Abstract
Anthropogenic elevated nitrogen (N) deposition has an accelerated terrestrial N cycle, shaping soil carbon dynamics and storage through altering soil organic carbon mineralization processes. However, it remains unclear how long-term high N deposition affects soil carbon mineralization in tropical forests. To address this question, we established a long-term N deposition experiment in an N-rich lowland tropical forest of Southern China with N additions such as NH4NO3 of 0 (Control), 50 (Low-N), 100 (Medium-N) and 150 (High-N) kg N ha−1 yr−1, and laboratory incubation experiment, used to explore the response of soil carbon mineralization to the N additions therein. The results showed that 15 years of N additions significantly decreased soil carbon mineralization rates. During the incubation period from the 14th day to 56th day, the average decreases in soil CO2 emission rates were 18%, 33% and 47% in the low-N, medium-N and high-N treatments, respectively, compared with the Control. These negative effects were primarily aroused by the reduced soil microbial biomass and modified microbial functions (e.g., a decrease in bacteria relative abundance), which could be attributed to N-addition-induced soil acidification and potential phosphorus limitation in this forest. We further found that N additions greatly increased soil-dissolved organic carbon (DOC), and there were significantly negative relationships between microbial biomass and soil DOC, indicating that microbial consumption on soil-soluble carbon pool may decrease. These results suggests that long-term N deposition can increase soil carbon stability and benefit carbon sequestration through decreased carbon mineralization in N-rich tropical forests. This study can help us understand how microbes control soil carbon cycling and carbon sink in the tropics under both elevated N deposition and carbon dioxide in the future.
1. Introduction
Anthropogenic-elevated nitrogen (N) deposition, significantly accelerating N cycle on Earth, has become an important driver of global change, especially in the tropics, in the coming decades [1,2]. Due to the coupled relationships between carbon (C) and N cycles, elevated N deposition will inevitably affect the stability of soil organic C (SOC) in terrestrial ecosystems [3], where soils are the largest C reservoir [4]. Soil C stability and sequent C sequestration are tightly related to SOC mineralization, one of the most important processes in the terrestrial ecosystem C cycle. A better understanding of the responses of SOC mineralization to elevated N deposition is essential for better C management under global changes.
Elevated N deposition can increase the terrestrial C sink through increasing net primary productivity in N-limited ecosystems, and even can stimulate soil C sequestration through impeding organic matter decomposition in temperate forests, where N is not limiting microbial growth [5,6,7,8]. In tropical forests, where ecosystems are more likely to be N-rich [9], soil C cycling has not been well-assessed. Tropical forests, with more than half of global forest C stocks, are a globally significant terrestrial C sink, and have a disproportionately large influence on the global C cycle [10,11,12]. Studies in subtropical successional forests showed that the contribution of heterotrophic soil respiration to total soil respiration reached 64% [13], indicating that microbial-driven soil heterotrophic respiration is a critical CO2 flux in the atmosphere in tropical ecosystems [14]. Many studies showed that high N inputs decreased soil respiration, and lower soil pH was suggested to be a key driver due to its negative priming effects [7,15,16,17], pointing to a further understanding of the role of soil C mineralization. In tropical forests, a few studies addressed the responses of organic C mineralization, using soils with one-time N addition [14,18,19] or soils from field N fertilization plots [20]. However, there are still large uncertainties regarding how and to what extent continuing N deposition affects the soil C mineralization process over a longer time-scale (e.g., >10 years), especially in regions with high background N deposition [1]. Understanding of N accumulation effect is urgent in the prediction of soil C stability and sequestration with the globalization of N deposition.
Here, we aim to explore how long-term N deposition (N accumulation effect) with varied N input rates affects soil C mineralization in N-rich tropical ecosystems. In 2002, we chose an N-rich primary tropical forest at the Dinghushan Biosphere Reserve in southern China (23°09′21″ N to 23°11′30″ N and 112°30′39″ E to 112°33′41″ E). The study site was naturally high in N status, as are many lowland tropical forests, and it was already affected by anthropogenic N deposition [21]. In 2009–2010, total atmospheric N deposition was about 48.6 kg N ha−1 y−1 [21]. Soils in the study site are lateritic red earths formed from sandstone, and are highly weathered, with poor soil-buffering capacity [22]. We hypothesize that long-term N additions decrease soil C mineralization in this N-rich forest, considering N-addition-induced soil acidification and alteration in microbial community composition in the studied site [22,23,24] and the global negative effects of nitrogen deposition on soil microbes [25].
2. Materials and Methods
2.1. Study Site
This study was conducted at the Dinghushan Biosphere Reserve (DHSBR), which is an UNESCO’s Man and the Biosphere (MAB) Programme site in the middle of Guangdong Province, southern China (112°10′ E, 23°10′ N). The DHSBR has a monsoon climate, and is in a subtropical/tropical moist forest zone. The annual average precipitation is 1748 mm, from 2002 to 2012, mainly concentrated from April to September [21]. Annual mean relative humidity is 80%. Mean annual temperature is 21.9 °C. The reserve has been experiencing high atmospheric N deposition in precipitation (e.g., commonly >30 kg N ha−1 y−1) since the 1990s. In 2009–2010, total atmospheric N deposition was about 48.6 kg N ha−1·y−1, with a wet N deposition of 34.4 kg N ha−1 y−1 (see Lu et al., 2018 for further references) [21].
We established our research site in 2002 in a mature monsoon evergreen broadleaf forest (primary forest), between 250 and 300 m above sea level. The forest was protected from disturbances related to land-use for >400 years [26], and supports a rich assemblage of plant species, most of which are natives of tropical and subtropical China, including Castanopsis chinensis Hance, Schima superba Chardn. and Champ., Cryptocarya chinensis (Hance) Hemsl., Machilus chinensis (Champ. Ex Benth.) Hemsl., Syzygium rehderianum Merr. & Perry, and Acmena acuminatissima (Blume) Merr. et Perry. Canopy closure is typically above 95% [27]. The topography is heterogeneous, with slopes ranging from 25° to 35°. Soils in the study site are lateritic red earths (Oxisols) formed from sandstone, and are highly weathered, with poor soil-buffering capacity [22]. The mature forest is a typically N-rich ecosystem, as are many lowland tropical forests [21].
2.2. Experimental Treatments
Nitrogen amendments were initiated in July 2003, with four rates: Control (0 N added), Low-N (50 kg N ha−1 y−1, close to the total background atmospheric N deposition), Medium-N (100 kg N ha−1 y−1) and High-N (150 kg N ha−1 y−1). These were based on the atmospheric N deposition rate of the 1990s [21], and the increase expected in the future due to the rapid development of agricultural and industrial activity [1]. In total, there were twelve 10-m × 20-m plots surrounded by buffer strips of at least 20 m in width, with treatments replicated in triplicate and randomly assigned. Monthly applications of NH4NO3 solution were administered by hand to the forest floor of these plots, in the form of 12 equal applications over the whole year. During each application, fertilizer was weighed, mixed with 20 L of deionized water, and applied to each plot, using a backpack sprayer below the canopy. Two passes were made across each plot to ensure an even distribution of fertilizer. The control plots received equal volumes (20 L) of water without N fertilizer.
2.3. Field Sampling and Laboratory Analysis
In December 2017, we took soil samples (upper 0–10 cm depth) from these plots, after removing litter layers. In each plot, soil samples were taken from six randomly selected points. In the laboratory, soils were sieved (2 mm) to remove roots and stones, and mixed thoroughly by hand for subsequent chemical analysis. Soil pH was measured using a soil to water ratio of 1:2.5 (10 g soil and 25 mL deionized water; pH meter: FE28-Standard FiveEasyPlus™, Mettler Toledo instruments Co., Ltd., Greifensee, Switzerland). Dissolved organic C was extracted by the addition of deionized water using fresh soils (soil to water ratio of 1:5). Soil inorganic N was extracted with 2M KCl (soil to water ratio of 1:5) and the filtrates were analyzed for NH4+-N and NO3−-N by colorimetric method using a flow-injection auto-analyzer (Lachat Quik-Chem 8000; Lachat Instrument, Mequon, WI, USA). The method for NH4+-N analysis is based on the Berthelot reaction (Lachat QuikChem Method: 10-107-06-1-C) and the method for NO3−-N analysis uses a copperized cadmium column to reduce nitrate to nitrite (Lachat QuikChem Method: 10-107-04-1-C). Soil microbial biomass C (MBC) and N (MBN) were determined by chloroform fumigation and extraction in 0.5 M K2SO4 by standard procedures [28,29]. The difference in organic C and total N between fumigated and unfumigated samples was assumed to originate from microbial biomass. Both MBC and MBN concentrations were corrected for unrecovered biomass using a k factor of 0.45 [30]. The amount of organic C and total N in the extracts were simultaneously measured with a Total Organic Carbon Analyzer (TOC-5000, Shimadzu Co. Ltd., Kyoto, Japan).
For laboratory incubation, soil subsamples with 20 g oven-dry equivalent weight were placed in each incubation unit (250 mL erlenmeyer flask). The soil water content was adjusted to 60% of field holding capacity, and soils were pre-incubated at 20 °C for 2 days prior to the start of the incubation. Soil CO2 fluxes were measured on days 1, 3, 5, 7, 14, 21, 28, 35, 44, and 58 days of the incubation. Incubation units were covered with white polyethylene plastic wrap to prevent water evaporation. After the polyethylene plastic wrap was removed, the upper air of the flasks was mixed so that the accumulated CO2 was removed. The CO2 concentrations, after the closure of a lid equipped with a syringe, were determined immediately using gas chromatography (Agilent 7890A; Agilent Technologies Inc., Santa Clara, CA, USA). The CO2 flux rate was calculated as the accumulation of CO2 over the given time period (20 min). We calculated microbial metabolic quotient (MMQ), microbial respiration per unit of biomass, which is a critically important parameter in the understanding of microbial controls for carbon cycling, particularly heterotrophic respiration [31].
2.4. Statistical Analyses
Repeated measures analysis of variance (ANOVA) was performed to examine the effects of N treatments on soil CO2 fluxes. One-way ANOVA with Fisher LSD test was employed to identify N-treatment effects on soil microbial and soil parameters. Linear regression analysis was also used to examine the relationships between these parameters. All analyses were conducted using SPSS 16.0 for Windows (SPSS, Chicago, IL, USA) at p < 0.05.
3. Results and Discussion
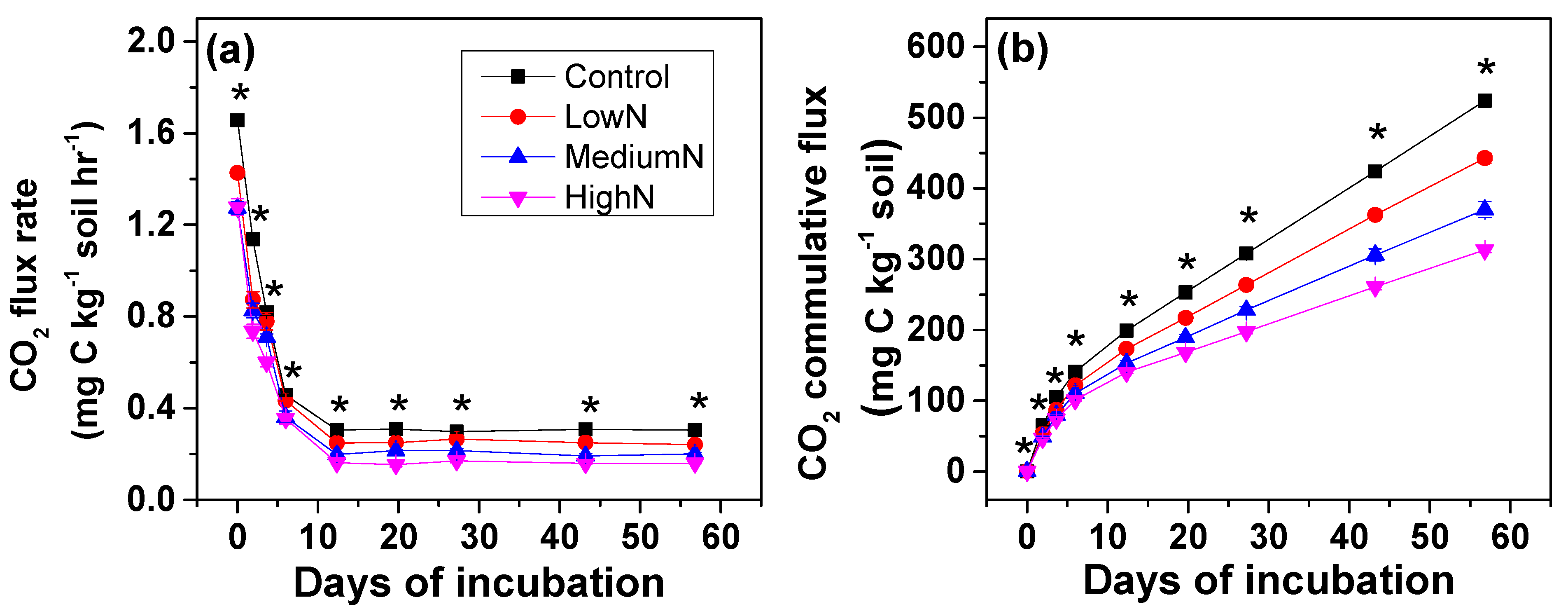
Repeated measures ANOVA further showed that long-term N additions significantly decreased soil C mineralization rates and cumulative CO2 emissions over the incubation period, with the lowest values found in the High-N treatments (Figure 1a,b). Compared to the control plots, soil CO2 emission rates at the beginning of incubation were significantly decreased by 14%–23% after 15 years of N additions (Figure 1a). The CO2 emission rates decreased rapidly in the first week and leveled off over subsequent days. During the period from 14th day to 56th day, the average decreases were 18%, 33% and 47% in the Low-N, Medium-N and High-N treatments, respectively. These findings supported our hypothesis, and gave a plausible explanation of our previous finding that N additions reduced soil respiration in the field monitoring of this N-rich site [15,24] (Table 1).
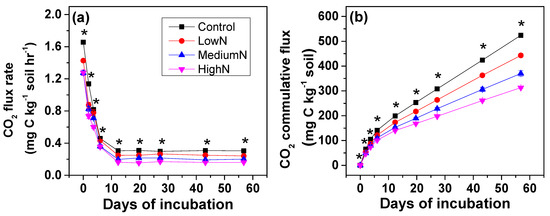
Figure 1.
Effects of long-term N additions on soil organic carbon mineralization rate (a) and CO2 cumulative flux (b) in the N-rich mature tropical forest at Dinghushan reserve. Note: Asterisks (*) means significant differences between Controls and N treatments using planned-contrast analysis; values are shown as means ± standard error (n = 3); h means hour.

Table 1.
Responses of soil carbon mineralization, soil microbial biomass, and soil properties at 0–10 cm layer to long-term N additions in the N-rich mature tropical forest of Southern China.
The soil C mineralization process is largely mediated by soil microbial activity, which depends on both microbial traits and soil environmental conditions. We found that long-term N addition did not alter specific microbial activity (MMQ; Table 1), indicating no changes in microbial C use efficiency [31]. Furthermore, microbial C:N ratios showed no response to N additions, which is consistent with the results of soil C:N ratios [23]. Carbon and N stoichiometric requirements for biomass production force microbial communities to adapt their foraging strategies to the available substrates, which affects the rate of microbial growth and respiration [32]. Hence, we could exclude the potential effect of soil C and N stoichiometry on soil microbes. Our previous study of the same site showed that thirteen years of N additions significantly decreased microbial biomass, with distinct shifts in microbial community composition leading to reductions in the relative abundance of bacteria as well as the genes responsible for cellulose and chitin degradation [23,24]. Hence, we suggest that an N-induced change in microbial biomass and community composition can dominate the soil C mineralization process in N-rich forests (Table 1).
We found that long-term N additions significantly increased soil available N and accelerated soil acidification, and there were significant negative relationships between N treatment rates and soil pH (Table 2). Ecosystem N saturation is a key reason to drive soil acidification under excess N inputs [22,33]. In general, a lower soil pH is known to restrict microbial growth [34,35] and to change the microbial community [23,24,36]. There were significantly positive relationships between microbial biomass and soil pH (Table 2), indicating that accelerated soil acidification contributed to the decline in MBC and MBN under long-term N inputs, which confirmed the results of our recent study [23]. Another reason for the decreased microbial biomass may be phosphorus (P) limitation, which is common for soil microbial processes in moist tropical forests [37,38,39]. Studying P leaching dynamics in this forest, we found that high N inputs enlarged the imbalance between N and P as influxes into soils [40]. Furthermore, experimental P additions significantly increased soil microbial biomass and soil respiration, suggesting that P availability is an important limiting factor for microbial growth in N-rich forests [41]. The decrease in microbial P utilization genes after N amendment suggested that N amendments exacerbated soil P deficiency in this study [24]. Meanwhile, bacterial relative abundance had the largest total effect on soil respiration in this site, and high N treatments decreased the abundances of genes responsible for labile and recalcitrant C [24]. A decrease in the bacteria’s relative abundance could inhibit the production of C degradation enzymes by soil bacterial biomass, providing a straightforward explanation for the measured decrease in CO2 emission under both field and incubation conditions (Table 1).

Table 2.
Pearson correlation coefficients between N treatment, soil carbon mineralization, soil microbial biomass, and soil properties across all the plots.
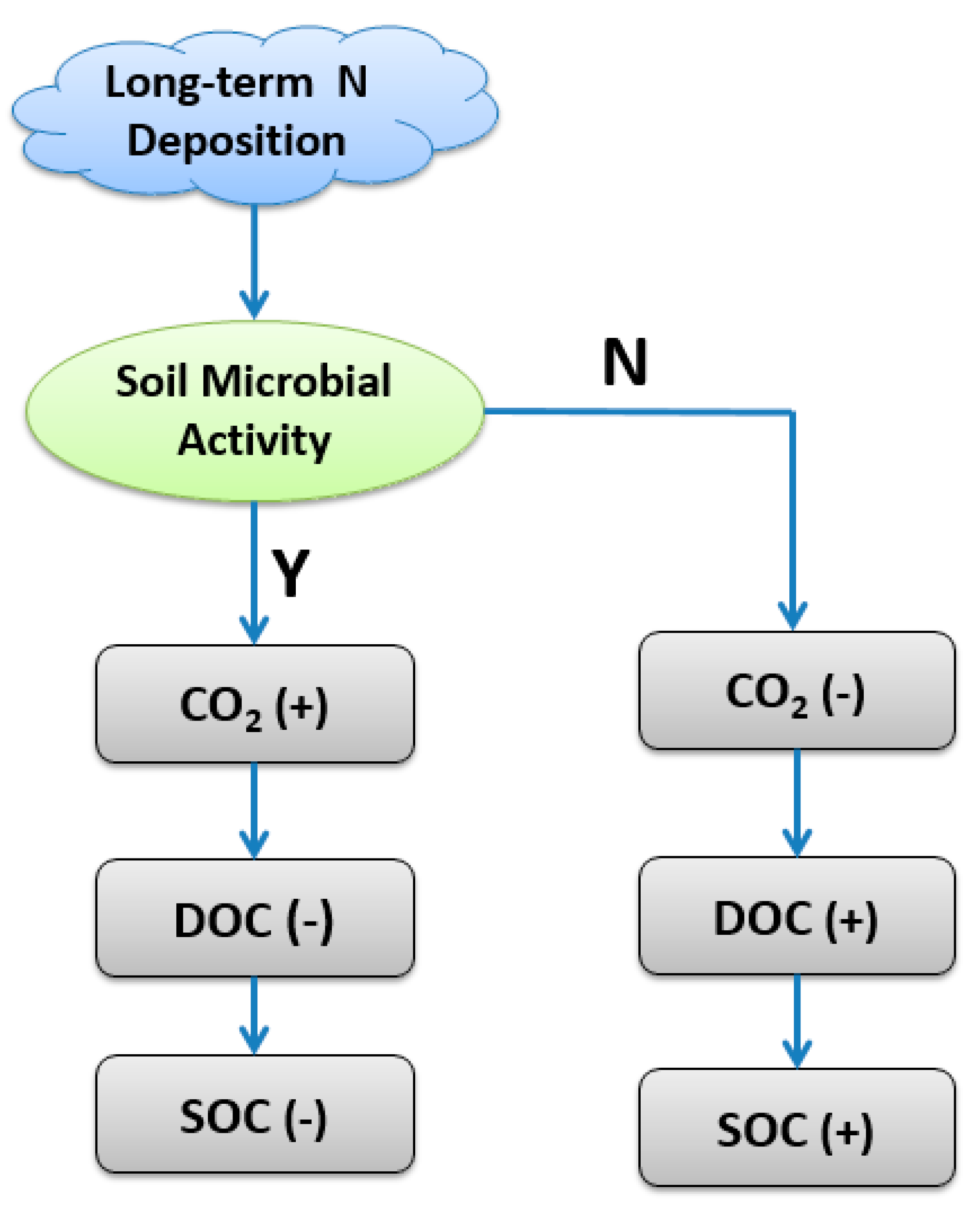
Interestingly, we found that long-term N additions significantly increased soil DOC contents (Table 1), which was a primary source of energy and cellular C for the soil microbial community. Considering that there were no changes in specific microbial activity (MMQ) among treatments (Table 1) and negative relationships between microbial biomass and soil DOC (Table 2), we suggested that decreases in the microbial consumption of soil-soluble C pool or retardation in organic C decomposition should be an important reason for increased soil DOC. DOC is a significant source of organic C in the mineral soil, and contributes to soil-forming processes though its downward movement and adsorption in mineral soil [42,43], which supports the increased SOC used under N treatments in this study (Table 1). Based on our findings and previous study, we developed a conceptual model hypothesis of how long-term N deposition affects soil C soil mineralization and storage through alterations in soil microbial activity, which will determine the fate of soil C and soil C storage potential (Figure 2). In this model, if N deposition increases soil microbial activity, such as microbial biomass and community functions related to C cycling, soil mineralization rates will increase, and more CO2 will be emitted; thus, more C sources, such as DOC, will be consumed by soil microbes, and finally, soil C storage will decrease. Alternatively, if N deposition decreases soil microbial activity (“N”), soil mineralization rates will decrease and CO2 emission will be inhibited, so that soil DOC and SOC will increase compared to the background conditions. Soil microorganisms play a key role in determining the longevity and stability of soil C, and C efflux from soils.
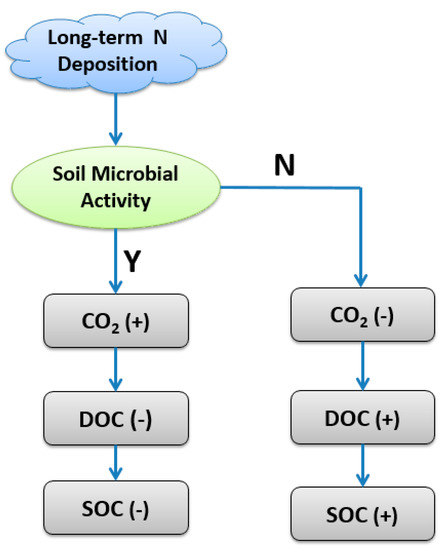
Figure 2.
A conceptual model on how long-term N deposition affects soil C soil mineralization and storage through altering soil microbial activity in both microbial biomass and community functions. Notes: “Y” means positive effects while “N” means negative effects; “+” and “−” mean increasing and decreasing, respectively: DOC, dissolved organic carbon; SOC, soil organic carbon.
4. Conclusions
In summary, we conclude that continuous high N deposition can inhibit soil C mineralization process at more than a decade scale in N-rich forests, which is primarily attributed to the decreased microbial activity in microbial biomass and community functions. This inhibiting effect is beneficial to soil C stabilization and accumulation in forest ecosystems. It is noteworthy that the inhibiting effect increased with elevated N addition, indicating that higher soil C storage may occur with increased N addition, as shown in Table 1. This study can help us to understand how microbes control soil carbon cycling and carbon sink in the tropics under both elevated N deposition and carbon dioxide. However, there are several limitations which need to be overcome in future studies. (1) We mainly focus on soils in the present study, but their interactions with plants and the resources they produce above- and belowground should be considered in the field conditions. (2) The DOC can be recalcitrant and not easily accessible to microbes, and a more detailed analysis of DOC chemistry is required in the future. (3) To confirm whether higher DOC under N fertilization leads to greater C retention, it is necessary to obtain more detailed measurements along the soil profile, over a longer period of time, to understand the fate of DOC. (4) It is worth exploring temporal and spatial (deeper soil layers) dynamics, because N addition may change plant phenology and there could be seasonal differences in resource availability and microbial activity, which could eventually lead to reversed patterns in other parts of the year. Finally, our findings require further verification in different forest ecosystems in the tropics, where intensifying industrial and agricultural activities will enhance atmospheric N deposition to much higher levels in the coming decades [1].
Author Contributions
Conceptualization, X.L.; formal analysis, X.L.; investigation, X.L., Q.M. and Z.W.; data curation, X.L.; writing—original draft preparation, X.L.; writing—review and editing, X.L., Q.M., Z.W., T.M., J.M., F.S. and Z.P.; visualization, X.L.; project administration, X.L. and Z.W.; funding acquisition, X.L. and J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 41922056, 41731176), Key Research and Development Program of Guangdong Province (2020B1111530004), and Youth Innovation Promotion Association CAS (No. Y201965).
Data Availability Statement
Data is available for use upon request.
Acknowledgments
We appreciate Shaoming Cai and Hui Mo, and Xiaoping Pan for their skillful assistance in laboratory and field work, and Dinghushan Forest Ecosystem Research Station for the support in the field work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Hietz, P.; Turner, B.L.; Wanek, W.; Richter, A.; Nock, C.A.; Wright, S.J. Long-Term Change in the Nitrogen Cycle of Tropical Forests. Science 2011, 334, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hou, E.; Guo, J.; Gilliam, F.S.; Li, J.; Tang, S.; Kuang, Y. Nitrogen addition stimulates soil aggregation and enhances carbon storage in terrestrial ecosystems of China: A meta-analysis. Glob. Chang. Biol. 2021, 27, 2780–2792. [Google Scholar] [CrossRef]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Reay, D.S.; Dentener, F.; Smith, P.; Grace, J.; Feely, R.A. Global nitrogen deposition and carbon sinks. Nat. Geosci. 2008, 1, 430–437. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Schulte-Uebbing, L.; de Vries, W. Global-scale impacts of nitrogen deposition on tree carbon sequestration in tropical, temperate, and boreal forests: A meta-analysis. Glob. Chang. Biol. 2018, 24, E416–E431. [Google Scholar] [CrossRef]
- Matson, P.A.; McDowell, W.H.; Townsend, A.R.; Vitousek, P.M. The globalization of N deposition: Ecosystem consequences in tropical environments. Biogeochemistry 1999, 46, 67–83. [Google Scholar] [CrossRef]
- Phillips, O.L.; Malhi, Y.; Higuchi, N.; Laurance, W.F.; Nunez, P.V.; Vasquez, R.M.; Laurance, S.G.; Ferreira, L.V.; Stern, M.; Brown, S.; et al. Changes in the carbon balance of tropical forests: Evidence from long-term plots. Science 1998, 282, 439–442. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Baccini, A.; Walker, W.; Carvalho, L.; Farina, M.; Sulla-Menashe, D.; Houghton, R.A. Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 2017, 358, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Han, T.; Liu, J.; Wang, G.; Zhou, G. Changes in soil respiration components and their specific respiration along three successional forests in the subtropics. Func. Ecol. 2016, 30, 1466–1474. [Google Scholar] [CrossRef]
- Fanin, N.; Barantal, S.; Fromin, N.; Schimann, H.; Schevin, P.; Haettenschwiler, S. Distinct Microbial Limitations in Litter and Underlying Soil Revealed by Carbon and Nutrient Fertilization in a Tropical Rainforest. PLoS ONE 2012, 7, e49990. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.; Zhu, W.; Gundersen, P.; Fang, Y.; Li, D.; Wang, H. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Chang. Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol. Lett. 2010, 13, 819–828. [Google Scholar] [CrossRef]
- Frey, S.D.; Ollinger, S.; Nadelhoffer, K.; Bowden, R.; Brzostek, E.; Burton, A.; Caldwell, B.A.; Crow, S.; Goodale, C.L.; Grandy, A.S.; et al. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 2014, 121, 305–316. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R. Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc. Natl. Acad. Sci. USA 2006, 103, 10316–10321. [Google Scholar] [CrossRef]
- Soong, J.L.; Maranon-Jimenez, S.; Cotrufo, M.F.; Boeckx, P.; Bode, S.; Guenet, B.; Penuelas, J.; Richter, A.; Stahl, C.; Verbruggen, E.; et al. Soil microbial CNP and respiration responses to organic matter and nutrient additions: Evidence from a tropical soil incubation. Soil Biol. Biochem. 2018, 122, 141–149. [Google Scholar] [CrossRef]
- Cusack, D.F.; Torn, M.S.; McDowell, W.H.; Silver, W.L. The response of heterotrophic activity and carbon cycling to nitrogen additions and warming in two tropical soils. Glob. Chang. Biol. 2010, 16, 2555–2572. [Google Scholar] [CrossRef]
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Zhou, G.; Zou, X.; Bai, E.; Scanlon, T.M.; Hou, E.; et al. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest. Proc. Natl. Acad. Sci. USA 2018, 115, 5187–5192. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Mo, J. Nitrogen deposition contributes to soil acidification in tropical ecosystems. Glob. Chang. Biol. 2014, 20, 3790–3801. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.; Mori, T.; Mao, Q.; Zhou, K.; Zhou, G.; Nie, Y.; Mo, J. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.A.J.; Lu, X.; Yang, Y.; Hartley, I.P.; Zhang, W.; Mo, J.; Yu, G.; Zhou, J.; Kuzyakov, Y. Long-term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest soil. Glob. Chang. Biol. 2019, 25, 3267–3281. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.Y.H.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Shen, C.D.; Liu, D.S.; Peng, S.L.; Sun, Y.M.; Jiang, M.T.; Yi, W.X.; Xing, C.P.; Gao, Q.Z.; Li, Z.; Zhou, G.Y. C-14 measurement of forest soils in Dinghushan Biosphere Reserve. Chin. Sci. Bull. 1999, 44, 251–256. [Google Scholar] [CrossRef]
- Lu, X.; Mo, J.; Gilliam, F.S.; Zhou, G.; Fang, Y. Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Glob. Chang. Biol. 2010, 16, 2688–2700. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil-nitrogen-a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass-c. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Brookes, P.C.; Powlson, D.S. Measuring soil microbial biomass. Soil Biol. Biochem. 2004, 36, 5–7. [Google Scholar] [CrossRef]
- Xu, X.; Schimel, J.P.; Janssens, I.A.; Song, X.; Song, C.; Yu, G.; Sinsabaugh, R.L.; Tang, D.; Zhang, X.; Thornton, P.E. Global pattern and controls of soil microbial metabolic quotient. Ecol. Monogr. 2017, 87, 429–441. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Aber, J.; Mcdowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; Mcnulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen Saturation in Temperate Forest Ecosystems. Bioscience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Nilsson, L.O.; Baath, E.; Falkengren-Grerup, U.; Wallander, H. Growth of ectomycorrhizal mycelia and composition of soil microbial communities in oak forest soils along a nitrogen deposition gradient. Oecologia 2007, 153, 375–384. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil pH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Kaspari, M.; Bujan, J.; Weiser, M.D.; Ning, D.; Michaletz, S.T.; He, Z.; Enquist, B.J.; Waide, R.B.; Zhou, J.; Turner, B.L.; et al. Biogeochemistry drives diversity in the prokaryotes, fungi, and invertebrates of a Panama forest. Ecology 2017, 98, 2019–2028. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Schmidt, S.K. Phosphorus limitation of microbial processes in moist tropical forests: Evidence from short-term laboratory incubations and field studies. Ecosystems 2002, 5, 680–691. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Chadwick, H.O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen—Phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Turner, B.L.; Stott, A.W.; Tanner, E.V.J. Nitrogen and phosphorus constrain labile and stable carbon turnover in lowland tropical forest soils. Soil Biol. Biochem. 2015, 80, 26–33. [Google Scholar] [CrossRef]
- Zhou, K.; Lu, X.; Mori, T.; Mao, Q.; Wang, C.; Zheng, M.; Mo, H.; Hou, E.; Mo, J. Effects of long-term nitrogen deposition on phosphorus leaching dynamics in a mature tropical forest. Biogeochemistry 2018, 138, 215–224. [Google Scholar] [CrossRef]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Neff, J.C.; Asner, G.P. Dissolved organic carbon in terrestrial ecosystems: Synthesis and a model. Ecosystems 2001, 4, 29–48. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schwesig, D.; Rethemeyer, J.; Matzner, E. Stabilization of dissolved organic matter by sorption to the mineral soil. Soil Biol. Biochem. 2005, 37, 1319–1331. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).