Both Mature Patches and Expanding Areas of Juniperus thurifera Forests Are Vulnerable to Climate Change But for Different Reasons
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Area
2.2. Sample Collection
2.3. Sample Processing
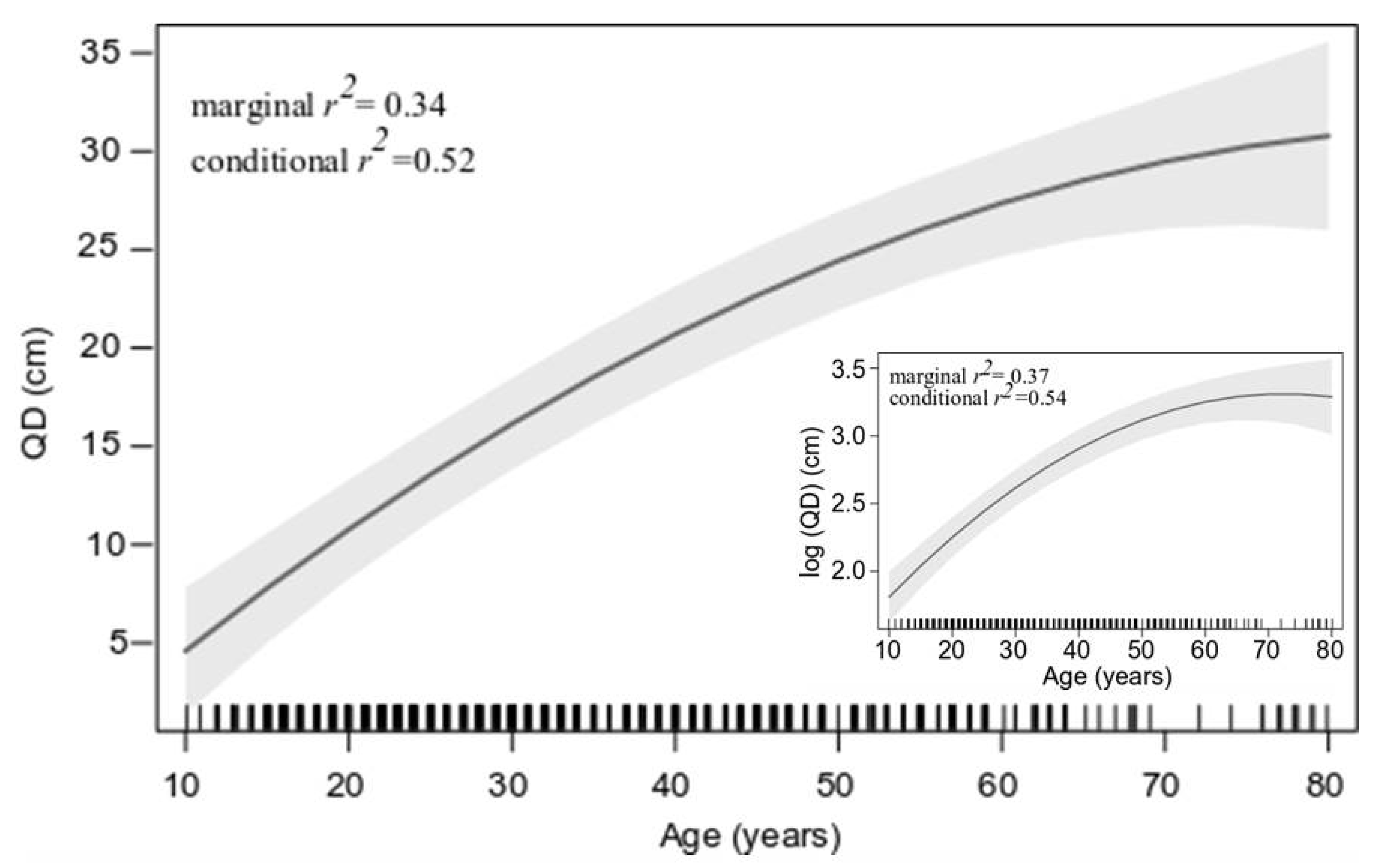
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- David, T.S.; Henriques, M.O.; Kurz-Besson, C.; Nunes, J.; Valente, F.; Vaz, M.; Pereira, J.S.; Siegwolf, R.; Chaves, M.M.; Gazarini, L.C.; et al. Water-use strategies in two co-occurring Mediterranean evergreen oaks: Surviving the summer drought. Tree Physiol. 2007, 27, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, S.; Valladares, F. Ecological and evolutionary responses of Mediterranean plants to global change. Environ. Exp. Bot. 2014, 103, 53–67. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 2008; ISBN 978-0-387-78340-6. [Google Scholar]
- Ehleringer, J.R. Variation in leaf carbon isotope discrimination in Encelia farinosa: Implications for growth, competition, and drought survival. Oecologia 1993, 95, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, X.; Walcroft, A.S.; Daudet, F.A.; Sinoquet, H.; Chaves, M.M.; Rodrigues, A.; Osorio, L. Photosynthetic light acclimation in peach leaves: Importance of changes in mass:area ratio, nitrogen concentration, and leaf nitrogen partitioning. Tree Physiol. 2001, 21, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.J.; Wullschleger, S.D.; Gunderson, C.A.; Johnson, D.W.; Ceulemans, R. Tree responses to rising CO2 in field experiments: Implications for the future forest. Plant Cell Environ. 1999, 22, 683–714. [Google Scholar] [CrossRef]
- Salazar-Tortosa, D.; Castro, J.; Villar-Salvador, P.; Viñegla, B.; Matías, L.; Michelsen, A.; de Casas, R.R.; Querejeta, J.I. The “isohydric trap”: A proposed feedback between water shortage, stomatal regulation, and nutrient acquisition drives differential growth and survival of European pines under climatic dryness. Glob. Change Biol. 2018, 24, 4069–4083. [Google Scholar] [CrossRef]
- Aranda, I.; Pardos, M.; Puertolas, J.; Jimenez, M.D.; Pardos, J.A. Water-use efficiency in cork oak (Quercus suber) is modified by the interaction of water and light availabilities. Tree Physiol. 2007, 27, 671–677. [Google Scholar] [CrossRef]
- Ceacero, C.J.; Díaz-Hernández, J.L.; de Campo, A.D.; Navarro-Cerrillo, R.M. Soil rock fragment is stronger driver of spatio-temporal soil water dynamics and efficiency of water use than cultural management in holm oak plantations. Soil Tillage Res. 2020, 197, 104495. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Valladares, F. Photosynthetic Acclimation to Simultaneous and Interacting Environmental Stresses Along Natural Light Gradients: Optimality and Constraints. Plant Biol. 2004, 6, 254–268. [Google Scholar] [CrossRef]
- Souza, M.L.; Duarte, A.A.; Lovato, M.B.; Fagundes, M.; Valladares, F.; Lemos-Filho, J.P. Climatic factors shaping intraspecific leaf trait variation of a neotropical tree along a rainfall gradient. PLoS ONE 2018, 13, e0208512. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Florit, A.; Vega, A.; Serrano, L.; Voltas, J. Δ13C and tree-ring width reflect different drought responses in Quercus ilex and Pinus halepensis. Oecologia 2003, 137, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Zalloni, E.; Battipaglia, G.; Cherubini, P.; Saurer, M.; De Micco, V. Wood Growth in Pure and Mixed Quercus ilex L. Forests: Drought Influence Depends on Site Conditions. Front. Plant Sci. 2019, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- de Andrés, E.G.; Camarero, J.J.; Blanco, J.A.; Imbert, J.B.; Lo, Y.-H.; Sangüesa-Barreda, G.; Castillo, F.J. Tree-to-tree competition in mixed European beech-Scots pine forests has different impacts on growth and water-use efficiency depending on site conditions. J. Ecol. 2018, 106, 59–75. [Google Scholar] [CrossRef]
- Gouveia, A.C.; Freitas, H. Intraspecific competition and water use efficiency in Quercus suber: Evidence of an optimum tree density? Trees 2008, 22, 521–530. [Google Scholar] [CrossRef]
- Ploughe, L.W.; Jacobs, E.M.; Frank, G.S.; Greenler, S.M.; Smith, M.D.; Dukes, J.S. Community Response to Extreme Drought (CRED): A framework for drought-induced shifts in plant–plant interactions. New Phytol. 2019, 222, 52–69. [Google Scholar] [CrossRef]
- Verwijmeren, M.; Rietkerk, M.; Wassen, M.J.; Smit, C. Interspecific facilitation and critical transitions in arid ecosystems. Oikos 2013, 122, 341–347. [Google Scholar] [CrossRef]
- Romero-Díaz, A.; Ruiz-Sinoga, J.D.; Robledano-Aymerich, F.; Brevik, E.C.; Cerdà, A. Ecosystem responses to land abandonment in Western Mediterranean Mountains. Catena 2017, 149, 824–835. [Google Scholar] [CrossRef]
- Keenan, R.J. Climate change impacts and adaptation in forest management: A review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef]
- Wilson, S.J.; Schelhas, J.; Grau, R.; Nanni, A.S.; Sloan, S. Forest ecosystem-service transitions: The ecological dimensions of the forest transition. Ecol. Soc. 2017, 22, 38. [Google Scholar] [CrossRef]
- Hoekstra, J.M.; Boucher, T.M.; Ricketts, T.H.; Roberts, C. Confronting a biome crisis: Global disparities of habitat loss and protection. Ecol. Lett. 2004, 8, 23–29. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; López-Moreno, J.I.; Vicente-Serrano, S.M.; Lasanta-Martínez, T.; Beguería, S. Mediterranean water resources in a global change scenario. Earth Sci. Rev. 2011, 105, 121–139. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Planelles, R.; Oliet, J.; Peñuelas-Rubira, J.L.; Jacobs, D.; González, M. Drought Tolerance and Transplanting Performance of Holm Oak (Quercus Ilex) Seedlings After Drought Hardening in the Nursery. Tree Physiol. 2004, 24, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Casado, M.; Costa, M.; Escribano, R.; García, M.; Génova, M.; Gómez, A.; Gómez, F.; Moreno, J.; Morla, C.; et al. Los Bosques Ibéricos: Una Interpretación Geobotánica; Editorial Planeta: Barcelona, Spain, 2005. [Google Scholar]
- Ponce, R.A.; Palomares, O.S.; Gómez, S.R. Las Estaciones Ecológicas Actuales y Potenciales de los Sabinares Albares Españoles; INIA—Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria: Madrid, Spain, 2010; ISBN 9788474985283. [Google Scholar]
- Gimeno, T.E.; Pías, B.; Martínez-Fernández, J.; Quiroga, D.L.; Escudero, A.; Valladares, F. The decreased competition in expanding versus mature juniper woodlands is counteracted by adverse climatic effects on growth. Eur. J. For. Res. 2012, 131, 977–987. [Google Scholar] [CrossRef]
- Gimeno, T.E.; Escudero, A.; Delgado, A.; Valladares, F. Previous Land Use Alters the Effect of Climate Change and Facilitation on Expanding Woodlands of Spanish Juniper. Ecosystems 2012, 15, 564–579. [Google Scholar] [CrossRef]
- Dickie, I.A.; Schnitzer, S.A.; Reich, P.B.; Hobbie, S.E. Is oak establishment in old-fields and savanna openings context dependent? J. Ecol. 2007, 95, 309–320. [Google Scholar] [CrossRef]
- Matías, L.; Zamora, R.; Castro, J. Repercussions of Simulated Climate Change on the Diversity of Woody-Recruit Bank in a Mediterranean-type Ecosystem. Ecosystems 2011, 14, 672–682. [Google Scholar] [CrossRef]
- Ponce, R.A.; Senespleda, E.L.; Palomares, O.S. A novel application of the ecological field theory to the definition of physiographic and climatic potential areas of forest species. Eur. J. For. Res. 2010, 129, 119–131. [Google Scholar] [CrossRef]
- Gauquelin, T.; Bertaudiere, V.; Montes, N.; Badri, W.; Asmode, J. Endangered stands of thuriferous juniper in the western Mediterranean basin: Ecological status, conservation and management. Biodivers. Conserv. 1999, 8, 1479–1498. [Google Scholar] [CrossRef]
- Montesinos, D.; Otto, R.; María, J.; Palacios, F. Bosques endémicos de Juniperus spp. In Bases Ecológicas Preliminares para la Conservación de los Tipos de Hábitat de Interés 549 Comunitario en España; Dirección General de Medio Natural, Ministerio de Medio Ambiente, y 550 Medio Rural y Marino: Madrid, Spain, 2009. [Google Scholar]
- Villellas, J.; Martín-Forés, I.; Mariette, S.; Massot, M.; Guichoux, E.; Acuña-Míguez, B.; Hampe, A.; Valladares, F. Functional distance is driven more strongly by environmental factors than by genetic relatedness in Juniperus thurifera L. expanding forest stands. Ann. For. Sci. 2020, 77, 1–18. [Google Scholar] [CrossRef]
- Stewart, J.L.; Salazar, R. A review of measurement options for multipurpose trees. Agrofor. Syst. 1992, 19, 173–183. [Google Scholar] [CrossRef]
- Barton, K. Package ‘MuMIn’ Title Multi-Model Inference; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2019; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using {lme4}. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. {lmerTest} Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix, Version 0.84; 2017. Available online: https://github.com/taiyun/corrplot (accessed on 15 June 2020).
- Mi, M.; Shao, M.; Liu, B. Effect of rock fragments content on water consumption, biomass and water-use efficiency of plants under different water conditions. Ecol. Eng. 2016, 94, 574–582. [Google Scholar] [CrossRef]
- Linares, J.C.; Delgado-Huertas, A.; Camarero, J.J.; Merino, J.; Carreira, J.A. Competition and drought limit the response of water-use efficiency to rising atmospheric carbon dioxide in the Mediterranean fir Abies pinsapo. Oecologia 2009, 161, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, T.E.; Escudero, A.; Valladares, F. Different intra- and interspecific facilitation mechanisms between two Mediterranean trees under a climate change scenario. Oecologia 2015, 177, 159–169. [Google Scholar] [CrossRef]
- Jeddi, K.; Cortina, J.; Chaieb, M. Acacia salicina, Pinus halepensis and Eucalyptus occidentalis improve soil surface conditions in arid southern Tunisia. J. Arid Environ. 2009, 73, 1005–1013. [Google Scholar] [CrossRef]
- Valladares, F.; Zaragoza-Castells, J.; Sánchez-Gómez, D.; Matesanz, S.; Alonso, B.; Portsmuth, A.; Delgado, A.; Atkin, O.K. Is shade beneficial for mediterranean shrubs experiencing periods of extreme drought and late-winter frosts? Ann. Bot. 2008, 102, 923–933. [Google Scholar] [CrossRef]
- Montesinos, D.; Fabado, J. Changes in land use and physiological transitions of a Juniperus thurifera forest: From decline to recovery. Can. J. For. Res. 2015, 45, 764–769. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sánchez, G.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef]
- Patterson, T.B.; Guy, R.D.; Dang, Q.L. Whole-plant nitrogen- and water-relations traits, and their associated trade-offs, in adjacent muskeg and upland boreal spruce species. Oecologia 1997, 110, 160–168. [Google Scholar] [CrossRef]
- Li, F.; Kang, S.; Zhang, J.; Cohen, S. Effects of atmospheric CO2 enrichment, water status and applied nitrogen on water- and nitrogen-use efficiencies of wheat. Plant Soil 2003, 254, 279–289. [Google Scholar] [CrossRef]
- Cramer, M.D.; Hawkins, H.-J.; Verboom, G.A. The importance of nutritional regulation of plant water flux. Oecologia 2009, 161, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, J.C.; van Bodegom, P.M.; Witte, J.-P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Otal-Laín, I.; Lasanta, T.; Sánchez-Navarrete, P.; Errea, P.; Cammeraat, E. Woody encroachment and soil carbon stocks in subalpine areas in the Central Spanish Pyrenees. Sci. Total Environ. 2018, 636, 727–736. [Google Scholar] [CrossRef]
- Aranda, I.; Forner, A.; Cuesta, B.; Valladares, F. Species-specific water use by forest tree species: From the tree to the stand. Agricult. Water Manag. 2012, 114, 67–77. [Google Scholar] [CrossRef]
- Montesinos, D.; Villar-Salvador, P.; García-Fayos, P.; Verdú, M. Genders in Juniperus thurifera have different functional responses to variations in nutrient availability. New Phytol. 2012, 193, 705–712. [Google Scholar] [CrossRef]
- Gimeno, T.E.; Camarero, J.J.; Granda, E.; Pias, B.; Valladares, F. Enhanced growth of Juniperus thurifera under a warmer climate is explained by a positive carbon gain under cold and drought. Tree Physiol. 2012, 32, 326–336. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carrer, M. Age-dependent xylogenesis in timberline conifers. New Phytol. 2007, 177, 199–208. [Google Scholar] [CrossRef]
- Castillo, A.C.; Goldfarb, B.; Johnsen, K.H.; Roberds, J.H.; Nelson, C.D. Genetic variation in water-use efficiency (WUE) and growth in mature longleaf pine. Forests 2018, 9, 727. [Google Scholar] [CrossRef]
- Granda, E.; Rossatto, D.R.; Camarero, J.J.; Voltas, J.; Valladares, F. Growth and carbon isotopes of Mediterranean trees reveal contrasting responses to increased carbon dioxide and drought. Oecologia 2014, 174, 307–317. [Google Scholar] [CrossRef]


| Gradient Stage | Site | |||||
|---|---|---|---|---|---|---|
| Mature Forest | Transition Zone | Expanding Front | Maranchón | Huertahernando | Ribarredonda | |
| QD (cm) | 22.80 ± 1.04 a | 15.80 ± 0.69 b | 13.15 ± 0.56 c | 16.84 ± 0.82 a | 17.86 ± 0.76 a | 16.35 ± 0.85 a |
| Height (m) | 5.76 ± 0.13 a | 4.42 ± 0.10 b | 4.09 ± 0.09 c | 4.50 ± 0.12 b | 4.71 ± 0.12 a | 5.04 ± 0.13 a |
| Average crown diameter (m) | 5.35 ± 0.16 a | 4.49 ± 0.12 b | 4.02 ± 0.11 c | 4.39 ± 0.12 b | 5.14 ± 0.14 a | 4.29 ± 0.15 b |
| Tree vigour (0–4) | 2.43 ± 0.06 c | 2.73 ± 0.06 b | 2.93 ± 0.07 a | 2.75 ± 0.06 a | 2.75 ± 0.07 a | 2.60 ± 0.07 a |
| Age (years) | 44.98 ± 1.40 a | 30.77 ± 1.01 b | 28.81 ± 1.13 b | 38.30 ± 1.26 a | 28.00 ± 0.97 b | 35.27 ± 1.40 a |
| C/N ratio | 38.86 ± 0.46 a | 36.85 ± 0.34 b | 38.91 ± 0.32 a | 37.75 ± 0.31 b | 37.04 ± 0.37 b | 40.02 ± 0.47 a |
| Bare soil (%) | 10.00 ± 0.84 a | 8.23 ± 0.52 a | 9.82 ± 0.73 a | 7.65 ± 0.61 c | 9.44 ± 0.69 b | 11.79 ± 0.76 a |
| Stoniness (%) | 32.05 ± 1.03 b | 42.09 ± 1.29 a | 43.51 ± 1.24 a | 40.10 ± 1.21 b | 47.93 ± 1.07 a | 29.30 ± 0.91 c |
| Juniperus thurifera L. (%) | 18.39 ± 1.17 a | 9.32 ± 0.84 b | 6.37 ± 0.88 c | 10.81 ± 0.88 a | 10.56 ± 1.09 a | 12.01 ± 1.21 a |
| Woody species (%) | 36.55 ± 1.44 a | 40.36 ± 1.28 a | 40.30 ± 1.25 a | 41.44 ± 1.18 b | 32.08 ± 0.98 c | 46.91 ± 1.45 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acuña-Míguez, B.; Valladares, F.; Martín-Forés, I. Both Mature Patches and Expanding Areas of Juniperus thurifera Forests Are Vulnerable to Climate Change But for Different Reasons. Forests 2020, 11, 960. https://doi.org/10.3390/f11090960
Acuña-Míguez B, Valladares F, Martín-Forés I. Both Mature Patches and Expanding Areas of Juniperus thurifera Forests Are Vulnerable to Climate Change But for Different Reasons. Forests. 2020; 11(9):960. https://doi.org/10.3390/f11090960
Chicago/Turabian StyleAcuña-Míguez, Belén, Fernando Valladares, and Irene Martín-Forés. 2020. "Both Mature Patches and Expanding Areas of Juniperus thurifera Forests Are Vulnerable to Climate Change But for Different Reasons" Forests 11, no. 9: 960. https://doi.org/10.3390/f11090960
APA StyleAcuña-Míguez, B., Valladares, F., & Martín-Forés, I. (2020). Both Mature Patches and Expanding Areas of Juniperus thurifera Forests Are Vulnerable to Climate Change But for Different Reasons. Forests, 11(9), 960. https://doi.org/10.3390/f11090960






