Leaf Bilateral Symmetry and the Scaling of the Perimeter vs. the Surface Area in 15 Vine Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Material
2.2. Data Acquisition
2.3. Leaf Symmetry and Leaf Shape Complexity Measures
2.4. Statistical Analysis
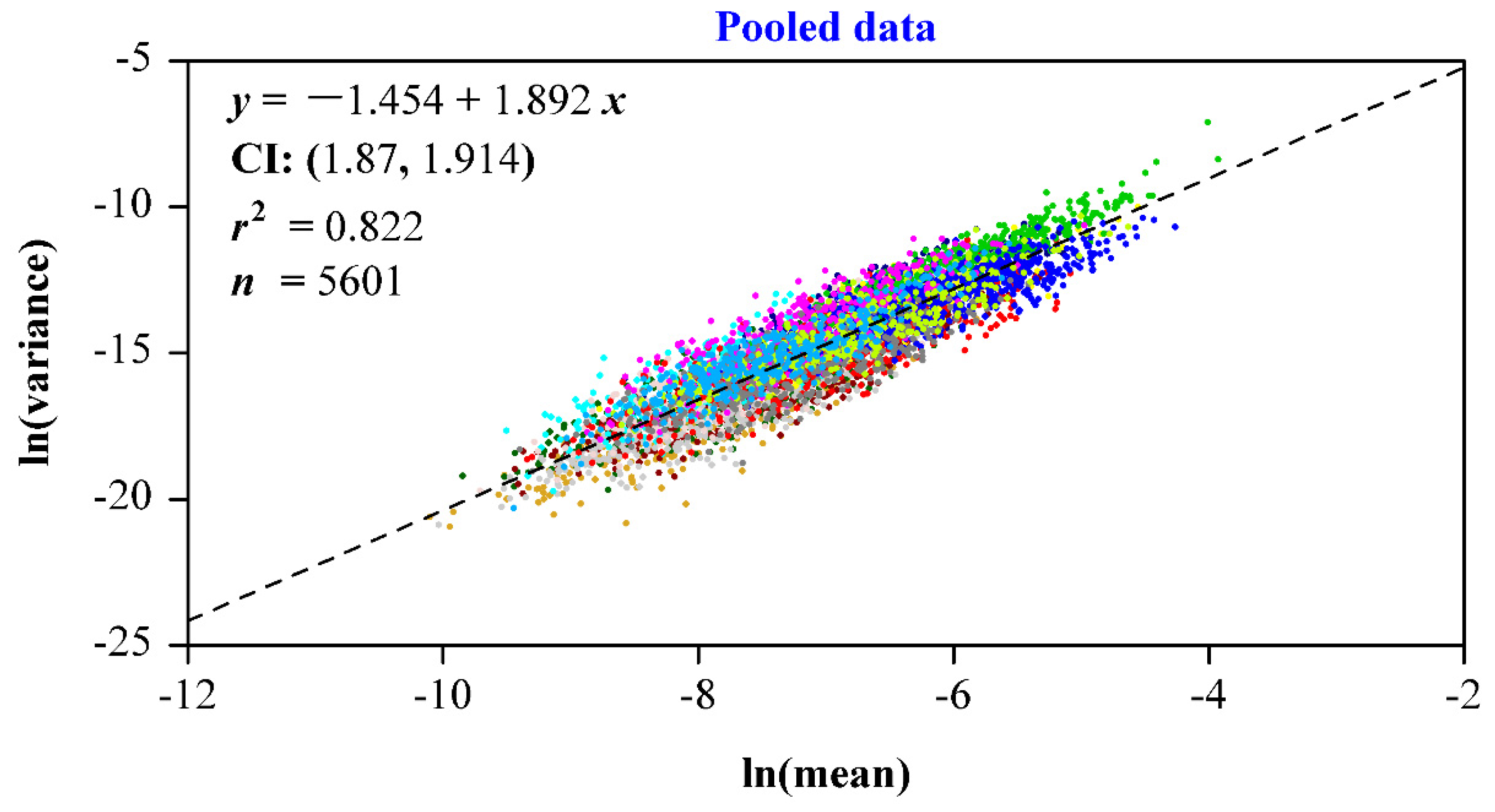
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niinemets, Ü.; Portsmuth, A.; Tobias, M. Leaf-shape and venation pattern alter the support investments within leaf lamina in temperate species: A neglected source of leaf physiological differentiation? Funct. Ecol. 2007, 21, 28–40. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Leigh, A.; Boyce, C.K.; Jones, C.S.; Niklas, K.J.; Royer, D.L.; Tsukaya, H. The evolution and functional significance of leaf shape in the angiosperms. Funct. Plant Biol. 2011, 38, 535–552. [Google Scholar] [CrossRef] [Green Version]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf form and photosynthesis: Do leaf structure and orientation interact to regulate internal light and carbon dioxide? BioScience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Royer, D.L.; Wilf, P. Why do toothed leaves correlate with cold climates? Gas exchange at leaf margins provides new insights into a classic paleotemperature proxy. Int. J. Plant Sci. 2006, 167, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Runions, A.; Fuhrer, M.; Lane, B.; Federl, P.; Rolland-Lagan, A.-G.; Prusinkiewicz, P. Modeling and visualization of leaf venation patterns. ACM Trans. Gr. 2005, 24, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Runions, A.; Tsiantis, M.; Prusinkiewicz, P. A common developmental program can produce diverse leaf shapes. New Phytol. 2017, 216, 401–418. [Google Scholar] [CrossRef] [Green Version]
- Brodribb, T.J.; Feild, T.S.; Jordan, G.J. Leaf maximum photosynthetic rate and venation area linked by hydraulics. Plant Physiol. 2007, 144, 1890–1898. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Niklas, K.J.; Wan, Y.; Hölscher, D.; Hui, C.; Ding, Y.; Shi, P. Leaf shape influences the scaling of leaf dry mass vs. area: A test case using bamboos. Ann. Forest Sci. 2020, 77, 11. [Google Scholar] [CrossRef] [Green Version]
- Piazza, P.; Jasinski, S.; Tsiantis, M. Evolution of leaf developmental mechanisms. New Phytol. 2005, 167, 693–710. [Google Scholar] [CrossRef]
- Bar, M.; Ori, N. Leaf development and morphogenesis. Development 2014, 141, 4219–4230. [Google Scholar] [CrossRef] [Green Version]
- Chitwood, D.H.; Ranjan, A.; Martinez, C.C.; Headland, L.R.; Thiem, T.; Kumar, R.; Covington, M.F.; Hatcher, T.; Naylor, D.T.; Zimmerman, S.; et al. A modern ampelography: A genetic basis for leaf shape and venation patterning in grape. Plant Physiol. 2014, 164, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.C.; Bazzaz, F.A. Elevated CO2 and leaf shape: Are dandelions getting toothier? Am. J. Bot. 1996, 83, 106–111. [Google Scholar] [CrossRef]
- Peppe, D.J.; Royer, D.L.; Gariglino, B.; Oliver, S.Y.; Newman, S.; Leight, E.; Enikolopov, G.; Fernandez-Burgos, M.; Herrera, F.; Adams, J.M.; et al. Sensitivity of leaf size and shape to climate: Global patterns and paleoclimatic applications. New Phytol. 2011, 190, 724–739. [Google Scholar] [CrossRef] [Green Version]
- Royer, D.L.; Peppe, D.J.; Wheeler, E.A.; Niinemets, Ü. Roles of climate and functional traits in controlling toothed vs. untoothed leaf margins. Am. J. Bot. 2012, 99, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Shi, P.; Hui, C.; Miao, L.; Liu, C.; Zhang, Q.; Feng, C. Effects of salt stress on the leaf shape and scaling of Pyrus betulifolia Bunge. Symmetry 2019, 11, 991. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Zhang, L.; Reddy, G.V.P.; Hui, C.; Gielis, J.; Ding, Y.; Shi, P. A geometrical model for testing bilateral symmetry of bamboo leaf with a simplified Gielis equation. Ecol. Evol. 2016, 6, 6798–6806. [Google Scholar] [CrossRef]
- Shi, P.; Xu, Q.; Sandhu, H.S.; Gielis, J.; Ding, Y.; Li, H.; Dong, X. Comparison of dwarf bamboos (Indocalamus sp.) leaf parameters to determine relationship between spatial density of plants and total leaf area per plant. Ecol. Evol. 2015, 5, 4578–4589. [Google Scholar] [CrossRef]
- Gielis, J. A generic geometric transformation that unifies a wide range of natural and abstract shapes. Am. J. Bot. 2003, 90, 333–338. [Google Scholar] [CrossRef]
- Shi, P.; Ratkowsky, D.A.; Li, Y.; Zhang, L.; Lin, S.; Gielis, J. General leaf-area geometric formula exists for plants—Evidence from the simplified Gielis equation. Forests 2018, 9, 714. [Google Scholar] [CrossRef] [Green Version]
- Kincaid, D.T.; Schneider, R.B. Quantification of leaf shape with a microcomputer and Fourier transform. Can. J. Bot. 1983, 61, 2333–2342. [Google Scholar] [CrossRef]
- Santiago, L.S.; Kim, S.-C. Correlated evolution of leaf shape and physiology in the woody Sonchus alliance (Asteraceae: Sonchinae) in Macaronesia. Int. J. Plant Sci. 2009, 170, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Baxes, G.A. Digital Image Processing: Principles and Applications; John Wiley and Sons, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Niinemets, Ü.; Cescatti, A.; Christian, R. Constraints on light interception efficiency due to shoot architecture in broad-leaved Nothofagus species. Tree Physiol. 2004, 24, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü.; Sparrow, A.; Cescatti, A. Light capture efficiency decreases with increasing tree age and size in the southern hemisphere gymnosperm Agathis australis. Trees Struct. Funct. 2005, 19, 177–190. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A.; Konrad, W. Fossil leaf traits as archives for the past—And lessons for the future? Flora 2019, 254, 59–70. [Google Scholar] [CrossRef]
- Milla, R.; Reich, P.B. The scaling of leaf area and mass: The cost of light interception increases with leaf size. Proc. R. Soc. Biol. Sci. 2007, 274, 2109–2114. [Google Scholar] [CrossRef] [Green Version]
- Niklas, K.J.; Cobb, E.D.; Niinemets, Ü.; Reich, P.B.; Sellin, A.; Shipley, B.; Wright, I.J. ‘Diminishing returns’ in the scaling of functional leaf traits across and within species groups. Proc. Natl. Acad. Sci. USA 2007, 104, 8891–8896. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Ratkowsky, D.A.; Hui, C.; Wang, P.; Su, J.; Shi, P. Leaf fresh weight versus dry weight: Which is better for describing the scaling relationship between leaf biomass and leaf area for broad-leaved plants? Forests 2019, 10, 256. [Google Scholar] [CrossRef] [Green Version]
- Price, C.A.; Symonova, O.; Mileyko, Y.; Hilley, T.; Weitz, J.S. Leaf extraction and analysis framework graphical user interface: Segmenting and analyzing the structure of leaf veins and areoles. Plant Physiol. 2011, 155, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Zheng, X.; Ratkowsky, D.A.; Li, Y.; Wang, P.; Cheng, L. A simple method for measuring the bilateral symmetry of leaves. Symmetry 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Niklas, K.J.; Huang, W.; Yu, X.; Yang, Y.; Shi, P. Lamina shape does not correlate with lamina surface area: An analysis based on the simplified Gielis equation. Glob. Ecol. Conserv. 2019, 19, e00666. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Bongers, F. The ecology of lianas and their role in forests. Trends Ecol. Evol. 2002, 17, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Appanah, S.; Gentry, A.H.; LaFrankie, J.V. Liana diversity and species richness of Malaysian rain forests. J. Trop. For. Sci. 1993, 6, 116–123. [Google Scholar]
- Li, W.; Cui, L.; Sun, B.; Zhao, X.; Gao, C.; Zhang, Y.; Zhang, M.; Pan, X.; Lei, Y.; Ma, W. Distribution patterns of plant communities and their associations with environmental soil factors on the eastern shore of Lake Taihu, China. Ecosyst. Health Sustain. 2017, 3, 1385004. [Google Scholar] [CrossRef]
- Gianoli, E. The behavioural ecology of climbing plants. AOB Plants 2015, 7, plv013. [Google Scholar] [CrossRef] [Green Version]
- Putz, F.E.; Holbrook, N.M. Strangler fig rooting habits and nutrient relations in the Llanos of Venezuela. Am. J. Bot. 1989, 76, 781–788. [Google Scholar] [CrossRef]
- Holbrook, N.M.; Putz, F.E. Water relations of epiphytic and terrestrially-rooted strangler figs in a Venezuelan palm savanna. Oecologia 1996, 106, 424–431. [Google Scholar] [CrossRef]
- McLellan, T. The roles of heterochrony and heteroblasty in the diversification of leaf shapes in Begonia dregei (Begoniaceae). Am. J. Bot. 1993, 80, 796–804. [Google Scholar] [CrossRef]
- Gianoli, E.; Carrasco-Urra, F. Leaf mimicry in a climbing plant protects against herbivory. Curr. Biol. 2014, 24, 984–987. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 1 June 2019).
- Hsu, J.C. Multiple Comparisons: Theory and Methods; Chapman and Hall/CRC: New York, NY, USA, 1996. [Google Scholar]
- Smith, R.J. Use and misuse of the reduced major axis for line-fitting. Am. J. Phys. Anthropol. 2009, 140, 476–486. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall/CRC: New York, NY, USA, 1993. [Google Scholar]
- Sandhu, H.S.; Shi, P.; Kuang, X.; Xue, F.; Ge, F. Applications of the bootstrap to insect physiology. Florida Entomol. 2011, 94, 1036–1041. [Google Scholar] [CrossRef]
- Ratkowsky, D.A. Handbook of Nonlinear Regression Models; Marcel Dekker: New York, NY, USA, 1990. [Google Scholar]
- Taylor, L.R. Aggregation, variance and the mean. Nature 1961, 189, 732–735. [Google Scholar] [CrossRef]
- Cohen, J.E.; Xu, M. Random sampling of skewed distributions implies Taylor’s power law of fluctuation scaling. Proc. Natl. Acad. Sci. USA 2015, 112, 7749–7754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giometto, A.; Formentin, M.; Rinaldo, A.; Cohen, J.E.; Maritan, A. Sample and population exponents of generalized Taylor’s law. Proc. Natl. Acad. Sci. USA 2015, 112, 7755–7760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, P.; Sandhu, H.S.; Reddy, G.V.P. Dispersal distance determines the exponent of the spatial Taylor’s power law. Ecol. Model. 2016, 335, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Ratkowsky, D.A.; Xiao, X.; Yu, X.; Su, J.; Zhang, L.; Shi, P. Taylor’s power law for leaf bilateral symmetry. Forests 2018, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Liu, M.; Ratkowsky, D.A.; Gielis, J.; Su, J.; Yu, X.; Wang, P.; Zhang, L.; Lin, Z.; Schrader, J. Leaf area-length allometry and its implications in leaf-shape evolution. Trees Struct. Funct. 2019, 33, 1073–1085. [Google Scholar] [CrossRef]
- Montgomery, E.G. Correlation Studies in Corn. In Annual Report no. 24; Agricultural Experimental Station: Lincoln, NB, USA, 1911; pp. 108–159. [Google Scholar]

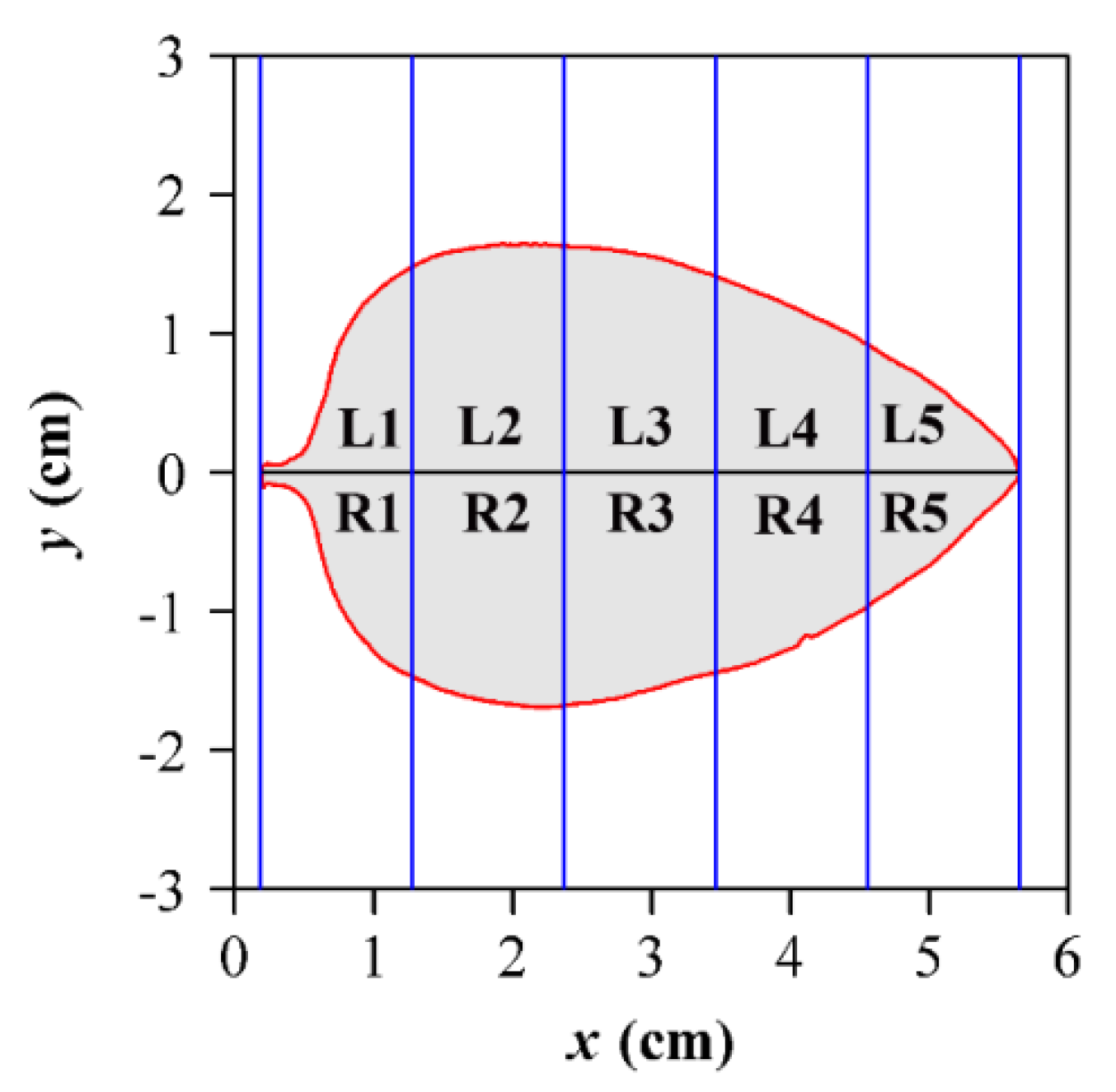

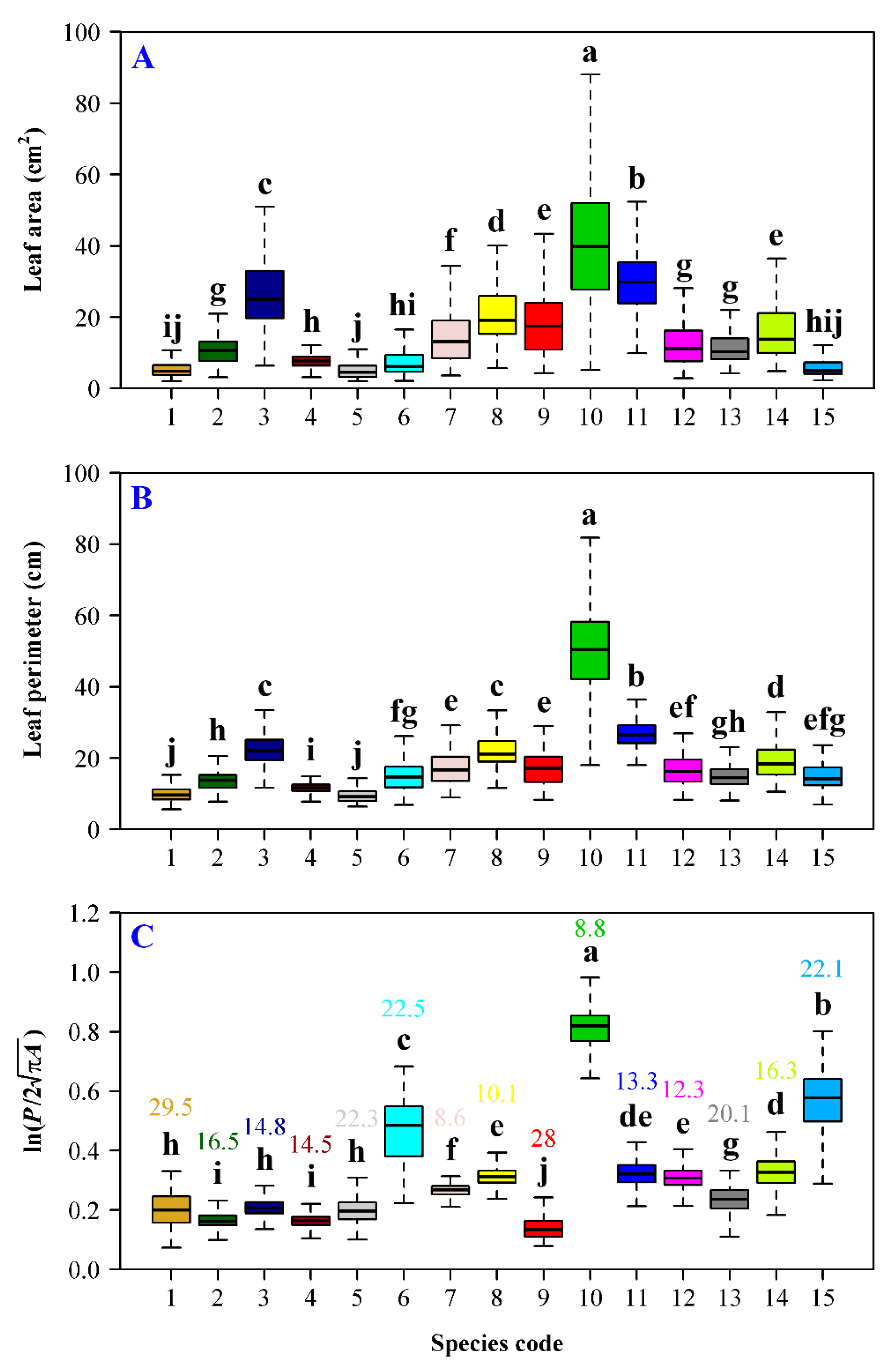

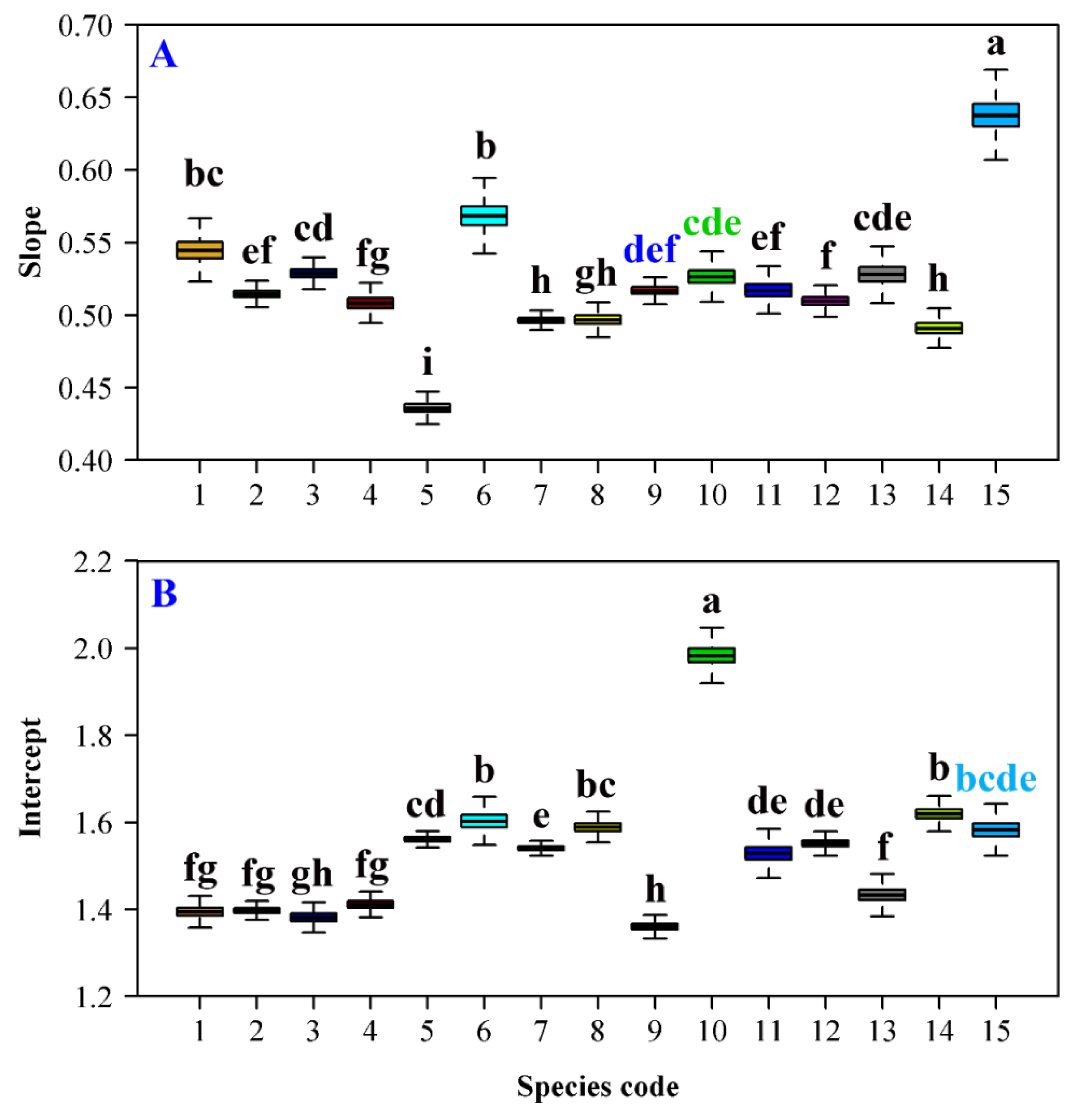
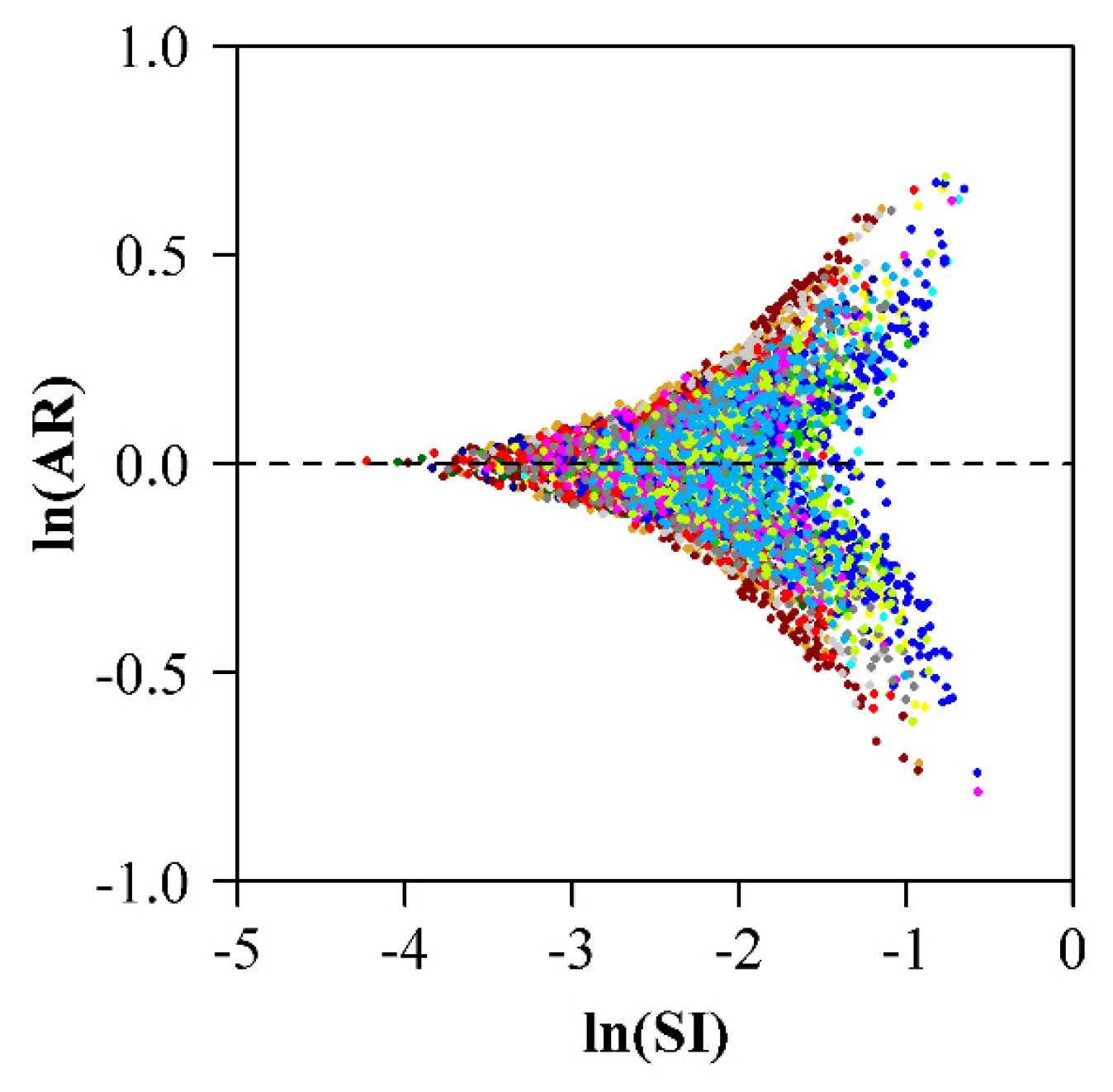
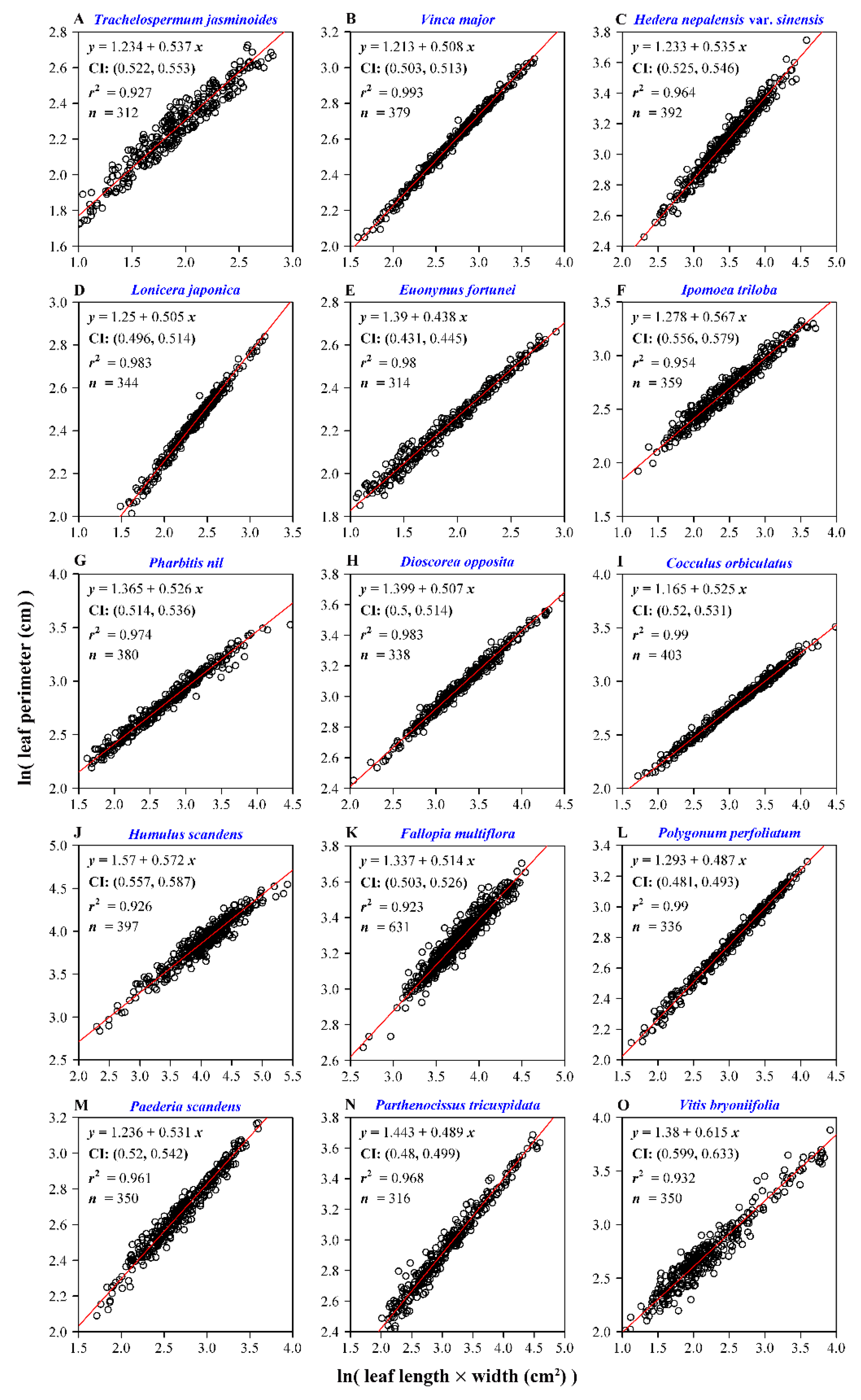
| Data Set | Family | Latin Name | Type | Sampling Time | Collection Location | Altitude (m) |
|---|---|---|---|---|---|---|
| 1 | Apocynaceae | Trachelospermum jasminoides (Lindl.) Lem. | Perennial woody | 20 July 2019 | 32°04′40″N, 118°48′30″E | 13 |
| 2 | Apocynaceae | Vinca major L. | Perennial woody | 15 October 2018 | 32°04′46″N, 118°48′32″E | 16 |
| 3 | Araliaceae | Hedera nepalensis K. Koch var. sinensis (Tobl.) Rehd. | Perennial woody | 23 October 2018 | 32°04′38″N, 118°48′32″E | 14 |
| 4 | Caprifoliaceae | Lonicera japonica Thunb. | Perennial woody | 20 July 2019 | 32°04′50″N, 118°48′34″E | 16 |
| 5 | Celastraceae | Euonymus fortunei (Turcz.) Hand.-Mazz. | Perennial woody | 13 August 2019 | 32°04′30″N, 118°48′33″E | 19 |
| 6 | Convolvulaceae | Ipomoea triloba L. | Annual herbaceous | 12 October 2018 | 31°36′08″N, 119°10′36″E | 37 |
| 7 | Convolvulaceae | Pharbitis nil (L.) Choisy | Annual herbaceous | 12 October 2018 | 31°36′07″N, 119°10′36″E | 37 |
| 8 | Dioscoreaceae | Dioscorea opposita Thunb. | Perennial herbaceous | 21 July 2019 | 32°04′48″N, 118°48′44″E | 17 |
| 9 | Menispermaceae | Cocculus orbiculatus (L.) DC. | Perennial woody | 12 October 2018 | 31°36′07″N, 119°10′43″E | 37 |
| 10 | Moraceae | Humulus scandens (Lour.) Merr. | Perennial herbaceous | 12 October 2018 | 31°36′07″N, 119°10′35″E | 37 |
| 11 | Polygonaceae | Fallopia multiflora (Thunb.) Harald. | Perennial herbaceous | 9 April 2019 | 32°05′03″N, 118°48′45″E | 25 |
| 12 | Polygonaceae | Polygonum perfoliatum L. | Annual herbaceous | 30 September 2018 | 31°36′19″N, 119°10′35″ E | 39 |
| 13 | Rubiaceae | Paederia scandens (Lour.) Merr. | Perennial herbaceous | 21 July 2019 | 32°04′44″N, 118°48′44″E | 20 |
| 14 | Vitaceae | Parthenocissus tricuspidata (S. et Z.) Planch. | Perennial woody | 13 August 2019 | 32°04′30″N, 118°48′33″E | 19 |
| 15 | Vitaceae | Vitis bryoniifolia Bunge | Perennial woody | 12 August 2019 | 31°36′07″N, 119°10′40″E | 35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, P.; Niinemets, Ü.; Hui, C.; Niklas, K.J.; Yu, X.; Hölscher, D. Leaf Bilateral Symmetry and the Scaling of the Perimeter vs. the Surface Area in 15 Vine Species. Forests 2020, 11, 246. https://doi.org/10.3390/f11020246
Shi P, Niinemets Ü, Hui C, Niklas KJ, Yu X, Hölscher D. Leaf Bilateral Symmetry and the Scaling of the Perimeter vs. the Surface Area in 15 Vine Species. Forests. 2020; 11(2):246. https://doi.org/10.3390/f11020246
Chicago/Turabian StyleShi, Peijian, Ülo Niinemets, Cang Hui, Karl J. Niklas, Xiaojing Yu, and Dirk Hölscher. 2020. "Leaf Bilateral Symmetry and the Scaling of the Perimeter vs. the Surface Area in 15 Vine Species" Forests 11, no. 2: 246. https://doi.org/10.3390/f11020246
APA StyleShi, P., Niinemets, Ü., Hui, C., Niklas, K. J., Yu, X., & Hölscher, D. (2020). Leaf Bilateral Symmetry and the Scaling of the Perimeter vs. the Surface Area in 15 Vine Species. Forests, 11(2), 246. https://doi.org/10.3390/f11020246







