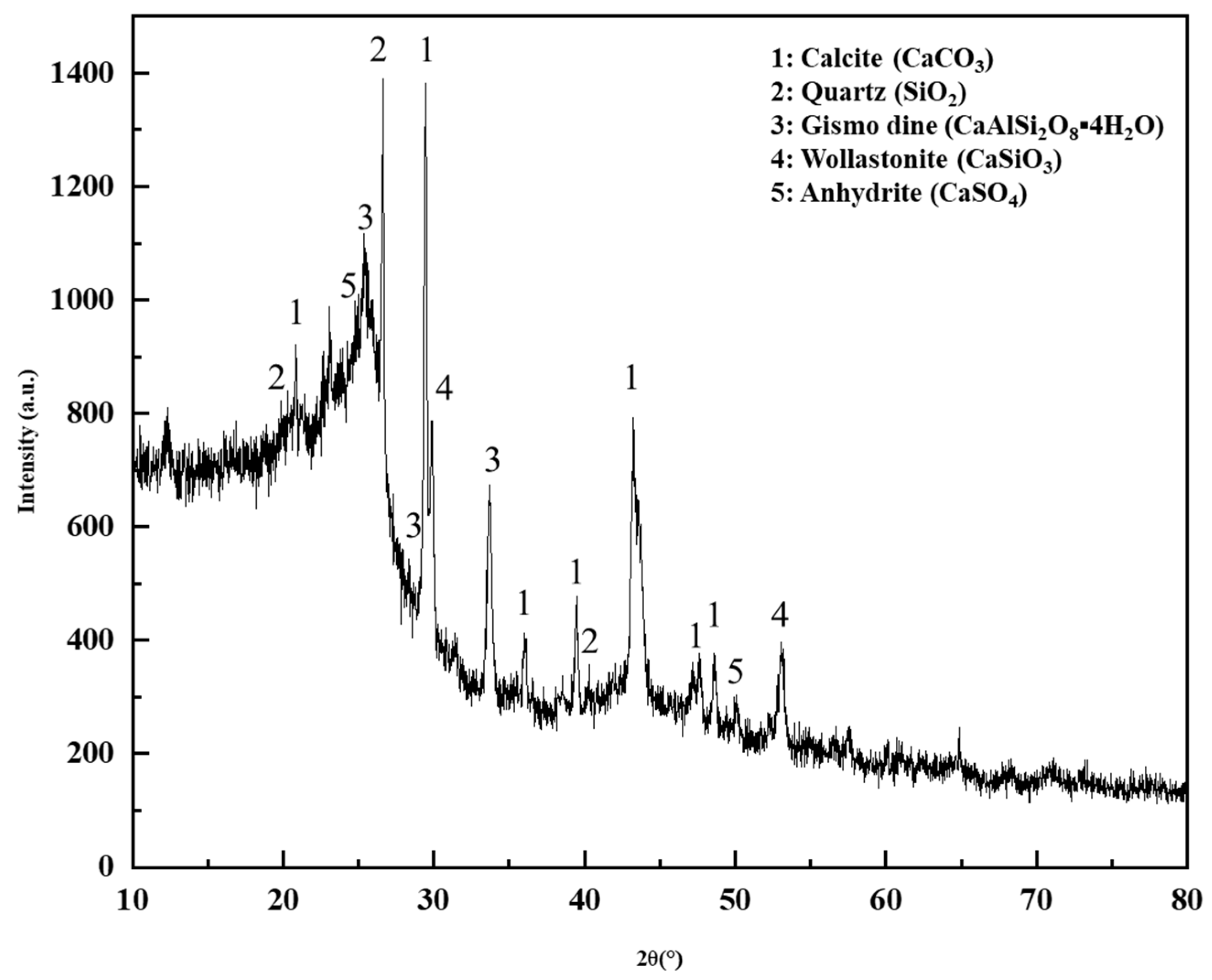
3.2.4. XRD Test Analysis of DCLR
3.3. The Influence of DCLR Content on the Performance of Modified Asphalt
3.3.1. The Influence of DCLR Content on the Conventional Performance of Modified Asphalt
3.3.2. The Influence of DCLR Content on the Rheological Properties of Modified Asphalt
3.4. Composition and Microstructure of High-Proportion DCLR-Modified Asphalt
3.4.1. High-Proportion DCLR-Modified Asphalt Component Recombination
3.4.2. Research on the Element Composition of High-Ratio DCLR-Modified Asphalt
3.4.3. The Molecular Weight Distribution of High-Proportion DCLR-Modified Asphalt
3.4.4. FTIR Spectrum Analysis of High-Proportion DCLR-Modified Asphalt
3.5. The Interaction Mechanism Between High-Proportion DCLR and Asphalt
3.6. Application of High-Ratio DCLR-Modified Asphalt
4. Conclusions
- (1)
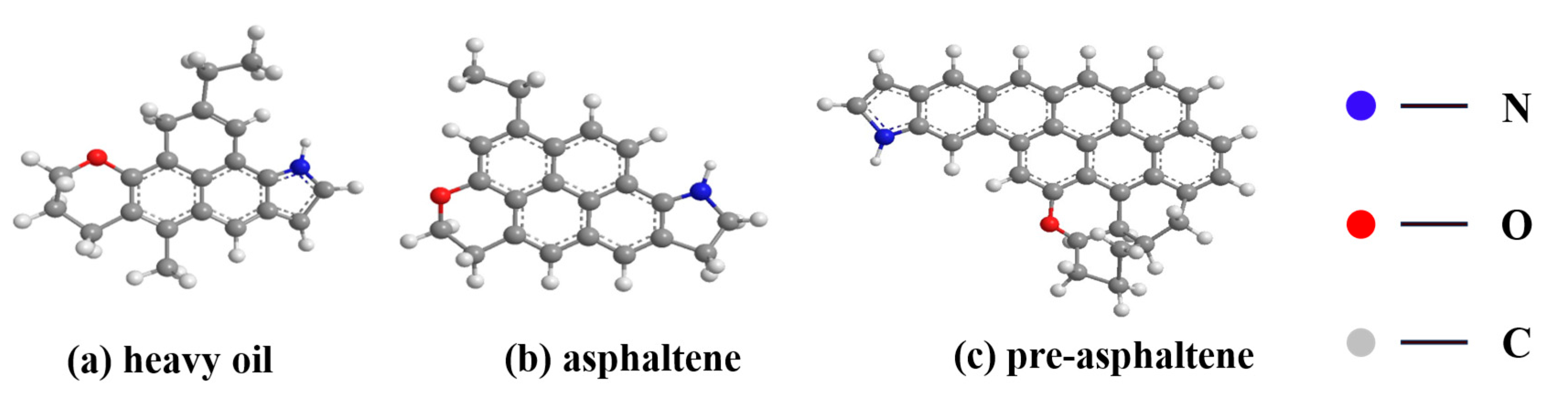
- DCLR and BRA exhibit significantly lower saturated and aromatic fractions compared to base asphalt, but substantially higher asphaltene content. DCLR comprises unsaturated hydrocarbon compound, including alkane-substituted benzene isomer, featuring unsaturated hydroxyl groups and an elevated C/H ratio. Its ash composition contains abundant heteroatoms (e.g., SiO2, CaSO4, Al2(SO4)3), contributing to strong polarity and colloidal instability. DCLR possesses a compact structure where asphaltene and heavy oil form a microporous, cross-linked network via hydrogen-bonding functional groups, exhibiting irregular pore morphology.
- (2)
- Both DCLR and BRA additives significantly improve the base asphalt’s high-temperature performance and PG grade, while reducing its low-temperature performance and PG grade. At equivalent dosages, DCLR demonstrates greater enhancement of high-temperature performance and more significant deterioration of low-temperature performance relative to BRA. The 40% high-proportion DCLR-modified asphalt formulation satisfies all technical specifications for high-modulus asphalt applications.
- (3)
- Under elevated temperatures with mechanical shear, DCLR absorbs asphalt’s light components (saturated and aromatics), undergoing dissolution and swelling that disrupts its highly associated network structure. Below 200 °C blending temperature, reactive -OH groups and other functionalities in DCLR’s condensed aromatic rings interact with asphalt’s active hydrogen, producing gaseous byproducts (CO2, SO2, and H2O). This process enhances DCLR swelling, accelerates components’ migration/recombination, and ultimately establishes a new modified colloidal system. At the macroscopic level, this modification results in increased hardness and substantially enhanced high-temperature performance. The newly formed colloidal equilibrium remains stable at DCLR concentrations below 15% (0.15 mm particle size). However, at DCLR concentrations exceeding 15%, increasing insolubility occurs in the matrix asphalt owing to DCLR’s ash content and high-molecular-weight components. Ultimately, density differences destabilize this colloidal system, leading to sedimentation and phase separation.
- (4)
- Building upon established dry-process DCLR research, contents exceeding 15% can be incorporated directly into asphalt mixtures via dry processing, replacing the corresponding proportion of base asphalt or modified asphalt for high-modulus asphalt concrete applications. Subsequent experimental validation of these mixture formulations is recommended.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.W.; Watson, J.; Liu, Z.D.; Wu, Y.L. Elemental migration and transformation during hydrothermal liquefaction of biomass. J. Hazard. Mater. 2022, 423, 126961. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zhang, S.Z.; Gong, X.Y.; Liang, W.B. Eddy Current Sensor’s Measurement and Protection Optimization Design of Axial Displacement in Turbine. Energy Sci. Technol. 2017, 15, 70–73. [Google Scholar]
- Mochida, I.; Okuma, O.; Yoon, S.H. Chemicals from Direct Coal Liquefaction. Chem. Rev. 2014, 114, 1637–1672. [Google Scholar] [CrossRef]
- Li, X.; Bai, Z.Q.; Li, W. Chemical transformation of sodium species during direct liquefaction of a sodium-rich Zhundong coal under different atmospheres and CO2 gasification of the direct coal liquefaction residue. Fuel 2018, 213, 144–149. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, S.J.; Lu, Z.Y.; Chen, Y.; Zhang, X.J. Research progress on the properties and high value-added applications of coal direct liquefaction residue. Clean Coal Technol. 2009, 15, 33–35. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Liu, Z. Pyrolysis behavior of direct coal liquefaction residues. J. Fuel Chem. Technol. 2010, 38, 385–390. [Google Scholar]
- Gu, X.H. Discussion on the Structural Characteristics of Shenhua Coal Direct Liquefaction Residue. Master’s Thesis, China Coal Research Institute, Xi’an, China, 2006. [Google Scholar]
- Lin, J.; Fridley, D.; Lu, H.Y.; Price, L.; Zhou, N. Has coal use peaked in China: Near-term trends in China’s coal consumption. Energy Policy 2018, 123, 208–214. [Google Scholar] [CrossRef]
- Wang, Z.C.; Ge, Y.; Shui, H.F.; Ren, S.B.; Pan, C.X.; Kang, S.G.; Lei, Z.P.; Zhao, Z.J.; Hu, J.C. Molecular structure and size of asphaltene and preasphaltene from direct coal liquefaction. Fuel Process. Technol. 2015, 137, 305–311. [Google Scholar] [CrossRef]
- Wang, Z.X.; Yang, J.L.; Liu, Z.N. Preliminary evaluation of the modification effect of coal direct liquefaction residue on road asphalt. J. Fuel Chem. Technol. 2007, 109–112. [Google Scholar]
- Zhu, W.P. Study on Direct Coal Liquefaction Residue as Asphalt. Energy Sci. Technol. 2009, 7, 68–71+85. [Google Scholar]
- Zhang, Y.R. Exploration and Research on Liquefied Residue Modified Road Asphalt. Master’s Thesis, Northwestern University, Evanston, IL, USA, 2013. [Google Scholar]
- Zheng, L.Z. Study on the Liquefaction Property of 10# Chenghe High Sulfur Coal and Preparation of Modified Asphalt from Chenghe Coal Liquefaction Residue. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2013. [Google Scholar]
- Fan, Y.Z. Exploratory Study on the Properties and Applications of Coal Direct Liquefaction Residues. Master’s Thesis, East China University of Science and Technology, Shanghai, China, 2011. [Google Scholar]
- Zhao, Y.S. Research on the Performance of Modified Asphalt and Its Slurry from Direct Coal Liquefaction Residue. Master’s Thesis, Beijing University of Civil Engineering and Architecture, Beijing, China, 2015. [Google Scholar]
- Song, Z.Z.; Sun, M.; Huang, Y.; LV, B.; Su, X.P.; Zhong, J.J.; Zhao, X.L.; Ma, X.X. Modified asphalt with the extract fractions of Shenhua direct coal liquefaction residue. Chem. Ind. Eng. Prog. 2017, 36, 3273–3279. [Google Scholar] [CrossRef]
- Chen, J. Modified Petroleum Asphalt from Coal Direct Liquefaction Residue Based on Aldehyde Crosslinking Agents. Master’s Thesis, Northwest University, Evanston, IL, USA, 2015. [Google Scholar]
- Ji, J.; Li, H.; Wang, J.N.; Suo, Z.; Xu, Y. Effect of compatibilizer on low-temperature performances of modified asphalts from direct coal liquefaction residue. J. Fuel Chem. Technol. 2019, 47, 925–933. [Google Scholar]
- Ji, J.; Li, H.; Xu, Y.; Shi, Y.F.; Suo, Z. The effect of tetrahydrofuran soluble substances in coal direct liquefaction residue on the rheological properties of asphalt. J. Chang. Univ. (Nat. Sci. Ed.) 2019, 39, 9–16. [Google Scholar] [CrossRef]
- Liu, W.J.; Mao, H.; Mao, H.Z.; Li, R.X. Study on the Effect of Coal Oil Residue on High Temperature-fatigue Rheological Properties of Asphalt Binder. Highw. Eng. 2021, 46, 73–80. [Google Scholar] [CrossRef]
- Ji, J.; Ma, R.D.; Zheng, W.H.; Suo, Z.; Xu, Y. The effect of coal direct liquefaction residue on asphalt aggregate adhesion. China J. Highw. Transp. 2018, 31, 27–33. [Google Scholar]
- Ji, J.; Zheng, W.H.; Shi, Y.F.; Suo, Z.; Xu, Y. Aging resistance of THFS modified asphalt. Pet. Asph. 2017, 31, 36–44. [Google Scholar]
- Sheng, Y.; Zhang, S.Z.; Liang, W.B.; Zhuo, J.D.; Wei, J.M. Study on the aging properties of coal direct liquefaction asphalt—Road modified asphalt. Clean Coal Technol. 2019, 25, 33–39. [Google Scholar] [CrossRef]
- Ji, J.; Suo, Z.; Zhang, R.; Li, H.; Han, B.; Wang, J.; You, Z. Effect of physical hardening on low temperature performance of DCLR modified asphalt. Constr. Build. Mater. 2021, 295, 123545. [Google Scholar] [CrossRef]
- Zhi, S.; Jie, J.; Ran, Z.; Zhe, W.; Hui, Y.; Jin, D.Z. Effects of Tire Pressures and Test Temperatures on Permanent Deformation of Direct Coal Liquefaction Residue Mixture. Front. Mater. 2020, 7, 246. [Google Scholar] [CrossRef]
- Ji, J.; Yao, H.; Wang, D.; Suo, Z.; Liu, L.H.; You, Z.P. Properties of Direct Coal Liquefaction Residue Modified Asphalt Mixture. Adv. Mater. Sci. Eng. 2017, 2017, 473283. [Google Scholar] [CrossRef]
- Ji, J.; Chen, M.; Suo, Z.; Wei, J.M.; Wang, J.N.; Chen, L. Rutting Prediction Model of Asphalt Mixture Based on the Triaxial Repeated Load Test. Adv. Civ. Eng. 2021, 2021, 238680. [Google Scholar] [CrossRef]
- Wu, H. Research on the Compatibility between Coal Direct Liquefaction Residue and Petroleum Asphalt. Master’s Thesis, Beijing University of Civil Engineering and Architecture, Beijing, China, 2019. [Google Scholar]
- Ji, J.; Wang, Z.; Li, P.F.; Zheng, W.H.; Xu, X.Q.; Wang, Z.H.; Han, B.Y.; Wei, J.M.; Li, H.M. A review on direct coal liquefaction residue applied in asphalt pavements. J. Clean. Prod. 2023, 395, 136273. [Google Scholar] [CrossRef]
- JTG E20-2011; Ministry of Transport of the Peoples Republic of China. Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering. China Communications Press: Beijing, China, 2011.
- GB/T 212-2008; China Coal Research Institute. Industrial Analysis Method of Coal; Standards Press of China: Beijing, China, 2008; p. 16.
- Sha, D.; Li, Y.; Zhou, X.; Yu, J. Influence of Volatile Content on the Explosion Characteristics of Coal Dust. ACS Omega 2021, 6, 27150–27157. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Li, G.; He, G.; Guo, Y.; Xu, L.; Fang, W. Impacts of hydrogen to carbon ratio (H/C) on fundamental properties and supercritical cracking performance of hydrocarbon fuels. Chem. Eng. J. 2016, 283, 1216–1223. [Google Scholar] [CrossRef]
- Ji, J.; Shi, Y.F.; Suo, Z.; Xu, S.F.; Yang, S.; Li, P.F. Comparison on properties of modified asphalt blended with DCLR and TLA. J. Fuel Chem. Technol. 2015, 43, 7. [Google Scholar]
- He, X.Q.; Mo, W.L.; Wang, Q.; Ma, Y.Y.; Ma, F.Y.; Fan, X.; Wei, X.Y. Effect of Swelling Treatment by Organic Solvent on the Structure and Pyrolysis Performance of the Direct Coal Liquefaction Residue. Energy Fuels 2020, 34, 8685–8696. [Google Scholar] [CrossRef]
- Song, Y.H.; Ma, Q.N.; He, W.J. Effect of Extracted Compositions of Liquefaction Residue on the Structure and Properties of the Formed-coke. In Proceedings of the Internationl Symposium on Materials Application and Engineering (SAME2016), Chiang Mai, Thailand, 20–21 August 2016. [Google Scholar] [CrossRef]
- Buttersack, C. Modeling of type IV and V sigmoidal adsorption isotherms. Phys. Chem. Chem. Phys. 2019, 21, 5614–5626. [Google Scholar] [CrossRef]
- Song, Y.; Yin, N.; Yao, D.; Ma, Q.; Zhou, J.; Lan, X. Co-pyrolysis characteristics and synergistic mechanism of low-rank coal and direct liquefaction residue. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 16. [Google Scholar] [CrossRef]
- Yang, L.H.; Takeuchi, M.; Chen, Y.; Ng, N.L. Characterization of thermal decomposition of oxygenated organic compounds in FIGAERO-CIMS. Aerosol Sci. Technol. J. Am. Assoc. Aerosol Res. 2021, 55, 1321–1342. [Google Scholar] [CrossRef]
- GB/T 38075-2019; State Administration for Market Regulation. Hard Road Petroleum Asphalt. State Administration for Market Regulation: Beijing, China, 2019.
- DB13T 2823-2018; Hebei Bureau of Quality and Technical Supervision. Technical Guidelines for Construction of High Modulus Asphalt Pavements on Highways. Hebei Bureau of Quality and Technical Supervision: Hebei, China, 2018.
- JTG F40-2004; Ministry of Transport of the Peoples Republic of China. Technical Specifications for Construction of Highway Asphalt Pavements; China Communications Press: Beijing, China, 2004; 196p.
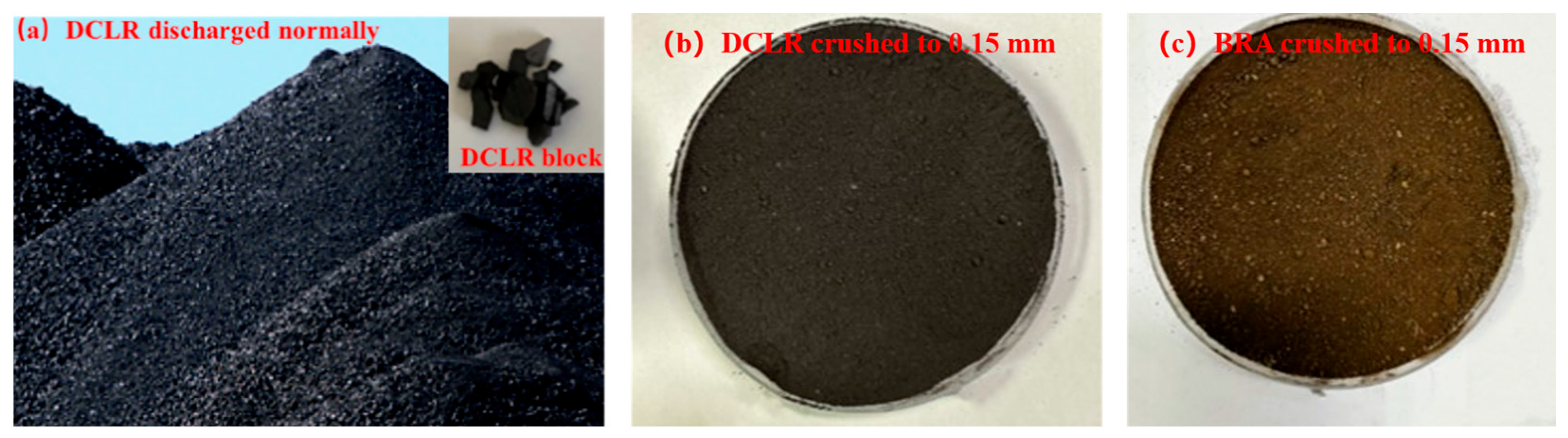
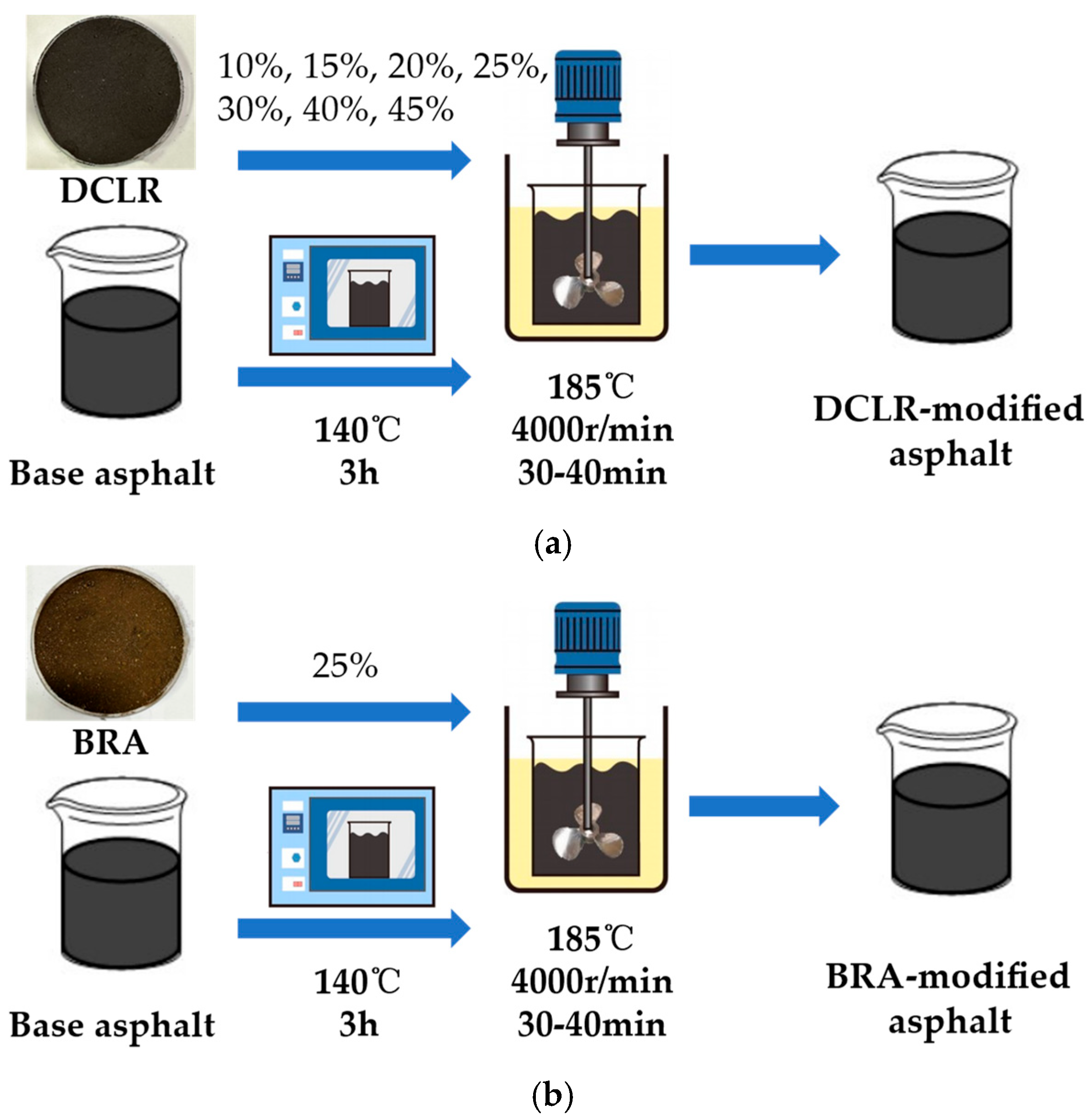

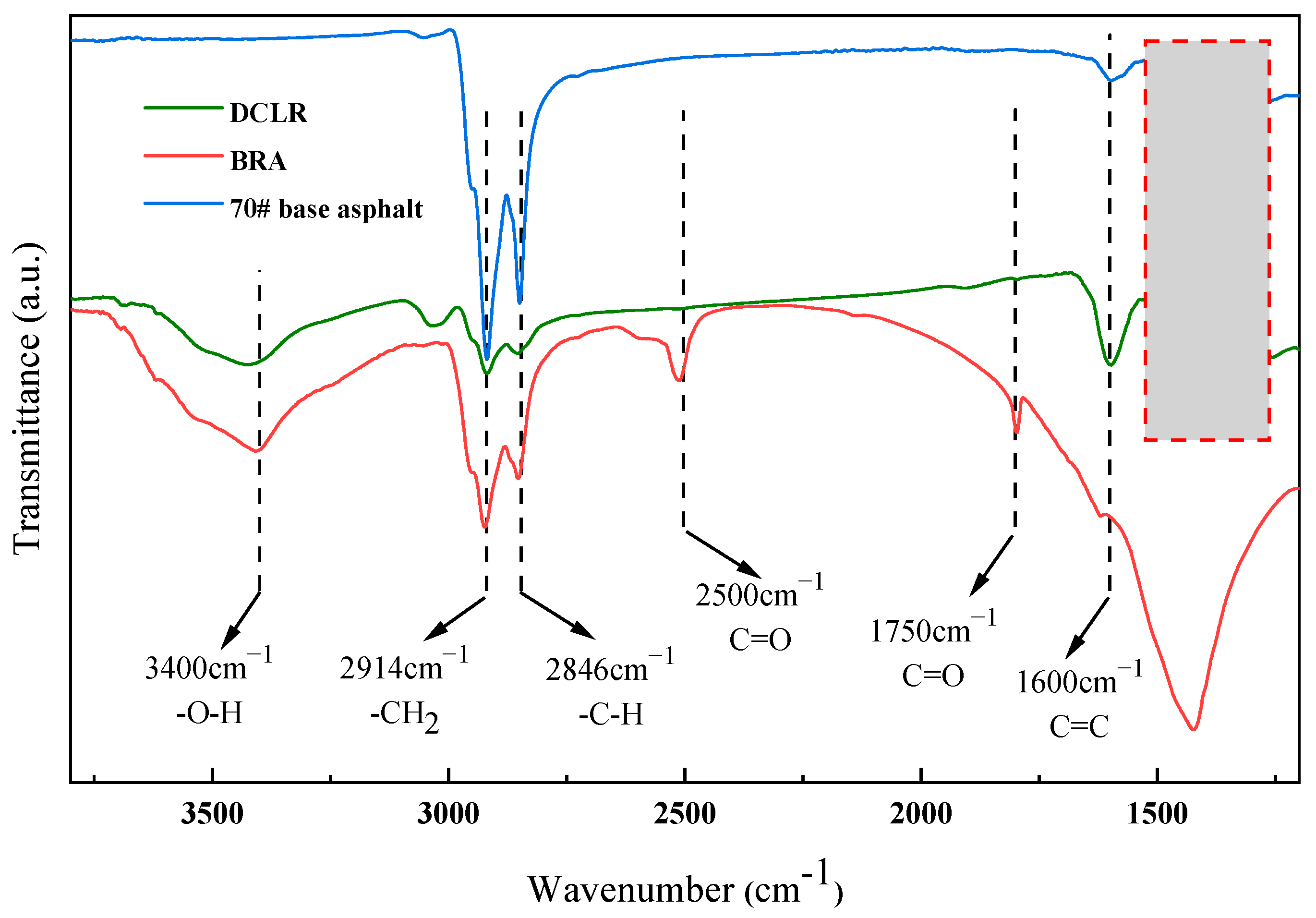







| Properties | Technical Requirements | Value |
|---|---|---|
| Penetration (25 °C) (0.1 mm) | 60~80 | 64.3 |
| Softening point (°C) | ≥45 | 47.8 |
| Ductility (15 °C) (cm) | ≥100 | >100 |
| Dynamic viscosity (60 °C) (Pa·s) | ≥160 | 185 |
| Mass loss (%) | ≤±0.8 | −0.403 |
| Penetration ratio (25 °C) (%) | ≥61 | 64.7 |
| Ductility (10 °C) (cm) | ≥6 | 8 |
| Properties | DCLR | BRA |
|---|---|---|
| Ash content (%) | 13.02 | 35 |
| Penetration (25 °C, 100 g, 5 s) (0.1 mm) | 2 | 2 |
| Softening point (5 °C) (°C) | 180 | 160 |
| Mass loss (%) | 0.374 | 0.58 |
| Flash point (°C) | 330 | 300 |
| Density (25 °C) (g/cm3) | 1.211 | 1.76 |
| Water content (%) | 1 | 0.2 |
| Solubility (%) | 48.5 | 45 |
| Specimen | ω (Saturate) (%) | ω (Aromatics) (%) | ω (Colloid) (%) | ω (Asphaltene) (%) | Colloid Unstable Coefficient (Ic) |
|---|---|---|---|---|---|
| DCLR | 1.1 | 3.9 | 2.7 | 92.3 | 14.15 |
| BRA | 2.5 | 7.8 | 2.2 | 87.5 | 9 |
| 70# base asphalt | 18.8 | 64.3 | 7.1 | 9.8 | 0.4 |
| Specimen | Industrial Analysis, m (%) | Elemental Analysis, m (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | C | H | S | N | O * | C/H | |
| DCLR | 0.08 | 14.24 | 34.63 | 51.05 | 78.76 | 4.036 | 1.768 | 1.63 | 9.985 | 19.51 |
| BRA | 0.77 | 48.91 | 50.32 | 0 | 26.54 | 2.255 | 2.29 | 0.95 | 27.753 | 11.77 |
| 70# base asphalt | 0.43 | 0.15 | 82.91 | 16.51 | 84.3 | 9.083 | 4.874 | 0.47 | 1.145 | 9.28 |
| Test Items | Instrument Model | Test Conditions |
|---|---|---|
| Softening point | SYD-2806F | 5 °C |
| Ductility | SYD-4508C | 5 cm/min, 25 °C or 15 °C |
| Penetration | SYD-2801E1 | 25 °C, 100 g, 5 s |
| TFOT | SYD-0610 | 15 r/min, 4000 mL/min, 163 °C |
| PAV | PAV-1 | 2.1 Mpa, 20 h, 50 g |
| Brookfield viscosity | NDJ-1C | 135 °C |
| DSR | DHR-10 | Strain value: 12%, angular frequency: 10 rad/s |
| Specimen | Mn | Mw | Mz | Mz+1 | PDI |
|---|---|---|---|---|---|
| DCLR | 248 | 471 | 1023 | 2051 | 1.89 |
| BRA | 970 | 3922 | 16,059 | 35,417 | 4.04 |
| 70# base asphalt | 874 | 1481 | 2766 | 4860 | 1.69 |
| Specimen | BET Surface Area (m2/g) | t-Plot Pore Volume (cm3/g) | Pore Diameter (Å) |
|---|---|---|---|
| DCLR | 14.352 | 0.000404 | 37.111 |
| BRA | 1.5082 | 0.000276 | 51.143 |
| Specimen | Pyrolysis Stage | Initial Temperature (°C) | Maximum Peak Temperature (°C) | Final Pyrolysis Temperature (°C) | Weight Loss Rate (%) |
|---|---|---|---|---|---|
| DCLR | 3 | 200 | 448 | 595 | 34 |
| Properties | DCLR Content | BRA Content | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 10% | 15% | 20% | 25% | 30% | 40% | 45% | 25% BRA | ||
| Softening point (5 °C) (°C) | 52.5 | 55.5 | 57 | 58.5 | 59.5 | 61.3 | 64.5 | 55.7 | |
| Penetration (25 °C, 100 g, 5 s) (0.1 mm) | 39 | 35 | 31 | 26 | 23 | 19 | 16 | 33.5 | |
| Ductility (5 cm/min, 25 °C) (cm) | 69 | 40 | 25 | 16 | 14 | 10 | 7 | 54.6 | |
| Ash content (%) | 1.5 | 1.7 | 2.4 | 3.1 | 3.8 | 4.1 | 4.5 | 3.9 | |
| Brookfield viscosity (135 °C) (Pa·s) | 0.56 | 0.613 | 0.693 | 0.752 | 0.826 | 0.975 | 1.256 | 0.915 | |
| Density (15 °C) | 1.052 | 1.066 | 1.103 | 1.081 | 1.130 | 1.145 | 1.157 | 1.081 | |
| Segregation (°C) | 0.5 | 1.2 | 2.7 | 3.8 | 5.2 | 7.1 | 8.5 | 7.4 | |
| After TFOT | Mass loss (%) | −0.403 | −0.264 | −0.122 | −0.01 | −0.087 | −0.062 | −0.023 | 0.197 |
| Penetration ratio (25 °C) (%) | 63 | 65 | 68 | 73 | 77 | 74 | 81 | 81 | |
| Ductility (5 cm/min, 25 °C) (cm) | 15 | 11 | 10 | 8 | 6 | 5 | 4 | 42.5 | |
| Properties | Grade of Asphalt | |||
|---|---|---|---|---|
| HA-15 | HA-25 | HA-35 | ||
| Softening point (5 °C) (℃) | ≥60 | ≥57 | ≥55 | |
| Penetration (25 °C, 100 g, 5 s) (0.1 mm) | 10–20 | 20–30 | 30–40 | |
| Ductility (5 cm/min, 25 °C) (cm) | ≥10 | ≥30 | ≥50 | |
| Ash content (%) | - | - | - | |
| Brookfield viscosity (135 °C) (Pa·s) | - | - | - | |
| Density (15 °C) | - | - | - | |
| After TFOT | Mass loss (%) | ≤±0.3 | ≤±0.3 | ≤±0.4 |
| Penetration ratio (25 °C) (%) | ≥70 | ≥67 | ≥65 | |
| Ductility (5 cm/min, 25 °C) (cm) | - | - | - | |
| Specimen | 70# Base Asphalt | 10% DCLR MA * | 15% DCLR MA | 20% DCLR MA | 25% DCLR MA | 30% DCLR MA | 40% DCLR MA | 45% DCLR MA | 25% BRA MA |
|---|---|---|---|---|---|---|---|---|---|
| PG grading | 58–22 | 70–16 | 70–16 | 70–16 | 70–10 | 76–10 | 76–10 | 76–10 | 76–10 |
| Specimen | C (%) | H (%) | S (%) | N (%) | O * (%) | C (%) |
|---|---|---|---|---|---|---|
| 70# base asphalt | 84.3 | 9.083 | 4.874 | 0.47 | 1.145 | 9.28 |
| 15% DCLR MA | 83.26 | 8.444 | 4.487 | 0.52 | 2.858 | 9.86 |
| 25% DCLR MA | 82.84 | 8.162 | 4.355 | 0.52 | 3.828 | 10.15 |
| 45% DCLR MA | 81.22 | 6.779 | 3.576 | 0.65 | 7.07 | 11.98 |
| 25% BRA MA | 76.54 | 8.237 | 4.637 | 0.48 | 8.524 | 9.29 |
| Properties | Technical Requirement | Test Result |
|---|---|---|
| Marshall stability (kN) | ≥8 | 15.16 |
| Flow value (mm) | 2~4.5 | 3.58 |
| Residual stability (%) | ≥85 | 95.4 |
| Freeze–thaw splitting strength ratio (%) | ≥80 | 90.7 |
| Dynamic stability (times/mm) | ≥3600 | 8580 |
| Permeability coefficient (mL/min) | ≤120 | 36 |
| Low-temperature bending failure strain (με) | ≥2500 | 2882 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Su, Q.; Liu, J.; Wang, Q.; Cao, D.; Zhang, G.; Shan, L. Preparation, Properties, and Interaction Mechanism of High-Ratio DCLR-Modified Asphalt. Materials 2025, 18, 1798. https://doi.org/10.3390/ma18081798
Xia L, Su Q, Liu J, Wang Q, Cao D, Zhang G, Shan L. Preparation, Properties, and Interaction Mechanism of High-Ratio DCLR-Modified Asphalt. Materials. 2025; 18(8):1798. https://doi.org/10.3390/ma18081798
Chicago/Turabian StyleXia, Lei, Qidong Su, Jian Liu, Qi Wang, Dongwei Cao, Gaoqiang Zhang, and Lingyan Shan. 2025. "Preparation, Properties, and Interaction Mechanism of High-Ratio DCLR-Modified Asphalt" Materials 18, no. 8: 1798. https://doi.org/10.3390/ma18081798
APA StyleXia, L., Su, Q., Liu, J., Wang, Q., Cao, D., Zhang, G., & Shan, L. (2025). Preparation, Properties, and Interaction Mechanism of High-Ratio DCLR-Modified Asphalt. Materials, 18(8), 1798. https://doi.org/10.3390/ma18081798






