Abstract
Organic acids are critical as secondary metabolites for plant adaption in a stressful situation. Oxalic acid, tartaric acid, and malic acid can improve plant tolerance under waterlogged conditions. Two prominent woody species (Taxodium distichum-Swamp cypress and Salix matsudana-Chinese willow) have been experiencing long-term winter submergence and summer drought in the Three Gorges Reservoir. The objectives of the present study were to explore the responses of the roots of two woody species during flooding as reflected by root tissue concentrations of organic acids. Potted sample plants were randomly divided into three treatment groups: control, moderate submergence, and deep submergence. The concentrations of oxalic acid, tartaric acid, and malic acid in the main root and lateral roots of the two species were determined at four stages. The results showed that T. distichum and S. matsudana adapted well to the water regimes of the reservoir, with a survival rate of 100% during the experiment period. After experiencing a cycle of submergence and emergence, the height and base diameter of the two species showed increasing trends. Changes in base diameter showed insignificant differences between submergence treatments, and only height was significant under deep submergence. The concentrations of three organic acids in the roots of two species were influenced by winter submergence. After emergence in spring, two species could adjust their organic acid metabolisms to the normal level. Among three organic acids, tartaric acid showed the most sensitive response to water submergence, which deserved more studies in the future. The exotic species, T. distichum, had a more stable metabolism of organic acids to winter flooding. However, the native species, S. matsudana, responded more actively to long-term winter flooding. Both species can be considered in vegetation restoration, but it needs more observations for planting around 165 m above sea level, where winter submergence is more than 200 days.
1. Introduction
Reservoirs have played an essential role in human development throughout the world for thousands of years by providing water and controlling flooding, as well as generating power and electricity [1,2]. The Three Gorges Dam Reservoir is one of the largest reservoirs globally, located in the Yangtze River upper reaches in China [3]. Annually, the water level in the reservoir fluctuates by 30 m from 145 m to 175 m above sea level (ASL), the hydro-fluctuation zone is saturated in winter and dry in summer [4,5,6]. Therefore, many native plants sensitive to winter submergence are dying out gradually [7], which leads to negative environmental effects such as soil degradation and erosion, geology disasters, and biodiversity loss [2,8,9]. Conducting vegetation restoration in the hydro-fluctuation zone is important to improve the ecological quality and ecosystem services. In recent years, many studies have proposed that reforestation in the zone between 165 m and 175 m ASL may be more effective and sustainable [4,10]. Understanding the physiological and ecological mechanisms of flood-tolerant plants would be the first step. Such knowledge could be useful for the restoration in the riparian of the Three Gorges Reservoir.
Plants are exposed to diverse stress conditions throughout their life, including biotic and abiotic stresses [11]. Abiotic challenges are metal-toxicity, drought stress, flooding-stress, salt-stress, high or low temperature-stress, nutrient-deprivation, and light-stress [12,13,14]. Among them, water environmental stresses, including drought and flooding, usually negatively affected the growth of plants [13,15,16]. The diffusion rate of gasses such as oxygen and carbon dioxide under submergence is approximately one-ten-thousandth of that in the air [17], which could disturb the photosynthetic and aerobic respiration activity and then restrict the growth of plants [18,19]. Flooding results in the increase of toxic substances such as Fe2+, Mn2+ in the soil, which can be absorbed by the plant; in addition, ethanol remaining in tissues can be converted into acetaldehyde when re-aeration after a period of O2 deprivation [20]. Thus, excessive accumulation of Fe2+, Mn2+, and ethanol in the hypoxic state results in increased plant pathology [21,22]. Moreover, plants can suffer severe oxidative stress due to high levels of reactive oxygen species (ROS) resulting from metabolic changes following the alternation of flood and drought [23]. Thus, plant growth and survival in the water-level fluctuation zone of Three Gorges Reservoir can be challenging.
Numerous investigations have focused on the water tolerance mechanisms of plants, such as the anatomy and physiological changes under waterlogging submergence conditions [24], rapid formation of adventitious roots and aerenchyma under flooding [25,26], changes in carbohydrate content, and antioxidant enzymatic activities [27,28], and photosynthetic physiological adaption to submergence [24,29]. Many studies have also focused on the physiology during the recovery period from submergence [29,30,31,32]. It is essential to examine the adaptation processes of plants exposed to long-term periodic submergence and drought. However, the physiological characteristics of plants to dynamic water levels of reservoir still remain unclear, as does whether flooding-tolerant plants respond similarly to submergence.
The root system is the main organ by which plants absorb water and nutrients, but it is susceptible to environmental changes. Low molecular weight organic acids are secondary metabolites produced in plants under environmental stress conditions that can remove free radicals and relieve toxicity [33,34,35]. It also strengthens disease resistance, activates insoluble soil phosphorus, chelates heavy metal in contaminated areas, and enhances antioxidant enzyme activity [36,37]. Oxalic acid, tartaric acid, and malic acid, as critical organic acids, can improve the tolerance of waterlogged plants to water stress [24,38,39,40]. How do these organic acids respond to the hydro-fluctuation in the Three Gorges Reservoir to help flooding-tolerant plants survive?
In the preliminary study, a batch of waterlogged plants screened out has been applied to reforest in the hydro-fluctuation zone. It was found that the woody plant species Taxodium distichum and Salix matsudana can grow well after repeated annual flooding [29,31,40,41]. T. distichum, native to Southeastern North America, has a specialized respiratory root structure for underwater conditions and was introduced into the hydro-fluctuation zone due to its flooding-resistant characteristics [42]. S. matsudana is a native tree in the Three Gorges Reservoir region. They both grow well in the hydro-fluctuation zone of the Three Gorges Reservoir, and both are considered candidates for the incorporation of reforestation. The objectives of this study were to explore the responses of the roots of two woody species during flooding conditions. The concentration of organic acids can reflect the reactions to dynamic water stress caused by submergence in winter and drought in summer. This research can recognize the physiological and ecological adaptation mechanisms of T. distichum and S. matsudana as reforestation species in the hydro-fluctuation zone of Three Gorges Reservoir. We hypothesized that: (1) Both species have strong waterlogging tolerance, but high-intensity waterlogging would inhibit plant growth to some degree. (2) Oxalic acid, tartaric acid, and malic acid in the two suitable plant roots will show increasing responses to a certain degree when suffering from the dynamic variation of soil moisture in the water-fluctuation zone. (3) The growth and adaptability of two woody plants are related to their characteristics of organic acid concentrations. The findings from the water rhythms in situ will enrich researcher knowledge about the adaptive tree species and benefit the reforestation practices in the Three Gorges Reservoir region.
2. Materials and Methods
2.1. Study Region
The experimental Plot (107°32′–108°14′ E, 30°03′–30°35′ N) is located along the Ruxi River, Shibaozhai, Gonghe Village, Zhong County, Chongqing Municipality, China. Ruxi River is a tributary of the Yangtze River, with a subtropical, southeastern monsoonal mountain climate. The region experiences an annual accumulative temperature of 5787 °C for days ≥10 °C, an annual average temperature of 18.2 °C, and highest and lowest temperatures of 40 °C and 0 °C, respectively. The highest temperatures mainly occur from July to September. There is a frost-free period of 341 days, total solar radiation of 83.7 × 4.18 kJ/cm−2, and an annual sunshine ratio of 29%. The mean annual precipitation is 1200 mm, and most rainfalls are in May and June. Plants suffer drought due to high air temperature and relatively fewer rainfalls in July and August [43,44].
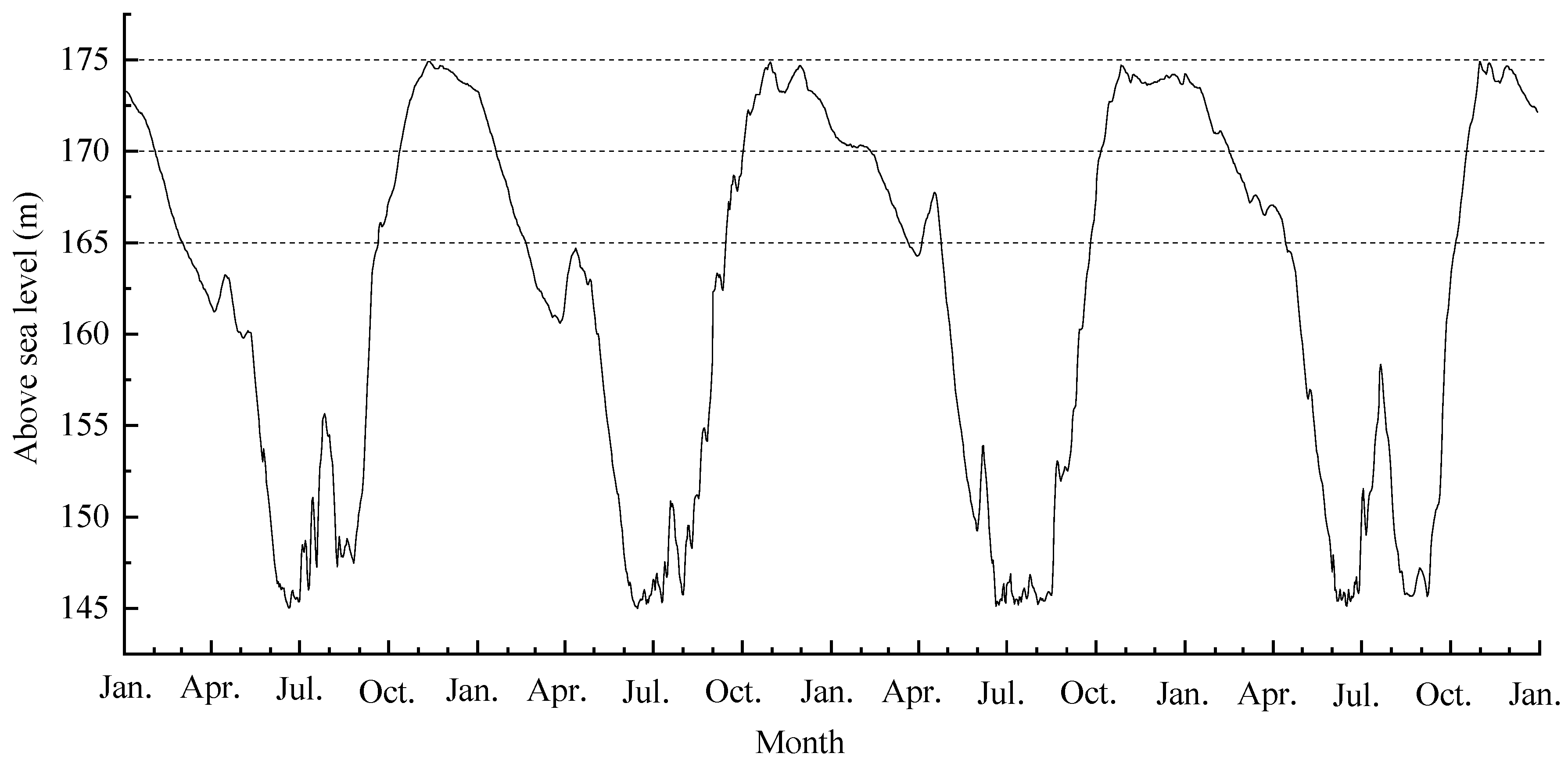
The plants are experiencing annual cycles of periodic submergence and drought growing in the hydro-fluctuation zone of the Three Gorges Dam (Figure 1). Water level gradually rises in the fluctuation zone from September to October. It attains the highest altitude (175 m ASL) from November through to January, then gradually decreases, reaching its lowest level at 145 m ASL from June to September.
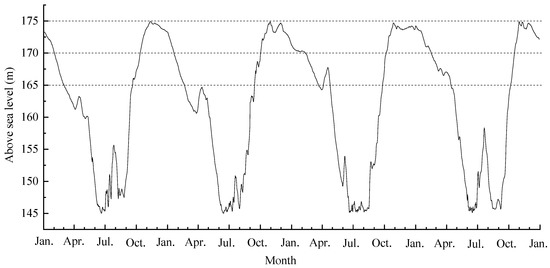
Figure 1.
The water-level changes in the hydro-fluctuation zone of the Three Gorges Reservoir from January 2013 to January 2017.
2.2. Experiment Design
Two-year-old potted seedlings of T. distichum and S. matsudana were cultivated in a greenhouse in Shibaozhai, Zhong County, Chongqing, China, close to the study area, with one plant per pot filled with 12.5 kg of purple soil from the water-level fluctuation zone of the Three Gorges Reservoir.
On 15 September 2015 (T0), for each species, 36 seedlings of uniform growth were randomly divided into three groups and placed in situ at an altitude of 175 m to 165 m ASL. The mean plant height and base diameter at T0 for T. distichum were 69.1 cm and 8.38 mm, compared with 136.25 cm and 17.82 mm for S. matsudana. The three treatment groups were shallow submergence, serving as control (shallow submergence, abbreviated as SS, 175m ASL), periodic moderate submergence (MS, 170 m ASL), and deep submergence (DS, 165 m ASL). The maximum submergence depth and submergence days in each treatment were shown in Table 1. At the initial stage of water withdrawal (T1, 16 April 2016), the stage of the recovery period (T2, 15 July 2016), and the beginning of the next submergence period (T3, 15 September 2016), 4 plants of T. distichum and S. matsudana were sampled, respectively, in each treatment for further measurements.

Table 1.
Water level changes in situ following treatments at different elevations of the Three Gorges Reservoir.
2.3. Sample Analysis
Before collecting each sample, plant height and base diameter of two species were measured using a height measuring rod and vernier calipers, respectively.
Primary roots were separated from the laterals root of each plant when collecting samples and immediately transported to the laboratory in an icebox. They were placed in an oven at 110 °C for 15 min after washing with deionized water, then adjusted to 80 °C to dry until constant weight [45,46], and finally ground into a powder then sifted through a 1 mm sieve.
A 0.1 g sample of root powder was placed in a 10 mL centrifuge tube with 5 mL of ultrapure water and ultrasonicated for 1 h, then cooled to room temperature. After centrifugation at 8000 rpm for 10 min, the supernatant was filtered through a 0.45 μm syringe filter (Millipore, Danvers, MA, USA) to determine the concentrations of oxalic acid, tartaric acid, and malic acid [47].
A high-performance liquid chromatography method was used to determine the organic acid concentration in dried roots by the Sepax Sapphire C18 column (4.6 mm × 250 mm, 5 μm). The mobile phase aqueous and organic components were 95% 20 mmol·L−1 KH2PO4 buffer (pH 2.5, adjusted with phosphoric acid) and 5% methanol. The HPLC instrument was coupled to an Agilent 1100 diode array multi-wavelength detector. The mobile phase flow rate was 0.9 mL·min−1, the detection wavelength was 210 nm, the column temperature was 30 °C, and the injection volume was 20 µL [47,48]. Organic acids in main and lateral roots were measured in milligrams of organic acid per gram of the mains root and lateral roots dry matter (mg.g−1, DW).
2.4. Statistical Analysis
SPSS 22.0 was used for statistical analyses. Paired sample t-tests were used to analyze the differences of growth between different experimental periods for the same treatment (Table 2). Tukey’s tests were applied to determine the significance of differences between different submerging treatment groups (Table 2). Repeated measures ANOVA was conducted to assess the effects of submergence, experimental period, and their interaction on the concentrations of organic acids for T. distichum and S. matsudana (Table 3). Repeated measurement analysis of variance and Bonferroni method were carried out to analyze the differences of organic acids among different experimental periods for the same plant [49] (Figure 2 and Figure 3). One-way ANOVA and Tukey’s tests were carried out to analyze the differences of organic acids among different treatment groups for the same plant (Figure 4). The p-value for statistical tests was 0.05.

Table 2.
Paired sample t-tests and Tukey’s tests for growth of Taxodium distichum and Salix matsudana.

Table 3.
Repeated measures ANOVA for organic acid concentrations in roots of T. distichum and S. matsudana.
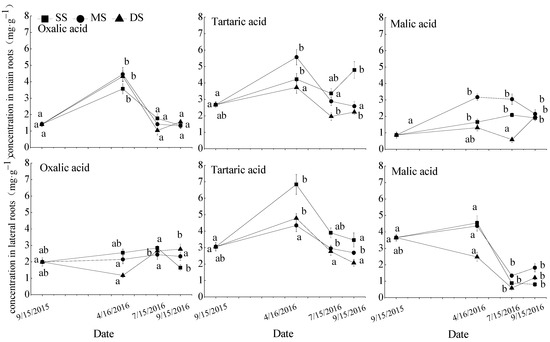
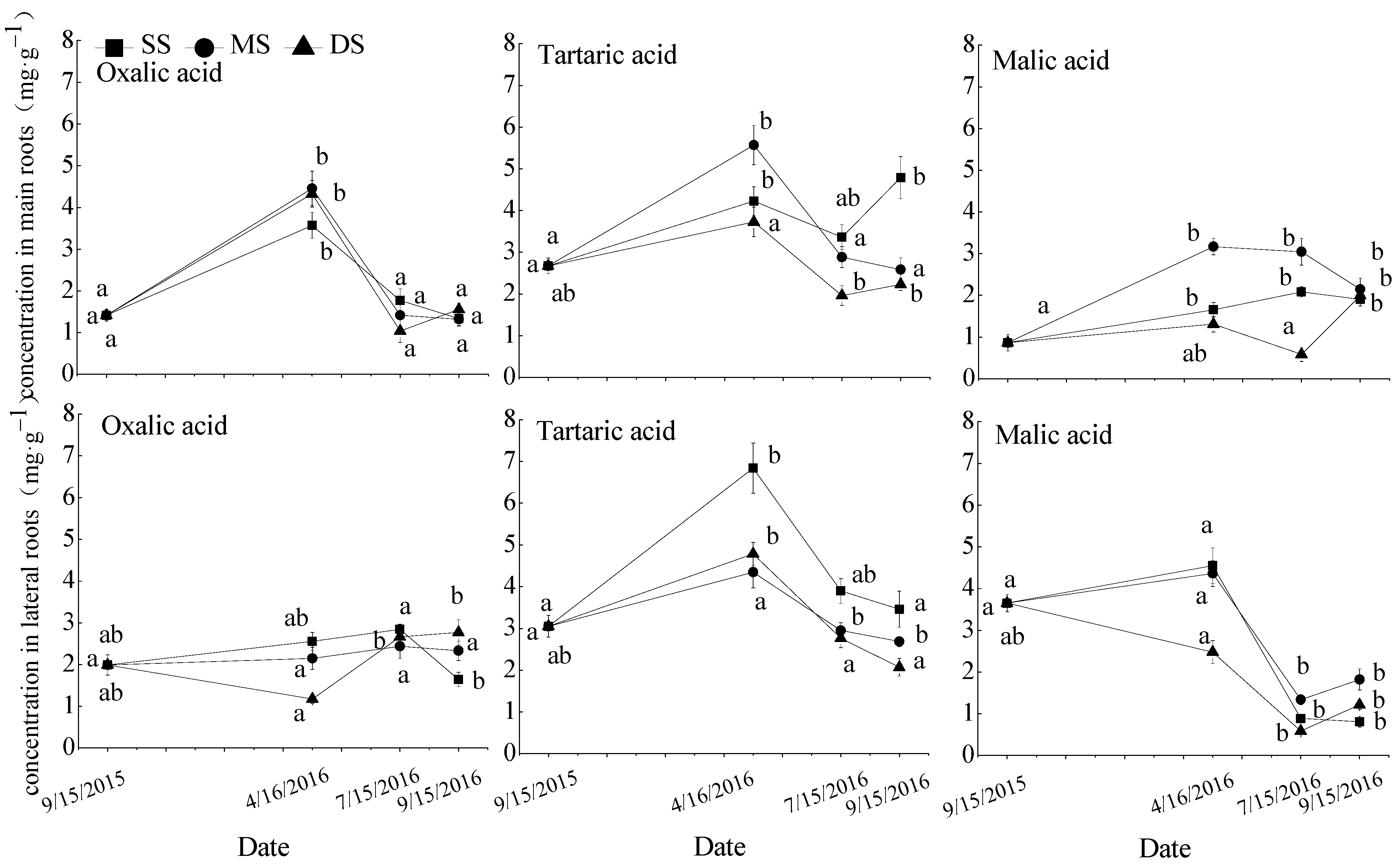
Figure 2.
Repeated measurement analysis of variance and Bonferroni method for oxalic acid, tartaric acid, and malic acid concentrations in roots of T. distichum. Data are shown as means ± SE (n = 4). SS, control; MS, moderate submergence; DS, deep submergence. Values with different letters indicate significant differences among different sampling times for the same treatment (p < 0.05).
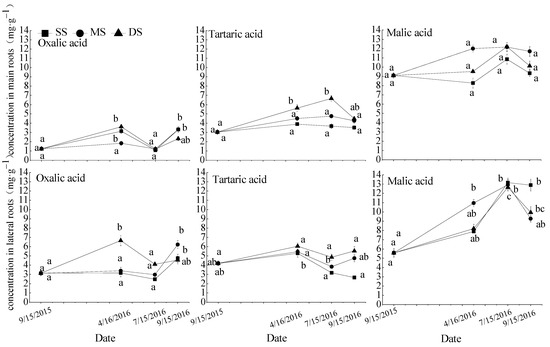
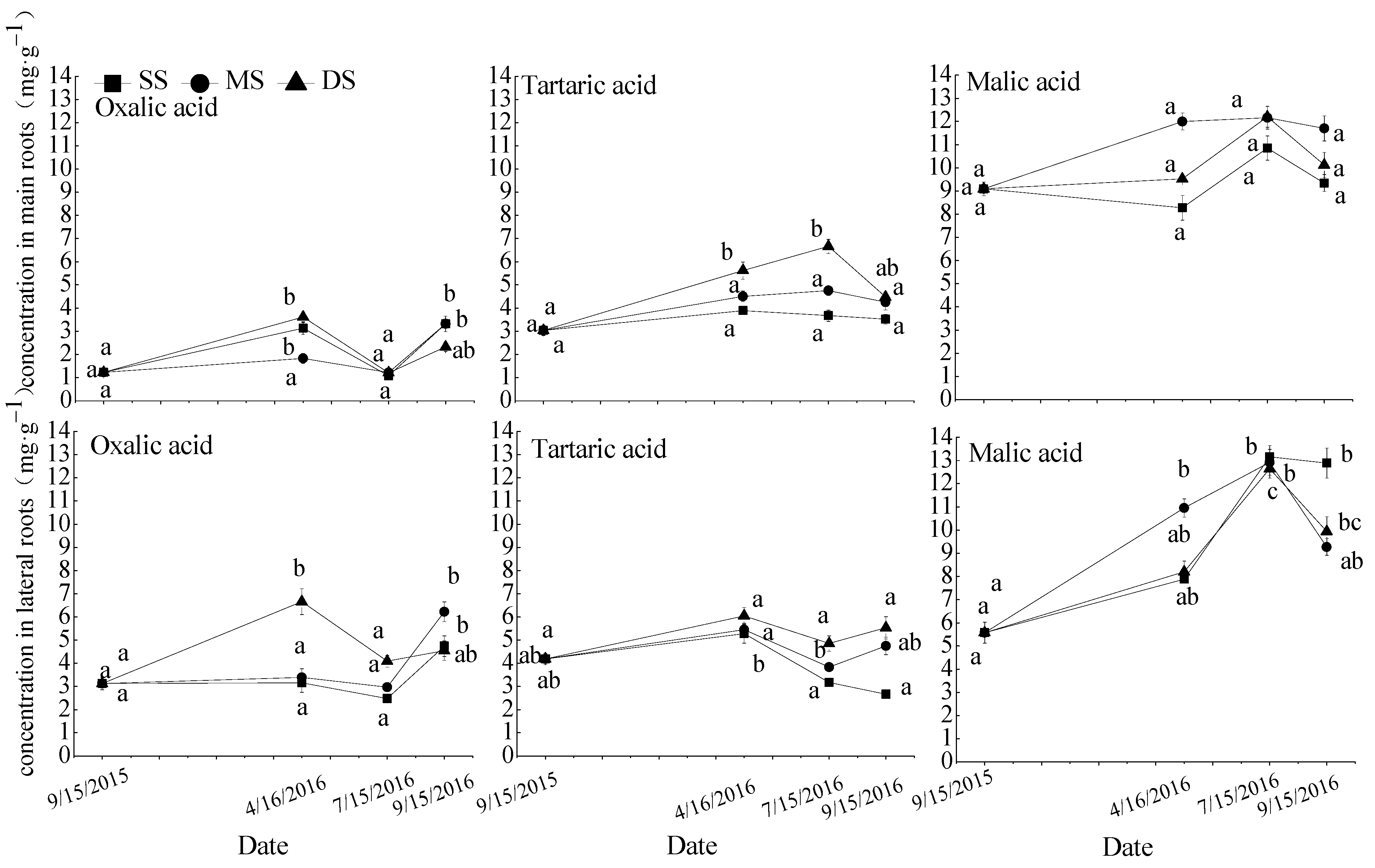
Figure 3.
Repeated measurement analysis of variance and Bonferroni method for oxalic acid, tartaric acid, and malic acid concentrations in roots of in S. matsudana. Data are shown as means ± SE (n = 4). SS, control; MS, moderate submergence; DS, deep submergence. Values with different letters indicate significant differences among different sampling times for the same treatment (p < 0.05).
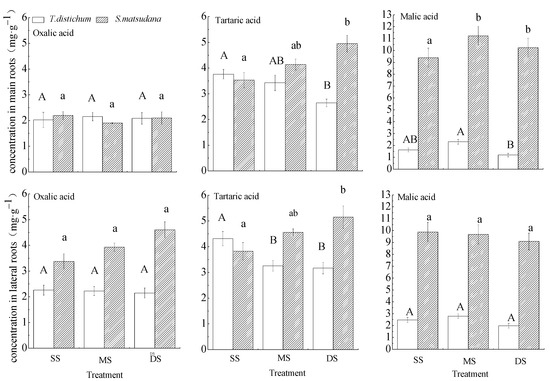
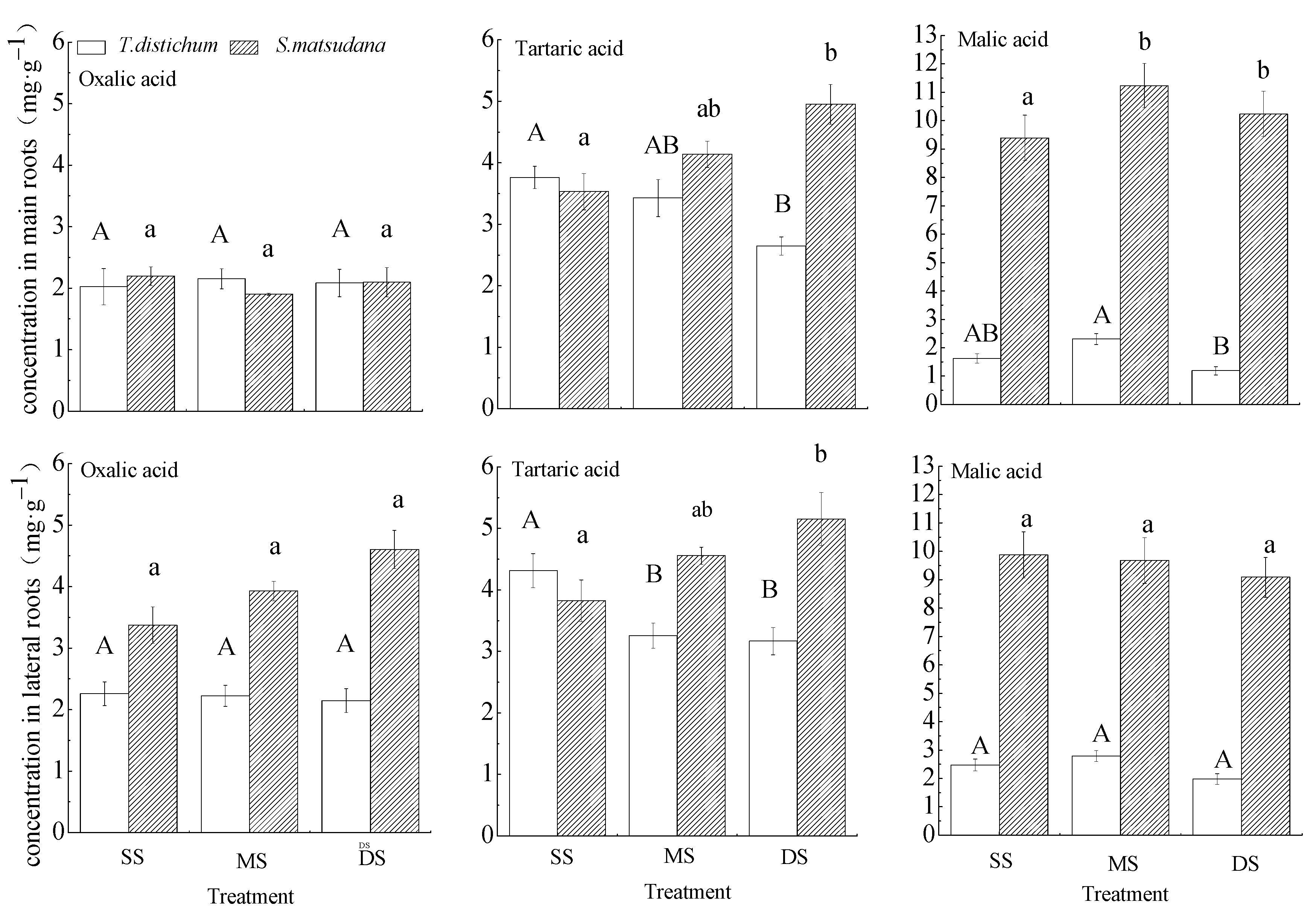
Figure 4.
One-way ANOVA and Tukey’s tests to compare organic acid concentrations in the roots of T. distichum and S. matsudana under different submergence treatments. Data are shown as means ± SE (n = 4). SS, control; MS, moderate submergence; DS, deep submergence. Values with different letters are significantly different among different submergence treatments. Capital letters represent T. distichum seedlings, and small letters indicate S. matsudana seedlings.
3. Results
3.1. Growth of T. distichum and S. matsudana Plants
The survival rate was 100% throughout the experiment for both species. The heights and base diameters of T. distichum and S. matsudana plants were increased after one year of growth (Table 2). Compared to the initial values at T0 (15 September 2015), the plant height of two species in the SS and MS groups and the base diameter of T. distichum in the SS group at T3 (15 September 2016) were significantly higher. Winter submergence significantly inhibited the growth height of plants in the DS groups. In contrast, the base diameter of T. distichum and S. matsudana plants showed fewer differences among different treatment groups.
3.2. Organic Acid Concentrations in T. distichum Roots
Table 3 shows the repeated measures of ANOVA results for organic acid concentrations in the roots of T. distichum following different water treatments, experimental periods, and their interaction. The experimental period had the most significant effect on the concentrations of organic acids in the root system. Different water treatments significantly affected tartaric acid in the main and lateral root and malic acid in the main root. In addition, tartaric acid and malic levels in the main root were significantly affected by treatment and experimental period interaction.
The response of the oxalic acid, tartaric acid, and malic acid concentrations in T. distichum roots to soil moisture was shown in Figure 2. The concentrations of three organic acids in the main and lateral roots displayed similar responses to the changes in soil moisture in the fluctuating water zone. Most of them increased during the flooding stress period (T0–T1), then decreased during the recovery growth period (T1–T2), and were no significant changes under mild drought stress (T2–T3). The concentrations of three organic acids in roots were influenced by winter submergence. The concentrations of tartaric acid in lateral roots in the MS and DS groups and malic acid in lateral roots in the DS group were significantly decreased compared with that in SS group. After emergence in spring, three organic acid concentrations had no differences among the three water treatments.
3.3. Organic Acid Concentrations in S. matsudana Roots
Different flooding treatments in the water fluctuation zone significantly affected oxalic acid concentration in the lateral roots and the tartaric acid concentrations in the main and lateral roots of S. matsudana (Table 3). Except for malic acid in the main roots, oxalic acid and tartaric acid in both the main and lateral roots, and malic acid in the lateral roots of S. matsudana were significantly affected by different experimental periods. The interaction between treatment and experimental periods had little effect on organic acids in the roots of S. matsudana. Only the oxalic acid concentration in lateral roots was significantly affected.
The response of the oxalic acid, tartaric acid, and malic acid concentrations in S. matsudana roots to soil moisture was shown in Figure 3. The concentrations of oxalic acid in the main and lateral roots and tartaric acid in the lateral roots were increased during the flooding stress period (T0–T1), then decreased during the recovery growth period (T1–T2), and were no significant changes under mild drought stress (T2–T3). The concentrations of malic acid in the main and lateral roots and tartaric acid in the main roots increased during the flooding stress period (T0–T1) and the recovery growth period (T1–T2), and then decreased under mild drought stress (T2–T3). Under water submergence in winter, the concentration of oxalic acid in the lateral roots and tartaric acid in the main roots were significantly increased. All acid concentrations in each treatment showed no significant difference after emergence from winter flooding.
3.4. Comparison between T. distichum and S. matsudana
Figure 4 showed the difference in mean concentrations of three organic acids in the whole experimental period between two species. The concentrations of organic acids in the roots of S. matsudana were higher than those of T. distichum generally.
The effects of different treatments on the concentrations of oxalic acid in the main and lateral roots of T. distichum and S. matsudana were similar. Under flooding stress, there were no significant differences in oxalic acid concentrations in the main and lateral roots of T. distichum and S. matsudana among the three treatment groups. The tartaric acid concentrations in the main and lateral roots of T. distichum gradually decreased with increasing flooding intensity, but it was the opposite trend for S. matsudana. The malic acid concentrations in the main roots of S. matsudana in MS and DS groups were significantly higher than those in the SS group. No significant differences in the concentrations of malic acid in lateral roots of T. distichum and S. matsudana were showed. The malic acid concentrations in the main and lateral roots were more sensitive to water flooding in S. matsudana than T. distichum.
4. Discussion
At present, adaptive woody plants such as T. distichum and S. matsudana were screened by simulated flooding tests and in situ planting analysis [23]. This study has shown that both T. distichum and S. matsudana adapt well to soil regimes in the water fluctuation zone of Three Gorges Reservoir. The seedlings of two species survived and grown well after winter submergence, although the flooding more than 200 days at 165 m ASL still inhibited the growth height of plants to a certain degree (Table 2). The plants planted around 165 m ASL should be paying more attention to their growth and survival after years of periodical winter flooding. The growth of T. distichum and S. matsudana at 170 m ASL recovered significantly after the emergence and showed no significant differences with plants at 175 m ASL.
Flooding is often accompanied by secondary stresses such as low temperature, low light, and low dissolved oxygen. The photosynthesis of plants is limited under flooding. Studies have shown that the survival of plants under flooding stress and the restoration of growth after flooding are closely related to the regulation of certain metabolic pathways [18]. Organic acids are secondary metabolites related to adaptation that play important roles in defenses against adverse conditions. The responses of oxalic acid, tartaric acid, and malic acid in roots of T. distichum and S. matsudana were different to winter flooding in the hydro-fluctuation zone. However, two species could adjust their organic acid metabolisms to the normal level after emergence in spring.
Under winter flooding stress, relative to the control group, the tartaric acid concentrations in the main and lateral roots and the malic acid concentration in the main roots of T. distichum in the DS group decreased significantly. However, the concentrations of tartaric acid in the main and lateral roots of S. matsudana in the DS group increased significantly, and the malic acid concentration in the main roots of the flooded group was increased significantly, while the other organic acids were not altered significantly (Figure 4). Thus, tartaric acid and malic acid responses in S. matsudana roots were more active than those in T. distichum roots.
Oxalic acid is one of the most important secondary metabolites in plants under adverse stress conditions, it can stimulate peroxidase activity and improves the antioxidant capacity [33]. Herein, the concentrations of oxalic acid in the roots of T. distichum and S. matsudana were greatly affected by the experimental period but not significantly affected by different flooding treatments. The concentration of oxalic acid in the main roots of T. distichum was significantly increased at the initial stage of water withdrawal (T1), and gradually decreased during the normal growth period; no significant changes showed under mild drought stress (Figure 2). The concentrations of oxalic acid in the main and lateral roots of S. matsudana increased gradually under water flooding stress, and remained high during the initial stages of plant reoxygenation, then decreased during the normal growth period, until the drought period (Figure 4), consistent with the simulation results [40]. Metabolic imbalance of active oxygen in flooding and the early stage of reoxygenation can result in severe oxidative stress. Thus, increased oxalic acid concentrations in the main roots of T. distichum and the main and lateral roots of S. matsudana may be associated with increased antioxidant capacity. During the recovery stage, reactive oxygen species production and elimination gradually achieve a balance; the oxalic acid concentration of roots may provide energy for plant growth through catabolic pathways, resulting in a decrease in oxalic acid concentration during recovery growth after emergence in spring. Throughout the whole experiment, the concentration of oxalic acid in the main roots of T. distichum was more sensitive than that in the lateral roots, while the concentration of oxalic acid in the lateral roots of S. matsudana was more sensitive than that in the main roots, but the roots of both plants responded positively to flooding in the water fluctuation zone. Relevant studies have pointed out that lateral root plays a more important role in responding to abiotic stress for plants [50], and adaptive plants can make full use of lateral root metabolism regulation to enhance the ability of water flooding tolerance [23].
The concentrations of tartaric acid in the main and lateral roots of S. matsudana was ordered by DS group > MS group > SS group. Some studies have previously reported that organic acids not only function as important intermediates in the energy balance and flow in plants but also participate in plant adaption to abiotic stress [51,52]. The DS group maintained a higher root tartaric acid concentration than the MS group. Therefore, we speculated that tartaric acid in the S. matsudana root system could respond positively to flooding stress in the water fluctuation zone [39]. The concentration of tartaric acid in S. matsudana differed between main and lateral roots, indicating different response strategies. Studies have shown that the change of organic acid concentration is closely related to the adaptation mechanism to environmental stress [52].In this study, changes in tartaric acid concentration in S. matsudana lateral roots over time were similar to those of oxalic acid in roots. The concentrations of oxalic acid and tartaric acid in roots are controlled by synthesis, decomposition, transport, and secretion [53], indicating that tartaric acid may perform similar physiological functions to oxalic acid upon changes in soil moisture in the water fluctuation zone. The synthesis of plant oxalic acid is mainly through the photorespiration glycolic acid pathway [54]. However, there is limited research work available on tartaric acid concentration mechanism under water-stressed conditions, and it is very important to be carried out further studies in this field.
5. Conclusions
The exotic species T. distichum and native species S. matsudana are two adaptive woody plants for reforestation in the hydro-fluctuation zone of the Three Gorges Reservoir. The two species can grow very well between 170 m to 175 m ASL in the hydro-fluctuation zone of Three Gorges Reservoir. It needs more observations for plants planted around 165 m ASL, where the duration of winter submergence is more than 200 days.
The concentrations of three organic acids in the roots of two species were influenced by winter submergence. After emergence in spring, two species could adjust their organic acid metabolisms to the normal level. Among three organic acids, tartaric acid was the most sensitive response to water submergence, which deserved more studies in the future. The exotic species, T. distichum, had more stable metabolism of organic acids to winter flooding. The native species, S. matsudana, responded more actively to long-term winter flooding. Two species showed the different organic acid regulation mechanisms facing water regimes, and both species can be applied in vegetation restoration.
Author Contributions
Funding acquisition, H.W.; investigation, X.H.; methodology, X.H., T.W., and K.W.; project administration, H.W.; resources, H.W.; software, X.H., K.W., and Y.Q; supervision, H.W. and M.A.; validation, X.H., H.W., and M.A.; visualization, X.H., P.W., and Y.Q.; writing—original draft preparation, X.H.; writing—review and editing, M.A. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was jointly supported by the National Key Research and Development Program of China (2017YFC0505305), the Program for the Follow-on Work of the Three Gorges: Ecological and Biological Diversity Conservation in the Reservoir Region (5000002013BB5200002), and the Key Research Projects in Forestry of Chongqing (Yu Lin Ke Yan 2015-6).
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Acknowledgments
The authors would like to acknowledge support from the Key Laboratory of Eco-environments in the Three Gorges Reservoir Region (Ministry of Education), the Chongqing Key Laboratory of Plant Ecology and Resources Research in Three Gorges Reservoir Region. We are grateful to Muhammad Tahir, Research Fellow at the Clausthal University of Technology, Germany, and Pinky Sanelisiwe Mzondi, Researcher at the Southwest University, China, for improving the language of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, J.G.; Huang, J.H.; Han, X.G.; Gao, X.M.; He, F.L.; Jiang, M.X.; Jiang, Z.G.; Primack, R.B.; Shen, Z.H. The Three Gorges Dam: An ecological perspective. Front. Ecol. Environ. 2004, 2, 241–248. [Google Scholar] [CrossRef]
- New, T.; Xie, Z. Impacts of large dams on riparian vegetation: Applying global experience to the case of China’s Three Gorges Dam. Biodivers Conserv. 2008, 17, 3149–3163. [Google Scholar] [CrossRef]
- Zhu, N.N.; Qin, A.L.; Guo, Q.S.; Zhu, L.; Xu, G.X.; Pei, S.X. Spatial heterogeneity of plant community in Zigui and Wushan typical hydro-fluctuation belt of Three Gorges Reservoir areas. For. Res. 2015, 28, 109–115. [Google Scholar] [CrossRef]
- Fan, D.Y.; Xiong, G.M.; Zhang, A.Y.; Li, X.; Xie, Z.G.; Li, Z.J. Effect of water-lever regulation on species selection for ecological restoration practice in the water-level fluctuation zone of Three Gorges Reservoir. Chin. J. Plant Ecol. 2015, 39, 416–432. [Google Scholar] [CrossRef]
- Ye, C.; Li, S.Y.; Zhang, Y.R.; Zhang, Q.F. Assessing soil heavy metal pollution in the water-level-fluctuation zone of the Three Gorges Reservoir, China. J. Hazard. Mater. 2011, 191, 366–372. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, X.Z.; Willison, J.H.M.; Zhang, Y.W.; Liu, H. Diversity and above-ground biomass patterns of vascular flora induced by flooding in the drawdown area of China’s Three Gorges Reservoir. PLoS ONE 2014, 9, e100889. [Google Scholar] [CrossRef] [PubMed]
- Garssen, A.G.; Baattrup-Pedersen, A.; Voesenek, L.A.C.J.; Verhoeven, J.T.A.; Soons, M.B. Riparian plant community responses to increased flooding: A meta-analysis. Glob. Chang. Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Mazaeva, O.A.; Kozyreva, E.A.; Babicheva, V.A.; Tyszkowski, S.; Rybchenko, A.A.; Brykała, D.; Bartczak, A.; Słowiński, M. Impact of large water level fluctuations on geomorphological processes and their interactions in the shore zone of a dam reservoir. J. Great Lakes Res. 2016, 42, 926–941. [Google Scholar] [CrossRef]
- Bao, Y.H.; Gao, P.; He, X.B. The water-level fluctuation zone of Three Gorges Reservoir—A unique geomorphological unit. Earth Sci. Rev. 2015, 150, 14–24. [Google Scholar] [CrossRef]
- Li, C.X.; Zhong, Z.C. Influences of mimic soil water change on the contents of malic acid and shikimic acid and root-biomasses of Taxodium distichum seedlings in the hydro-fluctuation belt of the Three Gorges reservoir region. Acta Ecol. Sin. 2007, 27, 4394–4402. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Yang, M.T.; Ma, W.X.; Sun, Y.J.; Chen, G.Y. Overexpression of SSBXoc, a Single-Stranded DNA-Binding Protein from Xanthomonas oryzae pv. oryzicola, Enhances Plant Growth and Disease and Salt Stress Tolerance in Transgenic Nicotiana benthamiana. Front. Plant Sci. 2018, 9, 953. [Google Scholar] [CrossRef] [PubMed]
- Dalal, V.K.; Tripathy, B.C. Water-stress induced downsizing of light-harvesting antenna complex protects developing rice seedlings from photo-oxidative damage. Sci. Rep. 2018, 8, 5955. [Google Scholar] [CrossRef]
- Bhusal, N.; Kim, H.S.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55. [Google Scholar] [CrossRef] [PubMed]
- Bangar, P.; Chaudhury, A.; Tiwari, B.; Kumar, S.; Kumari, R.; Bhat, K.V. Morphophysiological and biochemical response of mungbean [Vigna radiata (L.) Wilczek] varieties at different developmental stages under drought stress. Turk. J. Biol. 2019, 43, 58–69. [Google Scholar] [CrossRef]
- Peng, Y.J.; Zhou, Z.X.; Zhang, Z.; Yu, X.L.; Zhang, X.Y.; Du, K.B. Molecular and physiological responses in roots of two full-sib poplars uncover mechanisms that contribute to differences in partial submergence tolerance. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Colmer, T.D. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef]
- Fukao, T.; Bailey-Serres, J. Plant responses to hypoxia—is survival a balancing act? Trends Plant Sci. 2004, 9, 449–456. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Colmer, T.D.; Pierik, R.; Millenaar, F.F.; Peeters, A.J.M. How plants cope with complete submergence. New Phytol. 2006, 170, 213–226. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annl. Rev. Plant Biol. 2008, 59, 313. [Google Scholar] [CrossRef]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Armstrong, W. Rice: Sulphide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Ann. Bot. 2005, 96, 625–638. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Bhusal, N.; Kim, H.S.; Han, S.G.; Yoon, T.M. Photosynthetic traits and plant–water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- María, L.V.; Mignolli, F.; Aispuru, H.T.; Luis, A.M. Rapid formation of adventitious roots and partial ethylene sensitivity result in faster adaptation to flooding in the aerial roots (aer) mutant of tomato. Sci. Hortic. 2016, 201, 130–139. [Google Scholar] [CrossRef]
- Loreti, E.; Van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.T.; Zeng, B.; Xu, S.J.; Zhang, X.P. Response of basal metabolic rate to complete submergence of riparian species Salix variegata in the Three Gorges reservoir region. Sci. Rep. 2017, 7, 13885. [Google Scholar] [CrossRef]
- Miao, L.F.; Xiao, F.J.; Xu, W.; Yang, F. Reconstruction of Wetland Zones: Physiological and biochemical responses of Salix variegata to winter submergence—A case study from water level fluctuation zone of the Three Gorges Reservoir. Pol. J. Ecol. 2016, 64, 45–52. [Google Scholar] [CrossRef]
- Wang, C.Y.; Xie, Y.Z.; He, Y.Y.; Li, X.X.; Yang, W.H.; Li, C.X. Growth and physiological adaptation of Salix matsudana koidz. to periodic submergence in the hydro-fluctuation zone of the Three Gorges Dam Reservoir of China. Forests 2017, 8, 283. [Google Scholar] [CrossRef]
- He, Y.Y.; Wang, C.Y.; Yuan, Z.X.; Li, X.X.; Yang, W.H.; Song, H.; Li, C.X. Photosynthetic characteristics of Taxodium ascendens and Taxodium distichum under different submergence in the hydro-fluctuation belt of the Three Gorges Reservoir. Acta Ecol. Sin. 2018, 38, 2722–2731. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, C.X.; Wei, H.; Xie, Y.Z.; Han, W.J. Effects of long-term periodic submergence on photosynthesis and growth of Taxodium distichum and Taxodium ascendens saplings in the hydro-fluctuation zone of the Three Gorges Reservoir of China. PLoS ONE 2016, 11, e0162867. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Wei, H.; Geng, Y.H.; Schneider, R. Effects of submergence on photosynthesis and growth of Pterocarya stenoptera (Chinese wingnut) seedlings in the recently-created Three Gorges Reservoir region of China. Wetl. Ecol. Manag. 2010, 18, 485–494. [Google Scholar] [CrossRef]
- Magdziak, Z.; Mleczek, M.; Rutkowski, P.; Goliński, P. Diversity of low-molecular weight organic acids synthesized by Salix growing in soils characterized by different Cu, Pb and Zn concentrations. Acta Physiol. Plant. 2017, 39, 137. [Google Scholar] [CrossRef]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Huang, G.Y.; Guo, G.G.; Yao, S.Y.; Zhang, N.; Hu, H.Q. Organic acids, amino acids compositions in the root exudates and Cu-accumulation in castor (Ricinus communis L.) Under Cu stress. Int. J. Phytoremediat. 2016, 18, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.T.; Qi, Y.P.; Jiang, H.X.; Chen, L.S. Roles of organic acid anion secretion in aluminium tolerance of higher plants. BioMed Res. Int. 2013, 173682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Whalen, J.K.; Cao, Y.H.; Quan, Z.; Lu, C.Y.; Shi, Y. Kinetics of inorganic and organic phosphorus release influenced by low molecular weight organic acids in calcareous, neutral and acidic soils. J. Plant Nutr. Soil Sci. 2015, 178, 555–566. [Google Scholar] [CrossRef]
- Wang, T.; Wei, H.; Zhou, C.; Chen, H.C.; Li, R. Responses of root organic acids and nonstructural carbohydrates of Taxodium distichum to water-level changes in the hydro-fluctuation belt of the Three Gorges Reservoir. Acta Ecol. Sin. 2018, 38, 3004–3013. [Google Scholar] [CrossRef]
- Li, C.X.; Wei, H.; Lv, Q.; Zhang, Y. Effects of Water Stresses on Growth and Contents of Oxalate and Tartarate in the Roots of Chinese Wingnut (Pterocarya stenoptera) Seedlings. For. Res. 2010, 46, 81–88. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Z.X.; Qin, H.W.; Xiong, Y.; Xiang, L.X.; Liu, R.; Yang, Y.; Ma, R. Effects of winter submergence and waterlogging on growth and recovery growth of Salix babylonica. J. South. Agric. 2013, 44, 275–279. [Google Scholar]
- Wang, T.; Wei, H.; Ma, W.C.; Zhou, C.; Chen, H.C.; Li, R.; Li, S. Response of Taxodium distichum to winter submergence in the water-level-fluctuating zone of the Three Gorges Reservoir region. J. Freshw. Ecol. 2019, 34, 1–17. [Google Scholar] [CrossRef]
- Elcan, J.M.; Pezeshki, S.R. Effects of Flooding on Susceptibility of Taxodium distichum L. Seedlings to Drought. Photosynthetica. 2002, 40, 177–182. [Google Scholar] [CrossRef]
- Arif, M.; Zhang, S.L.; Zheng, J.; Wokadala, C.; Mzondi, P.S.; Li, C.X. Evaluating the Effects of Pressure Indicators on Riparian Zone Health Conditions in the Three Gorges Dam Reservoir, China. Forests 2020, 11, 214. [Google Scholar] [CrossRef]
- Ren, Q.S.; Song, H.; Yuan, Z.X.; Ni, X.L.; Li, C.X. Changes in soil enzyme activities and microbial biomass after revegetation in the Three Gorges Reservoir, China. Forests 2018, 9, 249. [Google Scholar] [CrossRef]
- Yamashita, N.; Tanabata, S.; Ohtake, N.; Sueyoshi, K.; Sato, T.; Higuchi, K.; Ohyama, T. Effects of Different Chemical Forms of Nitrogen on the Quick and Reversible Inhibition of Soybean Nodule Growth and Nitrogen Fixation Activity. Front. Plant Sci. 2019, 10, 131. [Google Scholar] [CrossRef]
- Park, S.; Cho, E.; Chung, H.; Cho, K.; Sa, S.; Balasubramanian, B.; Jeong, Y. Digestibility of phosphorous in cereals and co-products for animal feed. Saudi J. Biol. Sci. 2019, 26, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.X.; Zhou, G.M.; Huang, C.; Li, C.X.; Wang, L. Rapid determination of rudimental organic acids in root of Taxodium ascendens and Taxodium distichum by ion-suppression RP-HPLC. Chin. J. Pharm. Anal. 2005, 25, 1082–1085. [Google Scholar]
- Huang, T.Z.; Wang, S.J.; Liu, X.M.; Liu, H.; Wu, Y.Y.; Luo, X.Q. Rapid determination of eight organic acids in plant tissue by sequential extraction and high performance liquid chromatography. Chin. J. Chrom. 2014, 32, 1356–1361. [Google Scholar] [CrossRef][Green Version]
- Field, A.; Miles, J.; Field, Z. Discovering Statistics Using R; SAGE Publications Ltd.: London, UK, 2012. [Google Scholar]
- de Moraes Pontes, J.G.; Vendramini, P.H.; Fernandes, L.S.; de Souza, F.H.; Pilau, E.J.; Eberlin, M.N.; Magnani, R.F.; Wulff, N.A.; Fill, T.P. Mass spectrometry imaging as a potential technique for diagnostic of Huanglongbing disease using fast and simple sample preparation. Sci. Rep. 2020, 10, 13457. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.X.; Zhao, W.N.; Wang, Y.N.; Zhang, L.; Huang, S.C.; Lin, J.X. Metabolomics analysis reveals the alkali tolerance mechanism in puccinellia tenuiflora plants inoculated with arbuscular mycorrhizal fungi. Microorganisms 2020, 8, 327. [Google Scholar] [CrossRef]
- Li, C.X.; Wei, H.; Lv, Q.; Zhang, Y. Effects of different water treatments on growth and contents of secondary metabolites in roots of slash pine (Pinus elliottii Engelm.) seedlings. Acta Ecol. Sin. 2010, 30, 6154–6162. [Google Scholar]
- Liu, Y.H.; Peng, X.X.; Yu, L. Difference in oxalate content between buckwheat and soybean leaves and its possible cause. J. Plant Physiol. Mol. Biol. 2004, 20, 201–208. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, N.F.; Yang, H. Study on the organic acids during the grapes growing. Liquor Mak. 2004, 34, 69–71. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).