Initial Bonding Performance to CAD/CAM Restorative Materials: The Impact of Stepwise Concentration Variation in 8-Methacryloxyoctyl Trimethoxy Silane and 3-Methacryloxypropyl Trimethoxy Silane on Feldspathic Ceramic, Lithium Disilicate Glass-Ceramic, and Polymer-Infiltrated Ceramic
Abstract
:1. Introduction
2. Materials and Methods
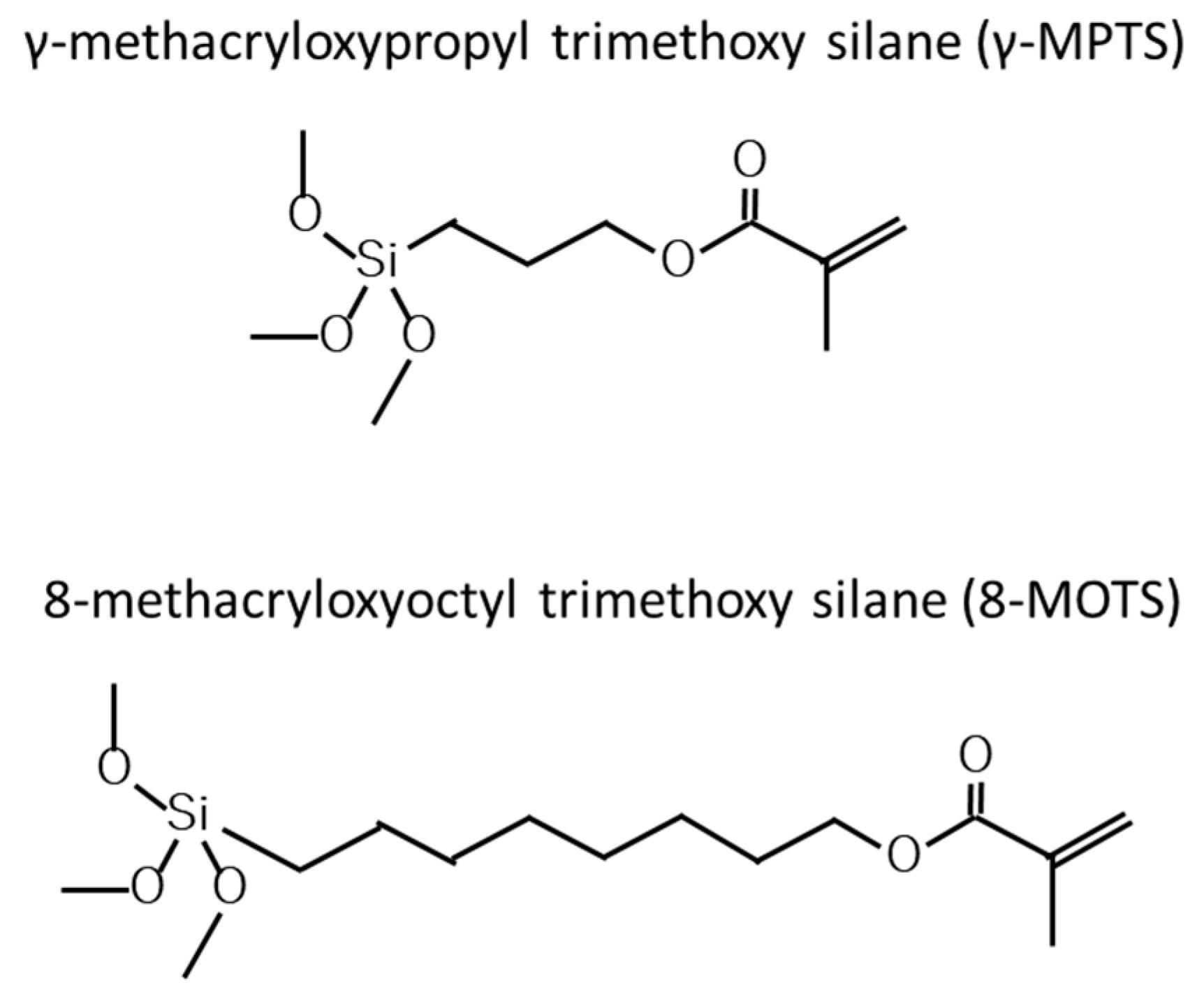
2.1. Surface Treatment
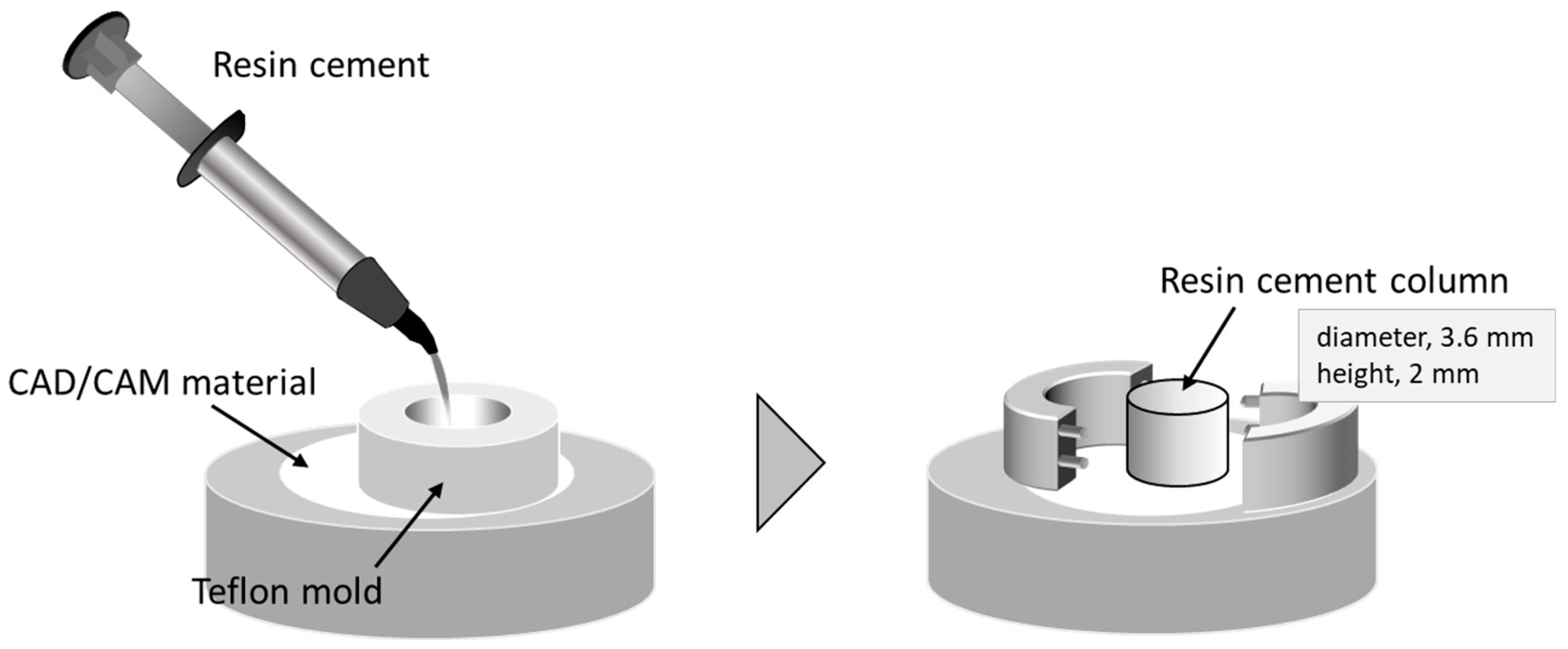
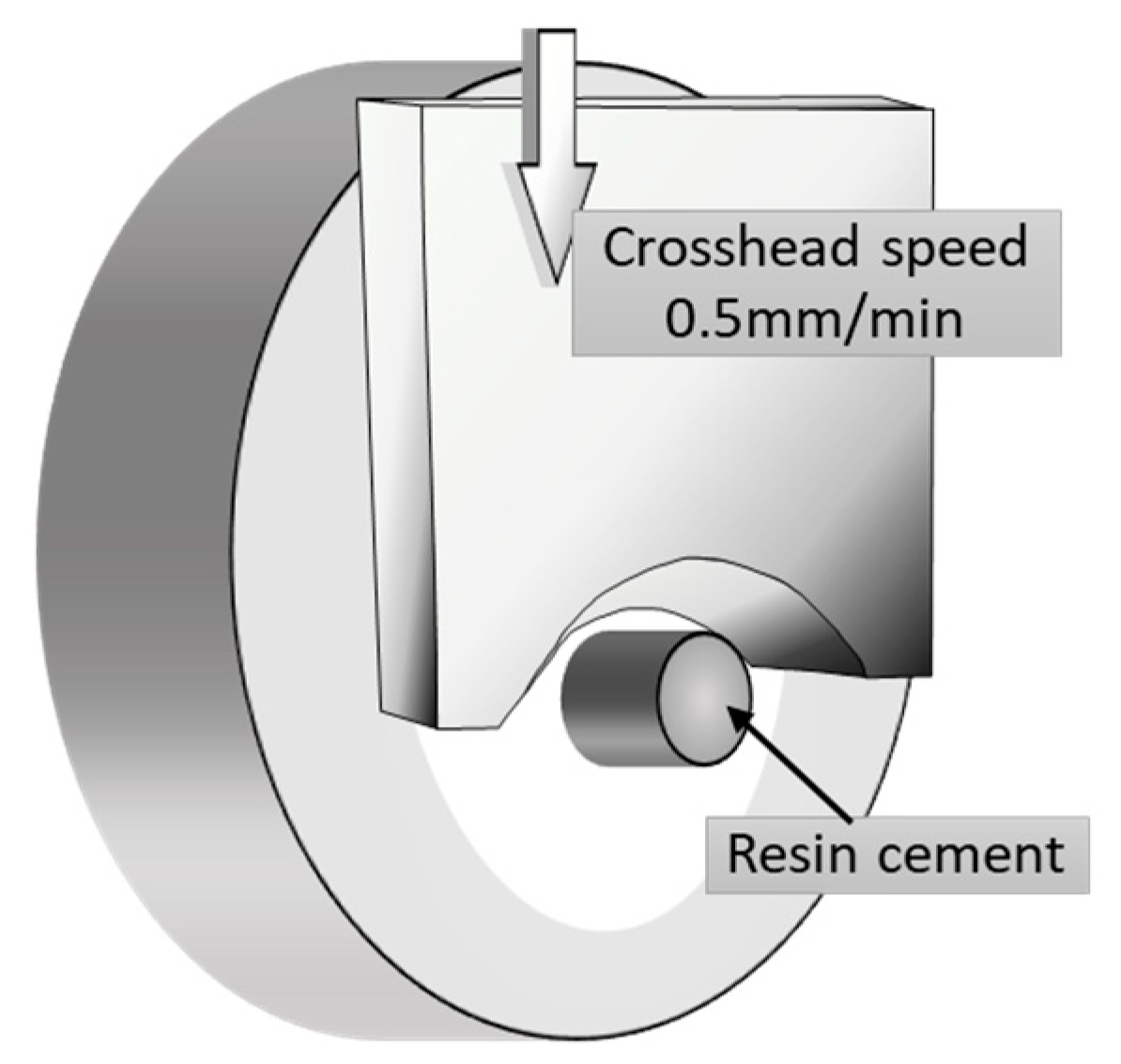
2.2. Shear Bond Strength Test
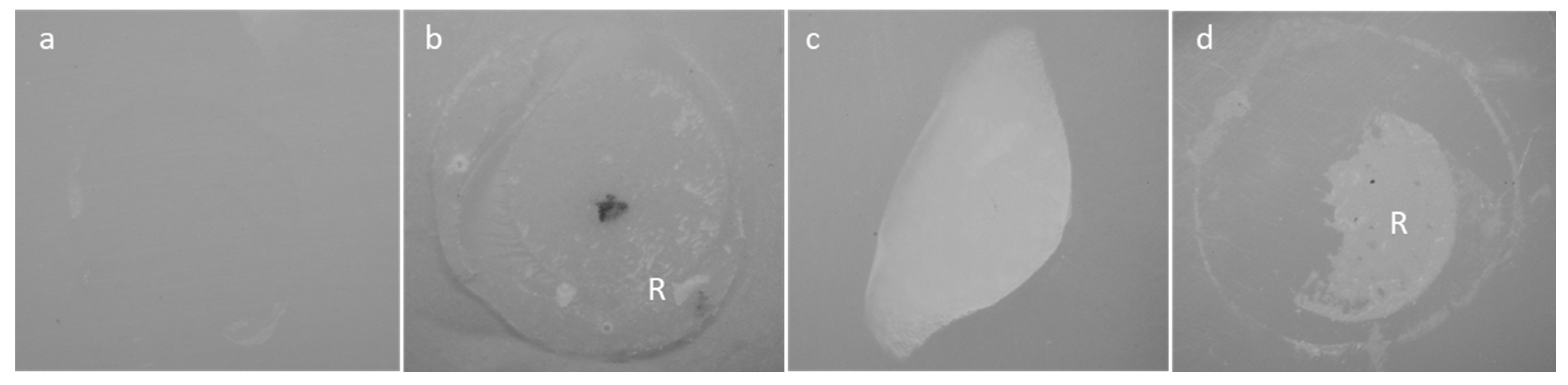
2.3. Failure Analysis and Statistical Analysis
3. Results
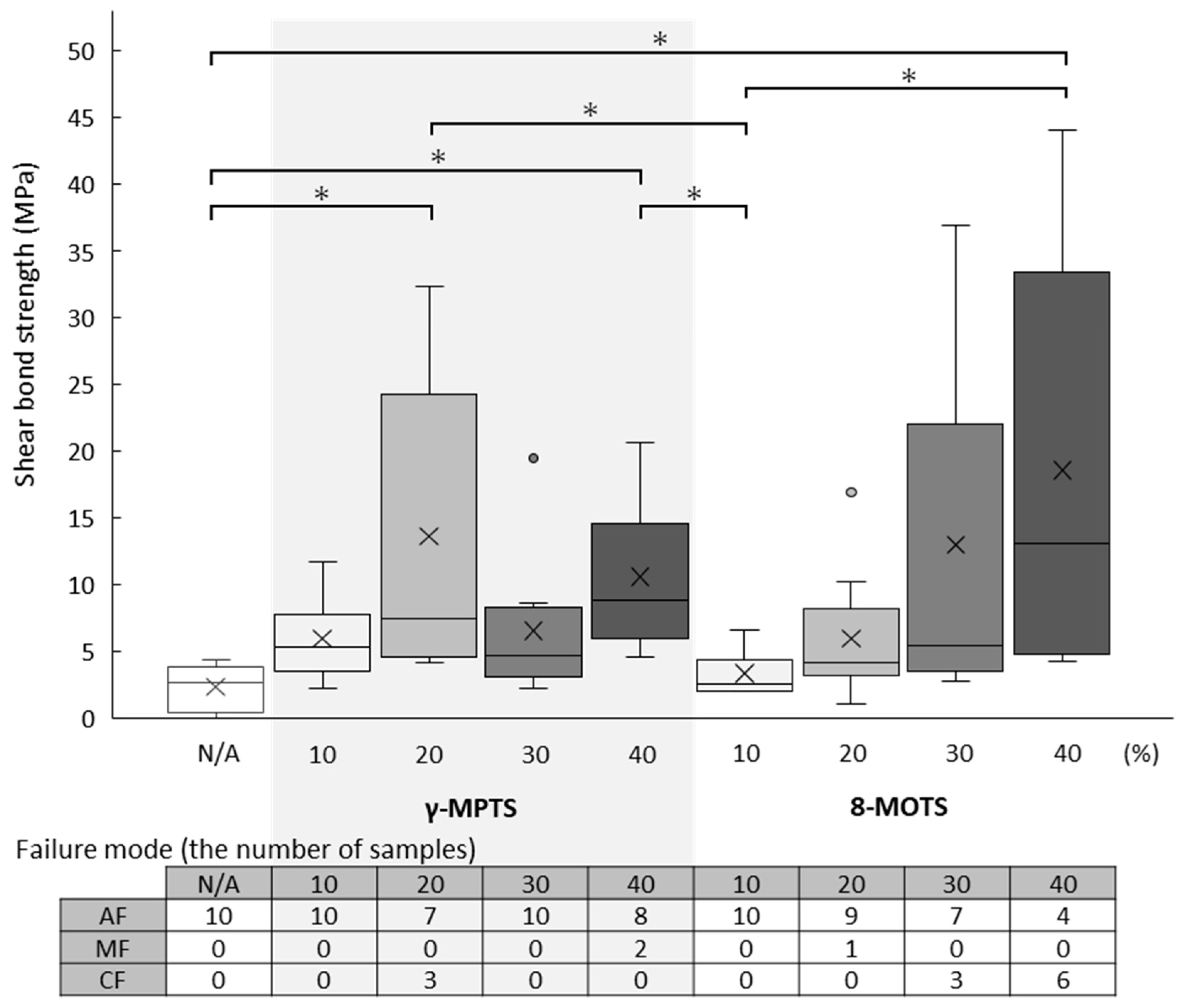
3.1. FC Shear Bond Strength
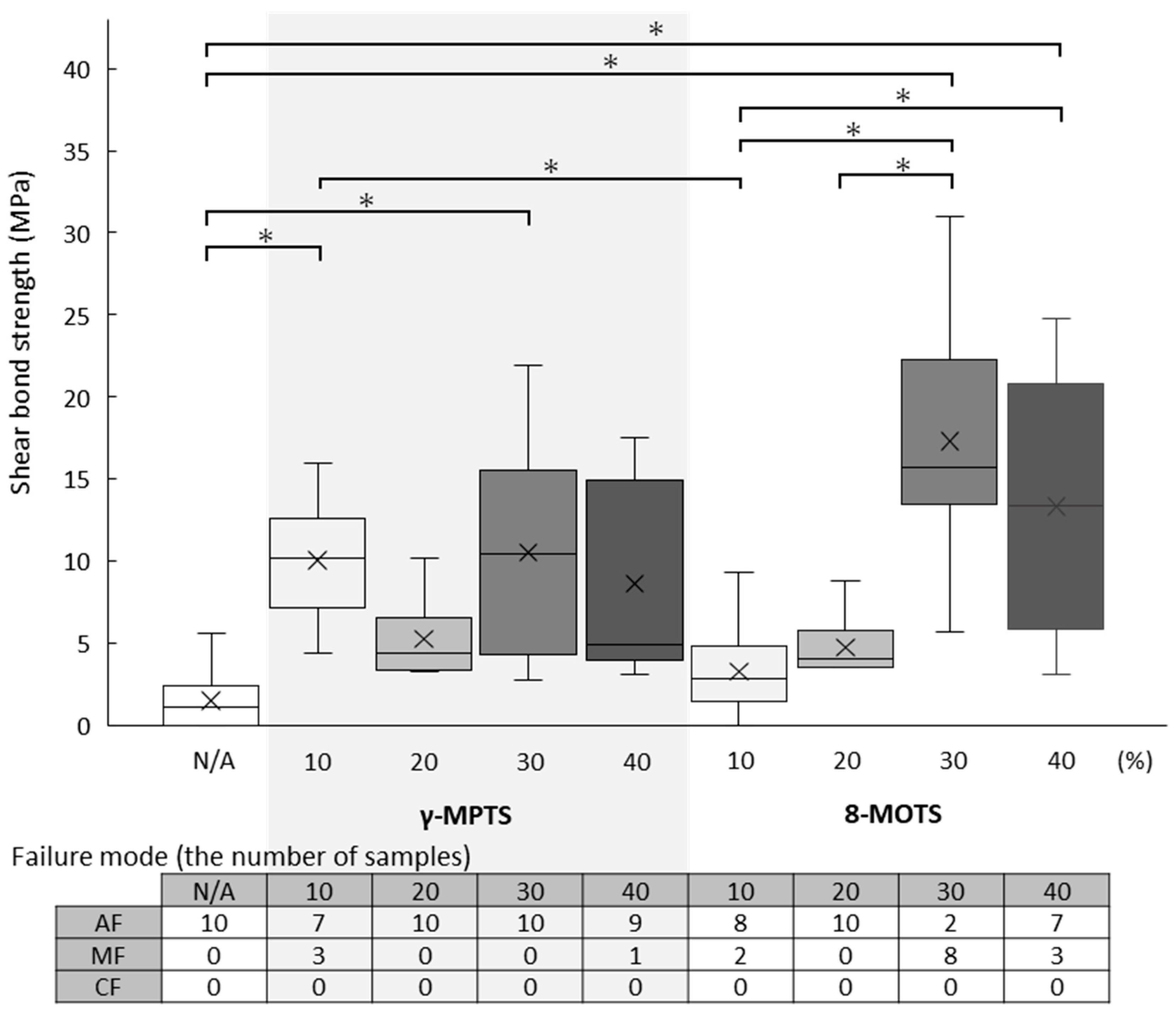
3.2. LD Shear Bond Strength
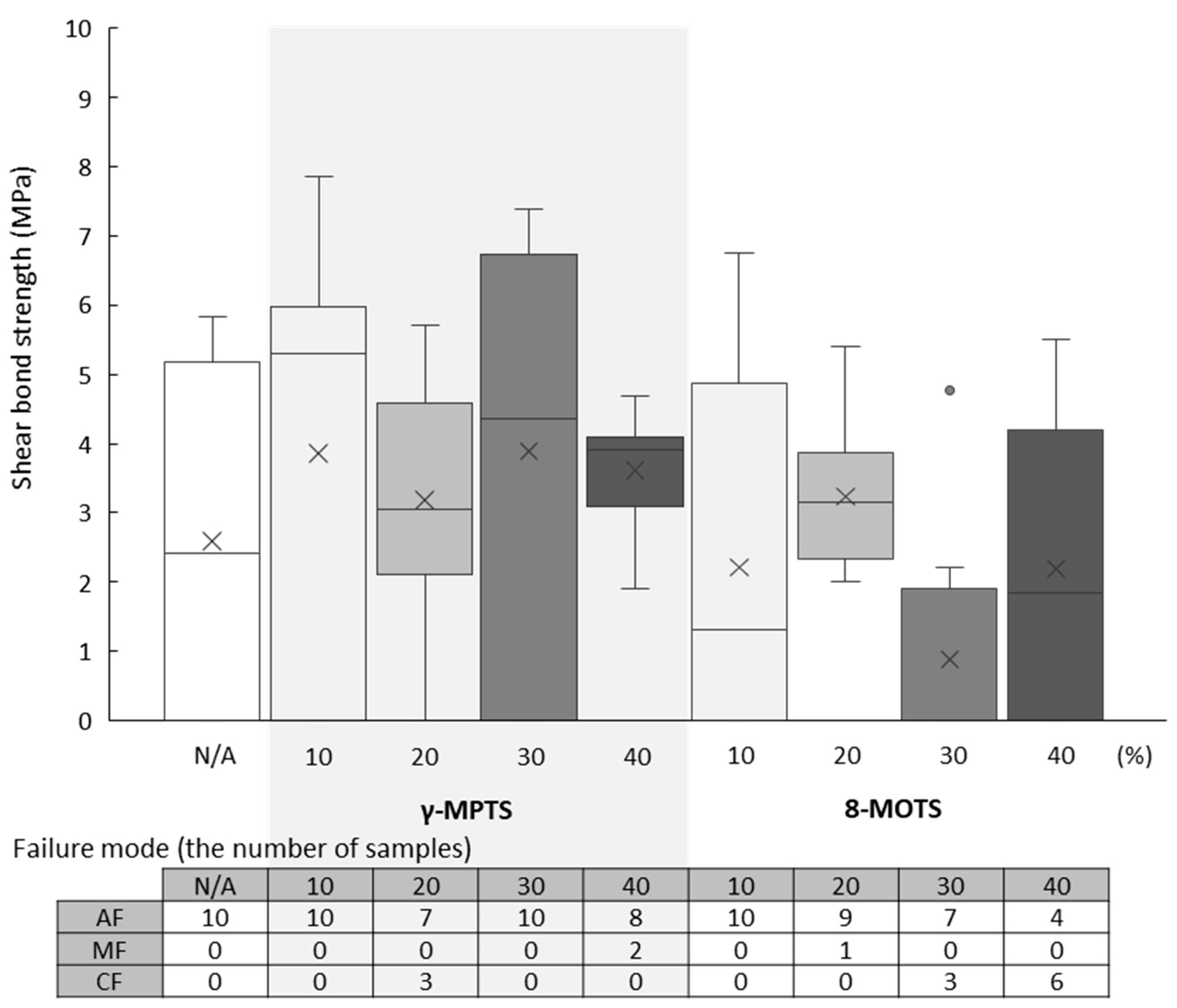
3.3. PIC Shear Bond Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mavriqi, L.; Valente, F.; Murmura, G.; Sinjari, B.; Macrì, M.; Trubiani, O.; Caputi, S.; Traini, T. Lithium disilicate and zirconia reinforced lithium silicate glass-ceramics for CAD/CAM dental restorations: Biocompatibility, mechanical and microstructural properties after crystallization. J. Dent. 2022, 119, 104054. [Google Scholar] [CrossRef]
- Vichi, A.; Louca, C.; Corciolani, G.; Ferrari, M. Color related to ceramic and zirconia restorations: A review. Dent. Mater. 2011, 27, 97–108. [Google Scholar] [CrossRef]
- Zarone, F.; Ruggiero, G.; Leone, R.; Breschi, L.; Leuci, S.; Sorrentino, R. Zirconia-reinforced lithium silicate (ZLS) mechanical and biological properties: A literature review. J. Dent. 2021, 109, 103661. [Google Scholar] [CrossRef]
- Joda, T.; Ferrari, M.; Brägger, U. Monolithic implant-supported lithium disilicate (LS2) crowns in a complete digital workflow: A prospective clinical trial with a 2 year follow-up. Clin. Implant Dent. Relat. Res. 2017, 19, 505–511. [Google Scholar] [CrossRef]
- Joda, T.; Brägger, U. Complete digital workflow for the production of implant-supported single-unit monolithic crowns. Clin. Oral Implants Res. 2014, 25, 1304–1306. [Google Scholar] [CrossRef]
- Abad-Coronel, C.; Ordoñez Balladares, A.; Fajardo, J.I.; Martín Biedma, B.J. Resistance to fracture of lithium disilicate feldspathic restorations manufactured using a CAD/CAM system and crystallized with different thermal units and programs. Materials 2021, 14, 3215. [Google Scholar] [CrossRef]
- Edelhoff, D.; Ozcan, M. To what extent does the longevity of fixed dental prostheses depend on the function of the cement? Working Group 4 materials: Cementation. Clin. Oral Implants Res. 2007, 18, 193–204. [Google Scholar] [CrossRef]
- Porto, T.S.; Medeiros da Silva, I.G.; de Freitas Vallerini, B.; Fernando de Goes, M. Different surface treatment strategies on etchable CAD-CAM materials: Part II-Effect on the bond strength. J. Prosthet. Dent. 2023, 130, 770–779. [Google Scholar] [CrossRef]
- Graiff, L.; Piovan, C.; Vigolo, P.; Mason, P.N. Shear bond strength between feldspathic CAD/CAM ceramic and human dentine for two adhesive cements. J. Prosthodont. 2008, 17, 294–299. [Google Scholar] [CrossRef]
- Shimakura, Y.; Hotta, Y.; Fujishima, A.; Kunii, J.; Miyazaki, T.; Kawawa, T. Bonding strength of resin cement to silicate glass ceramics for dental CAD/CAM systems is enhanced by combination treatment of the bonding surface. Dent. Mater. J. 2007, 26, 713–721. [Google Scholar] [CrossRef]
- Dapieve, K.S.; Machry, R.V.; Pereira, G.K.R.; Venturini, A.B.; Valcanaia, A.; Bottino, M.C.; Valandro, L.F. Alumina particle air-abrasion and aging effects: Fatigue behavior of CAD/CAM resin composite crowns and flexural strength evaluations. J. Mech. Behav. Biomed. Mater. 2021, 121, 104592. [Google Scholar] [CrossRef]
- Chen, B.; Lu, Z.; Meng, H.; Chen, Y.; Yang, L.; Zhang, H.; Xie, H.; Chen, C. Effectiveness of pre-silanization in improving bond performance of universal adhesives or self-adhesive resin cements to silica-based ceramics: Chemical and in vitro evidences. Dent. Mater. 2019, 35, 543–553. [Google Scholar] [CrossRef]
- Makishi, P.; André, C.B.; Silva, J.L.E.; Bacelar-Sá, R.; Correr-Sobrinho, L.; Giannini, M. Effect of storage time on bond strength performance of multimode adhesives to indirect resin composite and lithium disilicate glass ceramic. Oper. Dent. 2016, 41, 541–551. [Google Scholar] [CrossRef]
- Kern, M.; Thompson, V.P. Bonding to glass infiltrated alumina ceramic: Adhesive methods and their durability. J. Prosthet. Dent. 1995, 73, 240–249. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef]
- Ergun-Kunt, G.; Sasany, R.; Koca, M.F.; Özcan, M. Comparison of silane heat treatment by laser and various surface treatments on microtensile bond strength of composite resin/lithium disilicate. Materials 2021, 14, 7808. [Google Scholar] [CrossRef]
- Monteiro, J.B.; Prado, P.H.C.O.; Ribeiro Zucco, G.; Campos, T.M.B.; Machado, J.P.B.; Trava-Airoldi, V.J.; Melo, R.M. High-translucency zirconia following chemical vapor deposition with SiH4: Evidence of surface modifications and improved bonding. J. Adhes. Dent. 2023, 25, b3801051. [Google Scholar] [CrossRef]
- Queiroz, J.R.; Benetti, P.; Ozcan, M.; de Oliveira, L.F.; Della Bona, A.; Takahashi, F.E.; Bottino, M.A. Surface characterization of feldspathic ceramic using ATR FT-IR and ellipsometry after various silanization protocols. Dent. Mater. 2012, 28, 189–196. [Google Scholar] [CrossRef]
- Ishida, H.; Koenig, J. A fourier-transform infrared spectroscopic study of the hydrolytic stability of silane coupling agents on E-glass fibers. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 1931–1943. [Google Scholar] [CrossRef]
- Hooshmand, T.; van Noort, R.; Keshvad, A. Bond durability of the resin-bonded and silane treated ceramic surface. Dent. Mater. 2002, 18, 179–188. [Google Scholar] [CrossRef]
- Thadathil Varghese, J.; Cho, K.; Raju; Farrar, P.; Prentice, L.; Prusty, B.G. Influence of silane coupling agent on the mechanical performance of flowable fibre-reinforced dental composites. Dent. Mater. 2022, 38, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, K.; Hirota, M.; Nomoto, R.; Hayakawa, T. Bond strength and computational analysis for silane coupling treatments on the adhesion of resin block for CAD/CAM crowns. Dent. Mater. J. 2020, 39, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.T.; Cho, K.; Farrar, P.; Prentice, L.; Prusty, B.G. Effect of silane coupling agent and concentration on fracture toughness and water sorption behaviour of fibre-reinforced dental composites. Dent. Mater. 2023, 39, 362–371. [Google Scholar] [CrossRef]
- Arksornnukit, M.; Takahashi, H.; Nishiyama, N.; Pavasant, P. Effects of heat and pH in silanation process on flexural properties and hydrolytic durabilities of composite resin after hot water storage. Dent. Mater. J. 2004, 23, 175–179. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.F.; Martins, M.E.; de Queiroz, J.R.; Leite, F.P.; Ozcan, M. Influence of silane heat treatment on bond strength of resin cement to a feldspathic ceramic. Dent. Mater. J. 2011, 30, 392–397. [Google Scholar] [CrossRef]
- Silva, U.P.C.; Maia, A.P.; Silva, I.D.; Miranda, M.E.; Brandt, W.C. Influence of the multiple layers application and the heating of silane on the bond strength between lithium disilicate ceramics and resinous cement. Eur. J. Dent. 2021, 15, 720–726. [Google Scholar] [CrossRef]
- Abi-Rached, F.O.; Martins, S.B.; Almeida-Júnior, A.A.; Adabo, G.L.; Góes, M.S.; Fonseca, R.G. Air abrasion before and/or after zirconia sintering: Surface characterization, flexural strength, and resin cement bond strength. Oper. Dent. 2015, 40, E66–E75. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.; de Jager, N.; Campos, F.; Valandro, L.F.; Kleverlaan, C.J. Does luting strategy affect the fatigue behavior of bonded Y-TZP ceramic? J. Adhes. Dent. 2018, 20, 307–315. [Google Scholar] [CrossRef]
- Flinn, B.D.; deGroot, D.A.; Mancl, L.A.; Raigrodski, A.J. Accelerated aging characteristics of three yttria-stabilized tetragonal zirconia polycrystalline dental materials. J. Prosthet. Dent. 2012, 108, 223–230. [Google Scholar] [CrossRef]
| Brand Name | Description | Manufacturer |
|---|---|---|
| ARCTICA VITA Mark II (FC) | Feldspathic ceramic SiO2 (56–64%), Al2O3 (20–23%), Na2O (6–9%) and K2O (6–8%) | Vita Zahnfabrik Bad Säckingen, Germany |
| Litium discilicate ceramic (LD) | Lithium disilicate glass-ceramic SiO2 (57–80%), Li2O (11–19%), K2O (0–13%), P2O2 (0–11%), ZrO2 (0–8%), ZnO (0–8%), other oxides and ceramic pigments (0–10%) | Ivoclar Vivadent AG, Schaan, Liechtenstein |
| KATANA AVENCIA Block (PIC) | Polymer-infiltrated ceramic Al2O3 (20 nm) and SiO2 (40 nm) (62%), UDMA and TEGDMA | Kuraray Noritake Dental, Tokyo, Japan |
| Silane solution | γ-MPTS (10.0, 20.0, 30.0, 40.0 wt%) in ethanol 8-MOTS (10.0, 20.0, 30.0, 40.0 wt%) in ethanol | Shin-Etsu Chemical, Tokyo, Japan |
| Acetic acid solution | Acetic acid (2.0%), ethanol (70.0%) and water (28.0%) | Wako Pure Chemical Industries, Osaka, Japan |
| PANAVIA Veneer LC | Silanated spherical silica filler, UDMA, ytterbium trifluoride, TEGDMA, hydrophilic aliphatic dimethacrylate, hydrophilic amide monomer, accelerators, DL-Camphorquinone and pigments | Kuraray Noritake Dental, Tokyo, Japan |
| N/A | γ-MPTS | 8-MOTS | ||||||
|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 10 | 20 | 30 | 40 | |
| 2.6 | 5.3 | 7.4 | 4.7 | 8.8 | 2.6 | 4.1 | 5.4 | 13.1 |
| (0–4.3) | (2.2–11.7) | (4.2–32.4) | (2.2–19.5) | (4.6–20.6) | (2.0–6.6) | (1.1–16.9) | (2.8–36.9) | (4.3–44.1) |
| 1.1 | 10.2 | 4.4 | 10.5 | 5.0 | 2.9 | 4.1 | 15.8 | 13.4 |
| (0–5.6) | (4.4–16.0) | (3.3–10.2) | (2.8–21.9) | (3.1–17.5) | (0–9.3) | (3.5–8.8) | (5.7–31.0) | (3.1–24.8) |
| 2.4 | 5.3 | 3.1 | 4.4 | 3.9 | 1.3 | 3.2 | 0 | 1.8 |
| (0–5.8) | (0–7.9) | (0–5.7) | (0–7.4) | (1.9–4.7) | (0–6.8) | (2.0–5.4) | (0–4.8) | (0–5.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruo, Y.; Kuwahara, M.; Yoshihara, K.; Irie, M.; Nagaoka, N.; Yoshizane, M.; Matsumoto, T.; Akiyama, K. Initial Bonding Performance to CAD/CAM Restorative Materials: The Impact of Stepwise Concentration Variation in 8-Methacryloxyoctyl Trimethoxy Silane and 3-Methacryloxypropyl Trimethoxy Silane on Feldspathic Ceramic, Lithium Disilicate Glass-Ceramic, and Polymer-Infiltrated Ceramic. Materials 2025, 18, 1983. https://doi.org/10.3390/ma18091983
Maruo Y, Kuwahara M, Yoshihara K, Irie M, Nagaoka N, Yoshizane M, Matsumoto T, Akiyama K. Initial Bonding Performance to CAD/CAM Restorative Materials: The Impact of Stepwise Concentration Variation in 8-Methacryloxyoctyl Trimethoxy Silane and 3-Methacryloxypropyl Trimethoxy Silane on Feldspathic Ceramic, Lithium Disilicate Glass-Ceramic, and Polymer-Infiltrated Ceramic. Materials. 2025; 18(9):1983. https://doi.org/10.3390/ma18091983
Chicago/Turabian StyleMaruo, Yukinori, Miho Kuwahara, Kumiko Yoshihara, Masao Irie, Noriyuki Nagaoka, Mai Yoshizane, Takuya Matsumoto, and Kentaro Akiyama. 2025. "Initial Bonding Performance to CAD/CAM Restorative Materials: The Impact of Stepwise Concentration Variation in 8-Methacryloxyoctyl Trimethoxy Silane and 3-Methacryloxypropyl Trimethoxy Silane on Feldspathic Ceramic, Lithium Disilicate Glass-Ceramic, and Polymer-Infiltrated Ceramic" Materials 18, no. 9: 1983. https://doi.org/10.3390/ma18091983
APA StyleMaruo, Y., Kuwahara, M., Yoshihara, K., Irie, M., Nagaoka, N., Yoshizane, M., Matsumoto, T., & Akiyama, K. (2025). Initial Bonding Performance to CAD/CAM Restorative Materials: The Impact of Stepwise Concentration Variation in 8-Methacryloxyoctyl Trimethoxy Silane and 3-Methacryloxypropyl Trimethoxy Silane on Feldspathic Ceramic, Lithium Disilicate Glass-Ceramic, and Polymer-Infiltrated Ceramic. Materials, 18(9), 1983. https://doi.org/10.3390/ma18091983







