The Structure of Saproxylic Beetle Assemblages in View of Coarse Woody Debris Resources in Pine Stands of Western Poland
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baycheva-Merger, T.; Wolfslehner, B. Evaluating the implementation of the Pan-European Criteria and indicators for sustainable forest management—A SWOT analysis. Ecol. Indic. 2016, 60, 1192–1199. [Google Scholar] [CrossRef]
- Rykowski, K. Strategia ochrony różnorodności biologicznej w lasach-zarys koncepcji i propozycje działań. In Ocena Wpływu Praktyki Leśnej Na Różnorodność Biologiczną W Lasach W Europie Środkowej; Rykowski, K., Matuszewski, G., Lenart, E., Eds.; Instytut Badawczy Leśnictwa: Warszawa, Poland, 1999; pp. 365–389. [Google Scholar]
- Rykowski, K. Gospodarka leśna a różnorodność biologiczna. In Różnorodność Biologiczna Polski; Andrzejewski, R., Weigle, A., Eds.; Narodowa Fundacja Ochrona Środowiska: Warszawa, Poland, 2003; pp. 197–202. [Google Scholar]
- Weigle, A. Ochrona różnorodności biologicznej. In Agenda 21 W Polsce-Raport Okresowy 1992–2000; Podgajnik, T., Ed.; Narodowa Fundacja Ochrony Środowiska: Warszawa, Poland, 2000; pp. 103–110. [Google Scholar]
- Gutowski, J.M. Saproksyliczne chrząszcze. Kosmos 2006, 55, 53–73. [Google Scholar]
- Gimmel, M.L.; Ferro, M.L. Chapter 2. General Overview of Saproxylic Coleoptera. In Saproxylic Insects Diversity, Ecology and Conservation; Ulyshen, M.D., Ed.; Zoological Monographs 1; Springer: Cham, Switzerland, 2018; pp. 51–128. [Google Scholar] [CrossRef]
- Buchholz, L.; Ossowska, M. Entomofauna martwego drewna—Jej biocenotyczne znaczenie w środowisku leśnym oraz możliwości i problemy ochrony. Przegląd Przyr. 1995, 3–4, 93–105. [Google Scholar]
- Grove, S.J. Saproxylic Insect Ecology and the Sustainable Management of Forests. Annu. Rev. Ecol. Syst. 2002, 33, 1–23. [Google Scholar] [CrossRef]
- Gutowski, J.M.; Bobiec, A.; Pawlaczyk, P.; Zub, K. Drugie Życie Drzewa; WWF Polska: Warszawa, Poland; Hajnówka, Poland, 2004. [Google Scholar]
- Gutowski, J.M.; Buchholz, L.; Kubisz, D.; Ossowska, M.; Sućko, K. Chrząszcze saproksyliczne jako wskaźnik odkształceń ekosystemów leśnych borów sosnowych. Leśne Pr. Badaw. 2006, 4, 101–144. [Google Scholar]
- Byk, A.; Mokrzycki, T. Chrząszcze saproksyliczne jako wskaźnik antropogenicznych odkształceń Puszczy Białowieskiej. Studia I Mater. Cent. Edukac. Przyr.-Leśnej 2007, 9, 475–509. [Google Scholar]
- Lachat, T.; Wermelinger, B.; Gossner, M.M.; Bussler, H.; Isacsson, G.; Müller, J. Saproxylic beetles as indicator species for dead-wood amount and temperature in European beech forests. Ecol. Indic. 2012, 23, 323–331. [Google Scholar] [CrossRef]
- Seibold, S.; Bässler, C.; Brandl, R.; Gossner, M.M.; Thorn, S.; Ulyshen, M.D.; Müller, J. Experimental studies of dead-wood biodiversity—A review identifying global gaps in knowledge. Biol. Conserv. 2015, 191, 139–149. [Google Scholar] [CrossRef]
- Eckelt, A.; Müller, J.; Bense, U.; Brustel, H.; Bußler, H.; Chittaro, Y.; Cizek, L.; Frei, A.; Holzer, E.; Kadej, M.; et al. “Primeval forest relict beetles” of Central Europe: A set of 168 umbrella species for the protection of primeval forest remnants. J. Insect Conserv. 2017, 22, 15–28. [Google Scholar] [CrossRef]
- Parisi, F.; Di Febbraro, M.; Lombardi, F.; Biscaccianti, A.; Campanaro, A.; Tognetti, R.; Marchetti, M. Relationships between stand structural attributes and saproxylic beetle abundance in a Mediterranean broadleaved mixed forest. For. Ecol. Manag. 2019, 432, 957–966. [Google Scholar] [CrossRef]
- Samuelsson, J.; Gustafsson, L.; Ingelög, T. Dying and Dead Trees: A Review of Their Importance for Biodiversity; Swedish Threatened Species Unit: Uppsala, Sweden, 1994. [Google Scholar]
- Djupström, L.B.; Weslien, J.; Schroeder, L.M. Dead wood and saproxylic beetles in set-aside and non set-aside forests in a boreal region. For. Ecol. Manag. 2008, 255, 3340–3350. [Google Scholar] [CrossRef]
- Müller, J.; Bütler, R. A review of habitat thresholds for dead wood: A baseline for management recommendations in European forests. Eur. J. For. Res. 2010, 129, 981–992. [Google Scholar] [CrossRef]
- Jonsson, B.; Kruys, N.; Ranius, T. Ecology of species living on dead wood—Lessons for dead wood management. Silva Fenn. 2005, 39, 289–309. [Google Scholar] [CrossRef] [Green Version]
- Schiegg, K. Saproxylic insect diversity of beech: Limbs are richer than trunks. For. Ecol. Manag. 2001, 149, 295–304. [Google Scholar] [CrossRef]
- Schroeder, L.M.; Sahlin, E.; Paltto, H. Retention of aspen (Populus tremulae) at final cuttings—The effect of dead wood characteristics on saproxylic beetles. For. Ecol. Manag. 2011, 262, 853–862. [Google Scholar] [CrossRef]
- Müller, J.; Wende, B.; Strobl, C.; Eugster, M.; Gallenberger, I.; Floren, A.; Steffan-Dewenter, I.; Linsenmair, K.E.; Weisser, W.W.; Gossner, M.M. Forest management and regional tree composition drive the host preference of saproxylic beetle communities. J. Appl. Ecol. 2015, 52, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Parisi, F.; Lombardi, F.; Sciarretta, A.; Tognetti, R.; Campanaro, A.; Marchetti, M.; Trematerra, P. Spatial patterns of saproxylic beetles in a relic silver fir forest (Central Italy), relationships with forest structure and biodiversity indicators. For. Ecol. Manag. 2016, 381, 217–234. [Google Scholar] [CrossRef]
- Bouget, C.; Larrieu, L.; Brin, A. Key features for saproxylic beetle diversity derived from rapid habitat assessment in temperate forests. Ecol. Indic. 2014, 36, 656–664. [Google Scholar] [CrossRef]
- Seibold, S.; Bässler, C.; Brandl, R.; Büche, B.; Szallies, A.; Thorn, S.; Ulyshen, M.D.; Müller, J. Microclimate and habitat heterogeneity as the major drivers of beetle diversity in dead wood. J. Appl. Ecol. 2016, 53, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Janssen, P.; Cateau, E.; Fuhr, M.; Nusillard, B.; Brustel, H.; Bouget, C. Are biodiversity patterns of saproxylic beetles shaped by habitat limitation or dispersal limitation? A case study in unfragmented montane forests. Biodivers. Conserv. 2016, 25, 1167–1185. [Google Scholar] [CrossRef]
- Winter, M.-B.; Ammer, C.; Baier, R.; Donato, D.C.; Seibold, S.; Müller, J. Multi-taxon alpha diversity following bark beetle disturbance: Evaluating multi-decade persistence of a diverse early-seral phase. For. Ecol. Manag. 2015, 338, 32–45. [Google Scholar] [CrossRef]
- Wermelinger, B.; Moretti, M.; Duelli, P.; Lachat, T.; Pezzatti, G.B.; Obrist, M.K. Impact of windthrow and salvage-logging on taxonomic and functional diversity of forest arthropods. For. Ecol. Manag. 2017, 391, 9–18. [Google Scholar] [CrossRef]
- Boucher, J.; Azeria, E.T.; Ibarzabal, J.; Hébert, C. Saproxylic beetles in disturbed boreal forests: Temporal dynamics, habitat associations, and community structure. Écoscience 2012, 19, 328–343. [Google Scholar] [CrossRef]
- Hammond, H.J.; Langor, D.W.; Spence, J.R. Changes in saproxylic beetle (Insecta: Coleoptera) assemblages following wildfire and harvest in boreal Populus forests. For. Ecol. Manag. 2017, 401, 319–329. [Google Scholar] [CrossRef]
- Ranius, T.; Jansson, N. The influence of forest regrowth, original canopy cover and tree size on saproxylic beetles associated with old oaks. Biol. Conserv. 2000, 95, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Starzyk, J.R.; Grodzki, W.; Kosibowicz, M.; Michalcewicz, J.; Rossa, R. Old and dead trees as the site of occurrence and de-velopment of xylobiotic and dendrophilous beetles. Rocz. Bieszcz. 2008, 16, 325–348. [Google Scholar]
- Müller, J.; Noss, R.F.; Bussler, H.; Brandl, R. Learning from a “benign neglect strategy” in a national park: Response of saproxylic beetles to dead wood accumulation. Biol. Conserv. 2010, 143, 2559–2569. [Google Scholar] [CrossRef]
- Rubene, D.; Schroeder, M.; Ranius, T. Effectiveness of local conservation management is affected by landscape properties: Species richness and composition of saproxylic beetles in boreal forest clearcuts. For. Ecol. Manag. 2017, 399, 54–63. [Google Scholar] [CrossRef]
- Statistical Year Book of Forestry. Statistics Poland, Warsaw (2020). Forest Data Bank. Available online: https://www.bdl.lasy.gov.pl/portal/gus-lesnictwo (accessed on 29 June 2021).
- Czerepko, J.; Hilszczański, J.; Jabłoński, T. Martwe drewno—żywy problem. Studia I Mater. CEPL W Rogowie 2014, 41, 36–45. [Google Scholar]
- Czerepko, J.; Boczoń, A.; Cieśla, A.; Forycka, A.; Ksepko, M.; Obidziński, A.; Paluch, R.; Rodziewicz, A.; Różański, W.; Sokołowski, K.; et al. Stan Różnorodności Biologicznej Lasów W Polsce Na Podstawie Powierzchni Obserwacyjnych Monitoringu; Czerepko, J., Ed.; Instytut Badawczy Leśnictwa: Sękocin Stary, Poland, 2015. [Google Scholar]
- Johansson, T.; Gibb, H.; Hilszczański, J.; Pettersson, R.B.; Hjältén, J.; Atlegrim, O.; Ball, J.P.; Danell, K. Conservation-oriented manipulations of coarse woody debris affect its value as habitat for spruce-infesting bark and ambrosia beetles (Coleoptera: Scolytinae) in northern Sweden. Can. J. For. Res. 2006, 36, 174–185. [Google Scholar] [CrossRef]
- Foit, J. The occurrence of early-arriving saproxylic beetles on Scots pine logging residues generated by thinning. J. For. Sci. 2016, 61, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Väiäanen, R.; Biström, O.; Heliövaara, K. Sub-cortical Coleoptera in dead pines and spruces: Is primeval species composi-tion maintained in managed forests? Biodivers. Conserv. 1993, 2, 95–113. [Google Scholar] [CrossRef]
- Siitonen, J. Decaying wood and saproxylic Coleoptera in two old spruce forests: A comparison based on two sampling methods. Ann. Zool. Fennici. 1994, 31, 89–95. [Google Scholar]
- Økland, B.; Bakke, A.; Hågvar, S.; Kvamme, T. What factors influence the diversity of saproxylic beetles? A multiscaled study from a spruce forest in southern Norway. Biodivers. Conserv. 1996, 5, 75–100. [Google Scholar] [CrossRef]
- Similä, M.; Kouki, J.; Martikainen, P.; Uotila, A. Conservation of beetles in boreal pine forests: The effects of forest age and naturalness on species assemblages. Biol. Conserv. 2002, 106, 19–27. [Google Scholar] [CrossRef]
- Similä, M.; Kouki, J.; Martikainen, P. Saproxylic beetles in managed and seminatural Scots pine forests: Quality of dead wood matters. For. Ecol. Manag. 2003, 174, 365–381. [Google Scholar] [CrossRef]
- Jonsell, M.; Weslien, J. Felled or standing retained wood—it makes a difference for saproxylic beetles. For. Ecol. Manag. 2003, 175, 425–435. [Google Scholar] [CrossRef]
- McGeoch, M.A.; Schroeder, M.; Ekbom, B.; Larsson, S. Saproxylic beetle diversity in a managed boreal forest: Importance of stand characteristics and forestry conservation measures. Divers. Distrib. 2007, 13, 418–429. [Google Scholar] [CrossRef]
- Foit, J. Distribution of early-arriving saproxylic beetles on standing dead Scots pine trees. Agric. For. Èntomol. 2010, 12, 133–141. [Google Scholar] [CrossRef]
- Work, T.T.; Andersson, J.; Ranius, T.; Hjältén, J. Defining stump harvesting retention targets required to maintain saproxylic beetle biodiversity. For. Ecol. Manag. 2016, 371, 90–102. [Google Scholar] [CrossRef]
- Tylkowski, S. Sosnowe bory bagienne jako refugium występowania chrząszczy (Coleoptera) saproksylicznych. Studia I Mater. CEPL W Rogowie 2014, 16, 308–321. [Google Scholar]
- Gutowski, J.; Kubisz, D.; Sućko, K.; Zub, K. The succession of saproxylic beetles (Coleoptera) on windthrow areas in the Scots pine stands of the Piska Forest. For. Res. Pap. 2010, 71, 279–298. [Google Scholar] [CrossRef]
- Marczak, D. Chrząszcze Saproksyliczne Głównych Typów Siedliskowych Puszczy Kampinoskiej—Studium Faunistyczno-Ekologiczne; Instytut Badawczy Leśnictwa: Sękocin Stary, Poland, 2019. [Google Scholar]
- Byk, A.; Mokrzycki, T.; Perliński, S.; Rutkiewicz, A. Saproxylic beetles—monitoring of anthropogenic transformations of Białowieża Primeval Forest. In Zooindication-Based Monitoring of Anthropogenic Transformations in Białowieża Primeval Forest; Szujecki, A., Ed.; Warsaw Agricultural University Press: Warsaw, Poland, 2006; pp. 325–397. [Google Scholar]
- Gutowski, J.M.; Sućko, K.; Borowski, J.; Kubisz, D.; Mazur, M.A.; Melke, A.; Mokrzycki, T.; Plewa, R.; Żmihorski, M. Post-fire beetle succession in a biodiversity hotspot: Białowieża Primeval Forest. For. Ecol. Manag. 2020, 461, 117893. [Google Scholar] [CrossRef]
- Barzdajn, W.; Drogoszewski, B.; Zientarski, J.; Danielewicz, W.; Sienkiewicz, A. Wpływ sposobu zagospodarowania na trwałość i produkcyjność drzewostanów sosnowych. Cz. I. Założenia metodyczne i charakterystyka obiektu doświadczalnego w Nadleśnictwie Torzym. Rocz. Akad. Rol. W Pozn. 1991, 231, 3–26. [Google Scholar]
- Barzdajn, W.; Drogoszewski, B.; Zientarski, J.; Danielewicz, W.; Sienkiewicz, A. Wpływ sposobu zagospodarowania na trwałość i produkcyjność drzewostanów sosnowych. Cz. II. Charakterystyka powierzchni doświadczalnej w Nadleśnictwie Gubin. Rocz. Akad. Rol. W Pozn. 1993, 255, 3–22. [Google Scholar]
- Google Earth Pro 7.3.4.8248 (64-Bit) Data SIO, NOAA, U.S. Navy, NGA, GEBCO. Image Landasat/Copernicus. 2021. Available online: https://www.google.com/earth/ (accessed on 22 October 2021).
- Kwaśna, H.; Mazur, A.; Łabędzki, A.; Kuźmiński, R.; Łakomy, P. Communities of fungi in decomposed wood of oak and pine. For. Res. Pap. 2016, 77, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Kwaśna, H.; Mazur, A.; Kuźmiński, R.; Jaszczak, R.; Turski, M.; Behnke-Borowczyk, J.; Adamowicz, K.; Łakomy, P. Abundance and diversity of wood-decay fungi in managed and unmanaged stands in a Scots pine forest in western Poland. For. Ecol. Manag. 2017, 400, 438–446. [Google Scholar] [CrossRef]
- Adamowicz, K.; Jaszczak, R.; Kuźmiński, R.; Łabędzki, A.; Łakomy, P.; Mazur, A.; Starosta-Grala, M.; Szramka, H.; Turski, M.; Zientarski, J. An attempt at valuation of wood from dead trees in Polish forests. Acta Sci. Pol. Silvarum Colendarum Ratio Ind. Lignaria 2015, 14, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Bouget, C.; Brustel, H.; Brin, A.; Noblecourt, T. Sampling saproxylic beetles with window flight traps: Methodological insights. Rev. Écol. (Terre Vie) 2008, 63, 13–24. [Google Scholar]
- Mazur, A.; Klejdysz, T.; Dobrowolski, M.; Konwerski, S.; Królik, R.; Łabędzki, A.; Przewoźny, M. Saproxylic beetles of Karkonosze (Giant) Mountains. Part I—Checklist. Acta Sci. Pol. Silvarum Colendarum Ratio Ind. Lignaria 2015, 15, 269–295. [Google Scholar] [CrossRef] [Green Version]
- Mazur, A.; Kuźmiński, R.; Łabędzki, A.; Witkowski, R. Chrząszcze gnilikowate (Coleoptera, Histeridae) jako stały element fauny subkortykalnej lasów sosnowych i dębowych Wielkopolski. Studia I Mater. CEPL W Rogowie 2016, 18, 132–138. [Google Scholar]
- Tarnawski, D.; Buchholz, L. Sprężykowate—Elateridae. Część ogólna oraz podrodziny: Agrypninae, Negastriinae i Dominae. In Klucze do Oznaczania Owadów Polski, Part XIX 34a; Polskie Towarzystwo Entomologiczne: Toruń, Poland, 2008; pp. 5–125. [Google Scholar]
- Nunberg, M. Korniki—Scolytidae, Wyryniki—Platypodidae. In Klucze do Oznaczania Owadów Polski, Part XIX 99-100; Polskie Towarzystwo Entomologiczne: Warszawa, Poland; Wrocław, Poland, 1981; pp. 5–115. [Google Scholar]
- Szujecki, A. Kusakowate—Staphylinidae. In Klucze do Oznaczania Owadów Polski, Part XIX 24a; Polskie Towarzystwo Entomologiczne: Toruń, Poland, 2008; pp. 5–229. [Google Scholar]
- Lohse, G.A. Familie: Staphylinidae. In Die Käfer Mitteleuropas. Band 4 Staphylinidae I (Micropeplinae Bis Tachyporinae); Freude, H., Harde, K.W., Lohse, G.A., Eds.; Goecke & Evers Verlag: Krefeld, Germany, 1964; pp. 5–264. [Google Scholar]
- Assing, V.; Schülke, M. Freude-Harde-Lohse-Klausnitzer—Die Käfer Mitteleuropas. Band Staphylinidae I. Zweite neubearbeitete Auflage; Spectrum Akademischer Verlag I-XII: Heidelberg, Germany, 2011; pp. 1–560. [Google Scholar]
- Bense, U. Illustrated Key to the Cerambycidae and Vesperidae of Europe; Margraf Verlag: Weikersheim, Germany, 1995; pp. 1–512. [Google Scholar]
- Pfeffer, A. Zentral-und Westpaläarktische Borken-und Kernkäfer (Coleoptera, Scolytidae, Platypodidae). Entomol. Basilensia 1994, 17, 5–310. [Google Scholar]
- Mazur, S. Histeridae Gnilikowate (Insecta: Coleoptera). Fauna Poloniae; PWN: Warszawa, Poland, 1981; Volume 9, pp. 5–206. [Google Scholar]
- Löbl, I. Icones Insectorum Europae Centralis. Coleoptera: Staphylinidae Dasycerinae, Pselaphinae. Folia Heyrovskyana 2009, 10, 1–26. [Google Scholar]
- Jelínek, J. Icones Insectorum Europae Centralis. Coleoptera Sphindidae, Kateretidae, Nitidulidae. Folia Heyrovskyana 2014, 21, 1–29. [Google Scholar]
- Sienkiewicz, J. Concepts of biodiversity—Their dimensions and measures in the light of literature. Ochr. Sr. I Zasobów Nat. 2010, 45, 7–29. [Google Scholar]
- Shannon, C.A. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [Green Version]
- Szujecki, A. Wpływ rębni zupełnej na zgrupowania ściółkowych kusakowatych (Col.; Staphylinidae) borów sosnowych świeżych. Folia For. Pol. 1971, 18, 5–45. [Google Scholar]
- Szujecki, A. Ekologia Owadów Leśnych; PWN: Warszawa, Poland, 1980. [Google Scholar]
- Pawłowski, J. Reliktowe chrząszcze Coleoptera “Puszczy Karpackiej”. Rocz. Bieszcz. 2008, 16, 317–324. [Google Scholar]
- Byk, A.; Borowski, J.; Mazur, S.; Mokrzycki, T.; Rutkiewicz, A. Waloryzacja lasów Leśnego Kompleksu Promocyjnego “Lasy Spalsko-Rogowskie” na podstawie struktury zgrupowań chrząszczy saproksylicznych. Studia I Mater. CEPL W Rogowie 2013, 2, 82–128. [Google Scholar]
- Mokrzycki, T.; Borowski, J.; Byk, A.; Rutkiewicz, A. Waloryzacja ekosystemów Leśnego Kompleksu Promocyjnego “Lasy Spalsko-Rogowskie” na podstawie struktury zgrupowań chrząszczy (Coleoptera) zasiedlających pniaki. Studia I Mater. CEPL W Rogowie 2013, 2, 48–79. [Google Scholar]
- Biodiversity Map. Available online: https://baza.biomap.pl/pl/db (accessed on 29 June 2021).
- Alexander, K.N.A. Tree biology and saproxylic Coleoptera: Issues of definitions and conservation language. Rev. Écol. (Terre Vie) 2008, 63, 9–13. [Google Scholar]
- Nieto, A.; Alexander, K.N.A. European Red List of Saproxylic Beetles; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Kliczkowska, A.; Zielony, R. Regionalizacja Przyrodniczo-Leśna Polski; Generalna Dyrekcja Lasów Państwowych: Warszawa, Poland, 2012. [Google Scholar]
- Matuszkiewicz, W.; Sikorski, P.; Szwed, W.; Wierzba, M. (Eds.) Lasy I Zarośla. Zbiorowiska Roślinne Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2012; p. 520. [Google Scholar]
- Kuźmiński, R.; Chrzanowski, A.; Mazur, A.; Rutkowski, P.; Gwiazdowicz, D.J. Distribution and habitat preferences of the stag beetle Lucanus cervus (L.) in forested areas of Poland. Sci. Rep. 2020, 10, 1043. [Google Scholar] [CrossRef]
- Mokrzycki, T. Saproxylic Beetle Assemblages (Coleoptera) of Stumps of Chosen Tree Species—Comparative Study; Treatises and Monographs; Publications of Warsaw University of Life Sciences—SGGW: Warsaw, Poland, 2011. [Google Scholar]
- Butenko, K.O.; Gongalsky, K.B.; Korobushkin, D.I.; Ekschmitt, K.; Zaitsev, A.S. Forest fires alter the trophic structure of soil nematode communities. Soil Biol. Biochem. 2017, 109, 107–117. [Google Scholar] [CrossRef]
- Koltz, A.M.; Burkle, L.A.; Pressler, Y.; Dell, J.E.; Vidal, M.C.; Richards, L.A.; Murphy, S.M. Global change and the im-portance of fire for the ecology and evolution of insects. Curr. Opin. Insect Sci. 2018, 29, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bouget, C.; Larrieu, L.; Nusillard, B.; Parmain, G. In search of the best local habitat drivers for saproxylic beetle diversity in temperate deciduous forests. Biodivers. Conserv. 2013, 22, 2111–2130. [Google Scholar] [CrossRef]
- Floren, A.; Müller, T.; Dittrich, M.; Weiss, M.; Linsenmair, K.E. The influence of tree species, stratum and forest management on beetle assemblages responding to deadwood enrichment. For. Ecol. Manag. 2014, 323, 57–64. [Google Scholar] [CrossRef]
- As, S. Are habitat islands islands? Woodliving beetles (Coleoptera) in deciduous forest fragments in boreal forest. Ecography 1993, 16, 219–228. [Google Scholar] [CrossRef]
- Müller, J.; Goßner, M.M. Three-dimensional partitioning of diversity informs state-wide strategies for the conservation of saproxylic beetles. Biol. Conserv. 2010, 143, 625–633. [Google Scholar] [CrossRef]
- Knížek, M.; Zahradník, P. Bark and Wood Boring Beetles in the Pine Stands. In Methodology of Forest Insect and Disease Survey in Central Europe, Proceedings of the Second Workshop of the IUFRO WP 7.03.10, Sion-Chateauneuf, Switzerland, 20–23 April 1999; Forster, B., Knižek, M., Grodzki, W., Eds.; Swiss Federal Institute for Forest, Snow and Landscape Research (WSL): Birrnensdorf, Switzerland, 1999; pp. 54–59. [Google Scholar]
- Martikainen, P.; Viiri, H.; Räty, M. Beetles (Coleoptera) caught with pheromones of Gnathotrichus retusus and G. sulcatus (Col., Scolytidae) in southern Finland. Anz. Fur. Schdlingskunde 2001, 74, 7–10. [Google Scholar] [CrossRef]
- Ruchin, A.; Egorov, L.; Khapugin, A. Usage of Fermental Traps for the Study of the Species Diversity of Coleoptera. Insects 2021, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Hjältén, J.; Stenbacka, F.; Andersson, J. Saproxylic beetle assemblages on low stumps, high stumps and logs: Implications for environmental effects of stump harvesting. For. Ecol. Manag. 2010, 260, 1149–1155. [Google Scholar] [CrossRef]
- Plewa, R.; Jaworski, T.; Hilszczański, J. Dead wood and community structure of saproxylic beetles (Coleoptera) in oak stands. Studia i Materiały CEPL w Rogowie 2014, 16, 279–299. [Google Scholar]
- Vodka, Š.; Cizek, L. The effects of edge-interior and understorey-canopy gradients on the distribution of saproxylic beetles in a temperate lowland forest. For. Ecol. Manag. 2013, 304, 33–41. [Google Scholar] [CrossRef]
- Horák, J.; Kout, J.; Vodka, Š.; Donato, D.C. Dead wood dependent organisms in one of the oldest protected forests of Europe: Investigating the contrasting effects of within-stand variation in a highly diversified environment. For. Ecol. Manag. 2016, 363, 229–236. [Google Scholar] [CrossRef]
- Lindhe, A.; Lindelöw, Å. Cut high stumps of spruce, birch, aspen and oak as breeding substrates for saproxylic beetles. For. Ecol. Manag. 2004, 203, 1–20. [Google Scholar] [CrossRef]
- Peuhu, E.; Thomssen, P.-M.; Siitonen, J. Comparison of three trap types in sampling saproxylic beetles living in hollow urban trees. J. Insect Conserv. 2018, 23, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Wikars, L.-O.; Sahlin, E.; Ranius, T. A comparison of three methods to estimate species richness of saproxylic beetles (Coleoptera) in logs and high stumps of Norway spruce. Can. Èntomol. 2005, 137, 304–324. [Google Scholar] [CrossRef]
- Parmain, G.; Bouget, C. Large solitary oaks as keystone structures for saproxylic beetles in European agricultural landscapes. Insect Conserv. Divers. 2018, 11, 100–115. [Google Scholar] [CrossRef] [Green Version]
- Shavrin, A. Contribution to the knowledge of the fauna of the tribe Omaliini McLeay, 1825 (Coleoptera: Staphylinidae: Omaliinae) of the Baikal region and adjacent territories. Balt. J. Coleopterol. 2010, 10, 27–44. [Google Scholar]
- Hyvärinen, E.; Kouki, J.; Martikainen, P.; Lappalainen, H. Short-term effects of controlled burning and green-tree retention on beetle (Coleoptera) assemblages in managed boreal forests. For. Ecol. Manag. 2005, 212, 315–332. [Google Scholar] [CrossRef]
- Wermelinger, B.; Duelli, P.; Obrist, M.K. Dynamics of saproxylic beetles (Coleoptera) in windthrow areas in alpine spruce forests. For. Snow Landsc. Res. 2002, 77, 133–148. [Google Scholar]
- Niţu, E.; Olenici, N.; Popa, I.; Nae, A.; Biriş, I.A. Soil and saproxylic species (Coleoptera, Collembola, Araneae) in primeval forests from the northern part of South-Eastern Carpathians. Ann. For. Res. 2009, 52, 27–53. [Google Scholar]

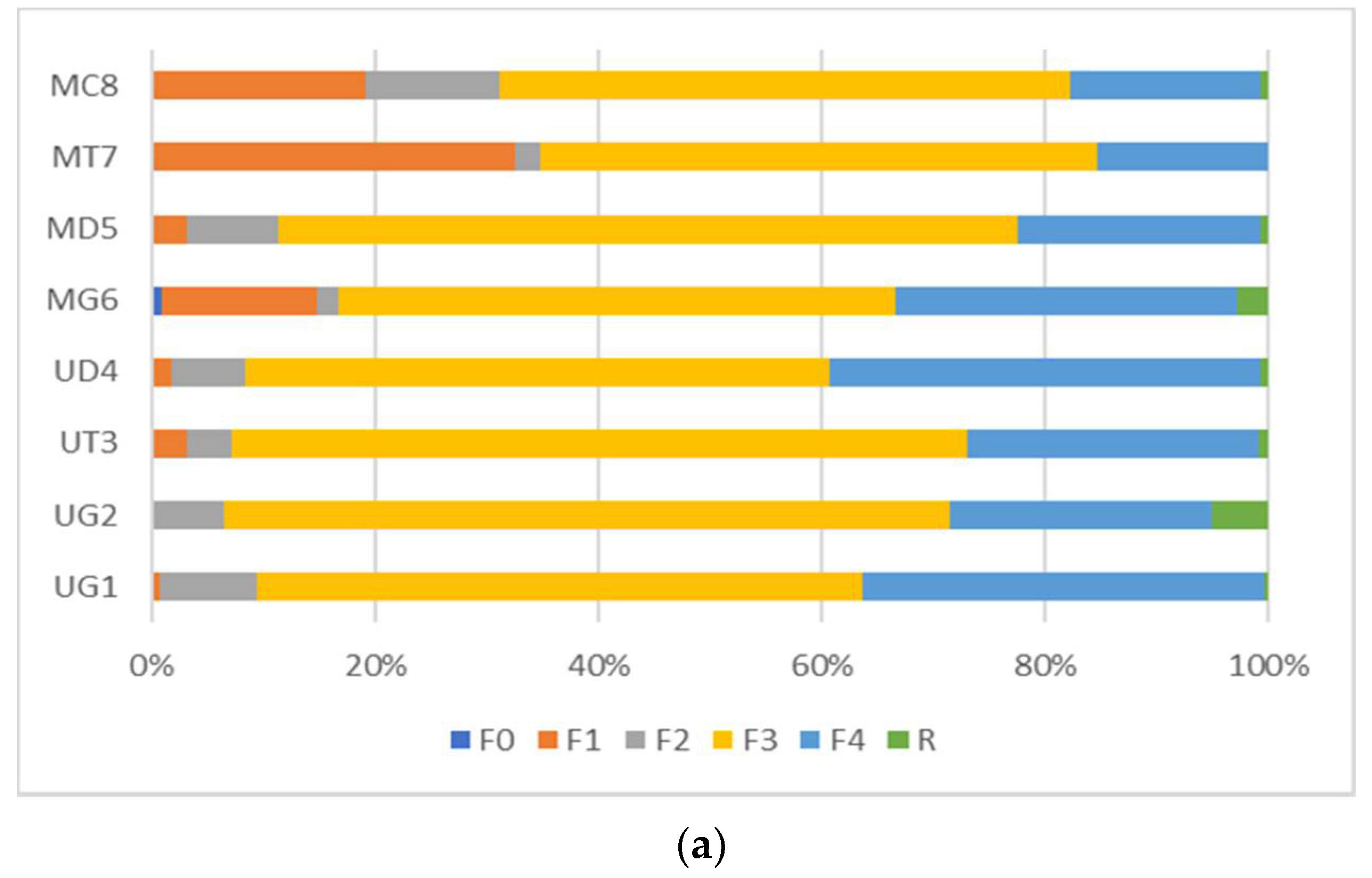

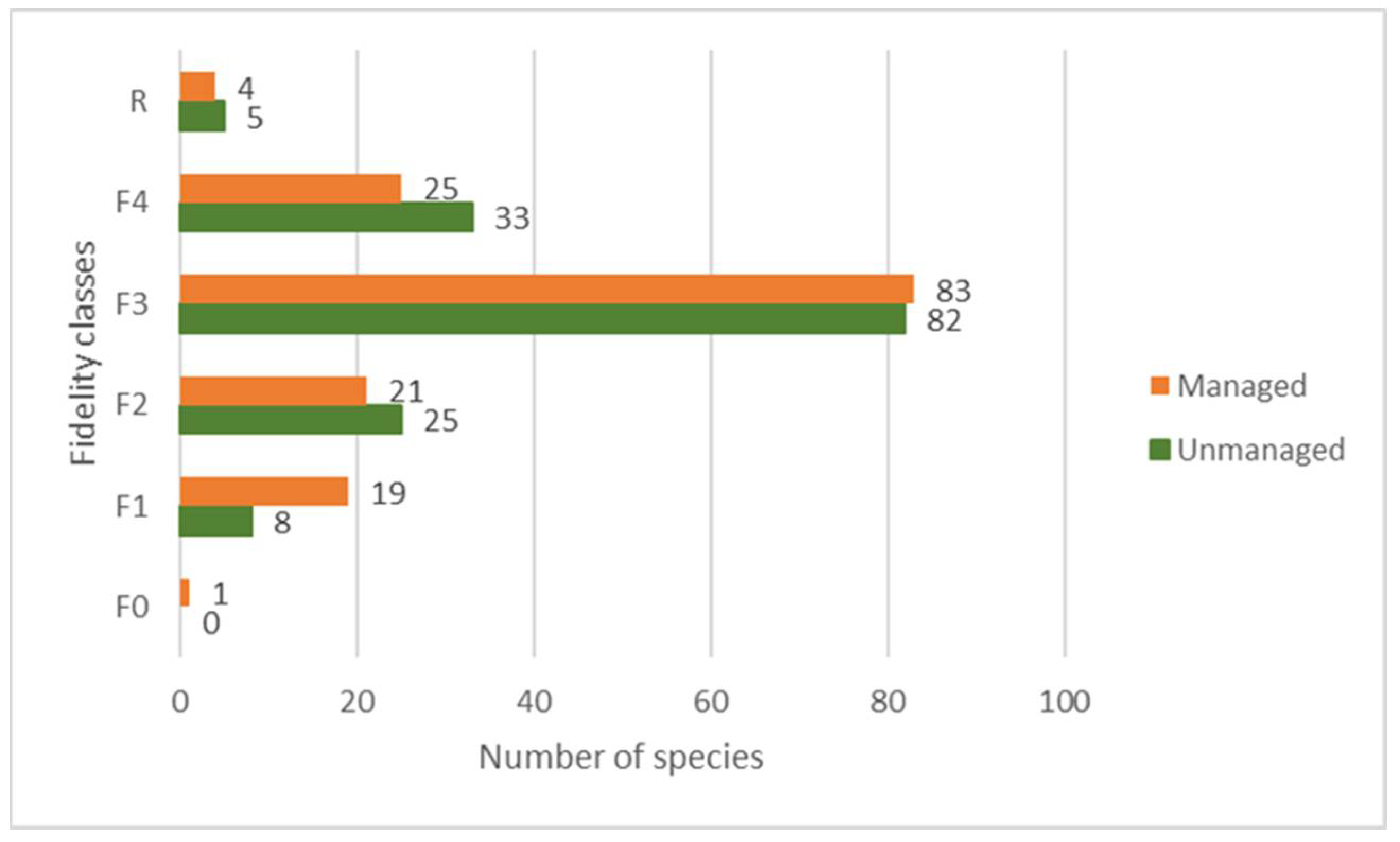
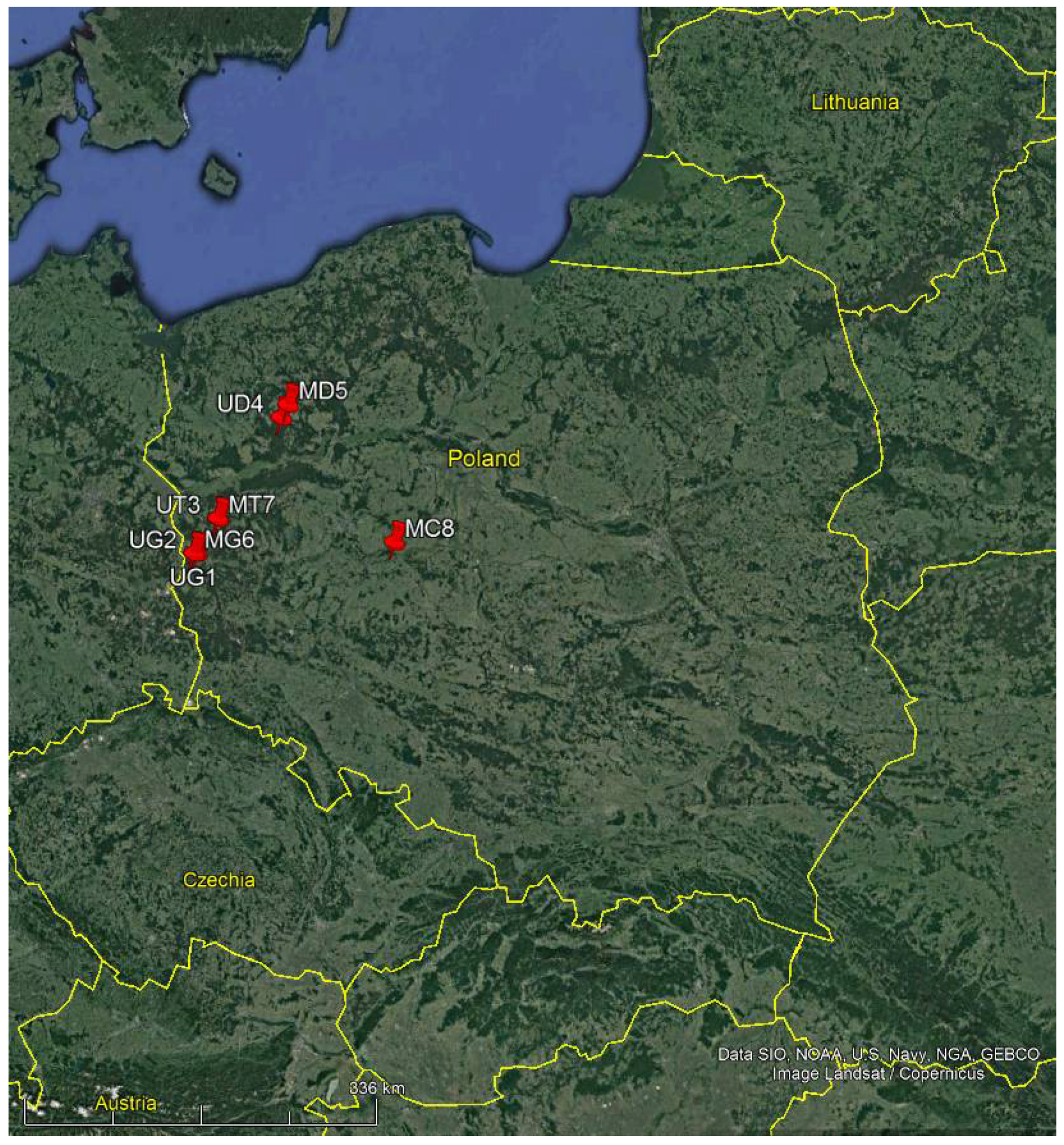
| Location and Description/Symbol of the Site | Geographical Coordinates WGS | Area (ha) | Stand Volume Excluding Dead Wood (m3/ha) | Volume of Dead Wood (CWD) (m3/ha) | Age | Share of Dead Wood/Growing Stock (%) |
|---|---|---|---|---|---|---|
| Unmanaged pine stands | ||||||
| Gubin Forest District compartment 73j—uneven-aged stand/UG1 | N: 51.9817 E: 14.8611 | 4.03 | 248 170 | 8.03 | 195 62 | 1.92 |
| Gubin Forest District compartment 57a/UG2 | N: 51.9737 E: 14.8195 | 20.18 | 317 | 16.51 | 92 | 5.45 |
| Torzym Forest District compartment 264a/UT3 | N: 52.2603 E: 15.1254 | 11.42 | 481 | 23.43 | 85 | 5.85 |
| Drawieński National Park, compartment 288h/UD4 | N: 53.0839 E: 15.9347 | 6.08 | 270 35 | 4.0 | 145 105 | 1.31 |
| Managed pine stands | ||||||
| Drawieński National Park, compartment 15b/MD5 | N: 53.1889 E: 16.0235 | 7.33 | 310 | 3.51 | 125 | 1.13 |
| Gubin Forest District compartment 56a/MG6 | N: 51.9758 E: 14.8229 | 20.14 | 303 | 3.7 | 113 | 1.17 |
| Torzym Forest District compartment 263a, c/MT7 | N: 52.2625 E: 15.1288 | 8.04 | 401 | 7.52 | 91 | 1.56 |
| Jarocin Forest District compartment 200a/MC8 | N: 52.1109 E: 17.4994 | 8.23 | 449 | 1.43 | 63 | 0.32 |
| Characteristics of Assemblages | Unmanaged | Managed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UG1 | UG2 | UT3 | UD4 | Mean (±SD) | MD5 | MG6 | MT7 | MC8 | Mean (±SD) | |
| Number of individuals (n) | 267 | 137 | 126 | 323 | 213.25 (±84.2) | 531 | 108 | 46 | 282 | 241.75 (±188.08) |
| Number of species (S) | 64 | 45 | 39 | 84 | 58 (±17.62) | 75 | 25 | 18 | 62 | 45 (±24.07) |
| Xylophagous species (%) | 14.1 | 13.3 | 15.4 | 22.6 | 16.3 (±3.7) | 28.0 | 16.0 | 16.7 | 22.6 | 20.8 (±4.9) |
| Mecytophagous species (%) | 15.6 | 22.2 | 23.1 | 14.3 | 18.8 (±3.9) | 16.0 | 10.7 | 16.7 | 27.4 | 17.7 (±6.1) |
| Zoophagous species (%) | 45.3 | 51.1 | 33.3 | 41.7 | 42.9 (±6.4) | 40.0 | 46.7 | 44.4 | 29.0 | 40.0 (±6.8) |
| Saproxylophagous species (%) | 9.4 | 8.9 | 7.7 | 6.0 | 8.0 (±1.3) | 8.0 | 6.7 | 5.6 | 9.7 | 7.5 (±1.5) |
| Species from other trophic groups (%) | 15.6 | 4.4 | 20.5 | 15.5 | 14.0 (±5.9) | 8.0 | 20.0 | 16.7 | 11.3 | 14.0 (±4.6) |
| Margalef’s index (d) | 11.28 | 8.94 | 7.86 | 14.37 | 10.61 (±2.5) | 11.79 | 5.13 | 4.44 | 10.99 | 8.09 (±3.32) |
| Shannon’s index (H’) | 3.49 | 3.32 | 2.80 | 3.92 | 3.38 (±0.4) | 2.56 | 2.77 | 2.50 | 3.35 | 2.8 (±0.33) |
| Species | Dominance Index in the Stand | |||||||
|---|---|---|---|---|---|---|---|---|
| UG1 | UG2 | UT3 | UD4 | MD5 | MG6 | MT7 | MC8 | |
| Enicmus rugosus | 16.5 | 16.1 | 7.1 | - | - | 5.6 | 6.5 | - |
| Dryophthorus corticalis | 9.0 | - | - | - | - | - | - | - |
| Placusa tachyporoides | 7.5 | - | - | - | - | 5.6 | - | - |
| Orthotomicus longicollis | 5.2 | - | - | - | - | - | - | - |
| Cerylon ferrugineum | - | 10.2 | - | - | - | - | - | - |
| Platydema violacea | - | 7.3 | - | - | - | - | - | - |
| Plegaderus caesus | - | 5.1 | - | - | - | - | - | - |
| Spondylis buprestoides | - | 5.1 | - | - | 6.4 | 10.2 | - | - |
| Phloeostiba lapponica | - | - | 34.9 | - | - | 12.1 | 10.9 | - |
| Paromalus parallelepipedus | - | - | 5.5 | - | - | - | - | - |
| Placus atrata | - | - | 5.5 | - | - | - | - | - |
| Phloeonomus pusillus | - | - | - | 8.4 | - | - | - | - |
| Rhizophagus depressus | - | - | - | 7.4 | - | - | - | - |
| Tomicus piniperda | - | - | - | 6.8 | - | - | - | 8.5 |
| Ampedus balteatus | - | - | - | - | 49.3 | - | - | - |
| Nicrophorus vespilloides | - | - | - | - | - | 13.9 | 28.2 | 9.6 |
| Crypturgus hispidulus | - | - | - | - | - | 12.9 | - | - |
| Epuraea thoracica | - | - | - | - | - | 7.4 | 8.7 | - |
| Abraeus perpusillus | - | - | - | - | - | - | 6.5 | - |
| Salpingus ruficollis | - | - | - | - | - | - | 6.5 | - |
| Melanotus villosus | - | - | - | - | - | - | - | 17.7 |
| Cerylon impressum | - | - | - | - | - | - | - | 5.3 |
| Trophic Group | Unmanaged Stands | Managed Stands | ||
|---|---|---|---|---|
| Mean Share of Species in the Trophic Group (%) | Mean Frequency of Individuals in the Trophic Group (%) | Mean Share of Species in the Trophic Group (%) | Mean Frequency of Individuals in the Trophic Group (%) | |
| Saproxylophagous | 8.0 (±1.3) | 5.7 (±4.1) | 7.5 (±1.5) | 15.5 (±20.0) |
| Xylophagous | 16.3 (±3.7) | 13.0 (±5.0) | 20.8 (±4.9) | 21.6 (±8.6) |
| Mycetophagous | 18.8 (±3.9) | 22.2 (±7.9) | 17.7 (±6.1) | 11.4 (±3.5) |
| Zoophagous | 42.9 (±6.4) | 52.5 (±6.2) | 40.0 (±6.8) | 31.6 (±9.8) |
| other | 14.0 (±6.6) | 6.6 (±3.2) | 14.0 (±4.6) | 20.0 (±11.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, A.; Witkowski, R.; Kuźmiński, R.; Jaszczak, R.; Turski, M.; Kwaśna, H.; Łakomy, P.; Szmyt, J.; Adamowicz, K.; Łabędzki, A. The Structure of Saproxylic Beetle Assemblages in View of Coarse Woody Debris Resources in Pine Stands of Western Poland. Forests 2021, 12, 1558. https://doi.org/10.3390/f12111558
Mazur A, Witkowski R, Kuźmiński R, Jaszczak R, Turski M, Kwaśna H, Łakomy P, Szmyt J, Adamowicz K, Łabędzki A. The Structure of Saproxylic Beetle Assemblages in View of Coarse Woody Debris Resources in Pine Stands of Western Poland. Forests. 2021; 12(11):1558. https://doi.org/10.3390/f12111558
Chicago/Turabian StyleMazur, Andrzej, Radosław Witkowski, Robert Kuźmiński, Roman Jaszczak, Mieczysław Turski, Hanna Kwaśna, Piotr Łakomy, Janusz Szmyt, Krzysztof Adamowicz, and Andrzej Łabędzki. 2021. "The Structure of Saproxylic Beetle Assemblages in View of Coarse Woody Debris Resources in Pine Stands of Western Poland" Forests 12, no. 11: 1558. https://doi.org/10.3390/f12111558







