Patterns of Diversity in the Symbiotic Mite Assemblage of the Mountain Pine Beetle, Dendroctonus Ponderosae Hopkins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mite Collection
2.2. Assessing Climatic Conditions
2.3. Mite Data Analysis
3. Results
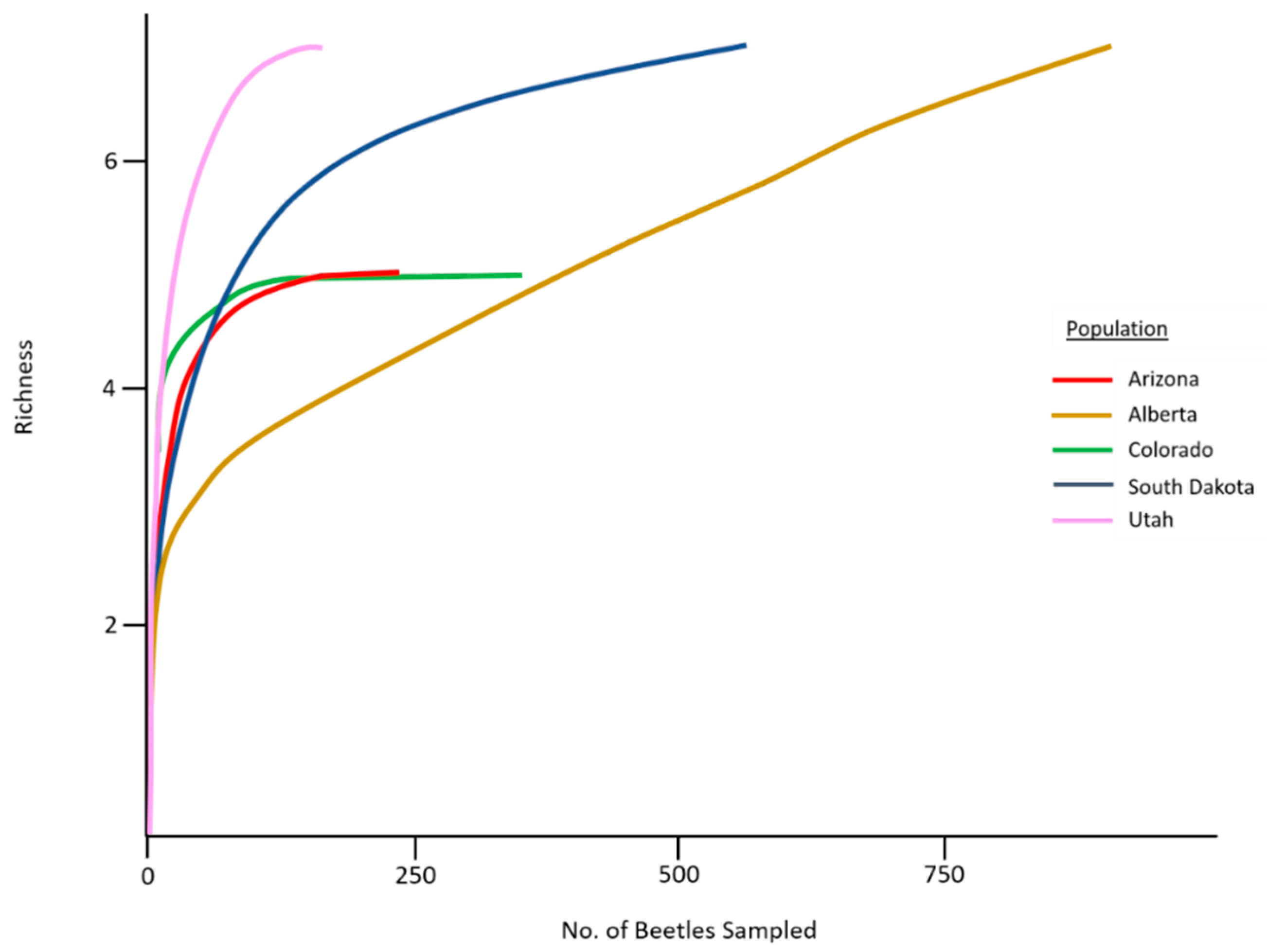
3.1. Mite Abundance, Taxa Found, and Species Richness
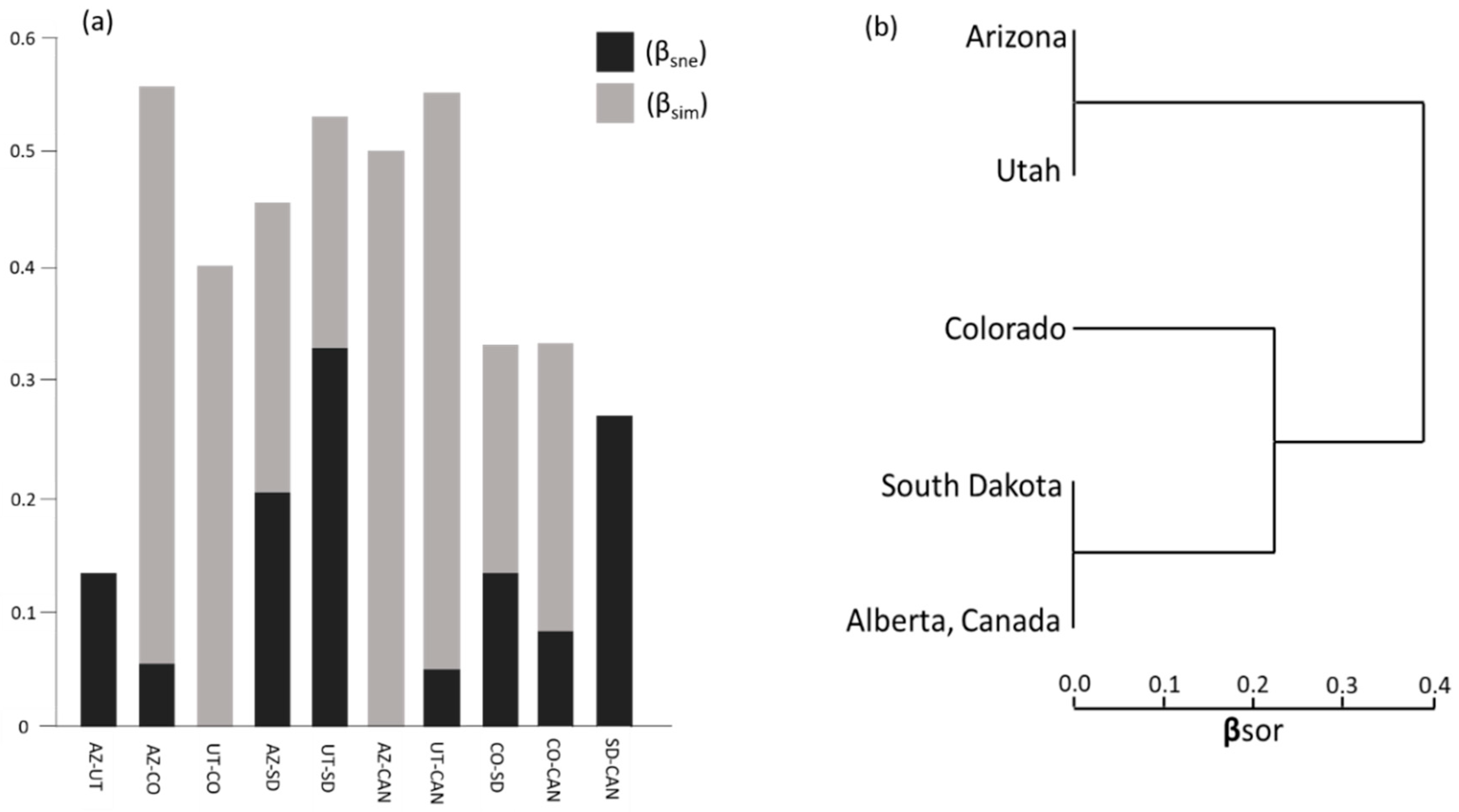
3.2. Partitioning Compositional Differences Using Measures of Beta Diversity
4. Discussion
4.1. Differences in Species Richness and Abundance
4.2. Beta Diversity Patterns
4.3. Implications for Shifts in Functional Groups of Mites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hart, S.J.; Veblen, T.T.; Schneider, D.; Molotch, N.P. Summer and winter drought drive the initiation and spread of spruce beetle outbreak. Ecology 2017, 98, 2698–2707. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, P.H.; Müller, J.; Grégoire, J.C.; Gruppe, A.; Hagge, J.; Hammerbacher, A.; Hofstetter, R.W.; Kandasamy, D.; Kolarik, M.; Kostovcik, M.; et al. Bark beetle population dynamics in the Anthropocene: Challenges and solutions. Trends Ecol. Evol. 2019, 34, 914–924. [Google Scholar] [CrossRef] [Green Version]
- De Groot, M.; Ogris, N. Short-term forecasting of bark beetle outbreaks on two economically important conifer tree species. For. Ecol. Manag. 2019, 450, e117495. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef] [Green Version]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, M.E.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. BioScience 2010, 60, 601–613. [Google Scholar] [CrossRef]
- Bentz, B.J.; Bracewell, R.R.; Mock, K.E.; Pfrender, M.E. Genetic architecture and phenotypic plasticity of thermally-regulated traits in an eruptive species, Dendroctonus ponderosae. Evol. Ecol. 2011, 25, 1269–1288. [Google Scholar] [CrossRef]
- Cullingham, C.I.; Cooke, J.E.K.; Dang, S.; Davis, C.S.; Cooke, B.J.; Coltman, D.W. Mountain pine beetle host-range expansion threatens the boreal forest. Mol. Ecol. 2011, 20, 2157–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffa, K.F.; Powell, E.N.; Townsend, P.A. Temperature-driven range expansion of an irruptive insect heightened by weakly coevolved plan defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 2193–2198. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.L. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytinae), a taxonomic monograph. Great Basin Nat. Mem. 1982, 6, 1–1356. [Google Scholar]
- Rosenberger, D.W.; Venette, R.C.; Maddox, M.P.; Aukema, B.H. Colonization behaviors of mountain pine beetle on novel hosts: Implications for range expansion into northeastern North America. PLoS ONE 2017, 12, e0176269. [Google Scholar] [CrossRef] [Green Version]
- Reid, R.W. Biology of the mountain pine beetle, Dendroctonus monticolae Hopkins, in the east Kootenay region of British Columbia, I. Life cycle, brood development, and flight periods. Can. Entomol. 1962, 94, 531–538. [Google Scholar] [CrossRef]
- Amman, G.D.; McGregor, M.D.; Dolph, R.E. Reprinted. (Updated 2002). Mountain pine beetle. USDA Forest Service. For. Insect Dis. Leafl. 1990, 2, 1–12. [Google Scholar]
- Six, D.L.; Bracewell, R. Dendroctonus. Bark Beetle; Elsevier: Amsterdam, The Netherlands, 2015; pp. 305–350. [Google Scholar]
- Hofstetter, R.W.; Dinkins-Bookwalter, J.; Davis, T.S.; Klepzig, K.D. Symbiotic associations of bark beetles. Bark Beetles; Elsevier: Amsterdam, The Netherlands, 2015; pp. 209–245. [Google Scholar]
- Vissa, S.; Hofstetter, R.W. The role of mites in bark and ambrosia beetle–fungal interactions. In Insect Physiology and Ecology; Intech Press: London, UK, 2017; pp. 135–156. [Google Scholar]
- Mercado, J.; Ortiz-Santana, B.; Kay, S. Fungal Frequency and Mite Load Trends Interact with a Declining Mountain Pine Beetle Population. Forests 2018, 9, 484. [Google Scholar] [CrossRef] [Green Version]
- Six, D.L.; Paine, T.D. Effects of Mycangial Fungi and Host Tree Species on Progeny Survival and Emergence of Dendroctonus ponderosae (Coleoptera: Scolytidae). Environ. Entomol. 1998, 27, 1393–1401. [Google Scholar] [CrossRef]
- Mock, K.E.; Bentz, B.J.; O’Neill, E.M.; Chong, J.P.; Orwin, J.; Pfrender, M.E. Landscape-scale genetic variation in a forest outbreak species, the mountain pine beetle (Dendroctonus ponderosae). Mol. Ecol. 2007, 16, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.W.A.; Borden, J.H.; Rahe, J.E.; Whitney, H.S. Nutrient-mediated germination of Beauveria bassiana conidia on the integument of the bark beetle Dendroctonus ponderosae (Coleoptera: Scolytidae). J. Invertebr. Pathol. 1984, 44, 304–314. [Google Scholar] [CrossRef]
- Wegensteiner, R. Pathogens in bark beetles. In Bark and Wood Boring Insects in Living Trees in Europe: A Synthesis; Springer: Dordrecht, The Netherlands, 2007; pp. 291–313. [Google Scholar]
- Mercado, J.E.; Hofstetter, R.W.; Reboletti, D.M.; Negro, F. Phoretic symbionts of the mountain pine beetle (Dendroctonus ponderosae Hopkins). For. Sci. 2014, 60, 512–526. [Google Scholar] [CrossRef] [Green Version]
- Klepzig, K.D.; Moser, J.C.; Lombardero, F.J.; Hofstetter, R.W.; Ayres, M.P. Symbiosis and competition: Complex interactions among beetles, fungi, and mites. Symbiosis 2001, 30, 83–96. [Google Scholar]
- Cardoza, Y.J.; Moser, J.C.; Klepzig, K.D.; Raffa, K.F. Multipartite symbioses among fungi, mites, nematodes, and the spruce beetle, Dendroctonus rufipennis. Environ. Entomol. 2008, 37, 956–963. [Google Scholar] [CrossRef]
- Knee, W.; Beaulieu, F.; Skevington, J.H.; Kelso, S.; Cognato, A.I.; Forbes, M.R. Species boundaries and host range of tortoise mites (Uropodoidea) phoretic on bark beetles (Scolytinae), using morphometric and molecular markers. PLoS ONE 2012, 7, e47243. [Google Scholar] [CrossRef]
- Knee, W.; Beaulieu, F.; Skevington, J.H.; Kelso, S.; Forbes, M.R. Cryptic species of mites (Uropodoidea: Uroobovella spp.) associated with burying beetles (Silphidae: Nicrophorus): The collapse of a host generalist revealed by molecular and morphological analyses. Mol. Phylogenet. Evol. 2012, 65, 276–286. [Google Scholar] [CrossRef]
- Moser, J.C. Mite predators of the southern pine beetle. Ann. Entomol. Soc. Am. 1975, 68, 1113–1116. [Google Scholar] [CrossRef]
- Moser, J.C. Phoretic carrying capacity of flying southern pine beetles (Coleoptera: Scolytidae). Can. Entomol. 1976, 108, 807–808. [Google Scholar] [CrossRef] [Green Version]
- Moser, J.C. Use of sporothecae by phoretic Tarsonemus mites to transport ascospores of coniferous bluestain fungi. Trans. Br. Mycol. Soc. 1985, 84, 750–753. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Moser, J.; Blomquist, S. Mites associated with bark beetles and their hyperphoretic ophiostomatoid fungi. Biodivers. Ser. 2014, 12, 165–176. [Google Scholar]
- Moser, J.C.; Perry, T.J.; Solheim, H. Ascospores hyperphoretic on mites associated with Ips typographus. Mycol. Res. 1989, 93, 513–517. [Google Scholar] [CrossRef]
- Moser, J.C.; Konrad, H.; Blomquist, S.R.; Kirisits, T. Do mites phoretic on elm bark beetles contribute to the transmission of Dutch elm disease? Naturwissenschaften 2010, 97, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Lindquist, E.E. New species of Tarsonemus (Acarina: Tarsonemidae) associated with bark beetles. Can. Entomol. 1969, 101, 1219–1314. [Google Scholar] [CrossRef]
- Kinn, D.; Witcosky, J. Variation in southern pine beetle attack height associated with phoretic Uropodid mites. Can. Entomol. 1978, 110, 249–251. [Google Scholar] [CrossRef]
- Wilson, D.S.; Knollenberg, W.G. Adaptive indirect effects: The fitness of burying beetles with and without their phoretic mites. Evol. Ecol. 1987, 1, 139–159. [Google Scholar] [CrossRef]
- Vissa, S.; Hofstetter, R.W.; Bonifácio, L.; Khaustov, A.; Knee, W.; Uhey, D.A. Phoretic mite communities associated with bark beetles in the maritime and stone pine forests of Setúbal, Portugal. Exp. Appl. Acarol. 2019, 77, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Lombardero, Â.J.; Klepzig, K.D.; Moser, J.C.; Ayres, M.P. Biology, demography and community interactions of Tarsonemus (Acarina: Tarsonemidae) mites phoretic on Dendroctonus frontalis (Coleoptera: Scolytidae). Agric. For. Entomol. 2001, 2, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Lombardero, M.J.; Ayres, M.P.; Hofstetter, R.W.; Moser, J.C.; Klepzig, K.D. Strong indirect interactions of Tarsonemus mites (Acarina: Tarsonemidae) and Dendroctonus frontalis (Coleoptera: Scolytidae). Oikos 2003, 102, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Hofstetter, R.W.; Dempsey, T.D.; Klepzig, K.D.; Ayres, M.P. Temperature-dependent effects on mutualistic and phoretic associations. Community Ecol. 2007, 8, 47–56. [Google Scholar] [CrossRef]
- Walter, D.E.; Proctor, H.C. Mites on plants. In Mites: Ecology, Evolution & Behavior; Springer: Dordrecht, The Netherlands, 2013; pp. 281–339. [Google Scholar]
- Walter, D.E.; Proctor, H.C. Mites: Ecology, Evolution and Behaviour; CABI Publishing: Wallingford, UK, 1999; pp. 52–55. [Google Scholar]
- Hofstetter, R.W.; Moser, J.C. The role of mites in insect-fungus associations. Ann. Rev. Entomol. 2014, 59, 537–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, B.A.; Proctor, H.C.; Walter, D.E.; Evenden, M.L. Phoretic mite associates of mountain pine beetle at the leading edge of an infestation in northwestern Alberta, Canada. Can. Entomol. 2011, 143, 44–55. [Google Scholar] [CrossRef]
- Lindgren, B.S. A multiple funnel trap for scolytid beetles (Coleoptera). Can. Entomol. 1983, 115, 299–302. [Google Scholar] [CrossRef]
- Reboletti, D.M. A Multi-Partite Mutualism: Bark Beetles, Fungi, and Mites. Ph.D. Thesis, Northern Arizona University, Flagstaff, AZ, USA, 2008. [Google Scholar]
- Davis, T.S.; Hofstetter, R.W. The effects of gallery density and ratio on the fitness and fecundity of two sympatric bark beetles. Environ. Entomol. 2009, 38, 639–650. [Google Scholar] [CrossRef]
- PRISM Climate Group, Oregon State University. Available online: http://prism.oregonstate.edu (accessed on 31 August 2020).
- Canada’s Changing Climate Report. Natural Resources Canada, Government of Canada, 2019. Available online: https://www.nrcan.gc.ca/maps-tools-publications/publications/climate-change-publications/canada-changing-climate-reports/canadas-changing-climate-report/21177 (accessed on 31 August 2020).
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S.S. The vegan package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Exploratory multivariate analysis. In Modern Applied Statistics with S; Springer: New York, NY, USA, 2002; pp. 301–330. [Google Scholar]
- Wang, Y.I.; Naumann, U.; Wright, S.T.; Warton, D.I. mvabund–an R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- Akaike, H. Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika 1973, 60, 255–265. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning abundance-based multiple-site dissimilarity into components: Balanced variation in abundance and abundance gradients. Methods Ecol. Evol 2017, 7, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Baselga, A.; Orme, C.D.L. Betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Peralta Vázquez, G.H. Ecology of Mite Phoresy on Mountain Pine Beetles. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 2018. [Google Scholar]
- Hofstetter, R.W.; Cronin, J.T.; Klepzig, K.D.; Moser, J.C.; Matthew, A.E.; Ayres, P. Antagonisms, mutualisms and commensalisms affect outbreak dynamics of the southern pine beetle. Oecologia 2006, 147, 679–691. [Google Scholar] [CrossRef]
- Pfammatter, J.A.; Malas, K.M.; Raffa, K.F. Behaviours of phoretic mites (Acari) associated with Ips pini and Ips grandicollis (Coleoptera: Curculionidae) during host-tree colonization. Agric. For. Entomol. 2016, 18, 108–118. [Google Scholar] [CrossRef]
- Wright, D.H.; Reeves, J.H. On the meaning and measurement of nestedness of species assemblages. Oecologia 1992, 92, 416–428. [Google Scholar] [CrossRef]
- Brown, J.H.; Ernest, S.K.M.; Parody, J.M.; Haskell, J.P. Regulation of diversity: Maintenance of species richness in changing environments. Oecologia 2001, 126, 321–332. [Google Scholar] [CrossRef]
- Archibald, S.B.; Greenwood, D.R.; Mathewes, R.W. Seasonality, montane beta diversity, and Eocene insects: Testing Janzen’s dispersal hypothesis in an equable world. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 371, 1–8. [Google Scholar] [CrossRef]
- Hill, M.J.; Heino, J.; Thornhill, I.; Ryves, D.B.; Wood, P.J. Effects of dispersal mode on the environmental and spatial correlates of nestedness and species turnover in pond communities. Oikos 2017, 126, 1575–1585. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, W.E.; Holzapfel, C.M. Genetic response to rapid climate change. Mol. Ecol. 2008, 17, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Jost, R.W.; Rice, A.V.; Langor, D.W.; Boluk, Y. Monoterpene emissions from lodgepole and jack pine bark inoculated with mountain pine beetle-associated fungi. J. Wood Chem. Technol. 2008, 28, 37–46. [Google Scholar] [CrossRef]
- Arango-Velez, A.; El Kayal, W.; Copeland, C.C.J.; Zaharia, L.I.; Lusebrink, I.; Cooke, J.E.K. Differences in defence responses of Pinus contorta and Pinus banksiana to the mountain pine beetle fungal associate Grosmannia clavigera are affected by water deficit. Plant Cell Environ. 2016, 39, 726–744. [Google Scholar] [CrossRef] [Green Version]
- Holeski, L.M.; Hillstrom, M.L.; Whitham, T.G.; Lindroth, R.L. Relative importance of genetic, ontogenetic, induction, and seasonal variation in producing a multivariate defense phenotype in a foundation tree species. Oecologia 2012, 170, 695–707. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, P.J.; Whitham, T.G. Plant genotype affects long-term herbivore population dynamics and extinction: Conservation implications. Ecology 2003, 84, 311–322. [Google Scholar] [CrossRef]
- Mittelbach, G.G.; Schemske, D.W.; Cornell, H.V.; Allen, A.P.; Brown, J.M.; Bush, M.B.; Harrison, S.P.; Hurlbert, A.H.; Knowlton, N.; Lessios, H.A.; et al. Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol. Lett. 2007, 10, 315–331. [Google Scholar] [CrossRef]
- Evans, L.M.; Hofstetter, R.W.; Ayres, M.P.; Klepzig, K.D. Temperature alters the relative abundance and population growth rates of species within the Dendroctonus frontalis (Coleoptera: Curculionidae) community. Environ. Entomol. 2011, 40, 824–834. [Google Scholar] [CrossRef]
- Swenson, N.G.; Enquist, B.J.; Pither, J.; Kerkhoff, A.J.; Boyle, B.; Weiser, M.D.; Elser, J.J.; Fagan, W.F.; Forero-Montaña, J.; Fyllas, N.; et al. The biogeography and filtering of woody plant functional diversity in North and South America. Glob. Ecol. Biogeogr. 2012, 21, 798–808. [Google Scholar] [CrossRef]
- Flores, O.; Seoane, J.; Hevia, V.; Azcárate, F.M. Spatial patterns of species richness and nestedness in ant assemblages along an elevational gradient in a Mediterranean mountain range. PLoS ONE 2018, 13, e0204787. [Google Scholar] [CrossRef] [Green Version]
- Villéger, S.; Grenouillet, G.; Brosse, S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in European fish assemblages. Glob. Ecol. 2013, 22, 671–681. [Google Scholar] [CrossRef]



| Population | Sampling Year(s) | Lat (N) N to S | Descriptive Latitude | Elevation (m) | MPB Attacked Host Tree | Year | MPB Population Phase | Descriptive Precipitation | # MPB | # mites | Average Mites per Beetle | Total No. of Mite Species |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grande Prairie, Alberta, Canada (CAN) | 2009 | 54.69 | northern | ~750 | Pinus contorta | 2009 | Epidemic | most dry | 910 | 801 | 0.88 | 3 |
| Black Hills, South Dakota (USA) | 2007 | 43.5 | middle | ~2200 | Pinus ponderosa | 2011 | Epidemic | dry | 350 | 1106 | 3.16 | 7 |
| Logan Canyon, Utah (USA) | 2016/2017 | 41.93 | middle | ~2300 | Pinus flexilis | 2016–2017 | Endemic | wet | 161 | 886 | 5.50 | 5 |
| Roosevelt N.F., Colorado (USA) | 2012/2013 | 40.4 | middle | ~2250 | Pinus ponderosa | 2012–2013 | Epidemic to Endemic | dry | 565 | 1460 | 2.58 | 5 |
| Lockett Meadow, Arizona (USA) | 2016/2017 | 35.36 | southern | ~2600 | Pinus strobiformis | 2016–2017 | Endemic | wettest | 239 | 595 | 2.49 | 5 |
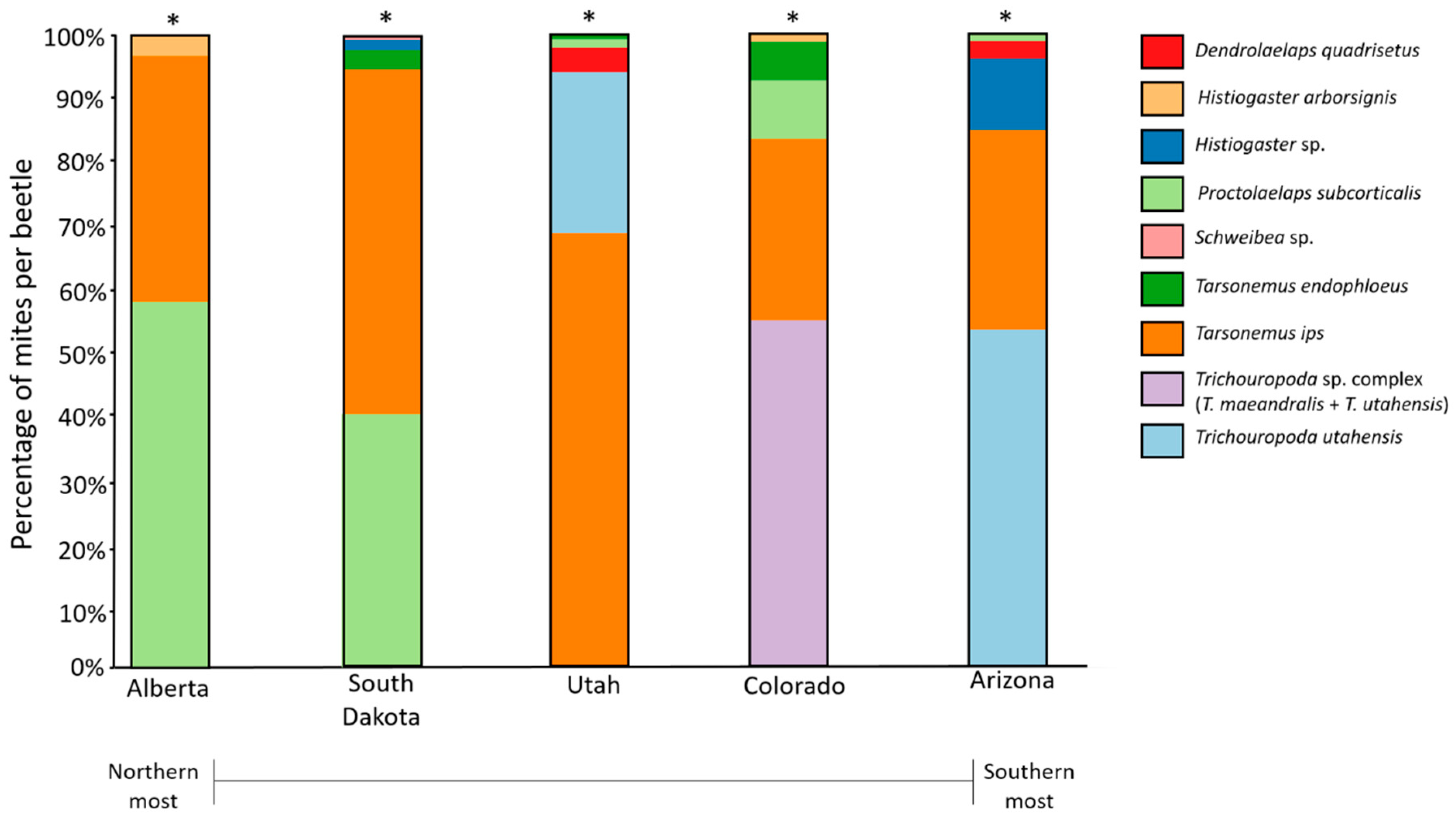
| Taxa Information | Average Abundance Per Beetle (±S.E.) | |||||
|---|---|---|---|---|---|---|
| Mite Species | Functional Group | Alberta | South Dakota | Utah | Colorado | Arizona |
| Tarsonemus ips Lindq. | Fungivore | 0.34 (0.03) | 3.26 (0.14) | 3.82 (0.46) | 1.19 (0.15) | 0.79 (0.1) * |
| Tarsonemus endophloeus Lindq. | Fungivore | 0.32 (0.01) | 0.04 (0.02) | 0.27 (0.05) * | ||
| Proctolaelaps subcorticalis Lindq | Predator | 0.5 (0.07) * | 1.73 (0.07) * | 0.03 (0.03) | 0.39 (0.07) * | 0.01 (0.01) |
| Dendrolaelaps quadrisetus Berl. | Predator | 0.22 (0.05) | 0.08 (0.02) | |||
| Trichouropoda sp. (T. utahensis Wis. and Hirch. in Utah, Arizona; and T. utahensis + T. maeandralis Hirsch. in Colorado) | Omnivore | 1.39 (0.22) * | 2.3 (0.35) * | 1.32 (0.16) | ||
| Nanacarus sp. | Omnivore | 0.01 (0.00) | ||||
| Histiogaster sp. | Detritivore | 0.43 (0.02) | 0.28 (0.19) * | |||
| Histiogaster arborsignis Woodr. | Detritivore | 0.04 (0.01) * | 0.02 (0.01) | |||
| Schweibea sp. | Unknown | 0.18 (0.01) * | ||||
| Parawinterschmidtia sp. | Unknown | 0.04 (0.00) | ||||
| Overall mites/beetle | 0.9 | 3.2 | 5.5 | 2.6 | 2.49 | |
| Total Species Richness | 3 | 7 | 5 | 5 | 5 | |
| Analysis of Deviance Table | ||
|---|---|---|
| Population | t-value | p-value |
| Intercept (Arizona) | 7.079 | <0.01 * |
| Alberta | −6.8 | <0.01 * |
| South Dakota | −1.542 | 0.1 |
| Colorado | 3.154 | <0.01 * |
| Utah | 4.091 | <0.01 * |
| Pairwise Comparisons | ||
| Population Pair | z-value | p-value |
| Arizona-Canada | 6.8 | <0.01 * |
| Arizona-Colorado | −3.154 | 0.01 * |
| Arizona-South Dakota | 1.542 | 0.53 |
| Arizona-Utah | −4.091 | <0.01 * |
| Canada-Colorado | −12.016 | <.01 |
| Canada-South Dakota | −6.685 | <.01 |
| Canada-Utah | −11.037 | <0.01 * |
| Colorado-South Dakota | 5.687 | <0.01 * |
| Colorado-Utah | −1.574 | 0.51 |
| South Dakota-Utah | −6.122 | <0.01 * |
| Population Pair | |t-Value| | Std. Error | p-Value |
|---|---|---|---|
| Arizona-Alberta | 0.164 | 0.01 | 0.99 |
| Arizona-Colorado | 0.797 | 0.01 | 0.93 |
| Arizona-South Dakota | 0.032 | 0.012 | 1 |
| Arizona-Utah | 1.412 | 0.01 | 0.62 |
| Alberta-Colorado | 1.089 | 0.01 | 0.81 |
| Alberta-South Dakota | 0.157 | 0.012 | 0.99 |
| Alberta-Utah | 1.706 | 0.01 | 0.43 |
| Colorado-South Dakota | 0.961 | 0.01 | 0.87 |
| Colorado-Utah | 0.762 | 0.012 | 0.94 |
| South Dakota-Utah | 1.604 | 0.01 | 0.5 |
| (A) Multivariate Test—Analysis of Deviance Table | ||||
|---|---|---|---|---|
| Df | Df Difference | LRT | p-value | |
| (Intercept) | 2030 | 4 | 7159 | 0.01 * |
| Population | 2026 | |||
| (B) Wald Test Statistics by Population | ||||
| Population | Wald Value | p-Value | ||
| Intercept (Arizona) | 6.311 | <0.01 * | ||
| Alberta | 6.646 | <0.01 * | ||
| South Dakota | 4.937 | <0.01 * | ||
| Colorado | 3.922 | <0.01 * | ||
| Utah | 5.31 | <0.01 * | ||
| (C) Univariate Comparisons of Species Abundances Across All Populations | ||||
| Mite Species | LRT | p-value | ||
| Tarsonemus ips Lindq. | 192.849 | <0.01 * | ||
| Tarsonemus endophloeus Lindq. | 116.192 | <0.01 * | ||
| Trichouropoda utahensis Wis. and Hirsch. | 223.699 | <0.01 * | ||
| Trichouropoda sp. complex (T. maeandralis Hirsch. + T. utahensis) | 459.59 | <0.01 * | ||
| Proctolaelaps subcorticalis Lindq. | 65.354 | <0.01 * | ||
| Histiogaster arborsignis Woodr. | 27.042 | <0.01 * | ||
| Histiogaster sp. | 24.011 | 0 | ||
| Schweibea sp. | 18.026 | <0.01 * | ||
| Other/Rare spp. | 9.689 | 0.04 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vissa, S.; Mercado, J.E.; Malesky, D.; Uhey, D.A.; Mori, B.A.; Knee, W.; Evenden, M.L.; Hofstetter, R.W. Patterns of Diversity in the Symbiotic Mite Assemblage of the Mountain Pine Beetle, Dendroctonus Ponderosae Hopkins. Forests 2020, 11, 1102. https://doi.org/10.3390/f11101102
Vissa S, Mercado JE, Malesky D, Uhey DA, Mori BA, Knee W, Evenden ML, Hofstetter RW. Patterns of Diversity in the Symbiotic Mite Assemblage of the Mountain Pine Beetle, Dendroctonus Ponderosae Hopkins. Forests. 2020; 11(10):1102. https://doi.org/10.3390/f11101102
Chicago/Turabian StyleVissa, Sneha, Javier E. Mercado, Danielle Malesky, Derek A. Uhey, Boyd A. Mori, Wayne Knee, Maya L. Evenden, and Richard W. Hofstetter. 2020. "Patterns of Diversity in the Symbiotic Mite Assemblage of the Mountain Pine Beetle, Dendroctonus Ponderosae Hopkins" Forests 11, no. 10: 1102. https://doi.org/10.3390/f11101102
APA StyleVissa, S., Mercado, J. E., Malesky, D., Uhey, D. A., Mori, B. A., Knee, W., Evenden, M. L., & Hofstetter, R. W. (2020). Patterns of Diversity in the Symbiotic Mite Assemblage of the Mountain Pine Beetle, Dendroctonus Ponderosae Hopkins. Forests, 11(10), 1102. https://doi.org/10.3390/f11101102







