Mixed-Species Plantation Effects on Soil Biological and Chemical Quality and Tree Growth of A Former Agricultural Land
Abstract
:1. Introduction
2. Methods
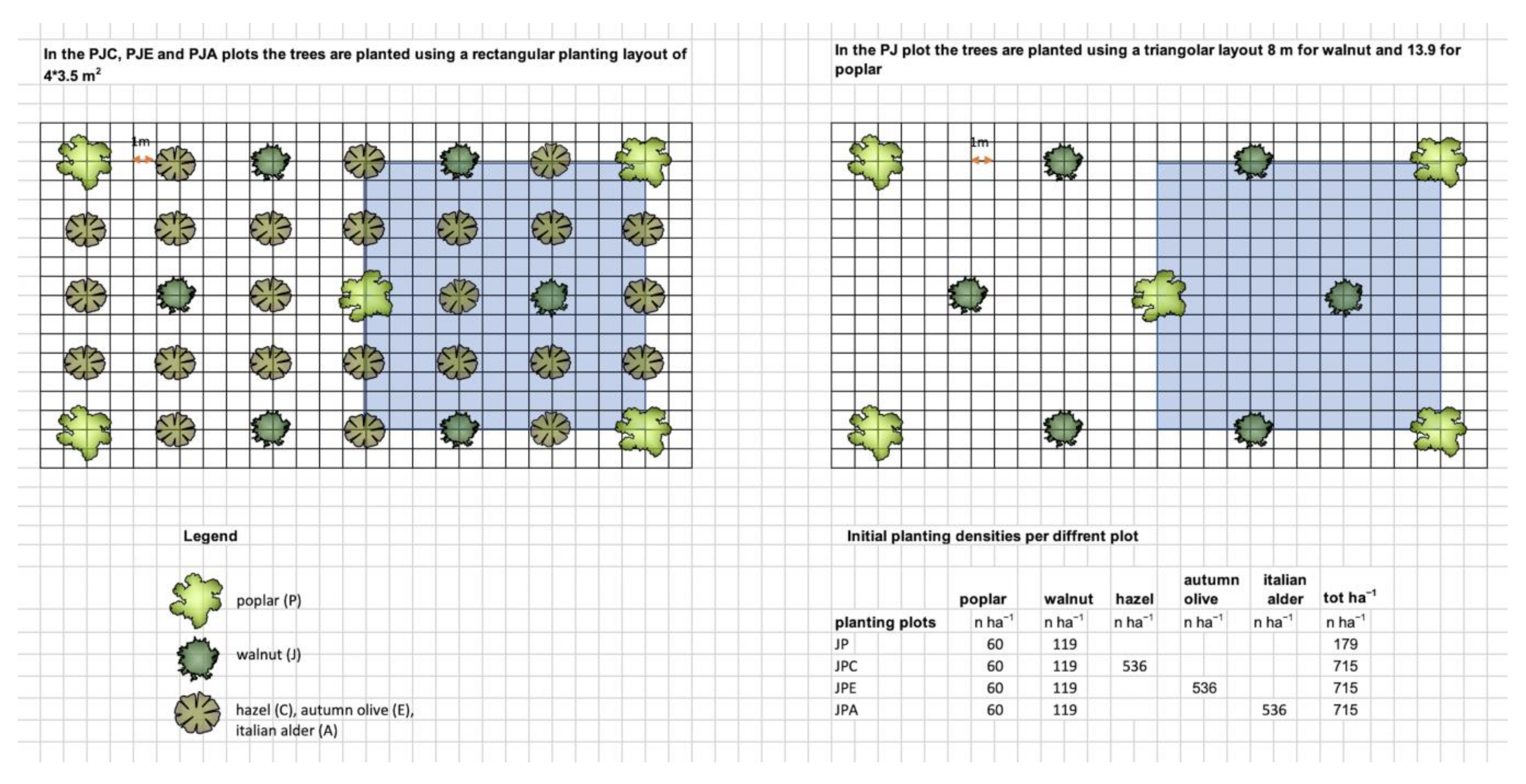
2.1. Study Site
- Poplar and walnut (plots PJ), planted in a high-density mixture.
- Poplar and walnut intercropped with hazel (Corylus avellana L., Betulaceae) (plots PJC), a native shrub frequent in the surrounding forests.
- Poplar and walnut intercropped with autumn olive (Elaeagnus umbellata Thunb., Elaeagnaceae) (plots PJE), an alien N-fixing shrub used in tree farming plantations for its high ability to fix nitrogen in the soil.
- Poplar and walnut intercropped with Italian alder (Alnus cordata (Loisel.) Duby, Betulaceae) (plots PJA), an N-fixing tree species common in Southern Italian forests and widely used in tree farming plantations.
2.2. Sampling
2.3. Physical and Chemical Soil Analysis
2.4. Biological Analysis
2.5. Nematodes
2.6. Tree-Ring Measurement and Basal Area Increment of Individual Trees at the Species Level
2.7. Statistical Analyses
3. Results
3.1. Soil Properties
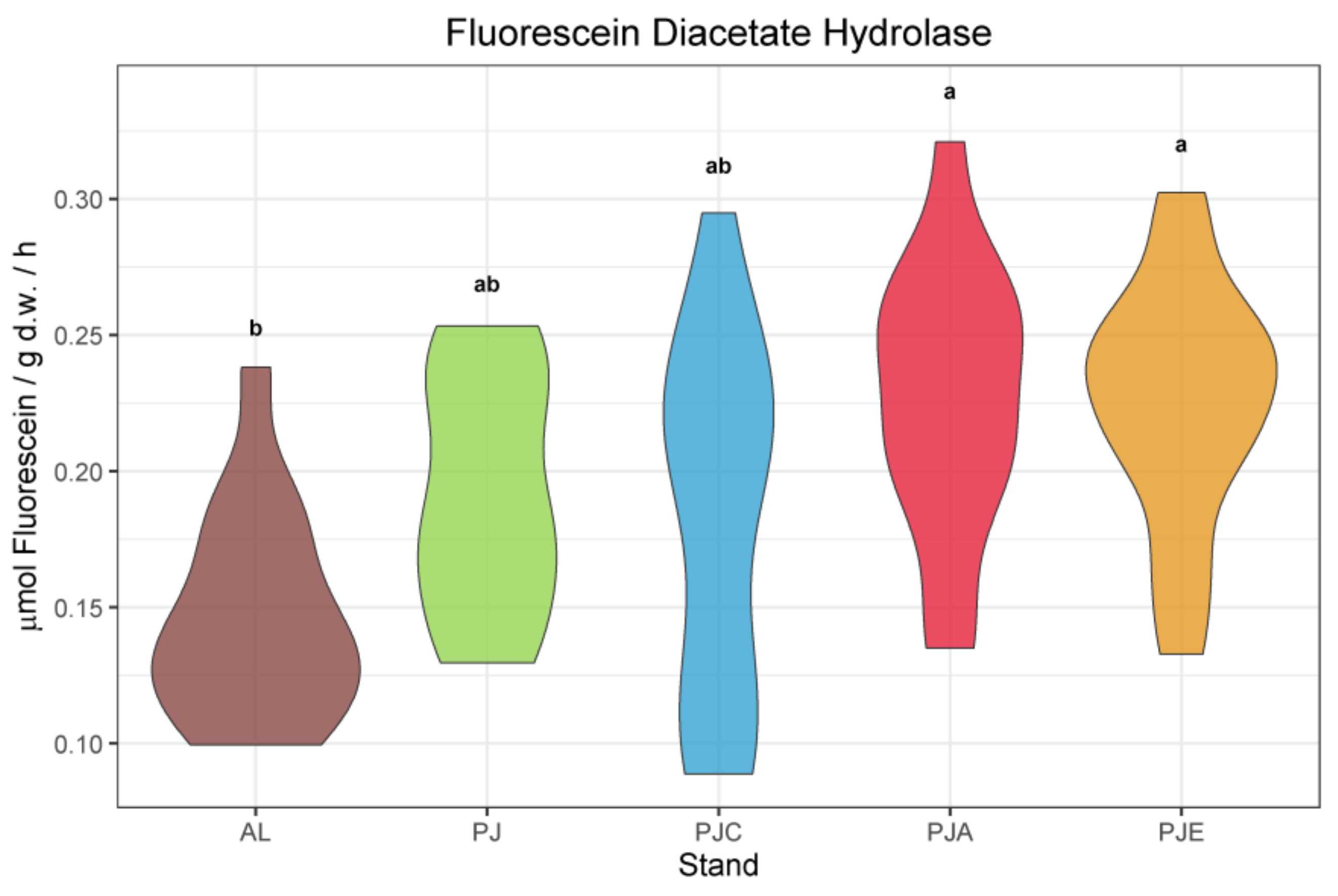
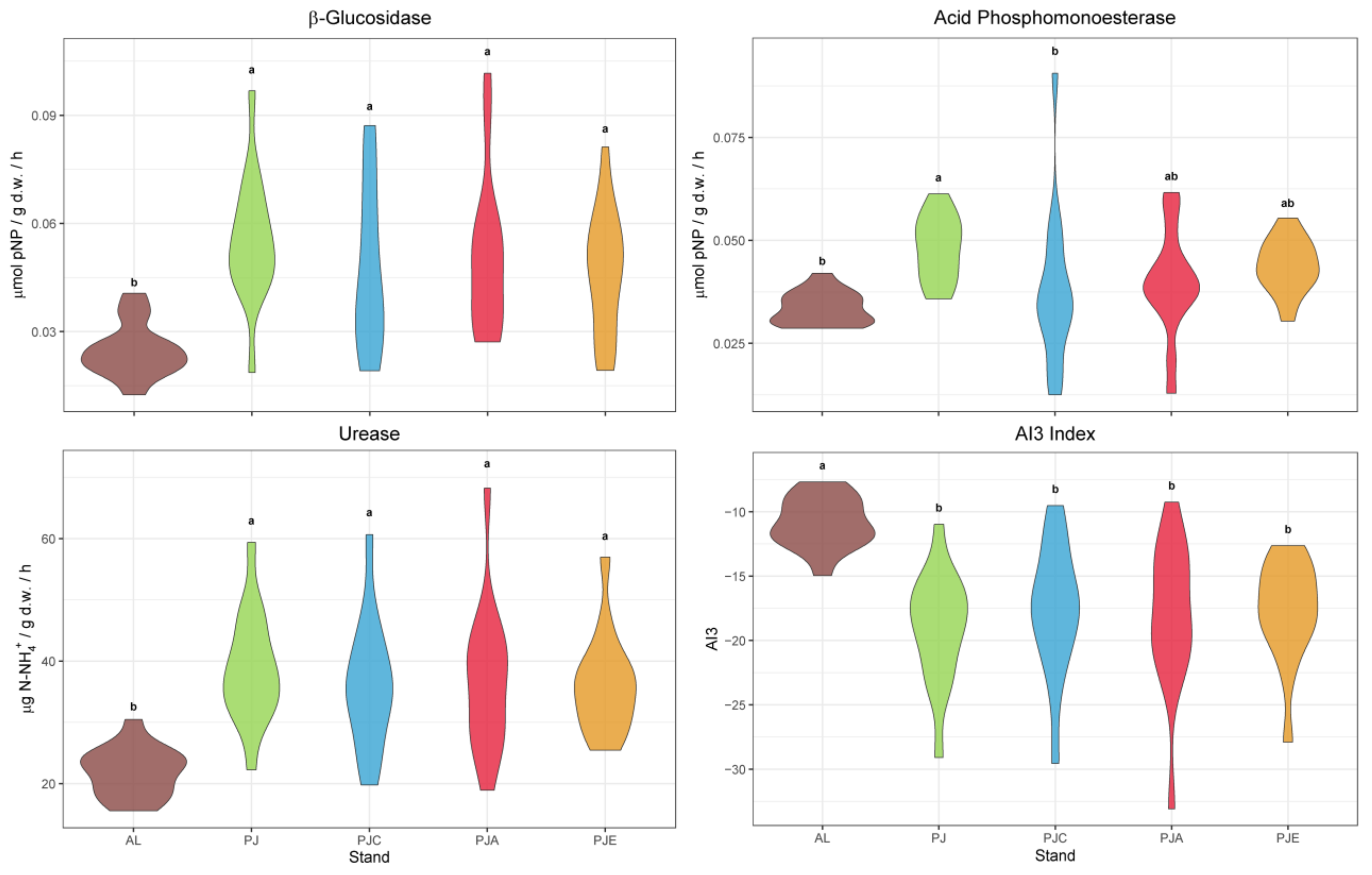
3.2. Soil Enzymatic Activities
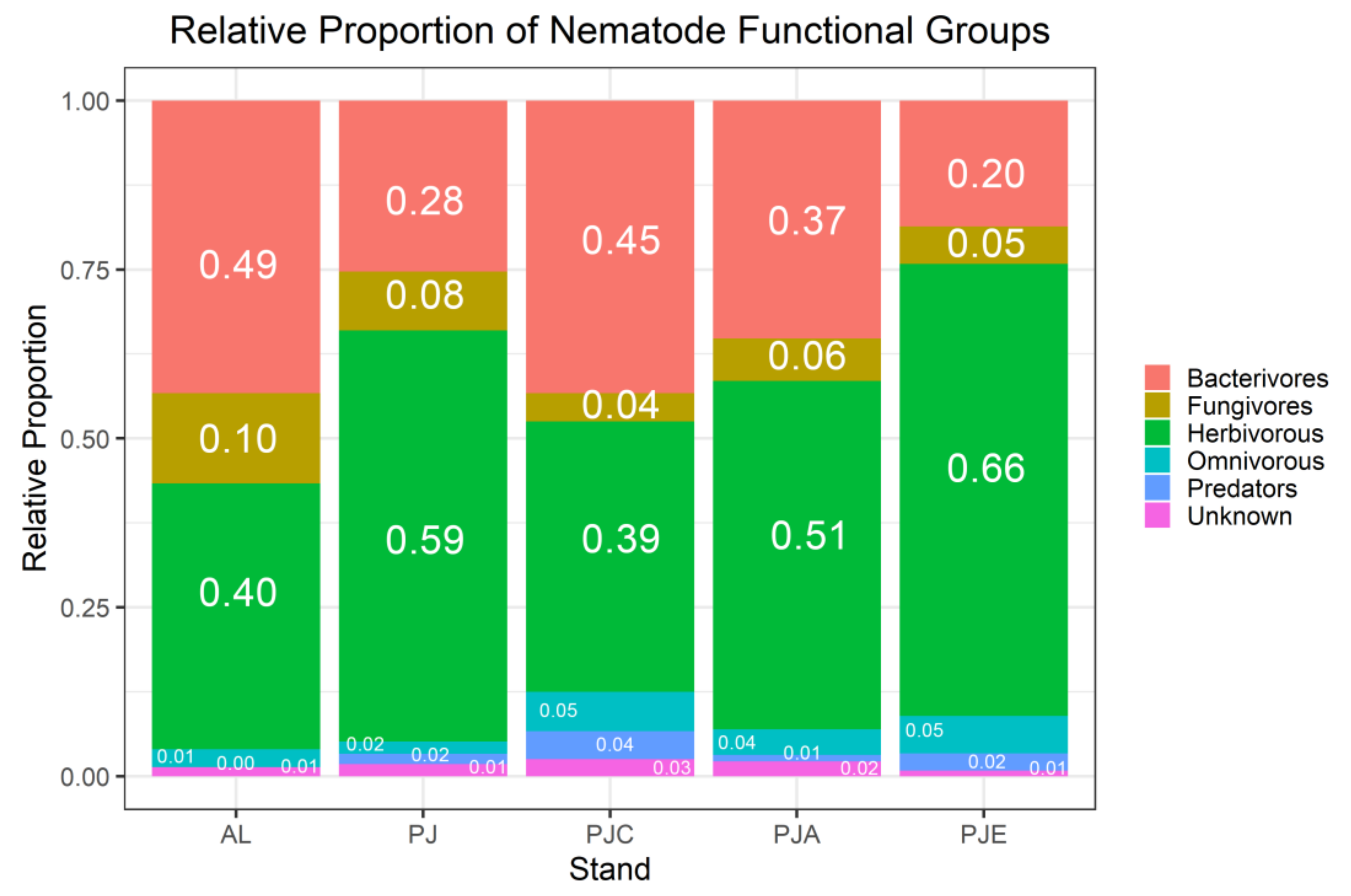
3.3. Nematodes
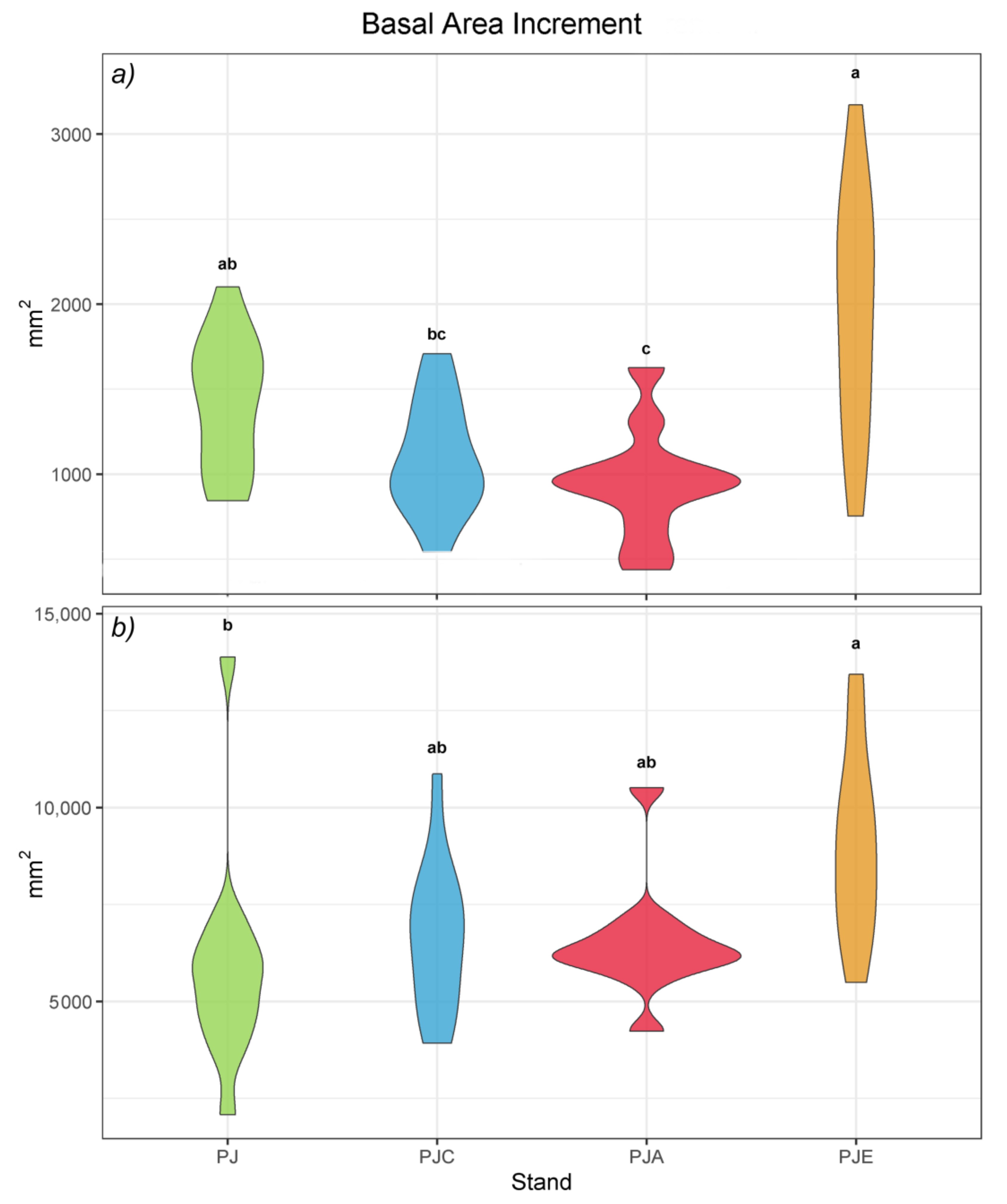
3.4. Basal Area Increment (BAI)
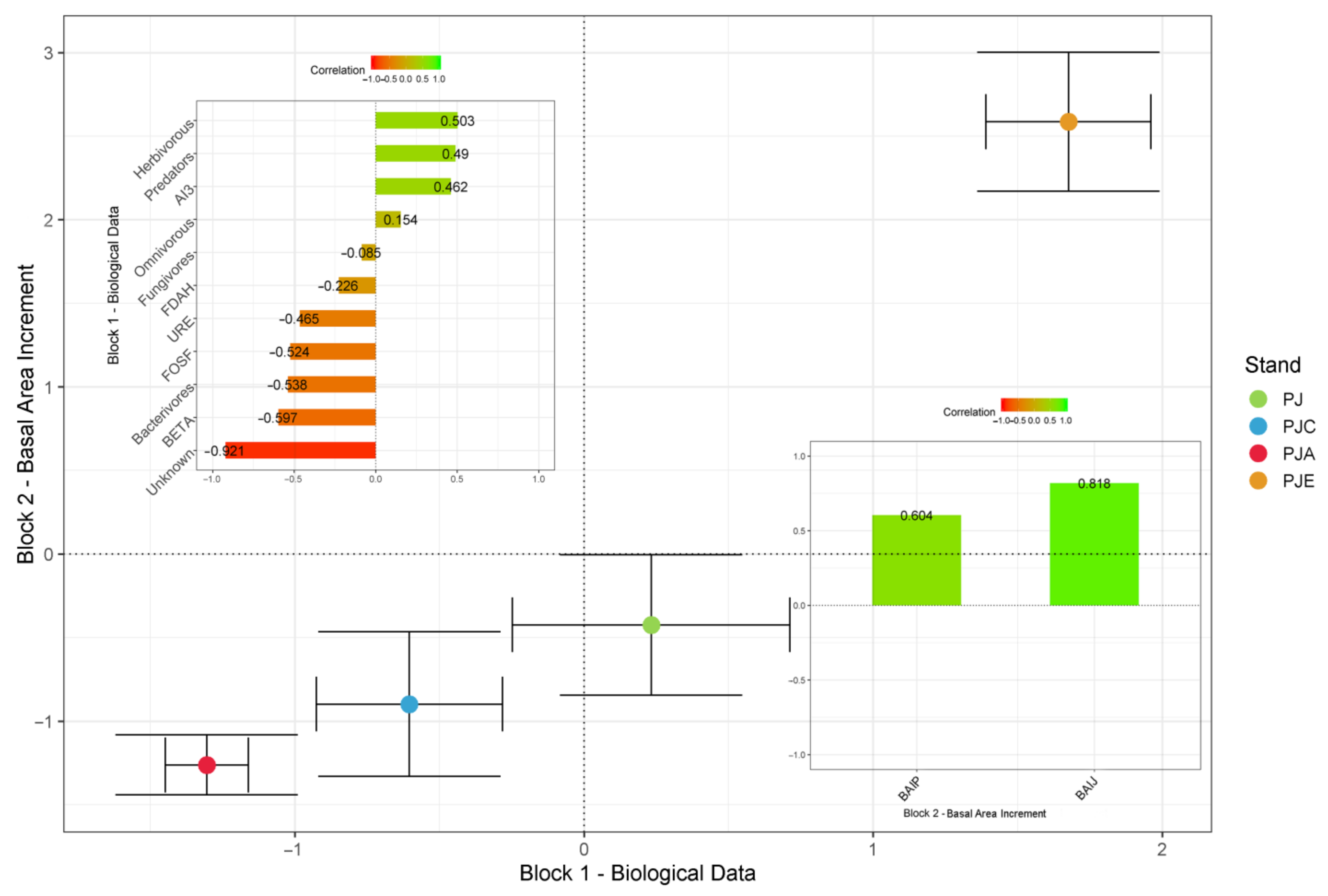
3.5. Two-Block Partial Least Squares Analysis
4. Discussion
4.1. Soil Properties
4.2. Enzymatic Activities and Soil Quality
4.3. Nematodes
4.4. Basal Area Increment
4.5. Overall Trends
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Zanden, E.H.; Verburg, P.H.; Schulp, C.J.E.; Verkerk Johannes, P. Trade-offs of European agricultural abandonment. Land Use Policy 2017, 62, 290–301. [Google Scholar] [CrossRef]
- Stoate, C.; Bàldi, A.; Beja, P.; Boatman, N.D.; Herzon, I.; van Doorn, A.; de Snoo, G.R.; Rakosy, L.; Ramwell, C. Ecological impacts of early 21st century agricultural change in Europe—A review. J. Environ. Manag. 2009, 91, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Keesstra, S.; Nunes, J.; Novara, A.; Finger, D.; Avelar, D.; Kalantari, Z.; Cerdà, A. The superior effect of nature based solutions in land management for enhancing ecosystem services. Sci. Total Environ. 2018, 610–611, 997–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Wal, A.; Van Veen, J.A.; Pijl, A.S.; Summerbell, R.C.; de Boer, W. Constraints on development of fungal biomass and decomposition processes during restoration of arable sandy soils. Soil Biol. Biochem. 2006, 38, 2890–2902. [Google Scholar] [CrossRef]
- Benayas, M.R.; Martins, A.; Nicolau, M.; Schulz, J.J. Abandonment of agricultural land : An overview of drivers and consequences. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pardos, M.; del Río, M.; Pretzsch, H.; Jactel, H.; Bielak, K.; Bravo, F.; Brazaitis, G.; Defossez, E.; Engel, M.; Godvod, K. The greater resilience of mixed forests to drought mainly depends on their composition: Analysis along a climate gradient across Europe. For. Ecol. Manag. 2020, 481, 118687. [Google Scholar] [CrossRef]
- Danise, T.; Innangi, M.; Curcio, E.; Fioretto, A.; Guggenberger, G. Fast Spectrophotometric Method as Alternative for CuO Oxidation to Assess Lignin in Soils with Different Tree Cover. Forests 2020, 11, 1262. [Google Scholar] [CrossRef]
- Marron, N.; Epron, D. Are mixed-tree plantations including a nitrogen-fixing species more productive than monocultures? For. Ecol. Manag. 2019, 441, 242–252. [Google Scholar] [CrossRef]
- Molina-Valero, J.A.; Camarero, J.J.; Álvarez-González, J.G.; Cerioni, M.; Hevia, A.; Sánchez-Salguero, R.; Martin-Benito, D.; Pérez-Cruzado, C. Mature forests hold maximum live biomass stocks. For. Ecol. Manag. 2021, 480, 118635. [Google Scholar] [CrossRef]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Battipaglia, G.; Pelleri, F.; Lombardi, F.; Altieri, S.; Vitone, A.; Conte, E.; Tognetti, R. Effects of associating Quercus robur L. and Alnus cordata Loisel. on plantation productivity and water use efficiency. For. Ecol. Manag. 2017, 391, 106–114. [Google Scholar] [CrossRef]
- Kelty, M.J. The role of species mixtures in plantation forestry. For. Ecol. Manag. 2006, 233, 195–204. [Google Scholar] [CrossRef]
- Chifflot, V.; Rivest, D.; Olivier, A.; Cogliastro, A.; Khasa, D. Molecular analysis of arbuscular mycorrhizal community structure and spores distribution in tree-based intercropping and forest systems. Agric. Ecosyst. Environ. 2009, 131, 32–39. [Google Scholar] [CrossRef]
- Yang, G.; Wagg, C.; Veresoglou, S.D.; Hempel, S.; Rillig, M.C. How soil biota drive ecosystem stability. Trends Plant. Sci. 2018, 23, 1057–1067. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Cook, R.; Yeates, G.W.; Denton, C.S. The influence of nematodes on below-ground processes in grassland ecosystems. Plant. Soil 1999, 212, 23–33. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Alternative soil quality indices for evaluating the effect of intensive cropping, fertilisation and manuring for 31 years in the semi-arid soils of India. Environ. Monit Assess. 2008, 136, 419–435. [Google Scholar] [CrossRef]
- van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; De Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Ayuke, F.O.; Brussaard, L.; Vanlauwe, B.; Six, J.; Lelei, D.K.; Kibunja, C.N.; Pulleman, M.M. Soil fertility management : Impacts on soil macrofauna, soil aggregation and soil organic matter allocation. Appl. Soil Ecol. 2011, 48, 53–62. [Google Scholar] [CrossRef]
- A’Bear, A.D.; Jones, T.H.; Boddy, L. Potential impacts of climate change on interactions among saprotrophic cord-forming fungal mycelia and grazing soil invertebrates. Fungal Ecol. 2014, 10, 34–43. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant—Soil feedbacks : The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Zou, K.; Thébault, E.; Lacroix, G.; Barot, S. Interactions between the green and brown food web determine ecosystem functioning. Funct. Ecol. 2016, 30, 1454–1465. [Google Scholar] [CrossRef] [Green Version]
- Mekonen, S.; Petros, I.; Hailemariam, M.; Conservation, B. The Role of Nematodes in the Processes of Soil Ecology and Their Use as Bioindicators. Agric. Biol. J. N. Am. 2017, 8, 132–140. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H.; Stevens, J.R.; Cobbold, S.M. Plant-soil feedbacks: A meta-analytical review. Ecol. Lett. 2008, 11, 980–992. [Google Scholar] [CrossRef]
- Vila`, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarosìk, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pysek, P. Ecological impacts of invasive alien plants : A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Pelleri, F.; Ravagni, S.; Bianchetto, E.; Bidini, C. Comparing growth rate in a mixed plantation (walnut, poplar and nurse trees) with different planting designs: Results from an experimental plantation in northern Italy. Ann. Silvic. Res. 2013, 37, 13–21. [Google Scholar]
- Cunningham, S.C.; Roxburgh, S.H.; Paul, K.I.; Patti, A.F.; Cavagnaro, T.R. Generating spatially and statistically representative maps of environmental variables to test the efficiency of alternative sampling protocols. Agric. Ecosyst. Environ. 2017, 243, 103–113. [Google Scholar] [CrossRef]
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- MIPAF. Metodi di Analisi Chimica del Suolo. In Collana di Metodi Analitici per L’agricoltura; Sequi, P., Ed.; Franco Angeli: Milan, Italy, 2000. [Google Scholar]
- Danise, T.; Innangi, M.; Curcio, E.; Fioretto, A. Covariation between plant biodiversity and soil systems in a European beech forest and a black pine plantation: The case of Mount Faito, (Campania, Southern Italy). J. For. Res. 2021. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Eivazi Phosphates in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Kandeler, E. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Soil Biol. Biochem. 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Green, V.S.; Stottb, D.E.; Diacka, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Puglisi, E.; Del Re, A.A.M.; Rao, M.A.; Gianfreda, L. Development and validation of numerical indexes integrating enzyme activities of soils. Soil Biol. Biochem. 2006, 38, 1673–1681. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; Goede, R.G.M.D.E.; Freckman, D.W.; Georgieva, S.S. Feeding Habits in Soil Nematode Families and Genera--An Outline for Soil Ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Carrascal, L.M.; Galván, I.; Gordo, O. Partial least squares regression as an alternative to current regression methods used in ecology. Oikos 2009, 118, 681–690. [Google Scholar] [CrossRef]
- Innangi, M.; Balestrieri, R.; Danise, T.; d’Alessandro, F.; Fioretto, A. From soil to bird community: A Partial Least Squares approach to investigate a natural wooded area surrounded by urban patchwork (Astroni crater, southern Italy). Ecol. Modell. 2019, 394, 1–10. [Google Scholar] [CrossRef]
- Innangi, M.; Danise, T.; d’Alessandro, F.; Curcio, E.; Fioretto, A. Dynamics of Organic Matter in Leaf Litter and Topsoil within an Italian Alder (Alnus cordata (Loisel.) Desf.) Ecosystem. Forests 2017, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2019. Available online: https://www.r-project.org/ (accessed on 5 March 2020).
- Hong, S.; Piao, S.; Chen, A.; Liu, Y.; Liu, L.; Peng, S.; Sardans, J.; Sun, Y.; Peñuelas, J.; Zeng, H. Afforestation neutralizes soil pH. Nat. Commun. 2018, 9, 520. [Google Scholar] [CrossRef] [Green Version]
- Laganiere, J.; Angers, D.A.; Pare, D. Carbon accumulation in agricultural soils after afforestation : A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Jobbágy, E.G.; Jackson, R.B. A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecol. Appl. 2009, 19, 2228–2241. [Google Scholar] [CrossRef] [Green Version]
- Liming, J. The Review of Mixtures of Nitrogen fixing and Non nitrogen fixing Tree Species. World For. Res. 1998, 11, 20–26. [Google Scholar]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Rao, C.S.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U. Soil organic carbon dynamics: Impact of land use changes and management practices: A review. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2019; Volume 156, pp. 1–107. ISBN 0065-2113. [Google Scholar]
- Kooch, Y.; Rostayee, F.; Hosseini, S.M. Effects of tree species on topsoil properties and nitrogen cycling in natural forest and tree plantations of northern Iran. Catena 2016, 144, 65–73. [Google Scholar] [CrossRef]
- Li, X.G.; Li, Y.K.; Li, F.M.; Ma, Q.; Zhang, P.L.; Yin, P. Changes in soil organic carbon, nutrients and aggregation after conversion of native desert soil into irrigated arable land. Soil Tillage Res. 2009, 104, 263–269. [Google Scholar] [CrossRef]
- Le Noë, J.; Matej, S.; Magerl, A.; Bhan, M.; Erb, K.; Gingrich, S. Modeling and empirical validation of long-term carbon sequestration in forests (France, 1850–2015). Glob. Chang. Biol. 2020, 26, 2421–2434. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 2014, 4, 6–11. [Google Scholar] [CrossRef]
- Chiti, T.; Certini, G.; Puglisi, A.; Sanesi, G.; Capperucci, A.; Forte, C. Effects of associating a N-fixer species to monotypic oak plantations on the quantity and quality of organic matter in minesoils. Geoderma 2007, 138, 162–169. [Google Scholar] [CrossRef]
- Chodak, M.; Niklinska, M. The effect of different tree species on the chemical and microbial properties of reclaimed mine soils. Biol Fertil Soils 2010, 46, 555–566. [Google Scholar] [CrossRef]
- Parsapour, M.K.; Kooch, Y.; Hosseini, S.M.; Alavi, S.J. Litter and topsoil in Alnus subcordata plantation on former degraded natural forest land : A synthesis of age-sequence. Soil Tillage Res. 2018, 179, 1–10. [Google Scholar] [CrossRef]
- Johnson, D.W.; Curtis, P.S. Effects of forest management on soil C and N storage: Meta analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Shi, S.; Han, P.; Zhang, P.; Ding, F.; Ma, C. The impact of afforestation on soil organic carbon sequestration on the Qinghai Plateau, China. PLoS ONE 2015, 10, e0116591. [Google Scholar] [CrossRef] [Green Version]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xiong, X.; Wu, J.; Zhao, J.; Zhao, M.; Chu, G.; Hui, D.; Zhou, G.; Deng, Q.; Zhang, D. Changes in Soil Microbial Biomass, Community Composition, and Enzyme Activities After Half-Century Forest Restoration in Degraded Tropical Lands. Forests 2019, 10, 1124. [Google Scholar] [CrossRef] [Green Version]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Boyle, S.A.; Yarwood, R.R.; Bottomley, P.J.; Myrold, D.D. Bacterial and fungal contributions to soil nitrogen cycling under Douglas fir and red alder at two sites in Oregon. Soil Biol. Biochem. 2008, 40, 443–451. [Google Scholar] [CrossRef]
- Bini, D.; Dos Santos, C.A.; Bouillet, J.-P.; de Morais Gonçalves, J.L.; Cardoso, E.J.B.N. Eucalyptus grandis and Acacia mangium in monoculture and intercropped plantations: Evolution of soil and litter microbial and chemical attributes during early stages of plant development. Appl. Soil Ecol. 2013, 63, 57–66. [Google Scholar] [CrossRef]
- Tan, X.; Chang, S.X.; Kabzems, R. Soil compaction and forest floor removal reduced microbial biomass and enzyme activities in a boreal aspen forest soil. Biol. Fertil. Soils 2008, 44, 471–479. [Google Scholar] [CrossRef]
- Raiesi, F.; Kabiri, V. Identification of soil quality indicators for assessing the effect of different tillage practices through a soil quality index in a semi-arid environment. Ecol. Indic. 2016, 71, 198–207. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6 (Suppl. 6707). [Google Scholar] [CrossRef]
- Neher, D.A. Ecology of plant and free-living nematodes in natural and agricultural soil. Annu. Rev. Phytopathol. 2010, 48, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Bjørnlund, L.; Liu, M.; Rønn, R.; Christensen, S.; Ekelund, F. Nematodes and protozoa affect plants differently, depending on soil nutrient status. Eur. J. Soil Biol. 2012, 50, 28–31. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T. Nematode diversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 113–135. [Google Scholar] [CrossRef]
- Armendáriz, I.; Arpin, P. Nematodes and their relationship to forest dynamics: I. Species and trophic groups. Biol. Fertil. soils 1996, 23, 405–413. [Google Scholar] [CrossRef]
- Urzelai, A.; Hernandez, J.A.; Pastor, J. Biotic indices based on soil nematode communities for assessing soil quality in terrestrial ecosystems. Sci. Total Environ. 2000, 247, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Dupont, S.T.; Ferris, H.; Horn, M. Van Effects of cover crop quality and quantity on nematode-based soil food webs and nutrient cycling. Appl. Soil Ecol. 2009, 41, 157–167. [Google Scholar] [CrossRef]
- Babst, F.; Alexander, M.R.; Szejner, P.; Bouriaud, O.; Klesse, S.; Roden, J.; Ciais, P.; Poulter, B.; Frank, D.; Moore, D.J.P. A tree-ring perspective on the terrestrial carbon cycle. Oecologia 2014, 176, 307–322. [Google Scholar] [CrossRef] [Green Version]
- de Paula, M.D.; Groeneveld, J.; Huth, A. The extent of edge effects in fragmented landscapes: Insights from satellite measurements of tree cover. Ecol. Indic. 2016, 69, 196–204. [Google Scholar] [CrossRef]
- Jingjing, L.; Crowther, T.W.; Picard, N.; Wiser, S.; Mo, Z.; Alberti, G.; Schulze, E.D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H. Positive biodiversity-productivity relationship predominant in global forests. Science 2021, 354, 196. [Google Scholar]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: A review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef] [Green Version]
- Guerrieri, R.; Mencuccini, M.; Sheppard, L.J.; Saurer, M.; Perks, M.P.; Levy, P.; Sutton, M.A.; Borghetti, M.; Grace, J. The legacy of enhanced N and S deposition as revealed by the combined analysis of δ13C, δ18O and δ15N in tree rings. Glob. Chang. Biol. 2011, 17, 1946–1962. [Google Scholar] [CrossRef] [Green Version]
- Khamzina, A.; Lamers, J.P.A.; Vlek, P.L.G. Nitrogen fixation by Elaeagnus angustifolia in the reclamation of degraded croplands of Central Asia. Tree Physiol. 2009, 29, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Paschke, M.W.; Dawson, J.O.; David, M.B. Soil nitrogen mineralization in plantations of Juglans nigra interplanted with actinorhizal Elaeagnus umbellata or Alnus glutinosa. Plant Soil 1989, 118, 33–42. [Google Scholar] [CrossRef]
- Hoogmoed, M.; Cunningham, S.C.; Baker, P.; Beringer, J.; Cavagnaro, T.R. N-fixing trees in restoration plantings: Effects on nitrogen supply and soil microbial communities. Soil Biol. Biochem. 2014, 77, 203–212. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Wang, Q.; Wang, L.; Liu, W.; Liu, X.; Huang, Y.; Christie, P. Response of soil enzymes and microbial communities to root extracts of the alien Alternanthera philoxeroides. Arch. Agron. Soil Sci. 2018, 64, 708–717. [Google Scholar] [CrossRef]
- Liang, J.; Reynolds, T.; Wassie, A.; Collins, C.; Wubalem, A. Effects of exotic Eucalyptus spp. plantations on soil properties in and around sacred natural sites in the northern Ethiopian Highlands. AIMS Agric. Food 2016, 1, 175–193. [Google Scholar] [CrossRef]
| Stand | Sand | Silt | Clay | Bulk Density | pHCaCl2 |
|---|---|---|---|---|---|
| % | % | % | kg dm−3 | ||
| PJC | 59.5 ± 5.5 a | 25.6 ± 7.9 a | 14.9 ± 2.6 a | 1.4 ± 0.1 a | 7.0 ± 0.2 a |
| PJE | 53.7 ± 3.8 a,b,c | 29.4 ± 1.4 ab | 16.9 ± 3.2 a | 1.5 ± 0.2 a | 7.1 ± 0.2 a |
| PJA | 37.5 ± 8.2 b | 42.4 ± 6.7 b | 20.0 ± 2.0 a | 1.4 ± 0.2 a | 7.0 ± 0.2 a |
| PJ | 43.7 ± 5.3 c | 38.0 ± 4.3 b | 18.3 ± 3.2 a | 1.4 ± 0.1 a | 7.0 ± 0.1 a |
| AL | 68.7 ± 6.4 a | 15.1 ± 8.2 a | 16.2 ± 1.9 a | 1.5 ± 0.1 a | 7.3 ± 0.2 b |
| Stand | SOM | TC | TN | TOC | Corg/N | TIC |
|---|---|---|---|---|---|---|
| mg g−1 | mg g−1 | mg g−1 | mg g−1 | mg g−1 | ||
| PJC | 34.6 ± 2.46 a | 32.6 ± 1.19 a,b | 1.3 ± 0.08 b | 17.4 ± 1.17 a,b | 13.4 ± 0.55 a | 15.1 ± 0.40 a,b,c |
| PJE | 34.0 ± 1.59 a | 28.7 ± 0.73 b,c | 1.4 ± 0.05 ab | 14.5 ± 0.82 b | 10.2 ± 0.27 b | 14.2 ± 0.34 b,c |
| PJA | 35.0 ± 1.21 a | 33.3 ± 1.86 a,b | 1.8 ± 0.12 a | 19.1 ± 1.83 a | 10.6 ± 0.28 b | 14.2 ± 0.85 c |
| PJ | 35.8 ± 1.20 a | 32.9 ± 0.87 a | 1.5 ± 0.07 ab | 16.4 ± 0.78 a,b | 11.0 ± 0.41 b | 16.5 ± 1.07 a,b |
| AL | 20.4 ± 1.06 b | 25.7 ± 0.59 c | 0.8 ± 0.03 c | 8.3 ± 0.22 c | 10.5 ± 0.29 b | 17.4 ± 0.73 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danise, T.; Andriuzzi, W.S.; Battipaglia, G.; Certini, G.; Guggenberger, G.; Innangi, M.; Mastrolonardo, G.; Niccoli, F.; Pelleri, F.; Fioretto, A. Mixed-Species Plantation Effects on Soil Biological and Chemical Quality and Tree Growth of A Former Agricultural Land. Forests 2021, 12, 842. https://doi.org/10.3390/f12070842
Danise T, Andriuzzi WS, Battipaglia G, Certini G, Guggenberger G, Innangi M, Mastrolonardo G, Niccoli F, Pelleri F, Fioretto A. Mixed-Species Plantation Effects on Soil Biological and Chemical Quality and Tree Growth of A Former Agricultural Land. Forests. 2021; 12(7):842. https://doi.org/10.3390/f12070842
Chicago/Turabian StyleDanise, Tiziana, Walter S. Andriuzzi, Giovanna Battipaglia, Giacomo Certini, Georg Guggenberger, Michele Innangi, Giovanni Mastrolonardo, Francesco Niccoli, Francesco Pelleri, and Antonietta Fioretto. 2021. "Mixed-Species Plantation Effects on Soil Biological and Chemical Quality and Tree Growth of A Former Agricultural Land" Forests 12, no. 7: 842. https://doi.org/10.3390/f12070842






