Study on Mass and Performance Deterioration of Concrete Under Multiple Corrosive Environments
Abstract
:1. Introduction
2. Experiment
2.1. Sample Preparation
2.1.1. Raw Materials
2.1.2. Mix Proportions
2.2. Experiment Methods
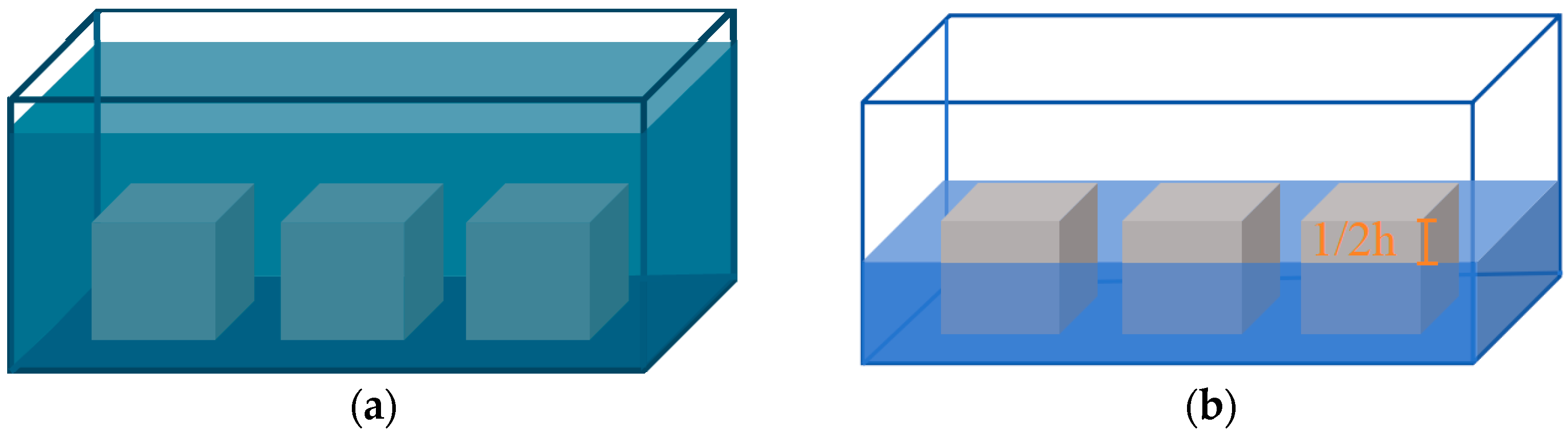
2.2.1. Corrosion Test Scheme
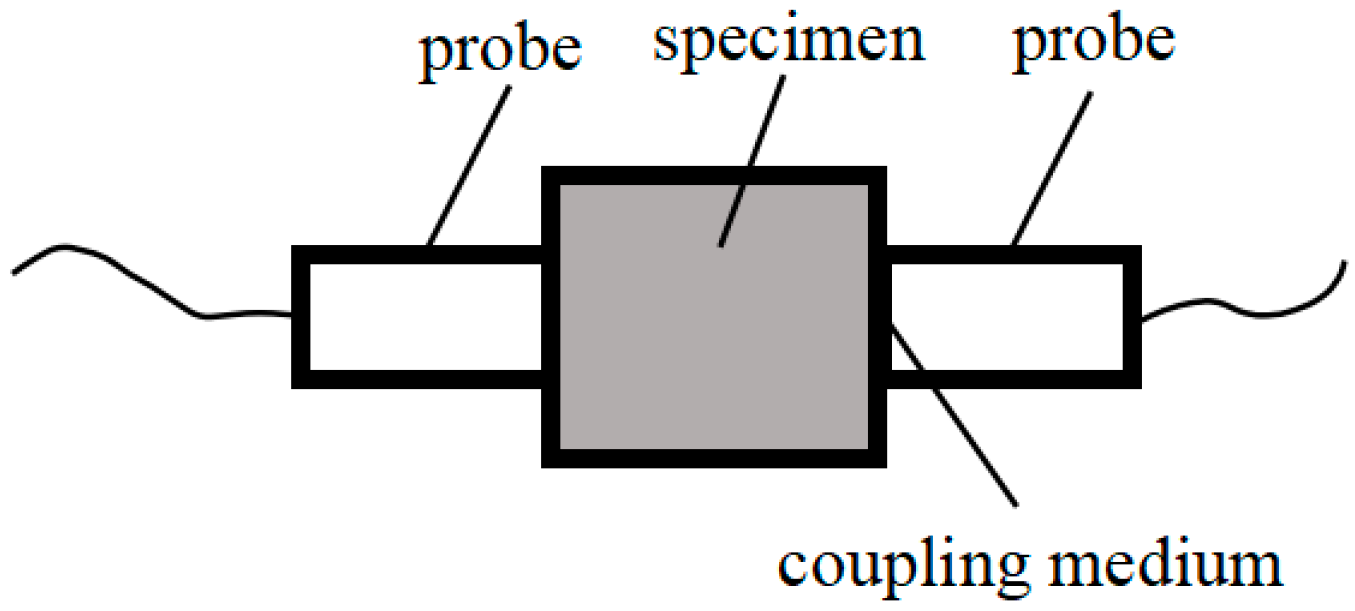
2.2.2. Experimental Testing Methods
- (1)
- Mass loss
- (2)
- Relative dynamic elastic modulus
3. Results and Discussion
3.1. Chemical Corrosion
3.1.1. Mass Change Under Chemical Corrosion Conditions
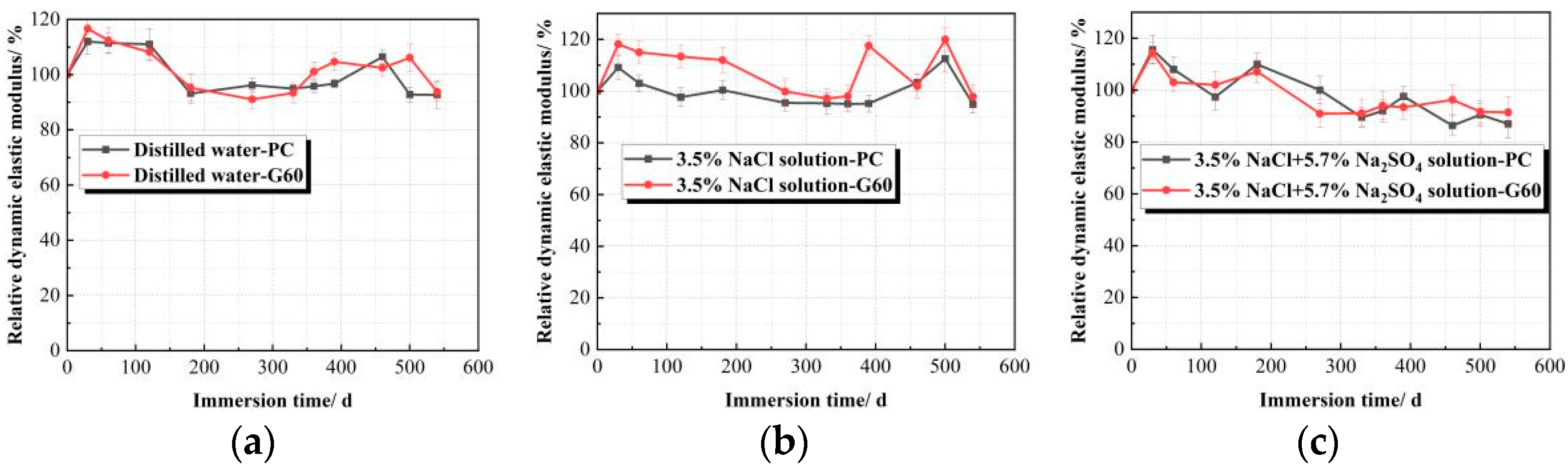
3.1.2. Relative Dynamic Elastic Modulus (Erd) Variation Under Chemical Corrosion Conditions
3.2. Salt Crystallization Corrosion Under Dry–Wet Cycling
3.2.1. Mass Changes Under Salt Crystallization Conditions
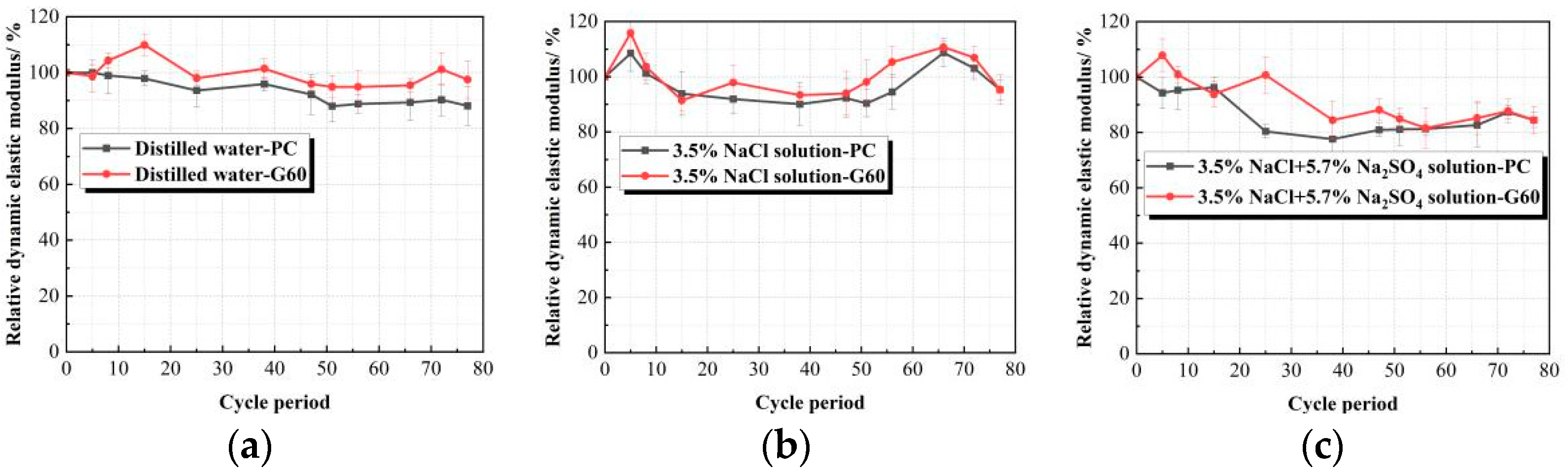
3.2.2. Relative Dynamic Elastic Modulus (Erd) Variation Under Salt Crystallization Conditions
4. Conclusions
- (1)
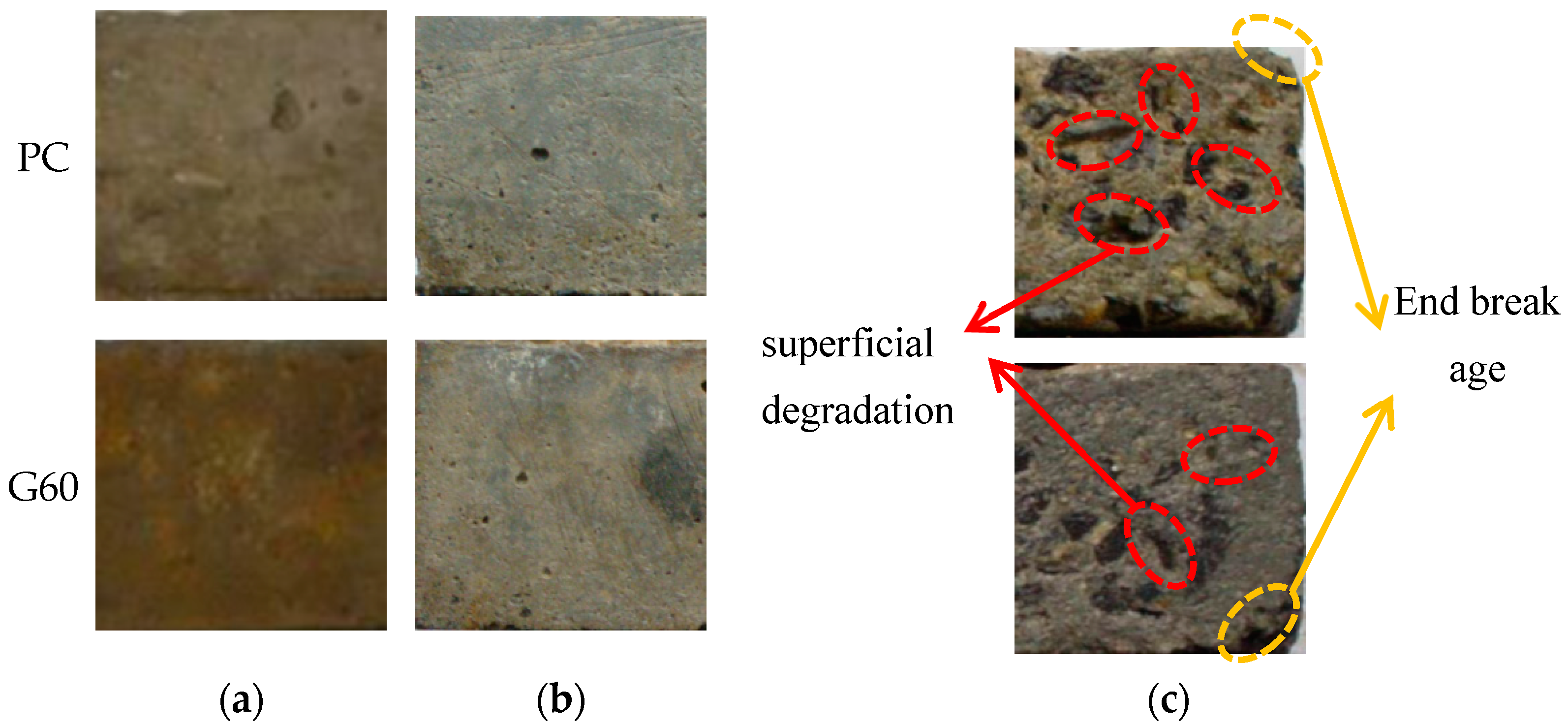
- Concrete specimens immersed in distilled water and 3.5% NaCl solution for 540 days showed almost no significant damage, with no obvious mass loss or reduction in the relative dynamic elastic modulus. However, in the composite solution, both PC and G60 exhibited significant spalling, with mass losses of 1.266% and 1.403%, and the relative dynamic elastic modulus decreased to 86.992% and 91.380%.
- (2)
- Chloride salt crystallization corrosion only caused mild surface spalling damage. After dry–wet cycling, the specimens’ surfaces showed whitening and powdering phenomena, but both mass and relative dynamic elastic modulus were well maintained. Under composite solution salt crystallization corrosion conditions, both types of concrete exhibited severe surface spalling, resulting in more mass loss and a greater reduction in relative dynamic elastic modulus.
- (3)
- Compared to solution corrosion, dry–wet cycling conditions accelerated the erosion of concrete by the composite solution, leading to greater mass loss and reduction in dynamic elastic modulus. The incorporation of slag improves concrete’s ability to resist ion corrosion in solution corrosion and salt crystallization corrosion, but the effect gradually diminishes with extended soaking time or increased cycling number.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Li, J.; Ren, Q.; Zheng, Q.; Jiang, Z. Durability of concrete coupled with life cycle assessment: Review and perspective. Cem. Concr. Compos. 2023, 139, 105041. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Wang, Q.; Shah, S.P. Effects of nano-kaolinite clay on the freeze–thaw resistance of concrete. Cem. Concr. Compos. 2015, 62, 1–12. [Google Scholar] [CrossRef]
- Rosenqvist, M.; Bertron, A.; Fridh, K.; Hassanzadeh, M. Concrete alteration due to 55 years of exposure to river water: Chemical and mineralogical characterisation. Cem. Concr. Res. 2017, 92, 110–120. [Google Scholar] [CrossRef]
- Ayora, C.; Chinchón, S.; Aguado, A.; Guirado, F. Weathering of iron sulfides and concrete alteration: Thermodynamic model and observation in dams from Central Pyrenees, Spain. Cem. Concr. Res. 1998, 28, 1223–1235. [Google Scholar] [CrossRef]
- Araujo, G.S.; Chinchón, S.; Aguado, A. Evaluation of the behavior of concrete gravity dams suffering from internal sulfate attack. Rev. Ibracon Estrut. Mater. 2008, 1, 84–112. [Google Scholar]
- Leemann, A.; Lothenbach, B.; Münch, B.; Campbell, T.; Dunlop, P. The “mica crisis” in Donegal, Ireland–A case of internal sulfate attack? Cem. Concr. Res. 2023, 168, 107149. [Google Scholar] [CrossRef]
- Brough, C.; Staniforth, B.; Garner, C.; Garside, R.; Colville, R.; Strongman, J.; Fletcher, J. High risk concrete blocks from County Donegal: The geology of defective aggregate and the wider implications. Constr. Build. Mater. 2023, 408, 133404. [Google Scholar] [CrossRef]
- Jana, D. Cracking of residential concrete foundations in eastern Connecticut, USA from oxidation of pyrrhotite. Case Stud. Constr. Mater. 2022, 16, e00909. [Google Scholar]
- Duchesne, J.; Rodrigues, A.; Fournier, B. Concrete damage due to oxidation of pyrrhotite-bearing aggregate: A review. RILEM Tech. Lett. 2021, 6, 82–92. [Google Scholar] [CrossRef]
- Ikumi, T.; Segura, I.; Cavalaro, S.H. Effects of biaxial confinement in mortars exposed to external sulfate attack. Cem. Concr. Compos. 2019, 95, 111–127. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Effects of gypsum formation on the performance of cement mortars during external sulfate attack. Cem. Concr. Res. 2003, 33, 325–332. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 1: Summary of experimental results. Cem. Concr. Res. 2002, 32, 915–921. [Google Scholar] [CrossRef]
- Gollop, R.S.; Taylor, H.F.W. Microstructural and microanalytical studies of sulfate attack III. Sulfate-resisting portland cement: Reactions with sodium and magnesium sulfate solutions. Cem. Concr. Res. 1995, 25, 1581–1590. [Google Scholar] [CrossRef]
- Yao, M.B.; Li, J.P. Distribution behavior of sulfate erosion in concrete piles under water pressure. Tongji Univ. 2019, 47, 1131–1136. [Google Scholar]
- Gao, J.; Yu, Z.; Song, L.; Wang, T.; Wei, S. Durability of concrete exposed to sulfate attack under flexural loading and drying–wetting cycles. Constr. Build. Mater. 2013, 39, 33–38. [Google Scholar] [CrossRef]
- Wang, Z.P.; Zhao, Y.T.; Yang, H.Y.; Xu, L.L. Research progress on resistance of calcium aluminate cement to seawater erosion. New Build. Mater. 2018, 45, 7–11. [Google Scholar]
- Ragoug, R.; Metalssi, O.O.; Barberon, F.; Torrenti, J.-M.; Roussel, N.; Divet, L.; de Lacaillerie, J.-B.D. Durability of cement pastes exposed to external sulfate attack and leaching: Physical and chemical aspects. Cem. Concr. Res. 2019, 116, 134–145. [Google Scholar] [CrossRef]
- Yin, G.-J.; Zuo, X.-B.; Sun, X.-H.; Tang, Y.-J. Macro-microscopically numerical analysis on expansion response of hardened cement paste under external sulfate attack. Constr. Build. Mater. 2019, 207, 600–615. [Google Scholar] [CrossRef]
- Nie, L.; Xu, J.; Bai, E. Dynamic stress-strain relationship of concrete subjected to chloride and sulfate attack. Constr. Build. Mater. 2018, 165, 232–240. [Google Scholar] [CrossRef]
- Chiker, T.; Aggoun, S.; Houari, H.; Siddique, R. Sodium sulfate and alternative combined sulfate/chloride action on ordinary and self-consolidating PLC-based concretes. Constr. Build. Mater. 2016, 106, 342–348. [Google Scholar] [CrossRef]
- Du, J.; Tang, Z.; Li, G.; Yang, H.; Li, L. Key inhibitory mechanism of external chloride ions on concrete sulfate attack. Constr. Build. Mater. 2019, 225, 611–619. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, W.; Feng, Y.; Castel, A.; Chen, X.; Liu, A. On the competitive antagonism effect in combined chloride-sulfate attack: A numerical exploration. Cem. Concr. Res. 2021, 144, 106406. [Google Scholar] [CrossRef]
- Wang, P.G.; Mo, R.; Sui, X.M.; Tian, S.; Xu, J.; Jin, Z.Q. Chemo-damage-transport model of combined chloride-sulfate attack in concrete. J. Chin. Ceram. Soc. 2022, 50, 512–521. [Google Scholar]
- Guo, J.J.; Liu, P.Q.; Wu, C.L.; Wang, K. Effect of dry–wet cycle periods on properties of concrete under sulfate attack. Appl. Sci. 2021, 11, 888. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, C.; Liu, H.; Zhang, W.; Hu, T. Deterioration mechanism of recycled composite powder concrete under dry-wet cycles of sulfate. J. Build. Mater. 2023, 388, 131718. [Google Scholar] [CrossRef]
- Metha, P.K.; Monteiro, P.J. Concrete, Structure, ProPerties and Materials, 3rd ed.; MeGraw-Hill: Englewood CliffS, NJ, USA, 2006. [Google Scholar]
- Thaulow, N.; Sahu, S. Mechanism of concrete deterioration due to salt crystallization. Mater. Charact. 2004, 53, 123–127. [Google Scholar] [CrossRef]
- Seherer, G.W. Factors affecting crystallization pressure. Intenational sulphate attaek. In Proceedings of the RILEM Workshop on Delayed Ettringite Formation, Villars, Switzerland, 4–6 September 2002. [Google Scholar]
- Tsui, N.; Flatt, R.J.; Seherer, G.W. Crystallization damage by sodium sulfate. J. Cult. Herit. 2003, 4, 109–115. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, Q. Effects of salt-crystallization of sodium sulfate on deterioration of concrete. J. Chin. Ceram. Soc. 2007, 35, 877. [Google Scholar]
- Wang, X.; Tian, Z.; Zhang, Y.; Su, X.; Liu, H.; Liu, T. Research on Corrosion Effect of Sulfate Ions on Concrete Under Wetting-Drying Cycle. J. Ocean Univ. China 2022, 21, 124–130. [Google Scholar] [CrossRef]
- Su, X.P.; Guo, J.H. Study on the Durability of Normal Strength Concrete under theAction of Salt Corrosion and Dry-Wet Cycles. Adv. Mater. Res. 2014, 919, 1849–1852. [Google Scholar] [CrossRef]
- Kuo, W.T.; Wang, H.Y.; Shu, C.Y. Engineering properties of cementless concrete produced from GGBFS and recycled desulfurization slag. Constr. Build. Mater. 2014, 63, 189–196. [Google Scholar]
- Hadjsadok, A.; Kenai, S.; Courard, L.; Michel, F.; Khatib, J. Durability of mortar and concretes containing slag with low hydraulic activity. Cem. Concr. Compos. 2012, 34, 671–677. [Google Scholar] [CrossRef]
- Singh, M.; Siddique, R. Compressive strength, drying shrinkage and chemical resistance of concrete incorporating coal bottom ash as partial or total replacement of sand. Constr. Build. Mater. 2014, 68, 39–48. [Google Scholar] [CrossRef]
- Chi, M.; Huang, R. Effect of circulating fluidized bed combustion ash on the properties of roller compacted concrete. Cem. Concr. Compos. 2014, 45, 148–156. [Google Scholar] [CrossRef]
- Kandasamy, S.; Shehata, M.H. Durability of ternary blends containing high calcium fly ash and slag against sodium sulphate attack. Constr. Build. Mater. 2014, 53, 267–272. [Google Scholar] [CrossRef]
- Duran, A.; Navarro-Blasco, I.; Fernández, J.; Alvarez, J. Long-term mechanical resistance and durability of air lime mortars with large additions of nanosilica. Constr. Build. Mater. 2014, 58, 147–158. [Google Scholar]
- Nielsen, P.; Nicolai, S.; Darimont, A.; Kestemont, X. Influence of cement and aggregate type on thaumasite formation in concrete. Cem. Concr. Compos. 2014, 53, 115–126. [Google Scholar] [CrossRef]
- Bentz, D.P.; Davis, J.M.; Peltz, M.A.; Snyder, K.A. Influence of internal curing and viscosity modifiers on resistance to sulfate attack. Mater. Struct. 2013, 47, 581–589. [Google Scholar] [CrossRef]
- Carsana, M.; Frassoni, M.; Bertolini, L. Comparison of ground waste glass with other supplementary cementitious materials. Cem. Concr. Compos. 2014, 45, 39–45. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zeng, H.; Wu, J.Y. A study on the macro and micro properties of concrete with LCD glass. Constr. Build. Mater. 2014, 50, 664–670. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.; Blanco-Varela, M. Use of barium carbonate to inhibit sulfate attack in cements. Cem. Concr. Res. 2015, 69, 96–104. [Google Scholar] [CrossRef]
- Singh, R.P.; Vanapalli, K.R.; Cheela, V.R.S.; Peddireddy, S.R.; Sharma, H.B.; Mohanty, B. Fly ash, GGBS, and silica fume based geopolymer concrete with recycled aggregates: Properties and environmental impacts. Constr. Build. Mater. 2023, 378, 131168. [Google Scholar] [CrossRef]
- Stroh, J.; Schlegel, M.-C.; Irassar, E.; Meng, B.; Emmerling, F. Applying high resolution SyXRD analysis on sulfate attacked concrete field samples. Cem. Concr. Res. 2014, 66, 19–26. [Google Scholar] [CrossRef]
- Li, C.C.; Yao, Y.; Wang, L. Accelerate Thaumasite Formation in Cement-limestone Powder Paste by internal adding method. Adv. Mater. Res. 2011, 250, 22–27. [Google Scholar] [CrossRef]
- Li, C.C.; Yao, Y.; Wang, L. Rapid quantitative identification of thaumasite. Mater. Sci. Forum 2013, 743, 186–192. [Google Scholar] [CrossRef]
- GB/T 50082-2009; Ministry of Construction of the People’s Republic of China, Standard for Test method for Long-Term Performance and Durability of Ordinary Concrete. Ministry of Construction of the People’s Republic of China: Beijing, China, 2009.
- Feldman, R.F.; Beaudoio, J.J.; Philipose, K.E. Effect of cement blends on chloride and sulfate ion diffusion in concrete. II Cem. 1991, 88, 3–18. [Google Scholar]
- Peng, G.F.; Liu, Y.F.; Hao, T.Y. Durability and Design in Concrete Structure in Coastal Area; Feng, X., Ed.; Communication Press: Beijing, China, 2004; pp. 287–296. (In Chinese) [Google Scholar]
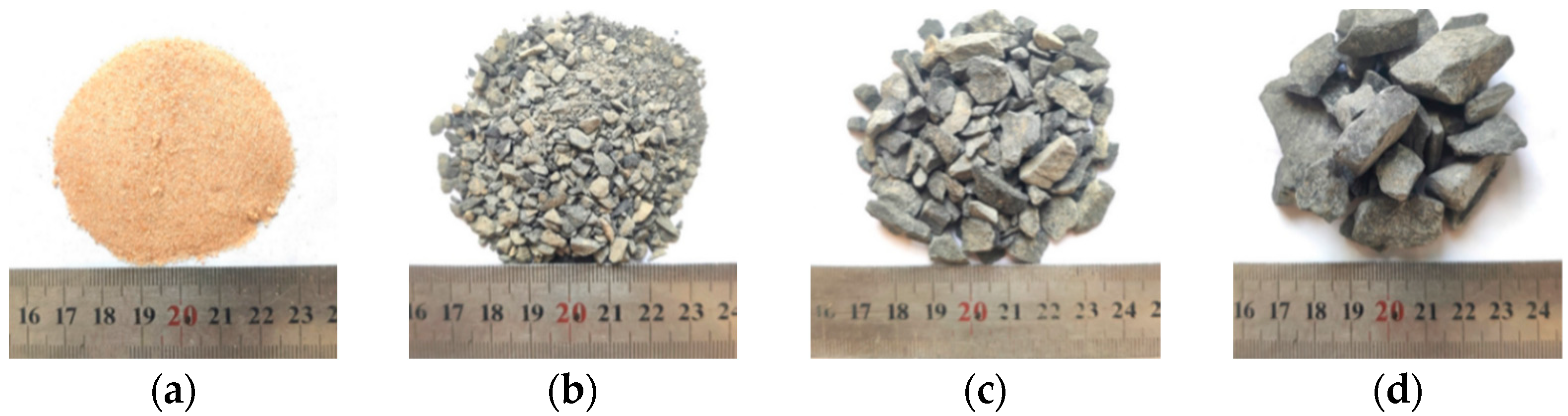

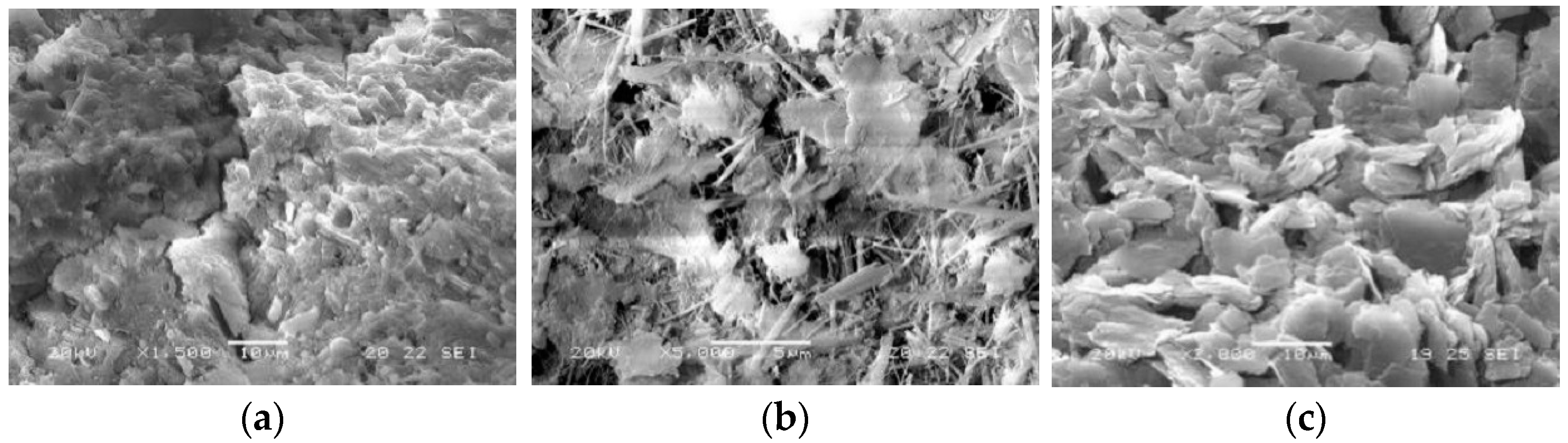

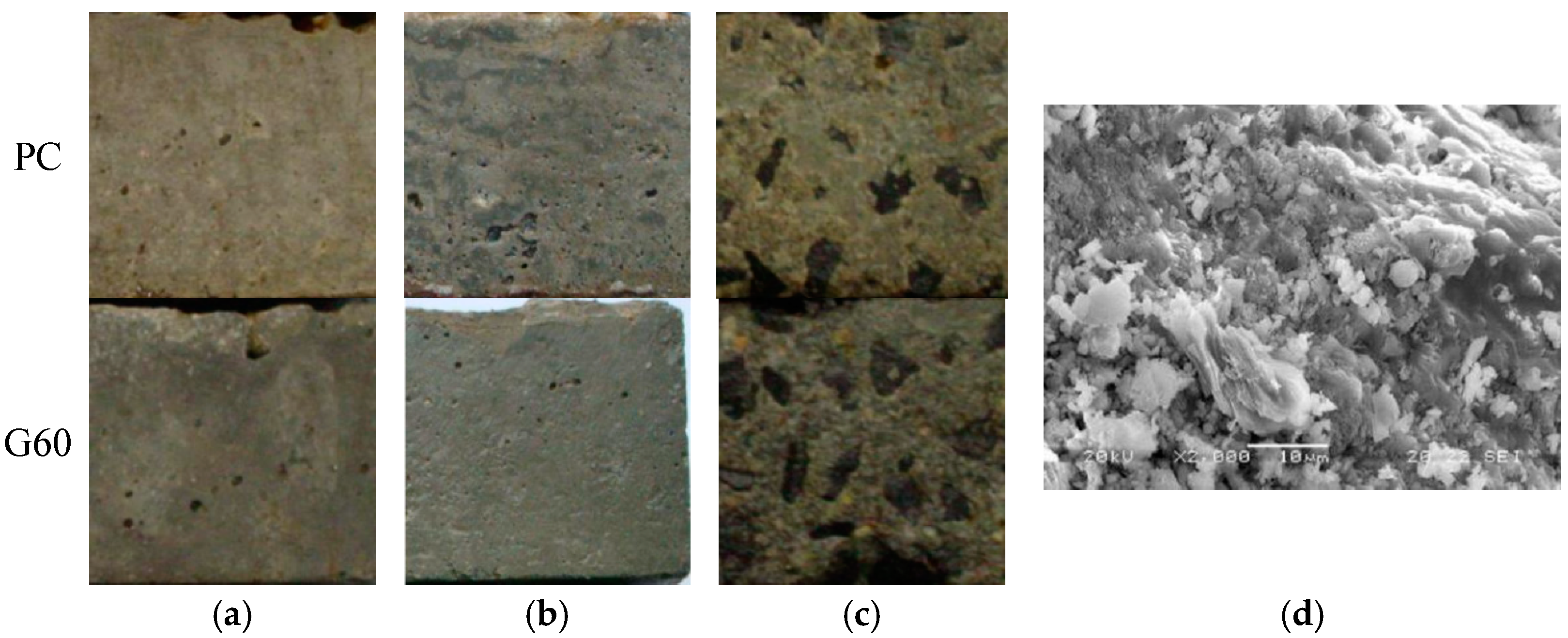

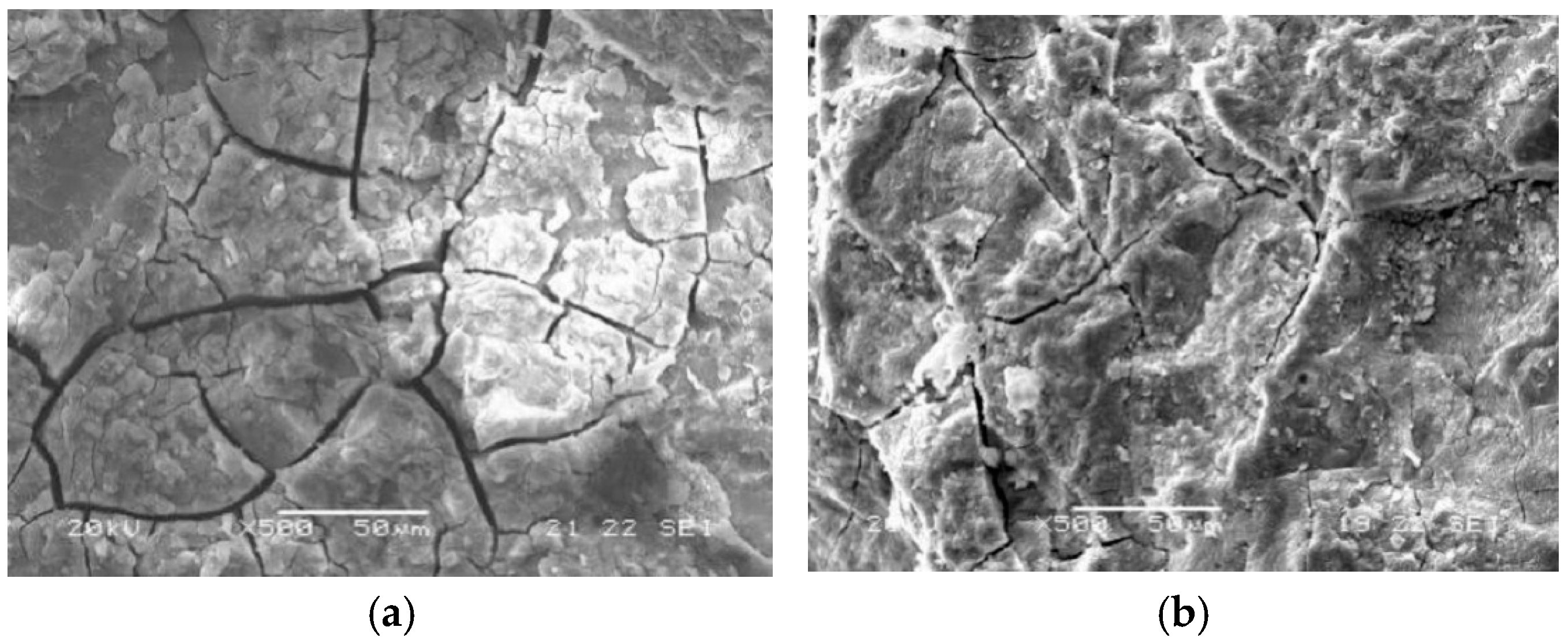
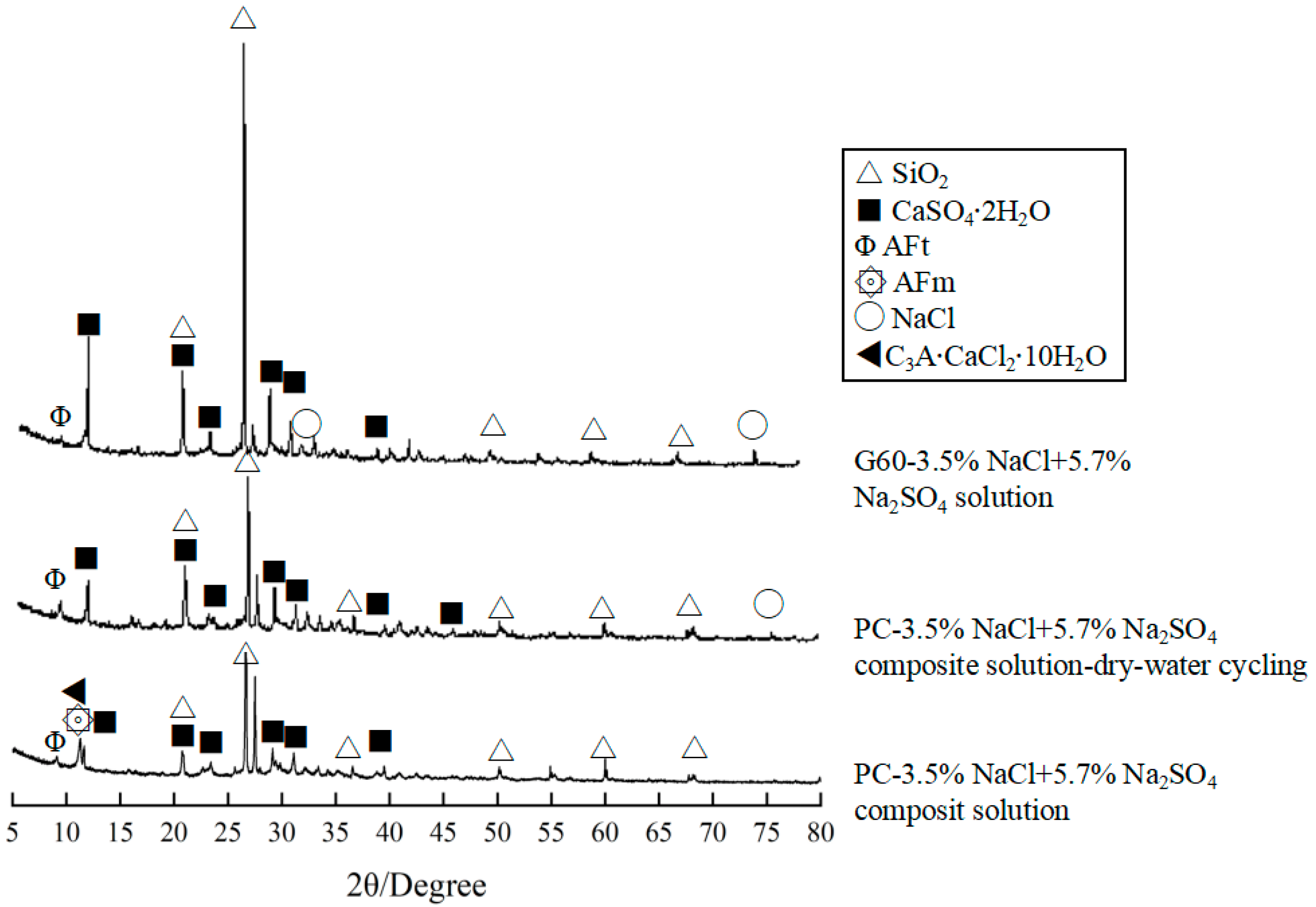
| Cement | SiO2 | Al2O3 | CaO | MgO | SO3 | Fe2O3 | Ignition Loss |
|---|---|---|---|---|---|---|---|
| P. II 52.5R | 20.60 | 5.03 | 65.06 | 0.55 | 2.24 | 4.38 | 1.30 |
| Specific Surface Area/m2·kg−1 | Setting Time/Min | Flexural Strength/MPa | Compressive Strength/MPa | |||
|---|---|---|---|---|---|---|
| First | Final | 3 d | 28 d | 3 d | 28 d | |
| 356 | 108 | 158 | 6.0 | 9.2 | 27.8 | 55.7 |
| Materials | SiO2 | Al2O3 | CaO | MgO | SO3 | Fe2O3 | TiO2 | K2O |
|---|---|---|---|---|---|---|---|---|
| SG | 32.86 | 13.21 | 40.34 | 2.72 | 5.59 | 1.90 | 1.22 | 0.94 |
| Aggregate | Bulk Density/ (kg·m−3) | Apparent Density/ (kg·m−3) | Crushing Value/(%) | Water Absorption Ratio/(%) |
|---|---|---|---|---|
| Fine sand | 1568 | 2633 | - | 0.8 |
| Manufactured sand | 1640 | 2953 | - | 0.9 |
| 3~8 mm crushed stones | 1634 | 2932 | 15.3 | 0.6 |
| 8~16 mm crushed stones | 1623 | 2938 | 14.5 | 0.4 |
| Type | Cement/ kg·m−3 | Slag/kg·m−3 | Fine Sand/kg·m−3 | 3~8 mm Crushed Stones/kg·m−3 | 8~16 mm Crushed Stones/kg·m−3 | Water/kg | Superplasticizer/ kg·m−3 | 28 d Compressive Strength/MPa |
|---|---|---|---|---|---|---|---|---|
| PC | 400 | - | 736 | 413 | 826 | 152 | 3.6 | 58.7 |
| G60 | 160 | 240 | 736 | 413 | 826 | 152 | 3.2 | 59.8 |
| Immersion Time (Days) | 0 d/% | 30 d/% | 60 d/% | 120 d/% | 180 d/% | 270 d/% | 330 d/% | 360 d/% | 390 d/% | 460 d/% | 500 d/% | 540 d/% | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distilled water | PC | 0 | −0.006 | 0.002 | 0.009 | −0.018 | 0.002 | 0.019 | 0.032 | 0.053 | 0.057 | 0.028 | 0.016 |
| G60 | 0 | −0.016 | 0.001 | 0.018 | 0.012 | 0.007 | 0.042 | 0.066 | 0.078 | 0.069 | 0.053 | 0.046 | |
| 3.5% NaCl solution | PC | 0 | −0.003 | 0.000 | 0.002 | −0.014 | 0.018 | 0.093 | 0.108 | 0.122 | 0.093 | 0.119 | 0.083 |
| G60 | 0 | 0.083 | 0.084 | 0.085 | 0.108 | 0.127 | 0.173 | 0.224 | 0.235 | 0.204 | 0.205 | 0.131 | |
| Composite solution | PC | 0 | 0.091 | 0.114 | 0.135 | 0.078 | −0.068 | −0.404 | −0.540 | −0.676 | −1.021 | −1.098 | −1.403 |
| G60 | 0 | −0.050 | −0.020 | 0.010 | −0.028 | −0.258 | −0.373 | −0.509 | −0.645 | −0.865 | −0.809 | −1.266 | |
| Type/Duration of Corrosive Environment | 0 d/% | 30 d/% | 60 d/% | 120 d/% | 180 d/% | 270 d/% | 330 d/% | 360 d/% | 390 d/% | 460 d/% | 500 d/% | 540 d/% | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distilled water | PC | 100 | 111.911 | 111.448 | 110.984 | 93.137 | 96.167 | 94.865 | 95.799 | 96.733 | 106.438 | 92.763 | 92.651 |
| G60 | 100 | 116.655 | 112.394 | 108.133 | 95.254 | 91.042 | 93.376 | 101.013 | 104.650 | 102.460 | 106.118 | 93.788 | |
| 3.5% NaCl solution | PC | 100 | 109.158 | 103.112 | 97.646 | 100.389 | 95.462 | 95.291 | 95.098 | 95.155 | 103.332 | 112.537 | 94.91 |
| G60 | 100 | 118.120 | 115.125 | 113.343 | 111.969 | 99.82 | 97.163 | 98.153 | 117.5757 | 101.911 | 119.957 | 97.847 | |
| Composite solution | PC | 100 | 115.573 | 108.065 | 97.336 | 109.956 | 100.008 | 89.482 | 92.563 | 97.552 | 86.348 | 90.501 | 86.992 |
| G60 | 100 | 114.337 | 103.056 | 102.131 | 107.132 | 90.918 | 91.033 | 94.442 | 93.392 | 96.264 | 91.676 | 91.380 | |
| Corrosion Environment Type/Dry–Wet Cycle Times | 0 Time/% | 5 Times/% | 8 Times/% | 15 Times/% | 25 Times/% | 38 Times/% | 47 Times/% | 51 Times/% | 56 Times/% | 66 Times/% | 72 Times/% | 77 Times/% | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distilled water | PC | 0 | −0.041 | −0.050 | −0.089 | −0.128 | −0.108 | −0.037 | −0.050 | −0.043 | −0.024 | −0.070 | −0.098 |
| G60 | 0 | 0.032 | 0.036 | 0.041 | −0.042 | 0.010 | 0.037 | 0.057 | 0.077 | 0.061 | 0.049 | 0.045 | |
| 3.5% NaCl solution | PC | 0 | −0.018 | −0.016 | −0.003 | −0.037 | −0.031 | 0.011 | 0.045 | 0.059 | 0.041 | 0.051 | 0.011 |
| G60 | 0 | 0.052 | 0.056 | 0.060 | 0.049 | 0.081 | 0.127 | 0.143 | 0.160 | 0.138 | 0.148 | 0.129 | |
| Composite solution | PC | 0.000 | 0.037 | 0.056 | 0.066 | 0.033 | −0.276 | −0.681 | −0.763 | −1.006 | −1.285 | −1.333 | −1.565 |
| G60 | 0.000 | 0.013 | 0.028 | 0.013 | −0.026 | −0.155 | −0.321 | −0.534 | −0.807 | −1.151 | −1.282 | −1.480 | |
| Corrosion Environment Type/Dry–Wet Cycle Times | 0 Time/% | 5 Times/% | 8 Times/% | 15 Times/% | 25 Times/% | 38 Times/% | 47 Times/% | 51 Times/% | 56 Times/% | 66 Times/% | 72 Times/% | 77 Times/% | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distilled water | PC | 100.000 | 100.004 | 98.954 | 97.903 | 93.631 | 95.875 | 92.166 | 87.982 | 88.797 | 89.334 | 90.266 | 88.081 |
| G60 | 100.000 | 98.741 | 104.314 | 109.887 | 97.995 | 101.442 | 95.923 | 94.908 | 94.893 | 95.465 | 101.205 | 97.505 | |
| 3.5% NaCl solution | PC | 100.000 | 108.480 | 101.192 | 93.903 | 91.900 | 90.049 | 92.244 | 90.357 | 94.469 | 108.679 | 103.029 | 95.245 |
| G60 | 100.000 | 115.814 | 103.608 | 91.402 | 97.878 | 93.336 | 93.966 | 98.135 | 105.304 | 110.688 | 106.891 | 95.263 | |
| Composite solution | PC | 100.000 | 94.277 | 95.259 | 96.240 | 80.388 | 77.601 | 80.923 | 81.098 | 81.272 | 82.643 | 87.317 | 84.454 |
| G60 | 100.000 | 107.846 | 100.811 | 93.776 | 100.707 | 84.399 | 88.12 | 84.853 | 81.584 | 85.192 | 87.711 | 84.463 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Liu, W.; Yu, H.; Wang, W.; Ma, H. Study on Mass and Performance Deterioration of Concrete Under Multiple Corrosive Environments. Materials 2025, 18, 1931. https://doi.org/10.3390/ma18091931
Yang H, Liu W, Yu H, Wang W, Ma H. Study on Mass and Performance Deterioration of Concrete Under Multiple Corrosive Environments. Materials. 2025; 18(9):1931. https://doi.org/10.3390/ma18091931
Chicago/Turabian StyleYang, Haicheng, Weifeng Liu, Hongfa Yu, Wei Wang, and Haiyan Ma. 2025. "Study on Mass and Performance Deterioration of Concrete Under Multiple Corrosive Environments" Materials 18, no. 9: 1931. https://doi.org/10.3390/ma18091931
APA StyleYang, H., Liu, W., Yu, H., Wang, W., & Ma, H. (2025). Study on Mass and Performance Deterioration of Concrete Under Multiple Corrosive Environments. Materials, 18(9), 1931. https://doi.org/10.3390/ma18091931






