Abstract
The aim of the present study was to assess fungal and oomycete communities in the irrigation water of forest nurseries, focusing on plant pathogens in the hope of getting a better understanding of potential pathogenic microorganisms and spreading routes in forest nurseries. The study sites were at Anykščiai, Dubrava, Kretinga and Trakai state forest nurseries in Lithuania. For the collection of microbial samples, at each nursery five 100-L water samples were collected from the irrigation ponds and filtered. Following DNA isolation from the irrigation water filtrate samples, these were individually amplified using ITS rDNA as a marker and subjected to PacBio high-throughput sequencing. Clustering in the SCATA pipeline and the taxonomic classification of 24,006 high-quality reads showed the presence of 1286 non-singleton taxa. Among those, 895 were representing fungi and oomycetes. The detected fungi were 57.3% Ascomycota, 38.1% Basidiomycota, 3.1% Chytridiomycota, 0.8% Mucoromycota and 0.7% Oomycota. The most common fungi were Malassezia restricta E. Guého, J. Guillot & Midgley (20.1% of all high-quality fungal sequences), Pezizella discreta (P. Karst.) Dennis (10.8%) and Epicoccum nigrum Link (4.9%). The most common oomycetes were Phytopythium cf. citrinum (B. Paul) Abad, de Cock, Bala, Robideau, Lodhi & Lévesque (0.4%), Phytophthora gallica T. Jung & J. Nechwatal (0.05%) and Peronospora sp. 4248_322 (0.05%). The results demonstrated that the irrigation water used by forest nurseries was inhabited by a species-rich but largely site-specific communities of fungi. Plant pathogens were relatively rare, but, under suitable conditions, these can develop rapidly, spread efficiently through the irrigation system and be a threat to the production of high-quality tree seedlings.
1. Introduction
The production of high-quality tree seedlings in forest nurseries is of key importance for forestry. Historically, artificial reforestation, i.e., the replanting or sowing of forest reproductive materials, has constantly increased [1,2], and today about 30% of the European Union (EU) forests are artificially reforested [3]. Besides, as ca. 30 million of forest tree seedlings are traded annually within the EU [2], the supply of healthy seedlings is a major challenge in order to prevent the spread and the introduction of fungal diseases to new areas [4].
Different abiotic and biotic factors may stress tree seedlings and predispose them to infections by pathogenic microorganisms [5]. Although a number of such pathogens are often air- or soil-borne, these can also spread through the irrigation water [6,7,8]. The irrigation water can either be from natural water sources, such as lakes, rivers, rainfall and groundwater, or from artificially created sources, such as reservoirs and ponds [9]. The risk posed by pathogenic microorganisms that are present in the irrigation water was recognized previously as a significant crop health issue [6,10]. Pathogens can enter irrigation systems at either the initial water source or along the distribution line [6,10,11]. Indeed, Zappia et al. [10] have shown that diverse fungi can occur in water that is used for irrigation. For example, ca. 3000 fungal species and 138 saprolegnialean species have been reported from aquatic habitats. The largest taxonomic group of fungi in aquatic habitats is comprised of meiosporic and mitosporic Ascomycota, followed by the Chytridiomycota [12,13,14]. Fungi that often dominate in aquatic ecosystems include genera Alternaria Nees, Botrytis P. Micheli ex Haller, Ascochyta Lib., Rhizoctonia DC., and Verticillium Nees that contain a number of plant pathogens [6,15]. Besides, the presence of oomycetes in the irrigation water can be another threat to the plants [8,10,16,17,18,19,20].
Oomycetes represent a diverse group of fungus-like eukaryotic microorganisms. They are globally distributed [21,22,23,24,25,26,27] and are known as water molds [28] that include both saprophytes and pathogens of vertebrate animals, fish, insects, plants, crustaceans and various microorganisms. The majority of saprophytic oomycetes inhabit primary aquatic and moist soil habitats [29]. Plant pathogenic oomycetes cause devastating diseases to numerous plants, crops, ornamental and forest trees [5,17,30,31,32]. In particular, oomycetes of the phylum Heterokontophyta, which includes genera Phytophthora de Bary and Pythium Pringsh., are pathogenic to different plants and trees, and these are well adapted for spread with the surface water [17,33,34]. The existence of oomycetes in the irrigation water was initially reported by Bewley and Buddin [35] who have discovered various pathogenic microorganisms in an uncovered irrigation supply tank, including P. cryptogea Pethybr. & Laff. and P. parasitica Dastur [6,10]. Since that time, oomycetes Pythium and Phytophthora have continually been recovered from the irrigation water [10]. The latter suggests that such irrigation water can be a primary, if not the sole, source of Phytophthora and Pythium inoculum in different growing environments. These findings pose great challenges in agriculture and forestry [6].
Indeed, in Europe, diseases caused by Phytophthora (e.g., P. citricola Sawada, P. cambivora (Petri) Buisman and P. cactorum (Lebert & Cohn) J. Schröt.) and Pythium are among the most economically important as these occur in forest nurseries, natural and managed forest ecosystems [36,37,38,39,40]. Symptoms include damping-off and root-rot, as well as stem cankers, shoot dieback, and foliar blight. If diseased nursery seedlings and/or infested potting soil are moved to new areas, this can become a major issue and subsequently be a source of new infections on other hosts [41,42]. As Phytophthora and Pythium are often associated with the surface water, careful assessment and management of the irrigation water used in forest nurseries is of key importance.
The aim of the present study was to assess fungal and oomycete communities in the irrigation water of forest nurseries, focusing on plant pathogens in the hope of getting a better understanding on potential pathogenic microorganisms and the spreading routes in forest nurseries in Lithuania, as, in the country, similar studies have not been done before, but is of considerable practical importance.
2. Materials and Methods
2.1. Study Sites and Sampling
The study sites were at Anykščiai, Dubrava, Kretinga and Trakai state forest nurseries in Lithuania. For each forest nursery, information on geographical position, the total land area, the number of seedlings produced annually and the area of the water pond, which is used for seedling irrigation, is in Table 1. All forest nurseries were situated within a radius of ca. 300 km. In all nurseries, seedlings are produced using a bare-root cultivation system. For the irrigation of seedlings, at Anykščiai, Dubrava and Trakai forest nurseries, the water is taken from ponds that are situated in the forest, while at Kretinga the water is taken from the dammed bog. Sampling was carried out during the dormancy period, i.e., between November 2017 and April 2018. At the time of sampling, the mean monthly temperature and precipitation were as presented in Table 1.

Table 1.
Characteristics of the study sites and the meteorological data obtained from the nearest meteorological stations.
For the collection of microbial samples, at each nursery, five 100-L water samples, which were collected at a 50-m distance from each other along the coast of the pond, were separately passed through a 90-mm diameter cellulose filter paper (particles ≥10µm are retained) (Ahlstrom, Falun, Sweden) placed in a holder under the funnel. Between different samples, the filtering equipment was cleaned using 70% ethyl alcohol. Water samples were taken at a depth of ca. 0.5–1.0 m below the water surface without disturbing the bottom sediment. In total, 20 filter papers with water residuals were collected, labelled, placed individually into the plastic zip lock bags, transported to the laboratory and kept at −20 °C before further analyses.
2.2. Molecular Analysis
Prior to isolation of DNA, individual filters with water filtrate samples were freeze-dried at −60 °C for 24 h. From each lyophilised filter, 1/4 part was taken (remaining was kept as a backup), cut into smaller pieces and placed in three 2-mL screw-cap centrifugation tubes (60 in total; 20 samples × 3 DNA extraction replicates) together with sterile glass beads and homogenised using a Precellys 24 tissue homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France). The total DNA was isolated from each sample by adding 1000 μL of CTAB buffer (0.5 M EDTA pH 8.0, 1 M Tris-HCL pH 8.0, 5 M NaCl, 3% CTAB) and incubating at 65 °C for 1 h. Following centrifugation at 8000 rpm for 5 min, the supernatant was transferred to a new 1.5-mL centrifugation tube, mixed by pipetting with an equal volume of chloroform, centrifuged for 8 min at 13,000 rpm and the upper phase was transferred to a new 1.5-mL centrifugation tube. Then, an equal volume of 2-propanol was added to precipitate the DNA that was pelleted by centrifugation at 13,000 rpm for 20 min. The DNA pellet was washed in 500 μL 70% ethanol by centrifugation at 13,000 rpm for 5 min, dried and dissolved in 50 μL of sterile milli-Q water. The DNA concentration of each sample was measured using a NanoDrop™ One spectrophotometer (Thermo Scientific, Rodchester, NY, USA) and if needed diluted to 1–10 ng/µL.
The amplification by PCR of the ITS rDNA region was done using forward ITS6 primer [43] and reverse ITS4 primer with barcodes [44]. The primer pair used was shown to amplify both fungi and oomycetes [43]. PCR reactions 50 μL in volume were performed using the following final concentrations: 200 μM of dNTPs; 750 μM of MgCl2; 200 nM of each primer; 0.025 μM DreamTaq Green polymerase (5 U/μL) (Thermo Scientific, Waltham, MA, USA); and 0.02 ng/μL of template DNA. Sterile milli-Q water was added to make the final volume (50.0 μL) of the reaction. Amplifications were done using the Applied Biosystems 2720 thermal cycler (Applied Biosystems, Foster City, CA, USA). The PCR program started with initial denaturation at 95 °C for 2 min, followed by 35 cycles of 95 °C for 30 s, annealing at 55 °C for 30 s and 72 °C for 1 min, followed by a final extension step at 72 °C for 7 min. The PCR products were assessed using gel electrophoresis on 1% agarose gels stained with GelRed (Biotium, Fremont, CA, USA). Following successful amplification, all three DNA extraction replicates of the same sample were pooled together, resulting in a total of 20 PCR samples. PCR products were purified using 3 M sodium acetate (pH 5.2) (AppliChem Gmbh, Darmstadt, Germany) and 96% ethanol mixture (1:25). After the quantification of PCR products using a Qubit fluorometer 4.0 (Life Technologies, Stockholm, Sweden), these were pooled in an equimolar mix and sequenced using a PacBio platform and one SMRT cell (SciLifeLab, Uppsala, Sweden).
2.3. Bioinformatics
The sequences obtained were analysed using the Sequence Clustering and Analysis of Tagged Amplicons (SCATA) next-generation sequencing (NGS) pipeline available at http://scata.mykopat.slu.se. Quality filtering included the removal of sequences shorter than 200 bp, sequences of low quality, homopolymers and primer dimers. Sequences lacking a tag or primer were also excluded. Following quality filtering, the primer and sample tags were removed from the sequence, but this information was stored as meta-data. The high-quality sequences were clustered into different OTUs using single-linkage clustering based on 98% sequence similarity. For each cluster, the most common genotype (a real sequence read) was used to represent each OTU. A consensus sequence was produced for clusters that were composed of only two sequences. Fungal OTUs were assigned taxonomic names using an Ribosomal Database Project (RDP) pipeline classifier at https://pyro.cme.msu.edu/index.jsp (centre for Microbial Ecology, Michigan State University, Michigan, MI, USA). Sequences of 80% of higher similarity to the phylum level were considered to be of fungal or oomycete origin and were retained, while the remaining sequences were considered to be of non-fungal or non-oomycete origin and excluded from further analyses. The 30 most common fungal taxa and all oomycete taxa from water filtrate samples were identified using GenBank (NCBI) database and the BLASTn algorithm. The criteria used for taxonomic identification were: sequence coverage > 80%; similarity to species level 98%–100%; and similarity to genus level 94%–97%. Sequences deviating from these criteria were considered unidentified to species or genus level and were given unique names as shown in Tables 3 and 4 and Table S1. Representative sequences of fungal and oomycete non-singletons are available from GenBank under accession numbers MT236332-MT237171.
2.4. Statistical Analyses
Rarefaction analysis was carried out using Analytical Rarefaction v.1.3 (http://www.uga.edu/strata/software/index.html). Differences in the richness of fungal taxa (for simplicity, here and onwards, oomycetes are referred to as fungi) in water filtrate samples among different forest nurseries were compared by nonparametric chi-square test [45]. As each of the datasets was subjected to multiple comparisons, confidence limits for the p-values of chi-square tests were reduced the corresponding number of times as required by the Bonferroni correction [46]. The Shannon diversity index, qualitative Sørensen similarity index and correspondence analysis (CA) in SAS v. 9.4 (SAS Institute, Cary, NC, USA) [45,47] were used to characterise the diversity and composition of fungal communities. The nonparametric Mann–Whitney test in SAS v. 9.4 was used to test if the Shannon diversity index differed among different forest nurseries.
3. Results
High-throughput sequencing of the 20 amplicon samples generated 35,179 reads. Following quality filtering, 24,006 high-quality reads (668 bp on average) were retained. Clustering analysis showed the presence of 1286 non-singleton taxa (Table 2 and Figure 1), while singletons were removed.

Table 2.
Generated high-quality sequences and detected diversity of fungal taxa in water filtrate samples from Anykščiai, Dubrava, Kretinga and Trakai forest nurseries in Lithuania.
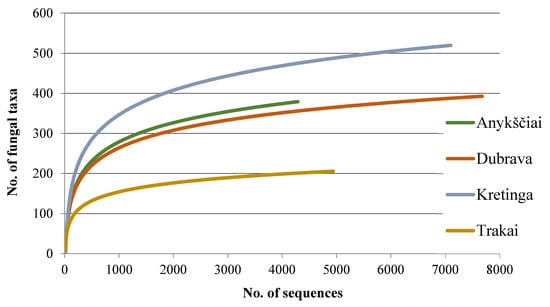
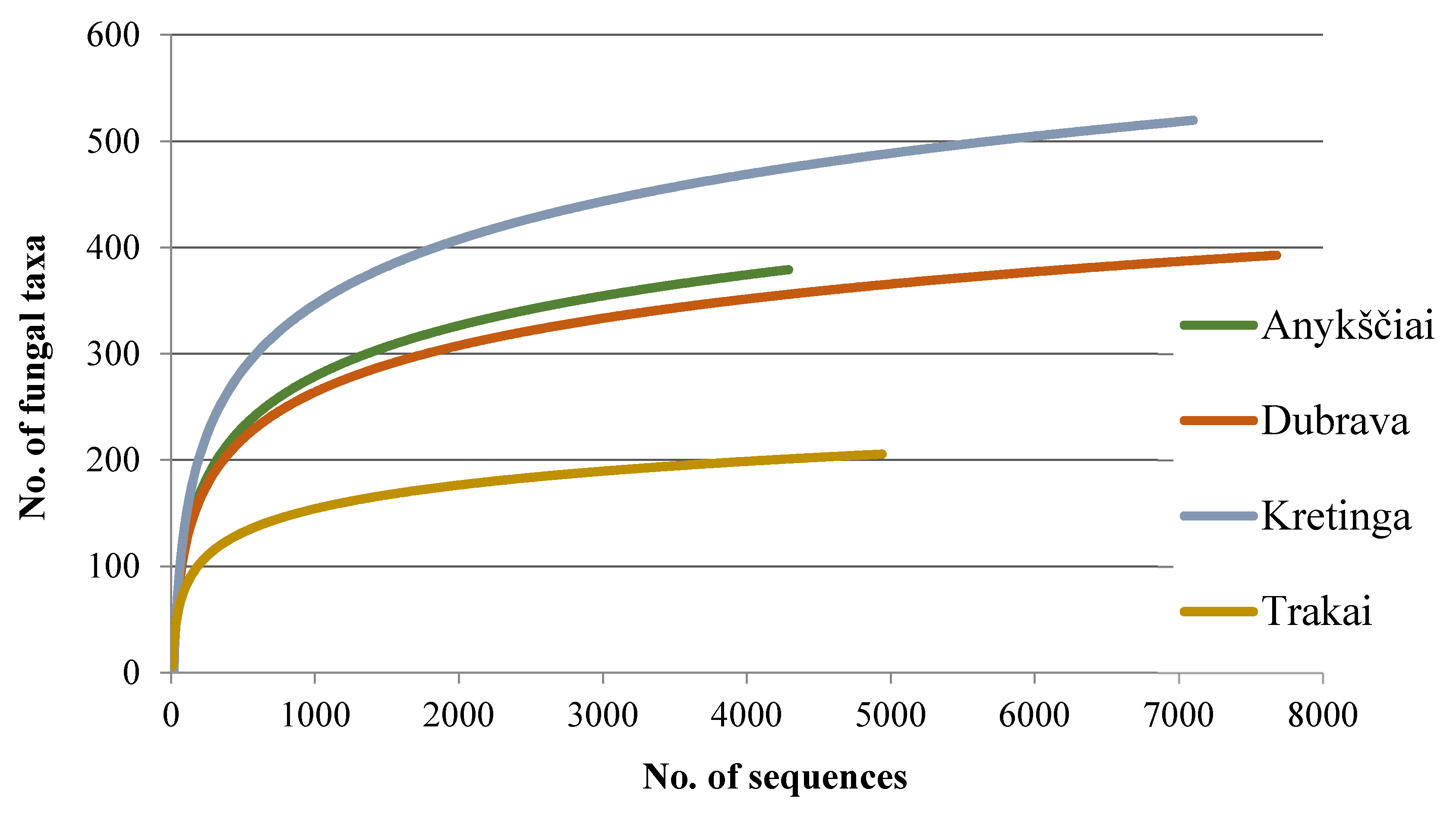
Figure 1.
Species accumulation curves showing the relationship between the cumulative number of taxa and the number of ITS rDNA sequences from four water ponds used for seedling irrigation in forest nurseries.
Among the non-singletons, 895 (69.6%) were representing fungi and oomycetes, and the remaining 391 (30.4%) were non-fungal, which were excluded. The number of high-quality sequences and fungal taxa from each study site are in Table 2. A plot of fungal taxa from four forest nurseries vs. the number of fungal sequences resulted in species accumulation curves that did not reach the species saturation (Figure 1), indicating that a potentially higher diversity of taxa could be detected by deeper sequencing. In this study, the detected fungi were 57.3% Ascomycota, 38.1% Basidiomycota, 3.1% Chytridiomycota, 0.8% Mucoromycota, and 0.7% Oomycota.
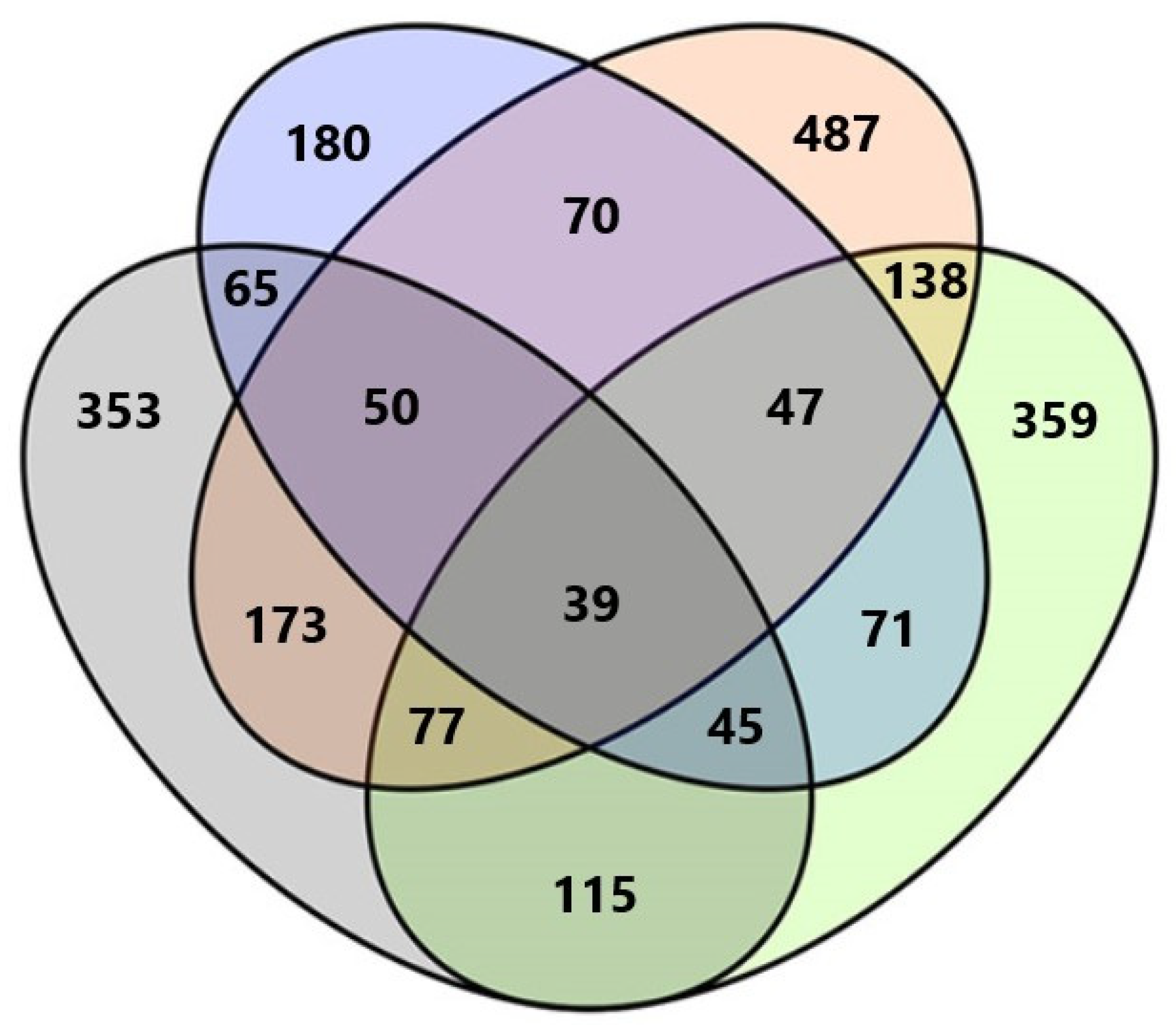
Among all fungal taxa, 148 (16.5%) were exclusively found in Anykščiai forest nursery, 124 (13.9%) in Dubrava, 221 (24.7%) in Kretinga, and 65 (7.3%) in Trakai. Only 39 (4.4%) fungal taxa were common to all forest nurseries (Figure 2). The highest number of shared taxa was between Kretinga and Dubrava, i.e., 173 taxa. The lowest number of shared taxa was between Trakai and Dubrava, i.e., 65 taxa (Figure 2).
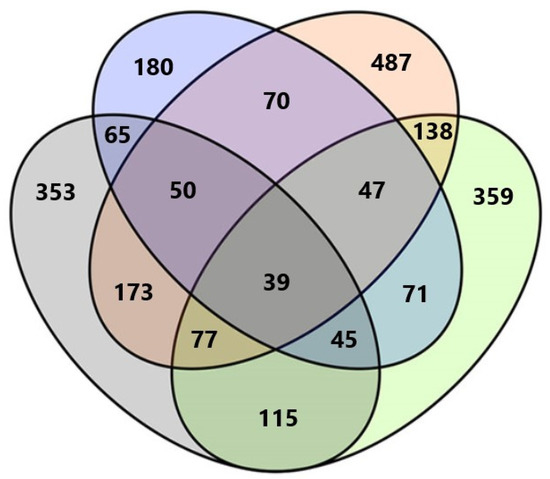
Figure 2.
Venn diagram showing the diversity and overlap of fungal taxa in water filtrate samples from four forest nurseries. Different colors represent different forest nurseries: Green—Anykščiai, Orange—Kretinga, Blue—Trakai, Gray—Dubrava.
Identification at least to genus level was possible for 475 (53.1%) out of 895 of fungal taxa. Information on the 30 most common fungal taxa representing 67.7% of all high-quality sequences is in Table 3. Among these, six taxa representing 7.4% of all high-quality sequences could not be identified to taxon or genus level (Table 3). The most common taxa were Malassezia restricta (20.1% of all high-quality sequences), Pezizella discreta (10.8%), and Epicoccum nigrum (4.9%). The most common oomycetes were Phytopythium cf. citrinum (0.4%), Phytophthora gallica (0.05%), and Peronospora sp. 4248_322 (0.05%) (Table 4).

Table 3.
Occurrence and relative abundance of the 30 most common fungal taxa (shown as a proportion of all high-quality fungal sequences) from irrigation water filtrate samples in Anykščiai, Kretinga, Trakai, and Dubrava forest nurseries. Within each row, values followed by the same letter do not differ significantly at p > 0.05.

Table 4.
Occurrence and relative abundance (shown as % of all high-quality fungal sequences) of oomycete taxa in irrigation water filtrate samples from Anykščiai, Dubrava, Kretinga and Trakai forest nurseries in Lithuania. Within each row, values followed by the same letter do not differ significantly at p > 0.05.
The chi-square test showed that among the 30 most common taxa, their relative abundance varied among different forest nurseries (Table 3). For example, P. discreta, Ramularia vizellae Crous, Unidentified sp. 4248_28 and Hypocreales sp. 4248_32 was significantly higher in the Dubrava forest nursery than in the Trakai forest nursery (p < 0.05). The abundance of Paraphaeosphaeria michotii (Westend.) O.E. Erikss. was significantly higher in the Kretinga forest nursery than in other three nurseries (p < 0.05). The abundance of M. restricta was significantly lower in the Kretinga than in the Trakai forest nursery. The three unidentified fungal taxa, i.e., Unidentified sp. 4248_13, Unidentified sp. 4248_17 and Unidentified sp. 4248_40, were exclusively found in the Trakai forest nursery (Table 3).
Twenty-three oomycete taxa were found in the irrigation water in all forest nurseries (Table 4). Among these, five taxa were from the genus Pythium, four from Phytophythium, three from Phytophthora, five from Saprolegnia Nees, and one was from each Achlya Nees and Peronospora Corda. The four taxa representing 0.03% of all high-quality sequences could not be identified to taxon or genus level. The most common oomycete taxon was P. cf. citrinum, which was detected in Anykščiai, Dubrava and Trakai, but not in the Kretinga forest nursery. Peronospora sp. 4248_322 occurred in the Kretinga forest nursery only (Table 4).
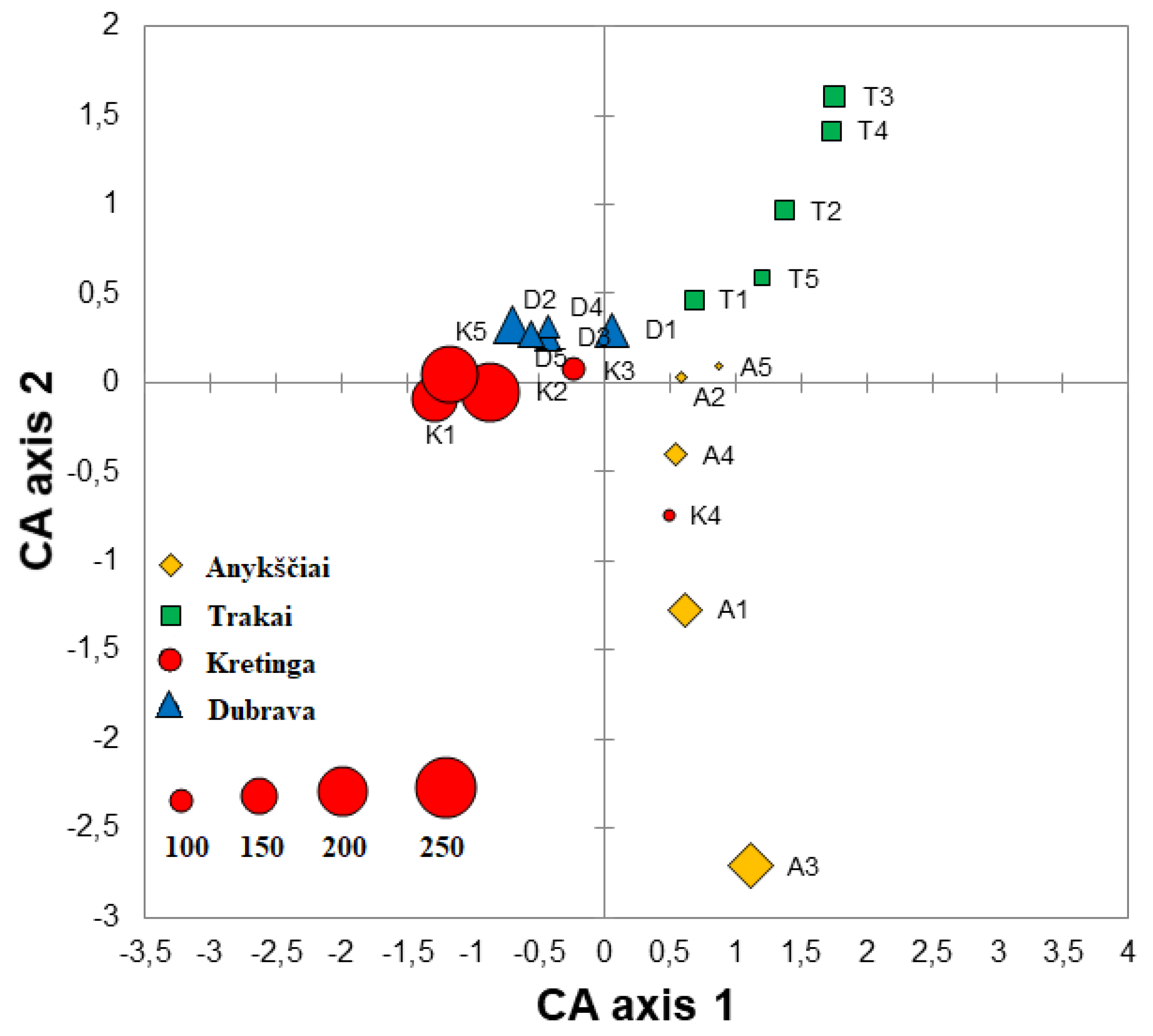
Correspondence analysis of fungal communities explained 13.6% variation on Axis 1 and 10.7% on Axis 2. The CA showed that different samples of the same forest nursery more or less well clustered together and were largely separated from other forest nurseries (Figure 3). An exception was the K4 sample from the Kretinga nursery, which clustered more closely with Anykščiai samples (Figure 3). The Sørensen similarity index of fungal communities ranged between 0.12 and 0.41 when the comparison was done among different forest nurseries, i.e., Anykščiai vs. Dubrava—0.34, Anykščiai vs. Kretinga—0.36, Anykščiai vs. Trakai—0.27, Dubrava vs. Kretinga—0.41, Dubrava vs. Trakai—0.26 and Kretinga vs. Trakai—0.12. In different samples and nurseries, the Shannon diversity index of fungal communities ranged between 1.72 and 4.27 (Table 2). The Shannon diversity index was significantly higher in the Kretinga forest nursery than in the Trakai forest nursery (p < 0.05), while no significant differences were found between other forest nurseries (p > 0.05).
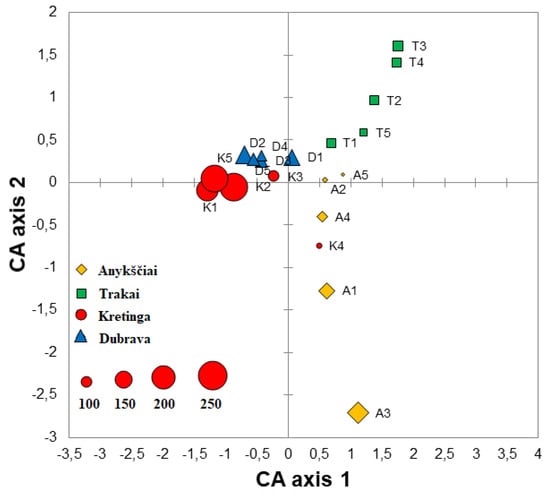
Figure 3.
Ordination diagram based on the correspondence analysis (CA) of fungal and oomycete communities in water filtrate samples from four forest nurseries: Anykščiai, Dubrava, Kretinga and Trakai. Each point in the diagram represents a single sample, and the size of each point reflects the richness of fungal taxa as shown in the lower left corner.
4. Discussion
The available knowledge indicates that water from open sources, such as rivers, canals, ponds, and reservoirs, may constitute a significant risk for disease spread to forest nurseries [11]. Some soil-inhabiting pathogenic fungi can enter the open water source together with the rainwater and, if a fine filtering of this water is lacking [48], these can be disseminated through the irrigation system. Runoff from the nursery can also carry plant pathogens back to the pond, thereby increasing the risk of new infections following irrigation [20,31]. Therefore, the irrigation water used in different cultivation systems can be one of the most efficient vehicles for the spread of plant pathogens [49,50]
Our results show that the richness of fungal taxa and the fungal community composition differed in water ponds of different forest nurseries. It appears that the characteristics of fungal communities in water filtrate samples can depend on specific parameters of each water body. In Kretinga, where water samples were from the damped bog, we found the highest diversity and the highest relative abundance of fungal taxa (Figure 2, Table 2). In Anykščiai, the pond was heavily overgrown by water plants, which could have also affected the diversity and composition of fungal communities in water [51]. In Dubrava, the pond is regularly refilled from a large lake, while, in Trakai, the pond is refilled using rainwater, which likely contributed to the observed lowest richness of fungal taxa as compared to other forest nurseries (Figure 2). The latter demonstrates that the heterogeneity of environmental conditions has likely contributed to the observed differences in richness and composition of fungal communities in different forest nurseries (Figure 1 and Figure 2; Table 2 and Table 3). This is in agreement with similar studies on aquatic fungi for which the diversity and composition may change depending on the source, location, and time of the year [52,53,54,55]. Indeed, as the sampling at different sites was carried out both in the autumn (Anykščiai, Kretinga and Dubrava) and in the spring (Trakai) (Table 1), the possibility should not be excluded that this has also contributed to the observed variation in richness and the composition of fungal communities among different forest nurseries. Besides, the fungal community structure may also vary significantly depending on both physical and chemical properties of water and physical properties of the habitat, e.g., the size and depth of the water body [56,57].
The detected principal taxonomic groups of fungi coincided with those in similar studies on freshwater habitats [12,58,59]. Among the 30 most commonly encountered fungi, several seedling pathogens have been identified, including Fusarium avenaceum (Fr.) Sacc., which is known as a cosmopolitan fungus occurring in most soils of the temperate climate zone. It is often considered as a soil-borne pathogen that causes numerous diseases, including seedling blights. Apart from soil, it can be transmitted with seeds and/or plant debris. Fusarium avenaceum has also been shown to be a problem in forest nurseries [60,61,62]. Nowadays, in Lithuanian forest nurseries, the use of chemical fungicides for seedling protection is limited due to the forest certification by the Forest Stewardship Council (FSC) [63]. Therefore, only five fungicides are registered by the State Plant Service [64] for the use in forest nurseries against a very narrow range of seedling diseases, such as needle cast, leaf mildew or rust. Therefore, seedlings can potentially be infected by various diseases, which are not controlled by these fungicides.
The second most common pathogen was Botrytis cinerea Pers. (Table 2), which is one of the main foliar pathogens in forest nurseries. Infections often appear after abiotic damages, such as caused by frost, fertilizers or herbicides [65], or environmental stress (e.g., high temperature or drought). This pathogen can also infect plants simultaneously with other fungi, or to colonize necroses caused by other diseases, such as pine twisting rust [66,67]. Petäistö [68] has found B. cinerea to be an important fungal pathogen showing frequent infection spread from diseased seedlings to healthy ones. The conidia of B. cinerea can be spread by insects (e.g., Bradysia spp.: [69]) or wind [65]. Our results provide additional information on the possible spread of this economically important fungal pathogen with the irrigation water. Generally, an infection by B. cinerea can take place just after three hours at 15–20 °C and 98% relative humidity in the presence of water on plant surfaces, while for Norway spruce it can be already at 6 °C and 80–90% relative humidity [68].
Gremmeniella abietina (Lagerb.) M. Morelet was the other pathogenic fungus, which is known as a causal agent of Scleroderris canker in conifer tree seedlings [70]. Alternaria alternata (Fr.) Keissl. was also detected and it is a destructive pathogen, which can affect sprouting seeds, first-year coniferous and deciduous tree seedlings [71]. The presence of Verticillium sp. has also indicated the potential threat to seedling production as these diseases are among the most devastating and can affect numerous plant species worldwide, ranging from herbaceous annuals to woody perennials [72]. Although the above-mentioned plant pathogens can cause a significant damage in forest nurseries worldwide, in our samples their abundance was relatively low.
The detected oomycete community included primarily plant pathogens (Pythopythium, Pythium, Phytophthora and Peronospora) and pathogens of fish and other aquatic organisms (Saprolengia) [6,73,74]. Interestingly, a similar oomycete community was reported by Redekar et al. [32] from the recycled irrigation water in a forest nursery using a containerized cultivation system. Nevertheless, during the last century, plant pathogenic oomycetes, such as Phytophthora and Phythium were detected in the irrigation water in different countries [10,30,32]. The latter repeatedly demonstrates the risk of pathogens spread with the irrigation water and highlights the importance of appropriate water management in plant production systems.
The detected diversity and abundance of oomycete taxa was relatively low, i.e., 23 taxa (Table 4), as compared to Redekar et al. [32] who, among other taxa in the irrigation water, detected 48 species of Phytophthora and 36 of Pythium. The lower diversity and relative abundance of oomycetes in our study could be due to climatic conditions as samples were taken during the dormancy period and oomycetes are known to be temperature dependant [10]. As sampling was carried out at the 0.5–1.0 m depth, this may have also affected the detected diversity of oomycetes as these are more common to the water surface. Besides, as structures of oomycetes are rather small, some of these could have passed through the filter, thereby affecting species richness and abundance estimates. Alternatively, this can partly be due to PCR biases that selected for shorter fragments of fungal DNA. Nevertheless, the oomycete diversity in the irrigation water may change depending on environmental conditions and the time of the year, as these factors may affect the survival and activity of individual taxa, and thus, may affect the degree of plant infections [6,16,52,53,54,55,75,76].
The oomycete genus Phytopythium was represented by four taxa, among which P. cf. citrinum was most common (Table 4). In Poland, P. cf. citrinum has been shown to be commonly isolated from the rhizosphere soil of oak stands with different health status [40]. As in our study, P. cf. citrinum predominated the oomycete community in Anykščiai, Dubrava and Trakai forest nurseries, it can be a threat to the seedlings of pedunculate oak, which comprises about 10% of the annual production in all of these forest nurseries [77].
The detected aggressive tree pathogens included Phytophthora cactorum (found in Kretinga and Trakai) and Phytophthora plurivora T. Jung & T.I. Burgess (found in Trakai). Phytophthora cactorum was previously reported from Finland, where it was isolated from the necrotic stem lesions of the container-grown seedlings of Betula pendula Roth. It is an omnivorous pathogen that can infect over 200 plant species in 160 genera, including Betula spp., Salix spp. and many other woody plants [30]. Phytophthora plurivora has been extensively isolated in Europe from natural forests and a variety of hosts in other parts of the world. It has been recovered from numerous hosts with symptoms of crown dieback, small-sized and often yellowish foliage, fine root dieback, root lesions, collar rots, cankers and shoot dieback [78]. Among the remaining oomycetes, Phytopythium litorale (Nechw.) Abad, de Cock, Bala, Robideau, Lodhi & Lévesque, Pythium mamillatum Meurs and Pythium dissotocum Drechsler have been reported as pathogens causing severe damping-off and root-rot diseases to different agricultural crops [54,79,80].
5. Conclusions
The irrigation water used by forest nurseries was inhabited by a species-rich but largely site-specific community of fungi. Although plant pathogens were relatively rare, under suitable conditions these can develop rapidly, spread efficiently through the irrigation system and be a threat to the production of high-quality tree seedlings. To prevent seedling infections and losses, the appropriate management of the irrigation water should be among the practices of integrated disease management in forest nurseries.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/4/459/s1, Table S1: Occurrence and relative abundance of fungal taxa (shown as a proportion of all fungal sequences) from irrigation water filtrate samples in Anykščiai, Kretinga, Trakai, and Dubrava forest nurseries in Lithuania.
Author Contributions
Conceptualization, J.L. and A.M. (Audrius Menkis); Data curation, D.M., J.L., A.G. and M.V.; Formal analysis, A.M. (Adas Marčiulynas); Investigation, A.M. (Adas Marčiulynas), D.M., J.L., A.G. and M.V.; Methodology, A.M. (Adas Marčiulynas), D.M., A.G., M.V. and A.M. (Audrius Menkis); Software, A.M. (Adas Marčiulynas); Supervision, A.M. (Audrius Menkis); Validation, A.M. (Adas Marčiulynas), D.M. and A.M. (Audrius Menkis); Visualization, J.L., M.V. and A.M. (Audrius Menkis); Writing—original draft, A.M. (Adas Marčiulynas) and J.L.; Writing—review & editing, D.M., A.G. and A.M. (Audrius Menkis). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Council of Lithuania, grant no. S-MIP-17-6.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Myking, T.; Rusanen, M.; Steffenrem, A.; Kjær, E.D.; Jansson, G. Historic transfer of forest reproductive material in the Nordic region: Drivers, scale and implications. Forestry 2016, 89, 325–337. [Google Scholar] [CrossRef]
- Jansen, S.; Konrad, H.; Geburek, T. Crossing borders–European forest reproductive material moving in trade. J. Environ. Manag. 2019, 233, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Forest Europe, 2015. State of Europe’s Forests 2015. Available online: http://www.foresteurope.org/docs/fullsoef2015.pdf (accessed on 19 March 2020).
- Stenlid, J.; Oliva, J.; Boberg, J.B.; Hopkins, A.J.M. Emerging diseases in European forest ecosystems and responses in society. Forests 2011, 2, 486–504. [Google Scholar] [CrossRef]
- Lilja, A.; Poteri, M.; Petäistö, R.-L.; Rikala, R.; Kurkela, T.; Kasanen, R. Fungal diseases in forest nurseries in Finland. Silva Fenn. 2010, 44, 525–545. [Google Scholar] [CrossRef]
- Hong, C.X.; Moorman, G.W. Plant pathogens in irrigation water: Challenges and opportunities. Crit. Rev. Plant Sci. 2005, 24, 189–208. [Google Scholar] [CrossRef]
- Oron, G.; Gillermana, L.; Buriakovskya, N.; Bickd, A.; Gargir, M.; Dolan, Y.; Manor, Y.; Katze, L.; Haginc, J. Membrane technology for advanced wastewater reclamation for sustainable agriculture production. Desalination 2008, 218, 170–180. [Google Scholar] [CrossRef]
- Da Machado, P.S.; Alfenas, A.C.; Coutinho, M.M.; Silva, C.M.; Mounteer, A.H.; Maffia, L.A.; Freitas, R.G.; da Freitas, C.S. Eradication of plant pathogens in forest nursery irrigation water. Plant Dis. 2013, 97, 780–788. [Google Scholar] [CrossRef]
- Oszako, T.; Sikora, K.; Belbahri, L.; Nowakowska, J.A. Molecular detection of oomycetes species in water courses. Folia For. Pol. 2016, 58, 246–251. [Google Scholar] [CrossRef]
- Zappia, R.E.; Huberli, D.; Hardy, G.E.S.J.; Bayliss, K.L. Fungi and oomycetes in open irrigation systems: Knowledge gaps and biosecurity implications. Plant Pathol. 2014, 63, 961–972. [Google Scholar] [CrossRef]
- Pettitt, T.R. Irrigation water and the health of nursery crops. In Biology, Detection, and Management of Plant Pathogens in Irrigation Water; Hong, C., Moorman, G.W., Wohanka, W., Büttner, C., Eds.; The American Phytopathological Society: Saint Paul, MN, USA, 2017; pp. 13–22. [Google Scholar]
- Shearer, C.A.; Descals, E.; Kohlmeyer, B.; Kohlmeyer, J.; Marvanova, L.; Padgett, D.; Porter, D.; Raja, H.A.; Schmit, J.P.; Thorton, H.A.; et al. Fungal biodiversity in aquatic habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar] [CrossRef]
- Friggens, N.L.; Taylor, J.E.; Koukol, O. Diversity and community composition of aquatic ascomycetes varies between freshwater, estuarine and marine habitats in western Scotland. Mycosphere 2017, 8, 1267–1287. [Google Scholar] [CrossRef]
- Grossart, H.; Van den Wyngaert, S.; Kagami, M. Fungi in aquatic ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Shokes, F.M.; McCarter, S.M. Occurrence, dissemination and survival of plant pathogens in surface irrigation ponds in Southern Georgia. Phytopathology 1979, 69, 510–516. [Google Scholar] [CrossRef]
- MacDonald, J.D.; Ali-Shtayeh, M.S.; Kabashima, J.; Stites, J. Occurrence of Phytophthora species in recirculated nursery irrigation. Plant Dis. 1994, 78, 607–611. [Google Scholar] [CrossRef]
- Kamoun, S. Molecular genetics of pathogenic oomycetes. Eukaryot. Cell 2003, 2, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Werres, S.; Wagner, S.; Brand, T.; Kaminski, K.; Seipp, D. Survival of Phytophtora ramorum in recirculating irrigation water and subsequent infection rhododendron and Viburnum. Plant Dis. 2007, 91, 1034–1044. [Google Scholar] [CrossRef]
- Moorman, G.W.; Gevens, A.J.; Granke, L.L.; Hausbeck, M.K.; Hendricks, K.; Roberts, P.D.; Pettitt, T.R. Sources and distribution systems of irrigation water and their potential risks for crop health. In Biology, Detection, and Management of Plant Pathogens in Irrigation Water; Hong, C., Moorman, G.W., Wohanka, W., Büttner, C., Eds.; The American Phytopathological Society: Saint Paul, MN, USA, 2017; pp. 3–11. [Google Scholar]
- Ivors, K.L.; Moorman, G.W. Oomycete plant pathogens in irrigation water. In Biology, Detection, and Management of Plant Pathogens in Irrigation Water; Hong, C., Moorman, G.W., Wohanka, W., Büttner, C., Eds.; The American Phytopathological Society: Saint Paul, MN, USA, 2017; pp. 57–64. [Google Scholar]
- Johnson, T.W.; Seymour, R.L. Aquatic fungi of Iceland: Comparative morphology of Achlya spiracaulis and Achlya papillosa. Nova Hedwig. 1974, 25, 433–449. [Google Scholar]
- Hughes, K.A.; Lawley, B.; Newsham, K.K. Solar UV-B radiation inhibits the growth of Antarctic terrestrial fungi. Appl. Environ. Microbiol. 2003, 69, 1488–1491. [Google Scholar] [CrossRef]
- Bridge, P.D.; Newsham, K.K.; Denton, G.J. Snow mould caused by a Pythium sp.: A potential vascular plant pathogen in the maritime Antarctic. Plant Pathol. 2008, 57, 1066–1072. [Google Scholar] [CrossRef]
- Tan, T.K.; Pek, C.L. Tropical mangrove leaf litter fungi in Singapore with an emphasis on Halophytophthora. Mycol. Res. 1997, 101, 165–168. [Google Scholar] [CrossRef]
- Nakagiri, A. Ecology and biodiversity of Halophytophthora species. Fungal Divers. 2000, 5, 153–164. [Google Scholar]
- Mirzaee, M.R.; Ploch, S.; Runge, F.; Telle, S.; Nigrelli, L.; Thines, M. A new presumably widespread species of Albugo parasitic to Strigosella spp. (Brassicaceae). Mycol. Prog. 2013, 12, 45–52. [Google Scholar] [CrossRef]
- Schubert, R.; Bahnweg, G.; Nechwatal, J.; Jung, T.; Cooke, D.E.; Duncan, J.M. Detection and quantification of Phytophthora species which are associated with root-rot diseases in European deciduous forests by species-specific polymerase chain reaction. For. Pathol. 1999, 29, 169–188. [Google Scholar]
- Bartnicki-Garcia, S. Cell wall chemistry, morphogenesis and taxonomy of fungi. Ann. Rev. Microbiol. 1968, 22, 87–108. [Google Scholar] [CrossRef]
- Nowakowska, J.A.; Oszako, T.; Borys, M.; Sikora, K.; Kubiak, K.; Olejarski, I. Genetic variability of Phytophthora community in natural water resources assessed with microsatellite DNA markers. Balt. For. 2012, 18, 56–64. [Google Scholar]
- Rytkönen, A.; Lilja, A.; Petäistö, R.L.; Hantula, J. Irrigation water and Phytophtora cactorum in a forest nursery. Scand. J. For. Res. 2008, 23, 404–411. [Google Scholar]
- Thines, M. Phylogeny and evolution of plant pathogenic oomycetes, a global overview. Eur. J. Plant Pathol. 2014, 138, 431–447. [Google Scholar] [CrossRef]
- Redekar, N.R.; Eberhart, J.L.; Parke, J.L. Diversity of Phytophtora, Pythium and Phytophythium species in recycled irrigation water in a container nursery. Phytobiomes J. 2019, 3, 31–45. [Google Scholar] [CrossRef]
- Thines, M.; Kamoun, S. Oomycete–plant coevolution: Recent advances and future prospects. Curr. Opin. Plant Biol. 2010, 13, 427–433. [Google Scholar] [CrossRef]
- Porter, L.D.; Johnson, D.A. Survival of Phytophthora infestans in surface water. Phytopathology 2004, 94, 380–387. [Google Scholar] [CrossRef]
- Bewley, W.F.; Buddin, W. On the fungus flora of glasshouse water supplies in relation to plant disease. Ann. Appl. Biol. 1921, 8, 10–19. [Google Scholar] [CrossRef]
- Jung, T.; Hudler, G.W.; Jensen-Tracy, S.L.; Griffiths, H.M.; Fleischmann, F.; Oßwald, W. Involvement of Phytophthora spp. in the decline of European beech in Europe and the USA. Mycologist 2005, 19, 159–166. [Google Scholar] [CrossRef]
- Jung, T. Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. For. Pathol. 2009, 39, 73–94. [Google Scholar] [CrossRef]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguın Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech1, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-naturaland horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2015, 46, 134–163. [Google Scholar] [CrossRef]
- Vitas, A.; Oszako, T.; Nowakowska, J.A.; Sikora, K.; Stankevičienė, A. First records of Phytophthora spp. based on DNA analysis in Lithuania. Folia For. Pol. Ser. A For. 2012, 54, 25–31. [Google Scholar]
- Jankowiak, R.; Stępniewska, H.; Bilański, P. Notes on some Phytopythium and Pythium species occurring in oak forests in southern Poland. Acta Mycol. 2015, 50, 1052. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Goss, E.M.; Press, C.M. Phytophthora ramorum: A pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals. Mol. Plant Pathol. 2008, 9, 729–740. [Google Scholar] [CrossRef]
- Ristvey, A.G.; Belayneh, B.E.; Lea-Cox, J.D. A Comparison of irrigation-water containment methods and management strategies between two ornamental production systems to minimize water security threats. Water 2019, 11, 2558. [Google Scholar] [CrossRef]
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bodeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandstrom-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region-evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Sokal, R.R. and Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Co.: New York, NY, USA, 1995. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Calvo-Bado, L.A.; Pettitt, T.R.; Parsons, N.; Petch, G.M.; Morgan, J.A.W.; Whipps, J.M. Spatial and temporal analysis of the microbial community in slow sand filters used for treating horticultural irrigation water. Appl. Environ. Microbiol. 2003, 69, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Ehert, D.L.; Alsanius, B.; Wohanka, W.; Menzies, J.G.; Utkhede, R. Disinfestation of recirculating nutrient solutions in greenhouse horticulture. Agronomie 2001, 21, 323–339. [Google Scholar]
- Wurzbacher, C.M.; Bärlocher, F.; Grossart, H.P.P. Fungi in lake ecosystems. Aquat. Microb. Ecol. 2010, 59, 125–149. [Google Scholar] [CrossRef]
- Cayanan, F.D.; Zhang, P.; Liu, W.; Dixon, M.; Zheng, Y. Efficacy of chlorine in controlling five common plant pathogens. Hort Sci. 2009, 44, 157–163. [Google Scholar] [CrossRef]
- Loyd, A.L.; Benson, D.M.; Ivors, K.L. Phytophtora populations in nursery irrigation water in relationship to pathogenicity and infection freguency of Rhododendron and Pieris. Plant Dis. 2014, 98, 1213–1220. [Google Scholar] [CrossRef]
- Parke, J.L.; Knaus, B.J.; Fieland, V.J.; Lewis, C.; Grunwald, N.J. Phytophtora community structure analyses in Oregon nurseries inform systems approaches to disease management. Phytopathology 2014, 104, 1052–1062. [Google Scholar] [CrossRef]
- Copes, W.E.; Yang, X.; Hong, C. Phytophtora species recovered from irrigation reservoirs in Mississipi and Alabama nurseries and pathogenicity of three new species. Plant Dis. 2015, 99, 1390–1395. [Google Scholar] [CrossRef]
- Choudhary, C.E.; Burgos-Garay, M.L.; Moorman, G.W.; Hong, C. Pythium and Phytophytium species in two Pennsylvania greenhouse irrigation water tanks. Plant Dis. 2016, 100, 926–932. [Google Scholar] [CrossRef]
- Baldy, V.; Chauvet, E.; Charcosset, J.Y.; Gessner, M.O. Microbial dynamics associated with leaves decomposing in the main stem and flood plain pond of a large river. Aquat. Microb. Ecol. 2002, 28, 25–36. [Google Scholar] [CrossRef]
- Mille-Lindblom, C.; Fischer, H.; Tranvik, L.J. Antagonism between bacteria and fungi: Substrate competition and a possible tradeoff between fungal growth and tolerance towards bacteria. OIKOS 2006, 113, 233–242. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Lacy, G.H.; Fox, J.A. Occurrece and population of Plasmodiospora brasisicae in sediments of irrigation water sources. Plant Dis. 1984, 68, 200–203. [Google Scholar] [CrossRef]
- Grech, N.M.; Rijkenberg, F.H.J. Injection of electrolytically generated chlorine into citrus microirrigation systems for the control of certain waterborne root pathogens. Plant Dis. 1992, 76, 457–461. [Google Scholar] [CrossRef]
- James, R.L.; Gilligan, C.J.; Reedy, V. Evaluation of Root Diseases of Containerized Conifer Seedlings at the Champion Timberlands Nursery, Plains, Montana; Report No. 88-11; Department of Agriculture, Forest Service Timber, Cooperative Forestry and Pest Management: Missoula, MT, USA, 1988; p. 88-11. [Google Scholar]
- Asiegbu, F.O.; Kacprzak, M.; Daniel, G.; Johansson, M.; Stenlid, J.; Mañka, M. Biochemical interactions of conifer seedling roots with Fusarium spp. Can. J. Microbiol. 1999, 45, 923–935. [Google Scholar] [CrossRef]
- Marek, S.M.; Yaghmour, M.A.; Bostock, R.M. Fusarium spp., Cylindrocarpon spp., and environmental stress in the etiology of a canker disease of cold-stored fruit and nut tree seedlings in California. Plant Dis. 2013, 97, 259–270. [Google Scholar] [CrossRef] [PubMed]
- FSC Pesticides Policy. 2020. Available online: https://fsc.org/en/document-centre/documents/resource/208 (accessed on 19 March 2020).
- The List os Pesticides (in Lithuanian). 2020. Available online: http://www.vatzum.lt/uploads/documents/20200213_fungicidai.pdf (accessed on 19 March 2020).
- Sutherland, J.R. Management of pathogens in seed orchards and nurseries. For. Chron. 1991, 67, 481–485. [Google Scholar] [CrossRef]
- Domanski, S.; Kowalski, T. Untypical dieback of the current season’s shoots of Pinus sylvestris in Poland. Eur. J. For. Pathol. 1988, 18, 157–160. [Google Scholar] [CrossRef]
- Hansen, E.M.; Hamm, P.B. Phythium species from forest and muskeg areas of Southeern Alaska. Trans. Br. Mycol. Soc. 1988, 91, 379–384. [Google Scholar] [CrossRef]
- Petäistö, R.L. Botrytis cinerea and Norway Spruce Seedlings in Cold Storage. Balt. For. 2006, 11, 24–33. [Google Scholar]
- James, R.L.; Dumroese, R.K.; Wenny, D.L. Botrytis cinerea carried by Adult Fungus Gnats (Diptera: Sciaridae) in Container Nurseries. Tree Plant. Notes 1995, 46, 48–53. [Google Scholar]
- Jeger, M.; Bragard, C.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Gregoire, J.C.; Miret, J.A.J.; MacLeod, A.; et al. Pest categorisation of Gremmeniella abietina. EFSA J. 2017, 15, 5030. [Google Scholar]
- Krol, E.; Machowicz-Stefaniak, Z.; Zimowska, B.; Abramczyk, B.; Zalewska, E. Fungi ihabiting seeds of selected forest tree species. Sylwan 2015, 159, 135–141. [Google Scholar]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Beakes, G.W.; Glockling, S.L.; Sekimoto, S. The evolutionary phylogeny of the oomycete “fungi”. Protoplasma 2012, 249, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Thines, M.; Choi, Y.J. Evolution, diversity, and taxonomy of the Peronosporaceae, with focus on the genus Peronospora. Phytopathology 2016, 106, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Bush, E.A.; Hong, C.X.; Stromberg, E.L. Fluctuations of Phytophthora and Pythium spp. in components of a recycling irrigation system. Plant Dis. 2003, 87, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.X.; Gallegly, M.E.; Richardson, P.A.; Kong, P.; Moorman, G.W. Phytophthora irrigata, a new species isolated from irrigation reservoirs and rivers in Eastern United States of America. FEMS Microbiol. Lett. 2008, 285, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lithuanian Statistical Yearbook of Forestry; Ministry of Environment, State Forest Service: Kaunas, Lithuania, 2017; pp. 108–109.
- Mrázková, M.; Čern, K.; Tomšovský, M.; Strnadová, V.; Gregorová, B.; Holub, V.; Pánek, M.; Havrdová, L.; Hejná, M. Occurrence of Phytophthora multivora and Phytophthora plurivora in the Czech Republic. Plant Prot. Sci. 2013, 49, 155–164. [Google Scholar] [CrossRef]
- Bates, M.L.; Stanghellini, M.E. Root rot of hydroponically grown spinach caused by Pythium aphanidermatum and P. dissotocum. Plant Dis. 1984, 68, 989–991. [Google Scholar] [CrossRef]
- Parkunan, V.; Ji, P. Isolation of Pythium litorale from irrigation ponds used for vegetable production and its pathogenicity on squash. Can. J. Plant Pathol. 2013, 35, 415–423. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).