Species Identity of Large Trees Affects the Composition and the Spatial Structure of Adjacent Trees
Abstract
:1. Introduction
2. Materials and Methods
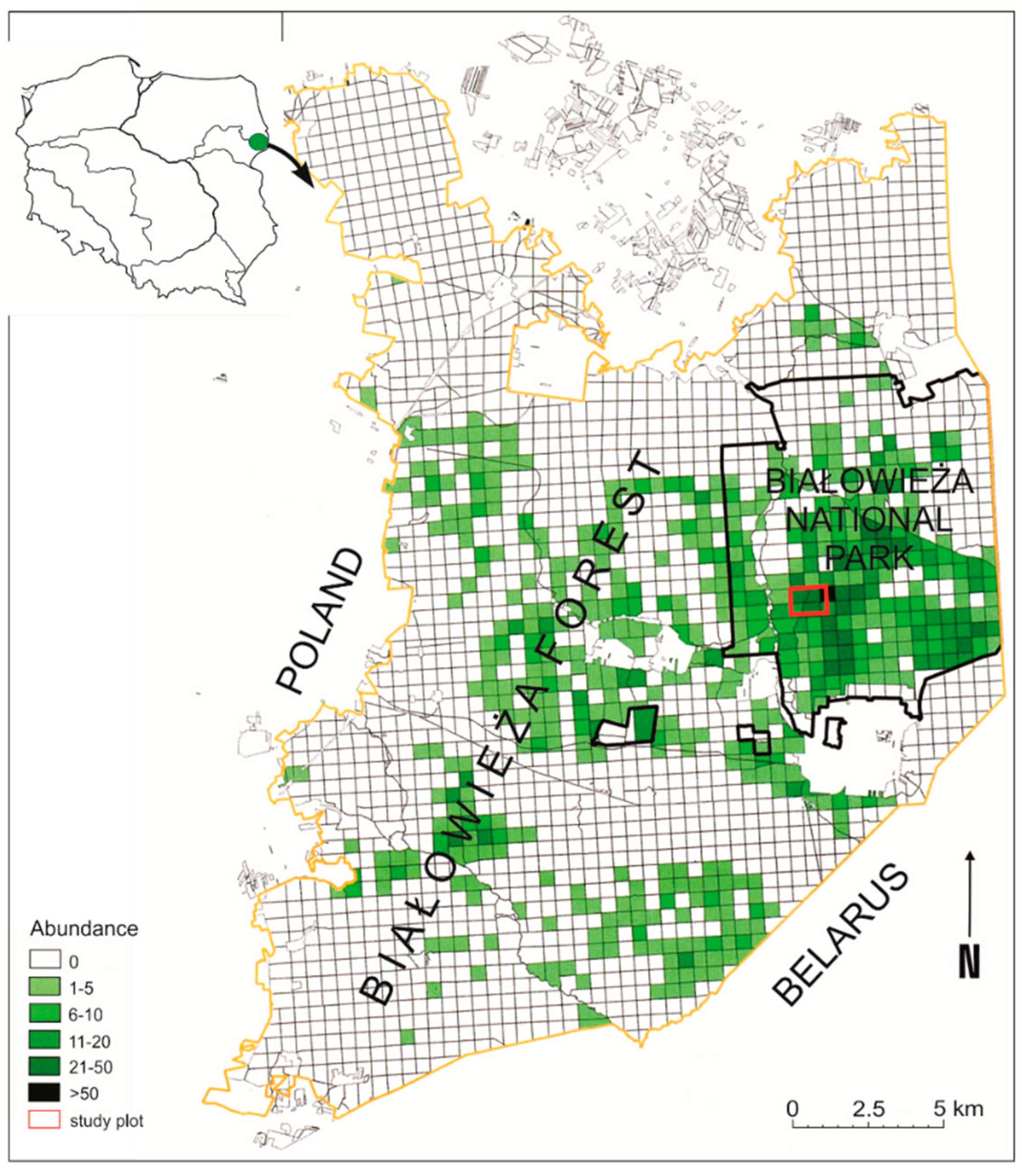
2.1. Study Site
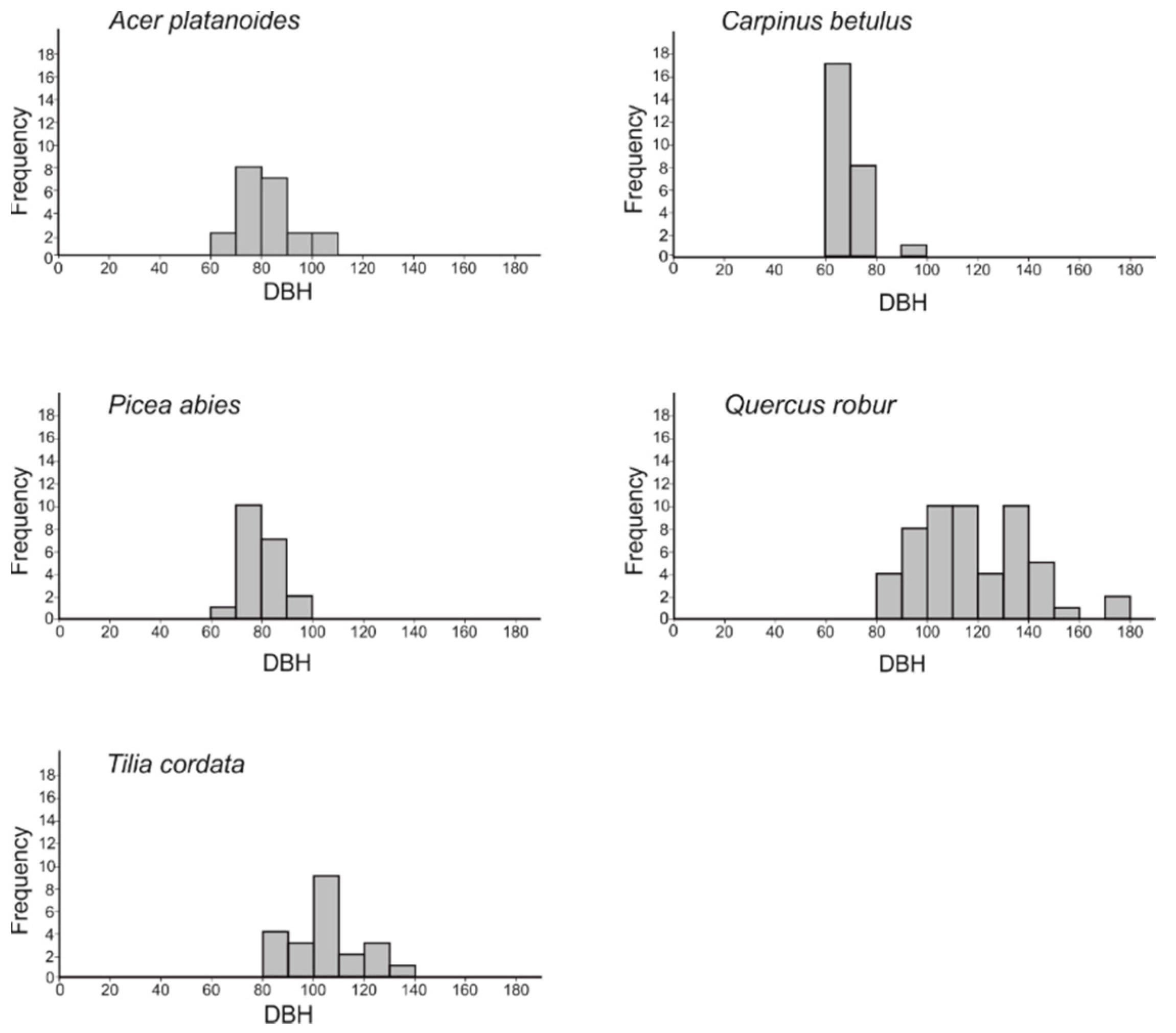
2.2. Field Measurements
2.3. Statistical Analyses
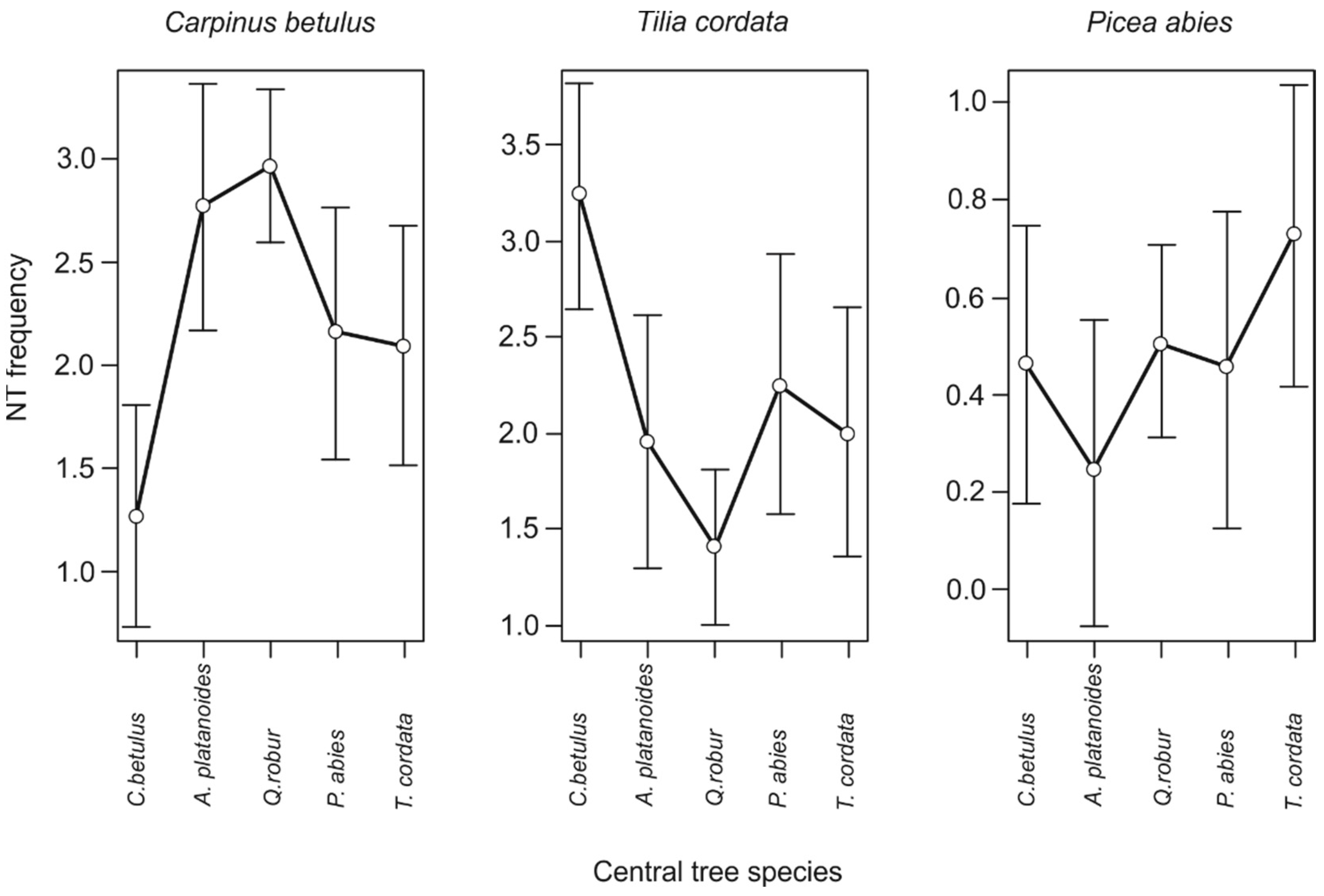
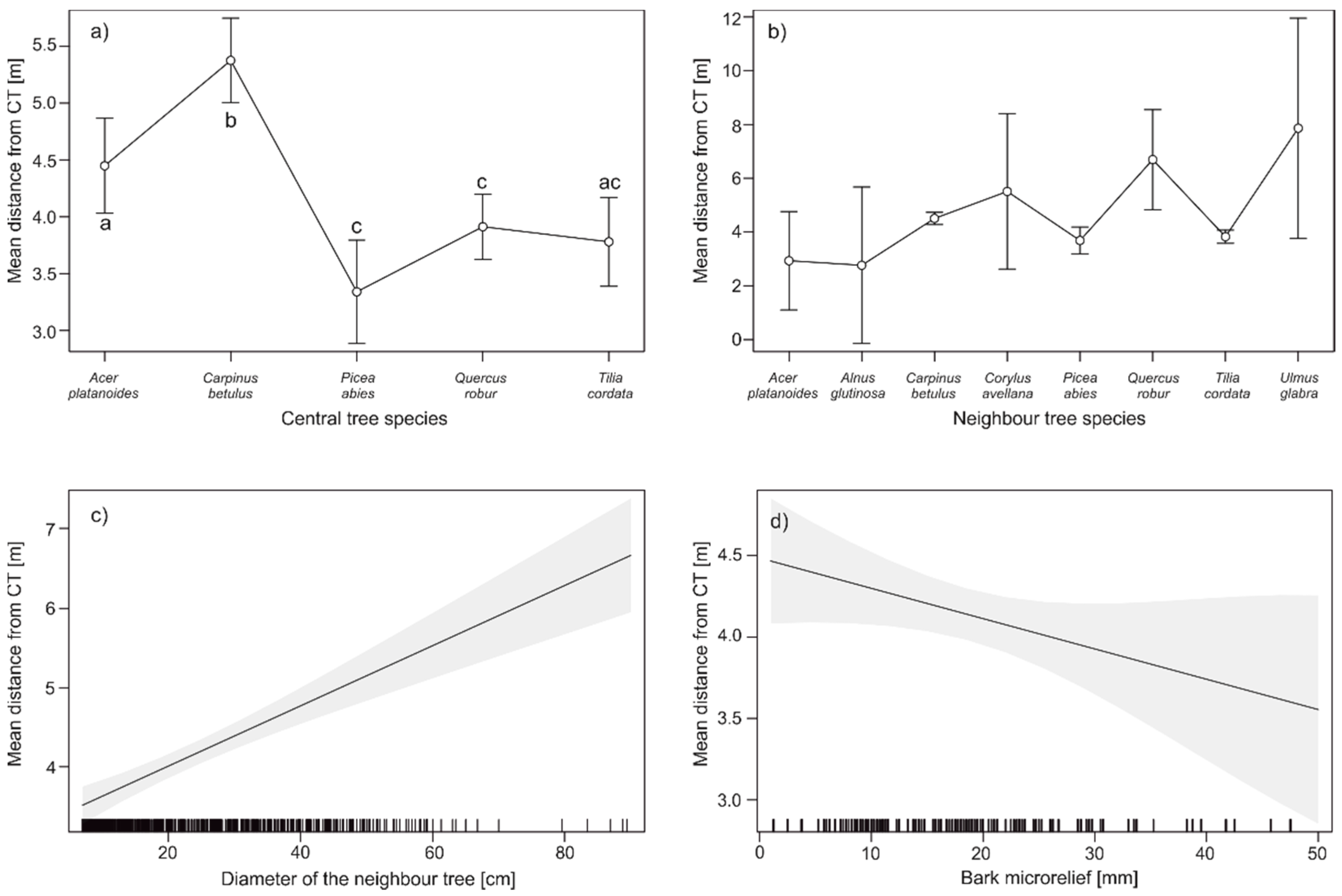
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| CT Species | N | CT DBH | NT DBH | CT–NT | Mi | NT SR |
|---|---|---|---|---|---|---|
| Acer platanoides | 21 | 81.96 (10.88) | 25.23 (16.91) | 4.66 (2.49) | 1.00 (0.00) | 2.1 (0.7) |
| Carpinus betulus | 26 | 68.69 (6.95) | 25.66 (17.37) | 5.32 (2.49) | 0.75 (0.30) | 1.9 (0.7) |
| Picea abies | 20 | 79.54 (8.25) | 21.17 (14.40) | 3.37 (1.85) | 0.90 (0.14) | 2.0 (0.5) |
| Quercus robur | 55 | 117.32 (22.10) | 22.42 (13.65) | 3.85 (2.08) | 0.99 (0.04) | 2.1 (0.7) |
| Tilia cordata | 22 | 104.33 (13.84) | 25.96 (15.17) | 3.78 (2.13) | 0.60 (0.31) | 2.3 (0.8) |
References
- Nolan, V.; Reader, T.; Gilbert, F.; Atkinson, N. The Ancient Tree Inventory: A Summary of the Results of a 15 Year Citizen Science Project Recording Ancient, Veteran and Notable Trees across the UK. Biodivers. Conserv. 2020, 29, 3103–3129. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F.; Likens, G.E.; Banks, S.C.; Blanchard, W.; Gibbons, P.; Ikin, K.; Blair, D.; McBurney, L.; et al. New Policies for Old Trees: Averting a Global Crisis in a Keystone Ecological Structure: Rapid Loss of Large Old Trees. Conserv. Lett. 2014, 7, 61–69. [Google Scholar] [CrossRef]
- Müller, J.; Jarzabek-Müller, A.; Bussler, H.; Gossner, M.M. Hollow Beech Trees Identified as Keystone Structures for Saproxylic Beetles by Analyses of Functional and Phylogenetic Diversity. Anim. Conserv. 2014, 17, 154–162. [Google Scholar] [CrossRef]
- Stagoll, K.; Lindenmayer, D.B.; Knight, E.; Fischer, J.; Manning, A.D. Large Trees Are Keystone Structures in Urban Parks. Conserv. Lett. 2012, 5, 115–122. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal Species Diversity Driven by Habitat Heterogeneity/Diversity: The Importance of Keystone Structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Hageneder, F. The Heritage of Trees: History, Culture and Symbolism; Floris: Edinburgh, UK, 2001; ISBN 978-0-86315-359-4. [Google Scholar]
- Dreslerová, J. Memorial Trees in The Czech Landscape. J. Landsc. Ecol. 2017, 10, 79–108. [Google Scholar] [CrossRef] [Green Version]
- Fay, N. Conservation Arboriculture Learning from Old Trees, Artists and Dead Poets. Arborists News 2011, 20, 53–59. [Google Scholar]
- Scipioni, M.C.; de Salomão, R.P.; Vibrans, A.C.; Uller, H.F. Decline in Giant Tree Numbers: Status Report for Santa Catarina State and Perspectives for Brazil. Floresta Ambient. 2019, 26, e20190039. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F. Global Decline in Large Old Trees. Science 2012, 338, 1305–1306. [Google Scholar] [CrossRef]
- Palik, B.J.; Ostry, M.E.; Venette, R.C.; Abdela, E. Fraxinus Nigra (Black Ash) Dieback in Minnesota: Regional Variation and Potential Contributing Factors. For. Ecol. Manag. 2011, 261, 128–135. [Google Scholar] [CrossRef]
- Simard, M.; Powell, E.N.; Raffa, K.F.; Turner, M.G. What Explains Landscape Patterns of Tree Mortality Caused by Bark Beetle Outbreaks in Greater Yellowstone?: Landscape Patterns of Bark Beetle Outbreaks. Glob. Ecol. Biogeogr. 2012, 21, 556–567. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Mazziotta, A.; Coletti, G.; Luiselli, L.; Audisio, P. Conflict between Insect Conservation and Public Safety: The Case Study of a Saproxylic Beetle (Osmoderma Eremita) in Urban Parks. J. Insect Conserv. 2010, 14, 555–565. [Google Scholar] [CrossRef]
- Miklín, J.; Čížek, L. Erasing a European Biodiversity Hot-Spot: Open Woodlands, Veteran Trees and Mature Forests Succumb to Forestry Intensification, Succession, and Logging in a UNESCO Biosphere Reserve. J. Nat. Conserv. 2014, 22, 35–41. [Google Scholar] [CrossRef]
- Lefèvre, F. Human Impacts on Forest Genetic Resources in the Temperate Zone: An Updated Review. For. Ecol. Manag. 2004, 197, 257–271. [Google Scholar] [CrossRef]
- Wojnicka-Półtorak, A.; Wachowiak, W.; Prus-Głowacki, W.; Celiński, K.; Korczyk, A. Genetic Heterogeneity in Age Classes of Naturally Regenerated Old Growth Forest of Picea abies (L.) Karst. Silvae Genet. 2014, 63, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Nowakowska, J.A.; Hsiang, T.; Patynek, P.; Stereńczak, K.; Olejarski, I.; Oszako, T. Health Assessment and Genetic Structure of Monumental Norway Spruce Trees during A Bark Beetle (Ips typographus L.) Outbreak in the Białowieża Forest District, Poland. Forests 2020, 11, 647. [Google Scholar] [CrossRef]
- Manning, A.D.; Fischer, J.; Lindenmayer, D.B. Scattered Trees Are Keystone Structures—Implications for Conservation. Biol. Conserv. 2006, 132, 311–321. [Google Scholar] [CrossRef]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lachat, T.; Michel, A.K.; Regnery, B.; Vandekerkhove, K. Tree Related Microhabitats in Temperate and Mediterranean European Forests: A Hierarchical Typology for Inventory Standardization. Ecol. Indic. 2018, 84, 194–207. [Google Scholar] [CrossRef]
- Paillet, Y.; Archaux, F.; Boulanger, V.; Debaive, N.; Fuhr, M.; Gilg, O.; Gosselin, F.; Guilbert, E. Snags and Large Trees Drive Higher Tree Microhabitat Densities in Strict Forest Reserves. For. Ecol. Manag. 2017, 389, 176–186. [Google Scholar] [CrossRef]
- Úradníček, L.; Šrámek, M.; Dreslerová, J. Checklist of Champion Trees in The Czech Republic. J. Landsc. Ecol. 2017, 10, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Siitonen, J.; Ranius, T. The importance of veteran trees for saproxylic insects. In Europe’s Changing Woods and Forests: From Wildwood to Managed Landscapes; Kirby, K.J., Watkins, C., Eds.; CABI: Wallingford, UK, 2015; pp. 140–153. ISBN 978-1-78064-337-3. [Google Scholar]
- Skarpaas, O.; Blumentrath, S.; Evju, M.; Sverdrup-Thygeson, A. Prediction of Biodiversity Hotspots in the Anthropocene: The Case of Veteran Oaks. Ecol. Evol. 2017, 7, 7987–7997. [Google Scholar] [CrossRef]
- Zapponi, L.; Mazza, G.; Farina, A.; Fedrigoli, L.; Mazzocchi, F.; Roversi, P.F.; Peverieri, G.S.; Mason, F. The Role of Monumental Trees for the Preservation of Saproxylic Biodiversity: Re-Thinking Their Management in Cultural Landscapes. Nat. Conserv. 2017, 19, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Pilskog, H.E.; Birkemoe, T.; Framstad, E.; Sverdrup-Thygeson, A. Effect of Habitat Size, Quality, and Isolation on Functional Groups of Beetles in Hollow Oaks. J. Insect. Sci. 2016, 16, 26. [Google Scholar] [CrossRef]
- Wetherbee, R.; Birkemoe, T.; Sverdrup-Thygeson, A. Veteran Trees Are a Source of Natural Enemies. Sci. Rep. 2020, 10, 18485. [Google Scholar] [CrossRef] [PubMed]
- Bastin, J.-F.; Barbier, N.; Réjou-Méchain, M.; Fayolle, A.; Gourlet-Fleury, S.; Maniatis, D.; de Haulleville, T.; Baya, F.; Beeckman, H.; Beina, D.; et al. Seeing Central African Forests through Their Largest Trees. Sci. Rep. 2015, 5, 13156. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.A.; Larson, A.J.; Swanson, M.E.; Freund, J.A. Ecological Importance of Large-Diameter Trees in a Temperate Mixed-Conifer Forest. PLoS ONE 2012, 7, e36131. [Google Scholar] [CrossRef]
- Cieśliński, S.; Tobolewski, Z. Porosty (Lichenes) Puszczy Białowieskiej i Jej Zachodniego Przedpola [Lichens of Białowieża Forest and Its Western Foreground]. Phytocoenosis 1988, 1, 1–216. [Google Scholar]
- Rostamian, M.; Kavosi, M.R. The Effect of Trees Diameter on Establishment, Diversity and Richness of Bracket Fungi in Golestan Province Forest, North of Iran. J. Biodivers. Ecol. Sci. 2013, 3, 99–105. [Google Scholar]
- Mildrexler, D.J.; Berner, L.T.; Law, B.E.; Birdsey, R.A.; Moomaw, W.R. Large Trees Dominate Carbon Storage in Forests East of the Cascade Crest in the United States Pacific Northwest. Front. For. Glob. Chang. 2020, 3, 594274. [Google Scholar] [CrossRef]
- Sist, P.; Mazzei, L.; Blanc, L.; Rutishauser, E. Large Trees as Key Elements of Carbon Storage and Dynamics after Selective Logging in the Eastern Amazon. For. Ecol. Manag. 2014, 318, 103–109. [Google Scholar] [CrossRef]
- Yuan, Z.; Ali, A.; Sanaei, A.; Ruiz-Benito, P.; Jucker, T.; Fang, L.; Bai, E.; Ye, J.; Lin, F.; Fang, S.; et al. Few Large Trees, Rather than Plant Diversity and Composition, Drive the above-Ground Biomass Stock and Dynamics of Temperate Forests in Northeast China. For. Ecol. Manag. 2021, 481, 118698. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.-D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-Growth Forests as Global Carbon Sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.L.; Das, A.J.; Condit, R.; Russo, S.E.; Baker, P.J.; Beckman, N.G.; Coomes, D.A.; Lines, E.R.; Morris, W.K.; Rüger, N.; et al. Rate of Tree Carbon Accumulation Increases Continuously with Tree Size. Nature 2014, 507, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Forrester, D.I. Does Individual-Tree Biomass Growth Increase Continuously with Tree Size? For. Ecol. Manag. 2021, 481, 118717. [Google Scholar] [CrossRef]
- Ali, A.; Lin, S.; He, J.; Kong, F.; Yu, J.; Jiang, H. Big-sized Trees Overrule Remaining Trees’ Attributes and Species Richness as Determinants of Aboveground Biomass in Tropical Forests. Glob. Chang. Biol. 2019, 25, 2810–2824. [Google Scholar] [CrossRef] [PubMed]
- Bastias, C.C.; Truchado, D.A.; Valladares, F.; Benavides, R.; Bouriaud, O.; Bruelheide, H.; Coppi, A.; Finér, L.; Gimeno, T.E.; Jaroszewicz, B.; et al. Species Richness Influences the Spatial Distribution of Trees in European Forests. Oikos 2020, 129, 380–390. [Google Scholar] [CrossRef]
- Gorman, G. Identifying the Presence of Woodpecker (Picidae) Species on the Basis of Their Holes and Signs. Aquila 1995, 102, 61–67. [Google Scholar]
- Winkler, H. Das Schmiedeverhalten Des Blutspechtes (Dendrocopos syriacus). Egretta 1967, 10, 1–8. [Google Scholar]
- Wiegand, T.; Gunatilleke, S.; Gunatilleke, N. Species Associations in a Heterogeneous Sri Lankan Dipterocarp Forest. Am. Nat. 2007, 170, E77–E95. [Google Scholar] [CrossRef] [Green Version]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in Nutrient Dynamics of Forest Ecosystems—A Review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Hane, E.N.; Hamburg, S.P.; Barber, A.L.; Plaut, J.A. Phytotoxicity of American Beech Leaf Leachate to Sugar Maple Seedlings in a Greenhouse Experiment. Can. J. For. Res. 2003, 33, 814–821. [Google Scholar] [CrossRef]
- Pigott, C.D. Natural Regeneration of Tilia Cordata in Relation to Forest-Structure in the Forest of Białowieża, Poland. Philos. Trans. R. Soc. Lond. B 1975, 270, 151–179. [Google Scholar] [CrossRef]
- Faliński, J.B. Vegetation Dynamics in Temperate Lowland Primeval Forests; Springer: Dordrecht, The Netherlands, 1986; ISBN 978-94-010-8631-8. [Google Scholar]
- McCarthy-Neumann, S.; Kobe, R.K. Conspecific and Heterospecific Plant-Soil Feedbacks Influence Survivorship and Growth of Temperate Tree Seedlings. J. Ecol. 2010, 98, 408–418. [Google Scholar] [CrossRef]
- Packer, A.; Clay, K. Soil Pathogens and Spatial Patterns of Seedling Mortality in a Temperate Tree. Nature 2000, 404, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Iwamoto, S.; Seiwa, K. Distance- and Density-Dependent Seedling Mortality Caused by Several Diseases in Eight Tree Species Co-Occurring in a Temperate Forest. Plant Ecol. 2009, 201, 181–196. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the Number of Tree Species in Tropical Forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J.H. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forests. In Dynamics of Populations; den Boer, P.J., Gradwell, G.R., Eds.; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1971; pp. 298–312. [Google Scholar]
- Comita, L.S.; Queenborough, S.A.; Murphy, S.J.; Eck, J.L.; Xu, K.; Krishnadas, M.; Beckman, N.; Zhu, Y. Testing Predictions of the J Anzen—C Onnell Hypothesis: A Meta-analysis of Experimental Evidence for Distance- and Density-dependent Seed and Seedling Survival. J. Ecol. 2014, 102, 845–856. [Google Scholar] [CrossRef]
- Pommerening, A.; Sánchez Meador, A.J. Tamm Review: Tree Interactions between Myth and Reality. For. Ecol. Manag. 2018, 424, 164–176. [Google Scholar] [CrossRef]
- Wang, H.; Peng, H.; Hui, G.; Hu, Y.; Zhao, Z. Large Trees are Surrounded by More Heterospecific Neighboring Trees in Korean Pine Broad-Leaved Natural Forests. Sci. Rep. 2018, 8, 9149. [Google Scholar] [CrossRef]
- Pommerening, A.; Uria-Diez, J. Do Large Forest Trees Tend towards High Species Mingling? Ecol. Inform. 2017, 42, 139–147. [Google Scholar] [CrossRef]
- Trogisch, S.; Liu, X.; Rutten, G.; Xue, K.; Bauhus, J.; Brose, U.; Bu, W.; Cesarz, S.; Chesters, D.; Connolly, J.; et al. The Significance of Tree-Tree Interactions for Forest Ecosystem Functioning. Basic Appl. Ecol. 2021. [Google Scholar] [CrossRef]
- Jaroszewicz, B.; Cholewińska, O.; Gutowski, J.M.; Samojlik, T.; Zimny, M.; Latałowa, M. Białowieża Forest—A Relic of the High Naturalness of European Forests. Forests 2019, 10, 849. [Google Scholar] [CrossRef] [Green Version]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where Are Europe’s Last Primary Forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef] [Green Version]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Upaccessd. Metz 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Boczoń, A. Charakterystyka warunków termiczno-pluwialnych w Puszczy Białowieskiej w latach 1950–2003. [Characteristics of thermal and pluvial conditions in the Bialowieza Primeval Forest between 1950 and 2003]. Leśne Prace Badawcze 2006, 1, 57–72. [Google Scholar]
- Niechoda, T.; Aleksiejczuk, A.; Chołuj, P.; Wróblewski, K. Puszcza Gigantów: Rzecz o Białowieskich Dębach; Biuro Ekspertyz Przyrodniczo-Leśnych: Warszawa, Poland, 2019; ISBN 978-83-953166-0-9. [Google Scholar]
- Grzywacz, A.; Keczyński, A.; Szczepkowski, A.; Bolibok, L.; Buraczyk, W.; Drozdowski, S.; Gawron, L.; Szeligowski, H.; Zajączkowski, J. Pomnikowe drzewa w Rezerwacie Ścisłym Białowieskiego Parku Narodowego. Sylwan 2018, 162, 915–926. [Google Scholar]
- Bobiec, A.; Zuyderduyn, C.; Haga, J.; Vlaanderen, B. Rich Deciduous Forests in Białowieża as a Dynamic Mosaic of Developmental Phases: Premises for Nature Conservation and Restoration Management. For. Ecol. Manag. 2000, 130, 159–175. [Google Scholar] [CrossRef]
- Gadow, K. Forest Structure and Diversity [Waldstruktur Und Diversität]. Allg. Forst-Und Jagdztg. 1999, 170, 117–122. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio PBC: Boston, MA, USA, 2021. [Google Scholar]
- Bartoń, K. Package ‘MuMIn’. In Multi-Model; R Package v. 1.43.17; 2020; Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 28 July 2021).
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Proceedings of the 2nd International Symposium on Information Theory, Tsahkadsor, Armenia, USSR, 2–8 September 1971; Petrov, B.N., Csaki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis; R Package Version 0.8.32; 2021; Available online: https://github.com/droglenc/FSA (accessed on 28 July 2021).
- Zar, J.H. Biostatistical Analysis: Books a La Carte Edition; Prentice Hall: Hoboken, NJ, USA, 2010; ISBN 978-0-321-65686-5. [Google Scholar]
- Oksanen, J.; Guillaume, B.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; 2020; Available online: https://CRAN.R-project.org/package=vegan (accessed on 28 July 2021).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE: Los Angeles, CA, USA, 2019; ISBN 978-1-5443-3647-3. [Google Scholar]
- Mangiafico, S. Rcompanion: Functions to Support. Extension Education Program. Evaluation; R Package Version 2.3.27; 2021; Available online: https://CRAN.R-project.org/package=rcompanion (accessed on 28 July 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Ledo, A. Nature and Age of Neighbours Matter: Interspecific Associations among Tree Species Exist and Vary across Life Stages in Tropical Forests. PLoS ONE 2015, 10, e0141387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punchi-Manage, R.; Wiegand, T.; Wiegand, K.; Getzin, S.; Huth, A.; Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N. Neighborhood Diversity of Large Trees Shows Independent Species Patterns in a Mixed Dipterocarp Forest in Sri Lanka. Ecology 2015, 96, 1823–1834. [Google Scholar] [CrossRef]
- De Jaegere, T.; Hein, S.; Claessens, H. A Review of the Characteristics of Small-Leaved Lime (Tilia Cordata Mill.) and Their Implications for Silviculture in a Changing Climate. Forests 2016, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Radoglou, K.; Dobrowolska, D.; Spyroglou, G.; Nicolescu, V.-N. A Review on the Ecology and Silviculture of Limes (Tilia Cordata Mill., Tilia Platyphyllos Scop. and Tilia Tomentosa Moench.) in Europe; EU COST Action: 2008; p. 29. Available online: http://www.valbro.uni-freiburg.de/re_publications.php (accessed on 28 July 2021).
- Chistyakova, A.A. Bolshoj Hziznennyj Cikl Tilia Cordata Mill. [High Life Cycle of Tilia Cordata Mill.]. Mosk. Obsc. Ispyt. Prir. Otd. Biol. 1979, 84, 85–98. [Google Scholar]
- Chistyakova, A.A. Biologicheskije Osobennosti Vegetativnogo Vozobnovienia Osnovnykh Porod v Shirokolistvennych Lesakh [Biological Features of Vegetative Reproduction of Basic Species of the Deciduous Forests]. Lesovedenye 1982, 2, 11–17. [Google Scholar]
- Pawlaczyk, P. Wegetatywne Odnowienie Lipy Drobnolistnej (Tilia Cordata Mill.) i Jego Znaczenie Ekologiczne w Grądzie w Białowieskim Parku Narodowym [Vegetative Regeneration of Small-Leaved Lime (Tilia Cordata Mill.) and Its Ecological Role in Mixed Deciduous Forest of the Białowieża National Park]. Phytocoenosis 1991, 3, 161–171. [Google Scholar]
- Szmyt, J. Structural Diversity of Plant Populations: Insight from Spatial Analyses. In Applications of Spatial Statistics; Hung, M.-C., Ed.; InTech: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Verheyen, K.; Baeten, L.; De Frenne, P.; Bernhardt-Römermann, M.; Brunet, J.; Cornelis, J.; Decocq, G.; Dierschke, H.; Eriksson, O.; Hédl, R.; et al. Driving Factors behind the Eutrophication Signal in Understorey Plant Communities of Deciduous Temperate Forests: Drivers of Change in Forest Understorey Vegetation. J. Ecol. 2012, 100, 352–365. [Google Scholar] [CrossRef]
- Brzeziecki, B.; Woods, K.; Bolibok, L.; Zajączkowski, J.; Drozdowski, S.; Bielak, K.; Żybura, H. Over 80 Years without Major Disturbance, Late-successional Białowieża Woodlands Exhibit Complex Dynamism, with Coherent Compositional Shifts towards True Old-growth Conditions. J. Ecol. 2020, 108, 1138–1154. [Google Scholar] [CrossRef]
- Bubnicki, J.W.; Churski, M.; Schmidt, K.; Diserens, T.A.; Kuijper, D.P. Linking Spatial Patterns of Terrestrial Herbivore Community Structure to Trophic Interactions. eLife 2019, 8, e44937. [Google Scholar] [CrossRef] [PubMed]
- Hedwall, P.-O.; Churski, M.; Jędrzejewska, B.; Miścicki, S.; Kuijper, D.P.J. Functional Composition of Temperate Forest Trees under Chronic Ungulate Herbivory. J. Veg. Sci. 2018, 29, 179–188. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Wright, S.J.; Hernández, A.; Reich, P.B. Does Relatedness Matter? Phylogenetic Density-Dependent Survival of Seedlings in a Tropical Forest. Ecology 2014, 95, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Bolibok, L.; Brzeziecki, B. Analiza Wybranych Zależności Allometrycznych Dla Głównych Gatunków Drzew Białowieskiego Parku Narodowego [An Analysis of Selected Allometric Relationships for Main Tree Species of the Białowieża National Park]. Sylwan 2000, 144, 73–81. [Google Scholar]

| Coefficients: | Estimate | Std Error | t Value | Pr (>|t|) | Significance |
|---|---|---|---|---|---|
| Intercept | 9.658 × 10−1 | 1.310 × 10−1 | 7.374 | 1.54 × 10−11 | *** |
| hor | 9.465 × 10−1 | 3.777 × 10−1 | 2.506 | 0.013 | * |
| map | 3.423 × 10−2 | 3.303 × 10−1 | 0.104 | 0.918 | - |
| spr | 8.282 × 10−2 | 4.170 × 10−1 | 0.199 | 0.843 | - |
| lim | −6.691 × 10−1 | 3.231 × 10−1 | −2.071 | 0.040 | * |
| CT diameter | 7.313 × 10−5 | 3.493 × 10−4 | 0.209 | 0.834 | - |
| hor:CT_DBH | −5.477 × 10−3 | 1.671 × 10−3 | −3.278 | 0.001 | ** |
| map:CT_DBH | −7.313 × 10−5 | 1.219 × 10−3 | −0.060 | 0.952 | - |
| spr:CT_DBH | −6.677 × 10−4 | 1.614 × 10−3 | −0.414 | 0.680 | - |
| lim:CT_DBH | 8.522 × 10−4 | 9.593 × 10−4 | 0.888 | 0.376 | - |
| Comparison (CT Species) | Mingling~CTspecies | CTdistance~CTspecies | ||
|---|---|---|---|---|
| Z | p adj. | Z | p adj. | |
| hor—lim | 2.0349 | 5.23217 × 10−2 | 4.4204 | 3.28456 × 10−5 |
| hor—map | −4.2700 | 4.88753 × 10−5 | −2.1248 | 4.80051 × 10−2 |
| hor—oak | −4.9893 | 2.01919 × 10−6 | 5.6384 | 8.58302 × 10−8 |
| hor—spr | −1.6514 | 1.09610 × 10−1 | 5.6490 | 1.61355 × 10−7 |
| map—lim | 6.0387 | 7.76991 × 10−9 | 2.1539 | 5.20860 × 10−2 |
| map—oak | 0.2546 | 7.98990 × 10−1 | 2.8011 | 1.01867 × 10−2 |
| map—spr | 2.4376 | 2.11211 × 10−2 | 3.3889 | 1.75471 × 10−3 |
| oak—lim | 7.0441 | 1.86731 × 10−11 | −0.2435 | 8.07623 × 10−1 |
| oak—spr | 2.6666 | 1.27717 × 10−2 | −1.3088 | 2.38232 × 10−1 |
| spr—lim | 3.4978 | 9.38288 × 10−4 | −1.3056 | 2.12976 × 10−1 |
| Comparison (CT Species) | Diff | lwr | upr | p adj. |
|---|---|---|---|---|
| Carpinus betulus frequency (NT species) | ||||
| hor—map | −1.278 | −2.514 | −0.043 | 0.039 |
| hor—oak | −1.831 | −2.833 | −0.829 | 0.00001 |
| hor—lim | −1.231 | −2.450 | −0.011 | 0.047 |
| Tilia cordata frequency (NT species) | ||||
| hor—map | 1.493 | 0.368 | 2.617 | 0.003 |
| hor—oak | 1.694 | 0.782 | 2.607 | 0.00001 |
| Coefficients: | Estimate | Std Error | t Value | Pr (>|t|) | Significance |
|---|---|---|---|---|---|
| Intercept | 21.51 | 1.79 | 12.007 | <2 × 10−16 | *** |
| CT_hor | 0.83 | 0.46 | 1.796 | 0.073 | - |
| CT_map | −0.01 | 0.50 | −0.009 | 0.993 | - |
| CT_spr | −1.18 | 0.55 | −2.167 | 0.031 | * |
| CT_lim | −0.89 | 0.45 | −1.981 | 0.048 | * |
| NT_A.glutinosa | −18.89 | 3.17 | −5.957 | 4.07 × 10−9 | *** |
| NT_C.betulus | −17.05 | 1.71 | −9.949 | <2 × 10−16 | *** |
| NT_C.avellana | −16.08 | 3.18 | −5.065 | 5.23 × 10−7 | *** |
| NT_P.abies | −17.89 | 1.75 | −10.212 | <2 × 10−16 | *** |
| NT_Q.robur | −15.02 | 2.41 | −6.233 | 7.87 × 10−10 | *** |
| NT_T.cordata | −17.70 | 1.72 | −10.317 | <2 × 10−16 | *** |
| NT_U.glabra | −13.62 | 4.17 | −3.269 | 0.001 | ** |
| NT_DBH | 0.04 | 0.01 | 3.882 | 0.0001 | *** |
| bark microrelief | −0.04 | 0.02 | −1.983 | 0.048 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholewińska, O.; Keczyński, A.; Kusińska, B.; Jaroszewicz, B. Species Identity of Large Trees Affects the Composition and the Spatial Structure of Adjacent Trees. Forests 2021, 12, 1162. https://doi.org/10.3390/f12091162
Cholewińska O, Keczyński A, Kusińska B, Jaroszewicz B. Species Identity of Large Trees Affects the Composition and the Spatial Structure of Adjacent Trees. Forests. 2021; 12(9):1162. https://doi.org/10.3390/f12091162
Chicago/Turabian StyleCholewińska, Olga, Andrzej Keczyński, Barbara Kusińska, and Bogdan Jaroszewicz. 2021. "Species Identity of Large Trees Affects the Composition and the Spatial Structure of Adjacent Trees" Forests 12, no. 9: 1162. https://doi.org/10.3390/f12091162
APA StyleCholewińska, O., Keczyński, A., Kusińska, B., & Jaroszewicz, B. (2021). Species Identity of Large Trees Affects the Composition and the Spatial Structure of Adjacent Trees. Forests, 12(9), 1162. https://doi.org/10.3390/f12091162







