The Possibility of Regenerating a Pine Stand through Natural Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Regeneration Potential of the Studied Stands
2.3. Species Diversity Sampling
2.4. Molecular Analyses
2.5. SSR Analysis
2.6. Statistical Analysis
3. Results
3.1. Reproductive Potential
- CG—15.63 seeds;
- Gr—17.45 seeds;
- S—14.54 seeds;
- Wi—14.54 seeds;
- W—11 seeds.
- CG—15.63 × 0.99 × 999 = 15,458 seedlings;
- Gr—17.45 × 0.99 × 1752 = 30,267 seedlings;
- S—14.54 × 0.99 × 2103 = 30,272 seedlings;
- Wi—14.54 × 0.99 × 2560 = 36,850 seedlings;
- W—11 × 0.99 × 1506 = 16,400 seedlings.
3.2. Species Variability of a Stand
3.3. Genetic Analysis
3.4. Correlations between Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Interpretation Manual. EUR28. pp. 113–122. Available online: https://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int Manual_EU28.pdf (accessed on 2 August 2021).
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- State Forest in Poland. Zasoby Leśne—Lasy Państwowe; State Forest in Poland. Available online: https://www. lasy.gov.pl (accessed on 2 August 2021).
- Miścicki, S. Changes in the stands of the Białowieża National Park from 2000 to 2015. For. Res. Pap. 2016, 77, 371–379. [Google Scholar] [CrossRef]
- Oleskog, G.; Sahlén, K. Effects of seedbed substrate on moisture conditions and germination of Scots pine (Pinus sylvestris) seeds in a mixed conifer stand. New For. 2000, 20, 119–133. [Google Scholar] [CrossRef]
- Hille, M.; den Ouden, J. Improved recruitment and early growth of Scots pine (Pinus sylvestris L.) seedlings after free and soil scarifcation. Eur. J. For. Res. 2004, 123, 213–218. [Google Scholar] [CrossRef]
- Nowakowska, J.A.; Oszako, T. Stan zdrowotny buków w Nadleśnictwie Siewierz a ich zróżnicowanie genetyczne określone na podstawie analiz chloroplastowego DNA. Sylwan 2008, 152, 11–20. [Google Scholar]
- Prus-Głowacki, W.; Godzik, S. Genetic structure of Picea abies trees tolerant and sensitive to industrial pollution. Silvae Genet. 1995, 44, 62–65. [Google Scholar]
- Oleksyn, J.; Prus-Głowacki, W.; Giertych, M.; Reich, P.B. Relation between genetic diversity and pollution impact in a 1912 experiment with East European Pinus sylvestris provenances. Can. J. For. Res. 1994, 24, 2390–2394. [Google Scholar] [CrossRef]
- Adams, W.T. Gene dispersal within forest tree populations. New For. 1992, 6, 217–240. [Google Scholar] [CrossRef]
- Farmer, R.E. Seed Ecophysiology of Temperate and Boreal Zone Forest Trees; Dispersal. St. Lucie Press: Delray Beach, FL, USA, 1997; pp. 45–65. [Google Scholar]
- Klisz, M.; Ukalski, K.; Ukalska, J.; Jastrzębowski, S.; Puchałka, R.; Przybylski, P.; Mionskowski, M.; Matras, M. What can we learn an erly test on the adaptation of silver fir populations to marginal envirments. Forest 2018, 9, 441. [Google Scholar] [CrossRef]
- Przybylski, P.; Mohytych, V.; Rutkowski, P.; Tereba, A.; Tyburski, Ł.; Fyalkowska, K. Relationships between Some Biodiversity Indicators and Crown Damage of Pinus sylvestris L. in Natural Old Growth Pine Forests. Sustainability 2021, 13, 1239. [Google Scholar] [CrossRef]
- Bergmann, F.; Scholz, F. Selection effects of air pollution in Norway spruce (Picea abies) populations. In Genetic Effects of Air Pollutants in Forest Tree Populations; Scholz, F., Gregorius, H.R., Rudin, D., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 143–162. [Google Scholar]
- Durel, C.E.; Bertin, P.; Kremer, A. Relationship between inbreeding depression and inbreeding coefficient in maritime pine (Pinus pinaster). Theor. Appl. Genet. 1996, 92, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.J.; van Heerwaarden, J.; Wegrzyn, J.L.; Nelson, C.D.; RossIbarra, J.; Gonzalez-Martınez, S.C.; Neale, D.B. Patterns of population structure and environmental associations to aridity across the range of loblolly pine (Pinus taeda L., Pinaceae). Genetics 2010, 185, 969–982. [Google Scholar] [CrossRef]
- Quesada, T.; Gopal, V.; Cumbie, W.P.; Eckert, A.J.; Wegrzyn, J.L.; Neale, D.B.; Goldfarb, B.; Huber, D.A.; Casella, G.; Davis, J.M. Association mapping of quantitative disease resistance in a natural population of loblolly pine (Pinus taeda L.). Genetics 2010, 186, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Rajora, O.P.; Rahman, M.H.; Buchert, G.P.; Dancik, B.P. Microsatellite DNA analysis of genetic effects of harvesting on old-growth eastern white pine (Pinus strobus L.) in Ontario. Can. Mol. Ecol. 2000, 9, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Klisz, M.; Ukalska, J.; Koprowski, M.; Tereba, A.; Puchałka, R.; Przybylski, P.; Jastrzębowski, S.; Nabais, C. Effect of provenance and climate in intra-annual density fluctuations of Norway spruce Picea abies (L.) Karst. Pol. Agric. Meteorol. 2019, 269–270, 145–156. [Google Scholar] [CrossRef]
- Williams, C. The peculiarities of pine genome. In Proceedings of the Plant and Animal Genome VII Conference, San Diego, CA, USA, 17–21 January 1999. [Google Scholar]
- Scalfi, M.; Piotti, A.; Rossi, M.; Piovani, P. Genetic variability of Italian southern Scots pine (Pinus sylvestris L.) populations: The rear edge of the range. Eur. J. For. Res. 2009, 128, 377. [Google Scholar] [CrossRef]
- Konecka, A.; Tereba, A.; Studnicki, M.; Nowakowska, J.A. Allele rzadkie i prywatne jako miara bogactwa puli genetycznej materiału sadzeniowego sosny zwyczajnej. Sylwan 2019, 163, 948–956. [Google Scholar] [CrossRef]
- Scotti, I.; Burelli, A.; Cattonaro, F.; Chagné, D.; Fuler, J.; Hedley, P.E.; Jansson, G.; Lalanne, C.; Madur, D.; Neale, D.; et al. Analysis of the distribution of marker classes in a genetic linkage map: A case study in Norway spruce (Picea abies Karst). Tree Genet. Genomes 2005, 1, 93–102. [Google Scholar] [CrossRef]
- Dąbrowski, M.J.; Pilot, M.; Kruczyk, M.; Żmihorski, M.; Umer, H.M.; Gliwicz, J. Reliability assessment of null allele detection: Inconsistencies between and within 2 different methods. Mol. Ecol. Resour. 2013, 14, 361–373. [Google Scholar] [CrossRef]
- Mason, W.L.; Edwards, C.; Hale, S.E. Survival and early seedling growth of conifers with different shade tolerance in a Sitka spruce spacing trial and relationship to understorey light climate. Silva Fenn. 2004, 38, 357–370. [Google Scholar] [CrossRef]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Ecology of seed germination of Pinus sylvestris L. at its southern. Mediterranean distribution range. Investig. Agrar. Sist. Recur. For. 2005, 14, 143–152. [Google Scholar] [CrossRef]
- Aleksandrowicz-Trzcińska, M.; Drozdowski, S.; Brzeziecki, B.; Rutkowska, P.; Jabłońska, B. Effect of different methods of site preparation on natural regeneration of Pinus sylvestris in eastern Poland. Dendrobiology 2014, 71, 73–81. [Google Scholar] [CrossRef]
- Czyżyk, K. The diversity of characteristics of the natural regeneration of Scots pine (Pinus sylvestris L.) in the clear-cutting. In Polish: Przestrzenne zróżnicowanie cech naturalnego odnowienia sosny zwyczajnej (Pinus sylvestris L.) w rębni zupełnej. Acta Sci. Pol. Form. Circumiectus 2017, 16, 59–70. [Google Scholar] [CrossRef]
- Bastien, C.; Alía, R. What might be useful measures of genetic variability for adaptive traits within populations of Scots pine? Investig. Agr. Sist. Recur. For. 2000, 9, 97–110. [Google Scholar]
- Grivet, D.; Sebastiani, F.; González-Martínez, S.C.; Vendramin, G.G. Patterns of polymorphism resulting from long-range colonization in the Mediterranean conifer Aleppo pine. New Phytol. 2009, 184, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Tyszkiewicz, S. Nasiennictwo Leśne; Forest Research Institute: Warsaw, Poland, 1949. [Google Scholar]
- ISTA. International Rules for Seed Testing. Intentional Seed Testing Association; ISTA: Bassersdorf, Switzerland, 2013. [Google Scholar]
- Soranzo, N.; Provan, J.; Powell, W. Characterization of microsatellite loci in Pinus sylvestris L. Mol. Ecol. 1998, 7, 1260–1261. [Google Scholar]
- Chagne, D.; Chaumeil, P.; Ramboer, A.; Collada, C.; Guevara, A.; Cervera, M.T.; Vendramin, G.G.; Garcia, V.; Frigerio, J.M.; Echt, C.; et al. Cross-species transferability and mapping of genomic and CDNA SSRs in pines. Theor. Appl. Genet. 2004, 109, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P. GENEALEX 6.5: Genetic Analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar]
- Goudet, J. FSTAT, a Program to Estimate and Test Gene Diversities and Fixation Indices (Version 2.9.4). Available online: http://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 21 November 2003).
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. MICRO-CHECKER—Software for identifying and correcting g enotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 15 February 2021).
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R package Version 1.3-3. 2020. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 15 February 2021).
- Wei, T.; Simko, V.R. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 15 February 2021).
- Chakraborty, R.; De Andrade, M.; Daiger, S.P.; Budowle, B. Apparent heterozygote deficiencies observed in DNA typing data and their implications in forensic applications. Ann. Hum. Genet. 1992, 56, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Brookfield, J.F.Y. A simple new method for estimating null allele frequency from heterozygote deficiency. Mol. Ecol. 1996, 5, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Kremer, F.; Vand der Stegen, J.; Gomez-Zamalloa, M.G.; Szedlak, T. Natura 2000 and Forests. Part I-II. European Commission. Technical Report—2015–088; Publications Office of the European Union: Luxembourg, 2015. [Google Scholar] [CrossRef]
- Andrzejewska, A. Klimat. Przyroda Kampinoskiego Parku Narodowego; Andrzejewski, R., Ed.; Kampinoski Park Narodowy: Izabelin, Poland, 2003; Volume 1, p. 728. [Google Scholar]
- Matula, R.; Řepka, R.; Šebesta, J.; Pettit, J.L.; Chamagne, J.; Šrámek, M.; Horgan, K.; Maděra, P. Resprouting trees drive understory vegetation dynamics following logging in a temperate forest. Sci. Rep. 2020, 10, 9231. [Google Scholar] [CrossRef]
- Enander, M. Val ar frötrad samt sortering av frö och plantar ar tall och gran, Svenska Skogsvfören. Tidskrift 1946, 44, 310–328. [Google Scholar]
- Ganatsas, P.; Tsakaldimi, M.; Thanos, C. Seed and cone diversity and seed germination of Pinus pinea in Strofylia Site of the Natura 2000 Network. Biodivers. Conserv. 2008, 17, 2427–2439. [Google Scholar] [CrossRef]
- Falińska, K. Przewodnik do Badań Biologii Populacji Roślin; PWN: Warsaw, Poland, 2002. [Google Scholar]
- Harper, J.L.; White, J. The dynamics of plant populations. In Proceedings of the Advanced Study Institute on Dynamics of Numbers in populations, Oosterbeek, The Netherlands, 7–18 September 1970; den Boer, P.J., Gradwell, G.R., Eds.; pp. 41–63. [Google Scholar]
- Sarukhán, J.; Harper, J.L. Studies on plant demography: Ranunculus repens L., R. bulbosus L. and R. acris L. I. Population flux and survivorship. J. Ecol. 1973, 61, 675–716. [Google Scholar] [CrossRef]
- Bergsten, U. Invigoration and IDS-sedimentation of Pinus sylvestris seeds from northern Finland. Silva Fenn. 1985, 22, 323–327. [Google Scholar] [CrossRef]
- Zentsch, W. Über Eigenschaften von Kiefernsaatgut aus verschiedenen Kronenregionen. Forstwiss. Cent. 1965, 80, 287–294. [Google Scholar] [CrossRef]
- Grzesiuk, S.; Kulka, K. Fizjologia i Biochemia Nasion; PWRiL: Warsaw, Poland, 1981. [Google Scholar]
- Jastrzębowski, S. Variability of Selected Traits of Scots Pine (Pinus Sylvestris L.) Preservation Stands’ Progeny in Poland. Ph.D. Thesis, Warsaw Agriculture University with Abstract in English, Warszawa, Poland, 2013, unpublished. [Google Scholar]
- Harju, A.M.; Kärkkäinen, K.; Ruotsalainen, S. Phenotypic and genetic variation in the seed maturity of Scots pine. Silvae Genet. 1996, 45, 205–211. [Google Scholar]
- Hilli, A.; Hokkanen, T.; Hyvönen, J.; Sutinen, M.L. Long-term variation in Scots pine seed crop size and quality in northern Finland. Scand. J. For. Res. 2008, 23, 395–403. [Google Scholar] [CrossRef]
- Sabor, J. Wpływ stosowanych zabiegów pielęgnacyjnych i rębni na zmianę struktury genetycznej drzewostanów. Sylwan 2003, 2, 39–48. [Google Scholar]
- Mitton, J.B. Apparent overdominance in natural plant populations. In: Concepts and breeding of heterosis in crop plants. Crop Sci. Soc. Am. 1998, 677, 57–69. [Google Scholar]
- Dzialuk, A.; Burczyk, J. Zmiany struktury genetycznej pomiędzy populacją rodzicielską a potomną w drzewostanie nasiennym sosny zwyczajnej (Pinus sylvestris L.). Sylwan 2006, 10, 30–38. [Google Scholar]
- Yazdani, R.; Muona, O.; Rudin, D.; Szmidt, A.E. Genetic Structure of a Pinus sylvestris L. Seed-Tree Stand and Naturally Regenerated Understory. For. Sci. 1985, 31, 430–436. [Google Scholar]
- Muona, O.; Yazdani, R.; Rudin, D. Genetic change between life stages in Pinus sylvestris L.: Allozyme variation in seeds and planted seedlings. Silvae Genet. 1987, 36, 39–42. [Google Scholar]
- Petit, C.; Fréville, H.; Mignot, A.; Colas, B.; Riba, M.; Imbert, E.; Hurtrez-Boussés, S.; Virevaire, M.; Olivieri, I. Gene flow and local adaptation in two endemic plant species. Biol. Conserv. 2001, 100, 21–34. [Google Scholar] [CrossRef]
- Nowakowska, J.A.; Zachara, T.; Konecka, A. Zmienność genetyczna odnowienia i drzewostanu macierzystego sosny (Pinus sylvestris L.) i świerka (Picea abies L. Karst.). Leśne Pr. Badaw. 2014, 75, 47–54. [Google Scholar]
- Rajora, O.P. Genetic biodiversity impacts of silvicultural practices and phenotypic selection in white spruce. Theor. Appl. Genet. 1999, 99, 954–961. [Google Scholar] [CrossRef]
- Kosińska, J.; Lewandowski, A.; Chalupka, W. Genetic variability of Scots pine maternal populations and their progenies. Silva Fenn. 2007, 41, 5–12. [Google Scholar] [CrossRef]
- Rutkowski, P.; Konatowska, M. Influence of human activity on forest plant communities, during the past 200 years, in the example of Zielonka Experimental Forest (Poland). In Conservation and Sustainable Use of Wild Plant Diversity; Andrianos, L., Sneep, J.W., Kenanidis, K., Eds.; Institute of Theology and Ecology Orthodox Academy of Crete: Chania, Greece, 2011. [Google Scholar]
- Kowalska, A.; Kołaczkowska, E. Regeneration-degeneration processes in the inland dune forests in protected areas of central Poland (Kampinos National Park). Misc. Geogr. Reg. Stud. Dev. 2016, 19, 56–63. [Google Scholar] [CrossRef][Green Version]
- Konatowska, M.; Rutkowski, P. Porównanie Zbiorowisk Roślinnych z Połowy XX i Początku XXI Wieku w Nadleśnictwie Doświadczalnym Zielonka (Comparison of Plant Communities of Zielonka Experimental Forest from the Half of the 20th and the Beginning of 21th Century); University of Life Sciences in Poznan: Poznan, The Netherlands, 2021; p. 526. [Google Scholar]
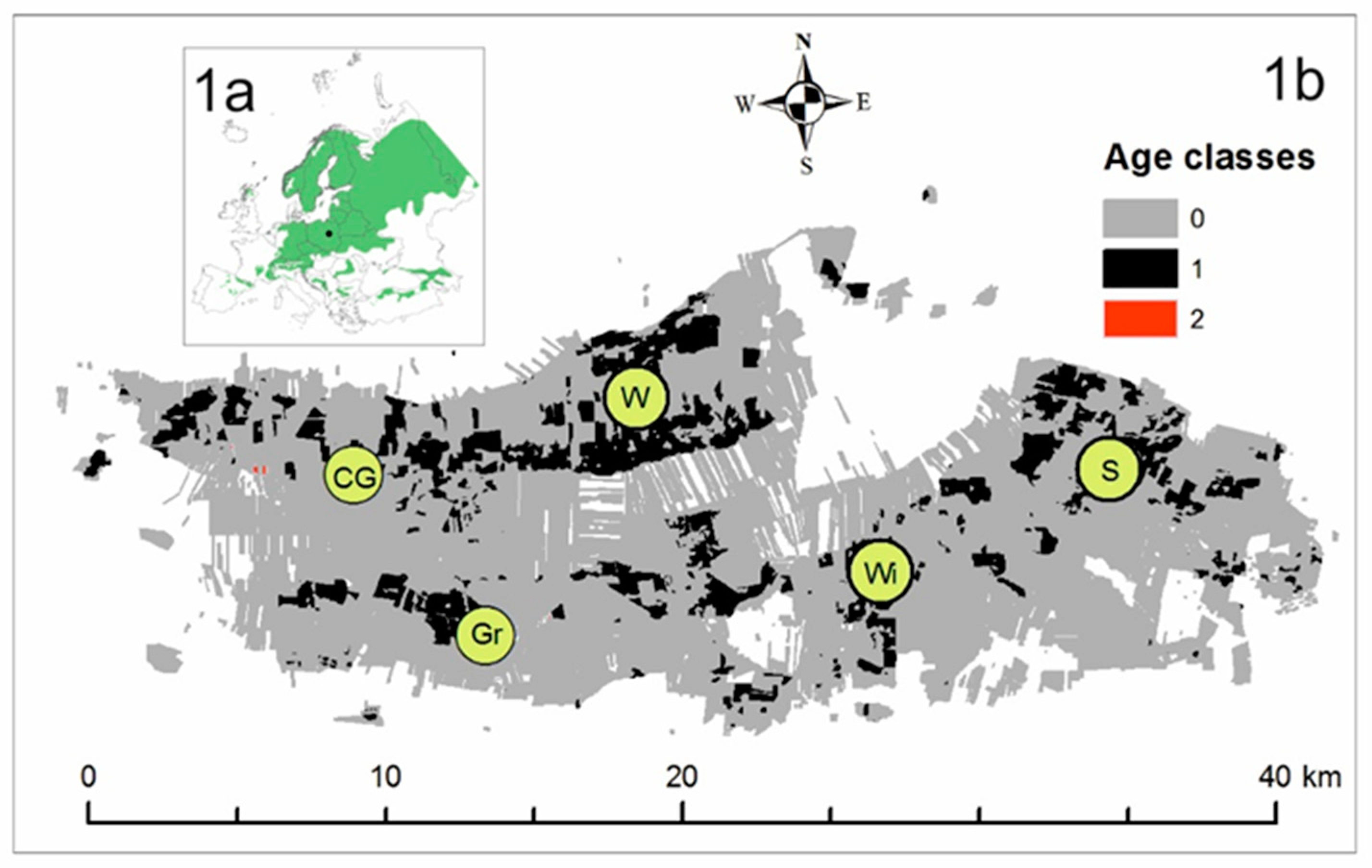
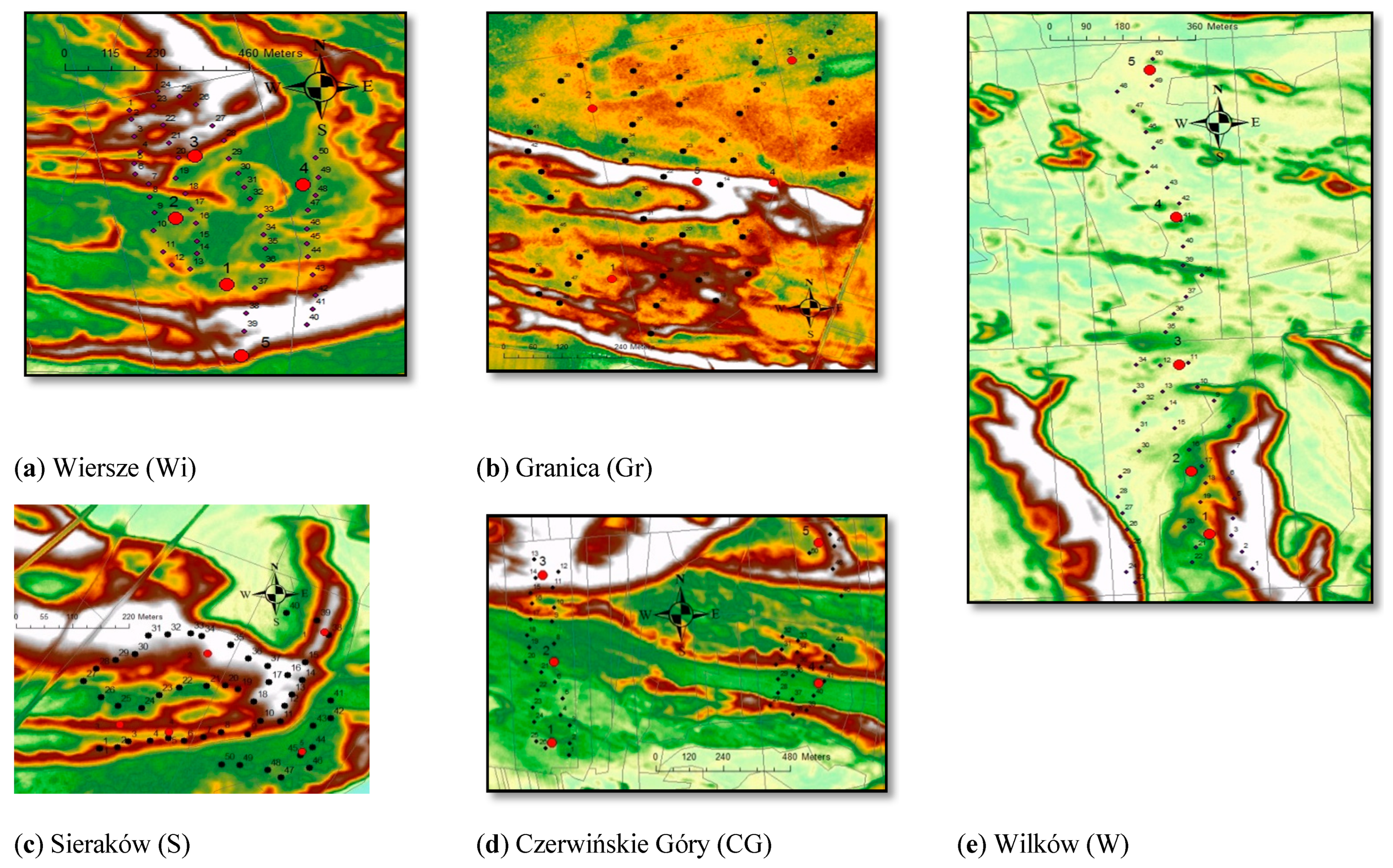
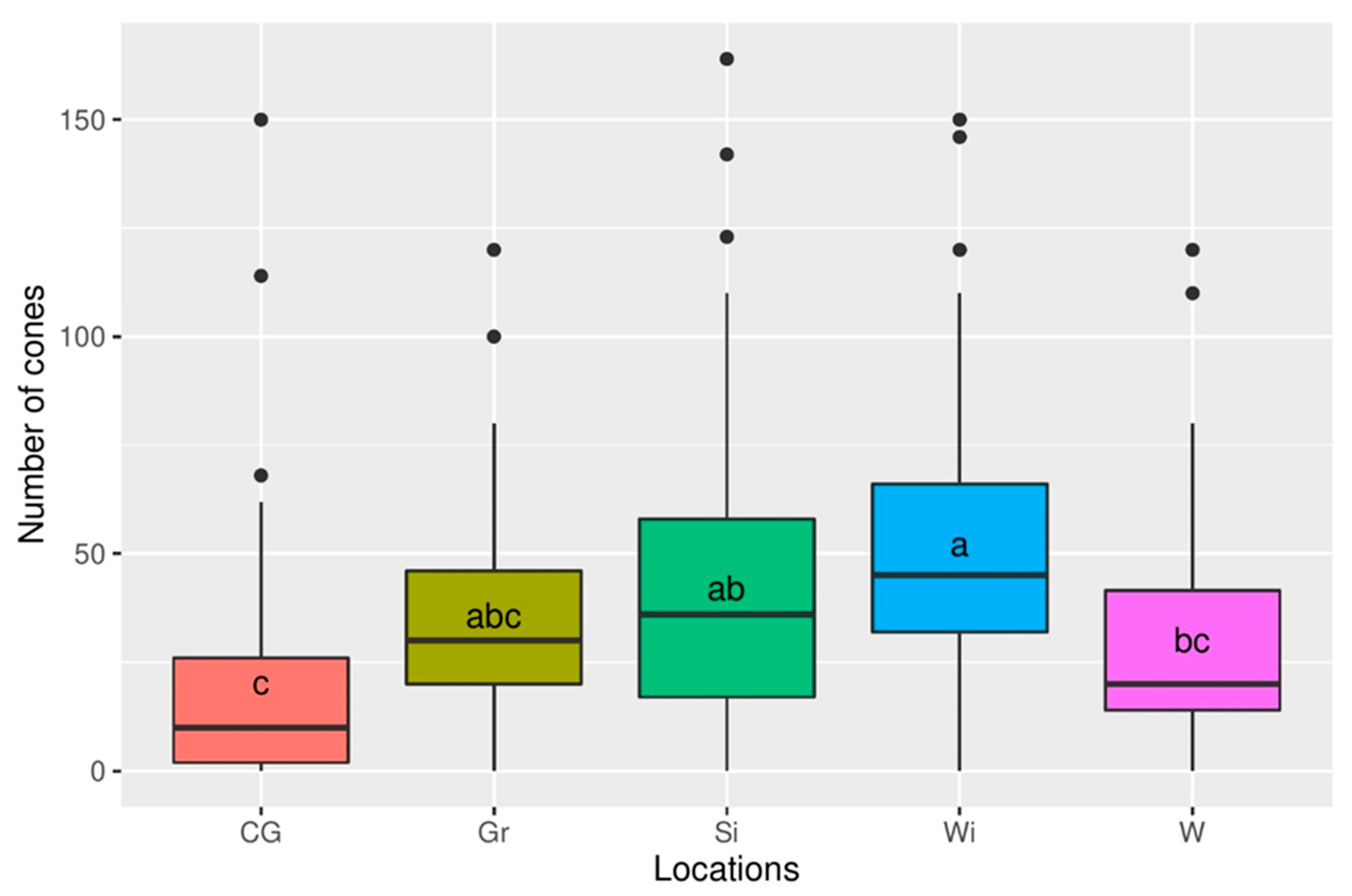
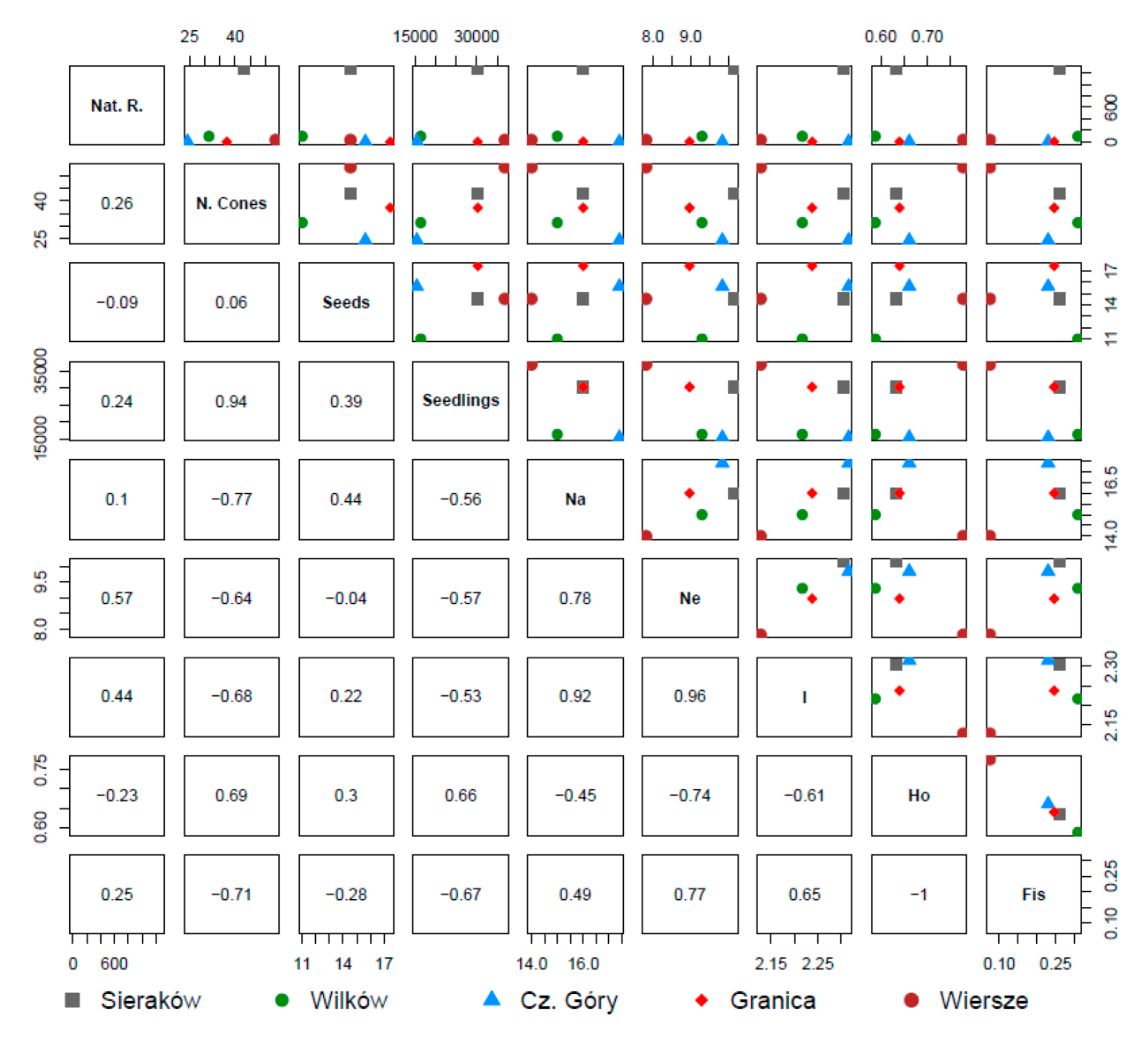
| Location | Czerwińskie Góry | Wilków | Granica | Sieraków | Wiersze |
|---|---|---|---|---|---|
| Abbreviation | CG | W | Gr | S | Wi |
| Coordinates | 20°23′36.67′′ E 52°20′27.693′′ N | 20°32′34.005′′ E 52°21′45.607′′ N | 20°27′50.019′′ E 52°17′21.605′′ N | 20°46′34.957′′ E 52°20′12.144′′ N | 20°39′45.706′′ E 52°18′34.157′′ N |
| Age * of the dominant P. sylvestris | 200–210 (avg.: 205) | 180–200 (avg.: 190) | 160–170 (avg.: 165) | 190–200 (avg.: 195) | app. 160 |
| Plant community | Querco roboris-Pinetum | Querco roboris-Pinetum. | SNFPC/Querco Carpinetum | Querco roboris-Pinetum | Querco roboris-Pinetum |
| Dominant soil type | AP, partially DBA | AP, partially DBA | AP, partially Lv | AP, partially DBA | AP, partially DBA |
| Loci | Repeat Motif. | Starter Sequences | Product Size |
|---|---|---|---|
| SPAG 7.14(VIC) | (TG)17(AG)21 | F: TTCGTAGGACTAAAAATGTGTG R: CAAAGTGGATTTTGACCG | 113–189 |
| SPAC 11.6(NED) | (CA)29(TA)7 | F: CTTCACAGGACTGATGTTCA R: TTACAGCGGTTGGTAAATG | 131–167 |
| NZPR 11.4(6-FAM) | (CA)15(CA)13(TA)22 | F: AAGATGACCCACATGAAGTTTGG R: GGAGCTTTATAACATATCTCGATGC | 180–236 |
| SsrPt_ctg4363(VIC) | (AT)10 | F: TAATAATTCAAGCCACCCCG R: AGCAGGCTAATAACAACACGC | 86–112 |
| PtTX3107(PET) | (CAT)14 | F: AAACAAGCCCACATCGTCAATC R: TCCCCTGGATCTGAGGA | 150–174 |
| Research Plots | CG | Gr | S | Wi | W | Total |
|---|---|---|---|---|---|---|
| Carpinus betulus | 1 | 1 | ||||
| Corylus avellana | 7 | 7 | ||||
| Frangula alnus | 34 | 19 | 26 | 5 | 84 | |
| Juniperus communis | 1 | 1 | ||||
| Pinus sylvestris | 32 | 1 | 3 | 36 | ||
| Pyrus pyraster | 1 | 1 | ||||
| Quercus robur | 6 | 2 | 5 | 10 | 3 | 26 |
| Sorbus aucuparia | 2 | 2 | ||||
| Viburnum opulus | 14 | 14 | ||||
| Total | 41 | 45 | 63 | 16 | 7 | 172 |
| Population | Samples | Na | Ne | I | Ho | Fis | |
|---|---|---|---|---|---|---|---|
| S | Mean | 50 | 16.000 | 10.141 | 2.304 | 0.634 | 0.260 * |
| SE | 3.507 | 3.205 | 0.311 | 0.089 | |||
| W | Mean | 50 | 15.000 | 9.295 | 2.217 | 0.588 | 0.309 * |
| SE | 3.606 | 2.764 | 0.321 | 0.056 | |||
| CG | Mean | 50 | 17.400 | 9.833 | 2.316 | 0.661 | 0.230 * |
| SE | 4.130 | 2.795 | 0.322 | 0.079 | |||
| Gr | Mean | 50 | 16.000 | 8.967 | 2.238 | 0.640 | 0.246 * |
| SE | 3.536 | 2.779 | 0.298 | 0.091 | |||
| Wi | Mean | 47 | 14.000 | 7.819 | 2.128 | 0.777 | 0.075 |
| SE | 3.755 | 2.133 | 0.308 | 0.081 |
| Locus | Null Present | Oosterhout et al., 2004 [38] | Chakraborty et al., 1992 [42] | Brookfield 1996a [43] | Brookfield 1996b [43] |
|---|---|---|---|---|---|
| PtTX3107 | no | 0.0926 | 0.1081 | 0.0764 | 0.5257 |
| SsrPt_ctg4363 | no | –0.1321 | –0.1065 | –0.0998 | 0.2675 |
| SPAG 7.14 | no | –0.0513 | –0.0393 | –0.0382 | 0.2629 |
| NZPR 11.4 | no | 0.0141 | 0.0125 | 0.0116 | 0.3135 |
| SPAC 11.6 | yes | 0.1569 | 0.1847 | 0.1498 | 0.4661 |
| Populations | S | W | CG | Gr | Wi |
|---|---|---|---|---|---|
| S | 0.000 | ns | Ns | ns | 0.000 |
| W | 0.000 | 0.000 | Ns | ns | 0.000 |
| CG | 0.001 | 0.003 | 0.000 | ns | 0.001 |
| Gr | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 |
| Wi | 0.026 | 0.026 | 0.019 | 0.017 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybylski, P.; Konatowska, M.; Jastrzębowski, S.; Tereba, A.; Mohytych, V.; Tyburski, Ł.; Rutkowski, P. The Possibility of Regenerating a Pine Stand through Natural Regeneration. Forests 2021, 12, 1055. https://doi.org/10.3390/f12081055
Przybylski P, Konatowska M, Jastrzębowski S, Tereba A, Mohytych V, Tyburski Ł, Rutkowski P. The Possibility of Regenerating a Pine Stand through Natural Regeneration. Forests. 2021; 12(8):1055. https://doi.org/10.3390/f12081055
Chicago/Turabian StylePrzybylski, Paweł, Monika Konatowska, Szymon Jastrzębowski, Anna Tereba, Vasyl Mohytych, Łukasz Tyburski, and Paweł Rutkowski. 2021. "The Possibility of Regenerating a Pine Stand through Natural Regeneration" Forests 12, no. 8: 1055. https://doi.org/10.3390/f12081055
APA StylePrzybylski, P., Konatowska, M., Jastrzębowski, S., Tereba, A., Mohytych, V., Tyburski, Ł., & Rutkowski, P. (2021). The Possibility of Regenerating a Pine Stand through Natural Regeneration. Forests, 12(8), 1055. https://doi.org/10.3390/f12081055






