Fabrication of Superhydrophobic Surfaces from Laser-Induced Graphene and Their Photothermally Driven Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
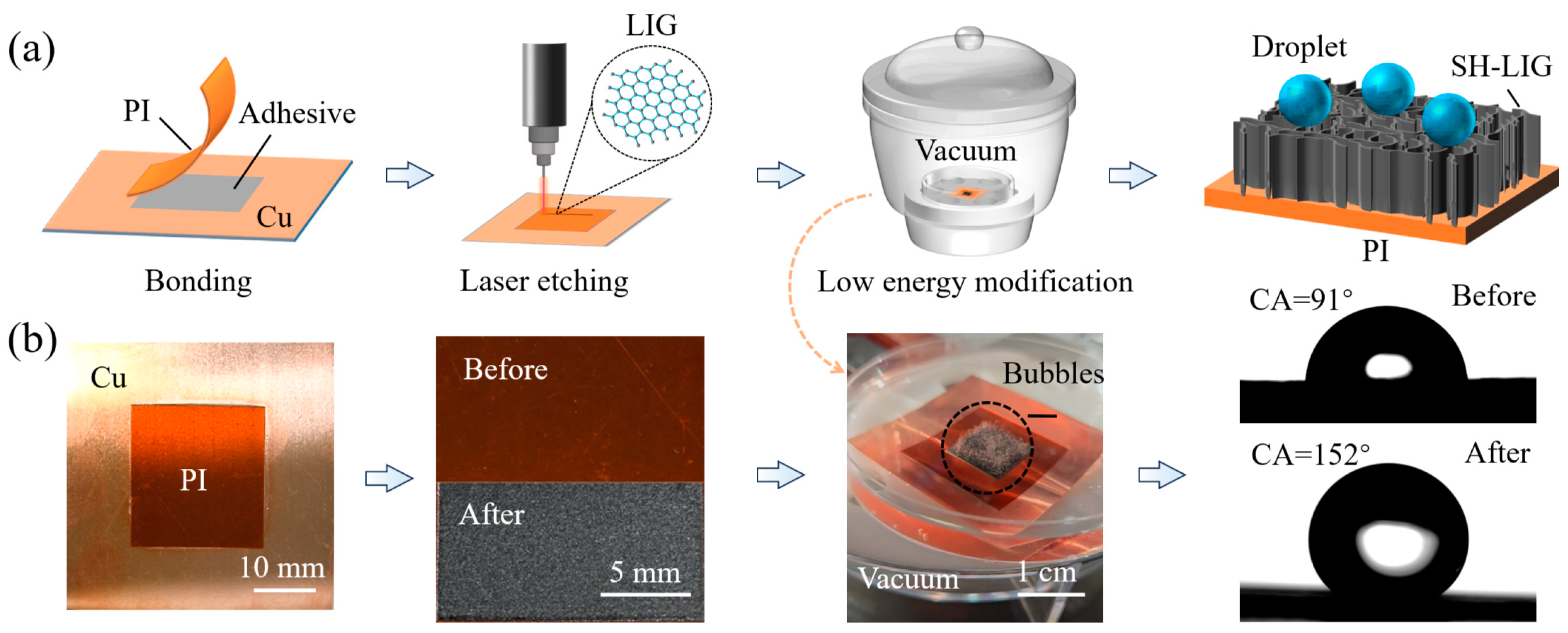
2.2. Fabrication of SH-LIG
2.3. Characterization
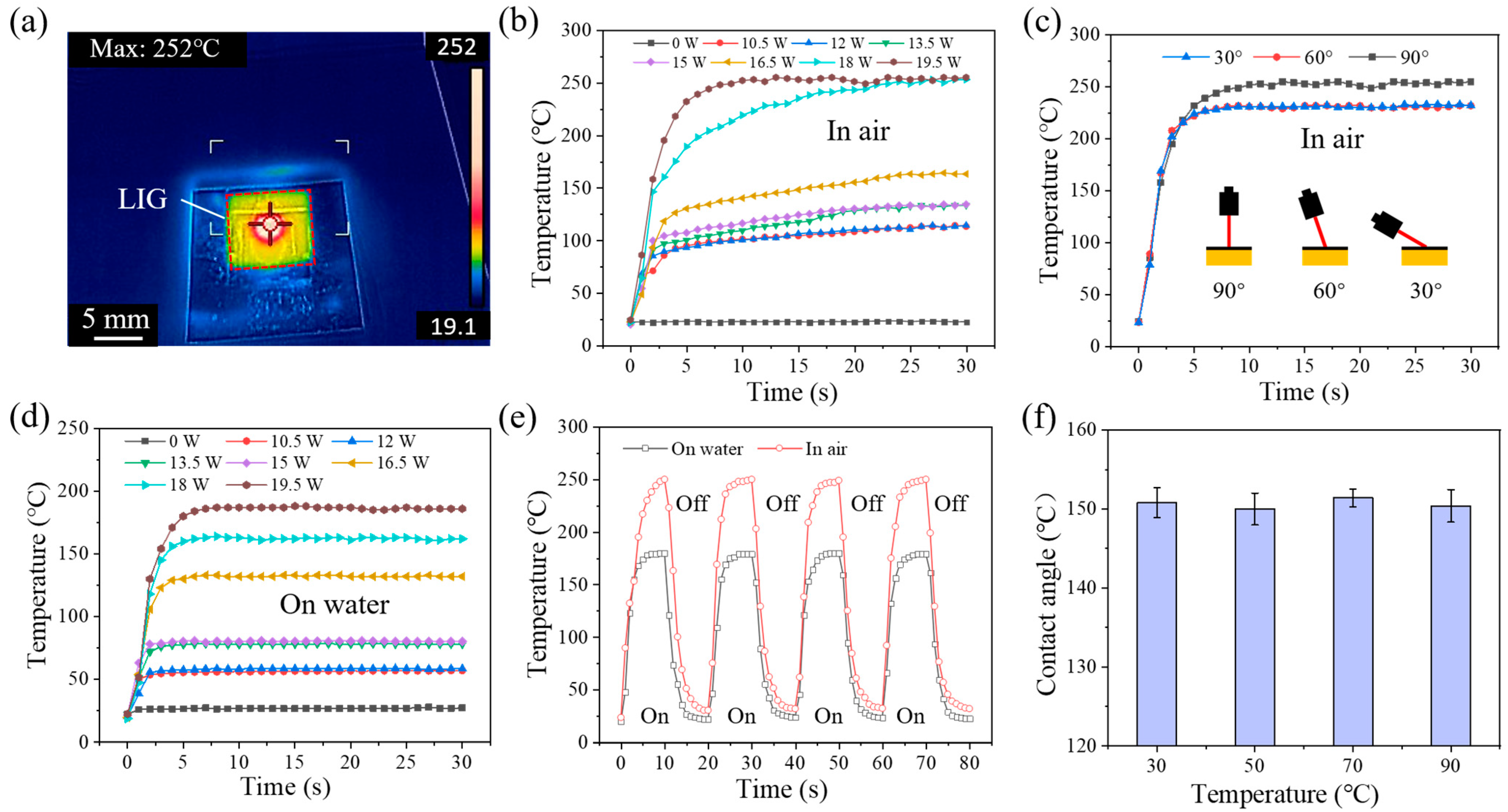
2.4. Photothermal Drive Experiment
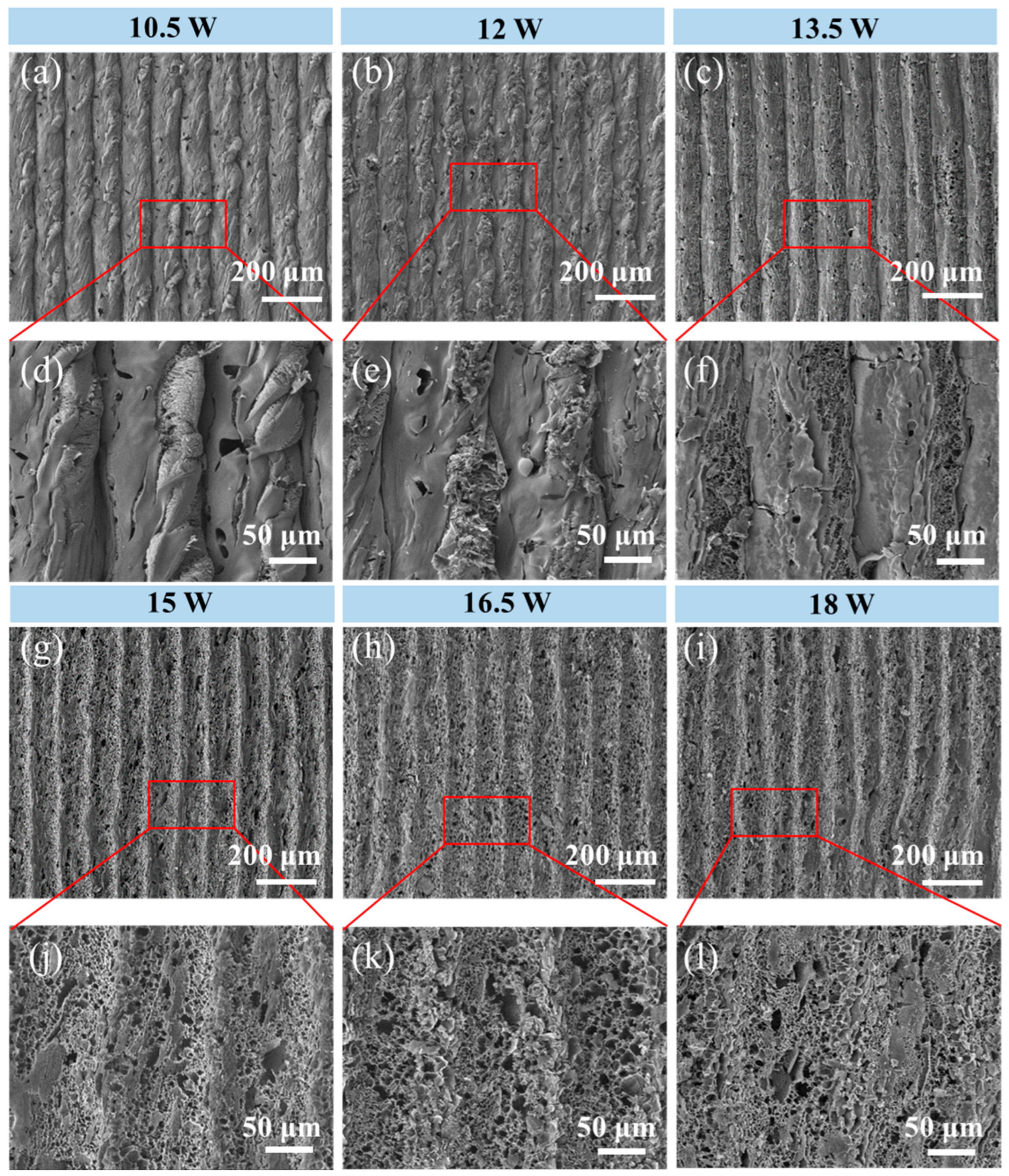
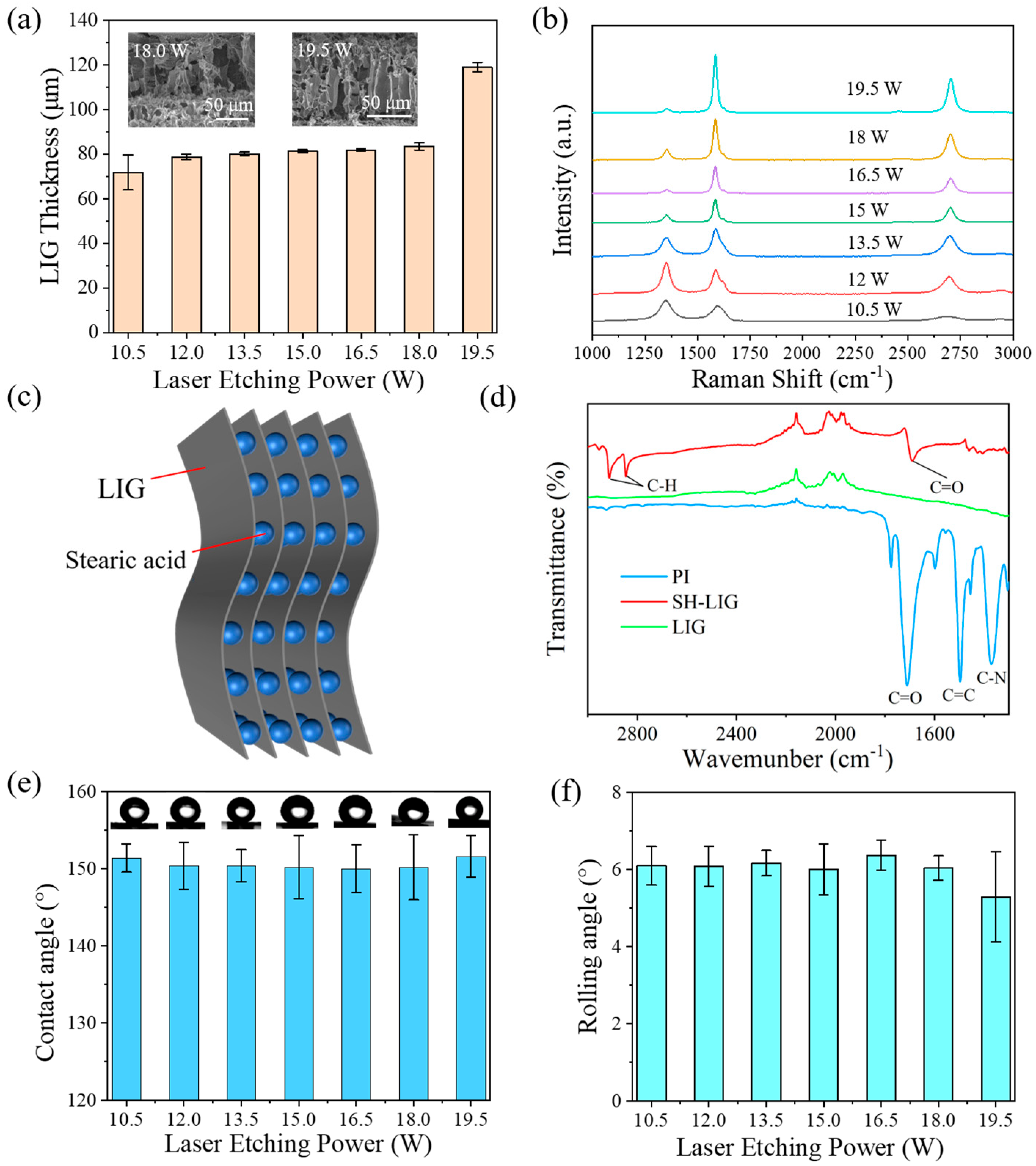
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Zhao, Z.; Liu, P.; Guo, X. A Soft and Stretchable Electronics Using Laser-Induced Graphene On Polyimide/PDMS Composite Substrate. NPJ Flex. Electron. 2022, 6, 26. [Google Scholar] [CrossRef]
- Zhao, P.; Bhattacharya, G.; Fishlock, S.J.; Guy, J.G.M.; Kumar, A.; Tsonos, C.; Yu, Z.; Raj, S.; McLaughlin, J.A.; Luo, J.; et al. Replacing the Metal Electrodes in Triboelectric Nanogenerators: High-Performance Laser-Induced Graphene Electrodes. Nano Energy 2020, 75, 104958. [Google Scholar] [CrossRef]
- Thaweeskulchai, T.; Sakdaphetsiri, K.; Schulte, A. Ten Years of Laser-Induced Graphene: Impact and Future Prospect On Biomedical, Healthcare, and Wearable Technology. Mikrochim. Acta 2024, 191, 292. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Long, Y.; Huang, Z.; Zi, Y.; Tao, L.; Li, C.; Sun, H.; Li, J. Laser-Induced Graphene (LIG)-Based Pressure Sensor and Triboelectric Nanogenerator Towards High-Performance Self-Powered Measurement-Control Combined System. Nano Energy 2022, 96, 107099. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Xia, Y.; Qiu, R.; Liu, W.; Wu, M.; Zhu, Y.; Zhu, S.; Jia, C.; Zhu, M.; et al. Flexible High-Resolution Triboelectric Sensor Array Based On Patterned Laser-Induced Graphene for Self-Powered Real-Time Tactile Sensing. Adv. Funct. Mater. 2021, 31, 2100709. [Google Scholar] [CrossRef]
- Yu, H.; Bian, J.; Chen, F.; Ji, J.; Huang, Y. Ultrathin, Graphene-in-Polyimide Strain Sensor Via Laser-Induced Interfacial Ablation of Polyimide. Adv. Electron. Mater. 2023, 9, 2201086. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, B.; Long, J.; Kuang, Y.; Chen, X.; Hou, M.; Gao, J.; Zhou, S.; Fan, B.; He, Y.; et al. Interfacial Laser-Induced Graphene Enabling High-Performance Liquid−Solid Triboelectric Nanogenerator. Adv. Mater. 2021, 33, 2104290. [Google Scholar] [CrossRef]
- Stanford, M.G.; Li, J.T.; Chyan, Y.; Wang, Z.; Wang, W.; Tour, J.M. Laser-Induced Graphene Triboelectric Nanogenerators. ACS Nano 2019, 13, 7166–7174. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Liu, Y.; Han, B.; Wang, H.; Han, D.; Wang, J.; Zhang, Y.; Sun, H. Direct Laser Writing of Superhydrophobic PDMS Elastomers for Controllable Manipulation Via Marangoni Effect. Adv. Funct. Mater. 2017, 27, 1702946. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, H.; Zhang, J.; Li, Y.; Mao, S.; Wang, Y.; Li, X.; Wang, Z.; Yuan, Z.; Liu, X. Magnetic Droplet-based Transporters Co-regulated by Wettability and Magnetic Field. Adv. Funct. Mater. 2025, 2504213. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-Induced Porous Graphene Films From Commercial Polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, H.; Zhang, W.; Zhang, Z.; Lu, J.; Xu, K.; Liu, Y.; Saetang, V. A Review of Laser-Induced Graphene: From Experimental and Theoretical Fabrication Processes to Emerging Applications. Carbon 2023, 214, 118356. [Google Scholar] [CrossRef]
- Du, Q.; Ai, J.; Qin, Z.; Liu, J.; Zeng, X. Fabrication of Superhydrophobic/Superhydrophilic Patterns On Polyimide Surface by Ultraviolet Laser Direct Texturing. J. Mater. Process. Technol. 2018, 251, 188–196. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Liu, W.; Hu, B.; Liu, J.; Zhang, Y. Patterned Laser-Induced Graphene for Terahertz Wave Modulation. J. Opt. Soc. Am. B 2020, 37, 546–551. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Fernandes, A.J.S.; Leitão, C.; Deuermeier, J.; Marques, A.C.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-Induced Graphene Strain Sensors Produced by Ultraviolet Irradiation of Polyimide. Adv. Funct. Mater. 2018, 28, 1805271. [Google Scholar] [CrossRef]
- Yang, R.; Zhu, Y.; Qin, D.; Xiong, Z. Light-Operated Dual-Mode Propulsion at the Liquid/Air Interface Using Flexible, Superhydrophobic, and Thermally Stable Photothermal Paper. ACS Appl. Mater. Interfaces 2020, 12, 1339–1347. [Google Scholar] [CrossRef]
- Tiliakos, A.; Ceaus, C.; Iordache, S.M.; Vasile, E.; Stamatin, I. Morphic Transitions of Nanocarbons Via Laser Pyrolysis of Polyimide Films. J. Anal. Appl. Pyrolysis 2016, 121, 275–286. [Google Scholar] [CrossRef]
- Duy, L.X.; Peng, Z.; Li, Y.; Zhang, J.; Ji, Y.; Tour, J.M. Laser-Induced Graphene Fibers. Carbon 2018, 126, 472–479. [Google Scholar] [CrossRef]
- Jiao, L.; Chua, Z.Y.; Moon, S.K.; Song, J.; Bi, G.; Zheng, H.; Lee, B.; Koo, J. Laser-Induced Graphene On Additive Manufacturing Parts. Nanomaterials 2019, 9, 90. [Google Scholar] [CrossRef]
- Wang, X.; Lin, D.; Zhou, Y.; Jiao, N.; Tung, S.; Liu, L. Multistimuli-Responsive Hydroplaning Superhydrophobic Microrobots with Programmable Motion and Multifunctional Applications. ACS Nano 2022, 16, 14895–14906. [Google Scholar] [CrossRef]
- Yan, D.; Lin, J.; Chen, Y.; Yang, X.; Lu, Y.; Song, J. High-Efficiency Water Collection of Superhydrophobic Condensation Absorber. Adv. Sci. 2025, 12, 2417024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Zhong, L.; Wang, J.; Jiang, Q.; Guo, X. Super-Hydrophobic Surface On Pure Magnesium Substrate by Wet Chemical Method. Appl. Surf. Sci. 2010, 256, 3837–3840. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, M.; Hao, J.; Wu, K.; Li, C.; Hu, C. Visible Light Laser-Induced Graphene From Phenolic Resin: A New Approach for Directly Writing Graphene-Based Electrochemical Devices On Various Substrates. Carbon 2018, 127, 287–296. [Google Scholar] [CrossRef]
- Nasser, J.; Lin, J.; Zhang, L.; Sodano, H.A. Laser Induced Graphene Printing of Spatially Controlled Super-Hydrophobic/Hydrophilic Surfaces. Carbon 2020, 162, 570–578. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Y.; Ji, F.; Zhu, J.; Ma, P.; Su, H.; Chen, P.; Feng, X.; Du, W.; Liu, B. Patterning Candle Soot for Light-Driven Actuator Via Marangoni Effect. Sens. Actuators B Chem. 2021, 347, 130613. [Google Scholar] [CrossRef]
- Wu, H.; Luo, J.; Huang, X.; Wang, L.; Guo, Z.; Liang, J.; Zhang, S.; Xue, H.; Gao, J. Superhydrophobic, Mechanically Durable Coatings for Controllable Light and Magnetism Driven Actuators. J. Colloid. Interface. Sci. 2021, 603, 282–290. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Muhammad Hafiz, S.; Ritikos, R.; Whitcher, T.J.; Md. Razib, N.; Bien, D.C.S.; Chanlek, N.; Nakajima, H.; Saisopa, T.; Songsiriritthigul, P.; Huang, N.M.; et al. A Practical Carbon Dioxide Gas Sensor Using Room-Temperature Hydrogen Plasma Reduced Graphene Oxide. Sens. Actuators B Chem. 2014, 193, 692–700. [Google Scholar] [CrossRef]
- Dwivedi, N.; Yeo, R.J.; Satyanarayana, N.; Kundu, S.; Tripathy, S.; Bhatia, C.S. Understanding the Role of Nitrogen in Plasma-Assisted Surface Modification of Magnetic Recording Media with and without Ultrathin Carbon Overcoats. Sci. Rep. 2015, 5, 7772. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zheng, H.; Zhao, Y.; Chen, Y.; Zhou, Y.; Liu, X. A Flexible Hybrid Generator for Efficient Dual Energy Conversion From Raindrops to Electricity. Adv. Sci. 2024, 11, 2404310. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, Y.; Su, X.; Wang, C.; Zhang, M.; Jian, M.; Xia, K.; Liang, X.; Lu, H.; et al. Laser Writing of Janus Graphene/Kevlar Textile for Intelligent Protective Clothing. ACS Nano 2020, 14, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Li, G. Direct Laser Writing of Graphene Electrodes. J. Appl. Phys. 2020, 127, 010901. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, D.; Liu, R.; Lu, Y.; Zhao, D.; Deng, X.; Song, J. Green Self-Propelling Swimmer Driven by Rain Droplets. Nano Energy 2022, 101, 107543. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, Y.; Chen, Y.; Fu, H.; Liu, H.; Song, J.; Liu, X. Fabrication of Superhydrophobic Surfaces from Laser-Induced Graphene and Their Photothermally Driven Properties. Materials 2025, 18, 1880. https://doi.org/10.3390/ma18081880
Zhao Y, Zhang Y, Chen Y, Fu H, Liu H, Song J, Liu X. Fabrication of Superhydrophobic Surfaces from Laser-Induced Graphene and Their Photothermally Driven Properties. Materials. 2025; 18(8):1880. https://doi.org/10.3390/ma18081880
Chicago/Turabian StyleZhao, Yue, Yonghui Zhang, Yang Chen, Haodong Fu, Hao Liu, Jinlong Song, and Xin Liu. 2025. "Fabrication of Superhydrophobic Surfaces from Laser-Induced Graphene and Their Photothermally Driven Properties" Materials 18, no. 8: 1880. https://doi.org/10.3390/ma18081880
APA StyleZhao, Y., Zhang, Y., Chen, Y., Fu, H., Liu, H., Song, J., & Liu, X. (2025). Fabrication of Superhydrophobic Surfaces from Laser-Induced Graphene and Their Photothermally Driven Properties. Materials, 18(8), 1880. https://doi.org/10.3390/ma18081880






