A Lithiophilic Artificial Li3P Interphase with High Li-Ion Conductivity via Solid-State Friction for Lithium Metal Anodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Li@P
2.3. Material Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
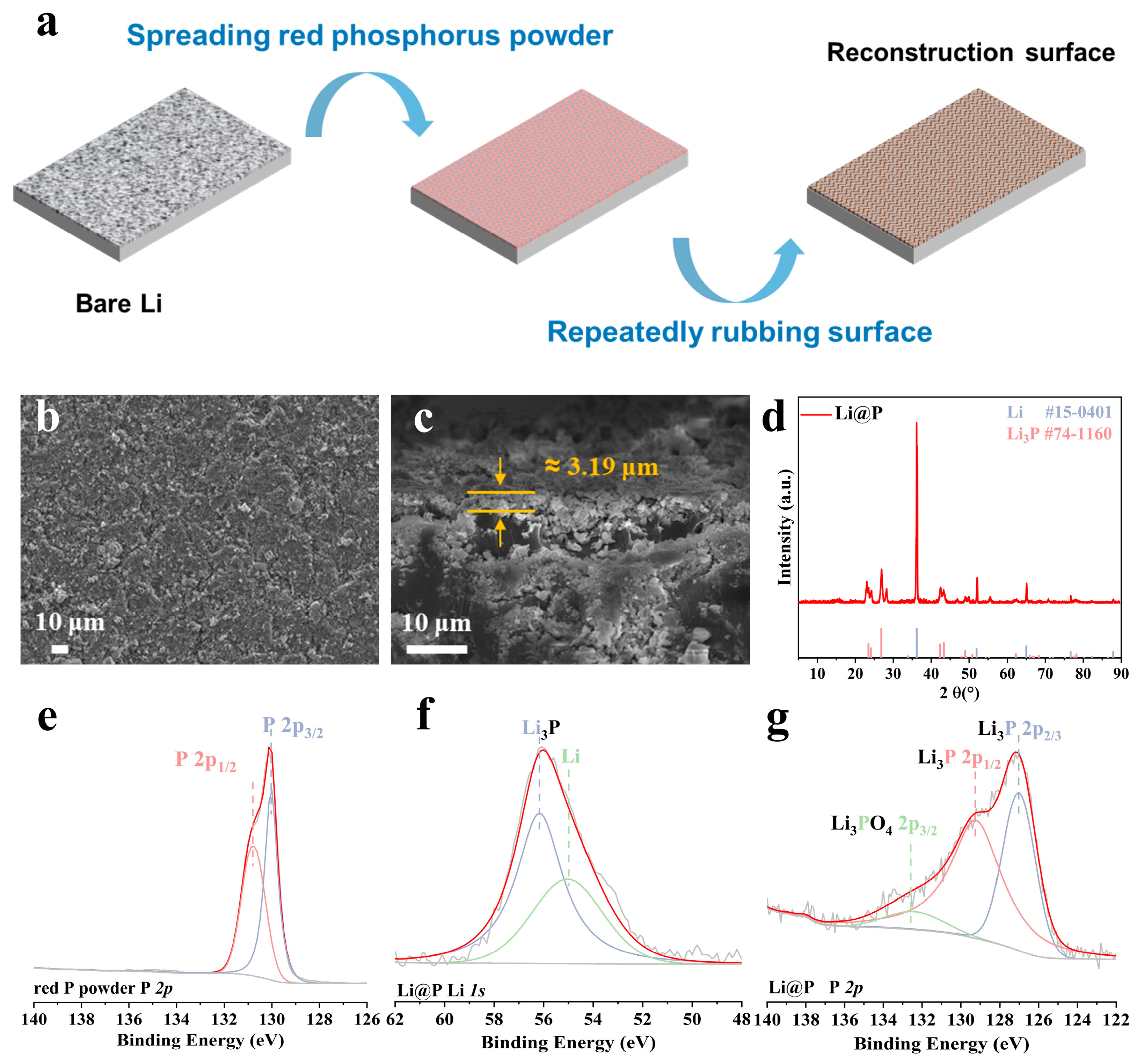
3.1. Compositional Analysis of Li3P Interphase via Solid-State Friction
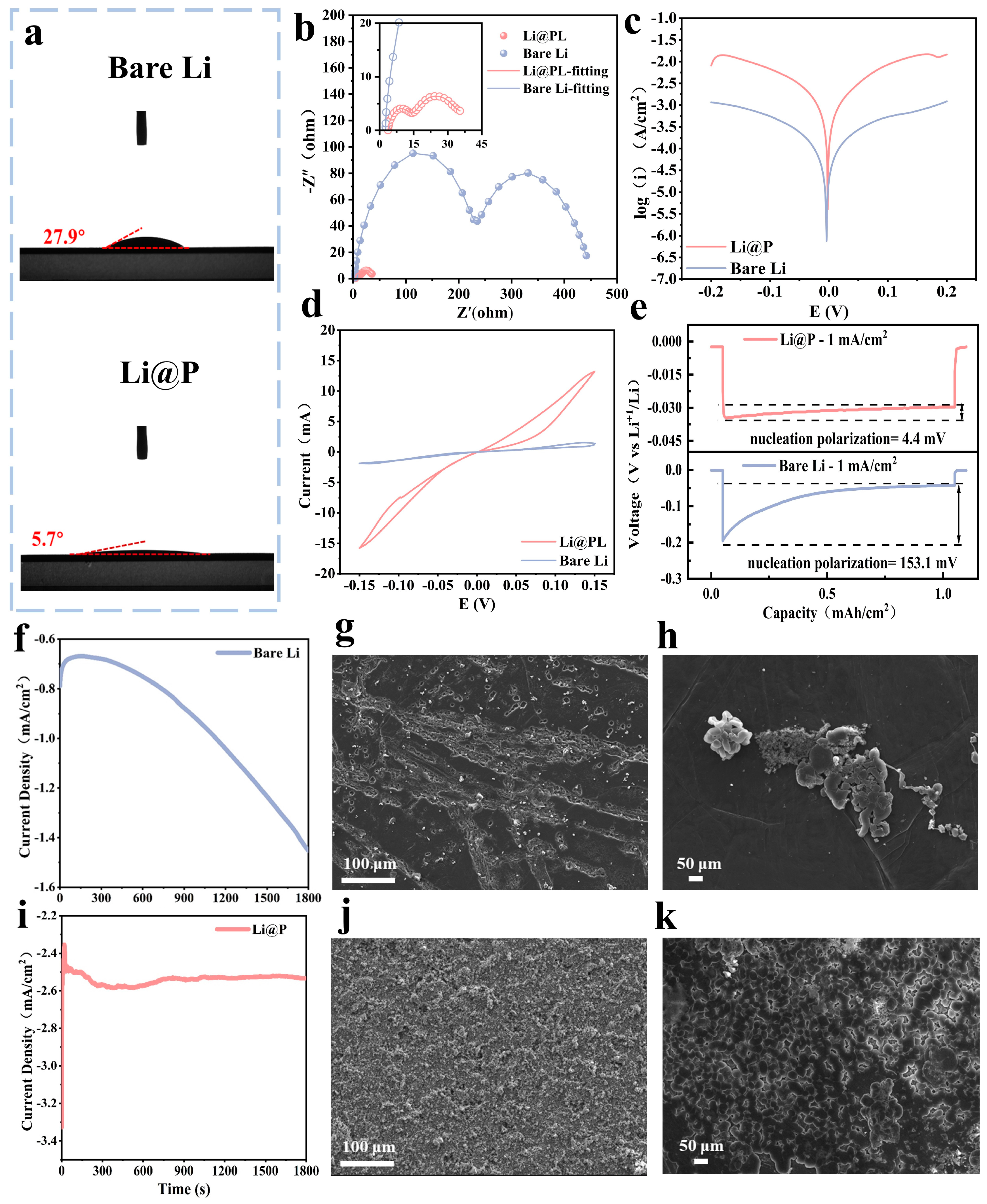
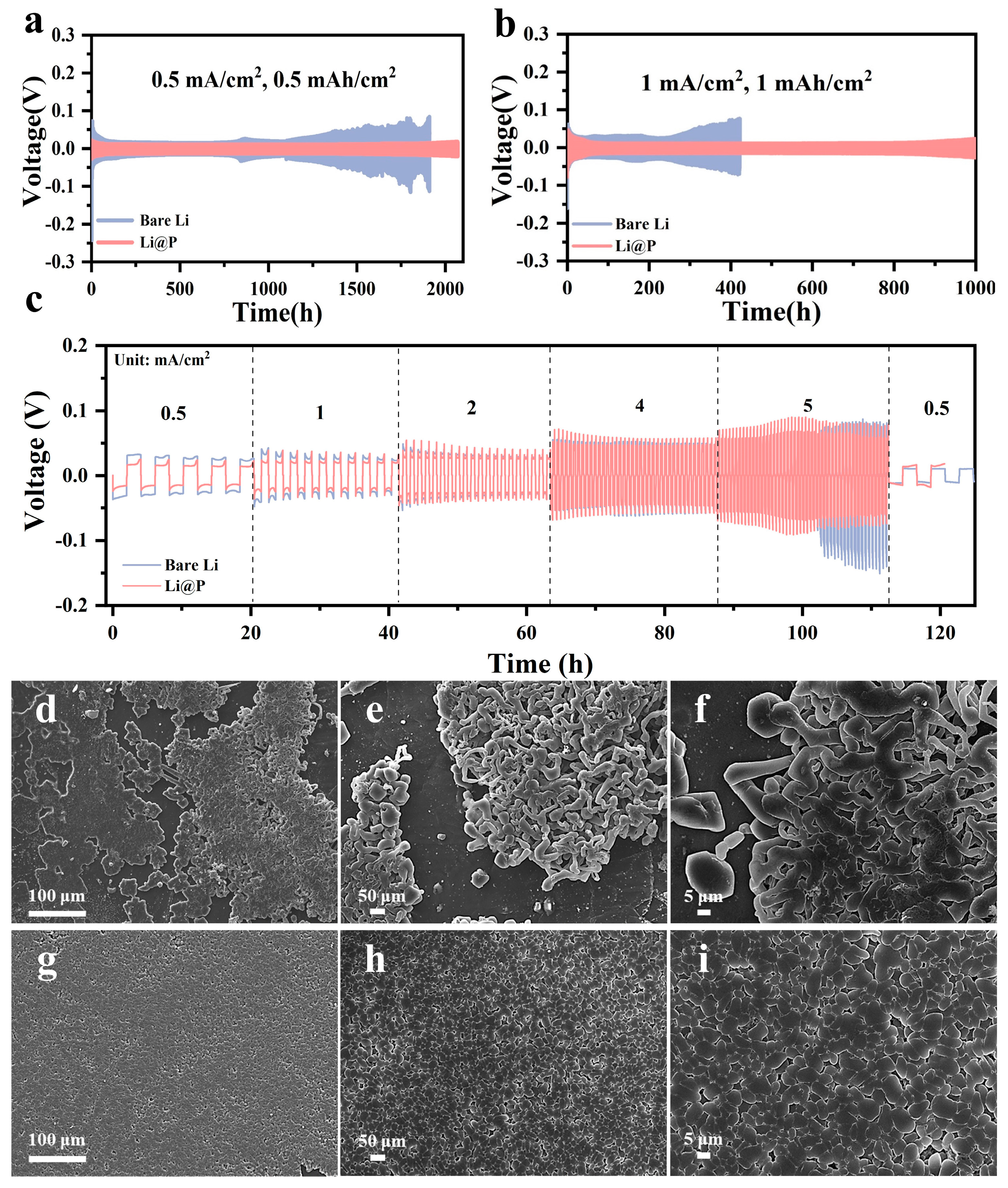
3.2. Electrochemical Properties of Li3P Interphase via Solid-State Friction
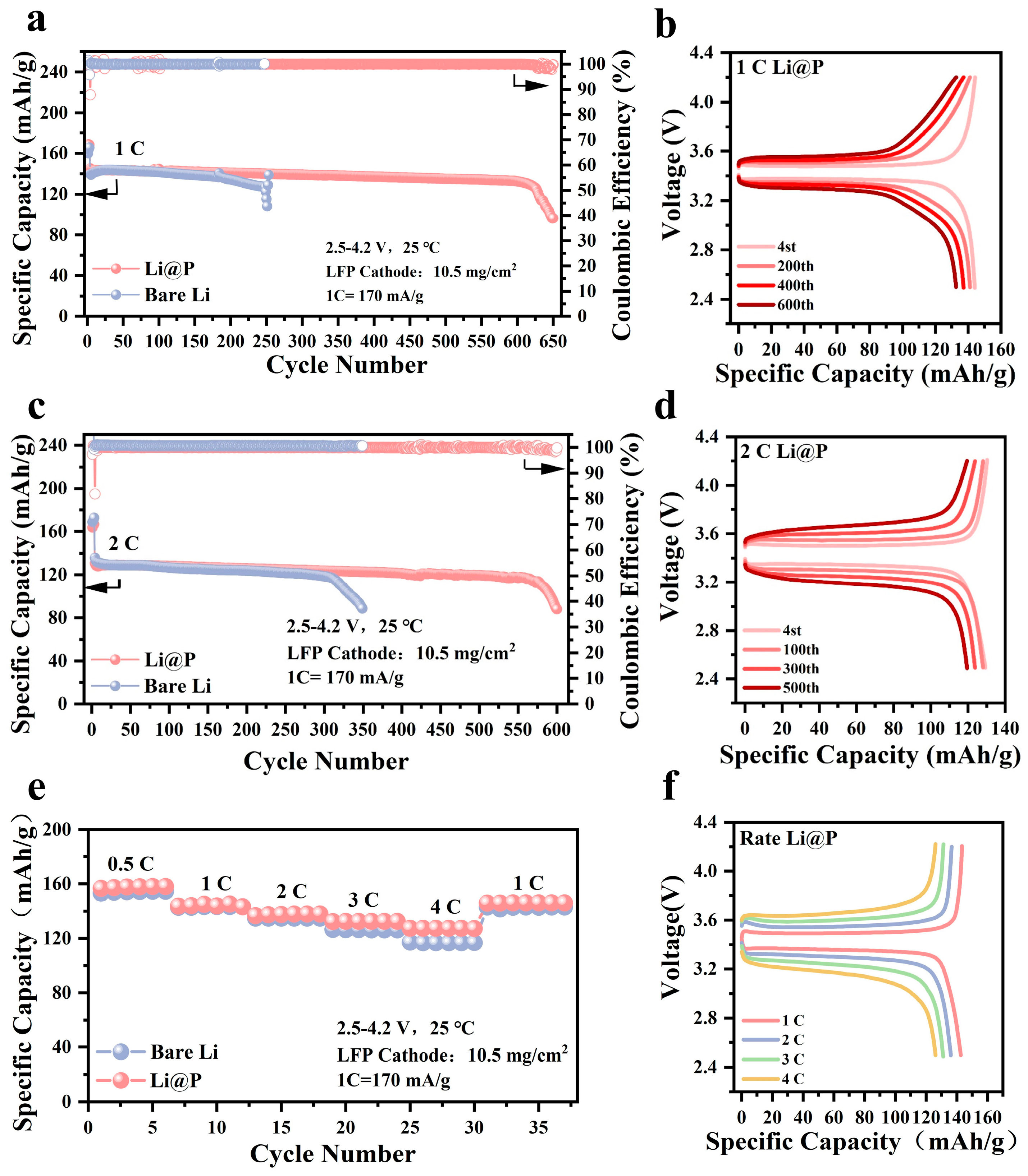
3.3. Full Cell Tests for Practical Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Wang, R.; Cui, W.; Chu, F.; Wu, F. Lithium metal anodes: Present and future. J. Energy Chem. 2020, 48, 145–159. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Zhai, T. Reviving Lithium-Metal Anodes for Next-Generation High-Energy Batteries. Adv. Mater. 2017, 29, 17000007. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Deng, Y.; Utomo, N.W.; Zheng, J.; Biswal, P.; Yin, J.; Archer, L.A. On the crystallography and reversibility of lithium electrodeposits at ultrahigh capacity. Nat. Commun. 2021, 12, 6034. [Google Scholar] [CrossRef]
- Gao, J.; Chen, C.; Dong, Q.; Dai, J.; Yao, Y.; Li, T.; Rundlett, A.; Wang, R.; Wang, C.; Hu, L. Stamping Flexible Li Alloy Anodes. Adv. Mater. 2021, 33, e2005305. [Google Scholar] [CrossRef]
- Ou, C.-H.; Pan, Y.-M.; Tang, H.-T. Electrochemically promoted N-heterocyclic carbene polymer-catalyzed cycloaddition of aldehyde with isocyanide acetate. Sci. China Chem. 2022, 65, 1873–1878. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, B. Multi-storey corridor structured host for a large area capacity and high rate metallic lithium anode. Electrochim. Acta 2021, 365, 137341. [Google Scholar] [CrossRef]
- Chazalviel, J.N. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 1990, 42, 7355–7367. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, X.; Liang, C.; Yu, Y.; Liu, W. Stabilized lithium metal anode by an efficient coating for high-performance Li–S batteries. Energy Storage Mater. 2020, 24, 329–335. [Google Scholar] [CrossRef]
- Lu, G.; Nai, J.; Luan, D.; Tao, X.; Lou, X.W. Surface engineering toward stable lithium metal anodes. Sci. Adv. 2023, 9, 1550. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, S.; Xie, Y.; Liu, J.; Shi, C.; Sun, M.; Peng, H.; Lan, J.; Deng, Y.-P.; Huang, L.; et al. Constructing an Artificial Interface as a Bifunctional Promoter for the Li Anode and the NCM Cathode in Lithium Metal Batteries. J. Am. Chem. Soc. 2024, 146, 31137–31149. [Google Scholar] [CrossRef]
- Wu, F.; Borodin, O.; Yushin, G. In situ surface protection for enhancing stability and performance of conversion-type cathodes. MRS Energy Sustain. 2017, 4, E9. [Google Scholar] [CrossRef]
- Ling, C.; Naren, T.; Liu, X.; Yang, J.; Xiao, P.; Wei, W.; Ji, X.; Kuang, G.-C.; Chen, L. In-situ polymerization induced phase separation to develop high-performance self-healable polymeric electrolytes for lithium metal battery. Mater. Today Energy 2023, 36, 101372. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Davey, K.; Li, J.; Li, G.; Zhang, S.; Mao, J.; Guo, Z. Recent progress in electrolyte design for advanced lithium metal batteries. SmartMat 2023, 4, e1185. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, C.; Xie, W.; Liu, X.; Lu, Y.; Hou, Y.; Ma, T.; Yan, Z.; Chen, J. Ether-Modified Nonflammable Phosphate Enabling Anion-Rich Electrolyte for High-Voltage Lithium Metal Batteries. Adv. Mater. 2024, 36, 2312302. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, H.; Cao, Y.; Xu, B.; Hong, Q.; Cai, W.; Yang, W. Multi-walled carbon nanotube interlayers with controllable thicknesses for high-capacity and long-life lithium metal anodes. J. Power Sources 2019, 412, 170–179. [Google Scholar] [CrossRef]
- Pan, L.; Luo, Z.; Zhang, Y.; Chen, W.; Zhao, Z.; Li, Y.; Wan, J.; Yu, D.; He, H.; Wang, D. Seed-Free Selective Deposition of Lithium Metal into Tough Graphene Framework for Stable Lithium Metal Anode. ACS Appl. Mater. Interfaces 2019, 11, 44383–44389. [Google Scholar] [CrossRef]
- Li, S.; Liu, Q.; Zhou, J.; Pan, T.; Gao, L.; Zhang, W.; Fan, L.; Lu, Y. Hierarchical Co3O4 Nanofiber–Carbon Sheet Skeleton with Superior Na/Li-Philic Property Enabling Highly Stable Alkali Metal Batteries. Adv. Funct. Mater. 2019, 29, 1808847. [Google Scholar] [CrossRef]
- Luo, Z.; Li, S.; Yang, L.; Tian, Y.; Xu, L.; Zou, G.; Hou, H.; Wei, W.; Chen, L.; Ji, X. Interfacially Redistributed charge for robust lithium metal anode. Nano Energy 2021, 87, 106212. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, C.; Adair, K.R.; Zhao, Y.; Goncharova, L.V.; Liang, J.; Wang, C.; Li, J.; Li, R.; Cai, M.; et al. Regulated lithium plating and stripping by a nano-scale gradient inorganic–organic coating for stable lithium metal anodes. Energy Environ. Sci. 2021, 14, 4085–4094. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, C.; Lv, H.; Xie, Y.; Zhou, S.; Ye, Y.; Zhou, E.; Zhu, T.; Xie, H.; Jiang, W.; et al. Bifunctional Interphase Promotes Li+ De-Solvation and Transportation Enabling Fast-Charging Graphite Anode at Low Temperature. Adv. Mater. 2023, 36, 2308675. [Google Scholar] [CrossRef]
- Fu, L.; Wang, X.; Zhang, B.; Chen, Z.; Li, Y.; Sun, Y. A Li3P nanoparticle dispersion strengthened ultrathin Li metal electrode for high energy density rechargeable batteries. Nano Res. 2024, 17, 4031–4038. [Google Scholar] [CrossRef]
- Tu, S.; Zhang, B.; Zhang, Y.; Chen, Z.; Wang, X.; Zhan, R.; Ou, Y.; Wang, W.; Liu, X.; Duan, X.; et al. Fast-charging capability of graphite-based lithium-ion batteries enabled by Li3P-based crystalline solid–electrolyte interphase. Nat. Energy 2023, 8, 1365–1374. [Google Scholar] [CrossRef]
- Li, J.; Liu, D.; Sun, H.; Qu, D.; Xie, Z.; Tang, H.; Liu, J. Mixed ion-electron conducting Li3P for efficient cathode prelithiation of all-solid-state Li-ion batteries. SmartMat 2023, 4, e1200. [Google Scholar] [CrossRef]
- Chen, W.; Salvatierra, R.V.; Li, J.T.; Luong, D.X.; Beckham, J.L.; Li, V.D.; La, N.; Xu, J.; Tour, J.M. Brushed Metals for Rechargeable Metal Batteries. Adv. Mater. 2022, 34, 2202668. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Wang, H.; Han, X.; Zhang, S.; Sun, J.; Zhang, Y.; Cao, Y.; Yao, Y.; Sun, J. The synthesis of greenish phosphorus on carbon substrates. Chem. Commun. 2021, 57, 3975–3978. [Google Scholar] [CrossRef]
- Chang, W.C.; Wu, J.H.; Chen, K.T.; Tuan, H.Y. Red Phosphorus Potassium-Ion Battery Anodes. Adv. Sci. 2019, 6, 1801354. [Google Scholar] [CrossRef]
- Zhao, Q.; Hao, X.; Su, S.; Ma, J.; Hu, Y.; Liu, Y.; Kang, F.; He, Y.-B. Expanded-graphite embedded in lithium metal as dendrite-free anode of lithium metal batteries. J. Mater. Chem. A 2019, 7, 15871–15879. [Google Scholar] [CrossRef]
- Zhu, P.; Jiang, Z.; Sun, W.; Yang, Y.; Silvester, D.S.; Hou, H.; Banks, C.E.; Hu, J.; Ji, X. Built-in anionic equilibrium for atom-economic recycling of spent lithium-ion batteries. Energy Environ. Sci. 2023, 16, 3564–3575. [Google Scholar] [CrossRef]
- He, X.; Hao, W.; Shi, Z.; Tan, Y.; Yue, X.; Xie, Y.; Yan, X.; Liang, Z. Colloid Electrolyte Containing Li3P Nanoparticles for Highly Stable 4.7 V Lithium Metal Batteries. ACS Nano 2024, 18, 22560–22571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lyu, R.; Lv, W.; Li, H.; Jiang, W.; Li, J.; Gu, S.; Zhou, G.; Huang, Z.; Zhang, Y.; et al. A Lightweight 3D Cu Nanowire Network with Phosphidation Gradient as Current Collector for High-Density Nucleation and Stable Deposition of Lithium. Adv. Mater. 2019, 31, 1904991. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Zhang, S.; Dong, S.; Wang, S.; Cui, Z.; Du, X.; Wang, C.; Xie, B.; Du, J.; et al. Highly Fluorinated Al-Centered Lithium Salt Boosting the Interfacial Compatibility of Li-Metal Batteries. ACS Energy Lett. 2022, 7, 591–598. [Google Scholar] [CrossRef]
- Krauskopf, T.; Richter, F.H.; Zeier, W.G.; Janek, J. Physicochemical Concepts of the Lithium Metal Anode in Solid-State Batteries. Chem. Rev. 2020, 120, 7745–7794. [Google Scholar] [CrossRef] [PubMed]
- Long, K.; Huang, S.; Wang, H.; Wang, A.; Chen, Y.; Liu, Z.; Zhang, Y.; Wu, Z.; Wang, W.; Chen, L. Green mechanochemical Li foil surface reconstruction toward long-life Li–metal pouch cells. Energy Environ. Sci. 2024, 17, 260–273. [Google Scholar] [CrossRef]
- Lai, Y.; Zhang, H.; Xia, G.; Yu, X. Long-term stable Li metal anode enabled by strengthened and protected lithiophilic LiZn alloys. J. Power Sources 2022, 543, 231839. [Google Scholar] [CrossRef]
- Long, K.; Liu, X.; Yang, J.; Wang, H.; Wang, A.; Chen, Y.; Mei, L.; Zhang, Y.; Wu, Z.; Wang, W.; et al. Homogeneously Planar-Exposure LiB Fiber Skeleton Toward Long-Lifespan Practical Li Metal Pouch Cells. Small 2024, 20, 2311193. [Google Scholar] [CrossRef]
- Xiong, X.; Yan, W.; Zhu, Y.; Liu, L.; Fu, L.; Chen, Y.; Yu, N.; Wu, Y.; Wang, B.; Xiao, R. Li4Ti5O12 Coating on Copper Foil as Ion Redistributor Layer for Stable Lithium Metal Anode. Adv. Energy Mater. 2022, 12, 2103112. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, H.; Yang, J.; Xu, Z.; Wang, J.; Hirano, S.-i.; Guo, Y.; Liang, C. Stable Lithium Metal Anode Enabled by a Lithiophilic and Electron/Ion Conductive Framework. ACS Nano 2020, 14, 5618–5627. [Google Scholar] [CrossRef]
- Lin, L.; Liu, F.; Zhang, Y.; Ke, C.; Zheng, H.; Ye, F.; Yan, X.; Lin, J.; Sa, B.; Wang, L.; et al. Adjustable Mixed Conductive Interphase for Dendrite-Free Lithium Metal Batteries. ACS Nano 2022, 16, 13101–13110. [Google Scholar] [CrossRef]
- Long, K.; Huang, S.; Wang, H.; Jin, Z.; Wang, A.; Wang, Z.; Qing, P.; Liu, Z.; Chen, L.; Mei, L.; et al. High interfacial capacitance enabled stable lithium metal anode for practical lithium metal pouch cells. Energy Storage Mater. 2023, 58, 142–154. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Pan, W.; Xiao, B.; Jin, Y.; Li, K.; Wang, A.; Li, H.; Wu, Z.; Chen, Y.; Huang, S.; et al. A Lithiophilic Artificial Li3P Interphase with High Li-Ion Conductivity via Solid-State Friction for Lithium Metal Anodes. Materials 2025, 18, 1930. https://doi.org/10.3390/ma18091930
Liu H, Pan W, Xiao B, Jin Y, Li K, Wang A, Li H, Wu Z, Chen Y, Huang S, et al. A Lithiophilic Artificial Li3P Interphase with High Li-Ion Conductivity via Solid-State Friction for Lithium Metal Anodes. Materials. 2025; 18(9):1930. https://doi.org/10.3390/ma18091930
Chicago/Turabian StyleLiu, Haoling, Wen Pan, Bo Xiao, Yunke Jin, Kun Li, An Wang, Huimiao Li, Zhibin Wu, Yuejiao Chen, Shaozhen Huang, and et al. 2025. "A Lithiophilic Artificial Li3P Interphase with High Li-Ion Conductivity via Solid-State Friction for Lithium Metal Anodes" Materials 18, no. 9: 1930. https://doi.org/10.3390/ma18091930
APA StyleLiu, H., Pan, W., Xiao, B., Jin, Y., Li, K., Wang, A., Li, H., Wu, Z., Chen, Y., Huang, S., Mei, L., & Chen, L. (2025). A Lithiophilic Artificial Li3P Interphase with High Li-Ion Conductivity via Solid-State Friction for Lithium Metal Anodes. Materials, 18(9), 1930. https://doi.org/10.3390/ma18091930








