Antibacterial Activity of GO-Based Composites Enhanced by Phosphonate-Functionalized Ionic Liquids and Silver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization
2.3. Preparation of GO
2.4. Preparation of GO@PEI
2.5. Preparation of GO@PEI-PFILOEt
2.6. Preparation of GO@PEI-PFIL-Ag+/Ag/AgBr and GO@PEI-PFIL-Mn+
2.7. Minimum Inhibitory Concentration (MIC) Assay
2.8. Agar Diffusion Test
2.9. Short-Term Antibacterial Activity Test
2.10. Long-Term Antibacterial Activity Test
2.11. Time-Killing Curve Assay
2.12. Activity of GO@PEI-PFIL-Ag+/Ag/AgBr After Repeated Use
2.13. Hemolytic Test
2.14. Cytotoxicity Assay
2.15. Inhibition and Destruction of Biofilm Assays
2.16. SEM Characterization of Bacterial Cells
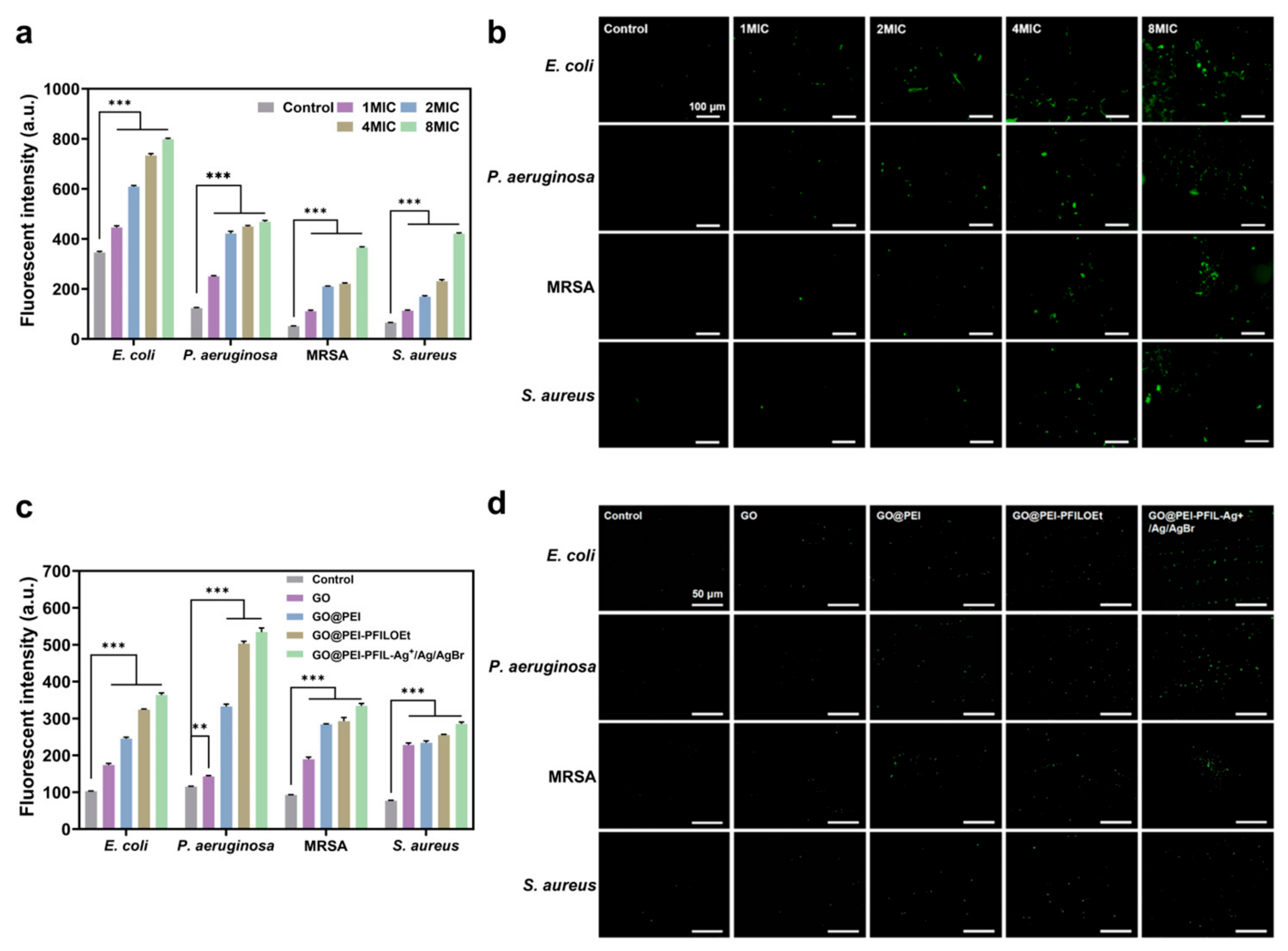
2.17. Live and Dead Bacteria Staining
2.18. Nucleotide Leakage Assay
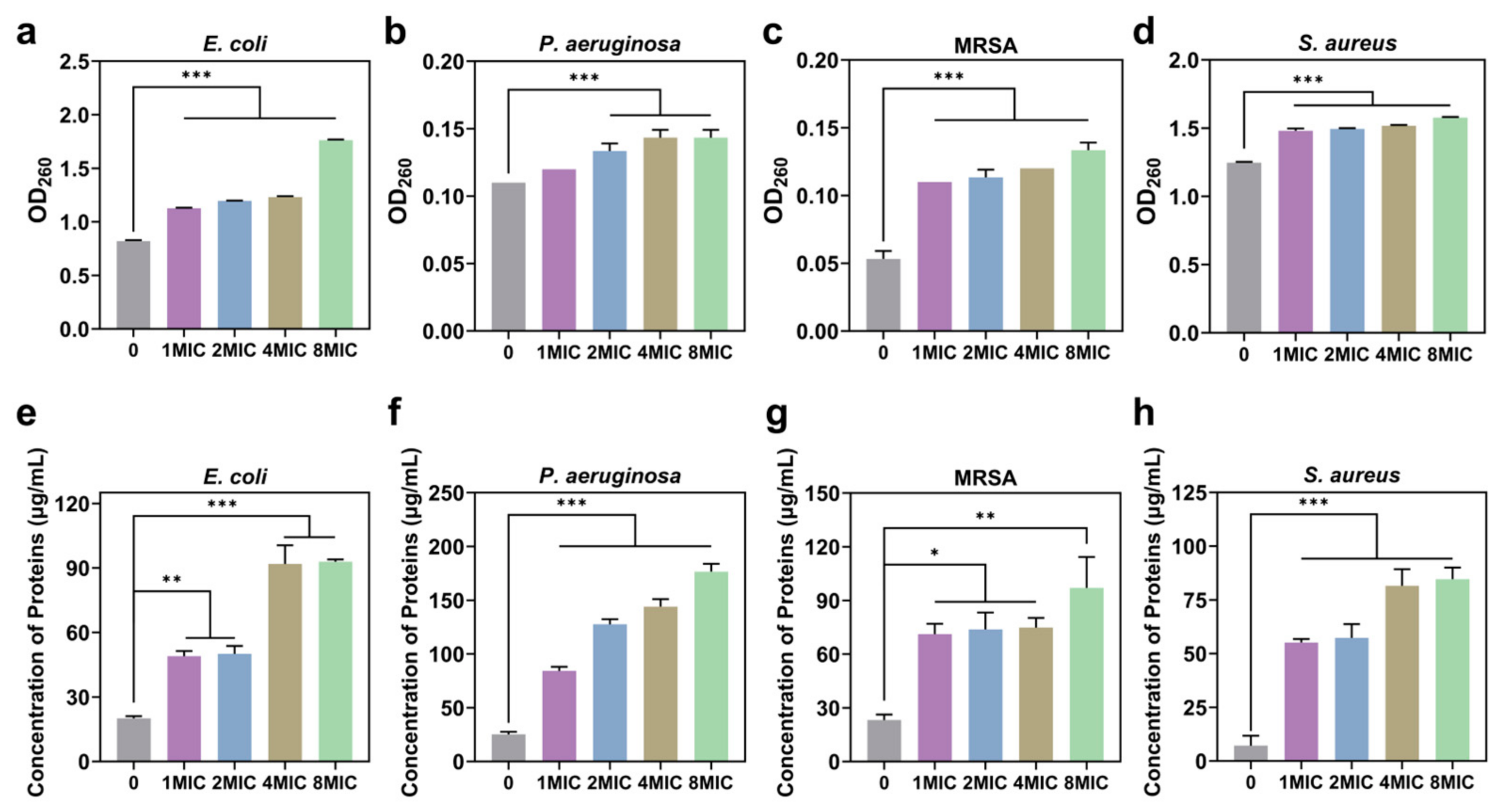
2.19. Protein Leakage Assay
2.20. Determination of Cellular Total Reactive Oxygen Species (ROS)
2.21. Statistical Analysis
3. Results and Discussion
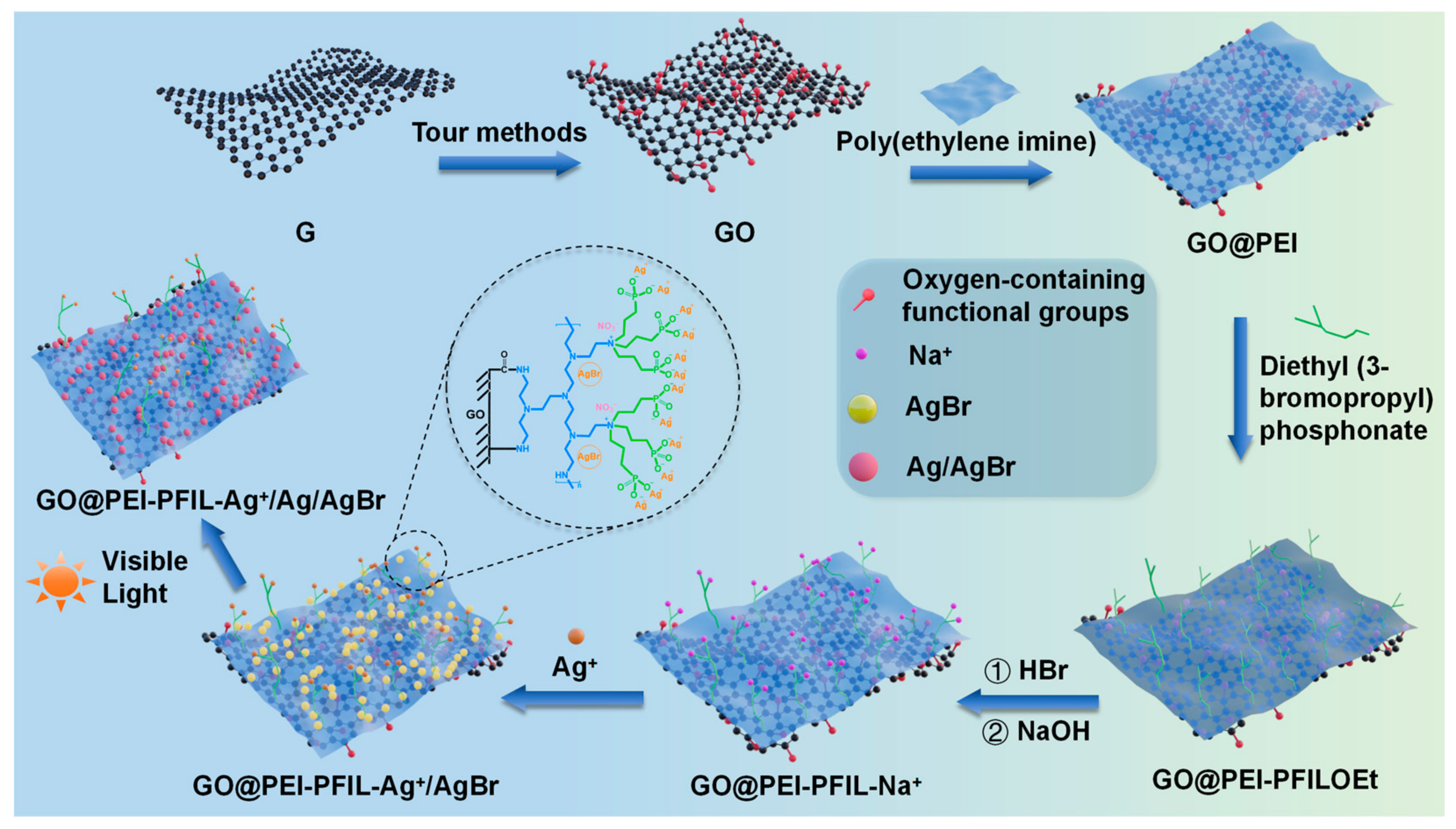
3.1. Synthesis of the GO@PEI-PFIL-Ag+/Ag/AgBr and GO@PEI-PFIL-Mn+ Composites
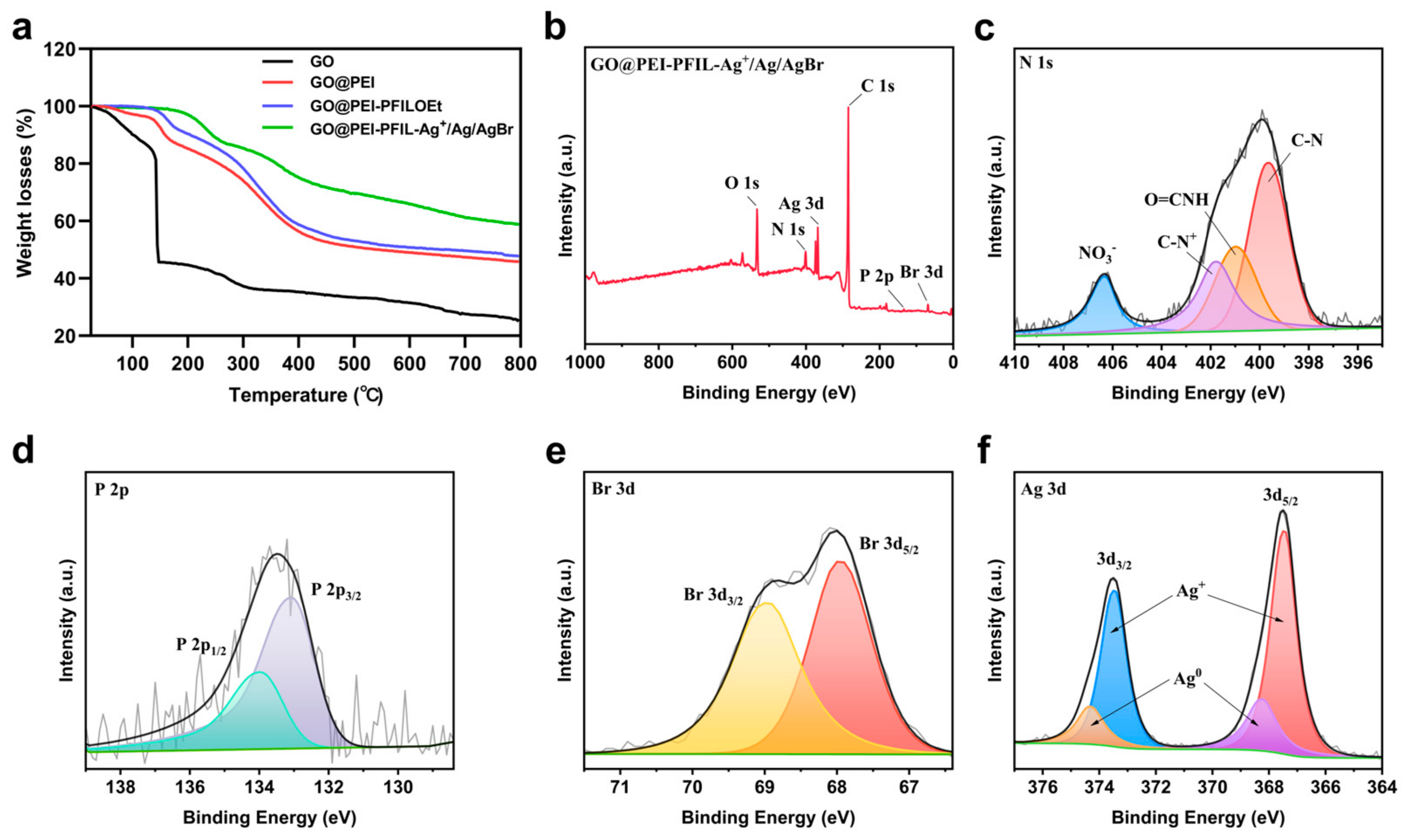
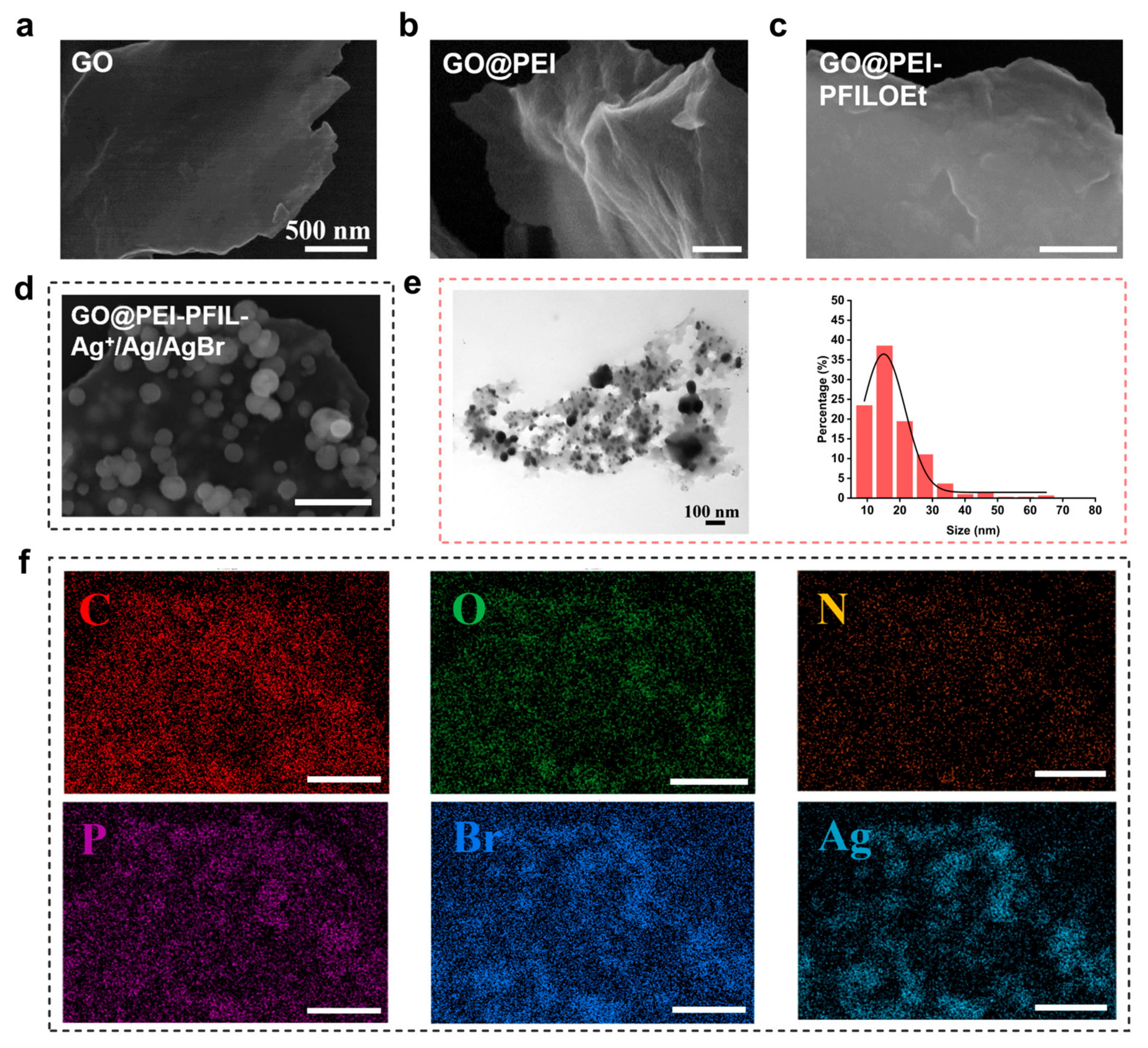
3.2. Characterization of the GO@PEI-PFIL-Ag+/Ag/AgBr Nanocomposite
3.3. Antimicrobial Activity of Nanocomposites
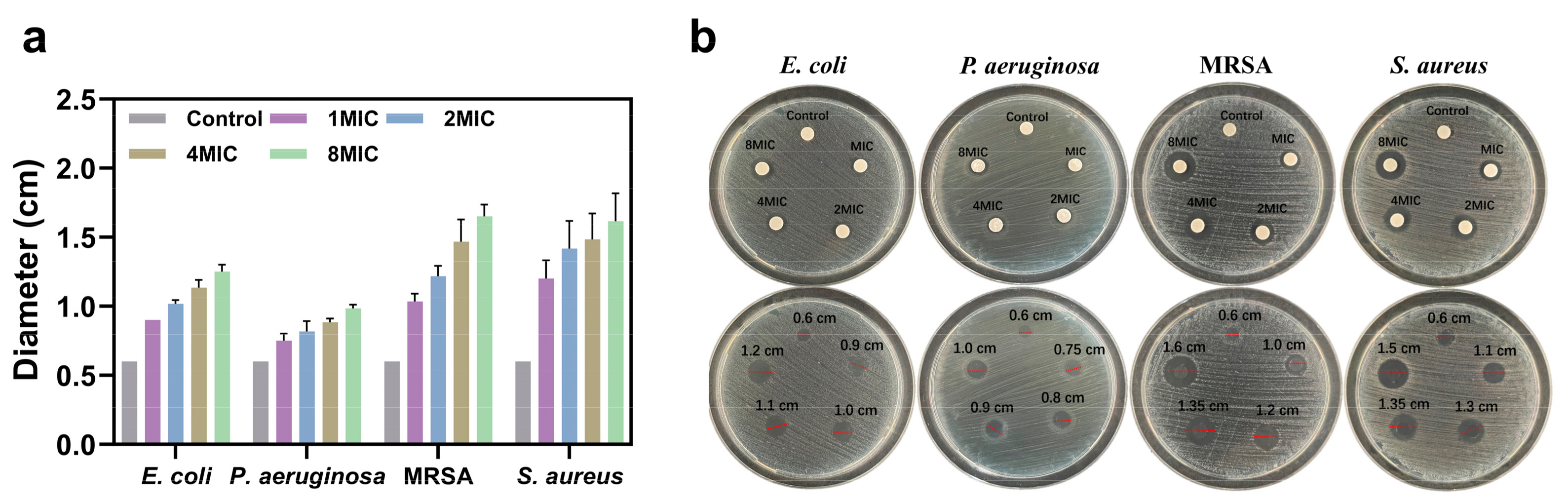
3.3.1. Antimicrobial Evaluation by MIC and Inhibition Zone Assays
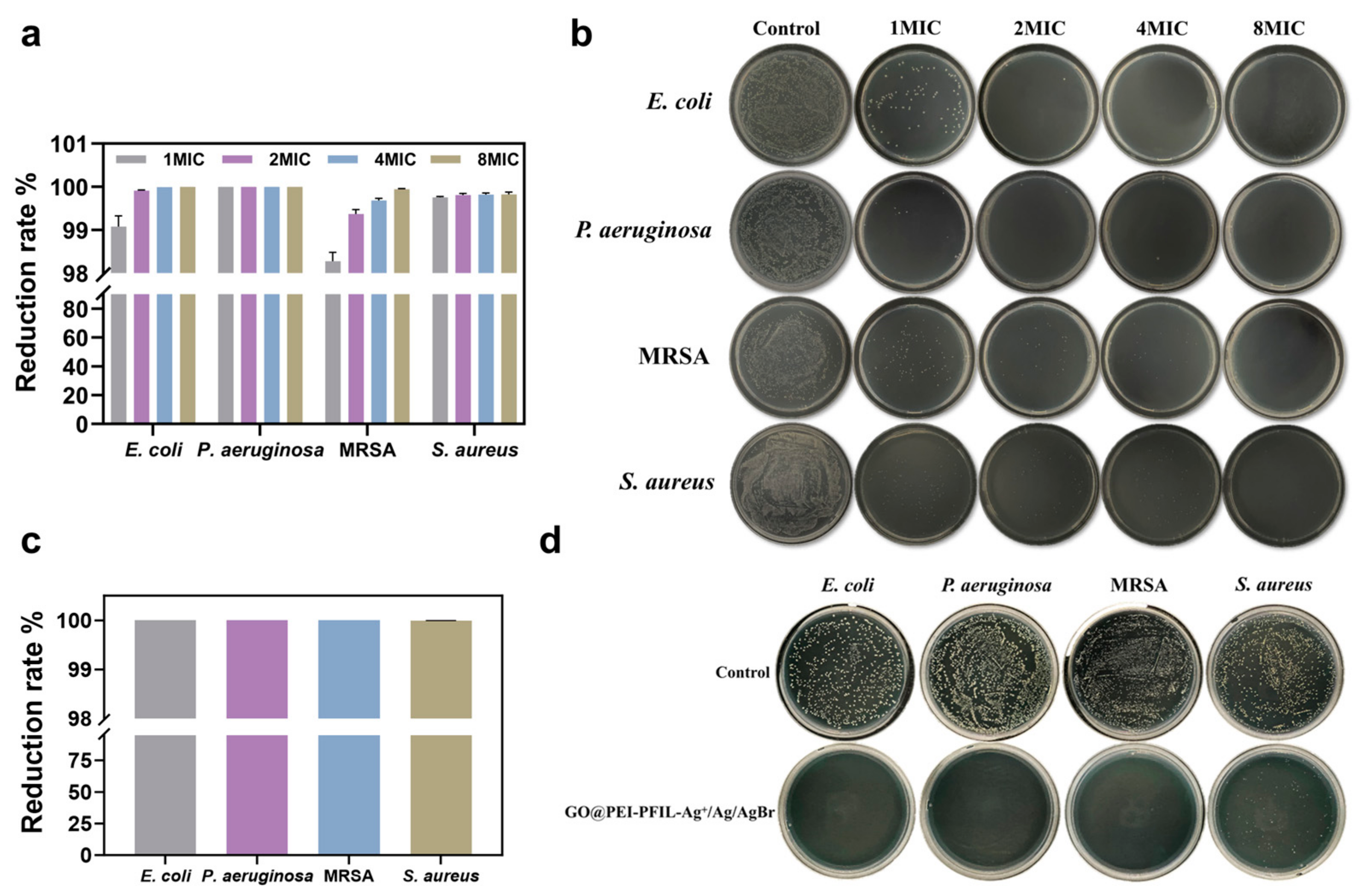
3.3.2. Short-Term and Long-Term Antimicrobial Activity
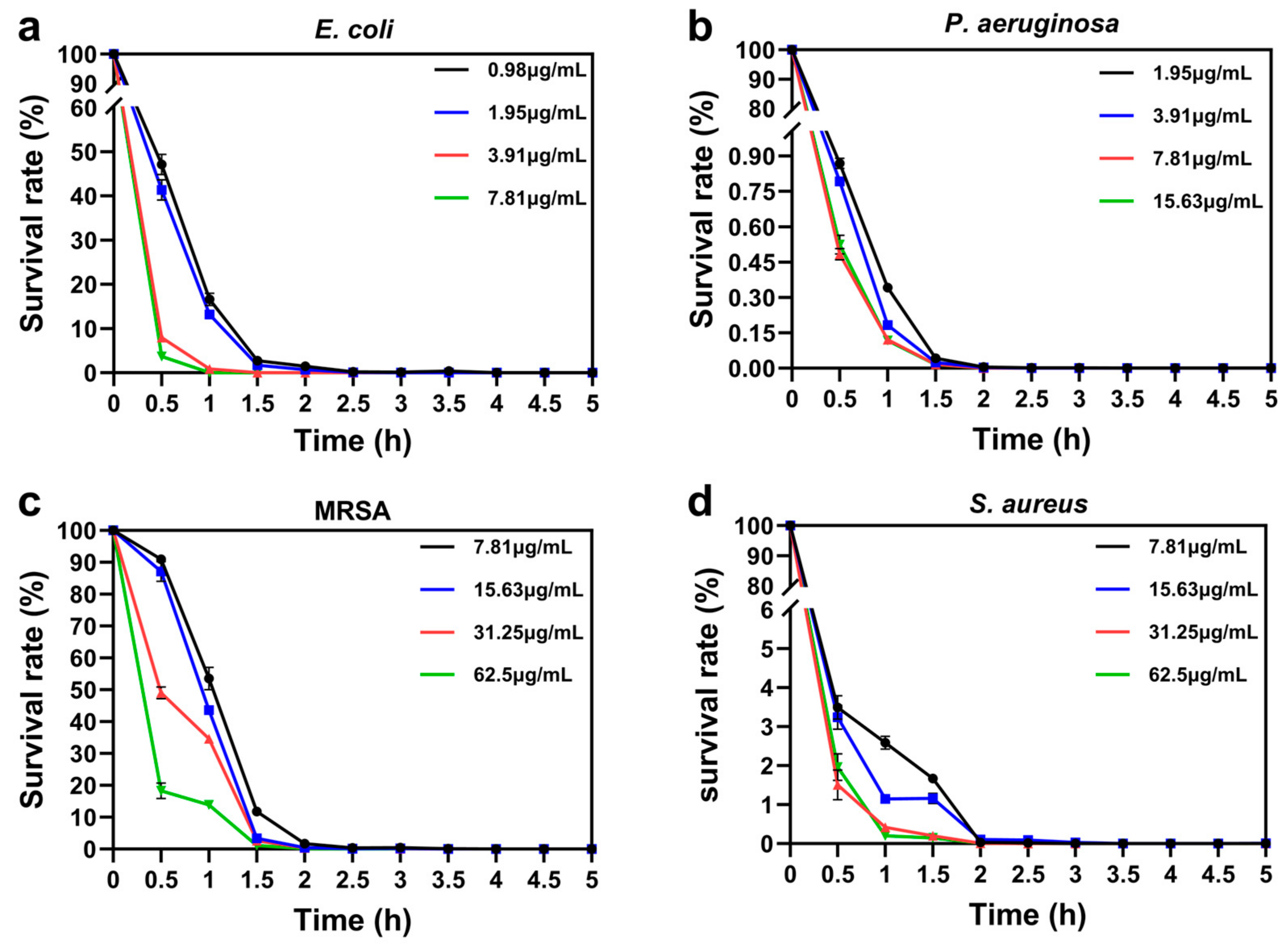
3.3.3. Bactericidal Kinetics and Reusability of GO@PEI-PFIL-Ag+/Ag/AgBr
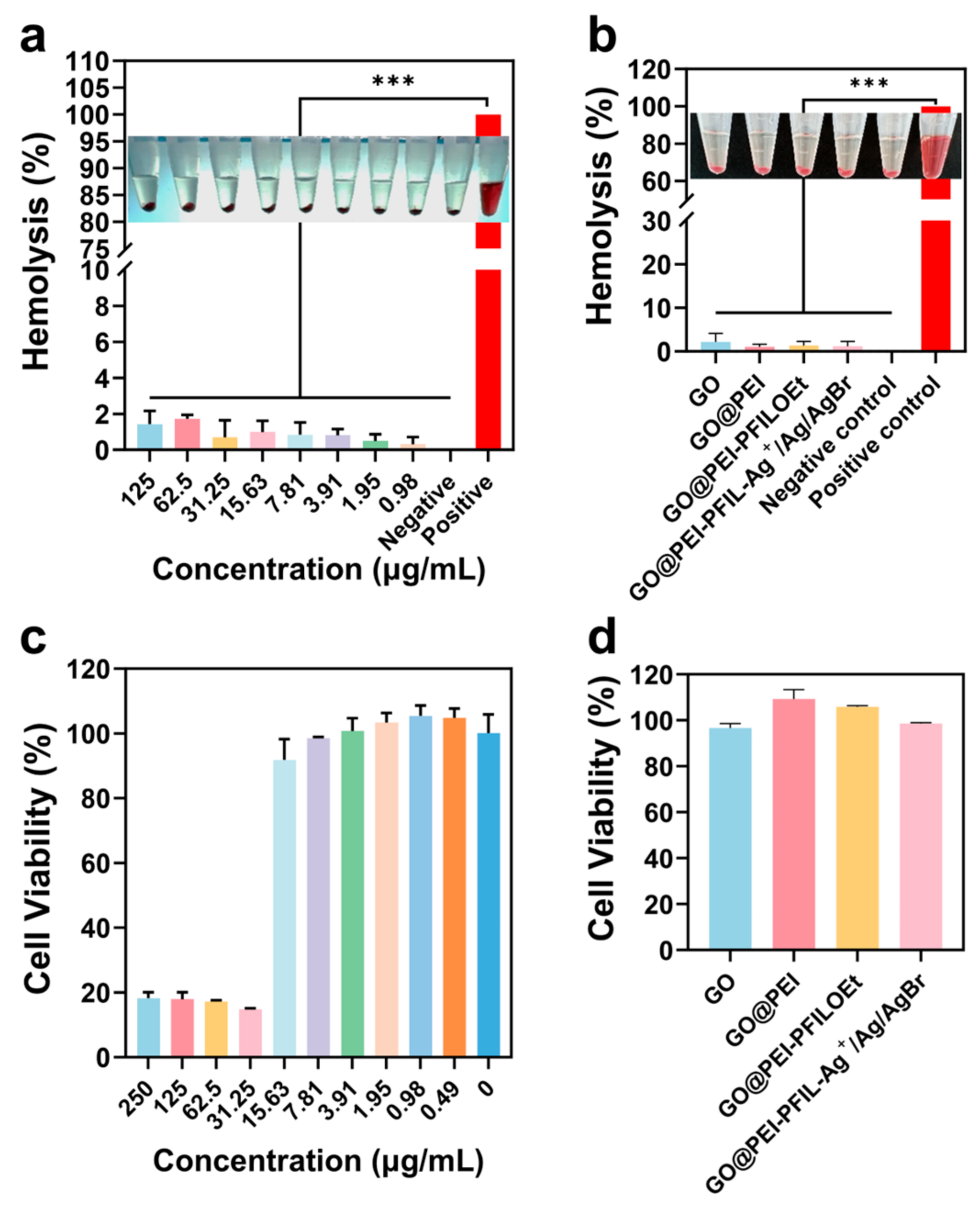
3.3.4. Hemolytic Activity and Cytotoxicity of GO@PEI-PFIL-Ag+/Ag/AgBr
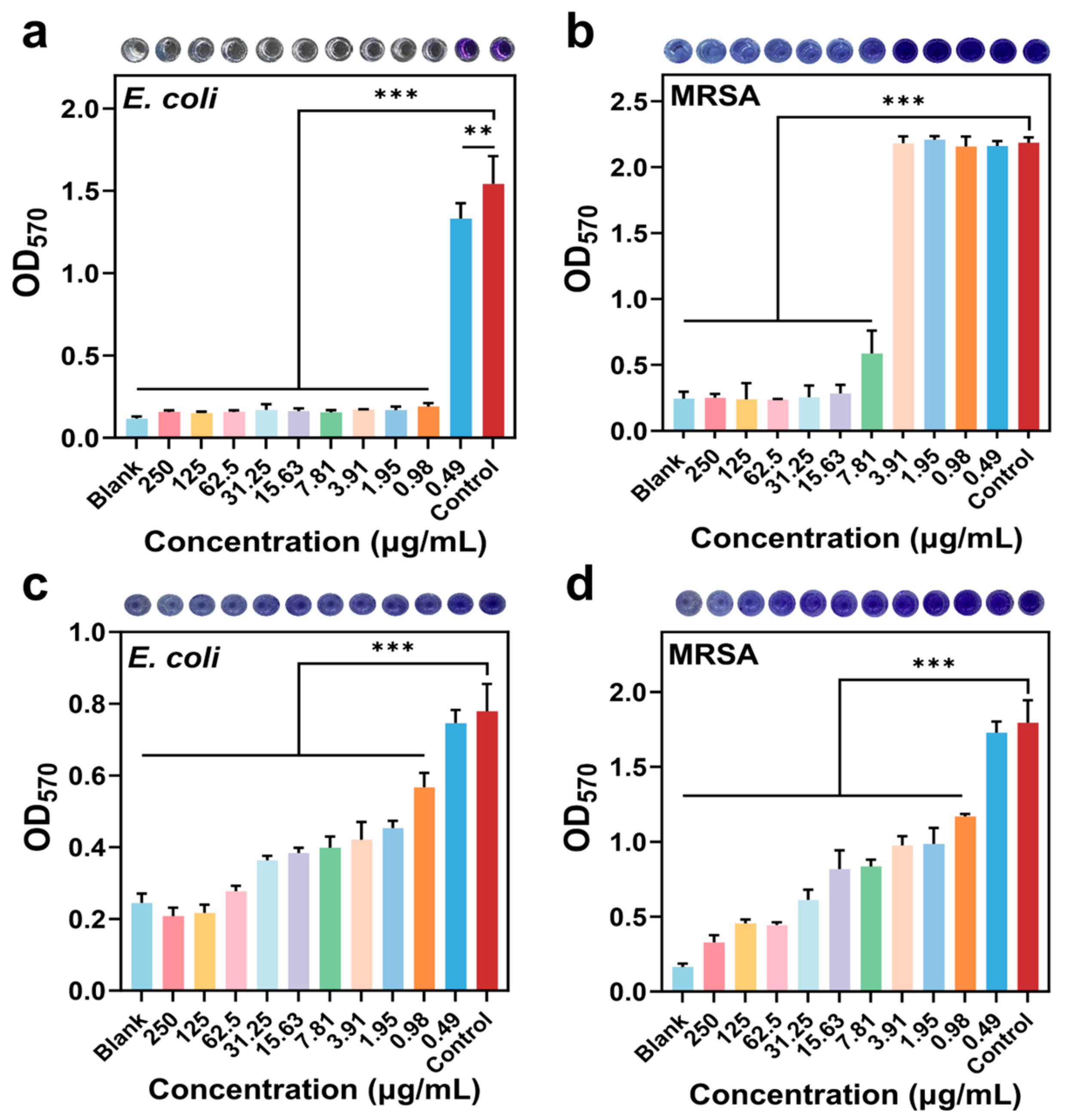
3.3.5. Inhibition and Disruption of Biofilms
3.4. Antimicrobial Mechanisms of GO@PEI-PFIL-Ag+/Ag/AgBr
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Roope, L.S.J.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V.; et al. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for Emerging Pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef]
- Li, G.Y.; Lai, Z.H.; Shan, A.S. Advances of Antimicrobial Peptide-Based Biomaterials for the Treatment of Bacterial Infections. Adv. Sci. 2023, 10, 2206602. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2010, 89, 475–492. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Khan, M.A.; Rossi, F.; Rossetti, A.; Zare, E.N.; et al. Antimicrobial Ionic Liquid-Based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Riduan, S.N.; Zhang, Y.G. Imidazolium salts and their polymeric materials for biological applications. Chem. Soc. Rev. 2013, 42, 9055–9070. [Google Scholar] [CrossRef]
- Rojas-Andrade, M.D.; Chata, G.; Rouholiman, D.; Liu, J.; Saltikov, C.; Chen, S. Antibacterial mechanisms of graphene-based composite nanomaterials. Nanoscale 2017, 9, 994–1006. [Google Scholar] [CrossRef]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z.; Ren, L. Surface Design for Antibacterial Materials: From Fundamentals to Advanced Strategies. Adv. Sci. 2021, 8, 2100368. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Qi, Y.; Zhong, D.; Xie, T.; Yao, K.; Yang, S.; Zhou, M. Cupriferous Silver Peroxysulfite Superpyramids as a Universal and Long-Lasting Agent to Eradicate Multidrug-Resistant Bacteria and Promote Wound Healing. ACS Appl. Bio Mater. 2021, 4, 3729–3738. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Antibacterial Action of Nanoparticle Loaded Nanocomposites Based on Graphene and Its Derivatives: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 3563. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Guo, Y.B.; Wang, D.G. PEI-RGO nanosheets as a nanoadditive for enhancing the tribological properties of water-based lubricants. Tribol. Int. 2019, 140, 105851. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Liang, H.; Liu, B.; Wang, Q.; Yan, Y.; Wang, T.; Jiang, Y.; Jiang, Y.; Qiu, H.; et al. Phosphonate-Functionalized Ionic Liquid: A New Surface Modifier Contributing to the Enhanced Enrichment of Phosphorylated Peptides. ACS Sustain. Chem. Eng. 2021, 9, 7930–7940. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Zhao, R.; Kong, W.; Sun, M.; Yang, Y.; Liu, W.; Lv, M.; Song, S.; Wang, L.; Song, H.; Hao, R. Highly Stable Graphene-Based Nanocomposite (GO-PEI-Ag) with Broad-Spectrum, Long-Term Antimicrobial Activity and Antibiofilm Effects. ACS Appl. Mater. Interfaces 2018, 10, 17617–17629. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, X.Y.; Qiu, H.D.; Liang, W.D.; Jiang, Y.L.; Wang, Q.; Wang, W.L.; Li, C.X.; Li, Y.; Han, B.W.; et al. Rational molecular design converting fascaplysin derivatives to potent broad-spectrum inhibitors against bacterial pathogens via targeting FtsZ. Eur. J. Med. Chem. 2024, 270, 116347. [Google Scholar] [CrossRef]
- Yu, R.X.; Chen, H.P.; He, J.; Zhang, Z.C.; Zhou, J.J.; Zheng, Q.Q.; Fu, Z.P.; Lu, C.Y.; Lin, Z.X.; Caruso, F.; et al. Engineering Antimicrobial Metal-Phenolic Network Nanoparticles with High Biocompatibility for Wound Healing. Adv. Mater. 2024, 36, e2307680. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Feng, D.; Kang, X.; Sun, Y.; Zhao, L.; Liang, H. Enhancing extraction ability by rational design of phosphoryl functionalized ionic liquids and mechanistic investigation on neodymium (III) extraction. J. Rare Earths 2016, 34, 83–90. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, T.R.; Qin, X.T.; Zhang, Z.Y.; Sun, Y.Y.; Liang, H.Z.; Li, H.R. Large-area flexible, transparent, and highly luminescent films containing lanthanide (III) complex-doped ionic liquids for efficiency enhancement of silicon-based heterojunction solar cell. Prog. Photovolt. 2017, 25, 1015–1021. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, X.Y.; Yang, D.Q.; Liang, H.Z.; Wang, Y.G. Luminescence enhancement of Europium(III) complexes by an ionic liquid. J. Lumin. 2019, 215, 116610. [Google Scholar] [CrossRef]
- Zhao, K.; Liang, H.Z.; Zhou, M.J.; Zhang, G.J.; Zhao, C.L.; Ge, J.W.; Xia, Y.G.; Gao, Z.H. Enhancing High-Rate Capability by Introducing Phosphonate Functionalized Imidazolium Ionic Liquid into Organic Carbonate Electrolyte. Chemistryselect 2018, 3, 4421–4424. [Google Scholar] [CrossRef]
- Ge, J.W.; Liang, H.Z.; Zhou, M.J.; Zhao, C.L.; Zheng, Z.; Yan, Y.H.; Zhao, L.L.; Tang, K.Q. Phosphonate-functionalized Ionic Liquid: A Novel Electrolyte Additive for Eenhanced Cyclic Stability and Rate Capability of LiCoO2 Cathode at High Voltage. Chemistryselect 2019, 4, 9959–9965. [Google Scholar] [CrossRef]
- Sang, K.M.; Wang, B.B.; Ge, J.W.; Zhu, T.F.; Jiang, Y.F.; Zhao, L.L.; Zhou, M.J.; Liang, H.Z. Bidentate Phosphonate-Functionalized Ionic Liquid Exhibiting Better Ability in Improving the Performance of Lithium-Ion Battery. Chemistryselect 2021, 6, 2607–2614. [Google Scholar] [CrossRef]
- Song, J.; Liao, K.; Si, J.; Zhao, C.; Wang, J.; Zhou, M.; Liang, H.; Gong, J.; Cheng, Y.-J.; Gao, J.; et al. Phosphonate-Functionalized Ionic Liquid Gel Polymer Electrolyte with High Safety for Dendrite-Free Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 2901–2910. [Google Scholar] [CrossRef]
- Liao, K.; Song, J.; Ge, J.; Si, J.; Cai, Y.; Luo, Z.; Zhou, M.; Liang, H.; Cheng, Y.-J.; Milanovic, M.; et al. Protective behavior of phosphonate-functionalized imidazolium ionic liquid and its impact on the Li-ion battery performance. Energy Mater. 2023, 3, 300044. [Google Scholar] [CrossRef]
- Xia, C.; Wang, Q.; Liang, W.; Wang, B.; Feng, Q.; Zhou, C.; Xie, Y.; Yan, Y.; Zhao, L.; Jiang, B.; et al. Superhydrophilic nanocomposite adsorbents modified via nitrogen-rich phosphonate-functionalized ionic liquid linkers: Enhanced phosphopeptide enrichment and phosphoproteome analysis of tau phosphorylation in the hippocampal lysate of Alzheimer’s transgenic mice. J. Mater. Chem. B 2022, 10, 7967–7978. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Xia, C.L.; Zhu, A.; Bao, Y.J.; Lu, J.N.; Chen, Y.; Xu, J.Y.; Wang, B.B.; Naman, C.B.; Li, L.P.; et al. Discrepancy of synaptic and microtubular protein phosphorylation in the hippocampus of APP/PS1 and MAPTxP301S transgenic mice at the early stage of Alzheimer’s disease. Metab. Brain Dis. 2023, 38, 1983–1997. [Google Scholar] [CrossRef] [PubMed]
- Kamerlin, S.C.L.; Sharma, P.K.; Prasad, R.B.; Warshel, A. Why nature really chose phosphate. Q. Rev. Biophys. 2013, 46, 1–132. [Google Scholar] [CrossRef] [PubMed]
- Galezowska, J.; Gumienna-Kontecka, E. Phosphonates, their complexes and bio-applications: A spectrum of surprising diversity. Coord. Chem. Rev. 2012, 256, 105–124. [Google Scholar] [CrossRef]
- Russell, R.G.G. Bisphosphonates: Mode of action and pharmacology. Pediatrics 2007, 119, S150–S162. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, H.D.; Yang, N.; Xie, H.J.; Liang, W.D.; Lin, J.Y.; Zhu, H.F.; Zhou, Y.; Wang, N.; Tan, X.Y.; et al. Fascaplysin derivatives binding to DNA via unique cationic five-ring coplanar backbone showed potent antimicrobial/antibiofilm activity against MRSA in vitro and in vivo. Eur. J. Med. Chem. 2022, 230, 114099. [Google Scholar] [CrossRef]
- Qiu, H.; Zhao, X.; Jiang, Y.; Liang, W.; Wang, W.; Jiang, X.; Jiang, M.; Wang, X.; Cui, W.; Li, Y.; et al. Design and synthesis of fascaplysin derivatives as inhibitors of FtsZ with potent antibacterial activity and mechanistic study. Eur. J. Med. Chem. 2023, 254, 115348. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, T.; Xing, Z.; Rodriguez, R.D.; Cheng, L.; Gao, Y.; Chen, Q.; Cheng, C. Enzymatic metal oxide/nanoparticle heterojunctions with mutually reinforced bifunctional chemotherapies for combating drug-resistant bacteria. Chem. Eng. J. 2024, 483, 149249. [Google Scholar] [CrossRef]
- He, J.; Ye, Y.; Zhang, D.; Tang, X.; Luo, C.; Chen, X.; Yao, K.; Zhou, M. Smartphone-triggered targeted inactivation of MRSA under SERS monitoring. Nano Today 2023, 53, 102012. [Google Scholar] [CrossRef]
- Zhou, W.; Bai, T.; Wang, L.; Cheng, Y.; Xia, D.; Yu, S.; Zheng, Y. Biomimetic AgNPs@antimicrobial peptide/silk fibroin coating for infection-trigger antibacterial capability and enhanced osseointegration. Bioact. Mater. 2023, 20, 64–80. [Google Scholar] [CrossRef]
- Han, J.W.; Gurunathan, S.; Jeong, J.K.; Choi, Y.J.; Kwon, D.N.; Park, J.K.; Kim, J.H. Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line. Nanoscale Res. Lett. 2014, 9, 459. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kim, E.; Han, J.W.; Park, J.H.; Kim, J.H. Green Chemistry Approach for Synthesis of Effective Anticancer Palladium Nanoparticles. Molecules 2015, 20, 22476–22498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Z.; Zhao, Q.; Huang, J.; Shen, H.; Zhang, Z. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide. Small 2011, 7, 460–464. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M.H. Ag@AgCl: A Highly Efficient and Stable Photocatalyst Active under Visible Light. Angew. Chem. Int. Ed. 2008, 47, 7931–7933. [Google Scholar] [CrossRef]
- Sarina, S.; Waclawik, E.R.; Zhu, H. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green. Chem. 2013, 15, 1814–1833. [Google Scholar] [CrossRef]
- Chen, W.; Wu, W.; Bai, Q.; Liu, J.; Zheng, C.; Gao, Q.; Hu, F.; Zhang, Y.; Lu, T. Photocatalytic Ag/AgBr-MBG for Rapid Antibacterial and Wound Repair. Acs Biomater. Sci. Eng. 2023, 9, 2470–2482. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.-N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Shen, J.F.; Hu, Y.Z.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M.X. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, L.; Chen, H.; Gao, C. Sol–gel fabrication of a non-laminated graphene oxide membrane for oil/water separation. J. Mater. Chem. A 2015, 3, 19517–19524. [Google Scholar] [CrossRef]
- Pan, N.; Li, L.; Ding, J.; Wang, R.; Jin, Y.; Xia, C. A Schiff base/quaternary ammonium salt bifunctional graphene oxide as an efficient adsorbent for removal of Th(IV)/U(VI). J. Colloid Interface Sci. 2017, 508, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, X.; Zhou, Y.; Huang, L.; Wang, Y.; Rong, Q.; Han, Z.; Qu, H.; Zhu, Z.; Xu, S.; et al. Bioinspired Graphene Oxide Membranes with pH-Responsive Nanochannels for High-Performance Nanofiltration. ACS Nano 2021, 15, 13178–13187. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Xu, Y.L.; Liu, K.; Wang, X.Q.; Mulder, F.M. High-Performance and Low-Cost Sodium-Ion Anode Based on a Facile Black Phosphorus-Carbon Nanocomposite. Chemelectrochem 2017, 4, 2140–2144. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, G.; Lee, H.-W.; Liu, N.; Wang, H.; Yao, H.; Yang, W.; Cui, Y. Formation of Stable Phosphorus–Carbon Bond for Enhanced Performance in Black Phosphorus Nanoparticle–Graphite Composite Battery Anodes. Nano Lett. 2014, 14, 4573–4580. [Google Scholar] [CrossRef]
- Liu, S.B.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Al-Sawarees, D.K.; Darwish, R.M.; Abu-Zurayk, R.; Masri, M.A. Assessing silver nanoparticle and antimicrobial combinations for antibacterial activity and biofilm prevention on surgical sutures. J. Appl. Microbiol. 2024, 135, lxae063. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, L.; Zhou, F.; Huang, J.; Yu, W.; Chen, H.; Gan, J.; Song, M.; Yang, X.; Zhu, R. Exploiting the advantages of cationic copolymers and AgBr nanoparticles to optimize the antibacterial activity of chitosan. Int. J. Biol. Macromol. 2024, 270, 132209. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.C.; Sun, D.D. Graphene oxide enwrapped Ag3PO4 composite: Towards a highly efficient and stable visible-light-induced photocatalyst for water purification. Catal. Sci. Technol. 2012, 2, 2525–2532. [Google Scholar] [CrossRef]
- Alahmadi, N.S.; Elshaarawy, R.F.M. Novel aminothiazolyl-functionalized phosphonium ionic liquid as a scavenger for toxic metal ions from aqueous media; mining to useful antibiotic candidates. J. Mol. Liq. 2019, 281, 451–460. [Google Scholar] [CrossRef]
- Khawaja, H.; Zahir, E.; Asghar, M.A.; Asghar, M.A. Graphene oxide, chitosan and silver nanocomposite as a highly effective antibacterial agent against pathogenic strains. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 246–255. [Google Scholar] [CrossRef]
- Chen, X.; Huang, X.; Zheng, C.; Liu, Y.; Xu, T.; Liu, J. Preparation of different sized nano-silver loaded on functionalized graphene oxide with highly effective antibacterial properties. J. Mater. Chem. B 2015, 3, 7020–7029. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Sun, T.; Xue, J.; Miao, Z.; Yan, X.; Fang, W.; Li, Q.; Tang, R.; Lu, Y.; Tang, L.; et al. Ag Nanoparticles Cluster with pH-Triggered Reassembly in Targeting Antimicrobial Applications. Adv. Funct. Mater. 2020, 30, 2000511. [Google Scholar] [CrossRef]
- Fan, Z.; Po, K.H.L.; Wong, K.K.; Chen, S.; Lau, S.P. Polyethylenimine-Modified Graphene Oxide as a Novel Antibacterial Agent and Its Synergistic Effect with Daptomycin for Methicillin-Resistant Staphylococcus aureus. ACS Appl. Nano Mater. 2018, 1, 1811–1818. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Z.; Sun, Y.; Chi, Z.; Qing, G. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J. Mater. Chem. B 2020, 8, 2951–2973. [Google Scholar] [CrossRef]
- Zheng, L.; Li, J.; Yu, M.; Jia, W.; Duan, S.; Cao, D.; Ding, X.; Yu, B.; Zhang, X.; Xu, F.-J. Molecular Sizes and Antibacterial Performance Relationships of Flexible Ionic Liquid Derivatives. J. Am. Chem. Soc. 2020, 142, 20257–20269. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Q.; Zheng, Z.; Zhou, S.; Mao, H.; Wang, B.; Yan, F. Intrinsically Antibacterial Poly(ionic liquid) Membranes: The Synergistic Effect of Anions. ACS Macro Lett. 2015, 4, 1094–1098. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef] [PubMed]
- Li, P.P.; Wu, H.X.; Dong, A. Ag/AgX nanostructures serving as antibacterial agents: Achievements and challenges. Rare Met. 2022, 41, 519–539. [Google Scholar] [CrossRef]
- Azócar, M.I.; Gómez, G.; Levín, P.; Paez, M.; Muñoz, H.; Dinamarca, N. Review: Antibacterial behavior of carboxylate silver(I) complexes. J. Coord. Chem. 2014, 67, 3840–3853. [Google Scholar] [CrossRef]
- Nomiya, K.; Tsuda, K.; Sudoh, T.; Oda, M. Ag(I)-N bond-containing compound showing wide spectra in effective antimicrobial activities: Polymeric silver(I) imidazolate. J. Inorg. Biochem. 1997, 68, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Weidenmaier, C.; Peschel, A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008, 6, 276–287. [Google Scholar] [CrossRef]
- Thomas, K.J.; Rice, C.V. Equilibrium binding behavior of magnesium to wall teichoic acid. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 1981–1987. [Google Scholar] [CrossRef]
- Podder, S.; Paul, S.; Basak, P.; Xie, B.W.; Fultwood, N.J.; Baldock, S.J.; Yang, Y.; Hardy, J.G.; Ghosh, C.K. Bioactive silver phosphate/polyindole nanocomposites. RSC Adv. 2020, 10, 11060–11073. [Google Scholar] [CrossRef] [PubMed]
- Lithgow, T.; Stubenrauch, C.J.; Stumpf, M.P.H. Surveying membrane landscapes: A new look at the bacterial cell surface. Nat. Rev. Microbiol. 2023, 21, 502–518. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.A.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugao, A.B. Advances in silver nanoparticles: A comprehensive review on their potential as antimicrobial agents and their mechanisms of action elucidated by proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Kaiser, K.G.; Delattre, V.; Frost, V.J.; Buck, G.W.; Phu, J.V.; Fernandez, T.G.; Pavel, I.E. Nanosilver: An Old Antibacterial Agent with Great Promise in the Fight against Antibiotic Resistance. Antibiotics 2023, 12, 1264. [Google Scholar] [CrossRef]
- Nor, Y.A.; Zhou, L.; Meka, A.K.; Xu, C.; Niu, Y.; Zhang, H.; Mitter, N.; Mahony, D.; Yu, C. Engineering Iron Oxide Hollow Nanospheres to Enhance Antimicrobial Property: Understanding the Cytotoxic Origin in Organic Rich Environment. Adv. Funct. Mater. 2016, 26, 5408–5418. [Google Scholar] [CrossRef]
- Khamaru, K.; Pal, U.; Shee, S.; Lo, R.; Seal, K.; Ghosh, P.; Maiti, N.C.; Banerji, B. Metal-Free Activation of Molecular Oxygen by Quaternary Ammonium-Based Ionic Liquid: A Detail Mechanistic Study. J. Am. Chem. Soc. 2024, 146, 6912–6925. [Google Scholar] [CrossRef]
- Wen, X.-J.; Shen, C.-H.; Fei, Z.-H.; Fang, D.; Liu, Z.-T.; Dai, J.-T.; Niu, C.-G. Recent developments on AgI based heterojunction photocatalytic systems in photocatalytic application. Chem. Eng. J. 2020, 383, 123083. [Google Scholar] [CrossRef]
- Tang, C.; Bai, H.; Liu, L.; Zan, X.; Gao, P.; Sun, D.D.; Yan, W. A green approach assembled multifunctional Ag/AgBr/TNF membrane for clean water production & disinfection of bacteria through utilizing visible light. Appl. Catal. B Environ. 2016, 196, 57–67. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; González-Ballesteros, N.; da Costa, A.; Machado, R.; Gomes, A.C.; Rodríguez-Argüelles, M.C. Antimicrobial and anti-biofilm activity of silver nanoparticles biosynthesized with Cystoseira algae extracts. JBIC J. Biol. Inorg. Chem. 2023, 28, 439–450. [Google Scholar] [CrossRef]


| MIC Values (μg/mL) | ||||
|---|---|---|---|---|
| Material | Gram-Negative Bacteria | Gram-Positive Bacteria | ||
| E. coli 1 | P. aeruginosa 2 | MRSA 3 | S. aureus 4 | |
| GO | >250 | >250 | >250 | >250 |
| GO@PEI | >250 | >250 | >250 | >250 |
| GO@PEI-PFILOEt | >250 | >250 | >250 | >250 |
| GO@PEI-PFIL-Cu2+ | >250 | >250 | >250 | ≤250 |
| GO@PEI-PFIL-Zn2+ | >250 | >250 | >250 | ≤250 |
| GO@PEI-PFIL-Fe3+ | ≤250 | >250 | >250 | >250 |
| GO@PEI-PFIL-Ag+/Ag/AgBr | ≤0.98 | ≤1.95 | ≤7.81 | ≤7.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhao, X.; Qiu, H.; Liang, W.; Liu, L.; Sun, Y.; Zhao, L.; Wang, X.; Liang, H. Antibacterial Activity of GO-Based Composites Enhanced by Phosphonate-Functionalized Ionic Liquids and Silver. Materials 2025, 18, 1889. https://doi.org/10.3390/ma18081889
Liu X, Zhao X, Qiu H, Liang W, Liu L, Sun Y, Zhao L, Wang X, Liang H. Antibacterial Activity of GO-Based Composites Enhanced by Phosphonate-Functionalized Ionic Liquids and Silver. Materials. 2025; 18(8):1889. https://doi.org/10.3390/ma18081889
Chicago/Turabian StyleLiu, Xinyu, Xing Zhao, Hongda Qiu, Weida Liang, Linlin Liu, Yunyu Sun, Lingling Zhao, Xiao Wang, and Hongze Liang. 2025. "Antibacterial Activity of GO-Based Composites Enhanced by Phosphonate-Functionalized Ionic Liquids and Silver" Materials 18, no. 8: 1889. https://doi.org/10.3390/ma18081889
APA StyleLiu, X., Zhao, X., Qiu, H., Liang, W., Liu, L., Sun, Y., Zhao, L., Wang, X., & Liang, H. (2025). Antibacterial Activity of GO-Based Composites Enhanced by Phosphonate-Functionalized Ionic Liquids and Silver. Materials, 18(8), 1889. https://doi.org/10.3390/ma18081889









