Structure Formation and Properties of Activated Supersulfate Cement
Abstract
:1. Introduction
2. Materials and Methods of Research
3. Results and Analysis of Test Results
3.1. Structure Formation of the SSC
3.2. Main Properties of Activated SSC
4. Conclusions
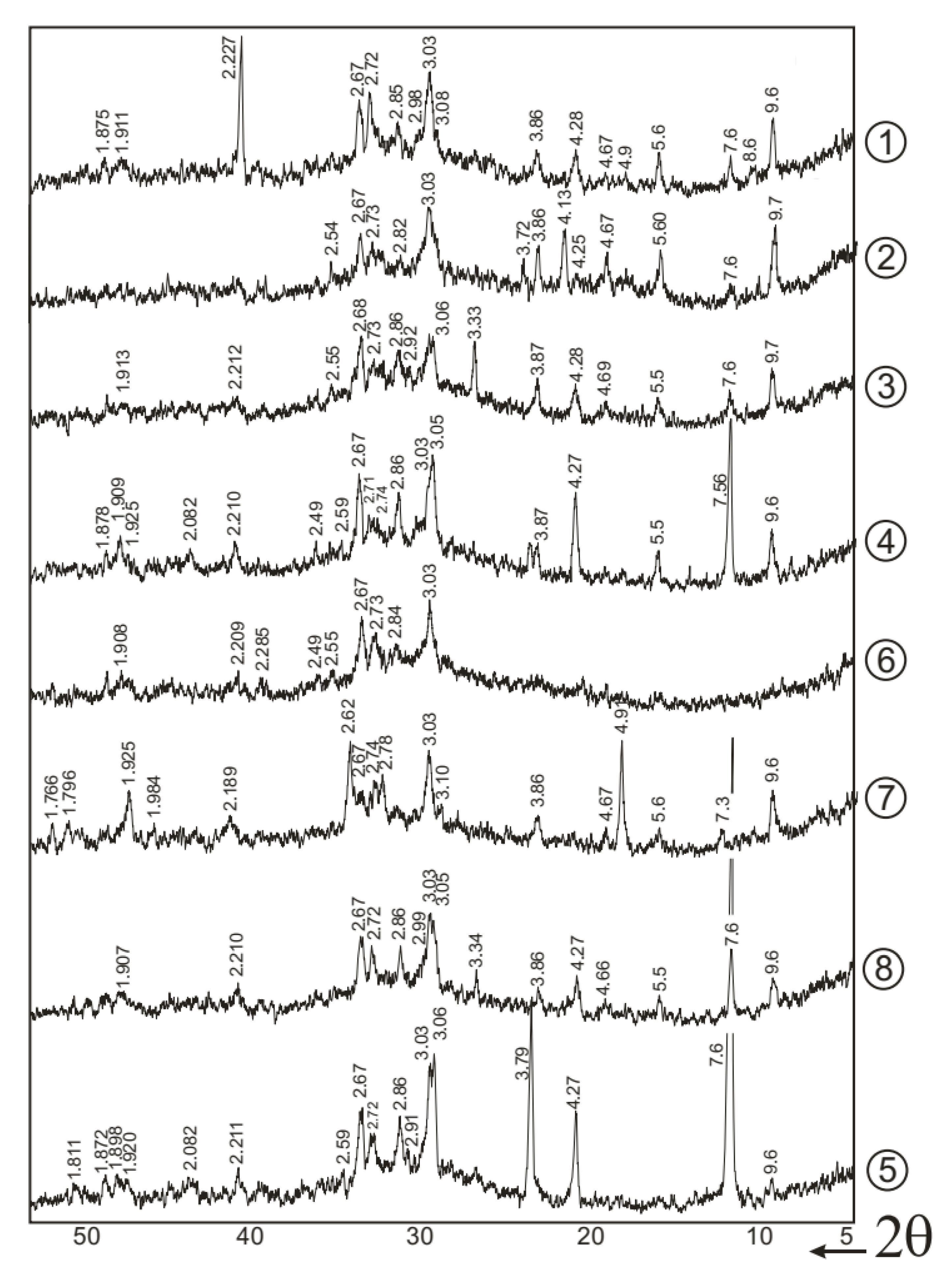
- The hardening process of SSCs based on low-alumina slag involves the formation of both low- and high-sulfate calcium aluminosilicate hydrates (CASH) and calcium hydrosilicates. CASH phases predominantly develop during the first 3–7 days, while calcium hydrosilicates form in the later stages of hydration.
- The use of a hardening accelerator in combination with a polycarboxylate-based superplasticizer significantly enhances the hydration kinetics of SSCs, resulting in notable increases in both early-age and 28-day compressive strength.
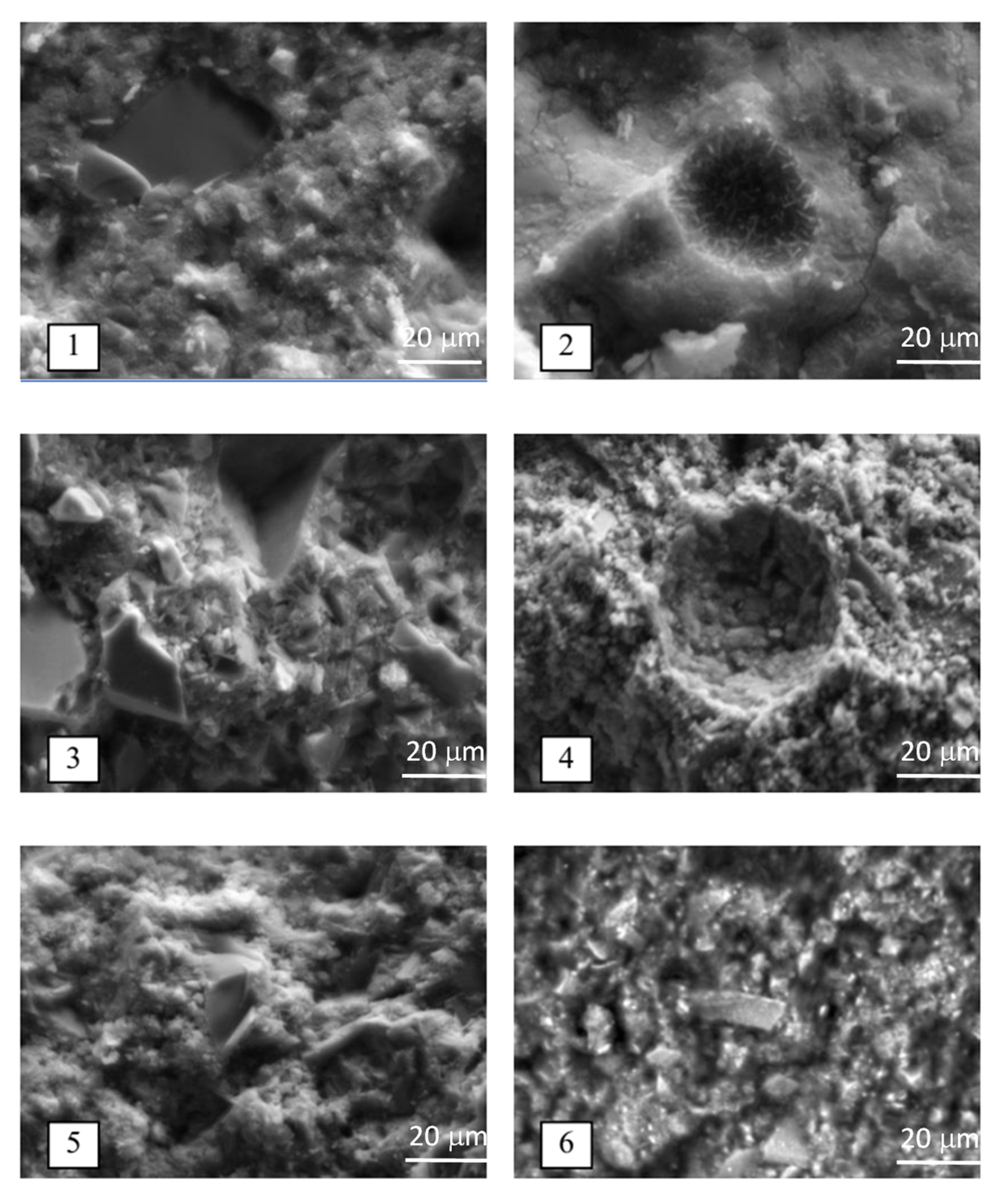
- The type of activator used in SSCs strongly influences the crystallinity and morphology of the hydration products. Over time, the quantity of needle-like and prismatic ettringite crystals increases, while the content of plate-like calcium sulfate dihydrate diminishes. The incorporation of superplasticizer and accelerator promotes the development of a fibrous microstructure dominated by low-basicity calcium hydrosilicates. However, increasing the phosphogypsum content beyond 10% leads to a greater amount of unbound calcium sulfate dihydrate.
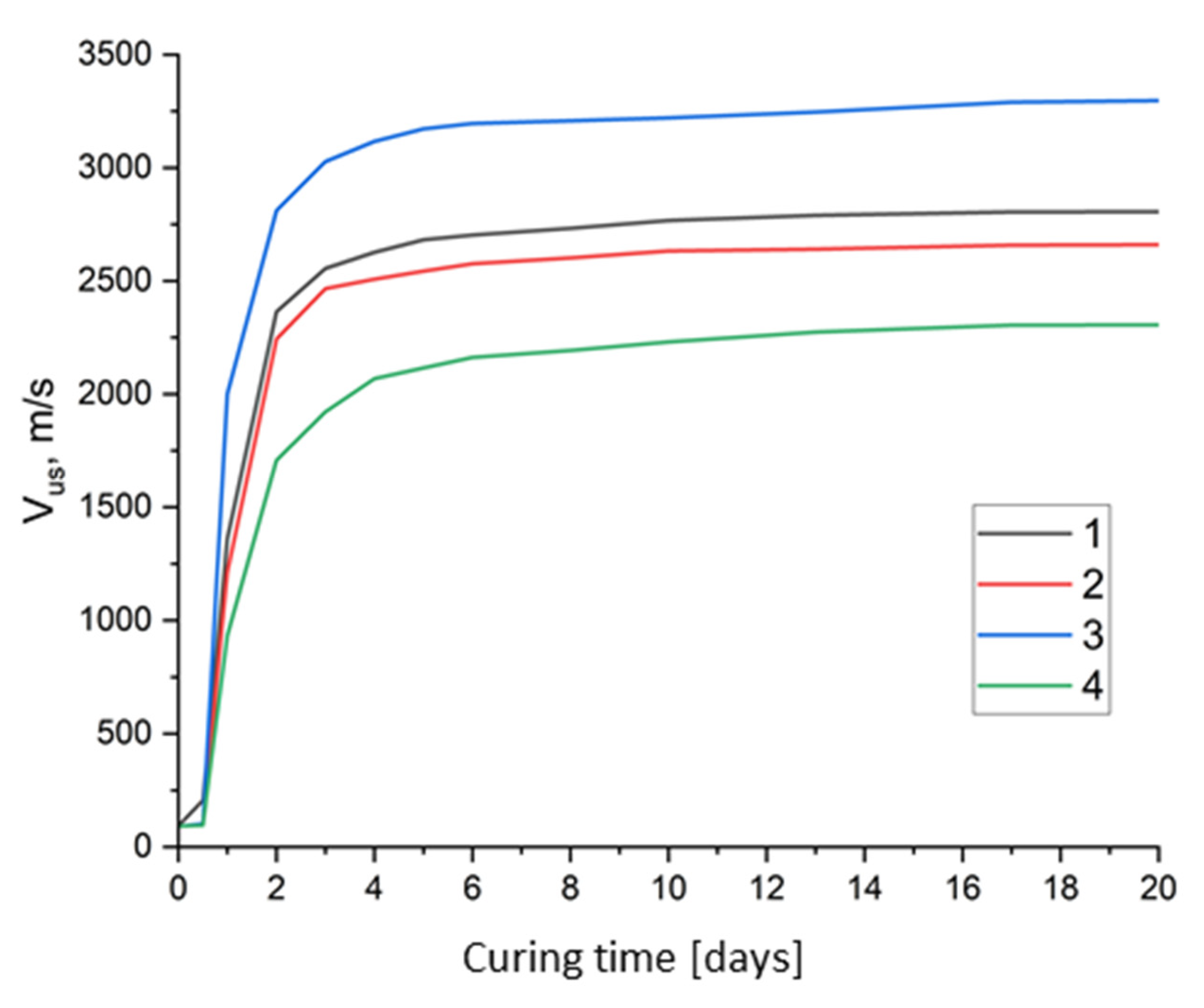
- Analysis of ultrasonic pulse velocity profiles reveals the key structural development stages of SSCs: an initial induction period, a growth phase of gel-like crystalline formations, and a final stage involving solid structure formation and recrystallization.
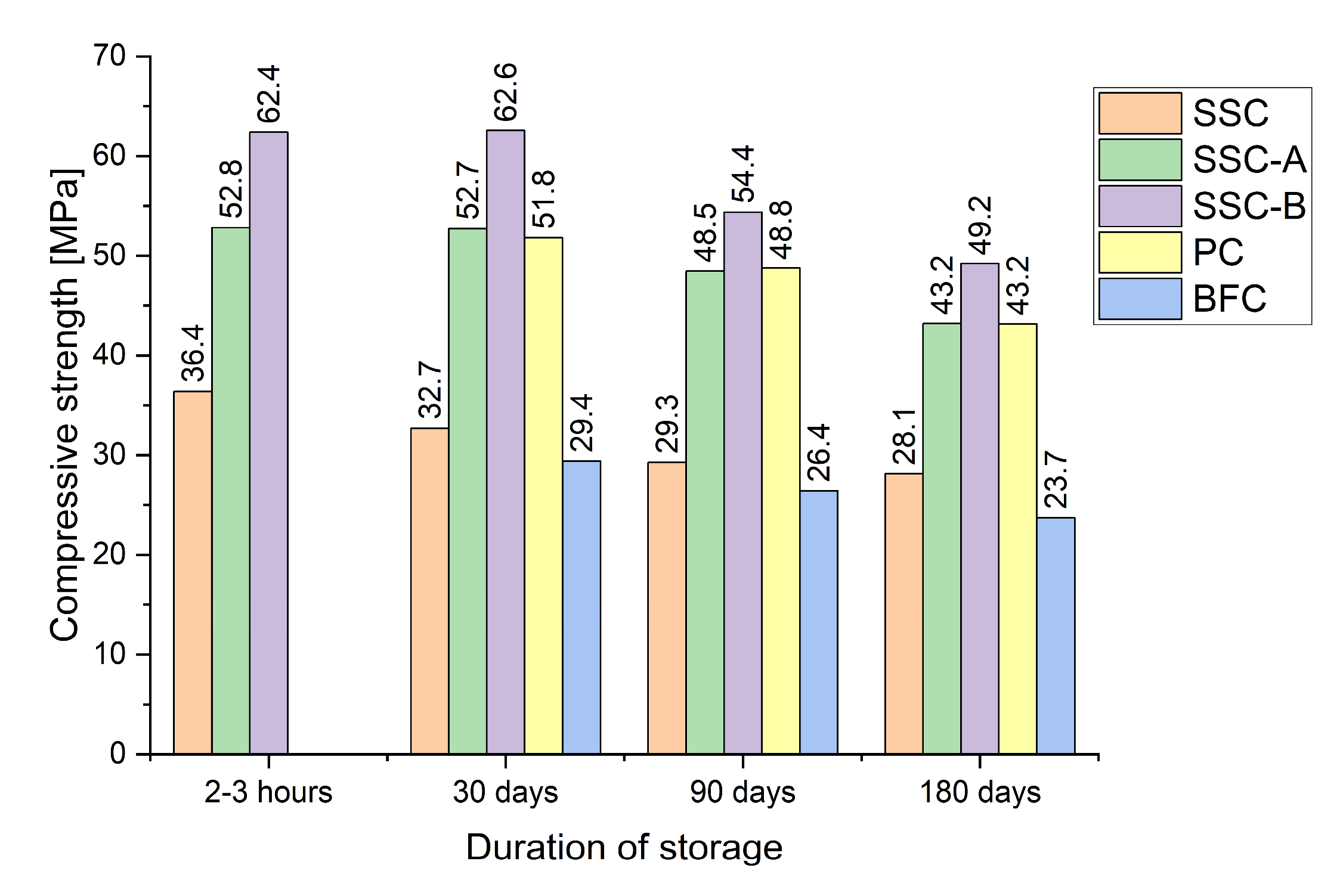
- Experimental data show that the rate of strength loss in SSCs during storage is composition-dependent. SSCs without accelerators or superplasticizers tend to lose strength more rapidly than Portland or slag-Portland cement. In contrast, SSCs modified with calcium chloride and polycarboxylate ether (PCE) retain their standard strength during the first 30 days of storage.
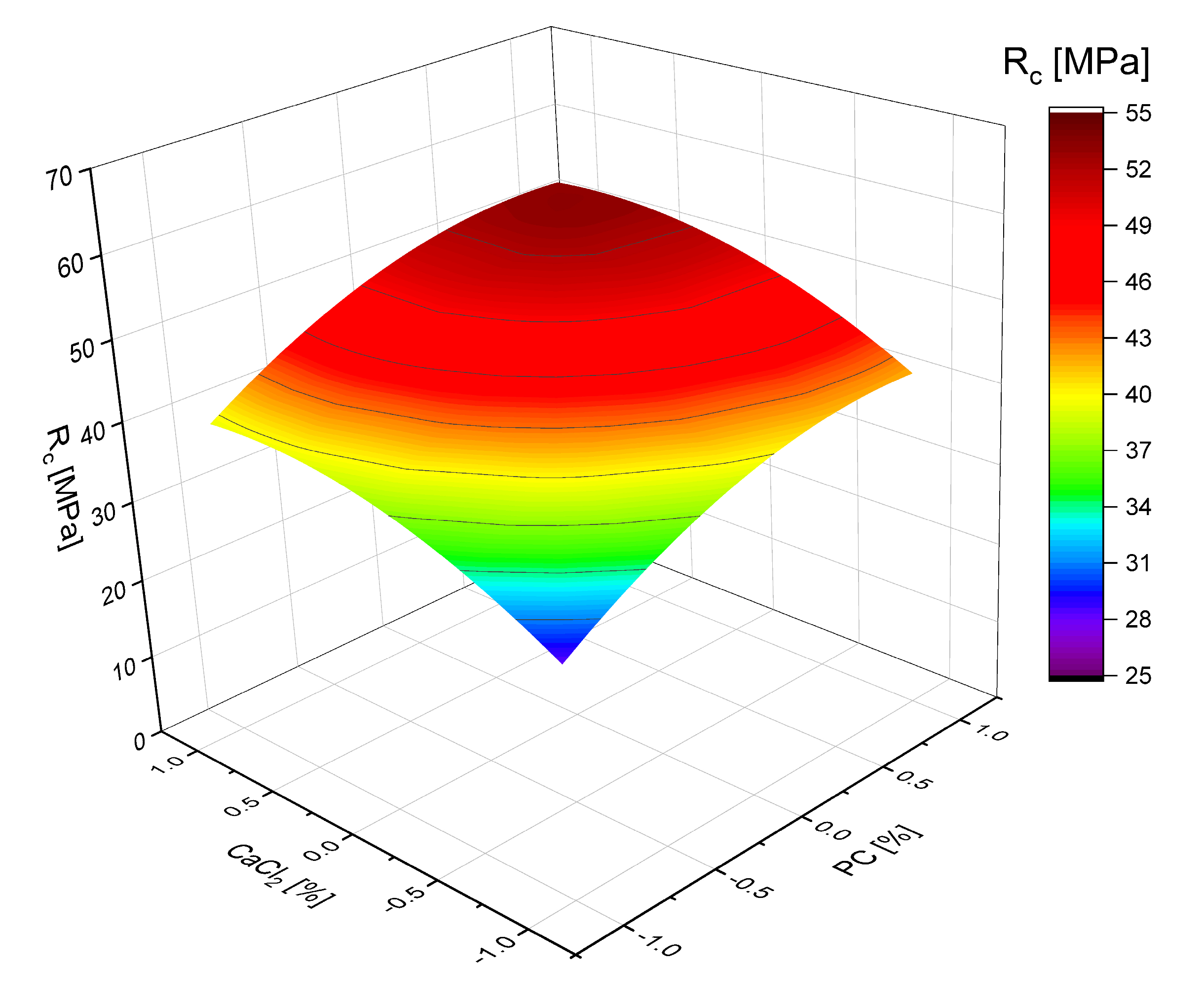
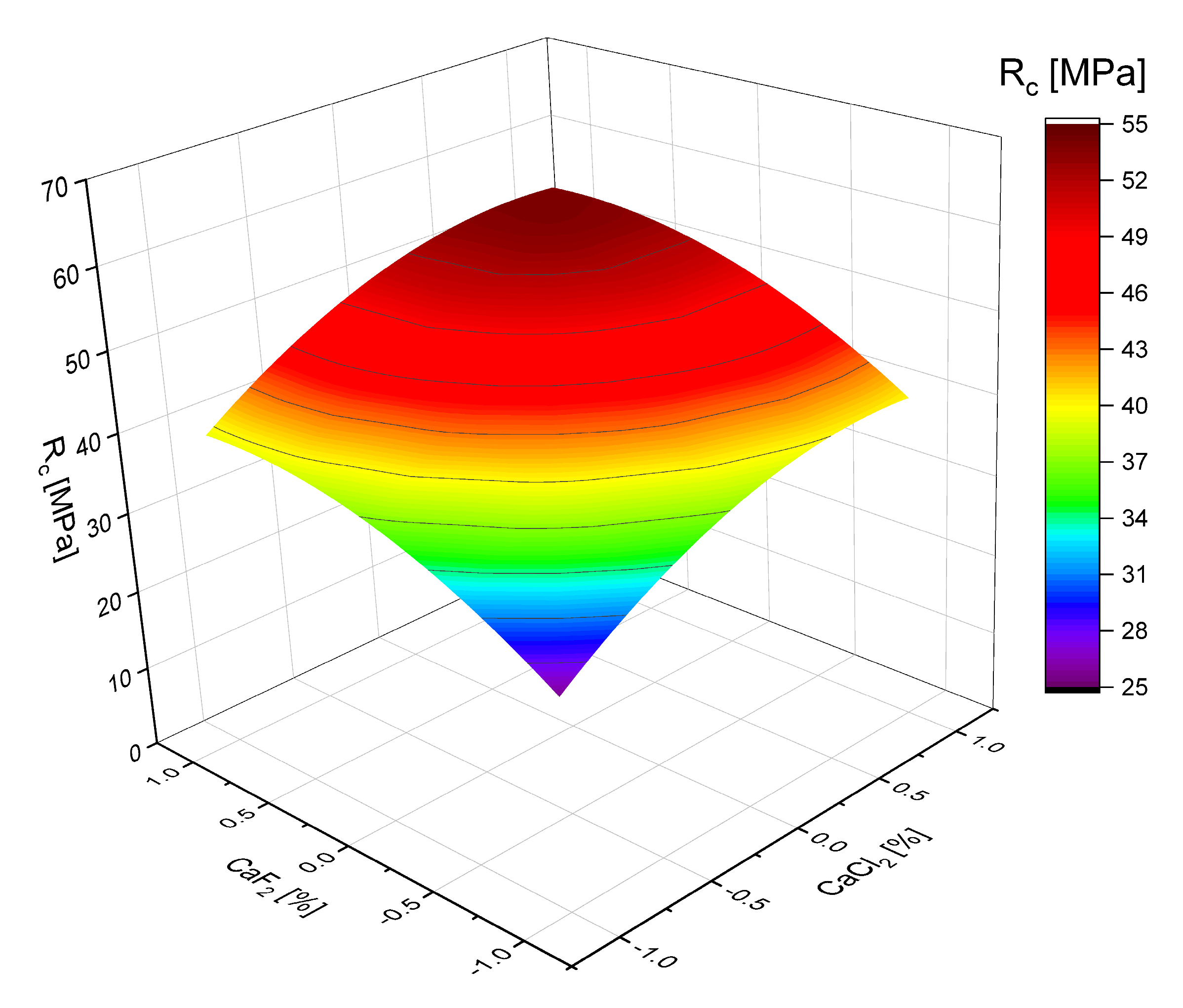
- Polynomial regression models developed for SSC compressive strength demonstrate the individual and synergistic effects of calcium chloride, PCE, and Portland cement content. These models confirm the positive contribution of these components to both compressive and flexural strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madlool, N.A.; Saidur, R.; Rahim, N.A.; Kamalisarvestani, M. An overview of energy savings measures for cement industries. Renew. Sustain. Energy Rev. 2013, 19, 18–29. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Xiang, Q.; Pan, H.; Ma, X.; Yang, M.; Lyu, Y.; Zhang, X.; Shui, W.; Liao, W.; Xiao, Y.; Wu, J.; et al. Impacts of energy-saving and emission-reduction on sustainability of cement production. Renew. Sustain. Energy Rev. 2024, 191, 114089. [Google Scholar] [CrossRef]
- Kühl, H. Cement and Process of Manufacturing the Same. U.S. Patent 900,939, 13 October 1908. Available online: https://patents.google.com/patent/US900939A/en (accessed on 12 April 2025).
- Svatovskaya, L.B.; Sychev, M.M. Aktivirovannoe Tverdenie Tsementov [Activated Hardening of Cements]; Stroiizdat: Leningrad, Russia, 1983; 160p. [Google Scholar]
- Budnikov, P.P. Khimija i Tekhnologija Silikatov [Chemistry and Technology of Silicates]; Academy of Sciences of the Ukrainian SSR: Kyiv, Ukraine, 1964; 612p. [Google Scholar]
- Erdem, E.; Ölmez, H. The mechanical properties of supersulphated cement containing phosphogypsum. Cem. Concr. Res. 1993, 23, 115–121. [Google Scholar] [CrossRef]
- Cai, T.; Hou, P.; Chen, H.; Zhao, P.; Du, P.; Wang, S.; Zhou, X.; Wang, X.; Cheng, X. Effects of nanosilica on supersulfated cements of different clinker-activation degree. Constr. Build. Mater. 2023, 365, 130118. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, T.; Shui, Z.; Ding, C.; Li, W. The impact of carbonation at different CO2 concentrations on the microstructure of phosphogypsum-based supersulfated cement paste. Constr. Build. Mater. 2022, 340, 127823. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Z.; Gao, F.; Li, X.; Chang, S.; Liu, S. Comparing study on the evolution characteristics of performance and micro-structure between Portland slag cement and supersulfated cement under chemical attacks. Constr. Build. Mater. 2024, 425, 135969. [Google Scholar] [CrossRef]
- Sun, Z.; Nie, S.; Zhou, J.; Li, H.; Chen, Z.; Xu, M.; Mu, R.; Wang, Y. Hydration mechanism of calcium sulfoaluminate-activated supersulfated cement. J. Clean. Prod. 2022, 333, 130094. [Google Scholar] [CrossRef]
- Dvorkin, L.Y.; Dvorkin, O.L.; Myronenko, A.V.; Polishchuk-Gerasimchuk, T.O.; Kundos, M.G. Modified Gypsum and Sulfate-Slag Binders and Materials Based on Them: Monograph; NUWM: Rivne, Ukraine, 2011; p. 188. [Google Scholar]
- EN 15743; Supersulfated Cement—Composition, Specification and Conformity Criteria. European Committee for Standardization: Brussels, Belgium, 2010.
- Wang, J.; Li, X.; Sun, R.; Zhao, Y.; Gong, F.; Huang, T.; Liu, Z.; Wang, D. Early-age carbonation mitigation of SSC by CxS minerals: Mechanism and performances. Constr. Build. Mater. 2024, 430, 136391. [Google Scholar] [CrossRef]
- Wu, Q.; Xue, Q.; Yu, Z. Research status of super sulfate cement. J. Clean. Prod. 2021, 294, 126228. [Google Scholar] [CrossRef]
- Zokaei, S.; Siad, H.; Lachemi, M.; Mahmoodi, O.; Ozcelikci, E.; Şahmaran, M. Engineered Cementitious Composites with Super-Sulfated Cement: Mechanical, Physical, and Durability Performance. Materials 2024, 17, 2240. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Gao, Y.; Yu, B.; Tang, W. Influence of fineness on hydration kinetics of supersulfated cement. Thermochim. Acta 2015, 605, 37–42. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Chen, H.; Li, Q.; Huang, Y.; Zhao, P.; Tchakouté, H.K.; Mapesu, P.; Hou, P.; Cheng, X. Improving the frost-resistance performance of supersulfated cement by reducing the crystalline-to-gel ratio through the addition of nano-SiO2. Constr. Build. Mater. 2024, 424, 135872. [Google Scholar] [CrossRef]
- Liao, Y.; Yao, J.; Deng, F.; Li, H.; Wang, K.; Tang, S. Hydration behavior and strength development of supersulfated cement prepared by calcined phosphogypsum and slaked lime. J. Build. Eng. 2023, 80, 108075. [Google Scholar] [CrossRef]
- Pinto, S.R.; da Luz, C.A.; Munhoz, G.S.; Medeiros-Junior, R.A. Durability of phosphogypsum-based supersulfated cement mortar against external attack by sodium and magnesium sulfate. Cem. Concr. Res. 2020, 136, 106172. [Google Scholar] [CrossRef]
- Masoudi, R.; Hooton, R. Examining the hydration mechanism of super-sulfated cements made with high and low-alumina slags. Cem. Concr. Compos. 2019, 103, 193–203. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Chen, L.; Huang, J.; Ma, T. The influence of two types of alkali activators on the microstructure and performance of supersulfated cement concrete: Mitigating the strength and carbonation resistance. Cem. Concr. Compos. 2021, 118, 103947. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Chang, T.P.; Shih, J.Y.; Chen, C.T. Influence of low calcium fly ash on compressive strength and hydration product of low energy super sulfated cement paste. Cem. Concr. Compos. 2019, 99, 40–48. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, Q.; Sun, Z. Effect of Calcium Aluminate and Carbide Slag on Mechanical Property and Hydration Mechanism of Supersulfated Cement. Buildings 2024, 14, 930. [Google Scholar] [CrossRef]
- Du, Y.; Chen, Q.; Wu, F.; Li, W.; Meng, L.; Liu, Y. Early Strength Enhancement Mechanism of CaO-Modified Electrolytic Manganese Residue-Based Supersulfate Cement. Materials 2025, 18, 270. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Winnefeld, F.; Figi, R.; Ko, S.-C.; Adler, M.; Mäder, U. Hydration mechanisms of super sulphated slag cement. Cem. Concr. Res. 2008, 38, 983–992. [Google Scholar] [CrossRef]
- Pashchenko, O.O.; Serbin, V.P.; Starchevska, O.O. Binders; Higher School: Kyiv, Ukraine, 1995; p. 416. [Google Scholar]
- EN 1015-11:2019; Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. Comité Européen de Normalisation: Brussels, Belgium, 2019.
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Discovery, and Innovation, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005; p. 655. [Google Scholar]
- Dvorkin, L.; Dvorkin, O.; Ribakov, Y. Mathematical Experiments Planning in Concrete Technology; Nova Science Publishers: New York, NY, USA, 2012; p. 172. [Google Scholar]
- Zhu, J.Y.; Sun, H.B. Monitoring hardening of concrete using ultrasonic guided waves. J. Acoust. Soc. Am. 2015, 138, 1885. [Google Scholar] [CrossRef]
- European Committee for Standardization (CEN). EN 196-3:2016-12; Methods of Testing Cement—Part 3: Determination of Setting Time and Soundness. CEN: Brussels, Belgium, 2016.
| Strength Class | Compressive Strength, MPa | Initial Setting Time, min | |||
|---|---|---|---|---|---|
| Early Strength | Standard Strength | ||||
| 2 Days | 7 Days | 28 Days | |||
| 32.5 L | - | ≥12.0 | ≥32.5 | ≤52.5 | ≥75 |
| 32.5 N | - | ≥16.0 | |||
| 42.5 L | - | ≥16.0 | ≥42.5 | ≤62.5 | ≥60 |
| 42.5 N | ≥10.0 | - | |||
| 52.5 L | ≥10.0 | - | ≥52.5 | - | ≥45 |
| 52.5 N | ≥20.0 | - | |||
| Oxide Content in Slag, wt. % | ||||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | MnO | LOI | Σ, % |
| 39.51 | 6.47 | 0.14 | 47.19 | 3.12 | 1.76 | 1.14 | 0.59 | 99.92 |
| Gypsum (Stone) (GS) | ||||||||
|---|---|---|---|---|---|---|---|---|
| LOI | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | CaSO4·2H2O | |
| 17.87 | 8.54 | 0.70 | 0.4 | 29.88 | 0.41 | 41.85 | 89.97 | |
| Phosphogypsum (PG) | ||||||||
| CaO | SO3 | P2O5 total | P2O5 water-soluble. | Fe2O3 | Al2O3 | F | MgO | Cl |
| 38.3 | 59.1 | 0.69 | 0.04 | 0.16 | 0.34 | 0.14 | 0.004 | 0.01 |
| No. | SSC Composition,% | Hardening Duration, Days | |||
|---|---|---|---|---|---|
| BFGS | Sulfate Component | Portland Cement | Additive | ||
| 1 | 90 | PG-10 | PC-5 | - | 7 |
| 2 | 90 | PG-10 | PC-5 | - | 180 |
| 3 | 90 | PG-10 | PC-5 | PCE—0.4%, CaCl2–2% | 7 |
| 4 | 85 | PG-15 | PC-5 | - | 7 |
| 5 | 80 | PG-20 | PC-5 | - | 7 |
| 6 | 90 | GS-10 | PC-5 | - | 7 |
| 7 | BFC | 7 | |||
| 8 | 80 | GS-20 | PC-5 | - | 180 |
| SSC Composition,% | Admixture, % | Standard Consistence, % | Setting Time, h-min | |||
|---|---|---|---|---|---|---|
| BFGS | Sulfate Component | Alkaline Component | ||||
| 85 | PG-10 | PC-5 | - | 26 | 3–10 | 7–40 |
| 85 | GS-10 | PC-5 | - | 26 | 3–30 | 7–50 |
| 88 | PG-10 | Lime-2 | - | 26 | 3–50 | 8–10 |
| 88 | GS-10 | Lime-2 | - | 26 | 3–50 | 8–20 |
| 85 | PG-10 | PC-5 | PCE—0.2% | 24 | 4–20 | 7–40 |
| 85 | PG-10 | PC-5 | PCE—0.4% | 22 | 4–40 | 7–10 |
| 85 | FG-10 | PC-5 | PCE—0.4% + CaCl2–2% | 22 | 3–20 | 6–50 |
| 85 | GS-10 | PC-5 | PCE—0.4% | 22 | 4–50 | 8–20 |
| Type of Binder | Additive | Compressive Strength After 28 Days with the Duration of Previous Storage of the Binder | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2–3 h | 30 Days | 90 Days | 180 Days | ||||||
| Mean | Standard Deviation (SD) | Mean | SD | Mean | SD | Mean | SD | ||
| SSC | - | 36.4 | 1.6 | 32.7 | 1.5 | 29.3 | 1.4 | 28.1 | 1 |
| SSC-A | PCE-0.4% | 52.8 | 1.4 | 52.7 | 1.8 | 48.5 | 2.0 | 43. 2 | 2.1 |
| SSC-B | PCE-0.6% CaCl2-2% | 62.4 | 1.4 | 62.6 | 1.1 | 54.4 | 1.6 | 49.2 | 1.5 |
| PC | - | - | - | 51.8 | 1.8 | 48.8 | 1.6 | 43.2 | 2.0 |
| BFC | - | - | - | 29.4 | 1.1 | 26.4 | 1.2 | 23.7 | 1.6 |
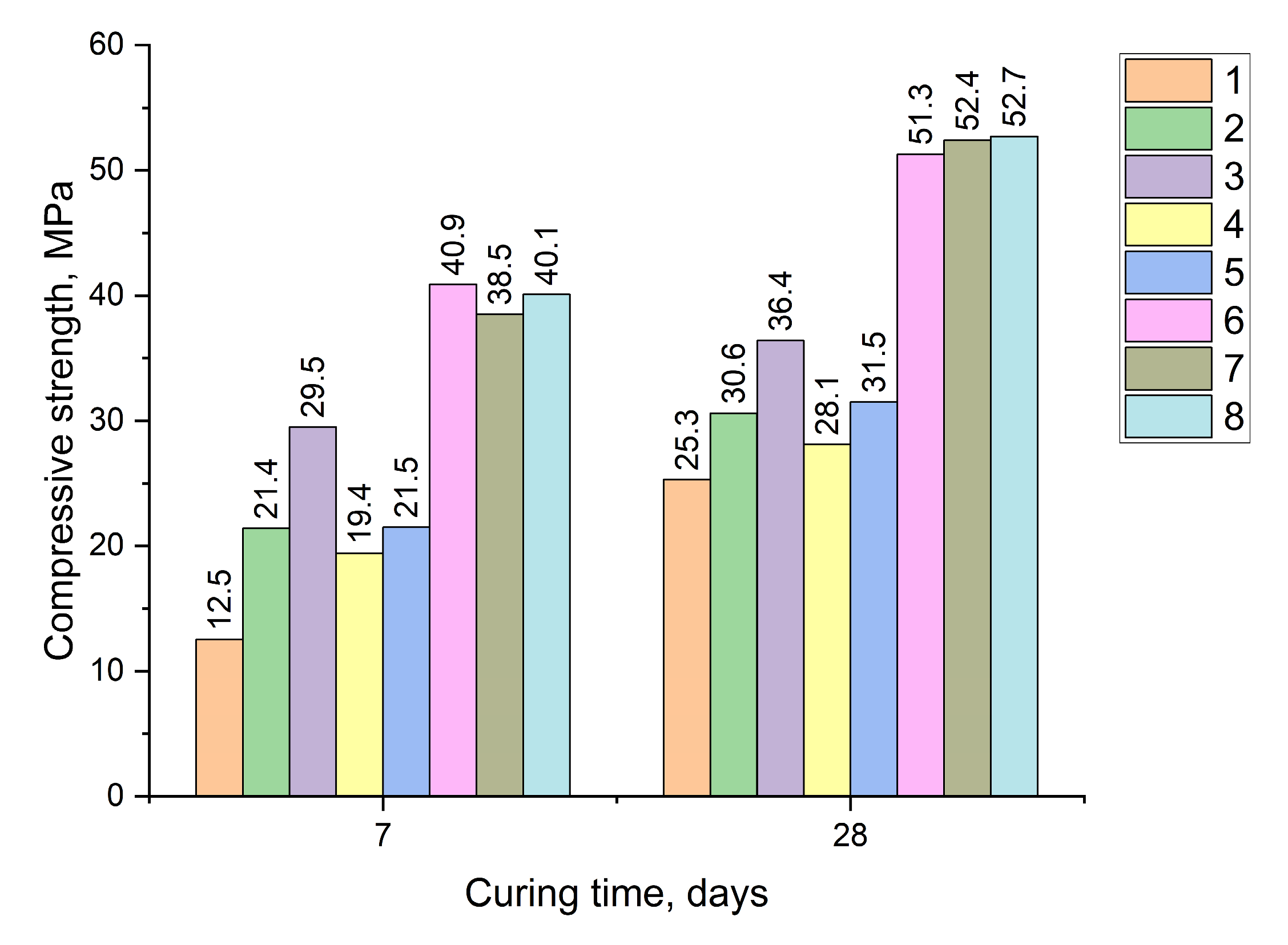
| # | SCC Composition, % | Specific Surface Area, m2/kg | Type/Content (%) of Modifying Admixtures | Compressive Strength, MPa | |||||
|---|---|---|---|---|---|---|---|---|---|
| BFGS | Sulfate Activator | PC | 7 Days | 28 Days | |||||
| Mean | SD | Mean | SD | ||||||
| 1 | 80 | PG/15 | 5 | 390 | - | 12.5 | 1.1 | 25.3 | 1.8 |
| 2 | 80 | PG/15 | 5 | 615 | - | 21.4 | 2.3 | 30.6 | 1.8 |
| 3 | 85 | PG/10 | 5 | 615 | - | 29.5 | 1.6 | 36.4 | 1.4 |
| 4 | 90 | PG/5 | 5 | 610 | - | 19.4 | 1.4 | 28.1 | 2.2 |
| 5 | 90 | GC/10 | 5 | 620 | 21.5 | 1.4 | 31.5 | 1.3 | |
| 6 | 90 | PG/10 | 5 | 610 | CaCl2/2 | 40.9 | 1.8 | 51.3 | 1.3 |
| 7 | 90 | PG/10 | 5 | 615 | CaCl2/2 | 38.5 | 2.0 | 52.4 | 1.4 |
| 8 | 90 | PG/10 | 5 | 610 | PCE/0.4 | 40.1 | 1.7 | 52.7 | 1.7 |
| Technological Factors | Levels of Variation | Variation Interval | |||
|---|---|---|---|---|---|
| Natural View | Coded View | −1 | 0 | +1 | |
| PC content, % | x1 | 1.0 | 2.0 | 3.0 | 1.0 |
| Content of CaCl2, % | x2 | 0 | 1.0 | 2.0 | 1.0 |
| Content of CaF2, % | x3 | 0 | 1.0 | 2.0 | 1.0 |
| Coded Factor Values | Strength, MPa | |||||
|---|---|---|---|---|---|---|
| x1 | x2 | x3 | Bending (Rb) | Compressive (Rc) | ||
| Mean | SD | Mean | SD | |||
| +1 | +1 | +1 | 11.52 | 0.21 | 59.44 | 0.99 |
| +1 | +1 | −1 | 8.51 | 0.11 | 39.58 | 1.92 |
| +1 | −1 | +1 | 8.93 | 0.38 | 40.62 | 1.53 |
| +1 | −1 | −1 | 7.50 | 0.95 | 26.39 | 2.03 |
| −1 | +1 | +1 | 9.57 | 0.46 | 43.27 | 1.64 |
| −1 | +1 | −1 | 7.73 | 0.85 | 28.58 | 1.11 |
| −1 | −1 | +1 | 7.76 | 0.41 | 29.24 | 1.86 |
| −1 | −1 | −1 | 7.50 | 1.01 | 20.20 | 1.51 |
| +1 | 0 | 0 | 9.97 | 0.66 | 50.23 | 1.55 |
| −1 | 0 | 0 | 8.91 | 0.18 | 39.01 | 1.81 |
| 0 | +1 | 0 | 10.04 | 0.51 | 51.09 | 1.8 |
| 0 | −1 | 0 | 8.61 | 0.22 | 37.58 | 1.83 |
| 0 | 0 | +1 | 10.12 | 0.32 | 51.00 | 1.62 |
| 0 | 0 | −1 | 8.51 | 0.81 | 36.57 | 1.98 |
| 0 | 0 | 0 | 9.70 | 0.33 | 48.43 | 2.05 |
| 0 | 0 | 0 | 9.66 | 0.48 | 48.37 | 1.56 |
| 0 | 0 | 0 | 9.74 | 0.71 | 48.41 | 1.77 |
| Output Parameters | Regression Equation (Confidence Probability (Importance Level) of 95%) |
|---|---|
| Bending strength SSC at 28 days, MPa | (5) |
| Standard deviation: 0.724 Mean quadratic errors: 0.256 Criterion of Fisher (calculated): 2.83 | |
| Compressive strength of SSC at 28 days, MPa | (6) |
| Standard deviation: 1.313 Mean quadratic errors: 0.464 Criterion of Fisher (calculated): 3.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvorkin, L.; Zhitkovsky, V.; Hager, I.; Tracz, T.; Zdeb, T. Structure Formation and Properties of Activated Supersulfate Cement. Materials 2025, 18, 1912. https://doi.org/10.3390/ma18091912
Dvorkin L, Zhitkovsky V, Hager I, Tracz T, Zdeb T. Structure Formation and Properties of Activated Supersulfate Cement. Materials. 2025; 18(9):1912. https://doi.org/10.3390/ma18091912
Chicago/Turabian StyleDvorkin, Leonid, Vadim Zhitkovsky, Izabela Hager, Tomasz Tracz, and Tomasz Zdeb. 2025. "Structure Formation and Properties of Activated Supersulfate Cement" Materials 18, no. 9: 1912. https://doi.org/10.3390/ma18091912
APA StyleDvorkin, L., Zhitkovsky, V., Hager, I., Tracz, T., & Zdeb, T. (2025). Structure Formation and Properties of Activated Supersulfate Cement. Materials, 18(9), 1912. https://doi.org/10.3390/ma18091912








