Exploring the Mechanism of Microstructural Changes in Ultra-High-Performance Concrete Under Microwave Influence: Experiments and Molecular Dynamics Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
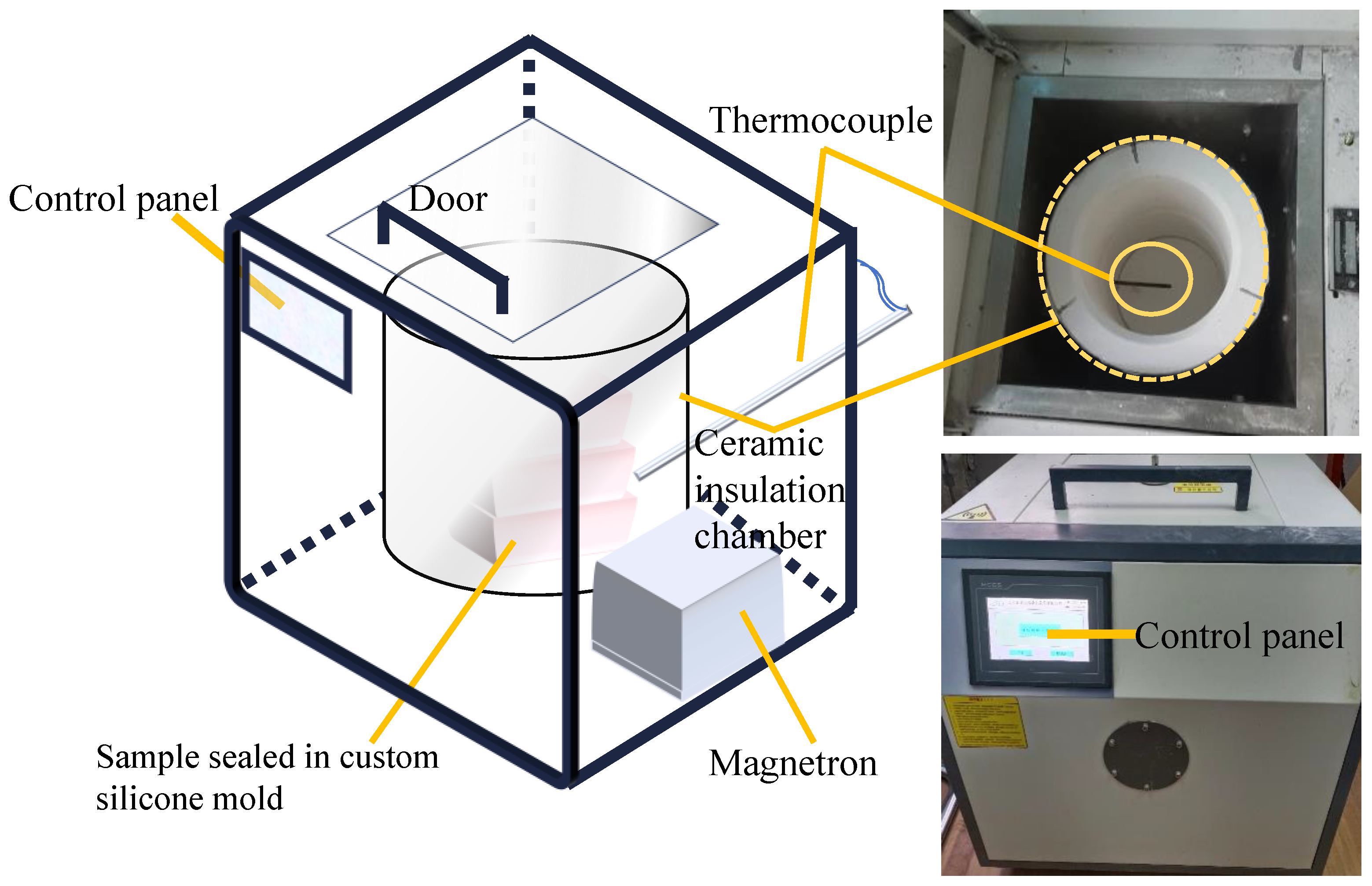
2.2. Curing Methods and Regimes
2.3. Microstructural Characterization
2.3.1. XRD
2.3.2. SEM
2.3.3. Pore Structure
2.4. Nanostructural Characterization
2.4.1. Establishment of Model
2.4.2. Molecular Dynamics Simulation
3. Results and Discussion
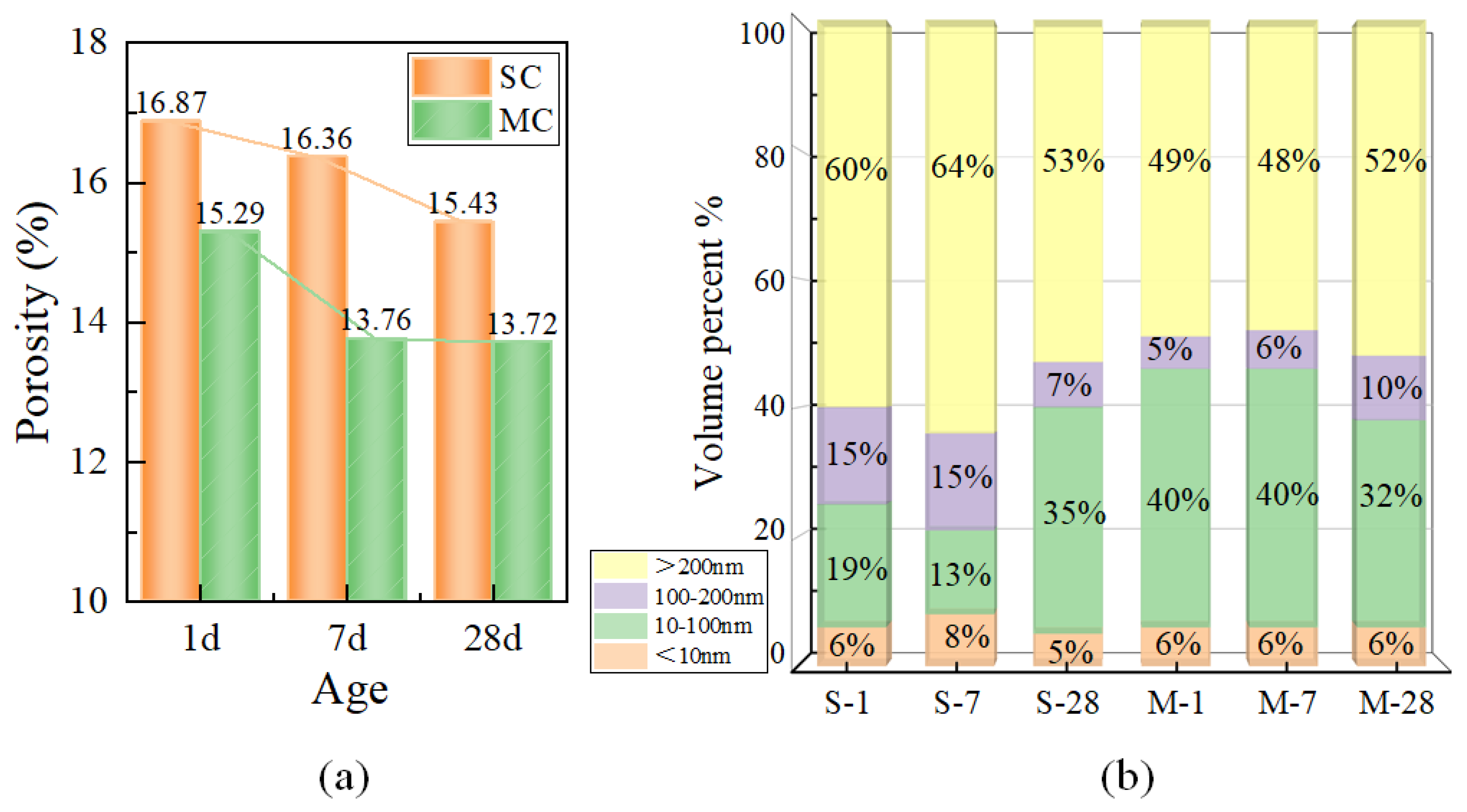
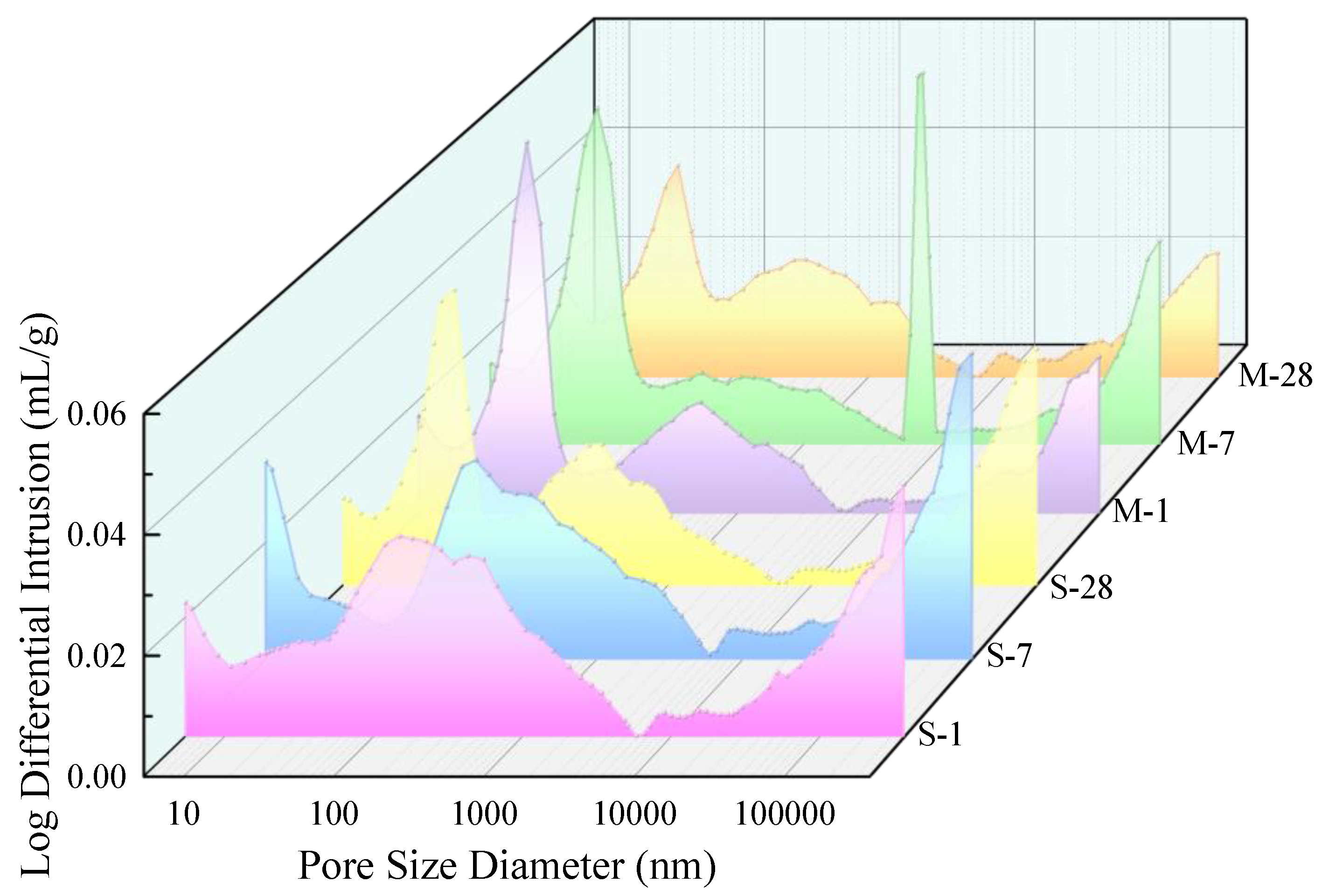
3.1. Pore Structure
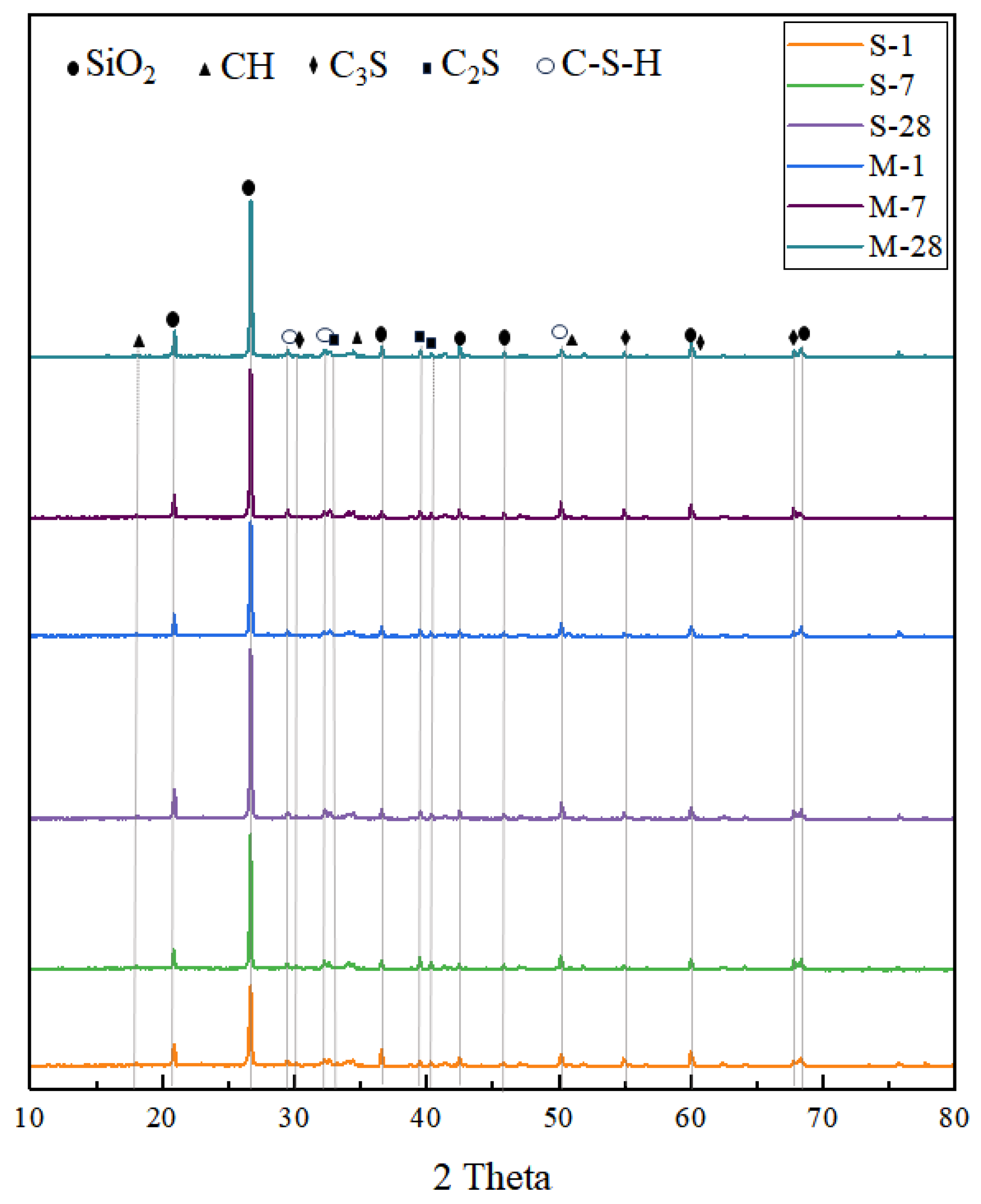
3.2. XRD Analysis
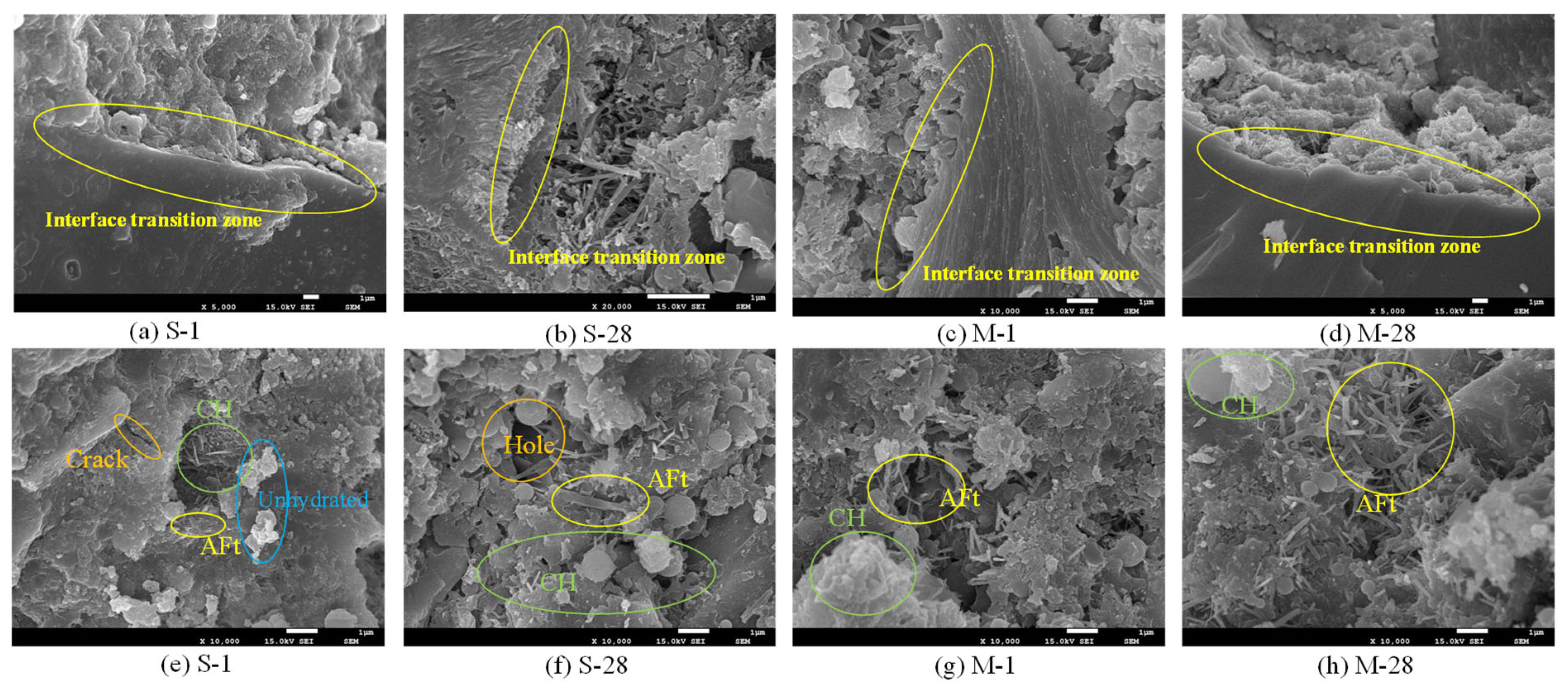
3.3. Microstructure
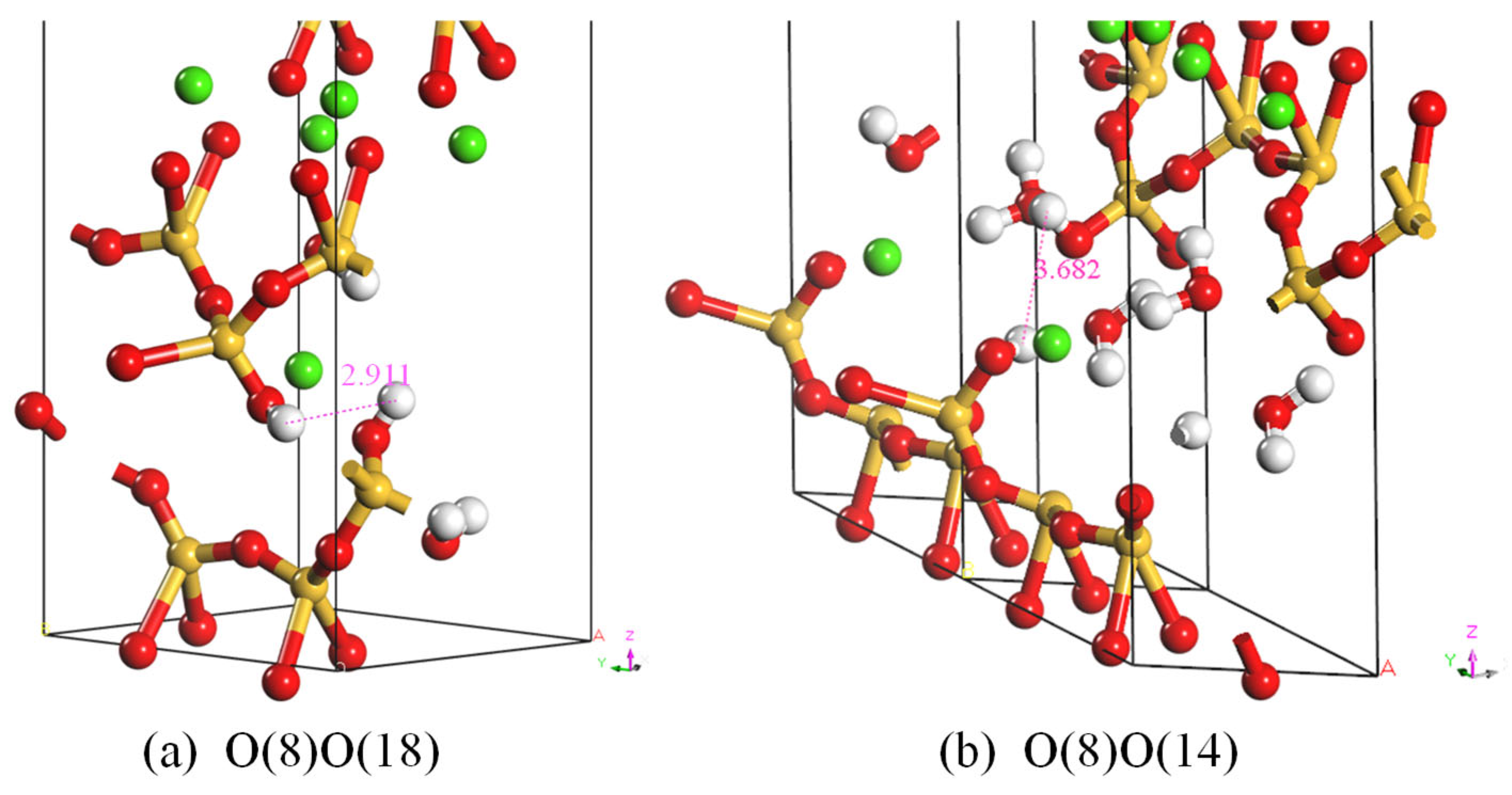
3.4. Nanostructure in Molecular Dynamics
3.4.1. Nanoscale Pores in the Structure
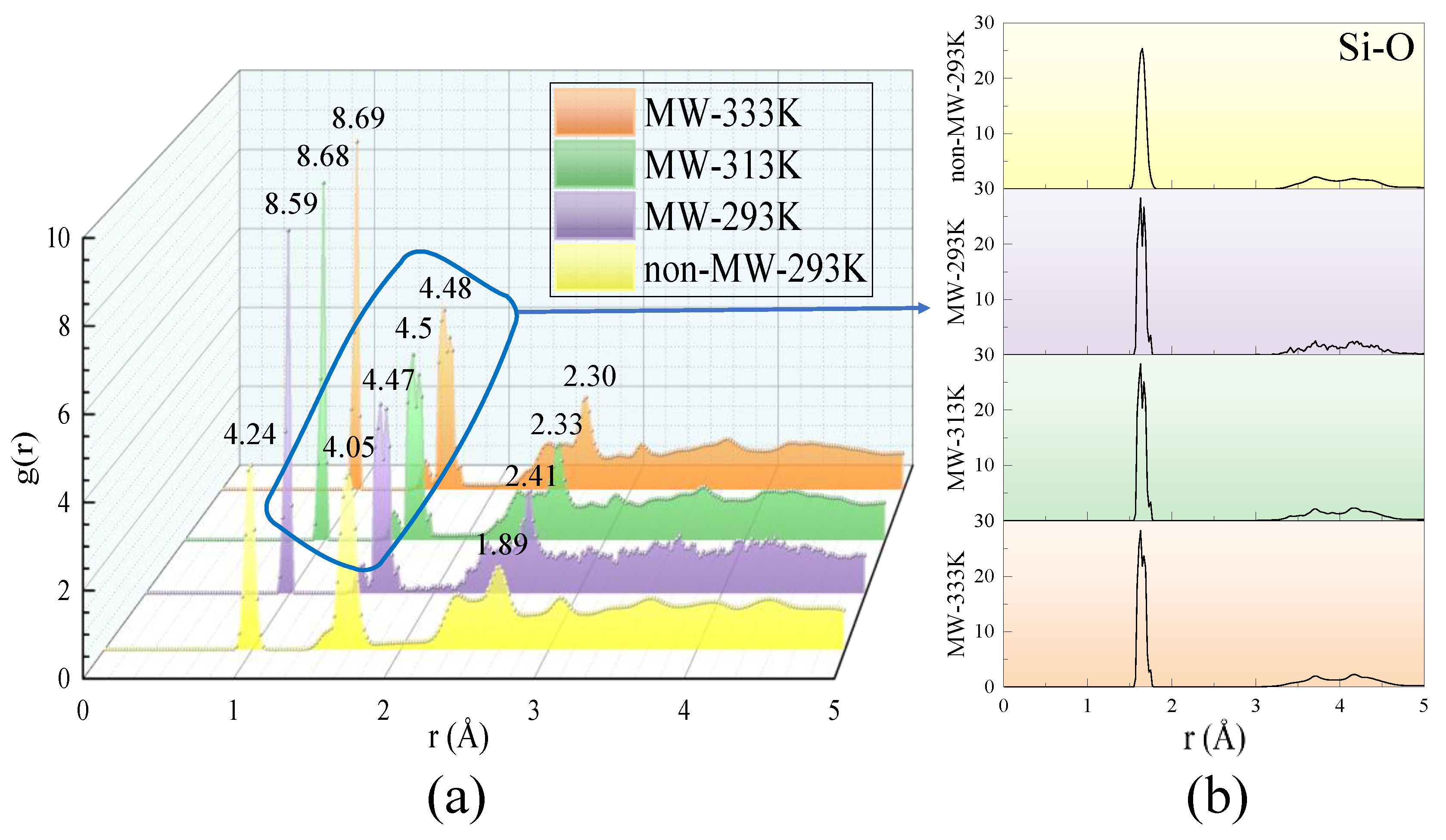
3.4.2. Radial Distribution
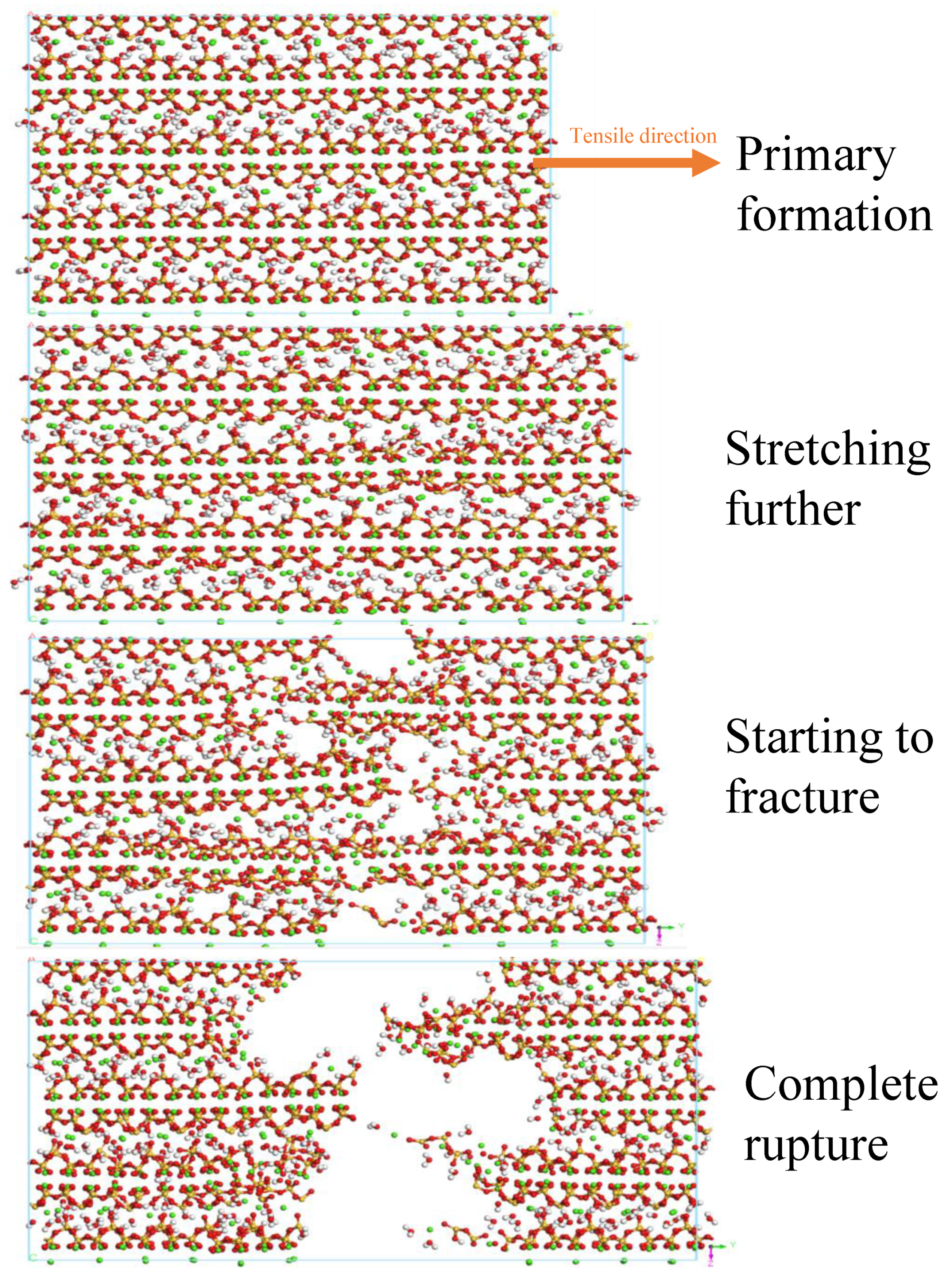
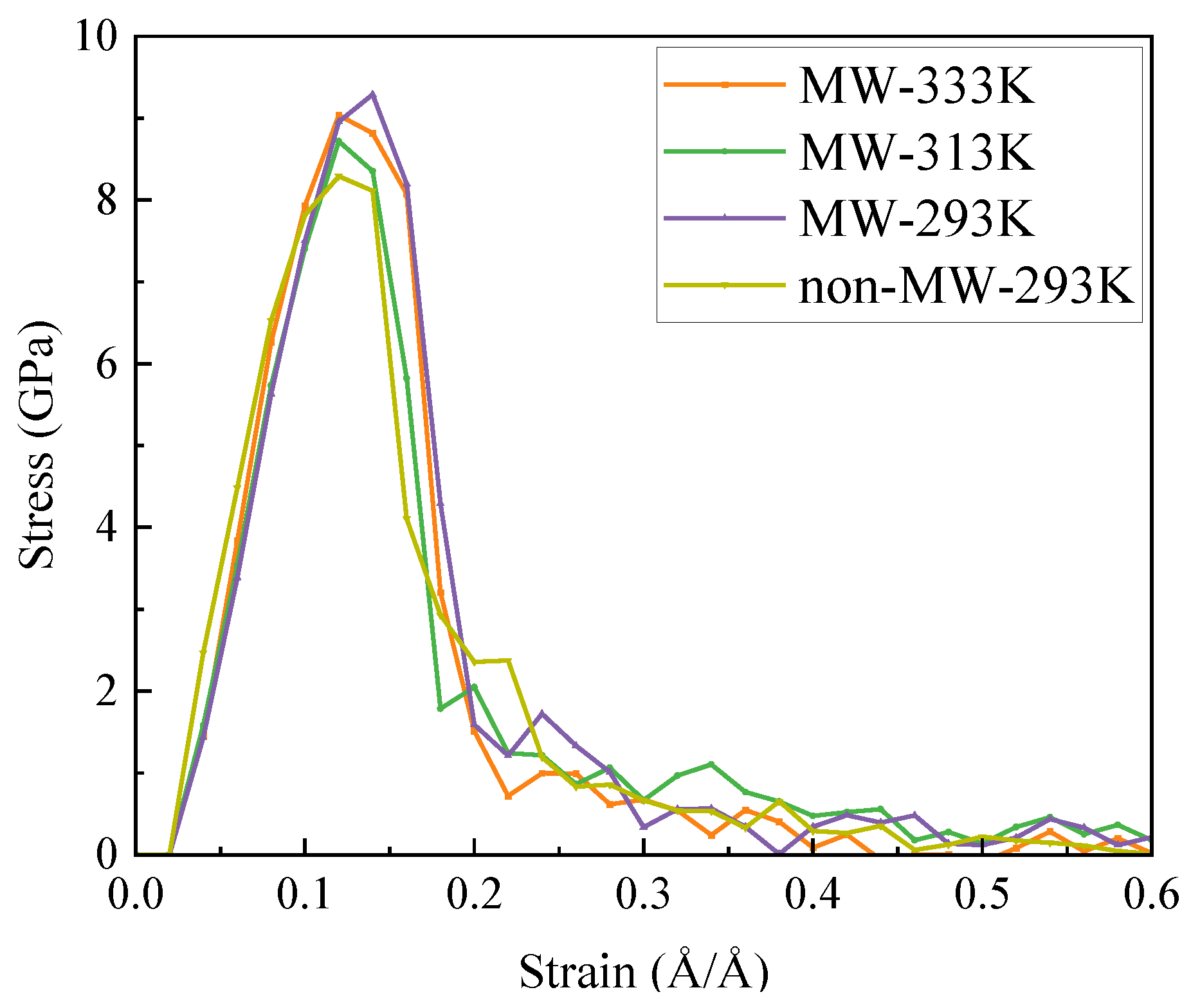
3.5. Uniaxial Tension and Tensile Fracture Simulation
4. Conclusions
- Microwave curing promotes the development of porosity in UHPC during the early stages and optimizes the pore size distribution. The average daily porosity reduction was 0.085% for the SC specimens in the first 7 days, while it was 0.15% for the MC specimens. Compared to the SC sample, the MC sample exhibits a higher proportion of small pores, indicating that microwave curing optimizes the pore structure. Microwave curing accelerates the hydration rate of concrete, resulting in a relatively smaller reduction in total porosity during the later stages. The pore size distribution at 28d demonstrates that microwave curing has no adverse effects on the development of the microstructure of concrete in the later stages.
- Microwave curing accelerates the formation of hydration products, such as AFt, which gradually fill the voids within the structure and form a dense cross-linked structure within 28 days. The formation of this structure further optimizes the internal void structure of UHPC, enhancing the density of its microstructure and thereby increasing structural strength. The significant reduction in CH content over 28 days suggests that microwave curing also accelerates the pozzolanic reaction process, consuming a substantial amount of CH.
- By simulating an ideal microwave field environment using computer modeling, it was found that the non-thermal effects of microwaves have a more significant impact on the structure than the thermal effects, providing a theoretical basis for optimizing the curing regime. Additionally, the mechanical oscillation effect improves the orderliness of molecular arrangement, compresses the free space within crystal cells, and promotes the densification of cement hydration products. However, the thermal effects have an adverse impact on pore structure optimization.
- The microwave field facilitates the enhancement of structural toughness by optimizing the internal architecture of the model, promoting closer interlayer structural connections which, in turn, lead to an increase in tensile strength of up to 1 GPa. Regarding the stress–strain behavior of the field model, the elastic stage is significantly longer, implying that the field model can maintain higher stress levels at the same strain level without entering the next stage.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Zhang, Y.; Zhao, W.; Yan, Z. Effect of multiscale fibers and cenospheres on the uniaxial tensile behavior of ultra-high performance concrete subjected to high temperatures. J. Build. Eng. 2024, 95, 110143. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jin, L.; Feng, Y.; Wang, Z.Y.; Yin, T.Y.; Liu, K.N.; Dong, E.L.; Yu, R. A low carbon embedded low water/binder cement-based composites (LW/BCC) based on steel slag powder and microwave pre-curing: Experiments and Life Cycle Assessment (LCA). Constr. Build. Mater. 2023, 400, 132778. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, S.; Fan, D.; Wu, Z.; Zhang, J.; Wang, Z.; Yin, T.; Yu, R. Coupling effects of steel slag powder and electromagnetic waves on the microstructure and hydration kinetics evolution of cementitious materials with ultra low water/binder ratio. J. Build. Eng. 2022, 58, 105036. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Tan, K.H.; Qian, H.; Liu, T. Microstructure-informed deep learning model for accurate prediction of multiple concrete properties. J. Build. Eng. 2024, 98, 111339. [Google Scholar] [CrossRef]
- Wang, T.; Fan, X.; Gao, C. Strength, pore characteristics, and characterization of fly ash-slag-based geopolymer mortar modified with silica fume. Structures 2024, 69, 107525. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z.; Shao, J.; Luo, S.; Yu, D. Novel soybean dregs biochar concrete: Characterization and evaluation of the mechanical properties and microstructure. Constr. Build. Mater. 2025, 458, 139512. [Google Scholar] [CrossRef]
- Wei, W.; Shao, Z.; Zhang, P.; Zhang, H.; Cheng, J.; Yuan, Y. Thermally Assisted Liberation of Concrete and Aggregate Recycling: Comparison between Microwave and Conventional Heating. J. Mater. Civ. Eng. 2021, 33, 04021370. [Google Scholar] [CrossRef]
- Jafari Foruzin, L.; Rezvani, Z. Microwave-assistant synthesis of Tartrazine/layered double hydroxide nano-hybrids with high photoluminescent properties: Influence of microwave energy. Mater. Chem. Phys. 2021, 267, 124716. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, S.; Wang, P. Effects of microwave curing on the compressive strength development and hydration of cement-granulated blast furnace slag composite system. Constr. Build. Mater. 2021, 270, 121432. [Google Scholar] [CrossRef]
- Xuequan, W.; Jianbgo, D.; Mingshu, T. Microwave curing technique in concrete manufacture. Cem. Concr. Res. 1987, 17, 205–210. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Xue, H.; Zhang, Q.; Dong, H. The effect of microwave curing on the static and dynamic mechanical properties and thermal brittleness of coal gangue concrete. Constr. Build. Mater. 2025, 463, 140025. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Jin, C.; Mu, J.; Shen, J. Research on the creep properties of cement paste after microwave pre-curing based on indentation test. Cem. Concr. Compos. 2024, 145, 105325. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Lin, H.; Liu, J. The experimental study on microwave-assisted preparation of Ultra-High Performance Geopolymer Concrete (UHPGC). Constr. Build. Mater. 2024, 414, 134934. [Google Scholar] [CrossRef]
- Xia, J.; Huang, Y.; Zhang, R.; Liu, J.; Ma, G. Low-carbon microwave curing of limestone calcined clay cement (LC3): Performance and mechanism. Constr. Build. Mater. 2024, 438, 137257. [Google Scholar] [CrossRef]
- Li, D.; Shi, Z.; Pan, Y.; Gao, X.; Li, S. Temporal effects on the micro- and nano-structural cement-slag pastes subjected to microwave curing over 7 years. Case Stud. Constr. Mater. 2024, 21, e03611. [Google Scholar] [CrossRef]
- Gao, X.; Li, S. Effects of microwave curing on the mechanical properties of ultra-high performance concrete and affecting mechanism. Mater. Rep. 2019, 33, 271–276. [Google Scholar]
- Gao, F.-F.; Zhao, Y.; Wang, W.-D.; Shi, Y.-L. Study on microstructure evolution mechanism of concrete containing carbon nanotubes subjected to different heating-cooling regimes: Experiments and molecular dynamics simulation. Constr. Build. Mater. 2025, 458, 139625. [Google Scholar] [CrossRef]
- Richardson, I.G. The nature of C-S-H in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- Stroeven, P.; Slowik, M. Economic and Reliable Estimation of Cementitious Material Properties on the Basis of Virtual Models. In Proceedings of the 12th International Symposium on Brittle Matrix Composites (BMC 2019), Warszawa, Poland, 23–24 September 2019; pp. 35–44. [Google Scholar]
- Yang, G.; Chen, X.; Xu, J. Molecular dynamics simulation of interfacial mechanical properties of crumb rubber concrete. Constr. Build. Mater. 2024, 438, 137336. [Google Scholar] [CrossRef]
- Li, W.; Xiong, C.; Zhou, Y.; Chen, W.; Zheng, Y.; Lin, W.; Xing, J. Insights on the mechanical properties and failure mechanisms of calcium silicate hydrates based on deep-learning potential molecular dynamics. Cem. Concr. Res. 2024, 186, 107690. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Wang, J.; Kirkpatrick, R.J. Molecular dynamics modeling of the structure, dynamics and energetics of mineral–water interfaces: Application to cement materials. Cem. Concr. Res. 2007, 37, 337–347. [Google Scholar] [CrossRef]
- Yu, Q.; Lin, Y.; Guo, T.; Wen, R.; Wang, C.; Tu, Y.; Sas, G.; Elfgren, L. Assessing the unsaturated transport and adsorption properties of ions in nanopores of realistic hydrated-calcium-silicate gel using molecular dynamics simulations. Comput. Mater. Sci. 2023, 222, 112121. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Gao, X.; Li, X. Study of the effects of microwave curing on ultra-high-performance concrete based on dielectric properties. Case Stud. Constr. Mater. 2024, 21, e04104. [Google Scholar] [CrossRef]
- GB/T 50081; Standard for Test Method of Physical and Mechanical Properties of Concrete. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2019. [CrossRef]
- Skinner, L.B.; Chae, S.R.; Benmore, C.J.; Wenk, H.R.; Monteiro, P.J.M. Nanostructure of Calcium Silicate Hydrates in Cements. Phys. Rev. Lett. 2010, 104, 195502. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Jiang, J. Insight into ions adsorption at the C-S-H gel-aqueous electrolyte interface: From atomic-scale mechanism to macroscopic phenomena. Constr. Build. Mater. 2022, 321, 126179. [Google Scholar] [CrossRef]
- Lushnikova, A.; Zaoui, A. Improving mechanical properties of C-S-H from inserted carbon nanotubes. J. Phys. Chem. Solids 2017, 105, 72–80. [Google Scholar] [CrossRef]
- Hou, D.; Li, Z. Molecular dynamics study of water and ions transport in nano-pore of layered structure: A case study of tobermorite. Microporous Mesoporous Mater. 2014, 195, 9–20. [Google Scholar] [CrossRef]
- Wang, S.; Peng, X.; Tang, L.; Zeng, L.; Lan, C. Influence of inorganic admixtures on the 11 Å-tobermorite formation prepared from steel slags: XRD and FTIR analysis. Constr. Build. Mater. 2014, 60, 42–47. [Google Scholar] [CrossRef]
- Hamid, S.A. The crystal structure of the 11Å natural tobermorite Ca2.25[Si3O7.5(OH)1.5] 1H2O. Z. Krist. Cryst. Mater. 1981, 154, 189–198. [Google Scholar] [CrossRef]
- Heinz, H.; Lin, T.-J.; Kishore Mishra, R.; Emami, F.S. Thermodynamically Consistent Force Fields for the Assembly of Inorganic, Organic, and Biological Nanostructures: The INTERFACE Force Field. Langmuir 2013, 29, 1754–1765. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohamed, A.K.; Geissbühler, D.; Manzano, H.; Jamil, T.; Shahsavari, R.; Kalinichev, A.G.; Galmarini, S.; Tao, L.; Heinz, H.; et al. cemff: A force field database for cementitious materials including validations, applications and opportunities. Cem. Concr. Res. 2017, 102, 68–89. [Google Scholar] [CrossRef]
- Fei, X. Thermodynamic Study on the Interaction between Aggressive Ions and C-S-H and C-A-S-H Gels in Harsh Environments. Master’s Thesis, Southeast University, Dhaka, Bangladesh, 2022. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, M. Microwave Heating of Water, Ice and Saline Solution: Molecular Dynamics Study. J. Chem. Phys. 2007, 126, 034509. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jia, G. Molecular dynamics simulation investigation of the microwave heating supercritical water. J. Mol. Liq. 2020, 297, 111440. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P. Monteiro, Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill: New York, NY, USA, 2014; Available online: https://cir.nii.ac.jp/crid/1130000797867624064 (accessed on 9 February 2025).
- Fan, D.Q.; Yu, R.; Shui, Z.H.; Wu, C.F.; Song, Q.L.; Liu, Z.J.; Sun, Y.; Gao, X.; He, Y.J. A new design approach of steel fibre reinforced ultra-high performance concrete composites: Experiments and modeling. Cem. Concr. Compos. 2020, 110, 103597. [Google Scholar] [CrossRef]
- He, R.; Lu, N. (Luna) Unveiling the dielectric property change of concrete during hardening process by ground penetrating radar with the antenna frequency of 1.6 GHz and 2.6 GHz. Cem. Concr. Compos. 2023, 144, 105279. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, R.; Shui, Z.; Liu, K. Hydration kinetics and microstructure development of Ultra-High Performance Concrete (UHPC) subjected to microwave pre-curing. Cem. Concr. Compos. 2022, 129, 104484. [Google Scholar] [CrossRef]
- Gallucci, E.; Zhang, X.; Scrivener, K.L. Effect of temperature on the microstructure of calcium silicate hydrate (C-S-H). Cem. Concr. Res. 2013, 53, 185–195. [Google Scholar] [CrossRef]



| Physical Indices | |
|---|---|
| Mesh size | 40–140 |
| Fineness (μm) | 109–380 |
| SiO2 (%) | ≥97 |
| Fe2O3 (%) | ≤0.1 |
| Apparent density (g/cm3) | 3.98 |
| Packing density (g/cm3) | 1.374 |
| Constituent | Mass (g) |
|---|---|
| Cement | 800 |
| Quartz sand | 880 |
| Silica fume | 201 |
| Water | 184 |
| PCE superplasticizer | 3 |
| Cell Parameter | Tobermorite11 Å | C-S-H, O(8)O(18) | C-S-H, O(8)O(14) |
|---|---|---|---|
| a/Å | 6.69 | 5.96 | 5.20 |
| b/Å | 7.39 | 6.91 | 6.80 |
| c/Å | 22.77 | 24.63 | 28.43 |
| α/° | 90.00 | 90.00 | 90.00 |
| β/° | 90.00 | 90.00 | 90.00 |
| γ/° | 123.46 | 125.278 | 131.31 |
| Total energy(kcal/mol) | — | −10,071.2 | −10,192.5 |
| Lengths (Å) | a | b | c |
|---|---|---|---|
| non-MW-293K | 13.44 | 61.50 | 45.25 |
| MW-293K | 13.36 | 61.13 | 44.97 |
| MW-313K | 13.36 | 61.14 | 44.98 |
| MW-333K | 13.39 | 61.20 | 45.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yu, K.; Li, S.; Liu, D. Exploring the Mechanism of Microstructural Changes in Ultra-High-Performance Concrete Under Microwave Influence: Experiments and Molecular Dynamics Simulation. Materials 2025, 18, 1892. https://doi.org/10.3390/ma18091892
Chen J, Yu K, Li S, Liu D. Exploring the Mechanism of Microstructural Changes in Ultra-High-Performance Concrete Under Microwave Influence: Experiments and Molecular Dynamics Simulation. Materials. 2025; 18(9):1892. https://doi.org/10.3390/ma18091892
Chicago/Turabian StyleChen, Jingyuan, Kunyang Yu, Shuangxin Li, and Dengao Liu. 2025. "Exploring the Mechanism of Microstructural Changes in Ultra-High-Performance Concrete Under Microwave Influence: Experiments and Molecular Dynamics Simulation" Materials 18, no. 9: 1892. https://doi.org/10.3390/ma18091892
APA StyleChen, J., Yu, K., Li, S., & Liu, D. (2025). Exploring the Mechanism of Microstructural Changes in Ultra-High-Performance Concrete Under Microwave Influence: Experiments and Molecular Dynamics Simulation. Materials, 18(9), 1892. https://doi.org/10.3390/ma18091892






