Effect of Co and Al Content on CrFeNiMo-System High Entropy Alloys Produced by Mechanical Alloying
Abstract
1. Introduction
2. Materials and Methods
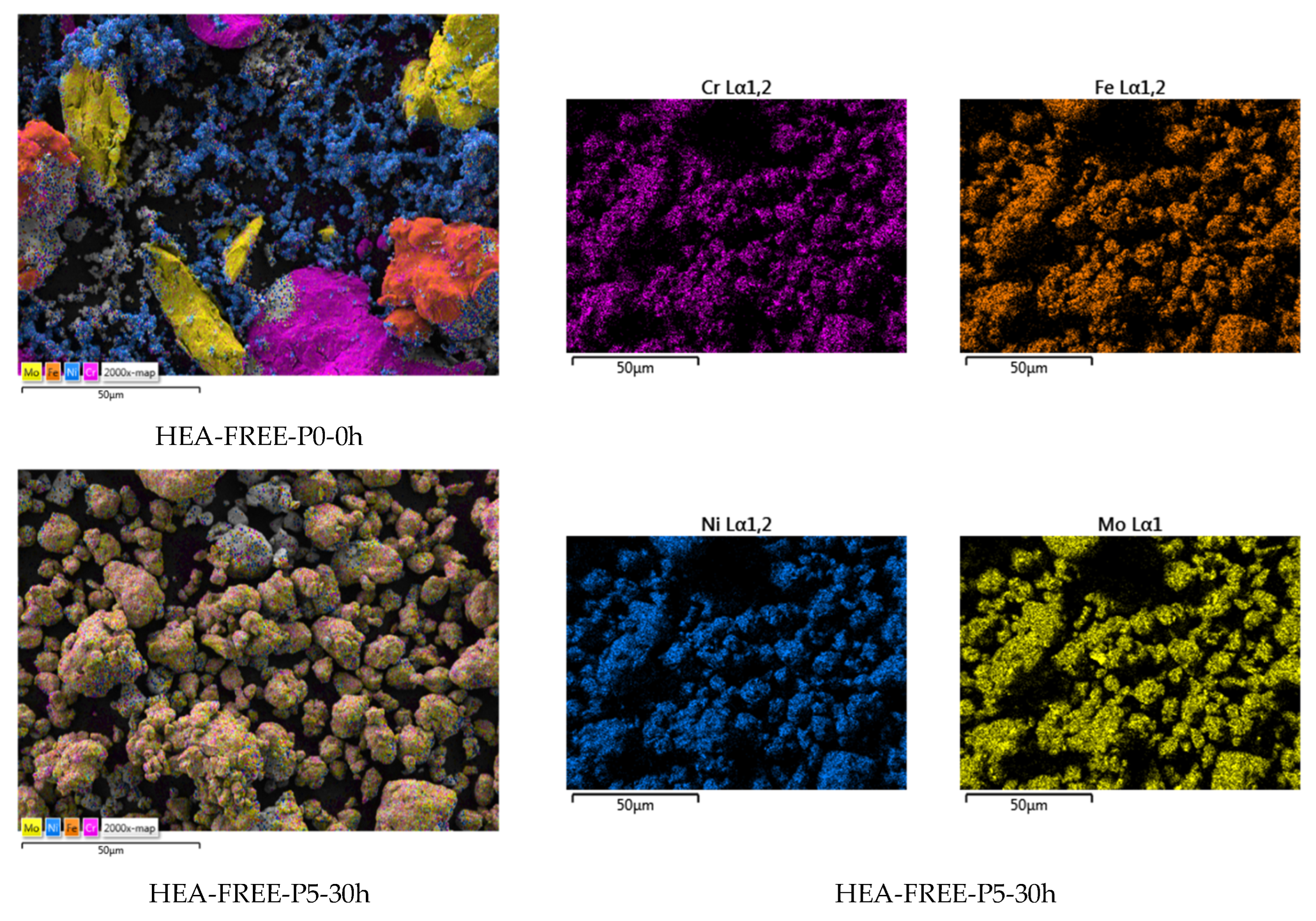
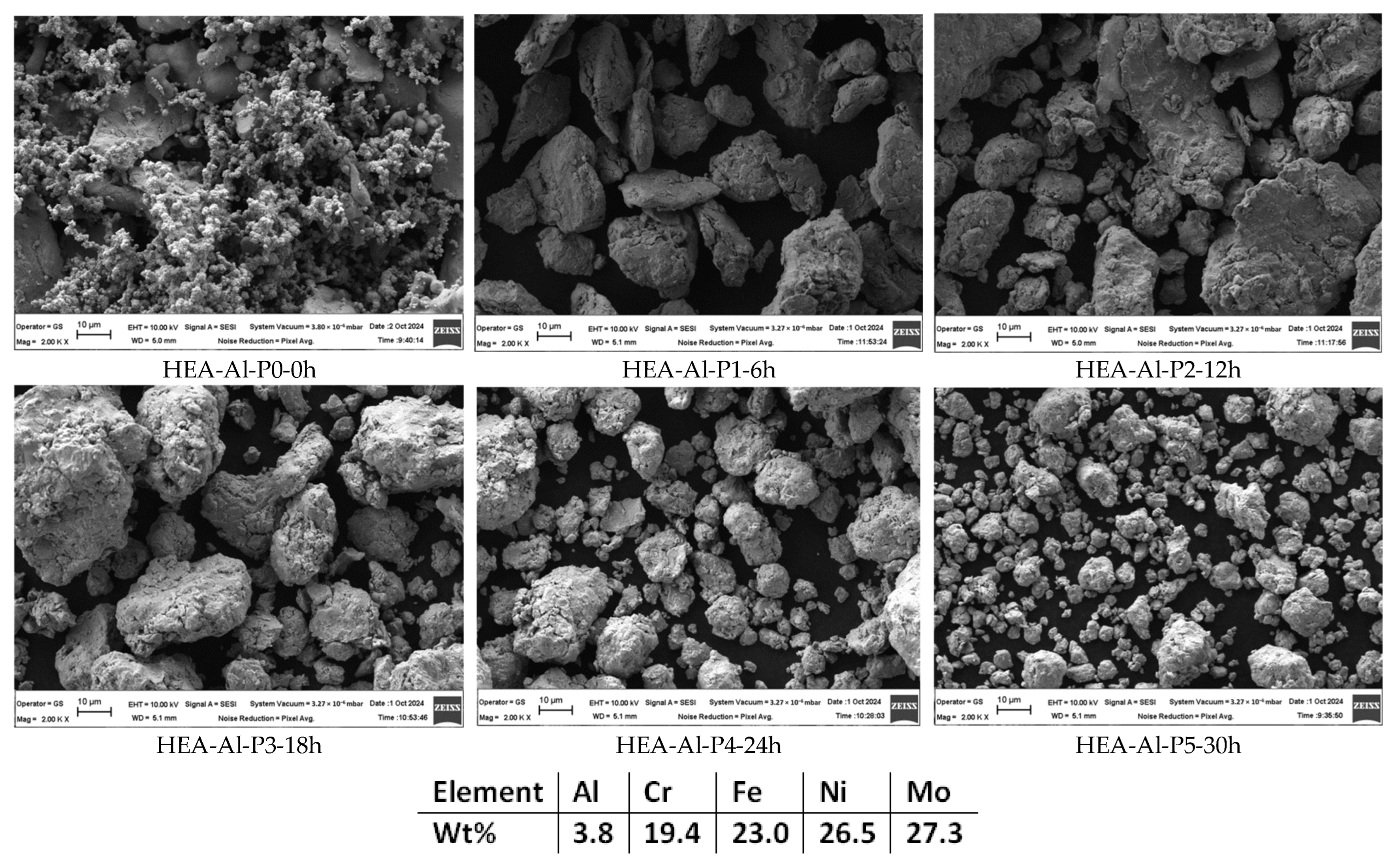
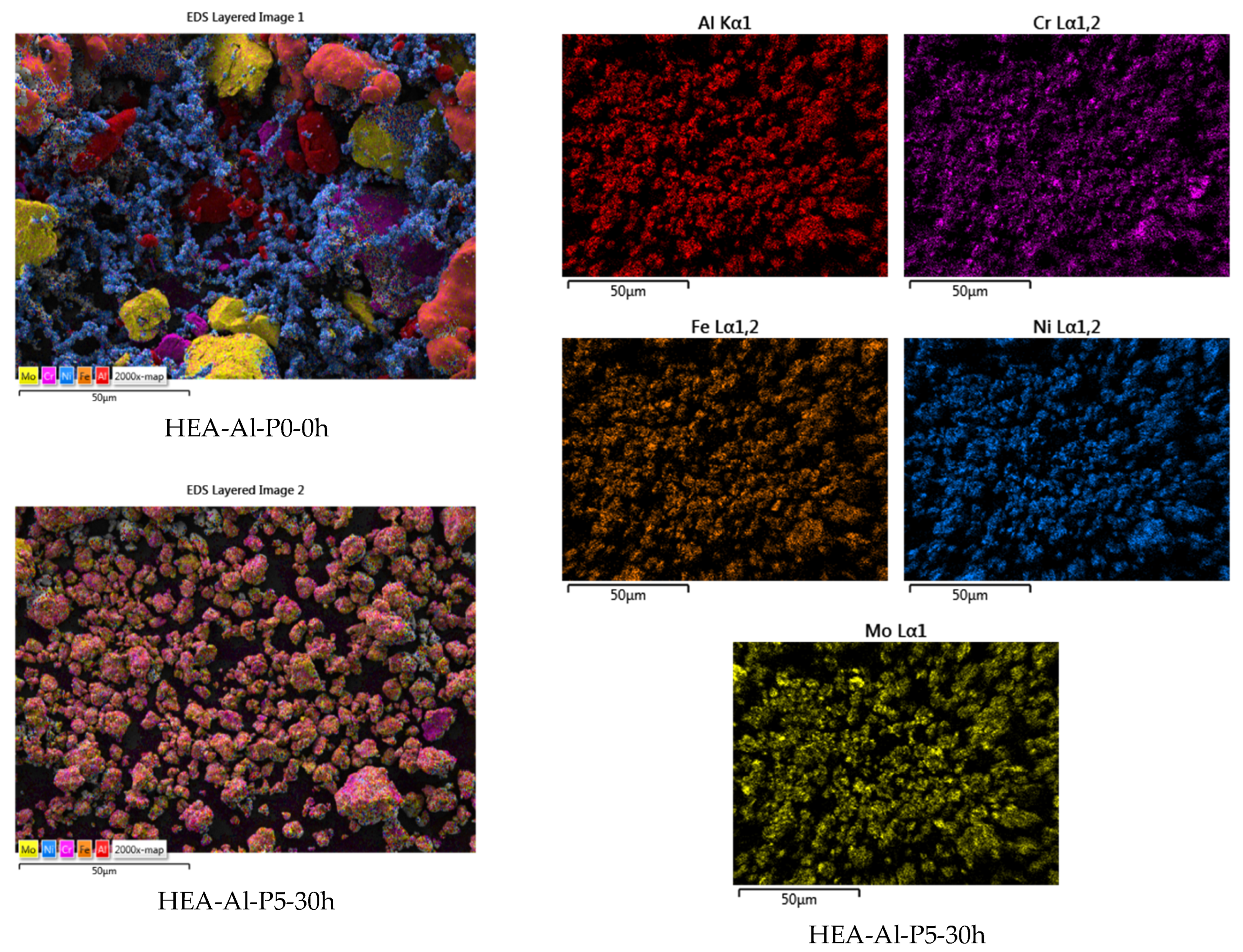
3. Results and Discussions
- Particle size;
- Particle size distribution;
- Particle shape.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, M.; Diao, G.; Yuan, J.F.; Fraser, D.; Li, J.; Chung, R.; Li, D.Y. Corrosion and corrosive wear of AlCrFeCoNi and Co-free AlCrFeNi-Tix (x = 0–1.5) high-entropy alloys in 3.5 wt % NaCl and H2SO4 (pH = 3) solutions. Wear 2023, 523, 204765. [Google Scholar] [CrossRef]
- Chang, T.; Zou, C.M.; Zhu, D.D.; Wang, X.H.; Wei, Z.J.; Wang, H.W.; Fang, N.; Chen, J.H. Microstructure and magnetic behaviors of FeCoNi (Al) alloys with incoherent nanoprecipitates prepared by high-pressure solidification. J. Alloys Compd. 2022, 894, 162501. [Google Scholar] [CrossRef]
- Sonal, S.; Lee, J. Recent advances in additive manufacturing of high entropy alloys and their nuclear and wear-resistant applications. Metals 2021, 11, 1980–1992. [Google Scholar] [CrossRef]
- He, Z.; Jia, N.; Wang, H.; Yan, H.; Shen, Y. Synergy effect of multi-strengthening mechanisms in FeMnCoCrN HEA at cryogenic temperature. J. Mater. Sci. Technol. 2021, 86, 158–170. [Google Scholar] [CrossRef]
- He, Z.F.; Jia, N.; Ma, D.; Yan, H.L.; Li, Z.M.; Raabe, D. Joint contribution of transformation and twinning to the high strength-ductility combination of a FeMnCoCr high entropy alloy at cryogenic temperatures. Mater. Sci. Eng. 2019, 759, 437–447. [Google Scholar] [CrossRef]
- Kumar, D.; Maulik, O.; Sharma, V.K.; Prasad, Y.V.S.S.; Kumar, V. Understanding the effect of tungsten on corrosion behavior of AlCuCrFeMnWx high-entropy alloys in 3.5 wt.% NaCl solution. J. Mater. Eng. Perform. 2018, 27, 4481–4488. [Google Scholar] [CrossRef]
- Kulkarni, R.; Murty, B.S.; Srinivas, V. Study of microstructure and magnetic properties of AlNiCo(CuFe) high entropy alloy. J. Alloys Compd. 2018, 746, 194–199. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Cieślak, G.; Stygar, M.; Mroczka, K.; Berent, K.; Kulik, T.; Danielewski, M. Influence of Cu content on high temperature oxidation behavior of AlCoCrCuxFeNi high entropy alloys (x = 0; 0.5; 1). Intermetallics 2017, 84, 52–61. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, N.; Komarasamy, M. Lattice strain framework for plastic deformation in complex concentrated alloys including high entropy alloys. Mater. Sci. Technol. 2015, 31, 1259–1263. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Y.; Ye, J. Early high-temperature oxidation behavior of Ti(C,N)-based cermets with multi-component AlCoCrFeNi high-entropy alloy binder. Int. J. Refract. Met. Hard Mater. 2014, 44, 35–41. [Google Scholar] [CrossRef]
- Gromova, V.E.; Shlyarovaa, Y.A.; Konovalovb, S.V.; Vorob’eva, S.V.; Peregudovc, O.A. Application of High-Entropy Alloys. Steel Transl. 2021, 51, 700–704. [Google Scholar] [CrossRef]
- Geambazu, L.E.; Voiculescu, I.; Manea, C.A.; Bololoi, R.V. Economic Efficiency of High-Entropy Alloy Corrosion-Resistant Coatings Designed for Geothermal Turbine Blades: A Case Study. Appl. Sci. 2022, 12, 7196. [Google Scholar] [CrossRef]
- Cui, C.; Wu, M.; He, R.; Jie, D.; Gong, Y.; Miao, X. Microstructure, wear and corrosion behavior of high-entropy alloy coatings: The concentration of Mo element and the dual effect of σ-CrMo phase. Surf. Coat. Technol. 2023, 467, 129726. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E. Pitting corrosion behaviour of austenitic stainless steels-combining effects of Mn and Mo additions. Corros. Sci. 2008, 50, 1796–1806. [Google Scholar] [CrossRef]
- Zhu, M.; Yao, L.J.; Chen, S.S.; Xu, J.F.; Zhang, L. The influence of partial replacement of Fe by Mo element on thermal stability and soft magnetic properties of the Fe-Nb- B amorphous alloys. Rare Metal Mater. Eng. 2016, 45, 715–719. [Google Scholar]
- Yu, W.Q.; Zeng, H.Q.; Sun, Y.M.; Hua, Z. Effect of Mo addition on the thermal stability, microstructure and magnetic property of FeCoZrBCu alloys. Vacuum 2017, 137, 175–182. [Google Scholar] [CrossRef]
- Shi, X.; Wang, C.; Huang, M.; Cui, H. Microstructure and wear resistance property of AlFeCrNiMox coatings by plasma cladding. Mater. Res. Express 2019, 6, 106537. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, Y.; Wang, P.W.; Li, X.; Malomo, B.; Yang, L. Enhancing corrosion resistance in CoCrFeNiTa high entropy alloys via Mo addition. Electrochim. Acta 2024, 480, 143951. [Google Scholar] [CrossRef]
- Liu, J.; Lv, Z.; Wu, Z.; Zhang, J.; Zheng, C.; Chen, C.; Ju, D.; Che, L. Research progress on the influence of alloying elements on the corrosion resistance of high-entropy alloys. J. Alloys Compd. 2024, 1002, 175394. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, N.; Zhu, S.; Qiao, Z.; Zhang, J.; Yang, J.; Liu, W. A novel Cu-doped high entropy alloy with excellent comprehensive performances for marine application. J. Mater. Sci. Technol. 2021, 69, 48–59. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, Y.; Wan, Y.; Chen, H.; Wang, Y.; Yang, J. Strengthening by Ti, Nb, and Zr doping on microstructure, mechanical, tribological, and corrosion properties of CoCrFeNi high-entropy alloys. J. Alloys Compd. 2024, 984, 173819. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Fan, J.; Li, G.; Liaw, P.K.; Liu, R. Microstructure and enhanced mechanical behavior of the Al7Co24Cr21Fe24Ni24 high-entropy alloy system by tuning the Cr content. Mater. Sci. Eng. 2018, 733, 299–306. [Google Scholar] [CrossRef]
- Jeong, H.U.; Park, N. TWIP and TRIP-associated mechanical behaviors of Fex (CoCrMnNi) 100-x medium-entropy ferrous alloys. Mater. Sci. Eng. 2020, 782, 138896. [Google Scholar] [CrossRef]
- Wu, P.; Gan, K.; Yan, D.; Fu, Z.; Li, Z. A non-equiatomic FeNiCoCr high-entropy alloy with excellent anti-corrosion performance and strength-ductility synergy. Corros. Sci. 2021, 183, 109341. [Google Scholar] [CrossRef]
- Kumar, S.; Patnaik, A.; Pradhan, A.K.; Kumar, V. Effect of Cobalt content on thermal, mechanical, and microstructural properties of Al 0.4 FeCrNiCox (x = 0, 0.25, 0.5, 1.0 mol) high-entropy alloys. J. Mater. Eng. Perform. 2019, 28, 4111–4119. [Google Scholar] [CrossRef]
- Kang, M.; Lim, K.R.; Won, J.W.; Na, Y.S. Effect of Co content on the mechanical properties of A2 and B2 phases in AlCoxCrFeNi high-entropy alloys. J. Alloys Compd. 2018, 769, 808–812. [Google Scholar] [CrossRef]
- Schweidler, S.; Botros, M.; Strauss, F.; Wang, Q.; Ma, Y.; Velasco, L.; Marques, G.C.; Sarkar, A.; Kübel, C.; Hahn, H. High-entropy materials for energy and electronic applications. Nat. Rev. Mater. 2024, 9, 266–281. [Google Scholar] [CrossRef]
- Boakye, G.O.; Straume, E.O.; Gunnarsson, B.G.; Kovalov, D.; Karlsdottir, S.N. Corrosion behaviour of HVOF developed Mo-based high entropy alloy coating and selected hard coatings for high temperature geothermal applications. Mater. Des. 2023, 235, 112431. [Google Scholar] [CrossRef]
- Tian, Q.W.; Zhang, G.J.; Yin, K.X.; Cheng, W.L.; Wang, Y.N.; Huang, J.C. Effect of Ni content on the phase formation, tensile properties and deformation mechanisms of the Ni-rich AlCoCrFeNix (x = 2, 3, 4) high entropy alloys. Mater. Charact. 2021, 176, 111148. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying: A critical review. Mater. Res. Lett. 2022, 10, 619–647. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Suhane, A. Mechanically alloyed high entropy alloys: Existing challenges and opportunities. J. Mater. Res. Technol. 2022, 17, 2431–2456. [Google Scholar] [CrossRef]
- Ju, R.; Su, J.J.; Berlanga, R.; Bonastre, J.; Escoda, L. The effects of process control agents on mechanical alloying behavior of a Fe-Zr based alloy. J. Alloys Compd. 2007, 434–435, 472–476. [Google Scholar]
- Nayan, N.; Singh, G.; Murty, S.V.S.N.; Jha, A.K.; Pant, B.; George, K.M.; Ramamurty, U. Hot deformation behavior and microstructure control in AlCrCuNiFeCo high entropy alloy. Intermetallics 2014, 55, 145–153. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Jayasree, R.; Mane, R.B.; Vijay, R.; Chakravarty, D. Effect of process control agents on mechanically alloyed Al0.3CoCrFeNi. Mater. Lett. 2021, 292, 129618. [Google Scholar] [CrossRef]
- Kotan, H.; Tekin, M.; Bayatlı, A.; Bayrak, K.G.; Kocabas, M.; Ayas, E. Effect of in-situ formed oxide and carbide phases on microstructure and corrosion behavior of Zr/Y doped CoCrFeNi high entropy alloys prepared by mechanical alloying and spark plasma sintering. Intermetallics 2023, 162, 107998. [Google Scholar] [CrossRef]






| Elemental Material | Particle Size (µm) | Purity (%) |
|---|---|---|
| Cobalt (Co) | 37 | 99.5 |
| Iron (Fe) | 60 | >99 |
| Chromium (Cr) | <45 | >99 |
| Nickel (Ni) | <50 | 99.7 |
| Aluminum (Al) | 15 | 99.5 |
| Molybdenum (Mo) | 53–90 | >99 |
| Metallic Powder | Free Flow Density (g/cm3) | Tap Density (g/cm3) | Packing Ratio (%) | Free Flow (g/s) | Slope Angle (°) |
|---|---|---|---|---|---|
| Al | 1.21 | 1.47 | 82.3 | 1.09 | 17.74 |
| Co | 2.08 | 2.6 | 80 | 0.57 | 34.21 |
| Cr | 2.45 | 3.2 | 76.6 | 1.2 | 31.79 |
| Fe | 3.12 | 4.05 | 77 | 0.85 | 9.64 |
| Ni | 4.54 | 5.1 | 89 | 12.19 | 17.74 |
| Mo | 4.16 | 4.63 | 89.8 | 9.61 | 21.8 |
| CrFeNiMo | 3.45 | 4.44 | 77.7 | 2.47 | 39.72 |
| Co0.5CrFeNiMo | 3.51 | 4.65 | 75.5 | 2.56 | 40.91 |
| Al0.5CrFeNiMo | 3.28 | 4.35 | 75.4 | 4.53 | 35.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geambazu, L.E.; Manea, C.A.; Mateș, I.M.; Pătroi, D.; Sbârcea, G.B.; Manta, E.; Semenescu, A. Effect of Co and Al Content on CrFeNiMo-System High Entropy Alloys Produced by Mechanical Alloying. Materials 2025, 18, 1936. https://doi.org/10.3390/ma18091936
Geambazu LE, Manea CA, Mateș IM, Pătroi D, Sbârcea GB, Manta E, Semenescu A. Effect of Co and Al Content on CrFeNiMo-System High Entropy Alloys Produced by Mechanical Alloying. Materials. 2025; 18(9):1936. https://doi.org/10.3390/ma18091936
Chicago/Turabian StyleGeambazu, Laura Elena, Ciprian Alexandru Manea, Ileana Mariana Mateș, Delia Pătroi, Gabriela Beatrice Sbârcea, Eugen Manta, and Augustin Semenescu. 2025. "Effect of Co and Al Content on CrFeNiMo-System High Entropy Alloys Produced by Mechanical Alloying" Materials 18, no. 9: 1936. https://doi.org/10.3390/ma18091936
APA StyleGeambazu, L. E., Manea, C. A., Mateș, I. M., Pătroi, D., Sbârcea, G. B., Manta, E., & Semenescu, A. (2025). Effect of Co and Al Content on CrFeNiMo-System High Entropy Alloys Produced by Mechanical Alloying. Materials, 18(9), 1936. https://doi.org/10.3390/ma18091936









