Mechanical Mastication Reduces Fuel Structure and Modelled Fire Behaviour in Australian Shrub Encroached Ecosystems
Abstract
:1. Introduction
- How does fuel load and structure change with mastication in shrub encroached woodlands and forests?
- Is mastication likely to affect wildfire rates of spread and flame heights?
- What is the impact of mastication on flora species richness and diversity?
2. Materials and Methods
2.1. Study Area
2.2. Fuel Treatment
2.3. Site Selection
2.4. Field Measurements
2.5. Data Analysis
2.6. Fire Modelling
3. Results
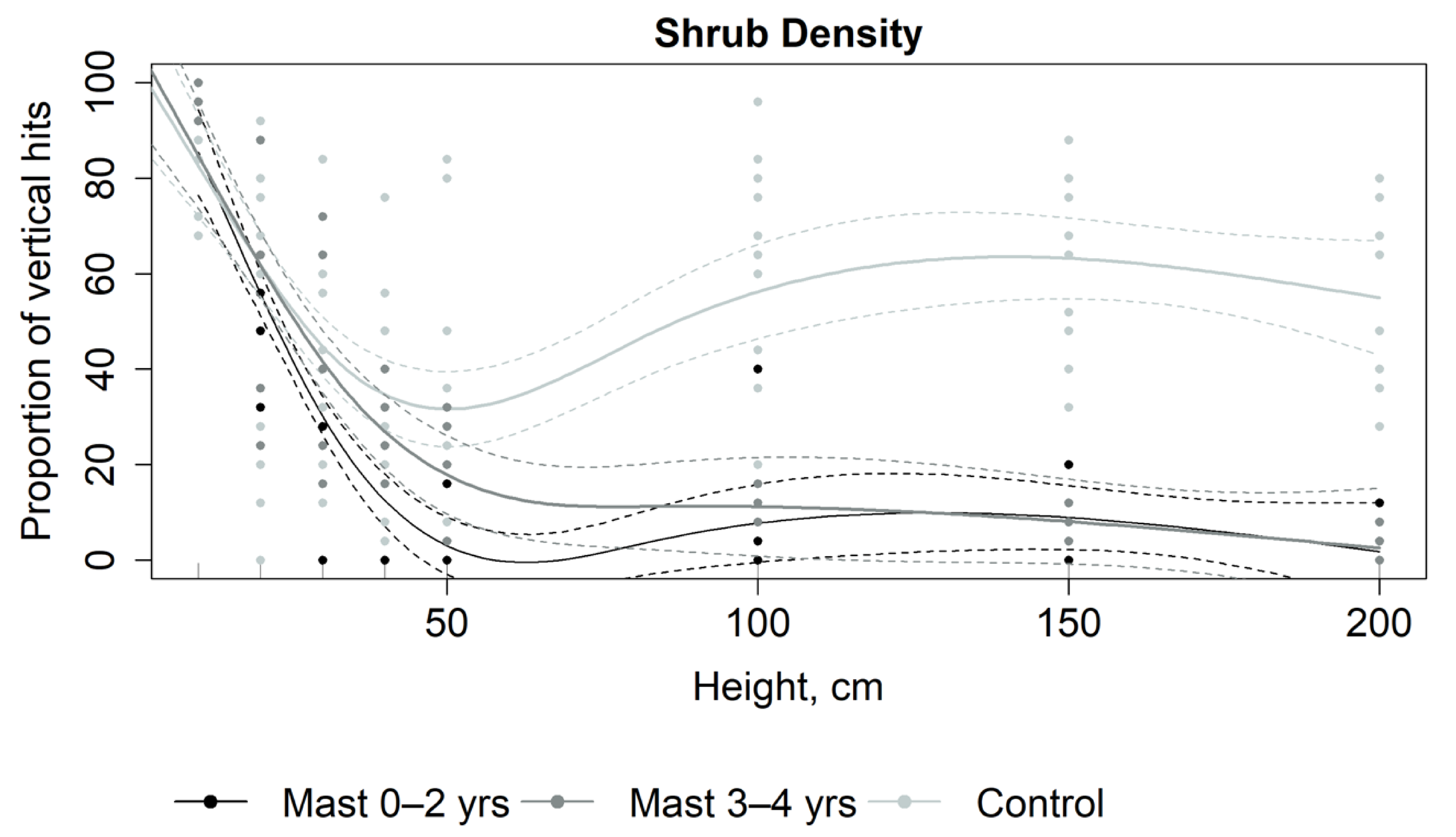
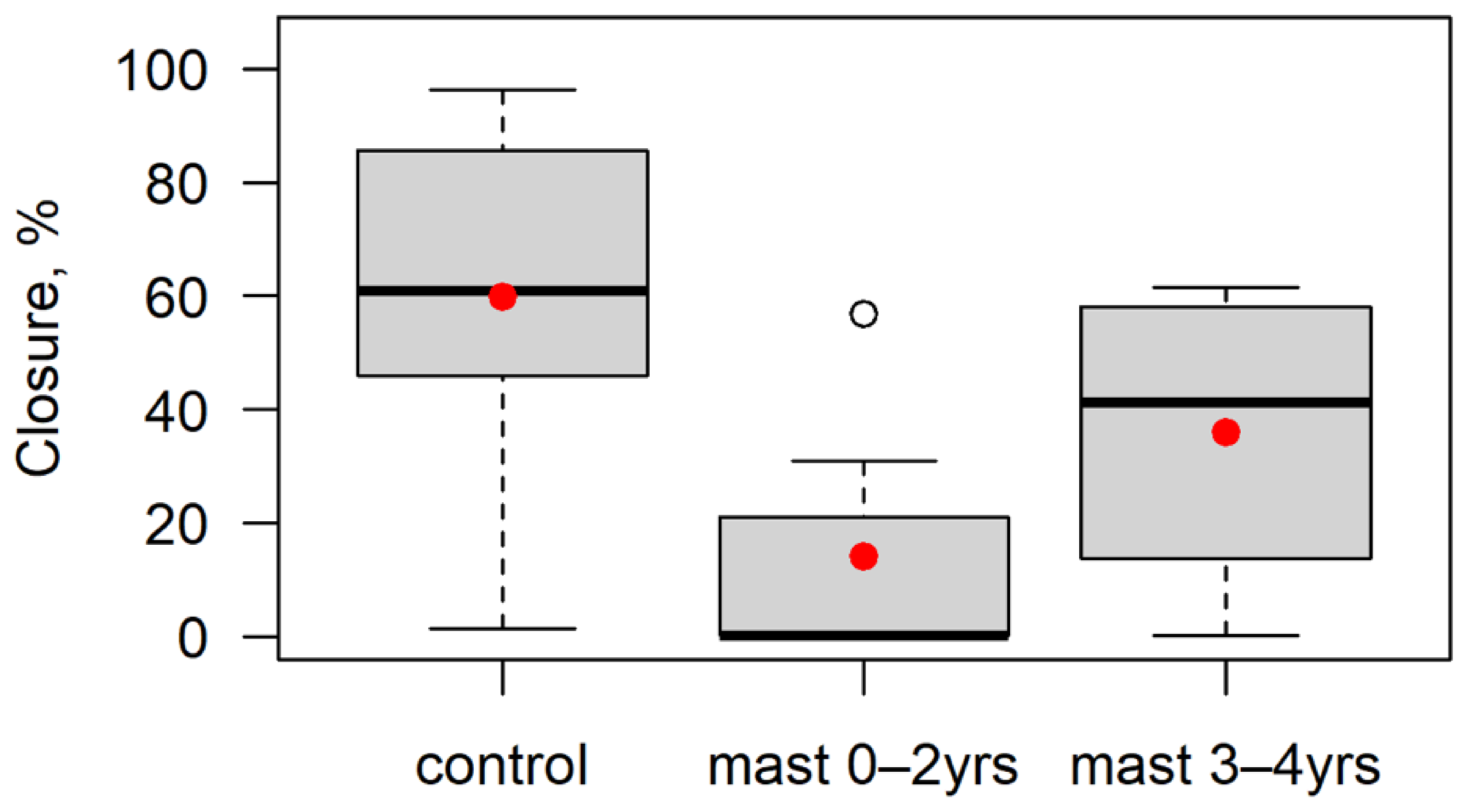
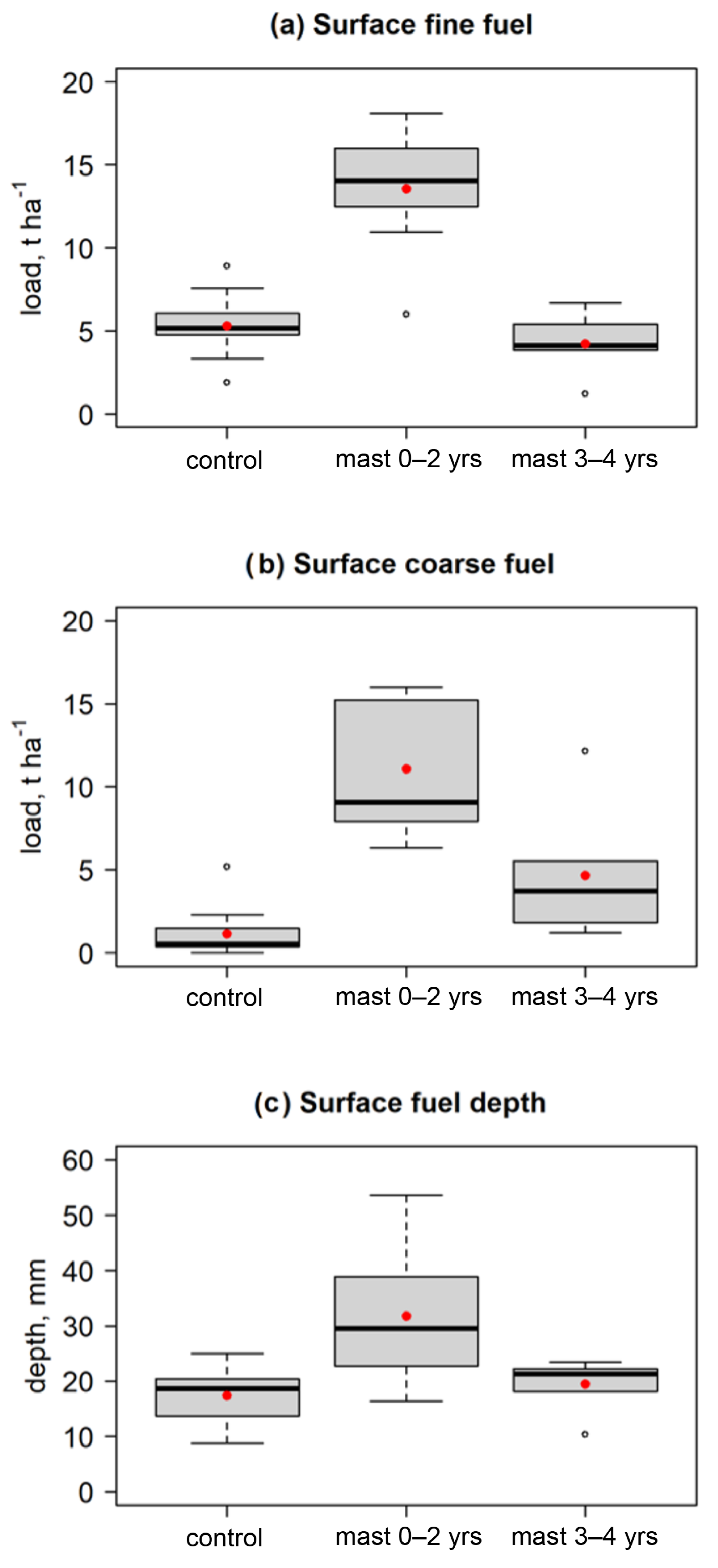
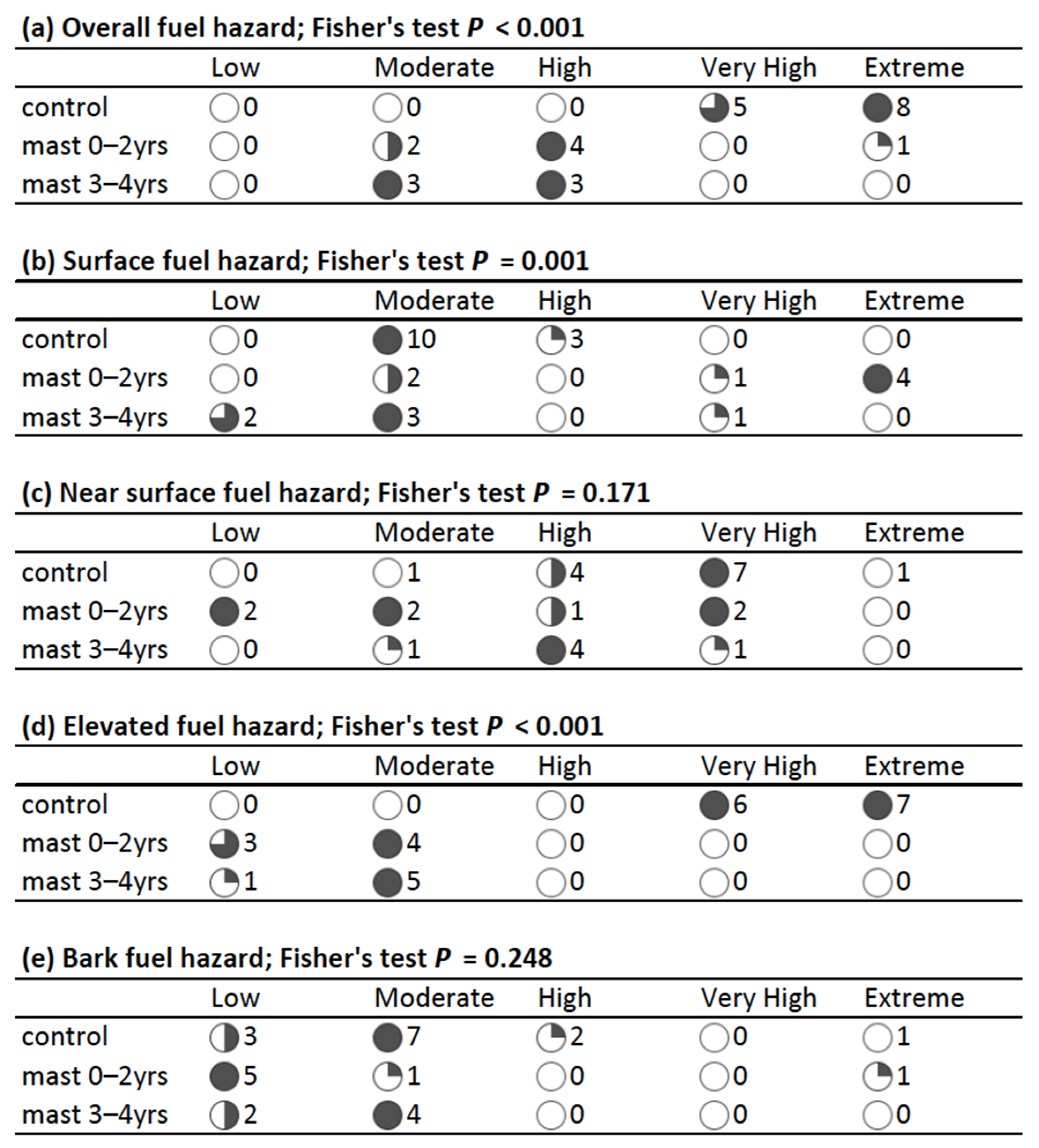
3.1. Fuel Properties
3.2. Wildfire Behaviour
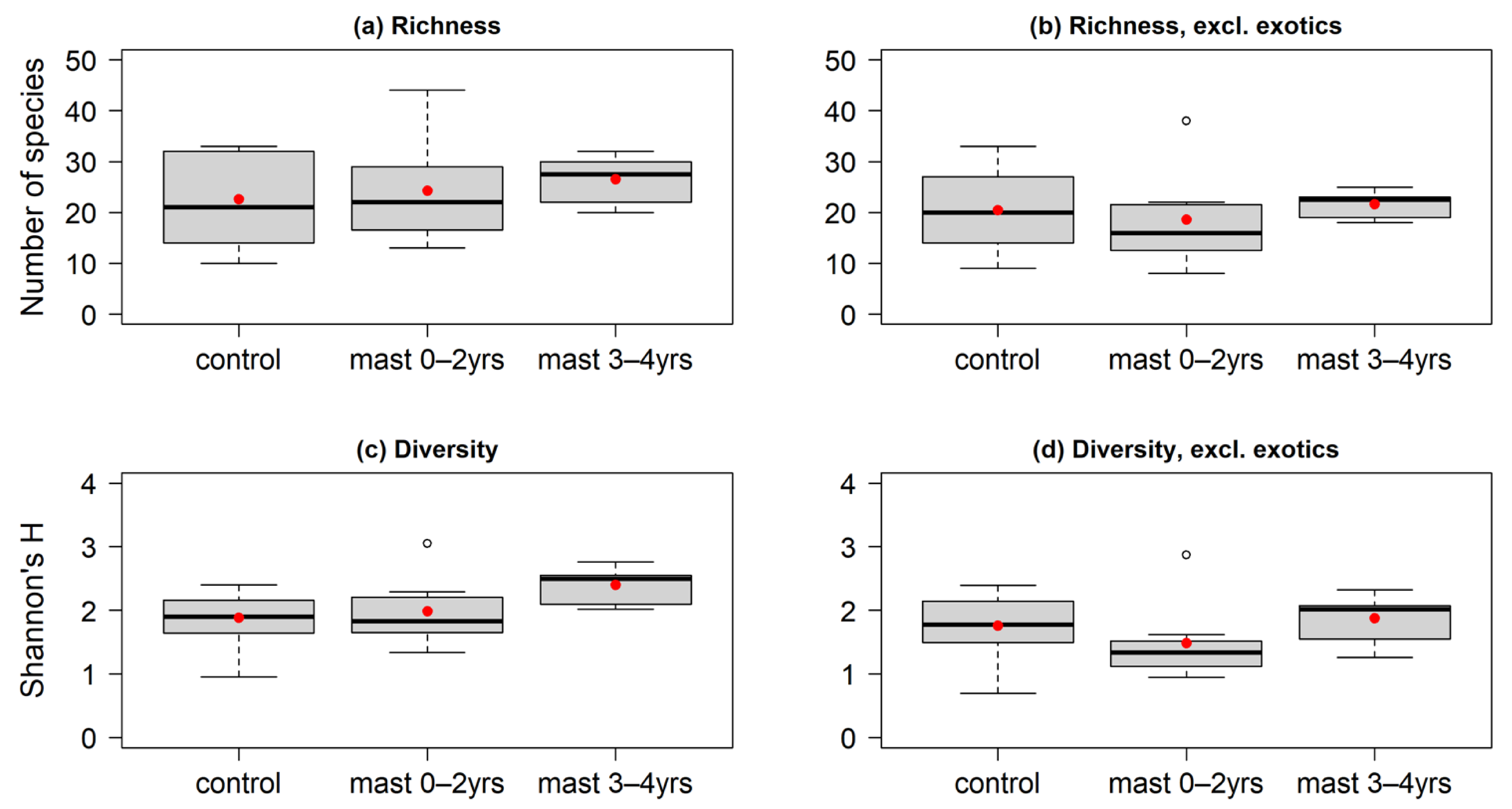
3.3. Floristic Diversity
4. Discussion
4.1. Fuel Load and Structure
4.2. Wildfire Behaviour
4.3. Species Richness and Diversity
4.4. Implications for Fire Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| df | F | p-Value | RP2P (Adjusted) | Deviance Explained | |
|---|---|---|---|---|---|
| control | 2.97 | 316.06 | <0.001 *** | 0.31 | 33% |
| mast 0–2 years | 2.98 | 349.97 | <0.001 *** | 0.79 | 74% |
| mast 3–4 years | 2.97 | 361.48 | <0.001 *** | 0.80 | 81% |
| Mast 0–2 yrs—Control | Mast 3–4 yrs—Control | |||||
|---|---|---|---|---|---|---|
| df | t | p-Value | df | t | p-Value | |
| Vegetation closure | 6 | −6.245 | 0.001 *** | 5 | −2.166 | 0.083 |
| Surface dead fine fuel load | 6 | 5.532 | 0.001 *** | 5 | −0.533 | 0.616 |
| Surface dead coarse fuel load | 6 | 6.871 | <0.001 *** | 5 | 21R W | 0.031 * |
| Surface fuel depth | 6 | 2.304 | 0.061 | 5 | 1.378 | 0.227 |
| Spread rate @ 0 km h−1 wind | 6 | −0.394 | 0.707 | 5 | −1.298 | 0.251 |
| Flame ht @ 0 km h−1 wind | 6 | −4.980 | 0.003 ** | 5 | −4.079 | 0.010 ** |
| Spread rate @ 30 km h−1 wind | 6 | −0.394 | 0.707 | 5 | −1.298 | 0.251 |
| Flame height @ 30 km h−1 wind | 6 | −4.368 | 0.005 ** | 5 | −3.707 | 0.014 * |
| Species richness | 6 | −0.131 | 0.900 | 5 | 1.845 | 0.124 |
| Species richness excl. exotics | 6 | −1.271 | 0.251 | 5 | 1.047 | 0.343 |
| Shannon’s H | 6 | 0.647 | 0.542 | 5 | 3.653 | 0.0147 |
| Shannon’s H excl. exotics | 6 | −1.127 | 0.303 | 5 | 0.454 | 0.669 |
| Cover exotic graminoids | – | 15R W | 0.059 | – | 15R W | 0.059 |
| Cover exotic herbs | 6 | 3.447 | 0.014 * | – | 15R W | 0.059 |
References
- Eldridge, D.J.; Bowker, M.A.; Maestre, F.T.; Roger, E.; Reynolds, J.F.; Whitford, W.G. Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 2011, 14, 709–722. [Google Scholar] [CrossRef]
- Watson, P.A.; Alexander, H.D.; Moczygemba, J.D. Coastal prairie recovery in response to shrub removal method and degree of shrub encroachment. Rangel. Ecol. Manag. 2019, 72, 275–282. [Google Scholar] [CrossRef]
- De Luis, M.; Baeza, M.; Raventos, J.; Hidalgo, J.C.G. Fuel characteristics and fire behaviour in mature Mediterranean gorse shrublands. Int. J. Wildland Fire 2004, 13, 79. [Google Scholar] [CrossRef] [Green Version]
- Gent, M.L.; Morgan, J.W. Changes in the stand structure (1975–2000) of coastal Banksia forest in the long absence of fire. Austral Ecol. 2007, 32, 239–244. [Google Scholar] [CrossRef]
- Emeny, J. Acacia Longifolia Spread in the Glenelg Plain Bioregion: Pattern and Process; Deakin University: Warrnambool, Australia, 2009. [Google Scholar]
- Bennett, L.T. The expansion of Leptospermum laevigatum on the Yanakie Isthmus, Wilson’s Promontory, under changes in the burning and grazing regimes. Aust. J. Bot. 1994, 42, 555–564. [Google Scholar] [CrossRef]
- Costello, D.A.; Lunt, I.D.; Williams, J.E. Effects of invasion by the indigenous shrub Acacia sophorae on plant composition of coastal grasslands in south-eastern Australia. Biol. Conserv. 2000, 96, 113–121. [Google Scholar] [CrossRef]
- Baeza, M.J.; De Luı́s, M.; Raventós, J.; Escarré, A. Factors influencing fire behaviour in shrublands of different stand ages and the implications for using prescribed burning to reduce wildfire risk. J. Environ. Manag. 2002, 65, 199–208. [Google Scholar] [CrossRef]
- Gordon, C.E.; Eldridge, D.J.; Ripple, W.J.; Crowther, M.S.; Moore, B.D.; Letnic, M. Shrub encroachment is linked to extirpation of an apex predator. J. Anim. Ecol. 2017, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Bowman, D. Conservation: Bring elephants to Australia? Nature 2012, 482, 30. [Google Scholar] [CrossRef]
- Archer, S.; Schimel, D.S.; Holland, E.A. Mechanisms of shrubland expansion: Land use, climate or CO2? Clim. Chang. 1995, 29, 91–99. [Google Scholar] [CrossRef]
- Kitzberger, T.; Perry, G.; Paritsis, J.; Gowda, J.; Tepley, A.; Holz, A.; Veblen, T. Fire–vegetation feedbacks and alternative states: Common mechanisms of temperate forest vulnerability to fire in southern South America and New Zealand. N. Z. J. Bot. 2016, 54, 247–272. [Google Scholar] [CrossRef] [Green Version]
- Price, J.N.; Morgan, J.W. Woody plant encroachment reduces species richness of herb-rich woodlands in southern Australia. Austral Ecol. 2008, 33, 278–289. [Google Scholar] [CrossRef]
- O’Loughlin, L.S.; Green, P.T.; Morgan, J.W. The rise and fall ofLeptospermum laevigatum: Plant community change associated with the invasion and senescence of a range-expanding native species. Appl. Veg. Sci. 2014, 18, 323–331. [Google Scholar] [CrossRef]
- Hradsky, B.A.; Loschiavo, J.; Hradsky, M.; Di Stefano, J. Shrub expansion alters forest structure but has little impact on native mammal occurrence. Austral Ecol. 2015, 40, 611–624. [Google Scholar] [CrossRef]
- Carlos, E.H.; Gibson, M. The habitat value of Gorse Ulex europaeus L. and Hawthorn Crataegus monogyna Jacq. for birds in Quarry Hills Bushland Park, Victoria. Vic. Nat. 2010, 127, 115–124. [Google Scholar]
- Anderson, W.R.; Cruz, M.G.; Fernandes, P.; McCaw, L.; Vega, J.A.; Bradstock, R.A.; Fogarty, L.; Gould, J.; McCarthy, G.; Marsden-Smedley, J.B.; et al. A generic, empirical-based model for predicting rate of fire spread in shrublands. Int. J. Wildland Fire 2015, 24, 443–460. [Google Scholar] [CrossRef] [Green Version]
- Moreira, F.; Vaz, P.; Catry, F.; Silva, J.S. Regional variations in wildfire susceptibility of land-cover types in Portugal: Implications for landscape management to minimize fire hazard. Int. J. Wildland Fire 2009, 18, 563–574. [Google Scholar] [CrossRef]
- Hines, F.; Hines, F.; Tolhurst, K.G.; Wilson, A.A.; McCarthy, G.J. Overall Fuel Hazard Assessment Guide; The State of Victoria, Department of Sustainability and Environment: Melbourne, Australia, 2010. [Google Scholar]
- Cheney, N.P.; Gould, J.S.; McCaw, W.L.; Anderson, W.R. Predicting fire behaviour in dry eucalypt forest in southern Australia. For. Ecol. Manag. 2012, 280, 120–131. [Google Scholar] [CrossRef]
- Gould, J.S.; McCaw, W.L.; Cheney, N.P. Quantifying fine fuel dynamics and structure in dry eucalypt forest (Eucalyptus marginata) in Western Australia for fire management. For. Ecol. Manag. 2011, 262, 531–546. [Google Scholar] [CrossRef]
- Weise, D.R.; Zhou, X.; Sun, L.; Mahalingam, S. Fire spread in chaparral—‘Go or no-go’. Int. J. Wildland Fire 2005, 14, 99–106. [Google Scholar] [CrossRef]
- Kreye, J.K.; Brewer, N.W.; Morgan, P.; Varner, J.M.; Smith, A.M.; Hoffman, C.; Ottmar, R.D. Fire behavior in masticated fuels: A review. For. Ecol. Manag. 2014, 314, 193–207. [Google Scholar] [CrossRef]
- Kane, J.M.; Varner, J.M.; Knapp, E.E. Novel fuelbed characteristics associated with mechanical mastication treatments in northern California and south-western Oregon, USA. Int. J. Wildland Fire 2009, 18, 686–697. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, M.A.; Rocca, M.E.; Rhoades, C.C.; Ryan, M.G. Surface fuel loadings within mulching treatments in Colorado coniferous forests. For. Ecol. Manag. 2010, 260, 1557–1566. [Google Scholar] [CrossRef]
- Kreye, J.K.; Kobziar, L. The effect of mastication on surface fire behaviour, fuels consumption and tree mortality in pine flatwoods of Florida, USA. Int. J. Wildland Fire 2015, 24, 573–579. [Google Scholar] [CrossRef]
- Lyon, Z.D.; Morgan, P.; Stevens-Rumann, C.S.; Sparks, A.M.; Keefe, R.F.; Smith, A.M.S. Fire behaviour in masticated forest fuels: Lab and prescribed fire experiments. Int. J. Wildland Fire 2018, 27, 280. [Google Scholar] [CrossRef]
- Hudak, A.T.; Rickert, I.; Morgan, P.; Strand, E.; Lewis, S.A.; Robichaud, P.R.; Hoffman, C.; Holden, Z.A. Review of Fuel Treatment Effectiveness in Forests and Rangelands and a Case Study from the 2007 Megafires in Central Idaho USA; General Technical Report RMRS-GTR-253; USDA Forest Service, Rocky Mountain Research Centre: Fort Collins, CO, USA, 2011. [Google Scholar]
- Graham, R.T.; Jain, T.B.; Loseke, M. Fuel Treatments, Fire Supression, and THEIR INTERACTIONS With Wildfire and Its Effects: The Warm Lake Experience During the Cascade Complex of Wildfires in Central Idaho, 2007; General Technical Report RMRS-GTR-229; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2009. [Google Scholar]
- Franco, J.A.; Morgan, J.W. Using historical records, aerial photography and dendroecological methods to determine vegetation changes in a grassy woodland since European settlement. Aust. J. Bot. 2007, 55, 1–9. [Google Scholar] [CrossRef]
- Lunt, I.D.; Winsemius, L.M.; McDonald, S.P.; Morgan, J.W.; DeHaan, R.L. How widespread is woody plant encroachment in temperate Australia? Changes in woody vegetation cover in lowland woodland and coastal ecosystems in Victoria from 1989 to 2005. J. Biogeogr. 2010, 37, 722–732. [Google Scholar] [CrossRef]
- Ximenes, F.; Stephens, M.; Brown, M.; Law, B.; Mylek, M.R.; Schirmer, J.; Sullivan, Å.; McGuffog, T. Mechanical fuel load reduction in Australia: A potential tool for bushfire mitigation. Aust. For. 2017, 80, 1–11. [Google Scholar] [CrossRef]
- Proctor, E.; McCarthy, G. Changes in fuel hazard following thinning operations in mixed-species forests in East Gippsland, Victoria. Aust. For. 2015, 78, 195–206. [Google Scholar] [CrossRef]
- Volkova, L.; Bi, H.; Hilton, J.; Weston, C.J. Impact of mechanical thinning on forest carbon, fuel hazard and simulated fire behaviour in Eucalyptus delegatensis forest of south-eastern Australia. For. Ecol. Manag. 2017, 405, 92–100. [Google Scholar] [CrossRef]
- Forest and Fire Planning. Impact of Mulching on Fire Behaviour: Skye—Blue Wren Rise Case Study; The State of Victoria, Department of Environment, Land, Water and Planning: Melbourne, Australia, 2018; (Unpublished, internal document). [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Sci. Discuss. 2007, 4, 429–473. [Google Scholar]
- Specht, R. Vegetation. In The Australian Environment; Leeper, G.W., Ed.; CSIRO & Melbourne University Press: Melbourne, Australia, 1970; pp. 44–67. [Google Scholar]
- Richardson, F.J.; Richardson, R.G.; Shepherd, R.C.H. Weeds of the South-East: An Identification Guide for Australia, 3rd ed.; CSIRO: Meredith, Australia, 2016. [Google Scholar]
- Beardsell, D.; Obrien, S.; Williams, E.; Knox, R.; Calder, D. Reproductive biology of Australian Myrtaceae. Aust. J. Bot. 1993, 41, 511–526. [Google Scholar] [CrossRef]
- Gibson, M.R.; Richardson, D.; Marchante, E.; Marchante, H.; Rodger, J.; Stone, G.N.; Byrne, M.; Fuentes-Ramírez, A.; George, N.; Harris, C.; et al. Reproductive biology of Australian acacias: Important mediator of invasiveness? Divers. Distrib. 2011, 17, 911–933. [Google Scholar] [CrossRef]
- Brown, K.L.; Bettnik, K.A. Swan Weeds: Management Notes, FloraBase—The Western Australian Flora. Available online: https://florabase.dpaw.wa.gov.au. (accessed on 31 March 2021).
- Department of Agriculture Water and the Environment. Australia—NVIS Major Vegetation Groups—Version 5.1; 2018, Bioregional Assessment Source Dataset. Available online: https://www.environment.gov.au/land/native-vegetation/national-vegetation-information-system (accessed on 19 August 2019).
- Coop, J.D.; Grant, T.A.; Magee, P.A.; Moore, E.A. Mastication treatment effects on vegetation and fuels in piñon-juniper woodlands of central Colorado, USA. For. Ecol. Manag. 2017, 396, 68–84. [Google Scholar] [CrossRef]
- McColl-Gausden, S.C.; Penman, T.D. Visual Assessment of Surface Fuel Loads Does Not Align with Destructively Sampled Surface Fuels. Forests 2017, 8, 408. [Google Scholar] [CrossRef] [Green Version]
- Department of Environment Land Water and Planning. Reducing Victoria’s Bushfire Risk on Public Land: Fuel Management Report 2014-15; The State of Victoria, Department of Environment Land Water and Planning: Melbourne, Australia, 2016. [Google Scholar]
- Watson, P.J.; Penman, S.H.; Bradstock, R.A. A comparison of bushfire fuel hazard assessors and assessment methods in dry sclerophyll forest near Sydney, Australia. Int. J. Wildland Fire 2012, 21, 755–763. [Google Scholar] [CrossRef]
- Yap, B.W.; Sim, C.H. Comparisons of various types of normality tests. J. Stat. Comput. Simul. 2011, 81, 2141–2155. [Google Scholar] [CrossRef]
- Wood, S.N.; Augustin, N.H. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecol. Model. 2002, 157, 157–177. [Google Scholar] [CrossRef] [Green Version]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Højsgaard, S.; Halekoh, U. doBy: Groupwise Statistics, LSmeans, Linear Contrasts, Utilities. 2018, CRAN R-Project. Available online: https://CRAN.R-project.org/package=doBy (accessed on 7 June 2019).
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. 2019, CRAN R-Project. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 7 June 2019).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical theory of Communication. 1949.; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Kutiel, P.; Kutiel, P. Spatial and Temporal Heterogeneity of Species Diversity in a Mediterranean Ecosystem Following Fire. Int. J. Wildland Fire 1997, 7, 307–315. [Google Scholar] [CrossRef]
- Cruz, M.G.; Gould, J.S.; Alexander, M.E.; Sullivan, A.L.; McCaw, W.L.; Matthews, S. A Guide to Rate of Fire Spread Models for Australian Vegetation; Canberra ACT and AFAC; CSIRO Land and Water Flagship: Melbourne, Australia, 2015; 123p. [Google Scholar]
- Cruz, M.G.; Alexander, M.E.; Sullivan, A.L.; Gould, J.S.; Kilinc, M. Assessing improvements in models used to operationally predict wildland fire rate of spread. Environ. Model. Softw. 2018, 105, 54–63. [Google Scholar] [CrossRef]
- Brennan, T.J.; Keeley, J.E. Effect of mastication and other mechanical treatments on fuel structure in chaparral. Int. J. Wildland Fire 2015, 24, 949. [Google Scholar] [CrossRef]
- Hood, S.; Wu, R. Estimating Fuel Bed Loadings in Masticated Areas. In Fuels Management—How to Measure Success: Conference Proceedings; Andrews, P.L., Butler, B.W., Eds.; Proceedings RMRS-P-41; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006; pp. 333–340. [Google Scholar]
- Potts, J.B.; Marino, E.; Stephens, S.L. Chaparral shrub recovery after fuel reduction: A comparison of prescribed fire and mastication techniques. Plant Ecol. 2010, 210, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Fernández, C.; Vega, J.A. Shrub recovery after fuel reduction treatments in a gorse shrubland in northern Spain. J. Environ. Manag. 2016, 166, 211–216. [Google Scholar] [CrossRef]
- Baeza, M.; Vallejo, V. Vegetation recovery after fuel management in Mediterranean shrublands. Appl. Veg. Sci. 2008, 11, 151–158. [Google Scholar] [CrossRef]
- Rothermel, R.C. A Mathematical Model for Predicting Fire Spread in Wildland Fuels; General Technical Report INT-207; USDA Forest Service, International Forest and Range Experiment Station: Ogden, UT, USA, 1972. [Google Scholar]
- Agee, J.K.; Skinner, C.N. Basic principles of forest fuel reduction treatments. For. Ecol. Manag. 2005, 211, 83–96. [Google Scholar] [CrossRef]
- Syphard, A.D.; Keeley, J.E.; Brennan, T.J. Factors affecting fuel break effectiveness in the control of large fires on the Los Padres National Forest, California. Int. J. Wildland Fire 2011, 20, 764–775. [Google Scholar] [CrossRef]
- Cheney, N.P.; Gould, J.S.; Knight, I. A Prescribed Burning Guide for Young Regrowth Forests of Silvertop Ash, Research Paper No. 16; Forestry Commission of New South Wales: Sydney, Australia, 1992. [Google Scholar]
- Buckley, A.J. Fuel Reducing Regrowth Forests with a Wiregrass Fuel Type: Fire Behaviour Guide and Prescriptions, Research Report No. 40; Fire Management Branch, Department of Conservation and Natural Resources: East Melbourne, Australia, 1993. [Google Scholar]
- Busse, M.D.; Hubbert, K.R.; Fiddler, G.O.; Shestak, C.J.; Powers, R.F. Lethal soil temperatures during burning of masticated forest residues. Int. J. Wildland Fire 2005, 14, 267. [Google Scholar] [CrossRef]
- Moon, K.; Duff, T.; Tolhurst, K. Sub-canopy forest winds: Understanding wind profiles for fire behaviour simulation. Fire Saf. J. 2019, 105, 320–329. [Google Scholar] [CrossRef]
- Schiks, T.; Thompson, D.K.; Wotton, B.M. Short-term effects of mastication on fuel moisture and thermal regime of boreal fuel beds. Can. J. For. Res. 2015, 45, 867–876. [Google Scholar] [CrossRef]
- Ross, M.R.; Castle, S.; Barger, N. Effects of fuels reductions on plant communities and soils in a Piñon-juniper woodland. J. Arid. Environ. 2012, 79, 84–92. [Google Scholar] [CrossRef]
- Kane, J.M.; Varner, J.M.; Knapp, E.E.; Powers, R.F. Understory vegetation response to mechanical mastication and other fuels treatments in a ponderosa pine forest. Appl. Veg. Sci. 2010, 13, 207–220. [Google Scholar] [CrossRef]
- Brennan, T.J.; Keeley, J.E. Impacts of Mastication Fuel Treatments on California, USA, Chaparral Vegetation Structure and Composition. Fire Ecol. 2017, 13, 120–138. [Google Scholar] [CrossRef] [Green Version]
- Fornwalt, P.; Rocca, M.E.; Battaglia, M.A.; Rhoades, C.C.; Ryan, M.G. Mulching fuels treatments promote understory plant communities in three Colorado, USA, coniferous forest types. For. Ecol. Manag. 2017, 385, 214–224. [Google Scholar] [CrossRef]
- Wolk, B.; Rocca, M.E. Thinning and chipping small-diameter ponderosa pine changes understory plant communities on the Colorado Front Range. For. Ecol. Manag. 2009, 257, 85–95. [Google Scholar] [CrossRef]
- Potts, J.B.; Stephens, S.L. Invasive and native plant responses to shrubland fuel reduction: Comparing prescribed fire, mastication, and treatment season. Biol. Conserv. 2009, 142, 1657–1664. [Google Scholar] [CrossRef]
- Davis, N.E.; Di Stefano, J.; Coulson, G.; Whelan, J.; Wright, J. Vegetation management influences habitat use by mammalian herbivores in shrub-encroached grassy woodland. Wildl. Res. 2016, 43, 438. [Google Scholar] [CrossRef]
- Ayers, D.; Melville, G.; Bean, J.; Beckers, D.; Ellis, M.; Mazzer, T.; Freudenberger, D. Woody Weeds, Biodiversity and Landscape Function in Western New South Wales; WEST 2000: Dubbo, Australia, 2001. [Google Scholar]
- Clarke, D.J.; White, J.G. Recolonisation of powerline corridor vegetation by small mammals: Timing and the influence of vegetation management. Landsc. Urban Plan. 2008, 87, 108–116. [Google Scholar] [CrossRef]
- Clarke, H.; Evans, J. Exploring the future change space for fire weather in southeast Australia. Theor. Appl. Clim. 2018, 136, 513–527. [Google Scholar] [CrossRef] [Green Version]
- Flannigan, M.D.; Wotton, B.M.; Marshall, G.A.; De Groot, W.J.; Johnston, J.; Jurko, N.; Cantin, A.S. Fuel moisture sensitivity to temperature and precipitation: Climate change implications. Clim. Chang. 2015, 134, 59–71. [Google Scholar] [CrossRef]
- Vitorelo, B.; Han, H.-S.; Varner, J. Masticators for fuel reduction treatment: Equipment options, effectiveness, costs, and environmental impacts. In Proceedings of the 2006 Council on Forest Engineering (COFE) meeting, Lake Tahoe, CA, USA, 15 June 2009. [Google Scholar]
- Penman, T.D.; Nicholson, A.E.; Bradstock, R.A.; Collins, L.; Penman, S.H.; Price, O.F. Reducing the risk of house loss due to wildfires. Environ. Model. Softw. 2015, 67, 12–25. [Google Scholar] [CrossRef]



| Site | UTM Grid Zones | Region | Year | Area | Plant | Follow Up | Shrub Species | Veg Class B |
|---|---|---|---|---|---|---|---|---|
| West-Soup Trk | H 55 E 0436507 N 5693030 | Wilsons Prom | 2018 | 0.5 ha | 110 Terex Positrack | N/A | Coast Teatree | Coastal shrubland |
| Pohlners Rd | H 54 E 0624050 N 5913495 | Grampians | 2018 | Unkn | 110 Terex Positrack | N/A | Sallow Wattle | Open forest |
| Tamarisk Dr | H 55 E 0339318 N 5779151 | Mornington Peninsula | 2018 | 2 ha | Skid Steer | N/A | Coast Teatree | Woodland |
| Tecoma Rd | H 54 E 0552896 N 5751114 | Far South West | 2017 | 0.5 ha | Unspec. | N/A | Coast Wattle | Woodland |
| Copper-mine Trk | H 54 E 0626409 N 5912772 | Grampians | 2017 | Unkn | 110 Terex Positrack | N/A | Sallow Wattle | Woodland |
| Pipeline Trk | H 55 E 0254627 N 5747230 | Otway Plain | 2017 | 8 ha | 100 HP Skid SteerS | N/A | Coast Wattle | Woodland |
| PMR Cave Rd | H 54 E 0499452 N 5795604 | Far South West | 2016 | 1.5 ha | Unspec. | N/A | Coast Wattle | Woodland |
| Mt Zero Rd | H 54 E 0623371 N 5917246 | Grampians | 2015 | Unkn | 110 Terex Positrack | Glyphosate Cut/Paint | Sallow Wattle | Woodland |
| Roses Gap Rd | H 54 E 0630116 N 5908325 | Grampians | 2015 | Unkn | 110 Terex Positrack | N/A | Sallow Wattle | Woodland |
| Darnley Trk | H 55 E 0340993 N 5779495 | Mornington Peninsula | 2015 | 7 ha | Skid Steer | Broad leaf Spot Spray | Coast Teatree | Woodland |
| Odonahue Rd | H 55 N 0252422 E 5742949 | Otway Plain | 2014 | 2 ha | 100 HP Skid Steer S | Glyphosate Cut/Paint | Coast Wattle | Woodland |
| Arthurs Seat | H 55 E 0320957 N 5753328 | Mornington Peninsula | 2014 | 1 ha | Walking excavator | Broad leaf Spot spray | Coast Teatree | Open forest |
| Waterfall Gully Rd | H 55 E 0319490 N 5750218 | Mornington Peninsula | 2014 | 7 ha | Walking excavator | Broad leaf Spot spray | Coast Teatree | Open forest |
| Variables | Units | Description |
|---|---|---|
| Shrub density | % | Percent of vertical fuel present at 8 height increments |
| Vegetation closure | % | Mean vegetation closure taken from a measurement at each corner per plot |
| Fuel hazard rating | – | Rating from low to extreme for each fuel strata (bark, elevated, near-surface, surface) and overall fuel hazard rating as per the OFH Assessment Guide [19] |
| Surface fuel depth | mm | Mean fuel depth taken from 12 measurements per plot |
| Surface fuel load | t ha−1 | Mean surface fuel load taken from four measurements per plot, two size classes: Dead fine (<6 mm diameter)/Dead coarse (6 ≥ diameter < 25 mm) |
| Species richness | – | Number of species recorded per plot |
| Shannon’s diversity(H’) | – | Index of species diversity derived from species cover abundance recorded per plot. It reflects the relative contribution of each species, where an even distribution of abundance among species receives a higher value of diversity [53,54]. |
| Species richness—excl. exotics | - | Number of species recorded per plot, excluding non-native species |
| Shannon’s diversity (H’)—excl. exotics | - | Index of species diversity derived from species cover recorded per plot, excluding non-native species |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grant, M.A.; Duff, T.J.; Penman, T.D.; Pickering, B.J.; Cawson, J.G. Mechanical Mastication Reduces Fuel Structure and Modelled Fire Behaviour in Australian Shrub Encroached Ecosystems. Forests 2021, 12, 812. https://doi.org/10.3390/f12060812
Grant MA, Duff TJ, Penman TD, Pickering BJ, Cawson JG. Mechanical Mastication Reduces Fuel Structure and Modelled Fire Behaviour in Australian Shrub Encroached Ecosystems. Forests. 2021; 12(6):812. https://doi.org/10.3390/f12060812
Chicago/Turabian StyleGrant, Madeleine A., Thomas J. Duff, Trent D. Penman, Bianca J. Pickering, and Jane G. Cawson. 2021. "Mechanical Mastication Reduces Fuel Structure and Modelled Fire Behaviour in Australian Shrub Encroached Ecosystems" Forests 12, no. 6: 812. https://doi.org/10.3390/f12060812
APA StyleGrant, M. A., Duff, T. J., Penman, T. D., Pickering, B. J., & Cawson, J. G. (2021). Mechanical Mastication Reduces Fuel Structure and Modelled Fire Behaviour in Australian Shrub Encroached Ecosystems. Forests, 12(6), 812. https://doi.org/10.3390/f12060812





