As-Cast Magnesium Alloys with Ca Addition as a Replacement for Magnesium Alloys with Rare Earth Metals
Abstract
1. Introduction
- Common Mg-Al alloys with the addition of zinc or manganese (AZ91, AM50, and AM60), which can be high-pressure- or gravity-cast (operating temperature up to 120 °C);
- Mg-Al-RE (AE44), Mg-Al-Ca (AXM53), Mg-Al-Sr (AJ62), and Mg-Al-Si (AS21) die-cast alloys and Mg-Sr-Mn and Mg-RE-Zn-Zr (EZ33) gravity-cast alloys (operating temperature up to 200 °C);
- Mg-Gd-Nd-Zr (Elektron 21), Mg-Y-RE-Zr (WE43), Mg-Ag-Nd-Zr (QE22), and Mg-Sc-Mn gravity-cast alloys (operating temperature up to 250 °C; alloys containing scandium up to 300 °C).
2. Materials and Methods
- Electrolytic polishing (double-stream) using a Tenupol-5 polisher: The electrolyte used was composed of 5.3 g of lithium chloride, 11.16 g of magnesium perchlorate, 500 mL of methanol, and 100 mL of 2-butoxy-ethanol. Polishing was performed at −45 °C at a voltage of 20 V.
- FIB method using a Quanta 3D 200i device: The following operating parameters were used: resolution in FIB mode at 7 nm at an accelerating voltage of 30 kV, beam current of 1 pA, and magnification range from 50× to 200,000×. SEM imaging was performed with a resolution down to 3 nm at an accelerating voltage of 30 kV in high vacuum (HiVac), 12 nm at an accelerating voltage of 3 kV in low vacuum (LowVac), and 3 nm at an accelerating voltage of 30 kV in environmental mode.
3. Results and Discussion
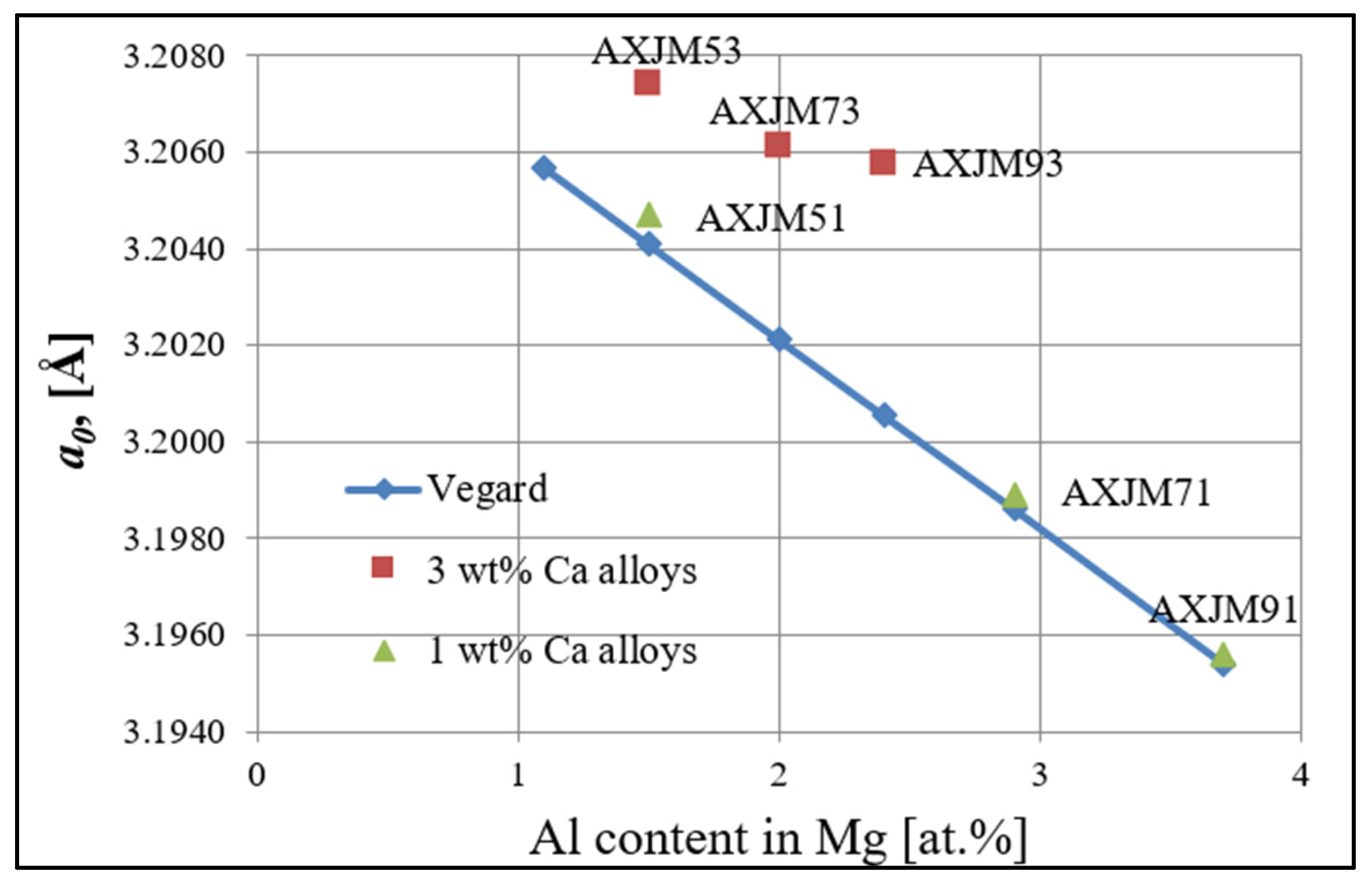
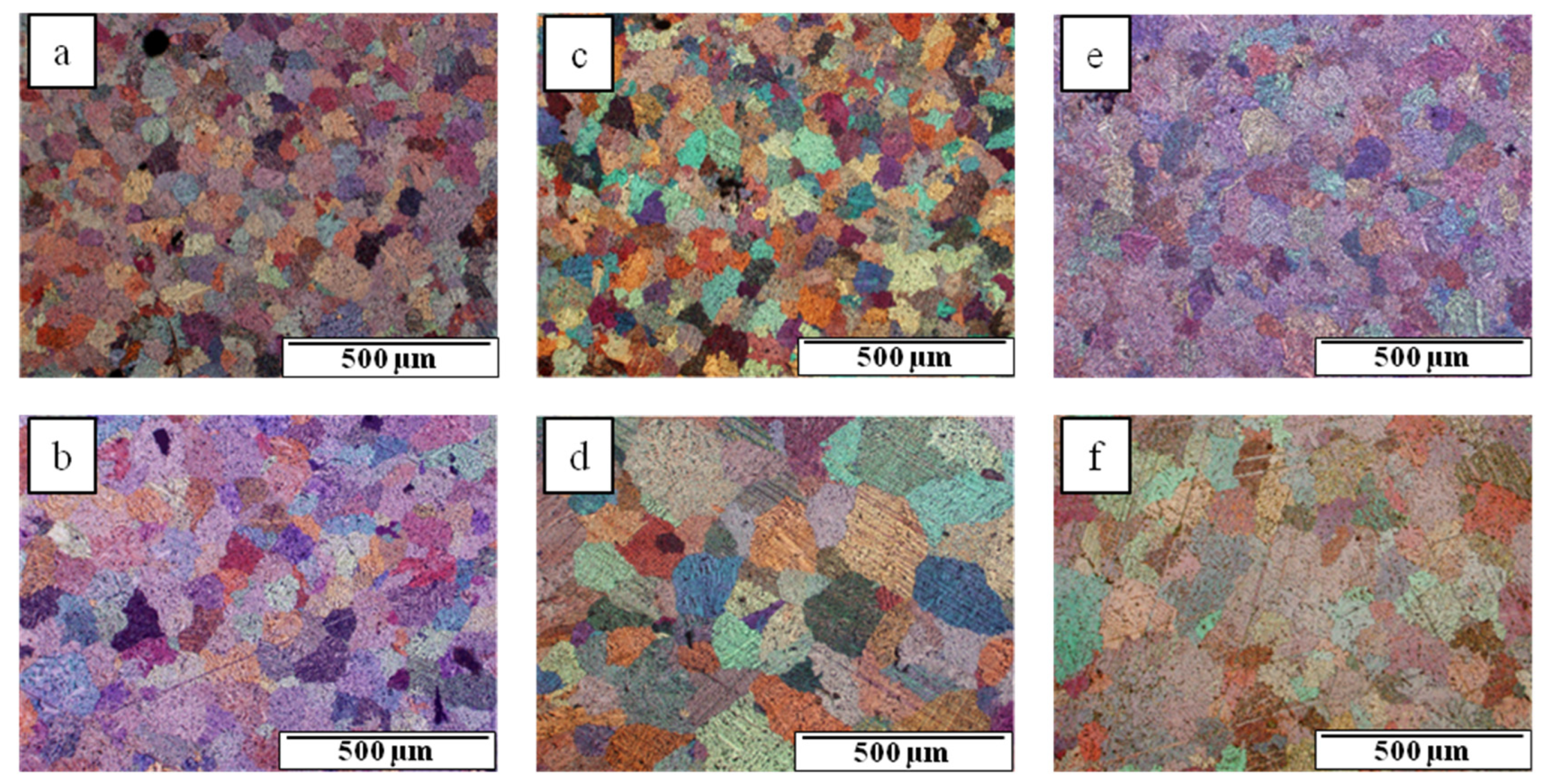
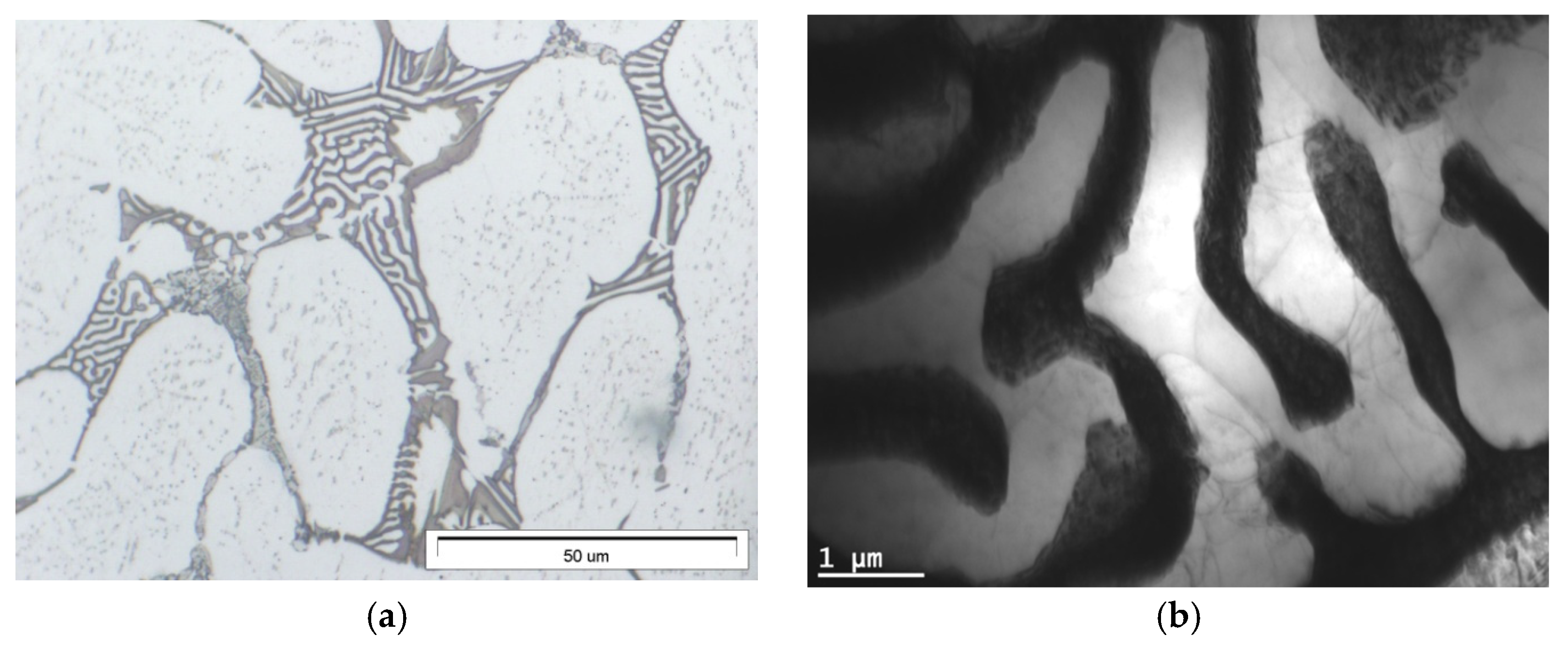
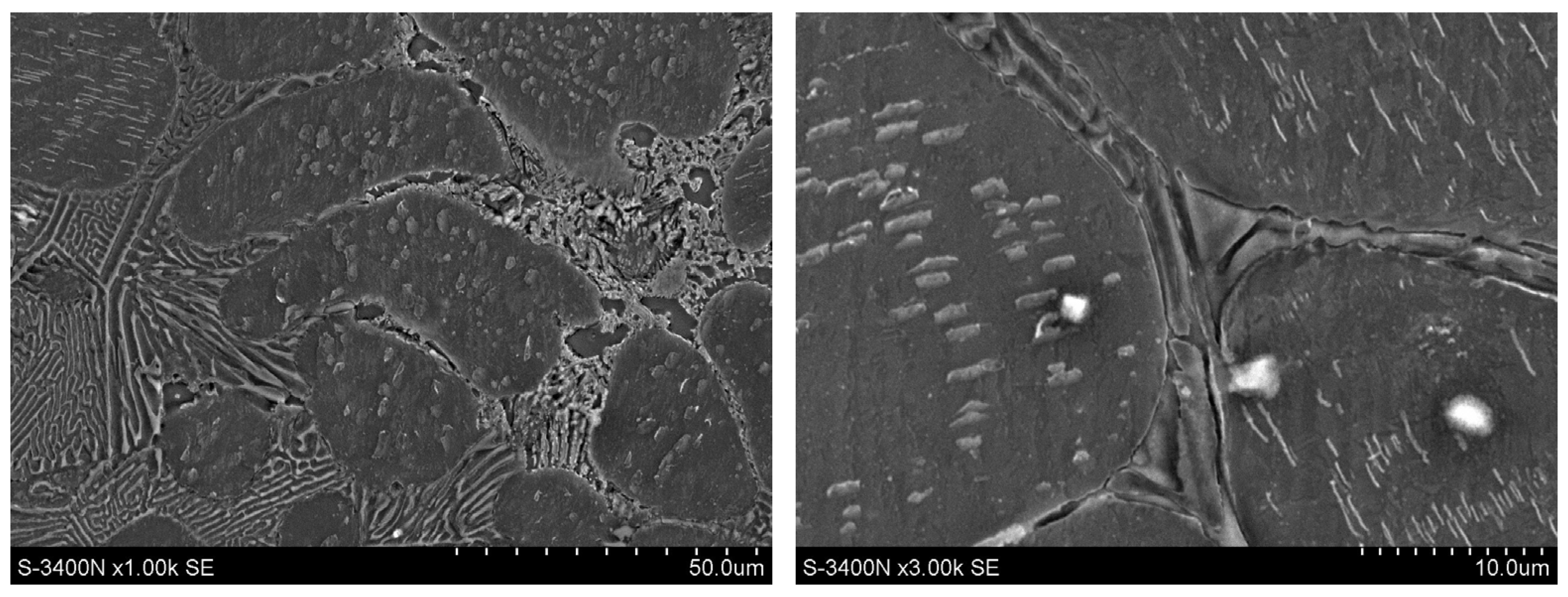
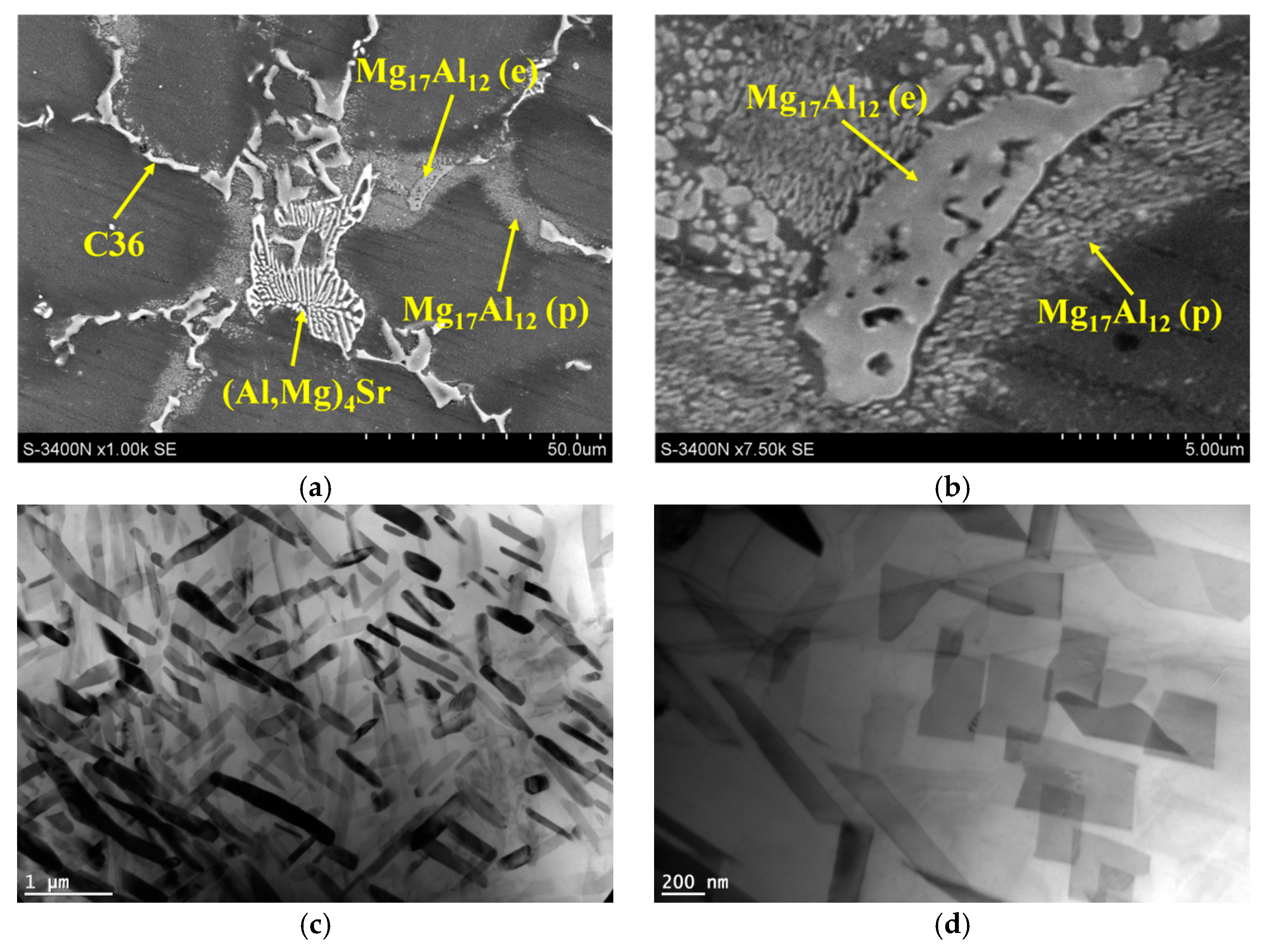
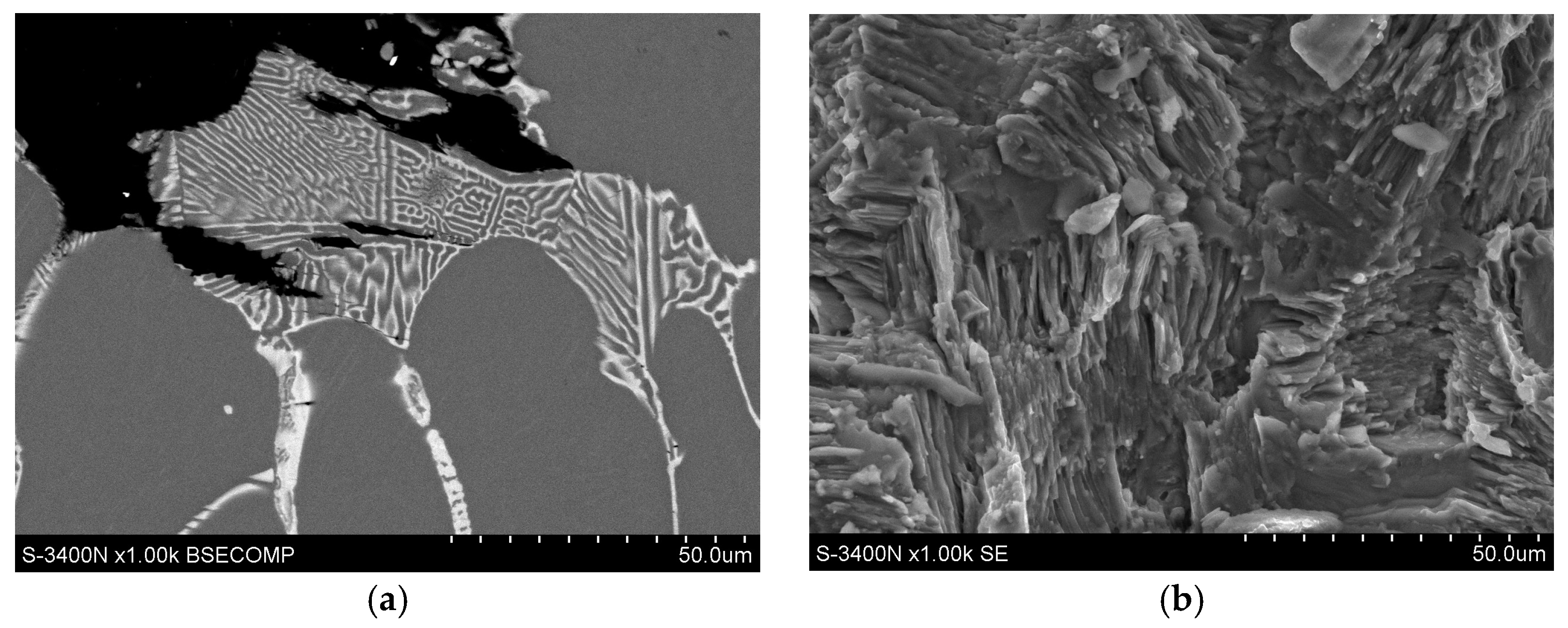
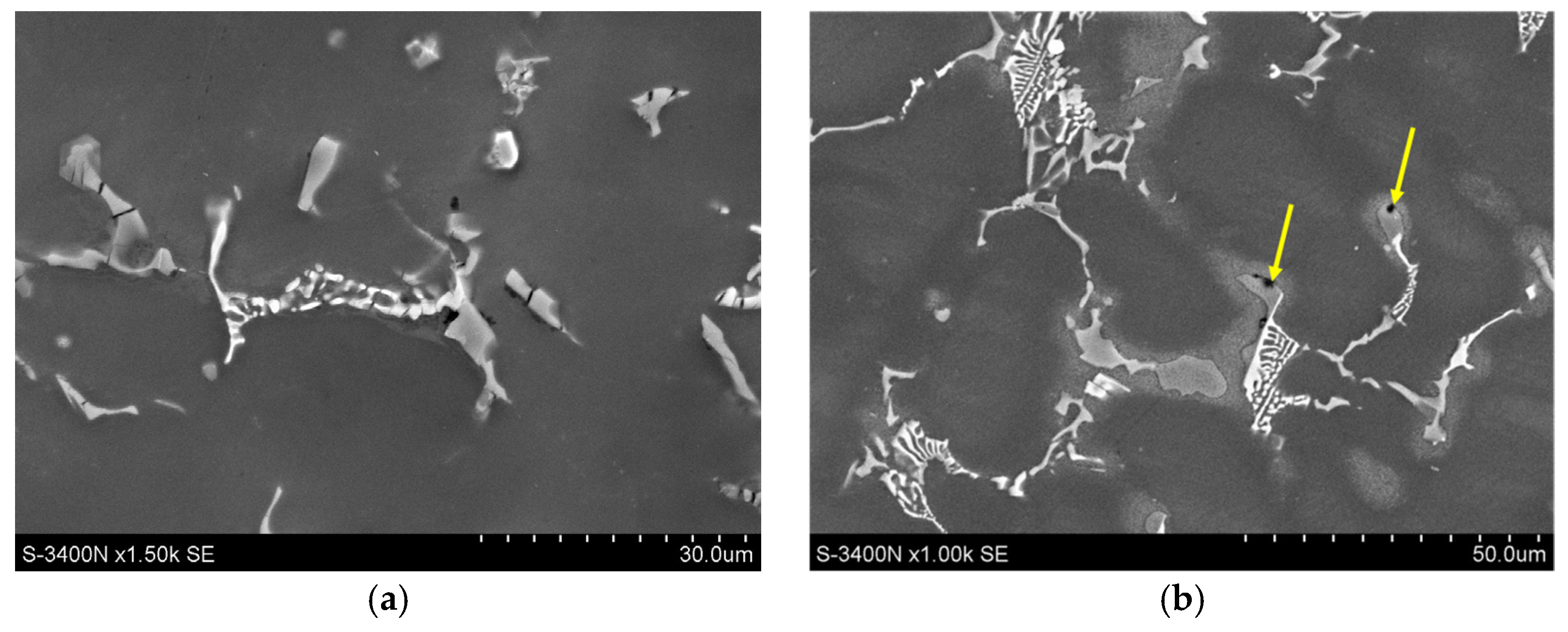
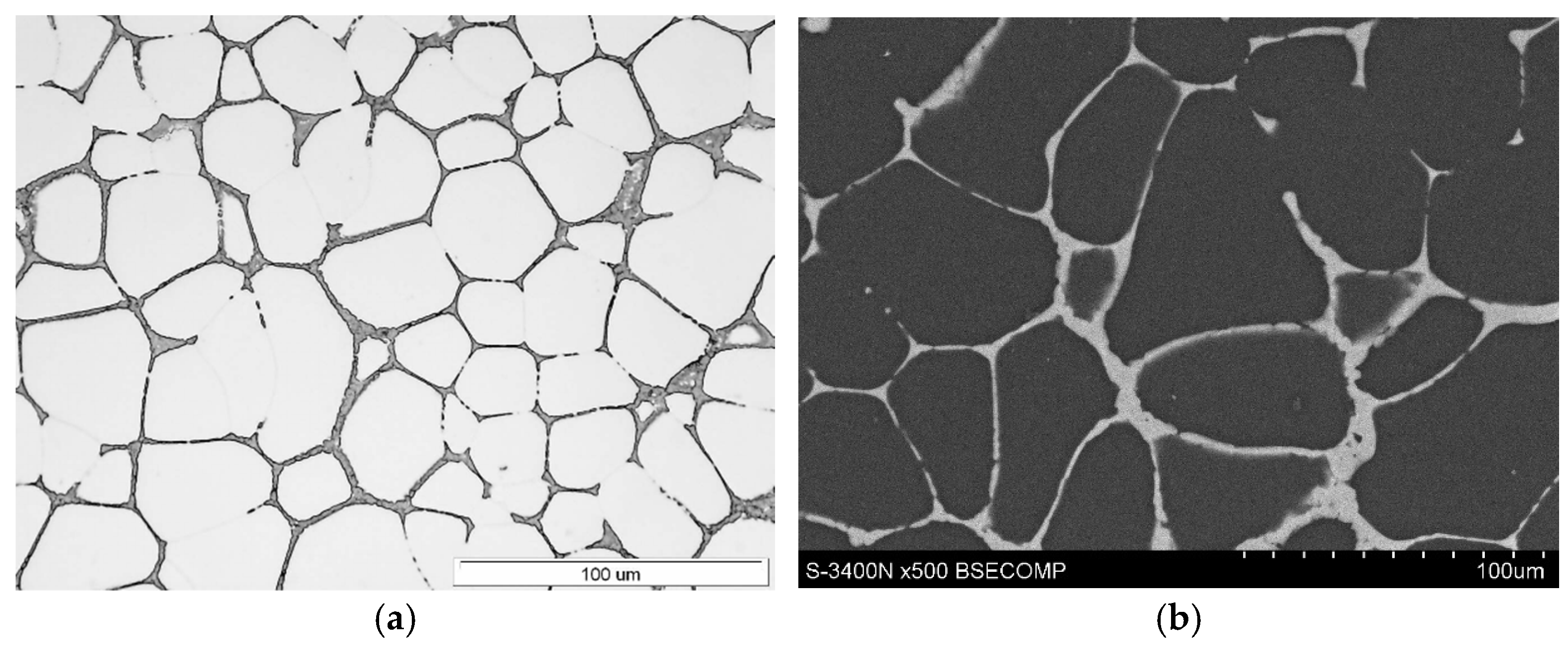
3.1. Microstructure of Mg-Al-Ca-Sr Alloys
3.2. Mechanical Properties of Mg-Al-Ca-Sr Alloys
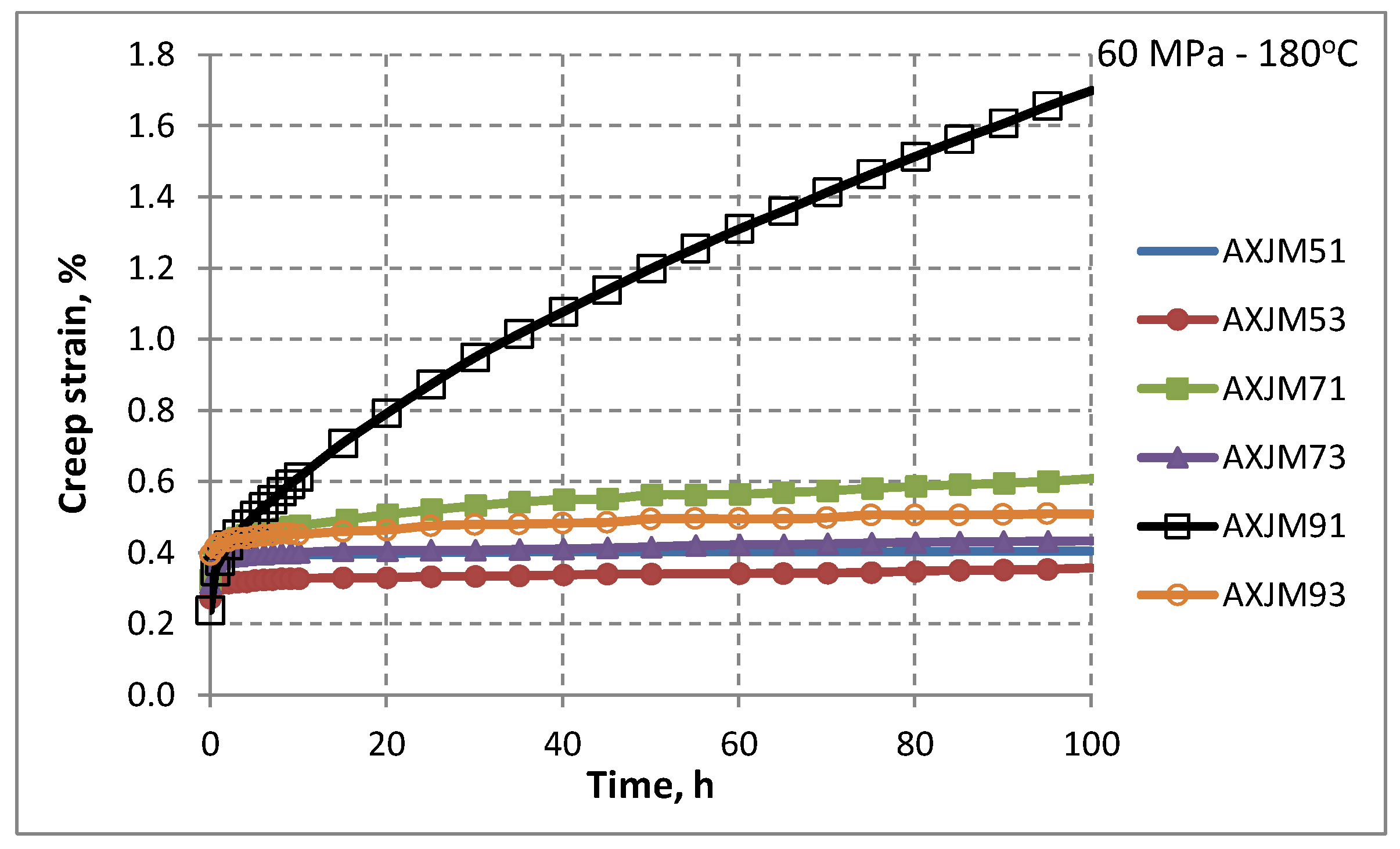
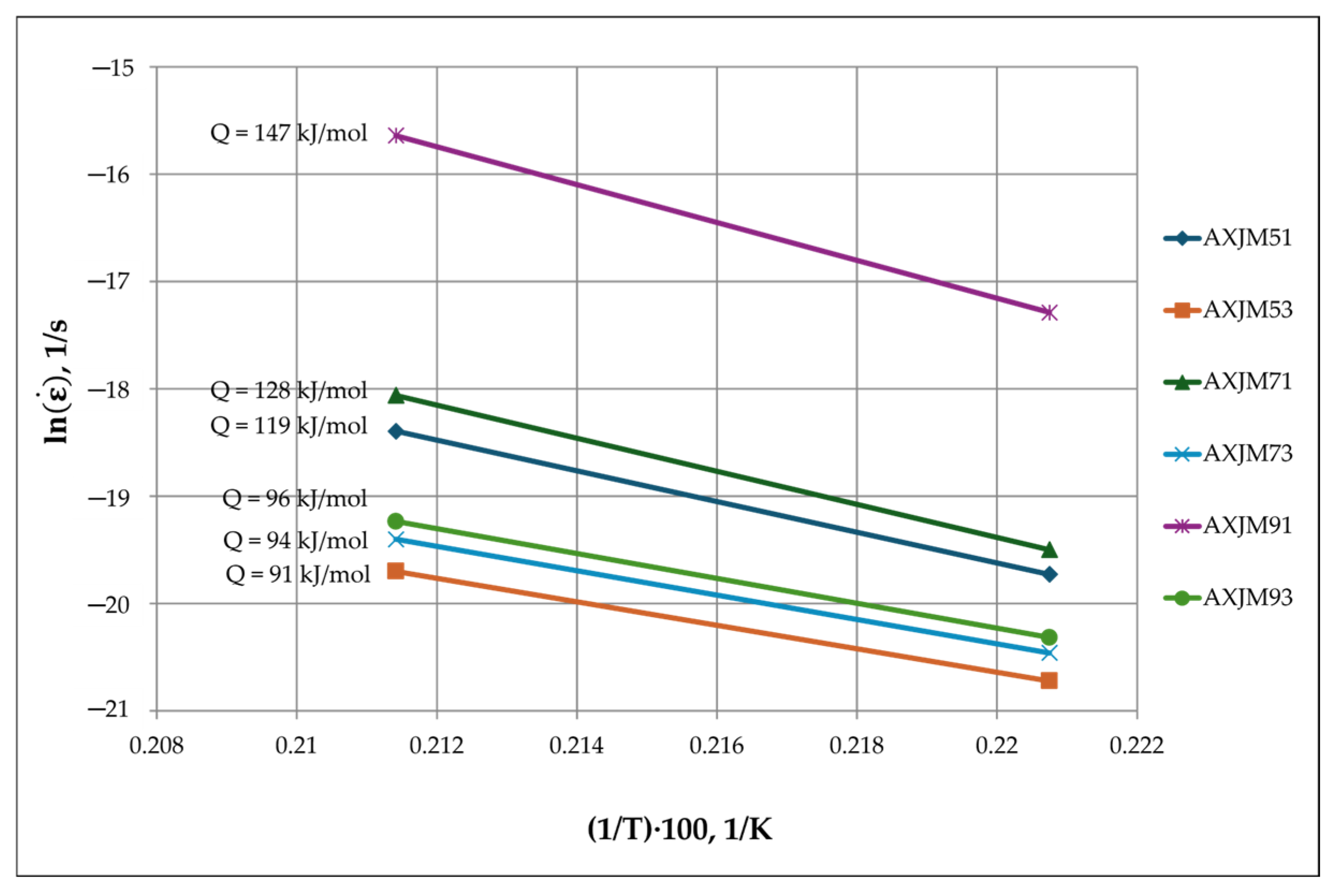
3.3. Creep Properties of Mg-Al-Ca-Sr Alloys
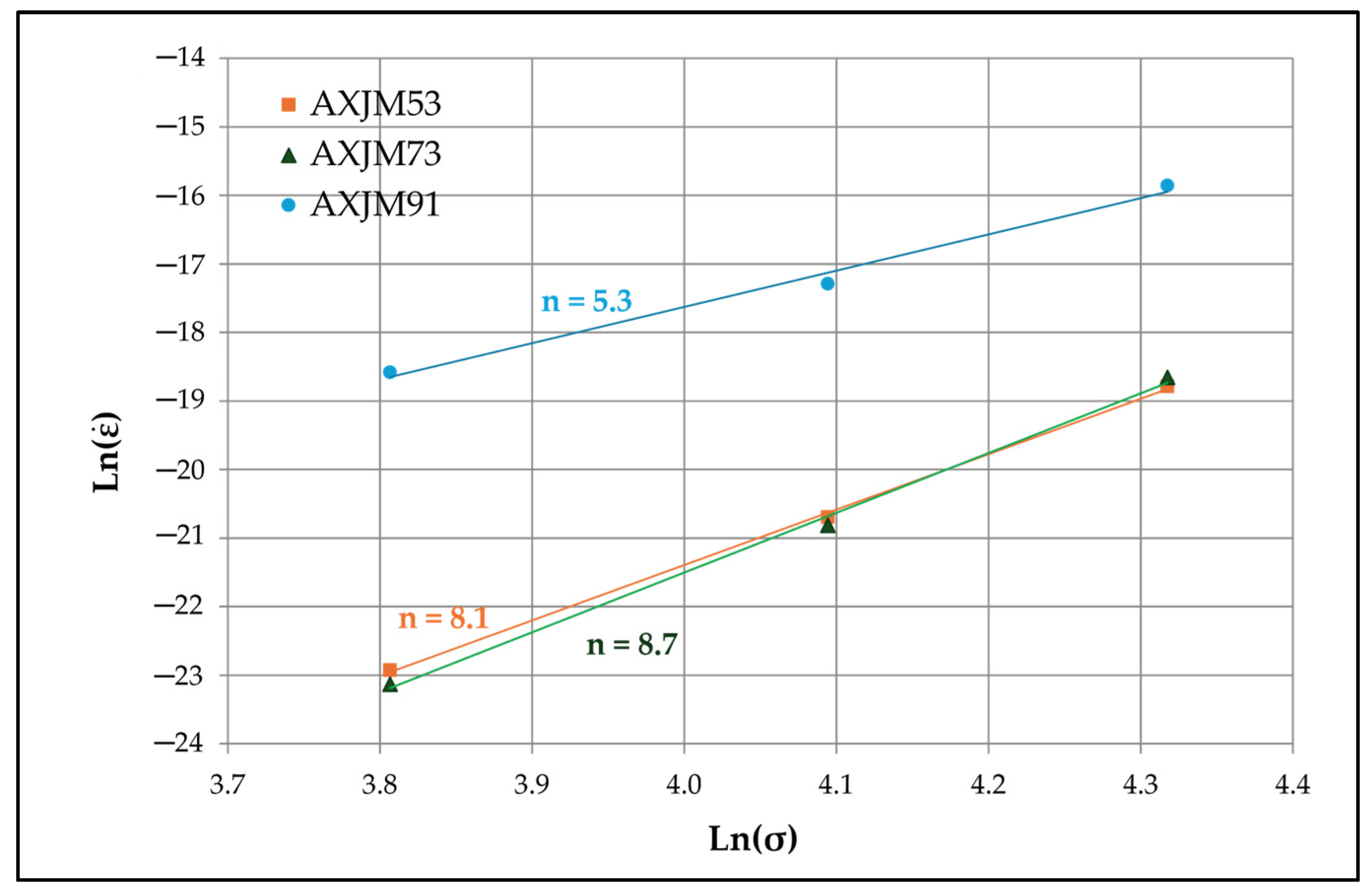
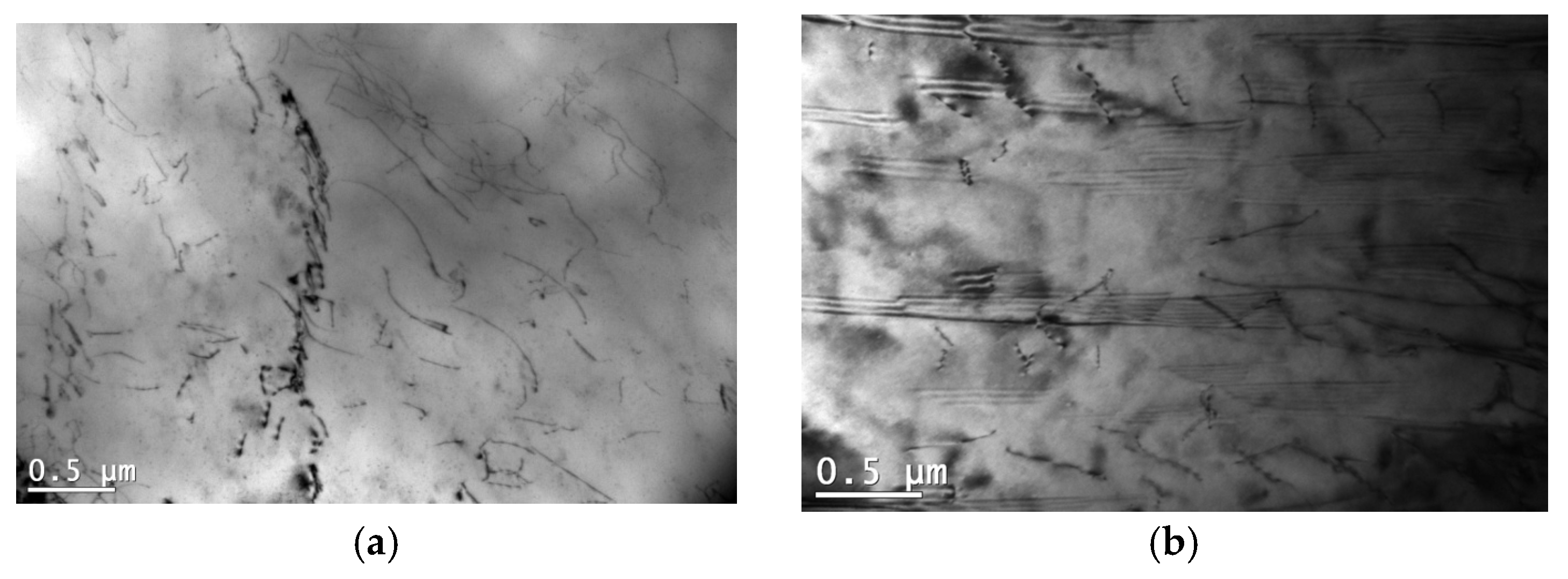
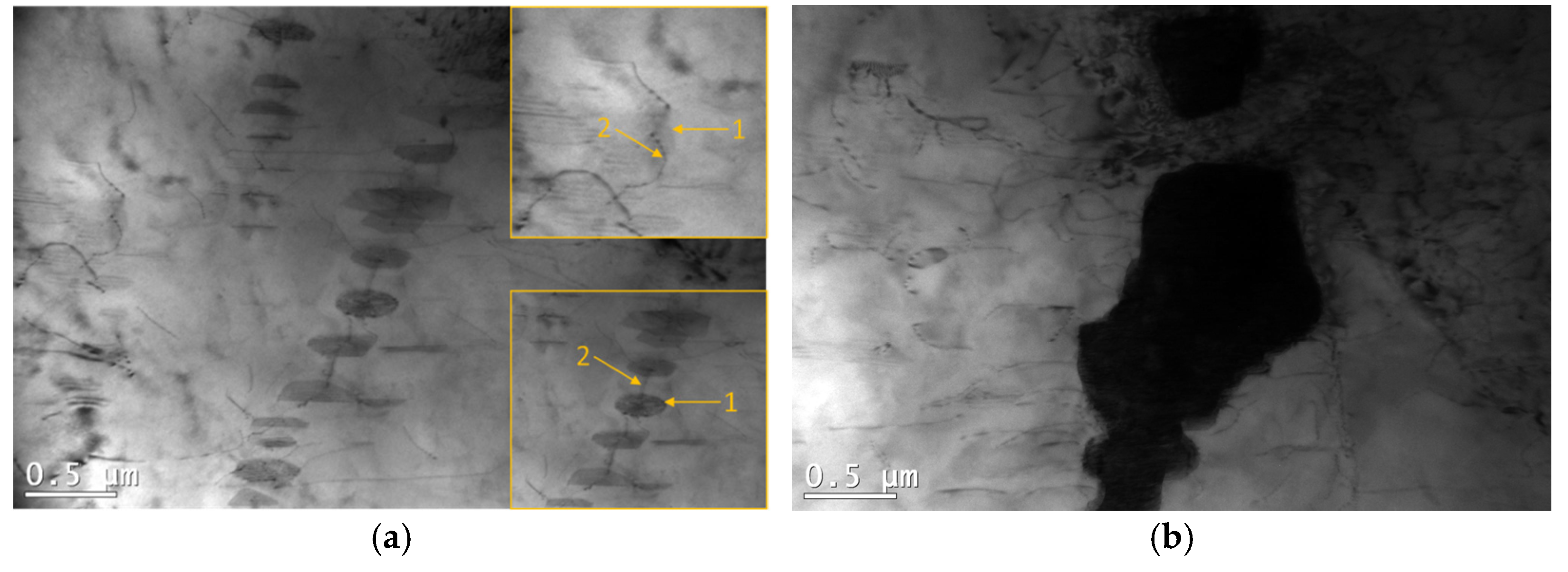
3.4. Influence of Microstructure on Creep Properties of Mg-Al-Ca-Sr Alloys
3.5. Comparison of Mg-Al-Ca-Sr Alloys with EZ33 Magnesium Alloy
4. Conclusions
- Increasing the Ca concentration in Mg-Al-Sr alloys causes a decrease in the volume fraction of the Mg17Al12 phase, the degree of supersaturation of the α-Mg solid solution, and the formation of Laves Ca-rich (Mg,Al)2(Ca,Sr) (C36) and (Mg,Al)2Ca (C14) phases and Sr-rich (Al,Mg)4Sr and (Mg,Al)17Sr2 phases. For large Ca/Al concentration ratios, additional plate-like precipitates of the Al2Ca (C15) Laves phase appear inside the dendrites of the α-Mg solid solution.
- Alloys containing 1% Ca are characterized by good mechanical properties at ambient temperature, but their creep resistance at 200 °C is insufficient. Increasing the Ca content to 3% causes a decrease in mechanical properties at ambient temperature while achieving satisfactory creep resistance at 200 °C. The most favorable mechanical properties at ambient and elevated temperatures are achieved by the AXJM93 alloy (Rm = 149 MPa, Rp0.2 = 102 MPa, A = 2.5%, creep properties at a temperature of 200 °C and at a stress of 60 MPa—~10−9 1/s, ε = 0.64%), which can be used as a substitute for the EZ33 alloy due to its comparable mechanical properties at ambient temperature, slightly better creep resistance at temperatures up to 200 °C, and lower density.
- Factors contributing to the improvement in the creep resistance of Mg-Al-Ca-Sr alloys at 200 °C include the following: solution strengthening caused by the dissolution of Al and Ca in α-Mg, precipitation strengthening resulting from the presence of plate-like precipitates of the Al2Ca (C15) phase with crystallographic orientation of the type {111}C15‖(0001)α-Mg [01]C15 ‖[010] α-Mg, and the presence of high-melting Laves phases in interdendritic areas. Conversely, the Mg17Al12 phase and the presence of supersaturated α-Mg solid solution, in which the Al content significantly exceeds the solubility limit of Al in Mg at 200 °C, exhibit an unfavorable effect on creep resistance.
- In the case of 1% Ca alloys, dislocation climbing was found to be the dominant creep mechanism at 180 °C. In the 3% Ca alloys, the steady-state creep activation energy (Qc) values indicate that creep deformation is controlled by pipe diffusion. Grain boundary slip, which is a significant contributor to creep deformation in die-cast Mg-Al-Ca-Sr alloys, plays a marginal role in sand-cast alloys due to the larger grain size.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedrich, H.E.; Mordike, B.L. Magnesium Technology. Metallurgy, Design Data, Applications; Springer-Verlag: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Avedesian, M.M.; Baker, H. ASM Specialty Handbook, Magnesium and Magnesium Alloys; ASM International: Novelty, OH, USA, 1999. [Google Scholar]
- Mordike, B.L.; Ebert, T. Magnesium, properties–applications–potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Friedrich, H.; Schumann, S. Research for a “new age of magnesium” in the automotive industry. J. Mater. Process. Technol. 2001, 117, 276–281. [Google Scholar] [CrossRef]
- Luo, A.A. Recent magnesium alloy development for elevated temperature applications. Int. Mater. Rev. 2004, 49, 13–30. [Google Scholar] [CrossRef]
- Luo, A.A. Applications: Aerospace, automotive and other structural applications of magnesium. In Advances in Wrought Magnesium Alloys; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 266–316. [Google Scholar]
- Aghion, E.; Bronfin, B.; von Buch, F.; Schumann, S.; Friedrich, H. Newly Developed Magnesium Alloys for Powertrain Applications. JOM 2003, 55, 30–33. [Google Scholar] [CrossRef]
- Aghion, E.; Bronfin, B.; Eliezer, D.; Schumann, S. The Art of Developing New Magnesium Alloys for High Temperature Applications. In Proceedings of the Second Osaka International Conference on Platform Science and Technology, Osaka, Japan, 26–30 January 2003; pp. 40–44. [Google Scholar]
- Elektron ZRE1 Datasheet 450. Available online: https://magnesium.elektron.com (accessed on 18 March 2025).
- Rzychoń, T.; Kiełbus, A. Optimisation of Hot-Chamber Die-Casting Process of AM60 Alloy Using Taguchi Method. Materials 2024, 17, 6256. [Google Scholar] [CrossRef]
- Tariq, H.M.R.; Ishtiaq, M.; Kang, H.-H.; Chaudry, U.M.; Jun, T.-S. A Critical Review on the Comparative Assessment of Rare-Earth and Non-Rare-Earth Alloying in Magnesium Alloys. Metals 2025, 15, 128. [Google Scholar] [CrossRef]
- Tuz, L.; Novák, V.; Tatíček, F. Evaluation of the Microstructure and Properties of As-Cast Magnesium Alloys with 9% Al and 9% Zn Additions. Materials 2025, 18, 10. [Google Scholar] [CrossRef]
- Al-Samman, T.; Letzig, D.; Yi, S. Microstructure–Mechanical Properties and Application of Magnesium Alloys. Metals 2021, 11, 1958. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of Magnesium and Its Alloys: A Review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, X.; Qiu, X. Microstructural Characteristics of High-Pressure Die Casting with High Strength–Ductility Synergy Properties: A Review. Materials 2023, 16, 1954. [Google Scholar] [CrossRef]
- Klupa, A. Cenniejsze niż złoto? Metale ziem rzadkich w światowej strategii gospodarczej. Przegląd Strateg. 2012, 1, 239–251. [Google Scholar] [CrossRef][Green Version]
- Koltygin, A.V.; Bazhenov, V.E.; Belova, E.A.; Nikitina, A.A. Development of a magnesium alloy with good casting characteristics on the basis of Mg-Al-Ca-Mn system, having Mg-Al2Ca structure. J. Magnes. Alloys 2013, 1, 224–229. [Google Scholar] [CrossRef]
- Rzychoń, T.; Kiełbus, A.; Szala, J. Microstructure, Castability, Microstructural Stability and Mechanical Properties of ZRE1 Magnesium Alloy. Arch. Metall. Mater. 2012, 57, 246–252. [Google Scholar] [CrossRef]
- Ahmad, R.; Sheggaf, Z.M.; Asmael, M.B.A.; Hamzah, M.Z. Effect of yttrium addition on microstructure and hardness of cast ZRE1 magnesium alloy. J. Eng. Appl. Sci. 2016, 11, 7689–7693. [Google Scholar]
- Tukiat, I.S.T.; Yusuf, N.K.; Khaireez, H.; Al-Alimi, S.; Lajis, M.A.; Shamsudin, S.; Ruhaizat, N.E. Microstructure and Mechanical Properties of Magnesium ZRE1 (Mg-Zn-Zr) Alloy with Rare Earth Element (Samarium) Addition. Int. J. Technol. 2024, 15, 49–62. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Breen, A.; Peng, Z.; Su, J.; Kürnsteiner, P.; Correa, M.J.D.; Lipińska-Chwałek, M.; Wang, H.; Holec, D.; et al. Elucidation of formation and transformation mechanisms of Ca-rich Laves phase in Mg-Al-Ca-Mn alloys. J. Alloys Compd. 2022, 928, 167–177. [Google Scholar] [CrossRef]
- Rzychoń, T. Characterization of Mg-rich clusters in the C36 phase of the Mg-5Al-3Ca-0.7Sr-0.2Mn alloy. J. Alloys Compd. 2014, 598, 95–105. [Google Scholar] [CrossRef]
- Suzuki, A.; Saddock, N.D.; Riester, L.; Lara-Curzio, E.; Jones, J.W.; Pollock, T.M. Effect of Sr Additions on the Microstructure and Strength of a Mg-Al-Ca Ternary Alloy. Metall. Mater. Trans. A 2007, 38, 420–427. [Google Scholar] [CrossRef]
- Bai, J.; Sun, Y.; Xue, F.; Xue, S.; Qiang, J.; Zhu, T. Effect of Al contents on microstructures, tensile and creep properties of Mg–Al–Sr–Ca alloy. J. Alloys Compd. 2007, 437, 247–253. [Google Scholar] [CrossRef]
- Kondori, B.; Mahmudi, R. Effect of Ca additions on the microstructure and creep properties of a cast Mg–Al–Mn magnesium alloy. Mater. Sci. Eng. A 2017, 700, 438–447. [Google Scholar] [CrossRef]
- Murray, J.L. Binary Alloy Phase Diagrams, 2nd ed.; Massalski, T.B., Ed.; ASM International: Materials Park, OH, USA, 1990; Volume 1, pp. 169–171. [Google Scholar]
- Ninomiya, R.; Yukawa, H.; Morinaga, M.; Kubota, K. An electronic approach to the prediction of the mechanical properties of magnesium alloys. J. Alloys Compd. 1994, 215, 315–323. [Google Scholar] [CrossRef]
- Burke, E.C. Solid solubility of calcium in magnesium. J. Met. Trans. AIME 1955, 203, 285–286. [Google Scholar] [CrossRef]
- Chartrand, P.; Pelton, A.D. Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the Al-Mg, Al-Sr, Mg-Sr, and Al-Mg-Sr systems. J. Phase Equilib. 1994, 15, 591–605. [Google Scholar] [CrossRef]
- Mezbahul-Islam, M.; Mostafa, A.O.; Medraj, M. Essential Magnesium Alloys Binary Phase Diagrams and Their Thermochemical Data. J. Mater. 2014, 2014, 1–33. [Google Scholar] [CrossRef]
- Jiang, B. Grain refinement of Ca addition in a twin-roll-cast Mg–3Al–1Zn alloy. Mater. Chem. Phys. 2012, 133, 611–616. [Google Scholar] [CrossRef]
- Nagasivamuni, B.; Ravi, K.R. An analytical approach to elucidate the mechanism of grain refinement in calcium-added Mg–Al alloys. J. Alloys Compd. 2015, 622, 789–795. [Google Scholar] [CrossRef]
- Liu, S.F.; Li, B.; Wang, X.H.; Su, W.; Han, H. Refinement effect of cerium, calcium and strontium in AZ91 magnesium alloy. J. Mater. Process. Technol. 2009, 209, 3999–4004. [Google Scholar] [CrossRef]
- Park, J.P.; Kim, M.G.; Yoon, U.S. Microstructures and mechanical properties of Mg-Al-Zn-Ca alloys fabricated by high-frequency electromagnetic casting method. J. Mater. Sci. 2009, 44, 47–54. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, N.E.; Yim, C.D.; Kim, B.K. Effect of calcium content on the microstructural evolution and mechanical properties of wrought Mg–3Al–1Zn alloy. Mater. Sci. Eng. A 2009, 525, 18–29. [Google Scholar] [CrossRef]
- Masoudpanah, S.M.; Mahmudi, R. Effects of rare-earth elements and Ca additions on the microstructure and mechanical properties of AZ31 magnesium alloy processed by ECAP. Mater. Sci. Eng. A 2009, 526, 22–30. [Google Scholar] [CrossRef]
- Cao, P.; Qian, M.; StJohn, D.H. Native grain refinement of magnesium alloys. Scr. Mater. 2005, 53, 841–844. [Google Scholar] [CrossRef]
- Lee, Y.C.; Dahle, A.K.; StJohn, D.H. The role of solute in grain refinement of magnesium. Metall. Mater. Trans. A 2000, 31A, 2895–2906. [Google Scholar] [CrossRef]
- Hirai, K.; Somekawa, H.; Takigawa, Y.; Higashi, K. Effects of Ca and Sr addition on mechanical properties of a cast AZ91 magnesium alloy at room and elevated temperature. Mater. Sci. Eng. A 2005, 403, 276–280. [Google Scholar] [CrossRef]
- Li, P.; Tang, B.; Kandalova, E.G. Microstructure and properties of AZ91D alloy with Ca additions. Mater. Lett. 2005, 59, 671–675. [Google Scholar] [CrossRef]
- StJohn, D.H.; Easton, M.A.; Qian, M.; Taylor, J.A. Grain Refinement of Magnesium Alloys: A Review of Recent Research, Theoretical Developments, and Their Application. Metall. Mater. Trans. A 2013, 44A, 2935–2949. [Google Scholar] [CrossRef]
- Pekguleryuz, M.; Kaya, A.A. Creep Resistant Magnesium Alloys for Powertrain Applications. Adv. Eng. Mater. 2003, 5, 866–878. [Google Scholar] [CrossRef]
- Wang, Q.D.; Chen, W.D.; Zeng, X.Q.; Lu, Y.Z.; Ding, W.J.; Zhu, Y.P.; Xu, X.P.; Mabuchi, M. Effects of Ca addition on the microstructure and mechanical properties of AZ91 magnesium alloy. J. Mater. Sci. 2001, 36, 3035–3040. [Google Scholar]
- Harandi, S.E.; Mirshahi, M.; Koleini, S.; Idris, M.H.; Jafari, H.; Kadir, M.R.A. Effect of calcium content on the microstructure, hardness and in-vitro corrosion behavior of biodegradable Mg–Ca binary alloy. Mater. Res. 2013, 16, 11–18. [Google Scholar] [CrossRef]
- Nie, J.F. Precipitation and Hardening in Magnesium Alloys. Metall. Mater. Trans. A 2012, 43, 3891–3939. [Google Scholar] [CrossRef]
- Peng, Z.; Qudong, W.; Chunquan, Z.; Yanping, Z. Effects of strontium and titanium on the microstructure, tensile properties and creep behavior of AM50 alloys. Mater. Sci. Eng. A 2007, 444, 318–326. [Google Scholar]
- Pekguleryuz, M.; Celikin, M. Creep Resistance in Magnesium Alloys. Int. Mater. Rev. 2010, 55, 197–217. [Google Scholar] [CrossRef]
- Shi, L.; Northwood, D.O. Strain-hardening and recovery during the creep of pure polycrystalline magnesium. Acta Metall. Mater. 1994, 42, 871–877. [Google Scholar] [CrossRef]
- Miller, W.K. Creep of die cast AZ91 magnesium at room temperature and low stress. Metall. Mater. Trans. A 1991, 22A, 873–877. [Google Scholar] [CrossRef]
- Regev, M.; Aghion, E.; Rosen, A.; Bamberger, M. Creep studies of coarse-grained AZ91D magnesium castings. Mater. Sci. Eng. A 1998, 252A, 6–16. [Google Scholar] [CrossRef]
- Regev, M.; Aghion, E.; Rosen, A. Creep studies of AZ91D pressure die casting. Mater. Sci. Eng. A 1997, 234, 123–126. [Google Scholar] [CrossRef]
- Terada, Y.; Itoh, D.; Sato, T. Creep rupture properties of die-cast Mg–Al–Ca alloys. Mater. Chem. Phys. 2009, 113, 503–506. [Google Scholar] [CrossRef]
- Celikin, M.; Kaya, A.A.; Pekguleryuz, M. Effect of manganese on the creep behavior of magnesium and the role of α-Mn precipitation during creep. Mater. Sci. Eng. A 2012, 534, 129–141. [Google Scholar] [CrossRef]
- Maruyama, K.; Suzuki, M.; Sato, H. Creep strength of magnesium-based alloys. Metall. Mater. Trans. A 2002, 33, 875–882. [Google Scholar] [CrossRef]
- Luo, A.A.; Balogh, M.P.; Powell, B.R. Creep and microstructure of magnesium–aluminum–calcium based alloys. Metall. Mater. Trans. A 2002, 33A, 567–574. [Google Scholar] [CrossRef]
- Kondori, B.; Mahmudi, R. Effect of Ca additions on the microstructure, thermal stability and mechanical properties of a cast AM60 magnesium alloy. Mater. Sci. Eng. A 2010, 527, 2014–2021. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Li, S.; Ding, N.; Liu, K.; Du, W. Hot Cracking Behaviors of Mg-Zn-Er Alloys with Different Er Contents. Materials 2023, 16, 3546. [Google Scholar] [CrossRef] [PubMed]
- Powell, B.R.; Luo, A.A.; Tiwari, B.L.; Rezhets, V. The Die Castability of Calcium Containing Magnesium Alloys: Thin-Wall Computer Case. In Magnesium Technology 2002; TMS: Pittsburgh, PA, USA, 2002; pp. 123–129. [Google Scholar]
- Cao, G.; Kou, S. Hot Tearing of Ternary Mg-Al-Ca Alloy Castings. Metall. Mater. Trans. A 2006, 37A, 3647–3663. [Google Scholar] [CrossRef]
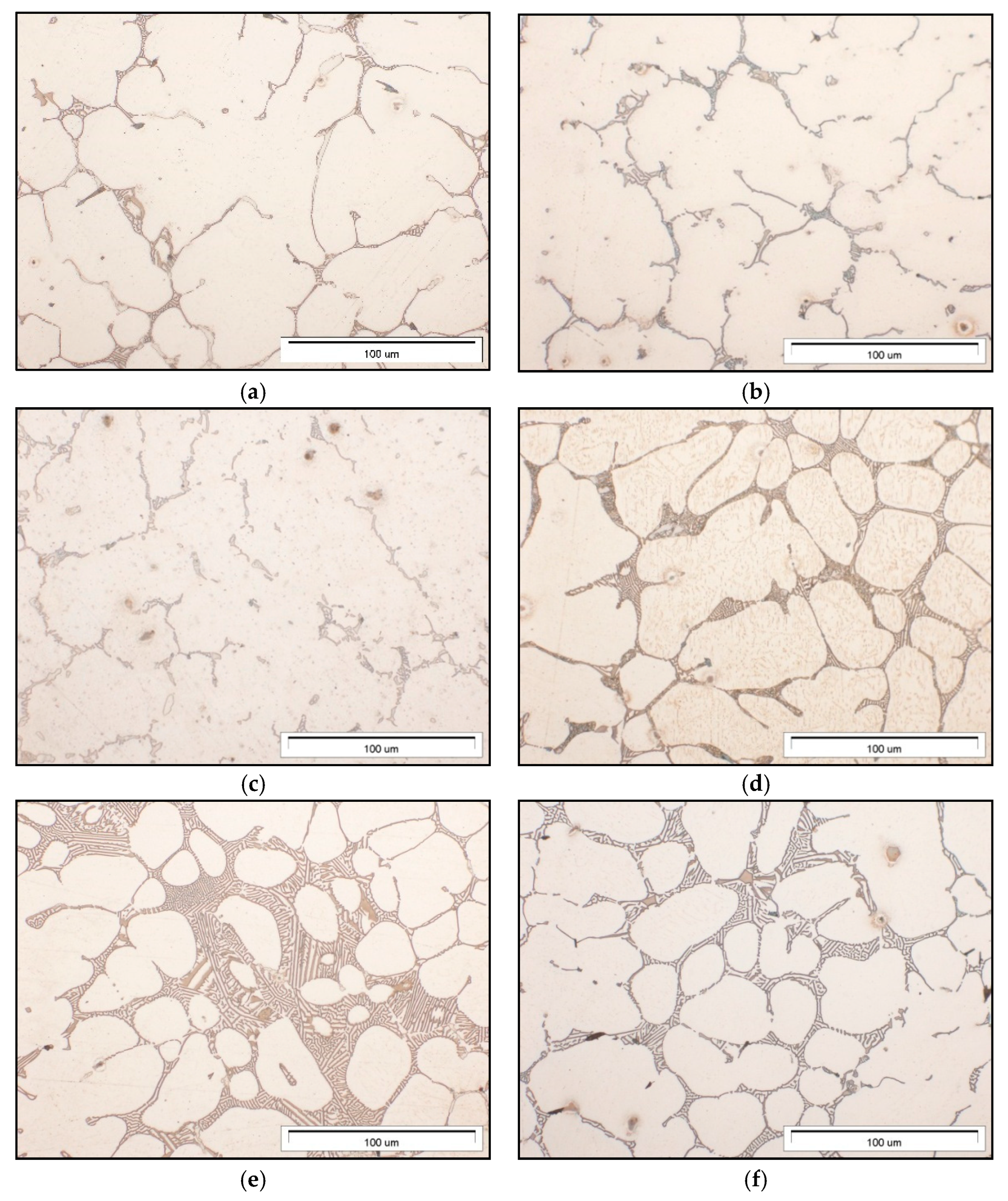
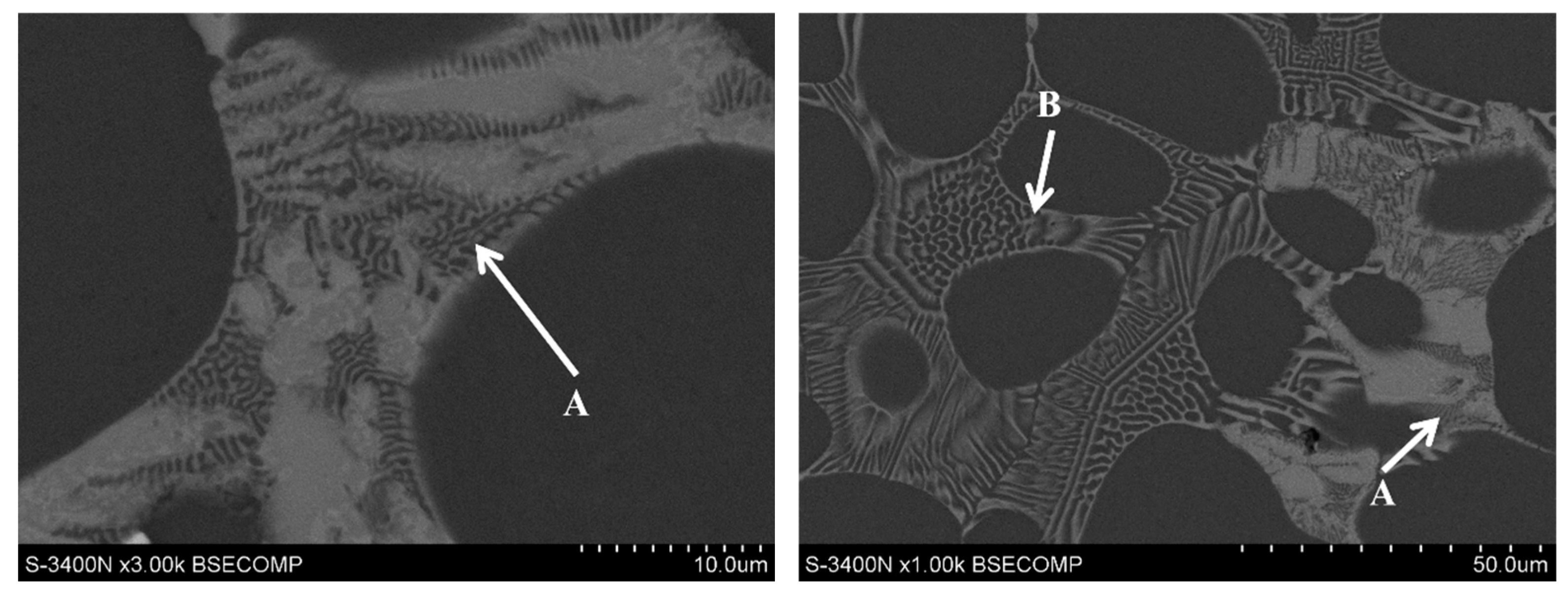
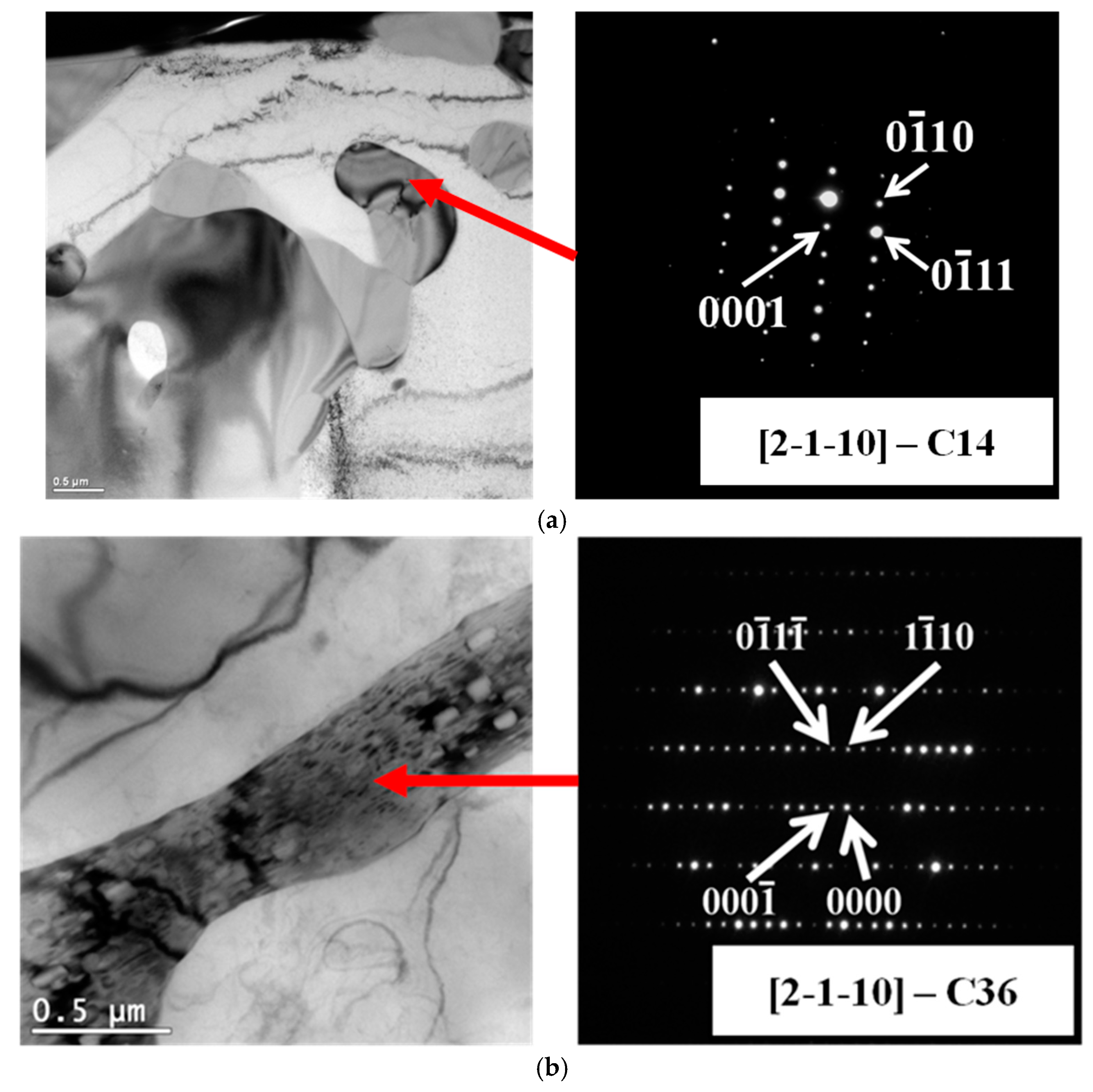
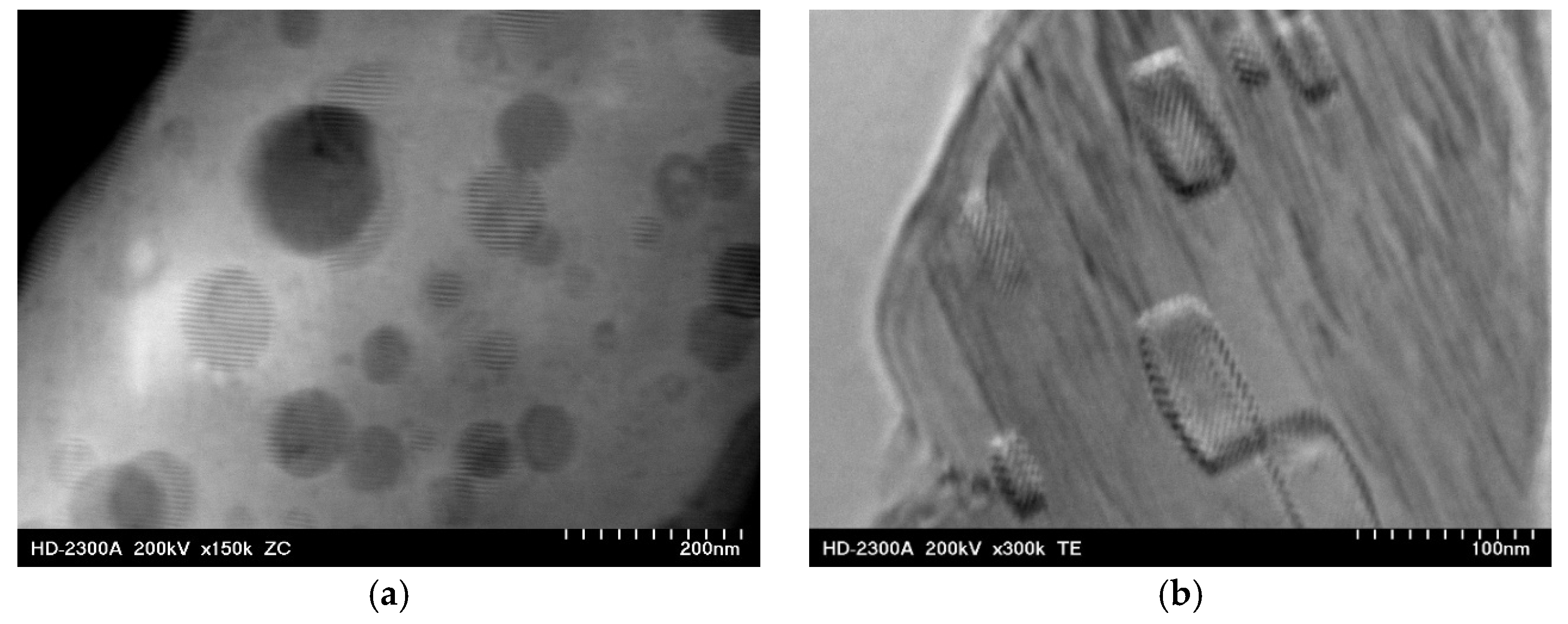
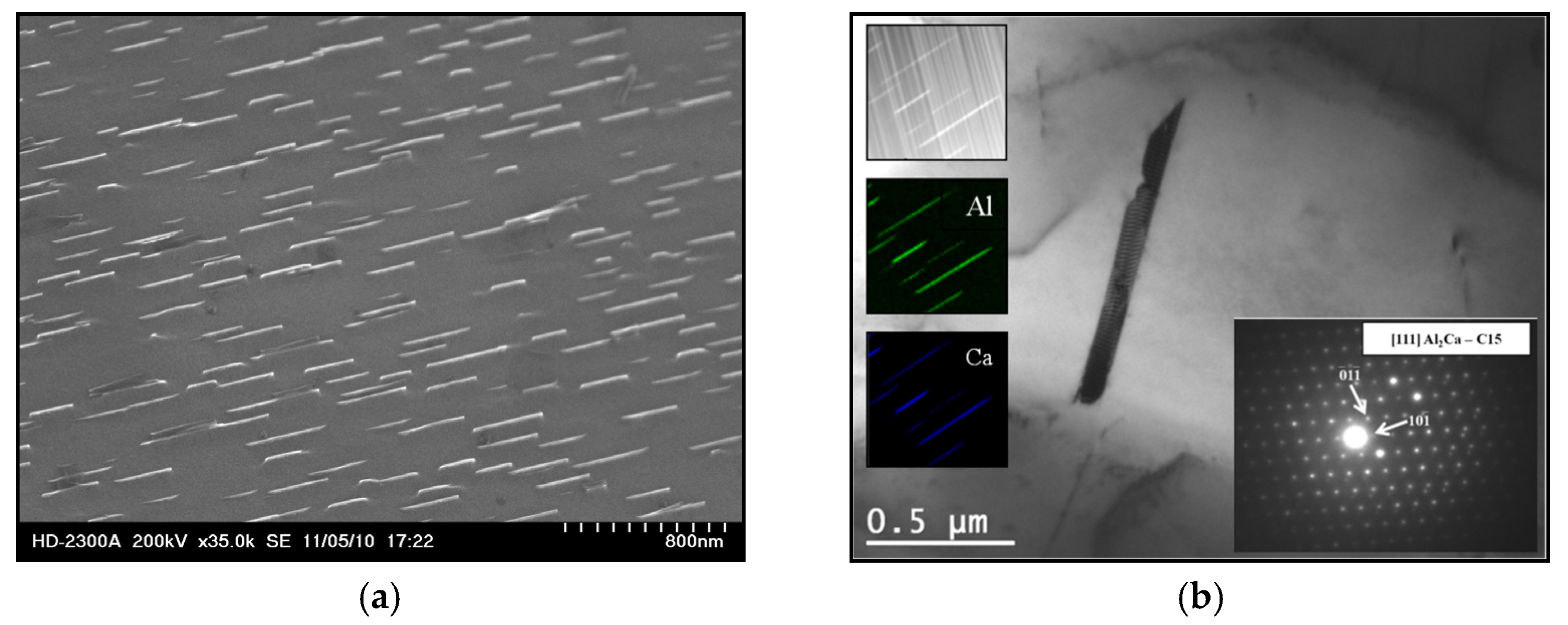
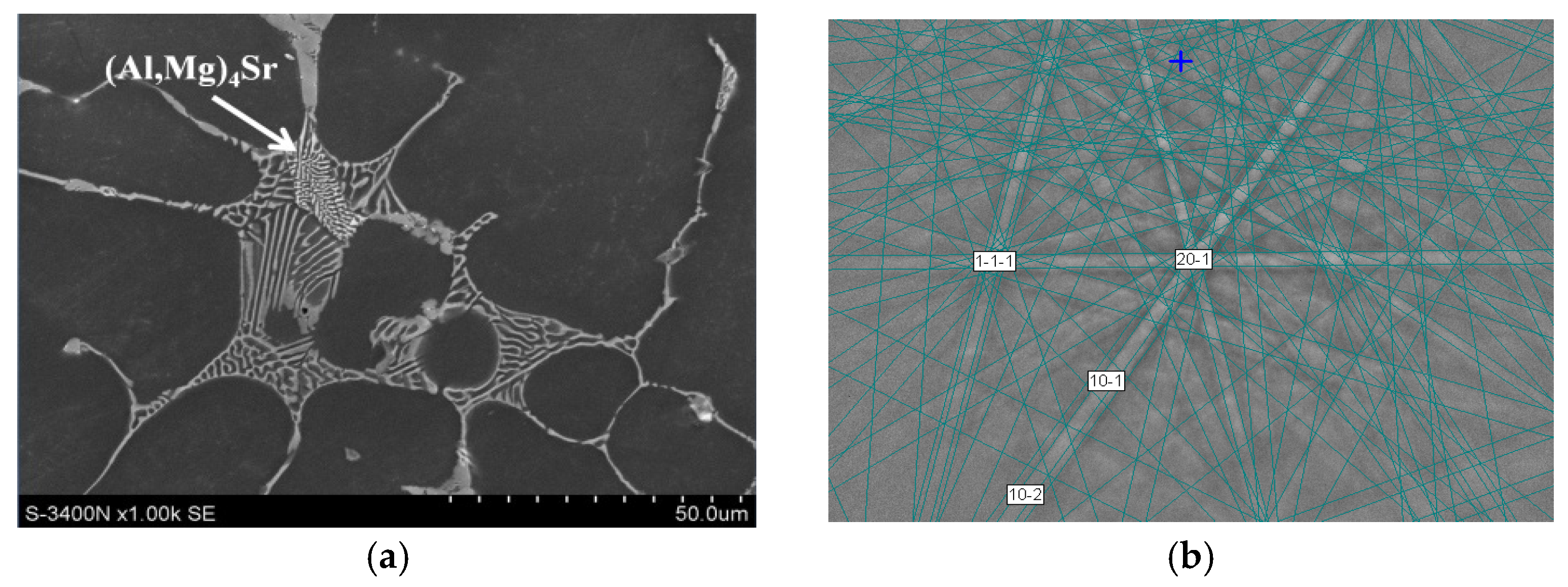
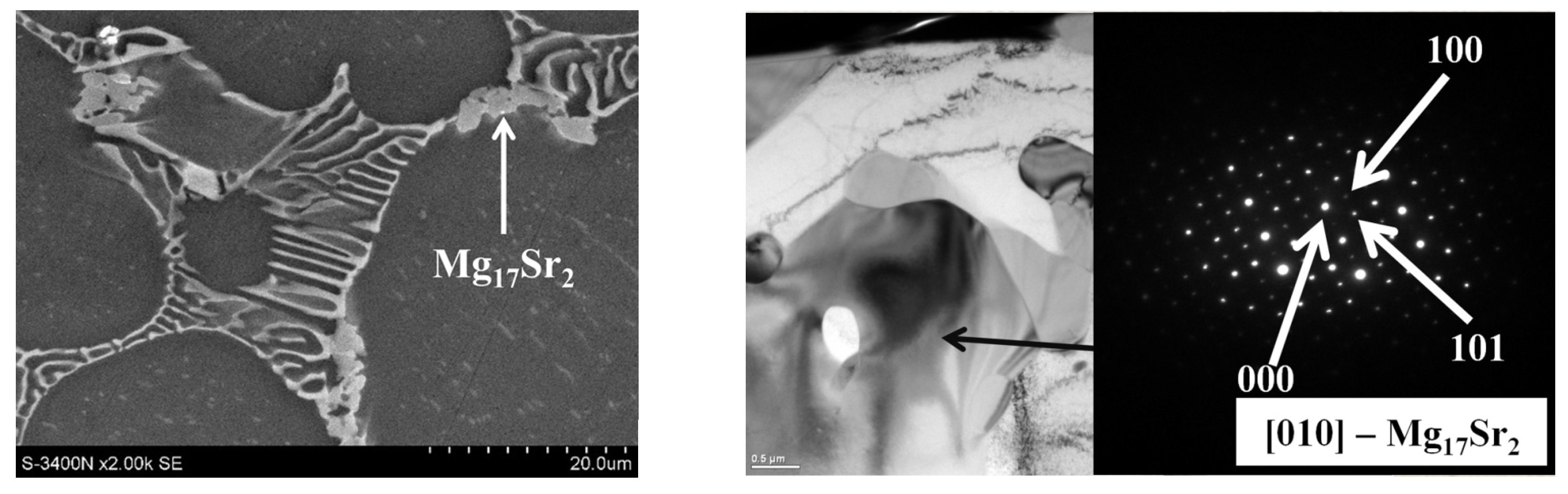

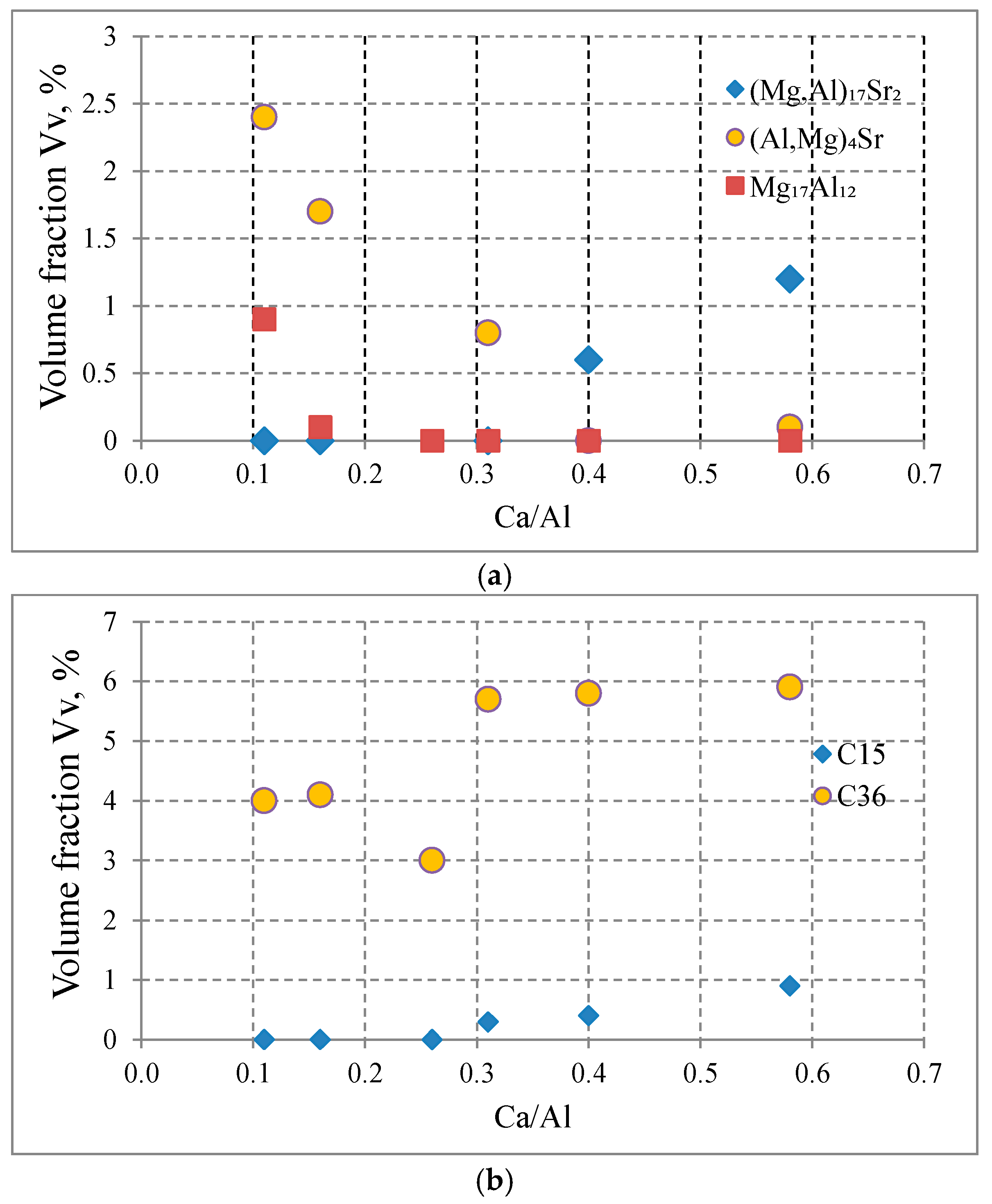





| Alloy | Al | Ca | Sr | Mn | Si | Fe | Mg |
| AXJM51 | 4.98 (0.38) | 1.24 (0.15) | 0.75 (0.12) | 0.19 (0.05) | 0.22 (0.06) | 0.0013 (0.002) | Balance |
| AXJM53 | 5.11 (0.19) | 3.01 (0.23) | 0.68 (0.22) | 0.16 (0.08) | 0.18 (0.07) | 0.0013 (0.001) | Balance |
| AXJM71 | 6.80 (0.32) | 1.22 (0.13) | 0.70 (0.16) | 0.13 (0.09) | 0.12 (0.1) | 0.0012 (0.0003) | Balance |
| AXJM73 | 7.09 (0.34) | 3.05 (0.24) | 0.61 (0.14) | 0.10 (0.07) | 0.10 (0.08) | 0.0007 (0.001) | Balance |
| AXJM91 | 9.01 (0.51) | 1.13 (0.24) | 0.81 (0.22) | 0.19 (0.08) | 0.26 (0.07) | 0.0011 (0.0011) | Balance |
| AXJM93 | 8.67 (0.50) | 2.89 (0.28) | 0.60 (0.1) | 0.16 (0.07) | 0.16 (0.05) | 0.001 (0.001) | Balance |
| Alloy | Zn | RE | Zr | Mn | Fe | Ni, Cu | Mg |
| EZ33 | 2.70 | 3.18 | 0.53 | 0.02 | 0.002 | <0.001 | Balance |
| Phase | Mg | Al | Ca | Sr | Mn |
|---|---|---|---|---|---|
| (Al,Mg)2Ca (C36) | 10.0 (1.7) | 57.6 (5.6) | 30.4 (2.8) | 2.0 (0.24) | - |
| (Mg,Al)2Ca (C14) | 17.5 (2.1) | 54.2 (3.7) | 26.5 (2.3) | 1.8 (0.19) | |
| (Mg,Al)17Sr2 | 71.8 (3.9) | 19.5 (1.8) | 2.5 (1.1) | 6.2 (0.13) | - |
| (Al,Mg)4Sr | 47.8 (3.9) | 41.2 (3.9) | 3.2 (1.2) | 7.8 (0.25) | - |
| Al2Ca (C15) | 75.2 (7.8) | 16.0 (2.4) | 8.8 (1.8) | - | - |
| Mg17Al12 | 70.7 (3.1) | 28.5 (1.9) | 0.7 (0.2) | 0.1 (0.11) | - |
| Alloy | Ca/Al Ratio | Al Content in α-Mg, at.% | α-Mg Grain Size, µm | Phase Composition | Volume Fraction, % |
|---|---|---|---|---|---|
| AXJM51 | 0.26 | 1.5 (0.18) | 58.1 (19.6) | (Al,Mg)2Ca—C36 | 3.0 (0.24) |
| (Mg,Al)17Sr2 | 2.0 (0.18) | ||||
| (Al,Mg)4Sr | 0.1 (0.07) | ||||
| Al8Mn5 | 0.1 (0.08) | ||||
| AXJM53 | 0.58 | 1.5 (0.16) | 75.2 (34.7) | (Al,Mg)2Ca—C36 | 5.9 (0.47) |
| Al2Ca—C15 | 0.9 (0.4) | ||||
| (Mg,Al)17Sr2 | 1.2 (0.11) | ||||
| (Al,Mg)4Sr | 0.1 (0.03) | ||||
| (Mg,Al)2Ca—C14 | 0.9 (0.05) | ||||
| Al8Mn5 | 0.1 (0.02) | ||||
| AXJM71 | 0.16 | 2.9 (0.25) | 59.0 (25.0) | (Al,Mg)2Ca—C36 | 4.1 (0.33) |
| (Al,Mg)4Sr | 1.7 (0.1) | ||||
| Al8Mn5 | 0.1 (0.06) | ||||
| Mg17Al12 | 0.1 (0.04) | ||||
| AXJM73 | 0.40 | 2.0 (0.32) | 113.7 (68.9) | (Al,Mg)2Ca—C36 | 5.8 (0.41) |
| Al2Ca—C15 | 0.4 (0.3) | ||||
| (Mg,Al)17Sr2 | 0.6 (0.04) | ||||
| (Mg,Al)2Ca—C14 | 0.3 (0.04) | ||||
| Al8Mn5 | 0.1 (0.01) | ||||
| AXJM91 | 0.11 | 3.7 (0.55) | 66.2 (25.9) | (Al,Mg)2Ca—C36 | 4.0 (0.28) |
| (Al,Mg)4Sr | 1.9 (0.12) | ||||
| Al8Mn5 | 0.1 (0.02) | ||||
| Mg17Al12 | 0.9 (0.16) | ||||
| AXJM93 | 0.31 | 2.4 (0.17) | 77.6 (28.9) | (Al,Mg)2Ca—C36 | 5.7 (0.45) |
| Al2Ca—C15 | 0.3 (0.1) | ||||
| (Al,Mg)4Sr | 0.8 (0.1) | ||||
| Al8Mn5 | 0.1 (0.06) |
| Alloy | HV2 | Hardness of α-Mg HV0.025 | 21 °C | 180 °C | ||||
|---|---|---|---|---|---|---|---|---|
| Rm, MPa | Rp0.2, MPa | A5, % | Rm, MPa | Rp0.2, MPa | A5, % | |||
| AXJM51 | 49(6) | 45(1) | 138(6) | 101(5) | 1.9(0.2) | 107(4) | 71(4) | 9.8(1.2) |
| AXJM53 | 58(1) | 50(2) | 135(4) | 104(3) | 2.1(0.3) | 123(8) | 84(7) | 3.2(0.6) |
| AXJM71 | 50(1) | 53(2) | 174(6) | 113(5) | 3.1(0.3) | 119(7) | 77(5) | 5.5(0.7) |
| AXJM73 | 60(6) | 52(2) | 137(5) | 105(4) | 2.1(0.2) | 119(3) | 88(5) | 2.9(0.4) |
| AXJM91 | 56(2) | 56(1) | 165(6) | 109(6) | 2.9(0.4) | 102(2) | 78(6) | 12.5(1.8) |
| AXJM93 | 60(7) | 54(1) | 149(7) | 102(4) | 2.5(0.3) | 115(7) | 81(3) | 3.9(0.9) |
| Alloy | Stress, MPa | Temp., °C | Time Test, h | Instant Strain εn, % | Creep Strain ε, % | 1/s |
|---|---|---|---|---|---|---|
| AXJM53 | 45 | 180 | 100 | 0.15 | 0.28 | 1.1 × 10−10 |
| AXJM73 | 45 | 180 | 100 | 0.16 | 0.27 | 9.0 × 10−11 |
| AXJM91 | 45 | 180 | 100 | 0.11 | 0.92 | 8.5 × 10−9 |
| AXJM51 | 60 | 180 | 100 | 0.57 | 0.94 | 2.7 × 10−9 |
| AXJM53 | 60 | 180 | 100 | 0.31 | 0.36 | 1.0 × 10−9 |
| AXJM71 | 60 | 180 | 100 | 0.32 | 0.61 | 3.4 × 10−9 |
| AXJM73 | 60 | 180 | 100 | 0.31 | 0.43 | 1.3 × 10−9 |
| AXJM91 | 60 | 180 | 100 | 0.24 | 1.7 | 3.1 × 10−8 |
| AXJM93 | 60 | 180 | 100 | 0.39 | 0.51 | 1.5 × 10−9 |
| AXJM51 | 75 | 180 | 100 | 1.03 | 3.56 | 4.0 × 10−8 |
| AXJM53 | 75 | 180 | 100 | 0.50 | 1.38 | 9.9 × 10−9 |
| AXJM71 | 75 | 180 | 100 | 0.61 | 5.59 | 1.0 × 10−7 |
| AXJM73 | 75 | 180 | 100 | 0.54 | 1.15 | 7.9 × 10−9 |
| AXJM91 | 75 | 180 | 100 | 0.44 | 6.1 | 1.3 × 10−7 |
| AXJM93 | 75 | 180 | 100 | 0.31 | 1.93 | 3.3 × 10−8 |
| AXJM53 | 90 | 180 | 16 | 1.37 | 5.17 | 4.9 × 10−7 |
| AXJM71 | 90 | 180 | 23 | 1.51 | 12.46 | 9.1 × 10−7 |
| AXJM73 | 90 | 180 | 31 | 1.47 | 6.1 | 8.4 × 10−8 |
| AXJM91 | 90 | 180 | 9 | 1.2 | 7.2 | 1.3 × 10−5 |
| AXJM51 | 60 | 200 | 100 | 0.63 | 1.27 | 1.03 × 10−8 |
| AXJM53 | 60 | 200 | 100 | 0.33 | 0.44 | 2.78 × 10−9 |
| AXJM71 | 60 | 200 | 100 | 0.34 | 0.98 | 1.43 × 10−8 |
| AXJM73 | 60 | 200 | 100 | 0.32 | 0.54 | 3.61 × 10−9 |
| AXJM91 | 60 | 200 | 100 | 0.28 | 6.22 | 1.61 × 10−7 |
| AXJM93 | 60 | 200 | 100 | 0.43 | 0.64 | 4.43 × 10−9 |
| Alloy | Alloy State | Dislocation Density ρ [1/m2] | Δρ [%] |
|---|---|---|---|
| AXJM53 | Sand-cast | 2.9 × 1013 (1.2 × 1012) | 21 |
| 60 MPa/180 °C | 3.5 × 1013 (1.9 × 1012) | ||
| AXJM73 | Sand-cast | 3.4 × 1013 (2.1 × 1012) | 18 |
| 60 MPa/180 °C | 4.0 × 1013 (1.7 × 1012) | ||
| AXJM91 | Sand-cast | 9.7 × 1013 (2.6 × 1012) | 3 |
| 60 MPa/180 °C | 1.0 × 1014 (3.7 × 1012) |
| Alloy | State | (Al,Mg)2Ca—C36 | Al2Ca—C15 | Mg17Al12 | (Mg,Al)17Sr2 | (Al,Mg)4Sr |
|---|---|---|---|---|---|---|
| AXJM51 | As-cast | 3.0 (0.24) | - | - | 2.0 (0.18) | 0.1 (0.07) |
| 60 MPa/180 °C | 3.2 (0.36) | - | - | 2.1 (0.45) | 0.1 (0.10) | |
| AXJM53 | As-cast | 5.9 (0.47) | 0.9 (0.5) | - | 1.2 (0.11) | 0.1 (0.03) |
| 60 MPa/180 °C | 5.7 (0.89) | 1.4 (0.5) | - | 1.4 (0.11) | 0.12 (0.1) | |
| AXJM71 | As-cast | 4.1 (0.33) | - | 0.1 (0.04) | - | 1.7 (0.1) |
| 60 MPa/180 °C | 4.2 (0.94) | - | 0.4 (0.08) | - | 1.7 (0.3) | |
| AXJM73 | As-cast | 5.8 (0.41) | 0.4 (0.3) | - | 0.6 (0.04) | - |
| 60 MPa/180 °C | 6.0 (0.92) | 0.9 (0.3) | - | 0.8 (0.15) | ||
| AXJM91 | As-cast | 4.0 (0.28) | - | 0.9 (0.16) | - | 1.9 (0.12) |
| 60 MPa/180 °C | 3.8 (0.38) | - | 1.9 (0.17) | - | 1.8 (0.25) | |
| AXJM93 | As-cast | 5.7 (0.45) | 0.3 (0.1) | - | - | 0.8 (0.1) |
| 60 MPa/180 °C | 5.9 (0.28) | 0.7 (0.3) | - | - | 0.9 (0.4) |
| Casting Method | Heat Treatment | Rm MPa | Rp0.2 MPa | Elongation A10, % |
|---|---|---|---|---|
| Sand casting | T5 | 119–140 | 80–95 | 3 |
| Alloy | Heat Treatment | Stress, MPa | Temperature, °C | Test Time, h | Creep Strain ε, % | , 1/s |
|---|---|---|---|---|---|---|
| EZ33 | - | 60 | 180 | 100 | 0.89 | 5.0 × 10−9 |
| EZ33 | - | 60 | 200 | 100 | 1.14 | 7.7 × 10−9 |
| EZ33 | - | 60 | 225 | 87 | 5.9 | 2.4 × 10−8 |
| EZ33 | T5 | 60 | 180 | 100 | 0.64 | 3.6 × 10−9 |
| EZ33 | T5 | 60 | 200 | 100 | 0.90 | 6.5 × 10−9 |
| EZ33 | T5 | 75 | 200 | 100 | 1.91 | 2.4 × 10−8 |
| EZ33 | T5 | 30 | 250 | 100 | 0.82 | 1.4 × 10−8 |
| EZ33 | T5 | 40 | 250 | 100 | 3.3 | 6.9 × 10−8 |
| Alloy | Density ρ, g/cm3 | Specific Strength Rm/ρ, MPa∙cm3/g | Specific Yield Strength Rp0.2/ρ, MPa∙cm3/g | Elongation, % | Creep Strain at 200 °C and 60 MPa After 100 h, % | , at 200 °C and 60 MPa After 100 h, 1/s |
|---|---|---|---|---|---|---|
| AXJM51 | 1.81 | 76.4 | 55.9 | 1.9 | 1.27 | 1.03 × 10−8 |
| AXJM71 | 1.82 | 95.5 | 62.0 | 3.1 | 0.98 | 1.43 × 10−8 |
| AXJM91 | 1.85 | 89.2 | 58.9 | 2.9 | 6.22 | 1.61 × 10−7 |
| AXJM53 | 1.80 | 75.0 | 57.8 | 2.1 | 0.44 | 2.78 × 10−9 |
| AXJM73 | 1.82 | 75.4 | 57.8 | 2.1 | 0.54 | 3.61 × 10−9 |
| AXJM93 | 1.84 | 81.2 | 55.6 | 2.5 | 0.64 | 4.43 × 10−9 |
| EZ33 * | 2.08 | 57.2 | 38.5 | 2.9 | 0.90 | 6.5 × 10−9 |
| EZ33 ** | 2.08 | 67.3 | 43.3 | 3.0 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzychoń, T.; Fornalczyk, A. As-Cast Magnesium Alloys with Ca Addition as a Replacement for Magnesium Alloys with Rare Earth Metals. Materials 2025, 18, 1860. https://doi.org/10.3390/ma18081860
Rzychoń T, Fornalczyk A. As-Cast Magnesium Alloys with Ca Addition as a Replacement for Magnesium Alloys with Rare Earth Metals. Materials. 2025; 18(8):1860. https://doi.org/10.3390/ma18081860
Chicago/Turabian StyleRzychoń, Tomasz, and Agnieszka Fornalczyk. 2025. "As-Cast Magnesium Alloys with Ca Addition as a Replacement for Magnesium Alloys with Rare Earth Metals" Materials 18, no. 8: 1860. https://doi.org/10.3390/ma18081860
APA StyleRzychoń, T., & Fornalczyk, A. (2025). As-Cast Magnesium Alloys with Ca Addition as a Replacement for Magnesium Alloys with Rare Earth Metals. Materials, 18(8), 1860. https://doi.org/10.3390/ma18081860







