Free Radical Copolymerization of N-Isopropylacrylamide and 2,3-Dihydroxypropyl Methacrylate: Reaction Kinetics and Characterizations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of P(NIPAm-co-DHPMA)
2.3. Structural Characterization
2.4. Molecular Molar Mass
2.5. Conversion of Monomers
2.6. Composition of the Copolymer
2.7. Reactivity Ratios
2.8. Cloud Point
2.9. In Vitro Cytotoxicity
3. Results and Discussion
3.1. Chemical Structure of the Copolymers
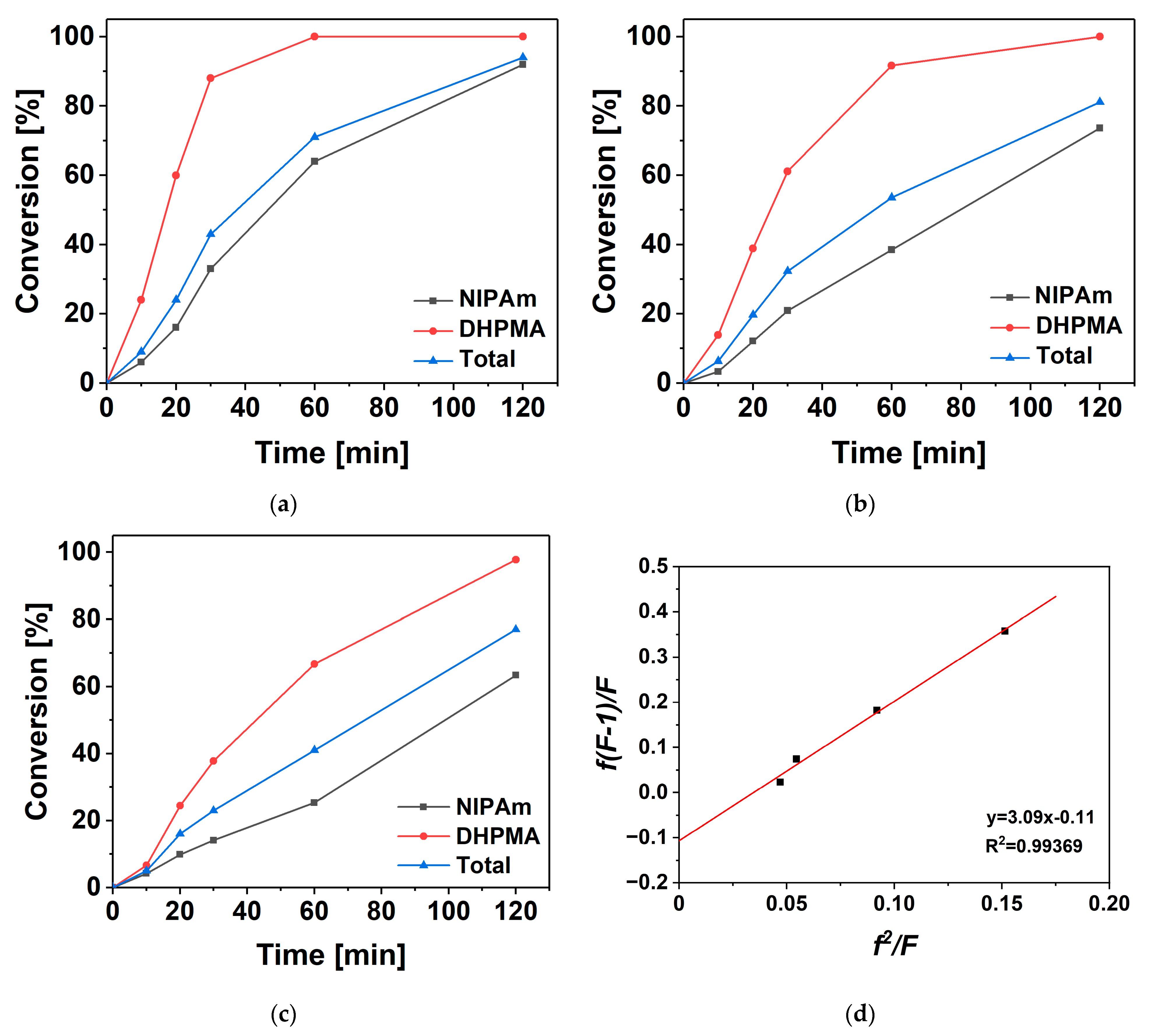
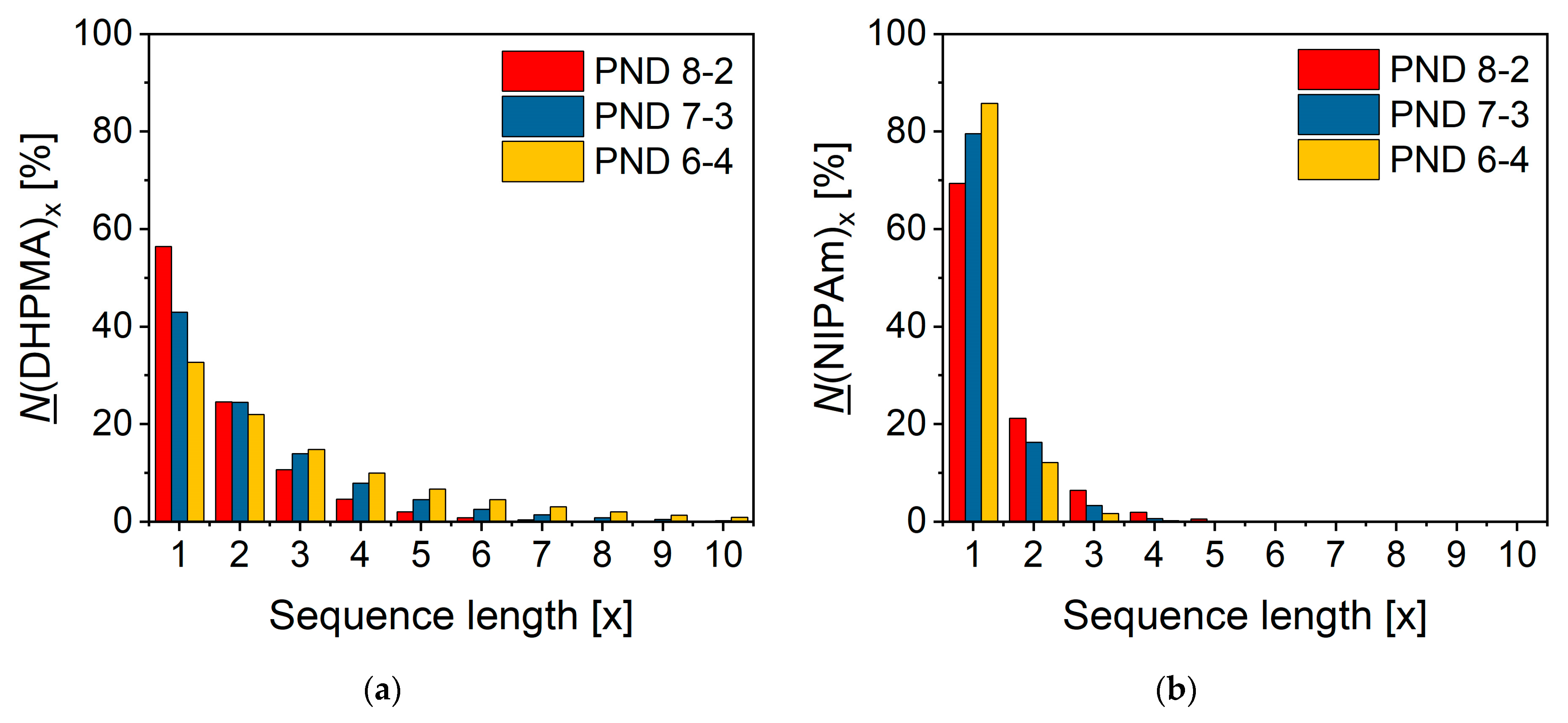
3.2. Kinetics of Copolymerization
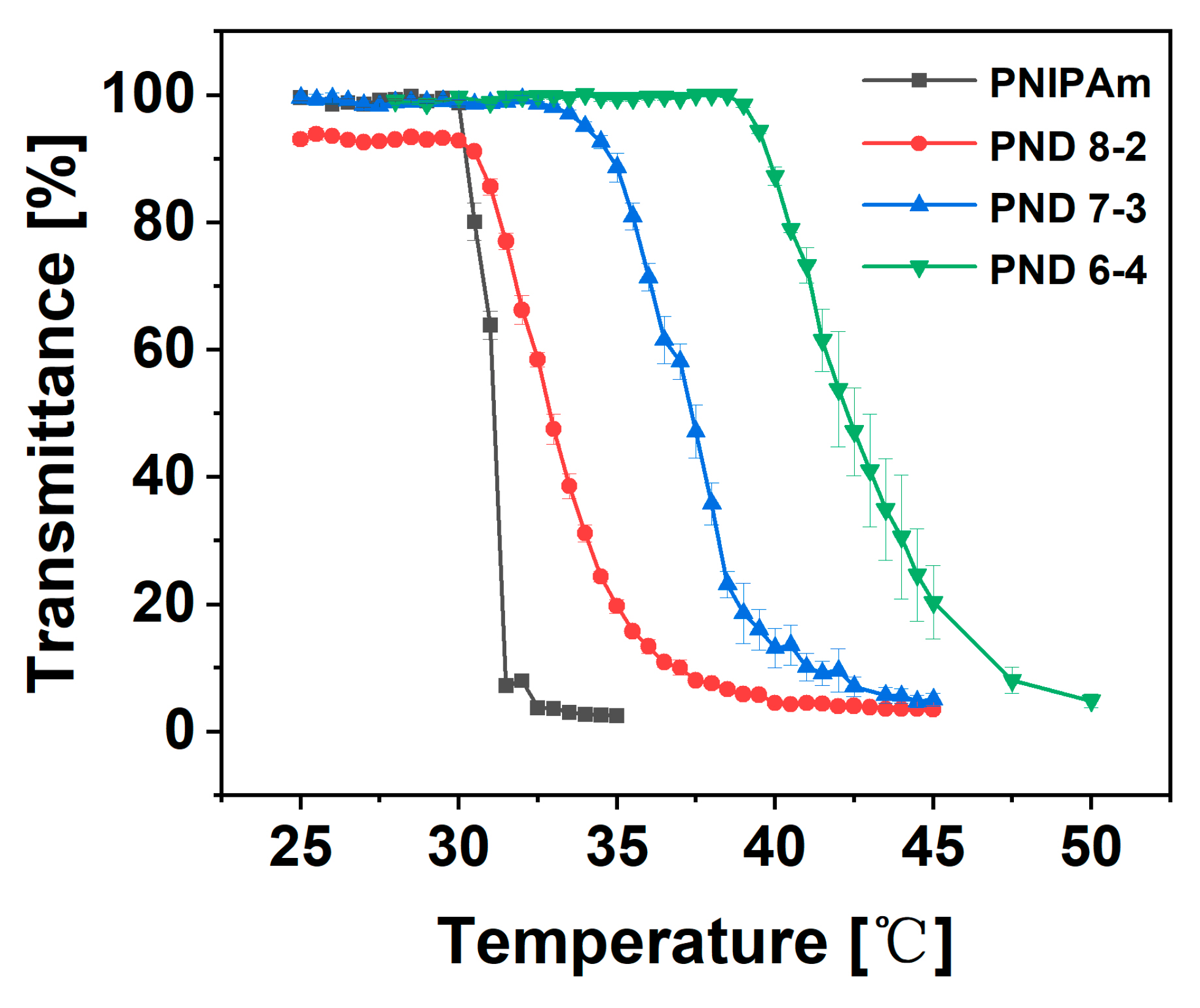
3.3. Phase Transition Behavior
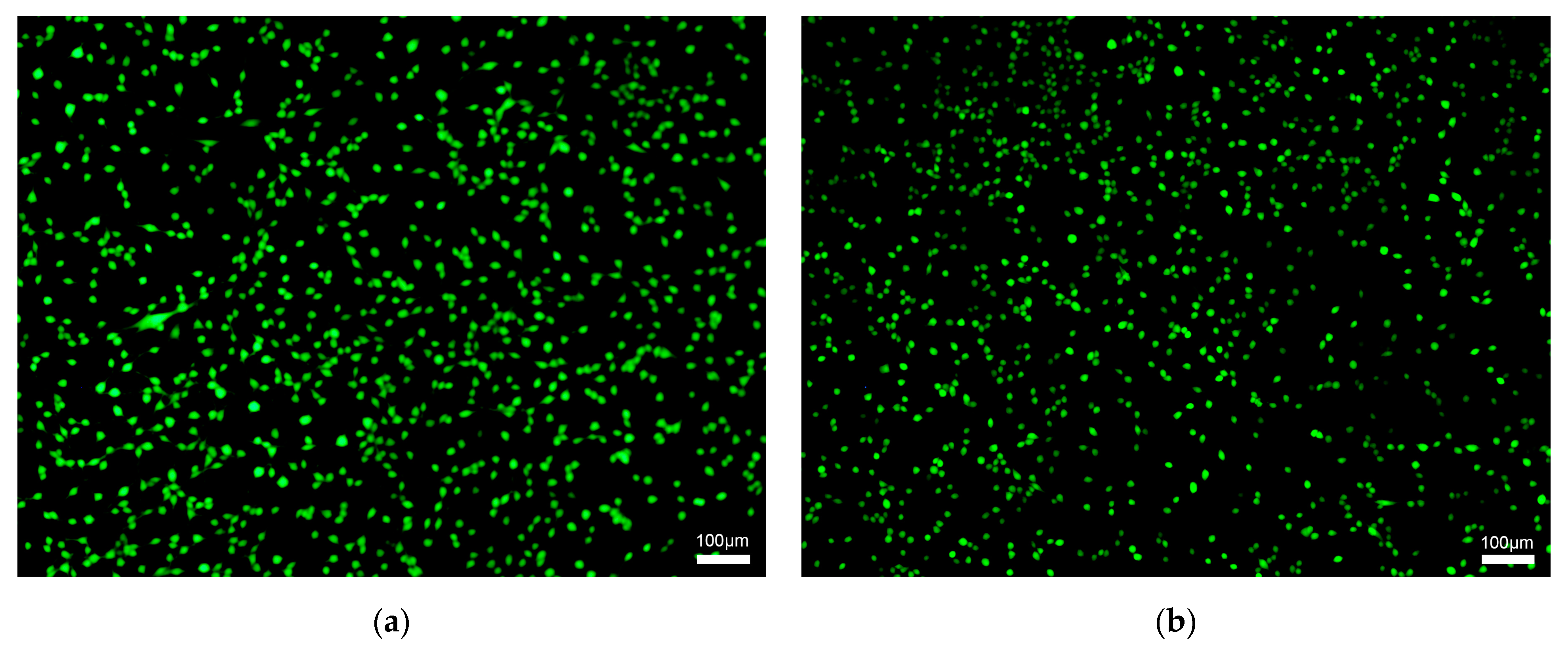
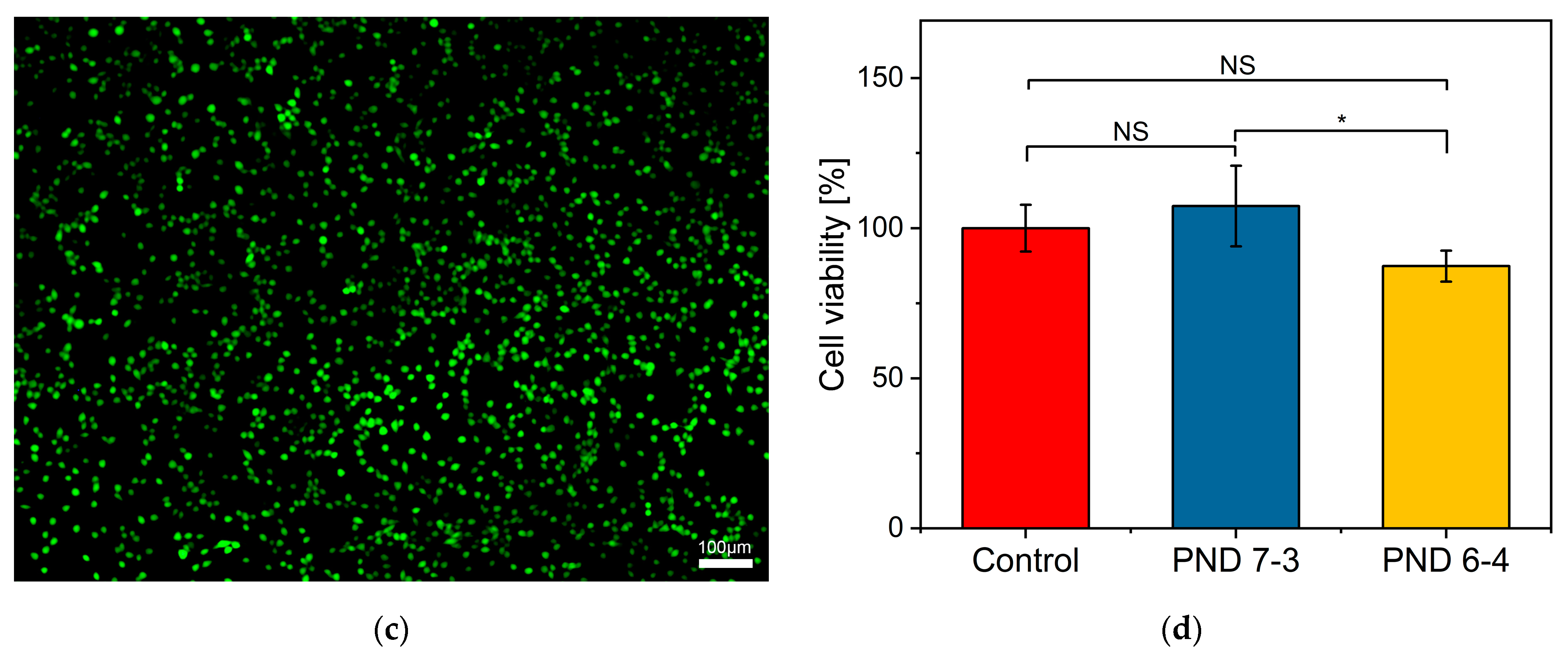
3.4. In Vitro Cytotoxicity
4. Conclusions
- The reaction kinetics of the free radical copolymerization of NIPAm and DHPMA were investigated. After two hours’ copolymerization, the conversion of DHPMA and NIPAm reached at least 90% and 60%, respectively. The reactivity ratios of DHPMA and NIPAm were 3.09 and 0.11, respectively. DHPMA with a lower isomer content had a higher preference for homopolymerization.
- The NIPAm-DHPMA-NIPAm and the DHPMA-NIPAm-DHPMA sequences were the most abundant in the copolymer, suggesting a preferred random distribution of the two structural units in the main chain of the copolymer.
- The cloud point of P(NIPAm-co-DHPMA) increased from 31 to 42 °C with an increase in the content of DHPMA from 0 to 58 mol%; these copolymers exhibited a wide phase transition temperature range.
- Finally, the good cytocompatibility of P(NIPAm-co-DHPMA) copolymers was confirmed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, A.; Babu, A.; Chakraborty, S.; Van Guyse, J.F.R.; Hoogenboom, R.; Maji, S. Poly(N-isopropylacrylamide) and its copolymers: A review on recent advances in the areas of sensing and biosensing. Adv. Funct. Mater. 2024, 34, 2402432. [Google Scholar] [CrossRef]
- Pelton, R. Poly(N-isopropylacrylamide) (PNIPAM) is never hydrophobic. J. Colloid Interface Sci. 2010, 348, 673–674. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, X.; Wu, C. Comparison of the coil-to-globule and the globule-to-coil transitions of a single poly(N-isopropylacrylamide) homopolymer chain in water. Macromolecules 1998, 31, 2972–2976. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, Z.; Zhu, X.X. Rational design of thermoresponsive polymers in aqueous solutions: A thermodynamics map. Prog. Polym. Sci. 2019, 90, 269–291. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Gregg, A.; De Volder, M.; Baumberg, J.J. Kinetics of light-responsive CNT/PNIPAM hydrogel microactuators. Small 2024, 20, 2305034. [Google Scholar] [CrossRef]
- Wang, K.; Chen, G.; Weng, S.; Hou, L.; Ye, D.; Jiang, X. Thermo-responsive poly(N-isopropylacrylamide)/hydroxypropylmethyl cellulose hydrogel with high luminous transmittance and solar modulation for smart windows. ACS Appl. Mater. Interfaces 2023, 15, 4385–4397. [Google Scholar] [CrossRef]
- Cui, X.; Liu, Z.; Zhang, B.; Tang, X.; Fan, F.; Fu, Y.; Zhang, J.; Wang, T.; Meng, F. Sponge-like, semi-interpenetrating self-sensory hydrogel for smart photothermal-responsive soft actuator with biomimetic self-diagnostic intelligence. Chem. Eng. J. 2023, 467, 143515. [Google Scholar] [CrossRef]
- Eklund, A.; Ikkala, O.; Zhang, H. Highly efficient switchable underwater adhesion in channeled hydrogel networks. Adv. Funct. Mater. 2024, 34, 2214091. [Google Scholar] [CrossRef]
- Li, F.; Wang, C.; Guo, W. Multifunctional poly-N-Isopropylacrylamide/DNAzyme microgels as highly efficient and recyclable catalysts for biosensing. Adv. Funct. Mater. 2018, 28, 1705876. [Google Scholar] [CrossRef]
- Kim, H.; Nam, S.; Durukan, M.B.; Unalan, H.E.; Lee, H.J. Self-charging dual-modal sensor for glucose monitoring based on piezoelectric nanowire/microgel hybrid film. Adv. Funct. Mater. 2024, 34, 2308086. [Google Scholar] [CrossRef]
- Li, B.-Y.; Lin, T.-Y.; Lai, Y.-J.; Chiu, T.-H.; Yeh, Y.-C. Engineering multiresponsive alginate/PNIPAM/carbon nanotube nanocomposite hydrogels as on-demand drug delivery platforms. Small 2025, 21, 2407420. [Google Scholar] [CrossRef]
- Lopez-Larrea, N.; Wustoni, S.; Peñas, M.I.; Uribe, J.; Dominguez-Alfaro, A.; Gallastegui, A.; Inal, S.; Mecerreyes, D. PNIPAM/PEDOT:PSS hydrogels for multifunctional organic electrochemical transistors. Adv. Funct. Mater. 2024, 34, 2403708. [Google Scholar] [CrossRef]
- Viola, M.; Valverde, M.G.; Bernal, P.N.; van Trijp, J.P.; Hak, J.; Marco, G.D.; Neumann, M.; Schuurmans, C.C.; van Nostrum, C.F.; Masereeuw, R.; et al. Thermal shrinking of biopolymeric hydrogels for high resolution 3D printing of kidney tubules. Adv. Funct. Mater. 2024, 34, 2406098. [Google Scholar] [CrossRef]
- Ding, H.; Li, B.; Liu, Z.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. Decoupled pH-and thermo-responsive injectable chitosan/PNIPAM hydrogel via thiol-ene click chemistry for potential applications in tissue engineering. Adv. Healthc. Mater. 2020, 9, 2000454. [Google Scholar] [CrossRef]
- Lerch, A.; Käfer, F.; Prévost, S.; Agarwal, S.; Karg, M. Structural insights into polymethacrylamide-based LCST polymers in solution: A small-angle neutron scattering study. Macromolecules 2021, 54, 7632–7641. [Google Scholar] [CrossRef]
- Kawano, S.; Lie, J.; Ohgi, R.; Shizuma, M.; Muraoka, M. Modulating polymeric amphiphiles using thermo- and pH-responsive copolymers with cyclodextrin pendant groups through molecular recognition of the lipophilic dye. Macromolecules 2021, 54, 5229–5240. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Zhang, S.; Zhang, G.; Tan, S.; Wu, Y.; Watanabe, M. Azobenzene molecular trigger controlling phase transitions of PNIPAm in ionic liquids and light-controlled adhesiveness. Macromolecules 2020, 53, 4901–4907. [Google Scholar] [CrossRef]
- Vishnevetskaya, N.S.; Hildebrand, V.; Niebuur, B.-J.; Grillo, I.; Filippov, S.K.; Laschewsky, A.; Müller-Buschbaum, P.; Papadakis, C.M. Aggregation behavior of doubly thermoresponsive polysulfobetaine-b-poly(N-isopropylacrylamide) diblock copolymers. Macromolecules 2016, 49, 6655–6668. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500. [Google Scholar] [CrossRef]
- Su, G.; Zhou, T.; Liu, X.; Ma, Y. Micro-dynamics mechanism of the phase transition behavior of poly(N-isopropylacrylamide-co-2-hydroxyethyl methacrylate) hydrogels revealed by two-dimensional correlation spectroscopy. Polym. Chem. 2017, 8, 865–878. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, L.; Zhao, S.; Li, X.; Zhao, B.; Zhou, Y.; Gong, S.; Chen, X.; Feng, X.; Pan, K. Study on polymerization kinetics of copolymerized flame-retardant polyamide 66: Apparent rate constant and reaction activation energy. Polymer 2024, 299, 126936. [Google Scholar] [CrossRef]
- Wu, Z.-C.; Liu, Y.; Wei, W.; Chen, F.-S.; Qiu, G.-X.; Xiong, H.-M. Reaction kinetics in anionic copolymerization: A revisit on Mayo-Lewis equation. Chin. J. Polym. Sci. 2016, 34, 431–438. [Google Scholar] [CrossRef]
- Ventura-Hunter, C.; Lechuga-Islas, V.D.; Ulbrich, J.; Kellner, C.; Schubert, U.S.; Saldívar-Guerra, E.; Rosales-Guzmán, M.; Guerrero-Sánchez, C. Glycerol methacrylate-based copolymers: Reactivity ratios, physicochemical characterization and cytotoxicity. Eur. Polym. J. 2022, 178, 111478. [Google Scholar] [CrossRef]
- Fineman, M.; Ross, S.D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Tidwell, P.W.; Mortimer, G.A. An improved method of calculating copolymerization reactivity ratios. J. Polym. Sci. Part A Gen. Pap. 1965, 3, 369–387. [Google Scholar] [CrossRef]
- Kucharski, M.; Ryttel, A. Copolymerization of alkyl acrylates with triallyl cyanurate: Kelen-Tüdös method applied for determining copolymerization reactivity ratios. Polymer 1984, 25, 555–558. [Google Scholar] [CrossRef]
- Save, M.; Weaver, J.V.M.; Armes, S.P.; McKenna, P. Atom transfer radical polymerization of hydroxy-functional methacrylates at ambient temperature: Comparison of glycerol monomethacrylate with 2-hydroxypropyl methacrylate. Macromolecules 2002, 35, 1152–1159. [Google Scholar] [CrossRef]
- Moreddu, R.; Vigolo, D.; Yetisen, A.K. Contact lens technology: From fundamentals to applications. Adv. Healthc. Mater. 2019, 8, 1900368. [Google Scholar] [CrossRef]
- Fan, X.; Torres-Luna, C.; Azadi, M.; Domszy, R.; Hu, N.; Yang, A.; David, A.E. Evaluation of commercial soft contact lenses for ocular drug delivery: A review. Acta Biomater. 2020, 115, 60–74. [Google Scholar] [CrossRef]
- Wang, C.; Yu, B.; Knudsen, B.; Harmon, J.; Moussy, F.; Moussy, Y. Synthesis and performance of novel hydrogels coatings for implantable glucose sensors. Biomacromolecules 2008, 9, 561–567. [Google Scholar] [CrossRef]
- Patrucco, E.; Ouasti, S.; Vo, C.D.; De Leonardis, P.; Pollicino, A.; Armes, S.P.; Scandola, M.; Tirelli, N. Surface-initiated ATRP modification of tissue culture substrates: Poly(glycerol monomethacrylate) as an antifouling surface. Biomacromolecules 2009, 10, 3130–3140. [Google Scholar] [CrossRef]
- Nalawade, A.C.; Ghorpade, R.V.; Shadbar, S.; Qureshi, M.S.; Chavan, N.N.; Khan, A.A.; Ponrathnam, S. Inverse high internal phase emulsion polymerization (i-HIPE) of GMMA, HEMA and GDMA for the preparation of superporous hydrogels as a tissue engineering scaffold. J. Mater. Chem. B 2016, 4, 450–460. [Google Scholar] [CrossRef]
- Robert-Nicoud, G.; Evans, R.; Vo, C.-D.; Cadman, C.J.; Tirelli, N. Synthesis, self-assembly and (absence of) protein interactions of poly(glycerol methacrylate)–silicone macro-amphiphiles. Polym. Chem. 2013, 4, 3458–3470. [Google Scholar] [CrossRef]
- Rundlöf, T.; Mathiasson, M.; Bekiroglu, S.; Hakkarainen, B.; Bowden, T.; Arvidsson, T. Survey and qualification of internal standards for quantification by 1H NMR spectroscopy. J. Pharm. Biomed. 2010, 52, 645–651. [Google Scholar] [CrossRef]
- Shaw, S.E.; Russo, T.; Solomon, D.H.; Qiao, G.G. An alternative pathway for the hydrolysis of epoxy ester compounds. Polymer 2006, 47, 8247–8252. [Google Scholar] [CrossRef]
- García, N.; Guzmán, J.; Riande, E. Migration phenomena in methacrylic esters and polymers derived from glycerol. Macromol. Chem. Phys. 2002, 203, 2225–2231. [Google Scholar] [CrossRef]
- Huang, Z.-S.; Shiu, J.-W.; Way, T.-F.; Rwei, S.-P. A thermo-responsive random copolymer of poly(NIPAm-co-FMA) for smart textile applications. Polymer 2019, 184, 121917. [Google Scholar] [CrossRef]
- Wolf, F.K.; Hofmann, A.N.; Frey, H. Poly(isoglycerol methacrylate)-b-poly(D or L-lactide) copolymers: A novel hydrophilic methacrylate as building block for supramolecular aggregates. Macromolecules 2010, 43, 3314–3324. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. TrAC-Trend Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Madsen, J.; Madden, G.; Themistou, E.; Warren, N.J.; Armes, S.P. pH-responsive diblock copolymers with two different fluorescent labels for simultaneous monitoring of micellar self-assembly and degree of protonation. Polym. Chem. 2018, 9, 2964–2976. [Google Scholar] [CrossRef]
- Kubo, M.; Higuchi, M.; Koshimura, T.; Shoji, E.; Tsukada, T. Control of the temperature responsiveness of poly(N-isopropylacrylamide-co-2-hydroxyethyl methacrylate) copolymer using ultrasonic irradiation. Ultrason. Sonochem. 2021, 79, 105752. [Google Scholar] [CrossRef]
- Chu, Y.; Wang, Q.; Lyu, S.; Yuan, B.; Huang, J.; Li, J.; Wang, Y. Thermal-responsive gel-based overheat limiter enabled intelligent photothermal therapy. Small 2024, 20, 2312140. [Google Scholar] [CrossRef]
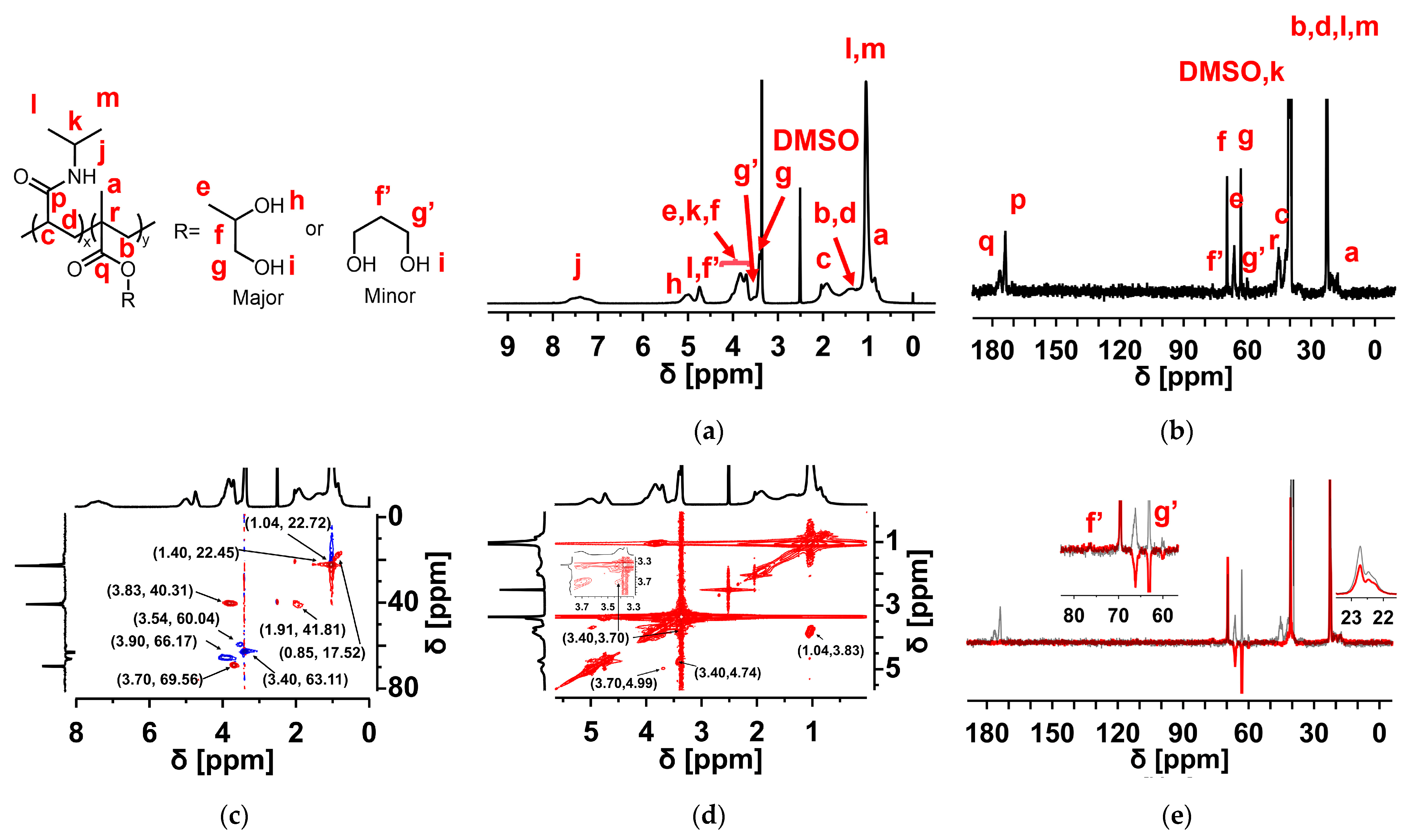
| 1H NMR | 13C NMR | ||
|---|---|---|---|
| Chemical Shift [ppm] | Attribution | Chemical Shift [ppm] | Attribution |
| 7.38 | 1H, –NH– [39] | 176.44 | C=ODHPMA |
| 4.99 | 1H, –CH2–CH–OH | 173.88 | C=ONIPAm |
| 4.74 | 1H, –CH–CH2–OH | 69.56 | –CH2–CH–OH |
| 3.90 | 2H, –O–CH2– | 66.17 | –O–CH2– |
| 3.83 | 1H, –CH–(CH3)2 | 63.11 | –CH–CH2–OH |
| 3.70 | 1H, –CH2–CH–OH | 60.04 | –CH(CH2OH)2 |
| 3.54 | –CH(CH2OH)2 | 45.33 | –C(CH3)–CH2– |
| 3.40 | 2H, –CH–CH2–OH | 41.81 | –CH–CH2– |
| 1.91 | 1H, –CH–CH2– | 40.31 | –CH–(CH3)2 |
| 1.40 | 2H, –CH–CH2– | 22.72 | –CH–(CH3)2 |
| 1.04 | 3H, –CH–(CH3)2 | 22.45 | –CH–CH2– |
| 0.85 | 3H, –C–CH3 | 17.52 | –C–CH3 |
| Polymer Name | Feeding Ratio ([M1]/[M2]) | DHPMA Content [mol%] | Phase Transition Range [°C] | Tcp [°C] |
|---|---|---|---|---|
| PNIPAm | 0 | 0 | 30.0–32.5 | 31.1 |
| PND 8-2 | 0.25 | 34.21 | 30.0–40.0 | 33.0 |
| PND 7-3 | 0.43 | 45.65 | 32.0–43.5 | 36.5 |
| PND 6-4 | 0.67 | 57.98 | 38.5–50.0 | 42.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhang, C. Free Radical Copolymerization of N-Isopropylacrylamide and 2,3-Dihydroxypropyl Methacrylate: Reaction Kinetics and Characterizations. Materials 2025, 18, 1614. https://doi.org/10.3390/ma18071614
Chen Z, Zhang C. Free Radical Copolymerization of N-Isopropylacrylamide and 2,3-Dihydroxypropyl Methacrylate: Reaction Kinetics and Characterizations. Materials. 2025; 18(7):1614. https://doi.org/10.3390/ma18071614
Chicago/Turabian StyleChen, Zhishu, and Chao Zhang. 2025. "Free Radical Copolymerization of N-Isopropylacrylamide and 2,3-Dihydroxypropyl Methacrylate: Reaction Kinetics and Characterizations" Materials 18, no. 7: 1614. https://doi.org/10.3390/ma18071614
APA StyleChen, Z., & Zhang, C. (2025). Free Radical Copolymerization of N-Isopropylacrylamide and 2,3-Dihydroxypropyl Methacrylate: Reaction Kinetics and Characterizations. Materials, 18(7), 1614. https://doi.org/10.3390/ma18071614






