Practical Implications of Different Phenotypic and Molecular Responses of Evergreen Conifer and Broadleaf Deciduous Forest Tree Species to Regulated Water Deficit in a Container Nursery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Material
2.3. Experimental Design
2.4. Microclimate
2.5. Regulated Water Deficit
2.6. Growth, Biomass Allocation, and Water Contents
2.7. Microscopic Observations
2.8. Determination of Leaf Proline
2.9. Expression of Stress-Related Genes
2.10. Statistical Analyses
3. Results
3.1. Seedling Water Status
3.2. Growth
3.3. Allocation
3.4. Leaf Proline Concentration and the Expression Levels of Selected Stress-Response Genes
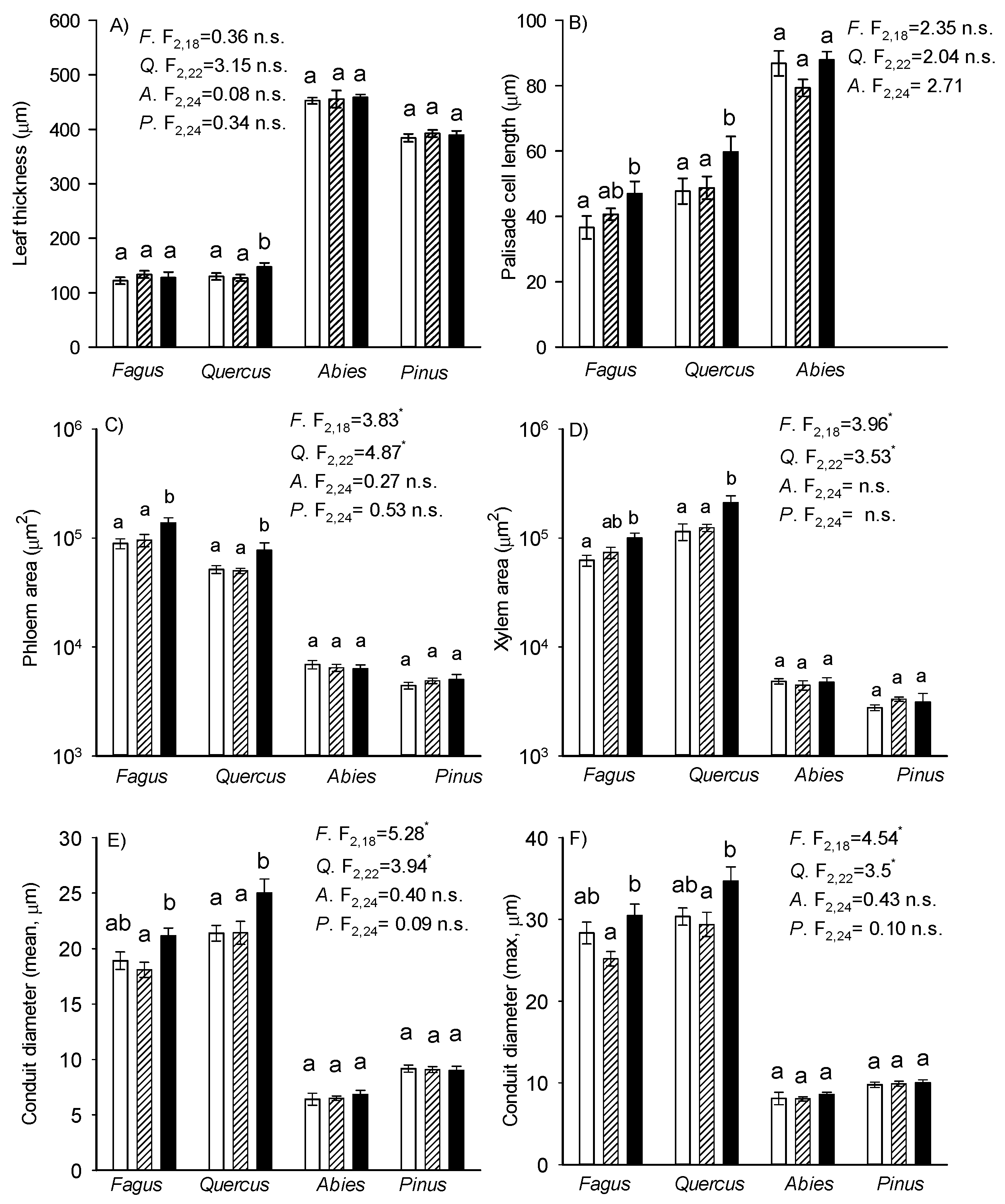
3.5. Anatomical Leaf Traits
4. Discussion
4.1. Species-Specific Growth Responses to Irrigation
4.2. Seedling Water Status
4.3. Biomass Allocation
4.4. Osmoregulation and Alterations in Gene Expression in Response to Regulated Water Deficit
4.5. Leaf Anatomy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Djalante, R.; Ebi, K.; Engelbrecht, F.; et al. Impacts of 1.5 °C Global Warming on Natural and Human Systems. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V.P., Zhai, H.O., Pörtner, D., Roberts, J., Skea, P.R., Shukla, A., Pirani, W., Moufouma-Okia, C., Péan, R., Pidcock, S., et al., Eds.; IPCC Secretariat: Geneva, Switzerland, 2018. [Google Scholar]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolstro, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Wang, Y.; Hogg, E.H.; Price, D.T.; Edward, J.; Williamson, T. Past and projected future changes in moisture conditions in the Canadian boreal forest. For. Chron. 2014, 90, 678–691. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Rich, R.L.; Hobbie, S.E.; Montgomery, R.A. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 2018. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; Mcdowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Maherali, H.; Pockman, W.T.; Jackson, R.B. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 2004, 85, 2184–2199. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, H. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Chang. Biol. 2013. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; Hillerislambers, J. Drought stress limits the geographic ranges of two tree species via different physiological mechanisms. Glob. Chang. Biol. 2015, 22, 1029–1045. [Google Scholar] [CrossRef]
- Leuzinger, S.; Zotz, G.; Asshoff, R.; Körner, C.H. Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiol. 2005, 25, 641–650. [Google Scholar] [CrossRef]
- Meier, I.C.; Leuschner, C. Genotypic variation and phenotypic plasticity in the drought response of the fine root system of European beech. Tree Physiol. 2008, 28, 297–309. [Google Scholar] [CrossRef]
- Metz, J.; Annighöffer, P.; Schall, P.; Zimmermann, J.; Kahll, T.; Schulze, E.-D.; Ammer, C.H. Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob. Chang. Biol. 2016, 22, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Sánchez-Gómez, D. Ecophysiological traits associated with drought in mediterranean tree seedlings: Individual responses versus interspecific trends in eleven species. Plant Biol. 2006, 8, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Taylor, S.F.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.D. Adaptations and responses to drought in Quercus species of North America. Tree Physiol. 1990, 7, 227–238. [Google Scholar] [CrossRef]
- Warren, C.R.; Bleby, T.; Adams, M.A. Changes in gas exchange versus leaf solutes as a means to cope with summer drought in Eucalyptus marginata. Oecologia 2007, 154, 1–10. [Google Scholar] [CrossRef]
- Volaire, F. A unified framework of plant adaptive strategies to drought: Crossing scales and disciplines. Glob. Chang. Biol. 2018, 24, 2929–2938. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- van Hess, A.F.M. Growth and morphology of pedunculate oak (Quercus robur L.) and beech (Fagus sylvatica L.) seedlings in relation to shading and drought. Ann. Sci. For. 1996, 54, 9–18. [Google Scholar] [CrossRef]
- Tognetti, R.; Johnson, J.D.; Michelozzi, M. The response of European beech (Fagus sylvatica L.) seedlings from two Italian populations to drought and recovery. Trees 1995, 9, 348–354. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analysis of interspecific variation and environmental control. New Phytol. 2011, 193, 30–50. [Google Scholar] [CrossRef]
- Galmés, J.; Ochogavía, J.M.; Gago, J.; Roldán, E.J.; Cifre, J.; Conesa, M.À. Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: Anatomical adaptations in relation to gas exchange parameters. Plant Cell Environ. 2013, 36, 920–935. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns of drought tolerance in major European temperate forest trees: Climatic drivers and levels of variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Binks, O.; Meir, P.; Rowland, L.; da Costa, A.C.L.; Vasconcelos, S.S.; de Oliveira, A.A.R.; Ferreira, L.; Christoffersen, B.; Nardini, A.; Mencuccini, M. Plasticity in leaf-level water relations of tropical rainforest trees in response to experimental drought. New Phytol. 2016, 211, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Tyree, M.; Davis, S.; Cochard, H. Biophysical perspectives of xylem evolution: Is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? Int. Assoc. Wood Anat. J. 1994, 15, 335–360. [Google Scholar] [CrossRef]
- Sperry, J.S.; Hacke, U.G.; Pittermann, J. Size and function in conifer tracheids and angiosperms vessels. Am. J. Bot. 2006, 93, 1490–1500. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Liu, C.h.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Reguera, M.; Peleg, Z.; Abdel-Tawab, Y.M.; Tumimbang, E.B.; Delatorre, C.A.; Blumwald, E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol. 2013, 163, 1609–1622. [Google Scholar] [CrossRef]
- Vandeleurs, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Markesteijn, L.; Poorter, L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J. Ecol. 2009, 97, 311–325. [Google Scholar] [CrossRef]
- Borghetti, M.; Cinnirella, S.; Magnani, F.; Saracino, A. Impact of long-term drought on xylem embolism and growth in Pinus halepensis Mill. Trees 1998, 12, 187–195. [Google Scholar] [CrossRef]
- Walter, J.; Nagy, L.; Heinb, R.; Rascher, U.; Beierkuhnlein, C.; Willner, E.; Jentsch, A. Do plants remember drought? Hints towards a drought-memory in grasses. Environ. Exp. Bot. 2011, 71, 34–40. [Google Scholar] [CrossRef]
- Fleta-Soriano, E.; Munné-Bosch, S. Stress memory and the inevitable effects of drought: A physiological perspective. Front. Plant Sci. 2016, 7, 143. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatall, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Helman, D.; Lensky, I.M.; Yakir, D.; Osem, Y. Forests growing under dry conditions have higher hydrological resilience to drought than do more humid forests. Glob. Chang. Biol. 2016, 23, 2801–2817. [Google Scholar] [CrossRef]
- Abrams, M.D. Comparative water relations of three successional hardwood species in central Wisconsin. Tree Physiol. 1988, 4, 263–273. [Google Scholar] [CrossRef]
- Arango-Velez, A.; Zwiazek, J.J.; Thomas, B.R.; Tyree, M.T. Stomatal factors and vulnerability of stem xylem to cavitation in poplars. Physiol. Plant. 2011, 143, 154–165. [Google Scholar] [CrossRef]
- Brzeziecki, B.; Kienast, F. Classifying the life-history strategies of trees on the basis of the Grimian model. For. Ecol. Manag. 1994, 69, 167–187. [Google Scholar] [CrossRef]
- Backes, K.; Leuschner, C. Leaf water relations of competitive Fagus sylvatica L. and Quercus petraea (Matt.) Liebl. trees during four years differing in soil drought. Can. J. For. Res. 2000, 30, 335–346. [Google Scholar] [CrossRef]
- Epron, D.; Dreyer, E. Long-term effects of drought on photosynthesis of adult oak trees (Quercus petraea (Matt.) Liebl. and Quercus robur L.) in a natural stand. New Phytol. 1993, 125, 381–389. [Google Scholar] [CrossRef]
- Epron, D.; Godard, D.; Cornic, G.; Genty, B. Limitations of net CO2 assimilation rates by internal resistances to CO2 transfer in leaves of two species (Fagus sylvatica L. and Castanea sativa Mill.). Plant Cell Environ. 1995, 18, 43–51. [Google Scholar] [CrossRef]
- Georgea, J.-P.; Schuelera, S.; Karanitsch-Ackerlb, S.; Mayerb, K.; Klumppc, R.T.; Grabnerb, M. Inter-and intra-specific variation in drought sensitivity in Abies spec. and its relation to wood density and growth traits. Agr. For. Meteorol. 2015, 214–215, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Toromani, E.; Sanxhaku, M.; Pasho, E. Growth responses to climate and drought in silver fir (Abies alba) along an altitudinal gradient in southern Kosowo. Can. J. For. Res. 2011, 41, 1795–1807. [Google Scholar] [CrossRef]
- Nourtier, M.; Chanzy, A.; Cailleret, M.; Yingge, X.; Huc, R.; Davi, H. Transpiration of silver fir (Abies alba Mill.) during and after drought in relation to soil properties in a Mediterranean mountain area. Ann. For. Sci. 2012, 1–13. [Google Scholar] [CrossRef]
- Bigler, C.h.; Braker, O.U.; Bugmann, H.; Dobbertin, M.; Rigling, A. Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems 2006, 9, 330–343. [Google Scholar] [CrossRef]
- Zarzycki, K.; Trzcińska-Tacik, H.; Różański, W.; Szeląg, Z.; Wołek, J.; Korzeniak, U. Ecological Indicator Values of Vascular Plants of Poland. Kraków. W. Szafer Inst. Bot. Pol. Acad. Sci. 2002, 2, 183. [Google Scholar]
- Pabian, R. Is a container nursery cost-effective? Las Polski [Pol. For.] 2014, 20. Available online: http://www.laspolski.pl/Czy_szkssnkarstwo_kontenerowe_sizo_opnaca_nr_202014_,strona-2633.html (accessed on 4 November 2018).
- Małek, S.; Banach, J.; Barszcz, J.; Dudek, K.; Durło, G.; Kormanek, M.; Skowrońska, I.; Jagiełło-Leńczuk, K.; Przybyłek, M.; Bierowiec, K. Optymalizacja Produkcji Sadzonek z Zakrytym Systemem Korzeniowym w Wybranych Szkółkach Kontenerowych (Optimalization of Cover-Root Seedling Production in Selected Container Nurseries); Final Report; 2017; pp. 293–318. Available online: https://tbr.lasy.gov.pl/apex/f?p=102:3:::NO::P3_TEMAT:3661 (accessed on 15 October 2019). (In Polish)
- Heiskanen, J. Favourable water and aeration for growth media in containerized tree seedling production: A review. Scand. J. For. Res. 1993, 8, 337–358. [Google Scholar] [CrossRef]
- Lamhamedi, M.S.; Lambany, G.; Margolis, H.A.; Renaud, M.; Veilleux, I.; Bernier, P.Y. Growth, physiology and leachate losses in Picea glauca seedlings (1 + 0) grown in air-slit container under different irrigation regimes. Can. J. For. Res. 2001, 31, 1968–1980. [Google Scholar] [CrossRef]
- Stowe, D.C.; Lamhamedi, M.S.; Margolis, H.A. Water relations, cuticular transpiration, and bud characteristics of air-slit containerized Picea glauca seedlings in response to controlled irrigation regimes. Can. J. For. Res. 2001, 31, 2200–2212. [Google Scholar] [CrossRef]
- Ramanjulu, S.; Bartels, D. Drought- and desiccation-induced modulation of gene expression in plants. Plant Cell Environ. 2002, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Knapik, D.; Sanz, M.A.; Peguero-Pina, J.J.; Niinemets, Ü.; Gil-Pelegrín, E. Changes of secondary metabolites in Pinus sylvestris L. needles under increasing soil water deficit. Ann. For. Sci. 2017, 74, 24. [Google Scholar] [CrossRef]
- Murray, B.G. Nuclear DNA amounts in Gymnosperms. Ann. Bot. 1998, 82, 3–15. [Google Scholar] [CrossRef]
- Bray, E.A. Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant Cell Environ. 2002, 25, 153–161. [Google Scholar] [CrossRef]
- Behringer, D.; Zimmermann, H.; Ziegenhagen, B.; Liepelt, S. Differential gene expression reveals candidate genes for drought stress response in Abies alba (Pinaceae). PLoS ONE 2015, 10, e0124564. [Google Scholar] [CrossRef]
- Bizet, F.; Bogeat-Triboulot, M.-B.; Montpied, P.; Christophe, A.; Ningre, N.; Cohen, D.; Hummel, I. Phenotypic plasticity toward water regime: Response of leaf growth and underlying candidate genes in Populus. Physiol. Plant. 2015, 154, 39–53. [Google Scholar] [CrossRef]
- Taeger, S.; Fussi, B.; Konnert, M.; Menzel, A. Large-scale genetic structure and drought-induced effects on European Scots pine (Pinus sylvestris L.) seedlings. Eur. J. For. Res. 2013, 132, 481. [Google Scholar] [CrossRef]
- Pierzgalski, E.; Tyszka, J.; Boczoń, J.; Wiśniewski, J.; Jeznach, J.; Żakowicz, S. Wytyczne Nawadniania Szkółek Leśnych na Powierzchniach Otwartych; Guidelines for Irrigation in Forest Nurseries; CILP: Warsaw, Poland, 2002; pp. 5–63. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, J.D. Rapid determination of proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Ledet-Jensen, J.; Ørntoft, T. Normalization of real-time quantitative RT-PCR data: A model based variance estimation approach to identify genes suited for normalization-applied to bladder-and colon-cancer data-sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Carsjens, C.; Ngoc, Q.N.; Guzy, J.; Knutzen, F.; Meier, I.C.H.; Müller, M.; Finkeldey, R.; Leuschner, C.h.; Polle, A. Intra-specific variations in expression of stress-related genes in beech progenies are stronger than drought-induced responses. Tree Physiol. 2014, 34, 1348–1361. [Google Scholar] [CrossRef]
- Makela, M.; Michael, P.; Theriault, G.; Nkongolo, K.K. High genetic variation among closely related red oak Quercus rubra populations in an ecosystem under metal stress: Analysis of gene regulation. Genes Genom. 2016, 38, 967–976. [Google Scholar] [CrossRef]
- Muilu-Mäkelä, R.; Vuosku, J.; Läärä, E.; Saarinen, M.; Heiskanen, J.; Häggman, H.; Sarjala, T. Water availability influences morphology, mycorrhizal associations, PSII efficiency and polyamine metabolism at early growth phase of Scots pine seedlings. Plant Physiol. Bioch. 2015, 88, 70–81. [Google Scholar]
- Bréda, N.; Cochard, H.; Dreyer, E.; Granier, A. Field comparison of transpiration, stomatal conductance and vulnerability to cavitation of Quercus petraea and Quercus robur under water stress. Ann. Sci. For. 1993, 50, 571–582. [Google Scholar] [CrossRef]
- Bréda, N.; Granier, A.; Aussnac, G. La sécheresse de 2003 dans le contexte climatique des 54 dernières années. Analyse écophysiologique et influence sur les arbres forstiers. (The 2003 drought in the climate context of the last 54 years-ecophysiological analysis and impact on forest trees). Rev. For. Fr. 2003, LVI, 109–131. (In French) [Google Scholar]
- Ponton, S.; Dupouey, J.-L.; Bréda, N.; Dreyer, E. Comparison of water use efficiency of seedlings from two sympatric oak species: Genotype x environment interactions. Tree Physiol. 2002, 22, 413–422. [Google Scholar] [CrossRef]
- Betch, P.; Bonal, D.; Bréda, N.; Montpied, P.; Peiffer, M.; Tuzet, A.; Granier, A. Drought effect on water relations in beech: The contributions of exchangeable water reservoirs. Agric. Forest Meteorol. 2011, 151, 531–543. [Google Scholar] [CrossRef]
- Michelot, A.; Bréda, N.; Damesin, C.; Dufrêne, E. Differing growth responses to climatic variations and soil water deficits of Fagus sylvatica, Quercus petraea, and Pinus sylvestris in a temperate forest. For. Ecol. Manag. 2012, 265, 161–171. [Google Scholar] [CrossRef]
- Edwards, C.E.; Ewers, B.E.; Weinig, C. Genotypic variation in biomass allocation in response to field drought has a greater effect on yield than gas exchange or phenology. BMC Plant Biol. 2016, 16, 185. [Google Scholar] [CrossRef] [PubMed]
- Tylkowski, T. Przedsiewne Traktowanie Nasion Drzew, Krzewów, Pnączy i Krzewinek (Pre-Sowing Treatment of Seeds of Trees, Shrubs, Climbers and Subshrubs); Information Centre of the State Forests; CILP: Warsaw, Poland, 2016; pp. 35–36, 185–186, 300–301, 343–344. (In Polish) [Google Scholar]
- Ahrends, B.; Penne, C. Modeling the impact of canopy structure on the spatial variability of net forest precipitation and interception loss in Scots pine stands. Open Geogr. J. 2010, 3, 115–124. [Google Scholar] [CrossRef]
- Staelens, J.; De Schrijver, A.; Verheyen, K.; Verhoest, N.E.C. Rainfall partitioning into throughfall, stemflow, and interception within a single beech (Fagus sylvatica L.) canopy: Influence of foliation, rain event characteristics, and meteorology. Hydrol. Process 2008, 22, 33–45. [Google Scholar] [CrossRef]
- Breshears, D.D.; McDowell, N.G.; Goddard, K.L.; Dayem, K.E.; Martens, S.N.; Meyer, C.W.; Brown, K.M. Foliar absorption of intercepted rainfall improves woody plants water status most during drought. Ecology 2008, 89, 41–47. [Google Scholar] [CrossRef]
- Irvine, J.; Perks, M.P.; Magnani, F.; Grace, J. The response of Pinus sylvestris to drought: Stomatal control of transpiration and hydraulic conductance. Tree Physiol. 1998, 18, 393–402. [Google Scholar] [CrossRef]
- Poyatos, R.; Llorens, P.; Piñol, J.; Rubio, C. Responses of Scots pine (Pinus sylvestris L.) and pubescent oak (Quercus pubescens Willd.) to soil and atmospheric water deficit under Mediterraenian mountain climate. Ann. For. Sci. 2008, 65, 1. [Google Scholar] [CrossRef]
- Cregg, B.M.; Zhang, J.W. Physiology and morphology of Pinus sylvestris seedlings from diverse sources under cyclic drought stress. For. Ecol. Manag. 2001, 154, 131–139. [Google Scholar] [CrossRef]
- Bergeron, O.; Lamhamedi, M.S.; Margolis, H.A.; Bernier, P.Y.; Stowe, D.C. Irrigation control and physiological responses of nursery-grown black spruce seedlings (1+0) cultivated in air-slit containers. HortScience 2004, 39, 599–605. [Google Scholar] [CrossRef]
- Singh, T.N.; Aspinall, D.; Paleg, L.G. Proline accumulation and varietal adaptability to drought in barley: A potential metabolic measure of drought resistance. Nat. New Biol. 1972, 236, 188–190. [Google Scholar] [CrossRef]
- Ahmed, C.B.; Rouina, B.B.; Sensoy, S.; Boukhriss, M.; Abdullah, F.B. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- Peuke, A.D.; Schraml, C.; Hartung, W.; Rennenberg, H. Identification of drought-sensitive beech ecotypes by physiological parameters. New Phytol. 2002, 154, 373–387. [Google Scholar] [CrossRef]
- Aasamaa, K.; Niinemets, Ü.; Sȏber, A. Leaf hydraulic conductance in relation to anatomical and functional traits during Populus tremula leaf ontogeny. Tree Physiol. 2005, 25, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Cyr, D.R.; Buxton, G.F.; Webb, D.P.; Dumbroff, E.B. Accumulation of free amino acids in the shoots and roots of three northern conifers during drought. Tree Physiol. 1990, 6, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Schiop, S.T.; Al Hassan, M.; Sestras, A.F.; Boscaiu, M.; Sestras, R.E.; Vicente, O. Biochemical responses to drought, at the seedling stage, of several Romanian Carpathian populations of Norway spruce (Picea abies L. Karst). Trees 2017, 31, 1479–1490. [Google Scholar] [CrossRef]
- Qureshi, M.K.; Sujeeth, N.; Gechev, T.S.; Hille, J. The zinc finger protein ZAT11 modulates paraquat-induced programmed cell death in Arabidopsis thaliana. Acta Physiol. Plant. 2013, 35, 1863–1871. [Google Scholar] [CrossRef]
- Liu, J.; Han, H.J.; Kim, S.H.; Lim, C.O.; Yun, D.J.; Chung, W.K. ZAT11, a zinc finger transcription factor, is a negative regulator of nickel ion tolerance in Arabidopsis. Plant Cell. Rep. 2014, 33, 2015–2021. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yu, J.-N.; Chen, T.; Zhang, Z.-G.; Hao, Y.-J.; Zhang, J.-S.; Chen, S.-Y. Functional analysis of a putative Ca2+ channel gene TaTPC1 from wheat. J. Exp. Bot. 2005, 56, 3051–3060. [Google Scholar] [CrossRef]
- Jia, J.; Li, S.; Cao, X.; Li, H.; Shi, W.; Polle, A.; Liu, T.X.; Peng, C.; Luo, Z.B. Physiological and transcriptional regulation in poplar roots and leaves during acclimation to high temperature and drought. Physiol. Plant. 2016, 157, 38–53. [Google Scholar] [CrossRef]
- Bussotti, F.; Bettini, D.; Grossoni, P.; Mansuino, S.; Nibbi, R.; Soda, C.; Tani, C. Structural and functional traits of Quercus ilex in response to water availability. Environ. Exp. Bot. 2002, 47, 11–23. [Google Scholar] [CrossRef]
- Stojnić, S.; Orlović, S.; Miljković, D.; Galić, Z.; Kebert, M.; von Wuehlisch, G. Provenance plasticity of European beech leaf traits under differing environmental conditions at two Serbian common garden sites. Eur. J. For. Res. 2015, 134, 1109. [Google Scholar] [CrossRef]
- Bresta, P.; Nikolopoulos, D.; Economou, G.; Vahamidis, P.; Lyra, D.; Karamanos, A.; Karabourniotis, G. Modification of water entry (xylem vessels) and water exit (stomata) orchestrates long term drought acclimation of wheat leaves. Plant Soil 2011, 347, 179–193. [Google Scholar] [CrossRef]
- Laajimi, N.O.; Boussadia, O.; Shkiri, F.H.; Teixeira da Silva, J.A.; Rezgui, S.; Hellali, R. Anatomical adaptations in vegetative structures of apricot tree (Prunus armeniaca L.) cv. ‘Amor El Euch’ grown under water stress. Fruit Veget. Cereal Sci. Biotech. 2011, 5, 46–51. [Google Scholar]
- Nardini, A.; Pedà, G.; La Rocca, N. Trade-offs between leaf hydraulic capacity and drought vulnerability: Morpho-anatomical bases, carbon costs and ecological consequences. New Phytol. 2012, 196, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U.G.; Spicer, R.; Schreiber, S.G.; Plavcová, L. An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ. 2017, 40, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Ivancich, H.S.; Lencinas, M.V.; Pastur, G.J.M.; Esteban, R.M.S.; Hernández, L.; Lindstrom, I. Foliar anatomical and morphological variation in Nothofagus pumulio seedlings under controlled irradiance and soil moisture levels. Tree Physiol. 2012, 32, 554–564. [Google Scholar] [CrossRef]
- Schramm, R. Über die anatomischen Jugendformen der Blätter einheimischer Holzpflanzen. Flora 1912, 104, 225–295. [Google Scholar] [CrossRef]




| Species | Irrigation Treatment (% Recommended Dose) | h (mm) | d (mm) | h/d |
|---|---|---|---|---|
| Fagus sylvatica | 25 | 104 ± 5 a | 1.88 ± 0.08 a | 57 ± 2 a |
| 50 | 124 ± 5 b | 2.13 ± 0.09 b | 59 ± 2 a | |
| 75 | 185 ± 7 c | 2.77 ± 0.08 c | 68 ± 3 b | |
| 100 | 225 ± 11 d | 3.37 ± 0.11 d | 67 ± 3 b | |
| F3, 156 | 62.5 *** | 51.4 *** | 6.0 ** | |
| Quercus petraea | 25 | 82 ± 3 a | 1.90 ± 0.07 a | 44 ± 2 a |
| 50 | 94 ± 3 b | 1.97 ± 0.06 a | 48 ± 2 ab | |
| 75 | 117 ± 7 c | 2.48 ± 0.08 b | 48 ± 3 ab | |
| 100 | 148 ± 8 d | 2.78 ± 0.08 c | 54 ± 3 b | |
| F3, 156 | 28.6 *** | 35.8 *** | 3.2 * | |
| Abies alba | 25 | 35 ± 1 a | 1.00 ± 0.03 a | 36 ± 1 a |
| 50 | 37 ± 1 a | 1.05 ± 0.03 a | 36 ± 1 a | |
| 75 | 38 ± 1 a | 1.03 ± 0.03 a | 38 ± 1 a | |
| 100 | 38 ± 1 a | 1.00 ± 0.02 a | 38 ± 1 a | |
| F3, 156 | n.s. | n.s. | n.s. | |
| Pinus sylvestris | 25 | 35 ± 1 a | 1.01 ± 0.03 a | 35 ± 1 a |
| 50 | 39 ± 1 b | 1.06 ± 0.03 a | 37 ± 1 a | |
| 75 | 48 ± 1 c | 1.17 ± 0.04 b | 43 ± 1 b | |
| 100 | 51 ± 2 c | 1.18 ± 0.04 b | 45 ± 2 b | |
| F3, 156 | 31.9 *** | 5.4 *** | 11.9 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robakowski, P.; Wyka, T.P.; Kowalkowski, W.; Barzdajn, W.; Pers-Kamczyc, E.; Jankowski, A.; Politycka, B. Practical Implications of Different Phenotypic and Molecular Responses of Evergreen Conifer and Broadleaf Deciduous Forest Tree Species to Regulated Water Deficit in a Container Nursery. Forests 2020, 11, 1011. https://doi.org/10.3390/f11091011
Robakowski P, Wyka TP, Kowalkowski W, Barzdajn W, Pers-Kamczyc E, Jankowski A, Politycka B. Practical Implications of Different Phenotypic and Molecular Responses of Evergreen Conifer and Broadleaf Deciduous Forest Tree Species to Regulated Water Deficit in a Container Nursery. Forests. 2020; 11(9):1011. https://doi.org/10.3390/f11091011
Chicago/Turabian StyleRobakowski, Piotr, Tomasz P. Wyka, Wojciech Kowalkowski, Władysław Barzdajn, Emilia Pers-Kamczyc, Artur Jankowski, and Barbara Politycka. 2020. "Practical Implications of Different Phenotypic and Molecular Responses of Evergreen Conifer and Broadleaf Deciduous Forest Tree Species to Regulated Water Deficit in a Container Nursery" Forests 11, no. 9: 1011. https://doi.org/10.3390/f11091011
APA StyleRobakowski, P., Wyka, T. P., Kowalkowski, W., Barzdajn, W., Pers-Kamczyc, E., Jankowski, A., & Politycka, B. (2020). Practical Implications of Different Phenotypic and Molecular Responses of Evergreen Conifer and Broadleaf Deciduous Forest Tree Species to Regulated Water Deficit in a Container Nursery. Forests, 11(9), 1011. https://doi.org/10.3390/f11091011





