How to Increase Biodiversity of Saproxylic Beetles in Commercial Stands through Integrated Forest Management in Central Europe
Abstract
:1. Introduction
- What is the optimal volume of deadwood?
- What provides effective enrichment?
- How to maintain the continuity of deadwood?
- Where is the best place to start enrichment?
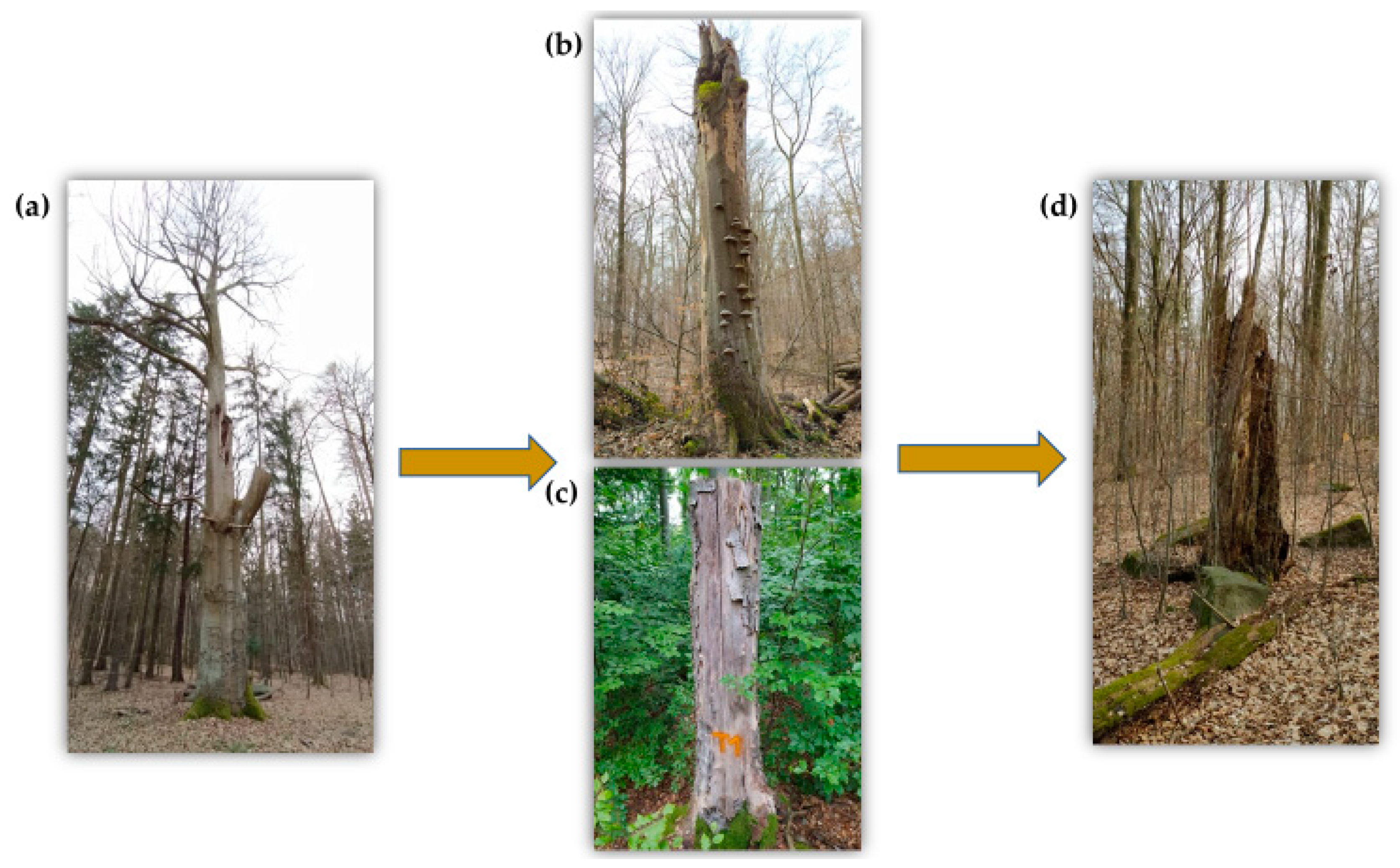
2. Analysis
2.1. What Is the Optimal Constant Volume of Deadwood?
2.2. How to Effectively Enrich the Stands
2.3. Maintaining Constant Volume Continuity
2.4. Where to Enrich the Stands?
3. Synthesis—A Case Study of Active Management
3.1. Norway Spruce
3.2. European Beech
3.3. Oak
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Bače, R.; Svoboda, M. Management Mrtvého Dřeva v Hospodářských Lesích; Certifikovaná metodika; VÚLHM: Strnady, Czechia, 2016; 44p. [Google Scholar]
- Vítková, L.; Bače, R.; Kjučukov, P.; Svoboda, M. Deadwood management in Central European forests: Key considerations for practical implementation. For. Ecol. Manag. 2018, 429, 394–405. [Google Scholar] [CrossRef]
- Doerfler, I.; Müller, J.; Gossner, M.M.; Hofner, B.; Weisser, W. Success of a deadwood enrichment strategy in production forests depends on stand type and management intensity. For. Ecol. Manag. 2017, 400, 607–620. [Google Scholar] [CrossRef]
- Doerfler, I.; Gossner, M.M.; Müller, J.; Seibold, S.; Weisser, W. Deadwood enrichment combining integrative and segregative conservation elements enhances biodiversity of multiple taxa in managed forests. Biol. Conserv. 2018, 228, 70–78. [Google Scholar] [CrossRef]
- Doerfler, I.; Cadotte, M.W.; Weisser, W.W.; Müller, J.; Gossner, M.M.; Heibl, C.; Bässler, C.; Thorn, S.; Seibold, S. Restoration-oriented forest management affects community assembly patterns of deadwood-dependent organisms. J. Appl. Ecol. 2020, 57, 2429–2440. [Google Scholar] [CrossRef]
- Roth, N.; Doerfler, I.; Bässler, C.; Blaschke, M.; Bussler, H.; Gossner, M.M.; Heideroth, A.; Thorn, S.; Weisser, W.W.; Müller, J. Decadal effects of landscape-wide enrichment of dead wood on saproxylic organisms in beech forests of different historic management intensity. Divers. Distribut. 2019, 25, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Leidinger, J.; Weisser, W.W.; Kienlein, S.; Blaschke, M.; Jung, K.; Kozak, J.; Fischer, A.; Mosandl, R.; Michler, B.; Ehrhardt, M.; et al. Formerly managed forest reserves complement integrative management for biodiversity conservation in temperate European forests. Biol. Conserv. 2020, 242, 108437. [Google Scholar] [CrossRef]
- Speight, M. Saproxylic invertebrates and their conservation. Council of Europe. Nat. Environ. Ser. 1989, 42, 79p. [Google Scholar]
- Alexander, K.N.A. Tree biology and saproxylic coleoptera: Issues of definitions, and conservation language. Revue d´Écologie la Terre et la vie 2008, 63 (Suppl. 10), 9–13. [Google Scholar]
- Jaworski, T.; Plewa, R.; Tarwacki, G.; Sućko, K.; Hilszczański, J.; Horák, J. Ecologically similar saproxylic beetles depend on diversified deadwood resources: From habitat requirements to management implications. For. Ecol. Manag. 2019, 449, 117462. [Google Scholar] [CrossRef]
- Stokland, J.N.; Tomter, S.M.; Söderberg, U. Development of dead wood indicators for biodiversity monitoring: Experiences from Scandinavia. Monit. Indic. For. Biodivers. Eur. Ideas Oper. 2004, 51, 207–226. [Google Scholar]
- Davies, Z.G.; Tyler, C.; Stewart, G.; Pullin, A. Are current management recommendations for saproxylic invertebrates effective? A systematic review. Biodivers. Conserv. 2008, 17, 209–234. [Google Scholar] [CrossRef] [Green Version]
- Boddy, L.; Watkinson, S.C. Wood decomposition, higher fungi, and their role in nutrient redistribution. Can. J. Botan. 1995, 73, 1377–1383. [Google Scholar] [CrossRef]
- Janovský, L.; Vágner, A.; Apltauer, J. The decomposition of wood mass under conditions of climax spruce stands and related mycoflora in the Krkonoše Mountains. J. For. Sci. 2002, 48, 70–79. [Google Scholar] [CrossRef]
- Baldrian, P.; Valášková, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weslien, J.; Djupström, L.; Schroeder, M.; Widenfalk, O. Long-term priority effects among insects and fungi colonizing decaying wood. J. Anim. Ecol. 2011, 80, 1155–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofstetter, R.; Dinkins-Bookwalter, J.; Davis, T.S.; Klepzig, K.D. Symbiotic Associations of Bark Beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 209–245. [Google Scholar] [CrossRef]
- Vogel, S.; Alvarez, B.; Bässler, C.; Müller, J.; Thorn, S. The Red-belted Bracket (Fomitopsis pinicola) colonizes spruce trees early after bark beetle attack and persists. Fungal Ecol. 2017, 27, 182–188. [Google Scholar] [CrossRef]
- Horák, J. Ochrana saproxylického hmyzu: Chceme řešit příčiny nebo pouze následky? In Brouci Vázaní na Dřeviny—Beetles Associated with Trees; Sborník referátů; Horák, J., Ed.; Česká Lesnická Společnost: Pardubice, Czech Republic, 2008; pp. 14–17. [Google Scholar]
- Nieto, A.; Alexander, K.N.A. European Red List of Saproxylic Beetles; Publications Office of the European Union: Luxembourg, 2010; 45p. [Google Scholar]
- Parisi, F.; Frate, L.; Lombardi, F.; Tognetti, R.; Campanaro, A.; Biscaccianti, A.B.; Marchetti, M. Diversity Patterns Of Coleoptera and Saproxylic Communities in Unmanaged Forests of Mediterranean Mountains. Ecol. Indic. 2020, 110. [Google Scholar] [CrossRef]
- Horák, J.; Kout, J.; Vodka, Š.; Donato, D. Dead wood dependent organisms in one of the oldest protected forests of Europe: Investigating the contrasting effects of within-stand variation in a highly diversified environment. For. Ecol. Manag. 2016, 363, 229–236. [Google Scholar] [CrossRef]
- Krása, A. Ochrana Saproxylického Hmyzu a Opatření na Jeho Podporu; Metodika AOPK ČR; vyd. –Agentura ochrany přírody a krajiny České republiky: Praha, Czechia, 2015; 156p, ISBN 978-80-88076-15-5. [Google Scholar]
- Vodka, S.; Konvicka, M.; Cizek, L. Habitat preferences of oak-feeding xylophagous beetles in a temperate woodland: Implications for forest history and management. J. Insect Conserv. 2009, 13, 553–562. [Google Scholar] [CrossRef]
- Vogel, S.; Bussler, H.; Finnberg, S.; Müller, J.; Stengel, E.; Thorn, S. Diversity and conservation of saproxylic beetles in 42 European tree species: An experimental approach using early successional stages of branches. Insect Conserv. Divers. 2021, 14, 132–143. [Google Scholar] [CrossRef]
- Ranius, T.; Jansson, N. The influence of forest regrowth, original canopy cover and tree size on saproxylic beetles associated with old oaks. Biol. Conser. 2000, 95, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Widerberg, M.K.; Ranius, T.; Drobyshev, I.; Nilsson, U.; Lindbladh, M. Increased openness around retained oaks increases species richness of saproxylic beetles. Biodivers. Conserv. 2012, 21, 3035–3059. [Google Scholar] [CrossRef]
- Horák, J.; Rébl, K. The species richness of click beetles in ancient pasture woodland benefits from a high level of sun exposure. J. Insect Conserv. 2013, 17, 307–318. [Google Scholar] [CrossRef]
- Sebek, P.; Vodka, S.; Bogusch, P.; Pech, P.; Tropek, R.; Weiss, M.; Zimova, K.; Cizek, L. Open-grown trees as key habitats for arthropods in temperate woodlands: The diversity, composition, and conservation value of associated communities. For. Ecol. Manag. 2016, 380, 172–181. [Google Scholar] [CrossRef]
- Mertlik, J. Review of the saproxylic click-beetles (Coleoptera: Elateridae) in Eastern Bohemia (Czech Republic), with special emphasis on species of the oak forests. Elateridarium 2017, 11, 17–110. [Google Scholar]
- Horak, J.; Vodka, S.; Kout, J.; Halda, J.P.; Bogusch, P.; Pech, P. Biodiversity of most dead wood-dependent organisms in thermophilic temperate oak woodlands thrives on diversity of open landscape structures. For. Ecol. Manag. 2014, 315, 80–85. [Google Scholar] [CrossRef]
- Horák, J.; Pavliček, J.; Kout, J.; Halda, J. Winners and losers in the wilderness: Response of biodiversity to the abandonment of ancient forest pastures. Biodivers. Conserv. 2018, 27, 3019–3029. [Google Scholar] [CrossRef]
- Gossner, M.M.; Lachat, T.; Brunet, J.; Isacsson, G.; Bouget, C.; Brustel, H.; Brandl, R.; Weisser, W.W.; Müller, J. Current Near-to-Nature Forest Management Effects on Functional Trait Composition of Saproxylic Beetles in Beech Forests. Conserv. Biol. 2013, 27, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, L.; Hannerz, M.; Koivula, M.; Shorohova, E.; Vanha-Majamaa, I.; Weslien, J. Research on retention forestry in Northern Europe. Ecol. Proces. 2020, 9, 3. [Google Scholar] [CrossRef]
- Aggestam, F.; Konczal, A.; Sotirov, M.; Wallin, I.; Paillet, Y.; Spinelli, R.; Lindner, M.; Derks, J.; Hanewinkel, M.; Winkel, G. Can nature conservation and wood production be reconciled in managed forests? A review of driving factors for integrated forest management in Europe. J. Environ. Manag. 2020, 268, 9. [Google Scholar] [CrossRef] [PubMed]
- Dieler, J.; Uhl, E.; Biber, P.; Müller, J.; Rötzer, T.; Pretzsch, H. Effect of forest stand management on species composition, structural diversity, and productivity in the temperate zoneof Europe. Eur. J. For. Res. 2017, 136, 739–766. [Google Scholar] [CrossRef]
- Stadelmann, G.; Bugmann, H.; Wermelinger, B.; Meier, F.; Bigler, C. A predictive framework to assess spatio-temporal variability of infestations by the European spruce bark beetle. Ecography 2013, 36, 1208–1217. [Google Scholar] [CrossRef]
- Netherer, S.; Matthews, B.; Katzensteiner, K.; Blackwell, E.; Henschke, P.; Hietz, P.; Pennerstorfer, J.; Rosner, S.; Kikuta, S.; Schume, H.; et al. Do water-limiting conditions predispose Norway spruce to bark beetle attack? New Phytol. 2015, 205, 1128–1141. [Google Scholar] [CrossRef]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.J.; Carroll, A.; Forster, B.; Grégoire, J.-C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce Forests. Ecography 2017, 40, 1426–1435. [Google Scholar] [CrossRef]
- Matthews, B.; Netherer, S.; Katzensteiner, K.; Pennerstorfer, J.; Blackwell, E.; Henschke, P.; Hietz, P.; Rosner, S.; Jansson, P.-E.; Schume, H.; et al. Transpiration deficits increase host susceptibility to bark beetle attack: Experimental observations and practical outcomes for Ips typographus hazard assessment. Agric. For. Meteorol. 2018, 263, 69–89. [Google Scholar] [CrossRef]
- Plieninger, T.; Hartel, T.; Martin-Lopez, B.; Beaufoy, G.; Bergmeier, E.; Kirby, K.; Montero, M.J.; Moreno, G.; Oteros-Rozas, E.; Van Uytvanck, J. Wood-pastures of Europe: Geographic coverage, social–ecological values, conservation management, and policy implications. Biol. Conserv. 2015, 190, 70–79. [Google Scholar] [CrossRef]
- Plieninger, T.; Levers, C.; Mantel, M.; Costa, A.; Schaich, H.; Kuemmerle, T. Patterns and Drivers of Scattered Tree Loss in Agricultural Landscapes: Orchard Meadows in Germany (1968–2009). PLoS ONE 2015, 10, e0126178. [Google Scholar] [CrossRef]
- Müller, J.; Bütler, R. A review of habitat thresholds for dead wood: A baseline for management recommendations in European forests. Eur. J. For. Res. 2010, 129, 981–992. [Google Scholar] [CrossRef]
- Thorn, S.; Seibold, S.; Leverkus, A.B.; Michler, T.; Müller, J.; Noss, R.F.; Stork, N.; Vogel, S.; Lindenmayer, D.B. The living dead: Acknowledging life after tree death to stop forest degradation. Front. Ecol. Environ. 2020, 18, 505–512. [Google Scholar] [CrossRef]
- Ranius, T.; Fahrig, L. Targets for maintenance of dead wood for biodiversity conservation based on extinction thresholds. Scand. J. For. Res. 2006, 21, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.; Brustel, H.; Brin, A.; Bussler, H.; Bouget, C.; Obermaier, E.; Heidinger, I.M.M.; Lachat, T.; Förster, B.; Horak, J.; et al. Increasing temperature may compensate for lower amounts of dead wood in driving richness of saproxylic beetles. Ecography 2015, 38, 499–509. [Google Scholar] [CrossRef]
- Lachat, T.; Chumak, M.; Chumak, V.; Jakoby, O.; Müller, J.; Tanadini, M.; Wermelinger, B.; Didham, R.; Jonsell, M. Influence of canopy gaps on saproxylic beetles in primeval beech forests: A case study from the Uholka-Shyrokyi Luh forest, Ukraine. Insect Conserv. Divers. 2016, 9, 559–573. [Google Scholar] [CrossRef]
- Brin, A.; Bouget, C.; Brustel, H.; Jactel, H. Diameter of downed woody debris does matter for saproxylic beetle assemblages in temperate oak and pine forests. J. Insect Conserv. 2011, 15, 653–669. [Google Scholar] [CrossRef]
- Brin, A.; Valladares, L.; Ladet, S.; Bouget, C. Effects of forest continuity on flying saproxylic beetle assemblages in small woodlots embedded in agricultural landscapes. Biodivers. Conserv. 2016, 25, 587–602. [Google Scholar] [CrossRef]
- Sandström, J.; Bernes, C.; Junninen, K.; Lõhmus, A.; Macdonald, E.; Müller, J.; Jonsson, B.G.; Mukul, S. Impacts of dead wood manipulation on the biodiversity of temperate and boreal forests. A systematic review. J. Appl. Ecol. 2019, 56, 1770–1781. [Google Scholar] [CrossRef] [Green Version]
- Cours, J.; Larrieu, L.; Lopez-Vaamonde, C.; Müller, J.; Parmain, G.; Thorn, S.; Bouget, C. Contrasting responses of habitat conditions and insect biodiversity to pest—Or climate-induced dieback in coniferous mountain forests. For. Ecol. Manag. 2021, 482. [Google Scholar] [CrossRef]
- Müller, J.; Engel, H.; Blaschke, M. Assemblages of woodinhabiting fungi related to silvicultural management intensity in beech forests in southern Germany. Eur. J. For. Res. 2007, 126, 513–527. [Google Scholar] [CrossRef]
- Atrena, A.; Banelytė, G.G.; Læssøe, T.; Riis-Hansen, R.; Bruun, H.H.; Rahbek, C.; Heilmann-Clausen, J. Quality of substrate and forest structure determine macrofungal richness along a gradient of management intensity in beech forests. For. Ecol. Manag. 2020, 478, 118512. [Google Scholar] [CrossRef]
- Økland, B.; Bakke, A.; Hagvar, S.; Kvamme, T. What factors influence the diversity of saproxylic beetles? A multi scaled study from a spruce forest in southern Norway. Biodivers. Conserv. 1996, 5, 75–100. [Google Scholar] [CrossRef]
- Sverdrup-Thygeson, A. Can ‘continuity indicator species’ predict species richness or red-listed species of saproxylic beetles? Biodivers. Conserv. 2001, 10, 815–832. [Google Scholar] [CrossRef]
- Janssen, P.; Fuhr, M.; Cateau, E.; Nusillard, B.; Bouget, C. Forest continuity acts congruently with stand maturity in structuring the functional composition of saproxylic beetles. Biol. Conserv. 2017, 205, 1–10. [Google Scholar] [CrossRef]
- Haase, V.; Topp, W.; Zach, P. Eichen-Totholz im Wirtschaftswald als Lebensraum fur xylobionte Insekten. Z. Okologie Nat. 1998, 7, 137–153. [Google Scholar]
- Procházka, J.; Schlaghamerský, J. Does dead wood volume affect saproxylic beetles in montane beechfir forests of Central Europe? J. Insect Conserv. 2019, 23, 157–173. [Google Scholar] [CrossRef]
- Penttilä, R.; Siitonen, J.; Kuusinen, M. Polypore diversity in managed and old-growth boreal Picea abies forests in southern Finland. Biol. Conserv. 2004, 117, 271283. [Google Scholar] [CrossRef]
- Müller, J.; Bußler, H.; Kneib, T. Saproxylic beetle assemblages related to silvicultural management intensity and stand structures in a beech forest in Southern Germany. J. Insect Conserv. 2008, 12, 107–124. [Google Scholar] [CrossRef]
- Friess, N.; Müller, J.C.; Aramendi, P.; Bässler, C.; Brändle, M.; Bouget, C.; Brin, A.; Bussler, H.; Georgiev, K.B.; Gil, R.; et al. Arthropod communities in fungal fruitbodies are weakly structured by climate and biogeography across European beech forests. For. Ecol. Manag. 2019, 25, 783–796. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.; Ulyshen, M.; Seibold, S.; Cadotte, M.; Chao, A.; Bässler, C.; Vogel, S.; Hagge, J.; Weiß, I.; Baldrian, P.; et al. Primary Determinants Of Communities In Deadwood Vary Among Taxa But Are Regionally Consistent. Oikos 2020, 129, 1579–1588. [Google Scholar] [CrossRef]
- Vogel, S.; Gossner, M.M.; Mergner, U.; Müller, J.; Thorn, S.; Cheng, L. Optimizing Enrichment Of Deadwood For Biodiversity By Varying Sun Exposure And Tree Species: An Experimental Approach. J. Appl. Ecol. 2020, 57, 2075–2085. [Google Scholar] [CrossRef]
- Lachat, T.; Wermelinger, B.; Gossner, M.M.; Bussler, H.; Isacsson, G.; Müller, J. Saproxylic Beetles As Indicator Species For Dead-Wood Amount And Temperature In European Beech Forests. Ecol. Indic. 2012, 23, 323–331. [Google Scholar] [CrossRef]
- Seibold, S.; Bässler, C.; Brandl, R.; Büche, B.; Szallies, A.; Thorn, S.; Ulyshen, M.D.; Müller, J.; Baraloto, C. Microclimate and Habitat Heterogeneity As The Major Drivers of Beetle Diversity In Dead Wood. J. Appl. Ecol. 2016, 53, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Přívětivý, T.; Adam, D.; Vrška, T. Decay Dynamics of Abies Alba And Picea Abies Deadwood in Relation to Environmental Conditions. For. Ecol. Manag. 2018, 427, 250–259. [Google Scholar] [CrossRef]
- Hararuk, O.; Kurz, W.A.; Didion, M. Dynamics of Dead Wood Decay In Swiss Forests. For. Ecosyst. 2020, 7. [Google Scholar] [CrossRef]
- Bouget, C.; Larrieu, L.; Brin, A. Key Features For Saproxylic Beetle Diversity Derived From Rapid Habitat Assessment In Temperate Forests. Ecol. Indic. 2014, 36, 656–664. [Google Scholar] [CrossRef]
- Kraus, D.; Krumm, F. Integrative Approaches as an Opportunity for the Conservation of Forest Biodiversity; European Forest Institute: Joensuu, Finland, 2013; 284p. [Google Scholar]
- Lassauce, A.; Lieutier, F.; Bouget, C. Woodfuel Harvesting And Biodiversity Conservation in Temperate Forests: Effects of Logging Residue Characteristics On Saproxylic Beetle Assemblages. Biol. Conserv. 2012, 147, 204–212. [Google Scholar] [CrossRef]
- Macagno, A.L.M.; Hardersen, S.; Nardi, G.; Lo Giudice, G.; Mason, F. Measuring Saproxylic Beetle Diversity In Small And Medium Diameter Dead Wood: The "Grab-And-Go" Method. Eur. J. Èntomol. 2015, 112, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Puletti, N.; Canullo, R.; Mattioli, W.; Gawryś, R.; Corona, P.; Czerepko, J. A Dataset Of Forest Volume Deadwood Estimates For Europe. Ann. For. Sci. 2019, 76, 68. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity Differences between Managed and Unmanaged Forests: Meta-Analysis of Species Richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef]
- Thorn, S.; Bässler, C.; Brandl, R.; Burton, P.J.; Cahall, R.; Campbell, J.L.; Castro, J.; Choi, C.-Y.; Cobb, T.; Donato, D.C.; et al. Impacts of salvage logging on biodiversity: A meta-analysis. J. Appl. Ecol. 2018, 55, 279–289. [Google Scholar] [CrossRef]
- Fridman, J.; Walheim, M. Amount, structure, and dynamics of dead wood on managed forestland in Sweden. For. Ecol. Manag. 2000, 131, 23–36. [Google Scholar] [CrossRef]
- Siitonen, I. Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol. Bull. 2001, 49, 11–41. [Google Scholar]
- Christensen, M.; Hahn, K.; Mountford, E.P.; Ódor, P.; Standovár, T.; Rozenbergar, D.; Diaci, J.; Wijdeven, S.; Meyer, P.; Winter, S.; et al. Dead wood in European beech (Fagus sylvatica) forest reserves. For. Ecol. Manag. 2005, 210, 267–282. [Google Scholar] [CrossRef]
- Vašíček, J. (Ed.) Národní Inventarizace Lesů v České Republice 2001–2004; ÚHUL: Brandýs nad Labem, Czech Republic, 2007. [Google Scholar]
- Kučera, M.; Adolt, R. (Eds.) Národní inventarizace lesů v České republice—výsledky druhého cyklu 2011–2015 [online]. Vydání první. Brandýs nad Labem: Ústav pro hospodářskou úpravu lesů Brandýs nad Labem. 2019. Available online: http://nil.uhul.cz/downloads/kniha_nil2_web.pdf (accessed on 20 June 2021).
- Bujoczek, L.; Bujoczek, M.; Zięba, S. How much, why and where? Deadwood in forest ecosystems: The case of Poland. Ecol. Indic. 2021, 121, 107027. [Google Scholar] [CrossRef]
- Haeler, E.; Bergamini, A.; Blaser, S.; Ginzler, C.; Hindenlang, K.; Keller, C.; Kiebacher, T.; Kormann, U.G.; Scheidegger, C.; Schmidt, R.; et al. Saproxylic species are linked to the amount and isolation of dead wood across spatial scales in a beech forest. Landsc. Ecol. 2021, 36, 89–104. [Google Scholar] [CrossRef]
- Jonsell, M.; Widenfalk, L.; Hellqvist, S. Substrate specificity among Diptera in decaying bioenergy wood: Can they be conserved by the same measures as are currently applied to beetles? Biodivers. Conserv. 2020, 29, 2623–2662. [Google Scholar] [CrossRef]
- Ettwein, A.; Korner, P.; Lanz, M.; Lachat, T.; Kokko, H.; Pasinelli, G. Habitat selection of an old-growth forest specialist in managed forests. Anim. Conserv. 2020, 23, 547–560. [Google Scholar] [CrossRef] [Green Version]
- Dudley, N.; Vallauri, D. Restoration of Deadwood as a Critical Microhabitat in Forest Landscapes. For. Restor. Landsc. 2006, 203–207. [Google Scholar] [CrossRef]
- Seibold, S.; Brandl, R.; Buse, J.; Hothorn, T.; Schmidl, J.; Thorn, S.; Müller, J. Association of extinction risk of saproxylic beetles with ecological degradation of forests in Europe. Conserv. Biol. 2015, 29, 382–390. [Google Scholar] [CrossRef]
- Kappes, H.; Topp, W. Coleoptera from dead wood in a managed broadleaved forest in Central Europe. Biodivers. Conserv. 2004, 13, 1905–1924. [Google Scholar] [CrossRef]
- Brin, A.; Brustel, H.; Jactel, H. Species variables or environmental variables as indicators of forest biodiversity: A case study using saproxylic beetles in Maritime pine plantations. Ann. For. Sci. 2009, 66, 306. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.; Bußler, H.; Bense, U.; Brustel, H.; Flechtner, G.; Fowles, A.; Kahlen, M.; Möller, G.; Mühle, H.; Schmidl, J.; et al. Urwald relict species—Saproxylic beetles indicating structural qualities and habitat tradition. Wald. Online 2005, 2, 106–113. [Google Scholar]
- Buse, J.; Ranius, T.; Assmann, T. An Endangered Longhorn Beetle Associated with Old Oaks and Its Possible Role as an Ecosystem Engineer. Conserv. Biol. 2008, 22, 329–337. [Google Scholar] [CrossRef]
- Cizek, L.; Schlaghamerský, J.; Bořucký, J.; Hauck, D.; Helešic, J. Range expansion of an endangered beetle: Alpine Longhorn Rosalia alpina (Coleoptera: Cerambycidae) spreads to the lowlands of Central Europe. EÈntomol. Fenn. 2009, 20, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Eckelt, A.; Müller, J.; Bense, U.; Brustel, H.; Bußler, H.; Chittaro, Y.; Cizek, L.; Frei, A.; Holzer, E.; Kadej, M.; et al. “Primeval forest relict beetles” of Central Europe: A set of 168 umbrella species for the protection of primeval forest remnants. J. Insect Conserv. 2017, 22, 15–28. [Google Scholar] [CrossRef]
- Kostanjsek, F.; Sebek, P.; Baranova, B.; Jelaska, L.S.; Riedl, V.; Cizek, L. Size matters! Habitat preferences of the wrinkled bark beetle, Rhysodes sulcatus, the relict species of European primeval forests. Insect Conserv. Divers. 2018, 11, 545–553. [Google Scholar] [CrossRef]
- Lonsdale, D.; Pautasso, M.; Holdenrieder, O. Wood-decaying fungi in the forest: Conservation needs and management options. Eur. J. For. Res. 2007, 127, 1–22. [Google Scholar] [CrossRef]
- Kirby, K.; Reid, C.; Thomas, R.; Goldsmith, F. Preliminary estimates of fallen dead wood and standing dead trees in managed and unmanaged forests in Britain. J. Appl. Ecol. 1998, 35, 148–155. [Google Scholar] [CrossRef]
- Hardersen, S.; Macagno, A.L.M.; Chiari, S.; Audisio, P.; Gasparini, P.; Giudice, G.L.; Nardi, G.; Mason, F. Forest management, canopy cover and geographical distance affect saproxylic beetle communities of small-diameter beech deadwood. For. Ecol. Manag. 2020, 467, 118152. [Google Scholar] [CrossRef]
- Schiegg, K. Saproxylic insect diversity of beech: Limbs are richer than trunks. For. Ecol. Manag. 2001, 149, 295–304. [Google Scholar] [CrossRef]
- Dufour-Pelletier, S.; Tremblay, J.A.; Hébert, C.; Lachat, T.; Ibarzabal, J. Testing the Effect of Snag and Cavity Supply on Deadwood-Associated Species in a Managed Boreal Forest. Forests 2020, 11, 424. [Google Scholar] [CrossRef] [Green Version]
- Jonsell, M.; Weslien, J. Felled or standing retained wood—it makes a difference for saproxylic beetles. For. Ecol. Manag. 2003, 175, 425–435. [Google Scholar] [CrossRef]
- Jonsell, M.; Nittérus, K.; Stighäll, K. Saproxylic beetles in natural and man-made deciduous high stumps retained for conservation. Biol. Conserv. 2004, 118, 163–173. [Google Scholar] [CrossRef]
- Lindhe, A.; Lindelöw, Å.; Åsenblad, N. Saproxylic Beetles in Standing Dead Wood Density in Relation to Substrate Sun-exposure and Diameter. Biodivers. Conserv. 2005, 14, 3033–3053. [Google Scholar] [CrossRef]
- Berg, A.; Ehnström, B.; Gustafsson, L.; Hallingbäck, T.; Jonsell, M.; Weslien, J. Threatened plant, animal, and fungus species in Swedish forests—Distribution andhabitat associations. Conserv. Biol. 1994, 8, 718–731. [Google Scholar] [CrossRef]
- Bouget, C.; Nusillard, B.; Pineau, X.; Ricou, C. Effect of deadwood position on saproxylic beetles in temperate forests and conservation interest of oak snags. Insect Conserv. Divers. 2011, 5, 264–278. [Google Scholar] [CrossRef]
- Paillet, Y.; Archaux, F.; Boulanger, V.; Debaive, N.; Fuhr, M.; Gilg, O.; Gosselin, F.; Guilbert, E. Snags and large trees drive higher tree microhabitat densities in strict forest reserves. For. Ecol. Manag. 2017, 389, 176–186. [Google Scholar] [CrossRef]
- Rosenvald, R.; Lõhmus, P.; Rannap, R.; Remm, L.; Rosenvald, K.; Runnel, K.; Lõhmus, A. Assessing long-term effectiveness of green-tree retention. For. Ecol. Manag. 2019, 448, 543–548. [Google Scholar] [CrossRef]
- Vuidot, A.; Paillet, Y.; Archaux, F.; Gosselin, F. Influence of tree characteristics and forest management on tree microhabitats. Biol. Conserv. 2011, 144, 441–450. [Google Scholar] [CrossRef]
- Taylor, S.L.; MacLean, D.A. Dead wood dynamics in declining balsam fir and spruce stands in New Brunswick, Canada. Can. J. For. Res. 2007, 37, 750–762. [Google Scholar] [CrossRef]
- Vacek, S.; Vacek, Z.; Bílek, L.; Hejcmanová, P.; Štícha, V.; Remeš, J. The dynamics and structure of dead wood in natural spruce-beech forest stand—A 40 year case study in the Krkonoše National Park. Dendrobiol. 2015, 73, 21–32. [Google Scholar] [CrossRef]
- Harmon, M.E.; Fasth, B.G.; Yatskov, M.; Kastendick, D.; Rock, J.; Woodall, C.W. Release of coarse woody detritus-related carbon: A synthesis across forest biomes. Carbon Balance Manag. 2020, 15, 1. [Google Scholar] [CrossRef]
- Sverdrup-Thygeson, A.; Skarpaas, O.; Ødegaard, F. Hollow oaks and beetle conservation: The significance of the surroundings. Biodivers. Conserv. 2009, 19, 837–852. [Google Scholar] [CrossRef]
- Pilskog, H.E.; Birkemoe, T.; Framstad, E.; Sverdrup-Thygeson, A. Effect of Habitat Size, Quality, and Isolation on Functional Groups of Beetles in Hollow Oaks. J. Insect Sci. 2016, 16, 26. [Google Scholar] [CrossRef]
- Parmain, G.; Bouget, C. Large solitary oaks as keystone structures for saproxylic beetles in European agricultural landscapes. Insect Conserv. Divers. 2018, 11, 100–115. [Google Scholar] [CrossRef] [Green Version]
- Hort, L.; Vrška, T. Podíl odumřelého dřeva v pralesovitých útvarech ČR. In Význam a Funkce Odumřelého Dřeva v Lesních Porostech: Česká Lesnická Společnost Pobočka Pro Silva Bohemica; VRŠKA, T., Ed.; Vydala Správa Národního parku Podyjí: Znojmo, Czechia, 1999; pp. 75–87. ISBN 80-238-4739-2. [Google Scholar]
- Larrieu, L.; Cabanettes, A.; Gouix, N.; Burnel, L.; Bouget, C.; Deconchat, M. Post-harvesting dynamics of the deadwood profile: The case of lowland beech-oak coppice-with-standards set-aside stands in France. Eur. J. For. Res. 2019, 138, 239–251. [Google Scholar] [CrossRef]
- Oettel, J.; Lapin, K.; Kindermann, G.; Steiner, H.; Schweinzer, K.-M.; Frank, G.; Essl, F. Patterns and drivers of deadwood volume and composition in different forest types of the Austrian natural forest reserves. For. Ecol. Manag. 2020, 463, 118016. [Google Scholar] [CrossRef]
- Floren, A.; Müller, T.; Dittrich, M.; Weiss, M.; Linsenmair, K.E. The influence of tree species, stratum and forest management on beetle assemblages responding to deadwood enrichment. For. Ecol. Manag. 2014, 323, 57–64. [Google Scholar] [CrossRef]
- Jacobsen, R.M.; Burner, R.C.; Olsen, S.L.; Skarpaas, O.; Sverdrup-Thygeson, A. Near-natural forests harbor richer saproxylic beetle communities than those in intensively managed forests. For. Ecol. Manag. 2020, 466, 118124. [Google Scholar] [CrossRef]
- Schiegg, K. Effects of dead wood volume and connectivity on saproxylic insect species diversity. Écoscience 2000, 7, 290–298. [Google Scholar] [CrossRef]
- Schiegg, K. Are the saproxylic beetle species characteristic of high dead wood connectivity? Ecography 2000, 23, 579–587. [Google Scholar] [CrossRef]
- Škorpík, M. Odumřelé dřevo jako mikrobiotop významných druhů hmyzu. In Význam a Funkce Odumřelého Dřeva v Lesních Porostech: Česká Lesnická Společnost Pobočka Pro Silva Bohemica; VRŠKA, T., Ed.; Vydala Správa Národního parku Podyjí: Znojmo, Czechia, 1999; pp. 107–119. ISBN 80-238-4739-2. [Google Scholar]
- Kraigher, H.; Jurc, D.; Kalan, P.; Kutnar, L.; Levanič, T.; Rupel, M.; Smolej, I. Beech coarse woody debris characteristics in two virgin forest reserves in southern Slovenia. Zb. Gozdarstva Lesar. 2002, 69, 91–134. [Google Scholar]
- Storaunet, K.O.; Rolstad, J. Time since death and fall of Norway spruce logs in old-growth and selectively cut boreal forest. Can. J. For. Res. 2002, 32, 1801–1812. [Google Scholar] [CrossRef]
- Zielonka, T. When does dead wood turn into a substrate for spruce replacement? J. Veg. Sci. 2006, 17, 739–746. [Google Scholar] [CrossRef]
- Lombardi, F.; Cherubini, P.; Lasserre, B.; Tognetti, R.; Marchetti, M. Tree rings used to assess time since death of deadwood of different decay classes in beech and silver fir forests in the central Apennines (Molise, Italy). Can. J. For. Res. 2008, 38, 821–833. [Google Scholar] [CrossRef]
- Šamonil, P.; Antolík, L.; Svoboda, M.; Adam, D. Dynamics of windthrow events in a natural fir-beech forest in the Carpathian mountains. For. Ecol. Manag. 2009, 257, 1148–1156. [Google Scholar] [CrossRef]
- Šebková, B.; Šamonil, P.; Janík, D.; Adam, D.; Král, K.; Vrška, T.; Hort, L.; Unar, P. Spatial and volume patterns of an unmanaged submontane mixed forest in Central Europe: 160 years of spontaneous dynamics. For. Ecol. Manag. 2011, 262, 873–885. [Google Scholar] [CrossRef]
- Herrmann, S.; Kahl, T.; Bauhus, J. Decomposition dynamics of coarse woody debris of three important central European tree species. For. Ecosyst. 2015, 2, 106. [Google Scholar] [CrossRef] [Green Version]
- Zumr, V.; Remeš, J. Saproxylic beetles as an indicator of forest biodiversity and the influence of forest management on their crucial life attributes: Review. Rep. For. Res. 2020, 65, 242–257. [Google Scholar]
- Míchal, I. Ponechávání odumřelého dřeva z hlediska péče o biologickou rozmanitost. In Význam a Funkce Odumřelého Dřeva v Lesních Porostech: Česká Lesnická Společnost Pobočka Pro Silva Bohemica; VRŠKA, T., Ed.; Vydala Správa Národního parku Podyjí: Znojmo, Czechia, 1999; pp. 9–19. ISBN 80-238-4739-2. [Google Scholar]
- Irmler, U.; Arp, H.; Nötzold, R. Species richness of saproxylic beetles in woodlands is affected by dispersion ability of species, age and stand size. J. Insect Conserv. 2009, 14, 227–235. [Google Scholar] [CrossRef]
- Ódor, P.; Heilmann-Clausen, J.; Christensen, M.; Aude, E.; van Dort, K.; Piltaver, A.; Siller, I.; Veerkamp, M.; Walleyn, R.; Standovár, T.; et al. Diversity of dead wood inhabiting fungi and bryophytes in semi-natural beech forests in Europe. Biol. Conserv. 2006, 131, 58–71. [Google Scholar] [CrossRef]
- Brunet, J.; Isacsson, G. Restoration of beech forest for saproxylic beetles—Effects of habitat fragmentation and substrate density on species diversity and distribution. Biodivers. Conserv. 2009, 18, 2387–2404. [Google Scholar] [CrossRef]
- Parisi, F.; Lombardi, F.; Sciarretta, A.; Tognetti, R.; Campanaro, A.; Marchetti, M.; Trematerra, P. Spatial patterns of saproxylic beetles in a relic silver fir forest (Central Italy), relationships with forest structure and biodiversity indicators. For. Ecol. Manag. 2016, 381, 217–234. [Google Scholar] [CrossRef]
- Bouget, C.; Larrieu, L.; Nusillard, B.; Parmain, G. In search of the best local habitat drivers for saproxylic beetle diversity in temperate deciduous forests. Biodivers. Conserv. 2013, 22, 2111–2130. [Google Scholar] [CrossRef]
- Mertlik, J. Faunistics of Crepidophorus mutilatus (Coleoptera: Elateridae) in the Czech Republic and Slovakia. Elateridarium 2014, 8, 36–56, ISSN 1802-4858. [Google Scholar]
- Mertlik, J. Faunistics of Ischnodes sanguinicollis (Coleoptera: Elateridae) in the Czechia and Slovakia. Elateridarium 2019, 13, 49–74, ISSN 1802-4858. [Google Scholar]
- Bílek, L.; Remes, J.; Zahradnik, D. Managed vs. unmanaged. Structure of beech forest stands (Fagus sylvatica L.) after 50 years of development, Central Bohemia. For. Syst. 2011, 20, 122. [Google Scholar] [CrossRef] [Green Version]
- Motta, R.; Berretti, R.; Castagneri, D.; Dukić, V.; Garbarino, M.; Govedar, Z.; Lingua, E.; Maunaga, Z.; Meloni, F. Toward a definition of the range of variability of central European mixed Fagus–Abies–Picea forests: The nearly steady-state forest of Lom (Bosnia and Herzegovina). Can. J. For. Res. 2011, 41, 1871–1884. [Google Scholar] [CrossRef] [Green Version]
- Motta, R.; Garbarino, M.; Berretti, R.; Meloni, F.; Nosenzo, A.; Vacchiano, G. Development of old-growth characteristics in uneven-aged forests of the Italian Alps. Eur. J. For. Res. 2015, 134, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Saniga, M.; Pittner, J.; Kucbel, S.; Filípek, M.; Jaloviar, P.; Sedmáková, D.; Vencurik, J. Dynamické Zmeny Štruktury, Regeneračné procesy a Zmena Objemu Mŕtveho Dreva v Rámci Vývojového Cyklu Bukového Pralesa NPR Stužica (Časová Študia); Technická univerzita vo Zvolene: Zvolen, Slovakia, 2019; 61p. [Google Scholar]
- Kraut, A.; Liira, J.; Lõhmus, A. Beyond a minimum substrate supply: Sustaining saproxylic beetles in semi-natural forest management. For. Ecol. Manag. 2016, 360, 9–19. [Google Scholar] [CrossRef]
- Slodičák, M.; Novák, J. Growth, Structure and Static Stability of Norway Spruce Stands with Different Thinning Regimes. Kostelec nad Černými Lesy; Lesnická práce; Folia Forestalia Bohemica: Brno, Czechia, 2007; 128p, ISBN 978-80-86386-91-1. [Google Scholar]
- Remeš, J.; Novák, J.; Štefančík, I.; Dušek, D.; Slodičák, M.; Bílek, L.; Pulkrab, K. Methods of Thinning for Silvicultural, Ecological and Economic Optimum of Beech Forest Stands in Forest Management Units 43 And 45; VÚLHM: Strnady, Czechia, 2016; ISBN 978-80-7417-123-9. [Google Scholar]
- Remeš, J.; Novák, J.; Štefančík, I.; Dušek, D.; Slodičák, M.; Bílek, L.; Pulkrab, K. Methods of Thinning for Silvicultural, Ecological and Economic Optimum of Spruce Forest Stands in Forest Management Units 43 And 45; VÚLHM: Strnady, Czechia, 2016; ISBN 978-80-7417-124-6. [Google Scholar]
- Černý, M.; Pařez, J.; Malík, Z. Yield and Mensurational Tables of the Principal Tree Species of the Czech Republic (Norway Spruce, Scots Pine, European Beech, Oak); Ústav pro Výzkum Lesních Ekosystémů, s.r.o.: Jílové u Prahy, Czech Republic, 1996; 245p. [Google Scholar]
- Černý, M.; Pařez, J.; Malík, Z. Yield and Mensurational Tables of Tree Species of the Czech Republic, Ústav pro Hospodářskou Úpravu lesů, Brandýs nad LABEM; Ústav pro Výzkum Lesních Ekosystémů, s.r.o.: Jílové u Prahy, Czech Republic, 1996; 156p. [Google Scholar]
- Weiss, M.; Kozel, P.; Zapletal, M.; Hauck, D.; Prochazka, J.; Benes, J.; Cizek, L.; Sebek, P. The Effect Of Coppicing On Insect Biodiversity. Small-Scale Mosaics Of Successional Stages Drive Community Turnover. For. Ecol. Manag. 2021, 483. [Google Scholar] [CrossRef]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Böhm, S.; et al. The impact of even-aged and uneven-aged forest management on regional biodiversity of multiple taxa in European beech forests. J. Appl. Ecol. 2017, 55, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.; Noss, R.F.; Bussler, H.; Brandl, R. Learning from a “benign neglect strategy” in a national park: Response of saproxylic beetles to dead wood accumulation. Biol. Conserv. 2010, 143, 2559–2569. [Google Scholar] [CrossRef]
- Fay, N. Environmental Arboriculture, Tree Ecology And Veteran Tree Management. Arboric. J. 2002, 26, 213–238. [Google Scholar] [CrossRef]
- Winter, S.; Möller, G.C. Microhabitats in lowland beech forests as monitoring tool for nature conservation. For. Ecol. Manag. 2008, 255, 1251–1261. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F. Global Decline in Large Old Trees. Science 2012, 338, 1305–1306. [Google Scholar] [CrossRef]
- Pilskog, H.E.; Birkemoe, T.; Evju, M.; Sverdrup-Thygeson, A. Species composition of beetles grouped by host association in hollow oaks reveals management-relevant patterns. J. Insect Conserv. 2020, 24, 65–86. [Google Scholar] [CrossRef]
- Laaksonen, M.; Punttila, P.; Siitonen, J. Early-successional saproxylic beetles inhabiting a common host-tree type can be sensitive to the spatiotemporal continuity of their substrate. Biodivers. Conserv. 2020, 29, 2883–2900. [Google Scholar] [CrossRef]
- Duncker, P.S.; Barreiro, S.M.; Hengeveld, G.M.; Lind, T.; Mason, W.L.; Ambrozy, S.; Spiecker, H. Classification of Forest Management Approaches: A New Conceptual Framework and Its Applicability to European Forestry. Ecol. Soc. 2012, 17, 51. [Google Scholar] [CrossRef]
- Pulkrab, K. Economic effectiveness of sustainable forest management. J. For. Sci. 2012, 52, 427–437. [Google Scholar] [CrossRef] [Green Version]



| Volume (m3/ha) | Tree Species | Country | |
|---|---|---|---|
| Fridman and Walheim (2000) [76] | 6.1 | Coniferous | Sweden |
| Siitonen (2001) [77] | 14 | Coniferous | Finland |
| Christensen et al. (2005) [78] | 10 | Beech | Europe |
| Vašíček (2007) [79] | 5.5–9 | Mix | Czech Republic |
| Vítková et al. (2018) [3] | 9.1 | Mix | Czech Republic |
| Puletti et al. (2019) [73] | 9.8 | Mix | Czech Republic |
| Roth et al. (2019) [7] | 18.9 | Beech | Germany |
| Kučera and Adolt (2019) [80] | 6.7–13.8 | Mix | Czech Republic |
| Leidinger et al. (2020) [8] | 19.3 | Beech-oak | Germany |
| Bujoczek et al. (2021) [81] | 4.1–15 | Mix | Poland |
| Volume (m3/ha) | Tree Species | Country | |
|---|---|---|---|
| Christensen et al. (2005) [78] | 100 *–220 | Beech | Europe |
| Dudley and Vallauri (2005) [85] | 40–200 | Broadleaved | Europe |
| Bílek et al. (2011) [137] | 48 * | Beech | Czech Republic |
| Motta et al. (2011, 2015) [138,139] | 327 | Mix | Bosnia & Herzegovina, Italy |
| Vacek et al. (2015) [108] | 170–242 * | Mix | Czech Republic |
| Saniga et al. (2019) [140] | 105–160 | Beech | Slovakia |
| Oettel et al. (2020) [115] | 109 | Mix | Austria |
| SI | Age | Volume DW m3/ha | Standing DW m3/ha | Mean DBH cm | Volume Stock m3/ha | Total Volume Production m3/ha | Loss in Volume Production % |
|---|---|---|---|---|---|---|---|
| 34 | 100/0 | 60 | 38 | ||||
| 55 | 20 | 25 | |||||
| 75 | 20 | 33 | |||||
| Σ | 100 | 827 | 1046 | 9.6 | |||
| 28 | 100/0 | 60 | 32 | ||||
| 55 | 20 | 20 | |||||
| 70 | 20 | 25 | |||||
| Σ | 100 | 620 | 737 | 13.6 | |||
| 16 | 100/0 | 64 | 24 | ||||
| 50 | 20 | 14 | |||||
| 70 | 16 | 20 | |||||
| Σ | 100 | 324 | 360 | 27.8 |
| ASI | Age | Volume DW m3/ha | Standing DW m3/ha | Mean DBH cm | Volume Stock m3/ha | Total Volume Production m3/ha | Loss in Volume Production % |
|---|---|---|---|---|---|---|---|
| 34 | 100/0 | 60 | 15 | 26 | |||
| 35 | 20 | 13 | |||||
| 45 | 20 | 19 | |||||
| 55 | 20 | 22 | |||||
| 70 | 30 | 15 | 28 | ||||
| Σ | 150 | 30 | 648 | 874 | 17.1 | ||
| 28 | 100/0 | 60 | 15 | 32 | |||
| 35 | 20 | 10 | |||||
| 45 | 20 | 14 | |||||
| 55 | 20 | 17 | |||||
| 70 | 30 | 15 | 18 | ||||
| Σ | 150 | 478 | 596 | 25.2 | |||
| 18 | 100/0 | 93 | 15 | 24 | |||
| 50 | 18 | 12 | |||||
| 65 | 19 | 17 | |||||
| 80 | 20 | 15 | 22 | ||||
| Σ | 150 | 30 | 260 | 317 | 47.3 |
| ASI | Age | Volume DW m3/ha | Standing DW m3/ha | Mean DBH cm | Volume Stock m3/ha | Total Volume Production m3/ha | Loss in Volume Production % |
|---|---|---|---|---|---|---|---|
| 30 | 130/0 | 30 | 10 | 41 | |||
| 65 | 10 | 20 | |||||
| 80 | 10 | 25 | |||||
| Σ | 50 | 10 | 622 | 789 | 6.3 | ||
| 24 | 130/0 | 30 | 10 | 52 | |||
| 70 | 10 | 27 | |||||
| 85 | 10 | 32 | |||||
| Σ | 50 | 10 | 424 | 516 | 9.7 | ||
| 14 | 130/0 | 34 | 10 | 23 | |||
| 55 | 11 | 12 | |||||
| 75 | 5 | 17 | |||||
| Σ | 50 | 10 | 182 | 201 | 24.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zumr, V.; Remeš, J.; Pulkrab, K. How to Increase Biodiversity of Saproxylic Beetles in Commercial Stands through Integrated Forest Management in Central Europe. Forests 2021, 12, 814. https://doi.org/10.3390/f12060814
Zumr V, Remeš J, Pulkrab K. How to Increase Biodiversity of Saproxylic Beetles in Commercial Stands through Integrated Forest Management in Central Europe. Forests. 2021; 12(6):814. https://doi.org/10.3390/f12060814
Chicago/Turabian StyleZumr, Václav, Jiří Remeš, and Karel Pulkrab. 2021. "How to Increase Biodiversity of Saproxylic Beetles in Commercial Stands through Integrated Forest Management in Central Europe" Forests 12, no. 6: 814. https://doi.org/10.3390/f12060814






