Biochar-Based Fertilizer Decreased Soil N2O Emission and Increased Soil CH4 Uptake in a Subtropical Typical Bamboo Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Production of Biochar-Based Fertilizer
2.4. Measuring Soil GHG Emissions
2.5. Measuring the Physicochemical Properties of Soil
2.6. Statistical Analyses
3. Results
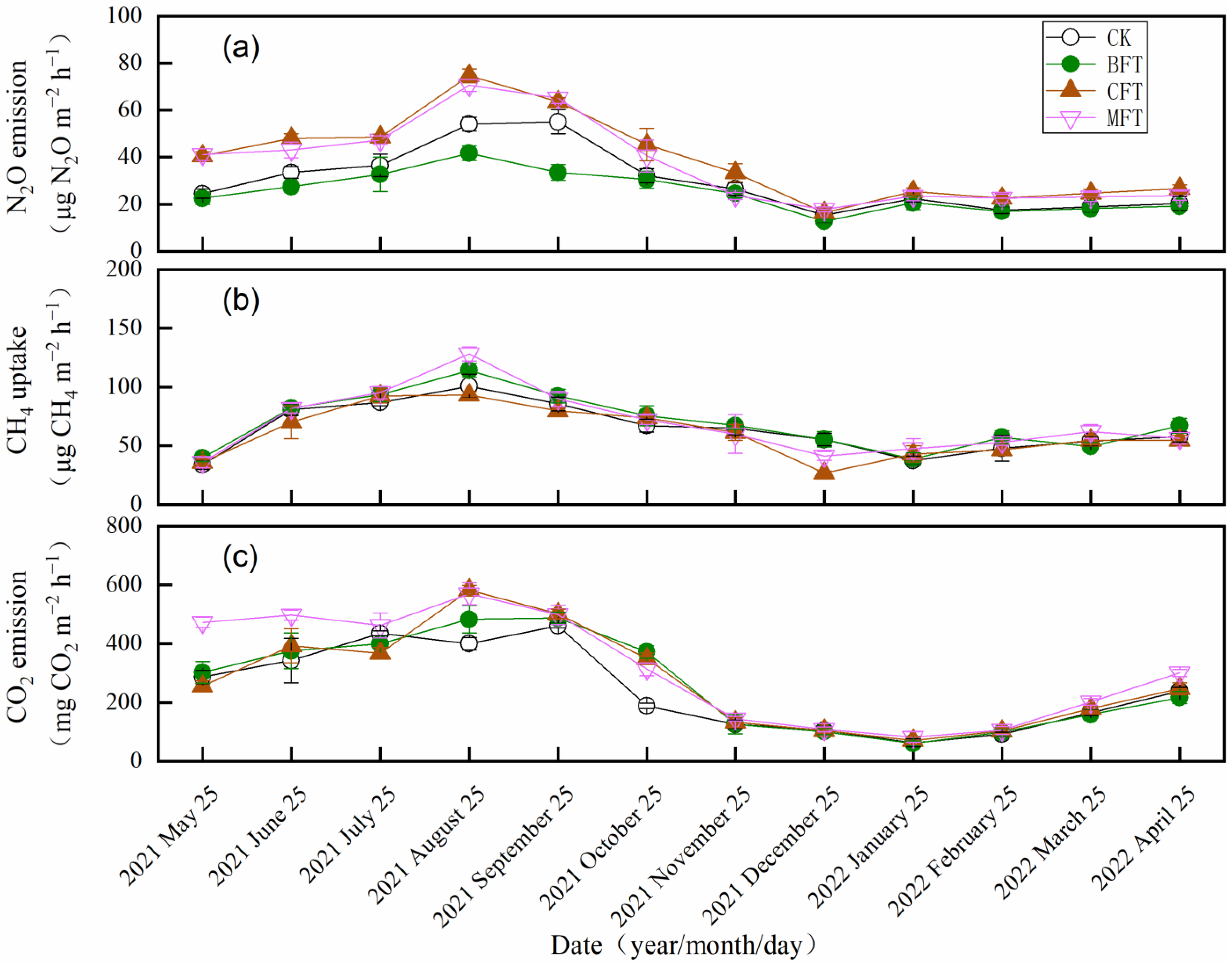
3.1. Soil GHG Emissions
3.2. Soil Environmental Factors
3.3. Relationship between Soil Environmental Factors and GHG Emissions
4. Discussion
4.1. Effects of Fertilization on Soil N2O Emission
4.2. Effects of Fertilization on Soil CH4 Uptake
4.3. Effects of Fertilization on Soil CO2 Emission
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vrrousek, P.M. Beyond Global Warming: Ecology and Global Change. Ecology 1994, 75, 1861–1876. [Google Scholar]
- Wardle, D.A.; Bardgett, R.D.; Callaway, R.M.; Van Der Putten, W.H. Terrestrial Ecosystem Responses to Species Gains and Losses. Science 2011, 332, 1273–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keenan, T.F.; Gray, J.; Friedl, M.A.; Toomey, M.; Bohrer, G.; Hollinger, D.Y.; Munger, J.W.; O’Keefe, J.; Schmid, H.P.; Wing, I.S. Net Carbon Uptake Has Increased through Warming-Induced Changes in Temperate Forest Phenology. Nat. Clim. Chang. 2014, 4, 598–604. [Google Scholar] [CrossRef]
- Fu, W.J.; Fu, Z.J.; Ge, H.L.; Ji, B.Y.; Jiang, P.K.; Li, Y.F.; Wu, J.S.; Zhao, K.L. Spatial Variation of Biomass Carbon Density in a Subtropical Region of Southeastern China. Forests 2015, 6, 1966–1981. [Google Scholar] [CrossRef] [Green Version]
- Dou, Y.; Yu, X.J. Development and Comparison of Bamboo Industry in the World. World Agric. 2008, 7, 18–21. [Google Scholar]
- Zhou, G.; Jiang, P.; Xu, Q. Carbon Sequestration and Transformation in Bamboo Forest Ecosystems, 1st ed.; Science Press: Beijing, China, 2010; pp. 2–3. [Google Scholar]
- Wu, H. Analysis on Fertilization Test of Pleioblastus amarus in Different Growth Periods. Green Sci. Technol. 2021, 23, 164–166. [Google Scholar]
- Shen, G.C.; Zhang, X.D.; Zhang, L.; Gao, S.H.; Zhang, R.; Zhu, W.S.; Tang, S.Q. Estimating the Carbon Stock and Carbon Sequestration of the Pleioblastus amarus Forest Ecosystem in Southern of Sichuan. Sci. Silvae Sin. 2013, 49, 78–84. [Google Scholar]
- He, Y.P.; Fei, S.M.; Jiang, J.M.; Chen, X.M.; Yu, Y.; Zhu, W.S.; Tang, S.Q. The Spatial Distribution of Organic Carbon in Phyllostachyspubescens and Pleioblastus amarus in Changning City. Sichuan For. Sci. Technol. 2007, 28, 10–14. [Google Scholar]
- Shen, G.C. Estimating the Carbon Sequestration of Phyllostachys Edulis and Pleioblastus amarus Plantations Based on Net Ecosystem Productivity. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2012. [Google Scholar]
- Xiong, Y.M.; Xia, H.P.; Li, Z.A.; Cai, X.A.; Fu, S.L. Impacts of Litter and Understory Removal on Soil Properties in a Subtropical Acacia Mangium Plantation in China. Plant Soil 2008, 304, 179–188. [Google Scholar]
- Zhang, M.; Fan, C.H.; Li, Q.L.; Li, B.; Zhu, Y.Y.; Xiong, Z.Q. A 2-Yr Field Assessment of the Effects of Chemical and Biological Nitrification Inhibitors on Nitrous Oxide Emissions and Nitrogen Use Efficiency in an Intensively Managed Vegetable Cropping System. Agric. Ecosyst. Environ. 2015, 201, 43–50. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhang, J.J.; Chang, S.X.; Jiang, P.K.; Zhou, G.M.; Fu, S.L.; Yan, E.R.; Wu, J.S.; Lin, L. Long-Term Intensive Management Effects on Soil Organic Carbon Pools and Chemical Composition in Moso Bamboo (Phyllostachys Pubescens) Forests in Subtropical China. For. Ecol. Manag. 2013, 303, 121–130. [Google Scholar] [CrossRef]
- Tian, H.Q.; Yang, J.; Xu, R.T.; Lu, C.Q.; Canadell, J.G.; Davidson, E.A.; Jackson, R.B.; Arneth, A.; Chang, J.F.; Ciais, P.; et al. Global soil nitrous oxide emissions since the preindustrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution, and uncertainty. Glob. Chang. Biol. 2019, 25, 640–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, D.L.; Murphy, D.V.; Khalid, M.; Ahmad, W.; Edwards-Jones, G.; DeLuca, T.H. Short-Term Biochar-Induced Increase in Soil CO2 Release Is Both Biotically and Abiotically Mediated. Soil Biol. Biochem. 2011, 43, 1723–1731. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, G.; Lv, C.Y.; Shao, H.B. Effects of Biochar Amendment on Soil Physical and Chemical Properties: Current Status and Knowledge Gaps. Adv. Earth Sci. 2014, 29, 68–79. [Google Scholar]
- Wu, M.X.; Feng, Q.B.; Sun, X.; Wang, H.L.; Gielen, G.; Wu, W.X. Rice (Oryza Sativa L.) Plantation Affects the Stability of Biochar in Paddy Soil. Sci. Rep. 2015, 5, 10001. [Google Scholar] [CrossRef] [Green Version]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Li, Y.F.; Hu, S.D.; Chen, J.H.; Müller, K.; Li, Y.C.; Fu, W.J.; Lin, Z.W.; Wang, H.L. Effects of Biochar Application in Forest Ecosystems on Soil Properties and Greenhouse Gas Emissions: A Review. J. Soils Sediments 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Ge, X.G.; Cao, Y.H.; Zhou, B.Z.; Wang, X.M.; Yang, Z.Y.; Li, M.H. Biochar Addition Increases Subsurface Soil Microbial Biomass but Has Limited Effects on Soil CO2 Emissions in Subtropical Moso Bamboo Plantations. Appl. Soil Ecol. 2019, 142, 155–165. [Google Scholar] [CrossRef]
- Xu, L.; Fang, H.Y.; Deng, X.; Ying, J.Y.; Lv, W.J.; Shi, Y.J.; Zhou, G.M.; Zhou, Y.F. Biochar Application Increased Ecosystem Carbon Sequestration Capacity in a Moso Bamboo Forest. For. Ecol. Manag. 2020, 475, 118447. [Google Scholar] [CrossRef]
- Song, Y.Z.; Li, Y.F.; Cai, Y.J.; Fu, S.L.; Luo, Y.; Wang, H.L.; Liang, C.F.; Lin, Z.W.; Hu, S.D.; Li, Y.C.; et al. Biochar Decreases Soil N2O Emissions in Moso Bamboo Plantations through Decreasing Labile N Concentrations, N-Cycling Enzyme Activities and Nitrification/Denitrification Rates. Geoderma 2019, 348, 135–145. [Google Scholar] [CrossRef]
- Hawthorne, I.; Johnson, M.S.; Jassal, R.S.; Black, T.A.; Grant, N.J.; Smukler, S.M. Application of Biochar and Nitrogen Influences Fluxes of CO2, CH4 and N2O in a Forest Soil. J. Environ. Manag. 2017, 192, 203–214. [Google Scholar] [CrossRef]
- Lin, Z.B.; Liu, Q.; Liu, G.; Annette, L.C.; Bei, Q.C.; Liu, B.J.; Wang, X.J.; Ma, J.; Zhu, J.G.; Xie, Z.B. Effects of Different Biochars on Pinus elliottii Growth, N Use Efficiency, Soil N2O and CH4 Emissions and C Storage in a Subtropical Area of China. Pedosphere 2017, 27, 248–261. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Zhang, Q.; Liu, X.; Xu, J.; Li, Y.; Di, H. Heterotrophic Nitrification and Denitrification Are the Main Sources of Nitrous Oxide in Two Paddy Soils. Plant Soil 2019, 445, 39–53. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.J.; Zhang, Y.H.; Hu, T.; Lin, Z.; Liu, G.; Wang, X.; Ma, J.; Wang, H.; Jin, H. Biochar Application as a Tool to Decrease Soil Nitrogen Losses (NH3 Volatilization, N2O Emissions, and N Leaching) from Croplands: Options and Mitigation Strength in a Global Perspective. Glob. Chang. Biol. 2019, 25, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Li, Y.F.; Singh, B.P.; Wang, H.L.; Cai, X.Q.; Chen, J.H.; Qin, H.; Li, Y.C.; Chang, S.X. Contrasting Short-Term Responses of Soil Heterotrophic and Autotrophic Respiration to Biochar-Based and Chemical Fertilizers in a Subtropical Moso Bamboo Plantation. Appl. Soil Ecol. 2021, 157, 103758. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.Y.; Tahmasbian, I.; Che, R.X.; Xu, Z.H.; Zhou, X.H.; Wallace, H.M.; Bai, S.H. Effects of Biochar on Soil Available Inorganic Nitrogen: A Review and Meta-Analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, Present, and Future of Biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Sim, D.H.H.; Tan, I.A.W.; Lim, L.L.P.; Hameed, B.H. Encapsulated Biochar-based Sustained Release Fertilizer for Precision Agriculture: A Review. J. Clean. Prod. 2021, 303, 127018. [Google Scholar] [CrossRef]
- Yi, S.; Chang, N.Y.; Imhoff, P.T. Predicting Water Retention of Biochar-amended Soil from Independent Measurements of Biochar and Soil Properties. Adv. Water Resour. 2020, 142, 103638. [Google Scholar] [CrossRef]
- Lin, Q.; Tan, X.F.; Almatrafi, E.; Yang, Y.; Wang, W.J.; Luo, H.Z.; Qin, F.Z.; Zhou, C.Y.; Zeng, G.M.; Zhang, C. Effects of Biochar-based Materials on the Bioavailability of Soil Organic Pollutants and Their Biological Impacts. Sci. Total Environ. 2022, 826, 153956. [Google Scholar] [CrossRef]
- Knoblauch, C.; Maarifat, A.A.; Pfeiffer, E.M.; Haefele, S.M. Degradability of Black Carbon and Its Impact on Trace Gas Fluxes and Carbon Turnover in Paddy Soils. Soil Biol. Biochem. 2011, 43, 1768–1778. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Y.J.; Zhou, G.M.; Xu, X.J.; Liu, E.B.; Zhou, Y.F.; Zhang, F.; Li, C.; Fang, H.Y.; Chen, L. Structural Development and Carbon Dynamics of Moso Bamboo Forests in Zhejiang Province, China. For. Ecol. Manag. 2018, 409, 479–488. [Google Scholar] [CrossRef]
- Wang, S.W.; Ma, S.; Shan, J.; Xia, Y.Q.; Lin, J.H.; Yan, X.Y. A 2-Year Study on the Effect of Biochar on Methane and Nitrous Oxide Emissions in an Intensive Rice–Wheat Cropping System. Biochar 2019, 1, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Lv, R.K. Soil Agrochemical Analysis Methods, 3rd ed.; China Agricultural Science and Technology Press: Beijing, China, 2000; pp. 1–638. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Eiol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Singh, B.P.; Hatton, B.J.; Singh, B.; Cowie, A.L.; Kathuria, A. Influence of Biochars on Nitrous Oxide Emission and Nitrogen Leaching from Two Contrasting Soils. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef]
- Yang, X.C.; Liu, D.P.; Fu, Q.; Li, T.X.; Hou, R.J.; Li, Q.L.; Li, M.; Meng, F.X. Characteristics of Greenhouse Gas Emissions from Farmland Soils Based on a Structural Equation Model: Regulation Mechanism of Biochar. Environ. Res. 2022, 206, 112303. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Y.F.; Chang, S.X.; Yang, Y.; Fu, S.; Jiang, P.; Luo, Y.; Yang, M.; Chen, Z.; Hu, S. Biochar Reduces Soil Heterotrophic Respiration in a Subtropical Plantation through Increasing Soil Organic Carbon Recalcitrancy and Decreasing Carbon-Degrading Microbial Activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Xu, L.; Deng, X.; Ying, J.Y.; Zhou, G.M.; Shi, Y.J. Silicate Fertilizer Application Reduces Soil Greenhouse Gas Emissions in a Moso Bamboo Forest. Sci. Total Environ. 2020, 747, 141380. [Google Scholar] [CrossRef]
- Tang, Z.M.; Liu, X.R.; Li, G.C.; Liu, X.W. Mechanism of Biochar on Nitrification and Denitrification to N2O Emissions Based on Isotope Characteristic Values. Environ. Res. 2022, 212, 113219. [Google Scholar] [CrossRef]
- Zhong, L.; Li, G.Y.; Qing, J.W.; Li, J.L.; Xue, J.M.; Yan, B.B.; Chen, G.Y.; Kang, X.M.; Rui, Y.C. Biochar Can Reduce N2O Production Potential from Rhizosphere of Fertilized Agricultural Soils by Suppressing Bacterial Denitrification. Eur. J. Soil Biol. 2022, 109, 103391. [Google Scholar] [CrossRef]
- Zwieten, L.V.; Singh, B.P.; Kimber, S.W.L.; Murphy, D.V.; Macdonald, L.M.; Rust, J.; Morris, S. An Incubation Study Investigating the Mechanisms that Impact N2O Flux from Soil Following Biochar Application. Agric. Ecosyst. Environ. 2014, 191, 53–62. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, Y.F.; Chang, S.X.; Jiang, P.K.; Zhou, G.M.; Liu, J.; Wu, J.S.; Shen, Z.M. Understory Vegetation Management Affected Greenhouse Gas Emissions and Labile Organic Carbon Pools in an Intensively Managed Chinese Chestnut Plantation. Plant Soil 2014, 376, 363–375. [Google Scholar] [CrossRef]
- Fracetto, F.J.C.; Fracetto, G.G.M.; Bertini, S.C.B.; Cerri, C.C.; Feigl, B.J.; Neto, M.S. Effect of Agricultural Management on N2O Emissions in the Brazilian Sugarcane Yield. Soil Biol. Biochem. 2017, 109, 205–213. [Google Scholar] [CrossRef]
- Zhou, J.S.; Qu, T.H.; Li, Y.F.; Van, Z.L.; Wang, H.; Chen, J.; Song, X.; Lin, Z.; Zhang, X.; Luo, Y.; et al. Biochar-based Fertilizer Decreased While Chemical Fertilizer Increased Soil N2O Emissions in a Subtropical Moso Bamboo Plantation. Catena 2021, 202, 105257. [Google Scholar] [CrossRef]
- Vargas, V.P.; Cantarella, H.; Martins, A.A.; Soares, J.R.; Carmo, J.B.; Andrade, C.A. Sugarcane Crop Residue Increases N2O and CO2 Emissions under High Soil Moisture Conditions. Sugar Technol. 2014, 16, 174–179. [Google Scholar] [CrossRef]
- Lu, X.H.; Li, Y.F.; Wang, H.L.; Singh, B.P.; Hu, S.D.; Luo, Y.; Li, J.W.; Xiao, Y.H.; Cai, X.Q.; Li, Y.C. Responses of Soil Greenhouse Gas Emissions to Different Application Rates of Biochar in a Subtropical Chinese Chestnut Plantation. Agric. For. Meteorol. 2019, 271, 168–179. [Google Scholar] [CrossRef]
- Fang, H.Y. Effects of Biochar Application on Carbon Sequestration and Greenhouse Gases Emission in Moso Bamboo Forests. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2019. [Google Scholar]
- Sonoki, T.; Furukawa, T.; Jindo, K.; Suto, K.; Aoyama, M.; Sánchez-Monedero, M.Á. Influence of Biochar Addition on Methane Metabolism during Thermophilic Phase of Composting. J. Basic Microbiol. 2013, 53, 617–621. [Google Scholar] [CrossRef]
- Powers, J.S.; Schlesinger, W.H. Relationships among Soil Carbon Distributions and Biophysical Factors at Nested Spatial Scales in Rain Forests of Northeastern Costa Rica. Geoderma 2002, 109, 165–190. [Google Scholar] [CrossRef]
- Liu, Y.X.; Yang, M.; Wu, Y.M.; Wang, H.L.; Chen, Y.X.; Wu, W.X. Reducing CH4 and CO2 Emissions from Waterlogged Paddy Soil with Biochar. J. Soils Sediments 2011, 11, 930–939. [Google Scholar] [CrossRef]
- Jia, J.X.; Li, B.; Chen, Z.Z.; Xie, Z.B.; Xiong, Z.Q. Effects of Biochar Application on Vegetable Production and Emissions of N2O and CH4. Soil Sci. Plant Nutr. 2012, 58, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Y.; Zhang, M.; Xiong, Z.Q.; Liu, P.L.; Pan, G.X. Effects of Biochar Addition on N2O and CO2 Emissions from Two Paddy Soils. Biol. Fertil. Soils 2011, 47, 887–896. [Google Scholar] [CrossRef]
- Troy, S.M.; Lawlor, P.G.; O’Flynn, C.J.; Healy, M.G. Impact of Biochar Addition to Soil on Greenhouse Gas Emissions Following Pig Manure Application. Soil Biol. Biochem. 2013, 60, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Kalu, S.; Kulmala, L.; Zrim, J.; Peltokangas, K.; Tammeorg, P.; Rasa, K.; Kitzler, B.; Pihlatie, M.; Karhu, K. Potential of Biochar to Reduce Greenhouse Gas Emissions and Increase Nitrogen Use Efficiency in Boreal Arable Soils in the Long-Term. Front. Environ. Sci. 2022, 10, 1–16. [Google Scholar] [CrossRef]
- Epron, D.; Ngao, J.; Granier, A. Interannual Variation of Soil Respiration in a Beech Forest Ecosystem over a Six-Year Study. Ann. For. Sci. 2004, 61, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Obia, A.; Cornelissen, G.; Mulder, J.; Dörsch, P. Effect of Soil PH Increase by Biochar on NO, N2O and N2 Production during Denitrification in Acid Soils. PLoS ONE 2015, 10, e0138781. [Google Scholar] [CrossRef]







| Properties | Surface Area (m2 g−1) | pH | N (g kg−1) | K (g kg−1) | P (g kg−1) |
|---|---|---|---|---|---|
| biochar | 11.4 | 9.07 | 5.6 | 8.6 | 0.64 |
| GHG | Treatment | Model | df | R2 | p |
|---|---|---|---|---|---|
| N2O | CK | Y = 0.827WSON | 48 | 0.678 | ** |
| Y = 0.614WSON + 0.342MBN Y = 0.777MBC Y = 0.547MBC + 0.486WSON Y = 0.467MBC + 0.401WSON +0.199MBN Y = 0.438MBC + 0.470WSON +0.252MBN + 0.164NH4+-N Y = 0.729T Y = 0.556T + 0.415MBC Y = 0.544T + 0.369MBC + 0.207M Y = 0.607T + 0.408MBC + 0.202M + 0.181NO3−-N | 48 | 0.745 | ** | ||
| BFT | 48 | 0.595 | ** | ||
| 48 | 0.777 | ** | |||
| 48 | 0.793 | ** | |||
| 48 | 0.807 | ** | |||
| CFT | 48 | 0.521 | ** | ||
| 48 | 0.659 | ** | |||
| 48 | 0.694 | ** | |||
| 48 | 0.714 | ** | |||
| Y = 0.638T + 0.250MBC + 0.109M + 0.363NO3−-N +0.379MBN | 48 | 0.769 | ** | ||
| Y = 0.649T + 0.248MBC + 0.393NO3−-N +0.436MBN | 48 | 0.764 | ** | ||
| MFT | Y = 0.857WSON | 48 | 0.729 | ** | |
| Y = 0.710WSON +0.331MBC | 48 | 0.814 | ** | ||
| Y = 0.681WSON +0.223MBC + 0.199MBN | 48 | 0.836 | ** |
| GHG | Treatment | Model | df | R2 | p |
|---|---|---|---|---|---|
| CH4 | CK | Y = 0.596WSOC Y = 0.476WSOC + 0.375WSON Y = 0.722MBC Y = 0.464MBC + 0.417WSON Y = 0.327MBC + 0.481WSON + 0.344WSOC Y = 0.399MBC + 0.587WSON + 0.325WSOC − 0.278NH4+-N Y = 0.523MBC + 0.646WSON + 0.255WSOC − 0.391NH4+-N − 0.227M Y = 0.538T | 48 | 0.341 | ** |
| 48 | 0.458 | ** | |||
| BFT | 48 | 0.511 | ** | ||
| 48 | 0.611 | ** | |||
| 48 | 0.716 | ** | |||
| 48 | 0.766 | ** | |||
| 48 | 0.796 | ** | |||
| CFT | 48 | 0.274 | ** | ||
| Y = 0.529T + 0.402WSOC | 48 | 0.427 | ** | ||
| Y = 0.658T + 0.386WSOC + 0.297NO3−-N | 48 | 0.490 | ** | ||
| Y = 0.630T + 0.326WSOC + 0.103NO3−-N +0.350MBN | 48 | 0.557 | ** | ||
| MFT | Y = 0.570WSON | 48 | 0.310 | ** | |
| Y = 0.561WSON + 0.495WSOC | 48 | 0.550 | ** | ||
| Y = 0.836WSON + 0.487WSOC + 0.120NH4+-N | 48 | 0.648 | ** |
| GHG | Treatment | Model | df | R2 | p |
|---|---|---|---|---|---|
| CO2 | CK | Y = 0.942T Y = 1.028T−0.200NO3−-N Y = 0.982T−0.198NO3−-N +0.116WSOC Y = 0.920T Y = 0.772T + 0.272MBC Y = 0.637T + 0.216MBC + 0.209WSON Y = 0.667T + 0.206MBC + 0.266WSON−0.160NO3−-N Y = 0.900T | 48 | 0.885 | ** |
| 48 | 0.916 | ** | |||
| 48 | 0.927 | ** | |||
| BFT | 48 | 0.844 | ** | ||
| 48 | 0.895 | ** | |||
| 48 | 0.907 | ** | |||
| 48 | 0.927 | ** | |||
| CFT | 48 | 0.806 | ** | ||
| Y = 0.868T + 0.212M | 48 | 0.847 | ** | ||
| Y = 0.862T + 0.224M + 0.177WSOC | 48 | 0.877 | ** | ||
| Y = 0.941T + 0.226M + 0.166WSOC + 0.182NO3−-N | 48 | 0.904 | ** | ||
| Y = 0.934T + 0.149M + 0.122WSOC + 0.317NO3−-N +0.230MBC | 48 | 0.930 | ** | ||
| MFT | Y = 0.966T | 48 | 0.932 | ** | |
| Y = 0.934T + 0.135M | 48 | 0.948 | ** | ||
| Y = 0.984T + 0.136M + 0.085NO3−-N | 48 | 0.952 | ** | ||
| Y = 0.951T + 0.127M + 0.103NO3−-N +0.083NH4+-N | 48 | 0.956 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.; Yuan, N.; Lv, S.; Tang, X.; Wang, G.; Wu, L.; Zhou, Y.; Zhou, G.; Shi, Y.; Xu, L. Biochar-Based Fertilizer Decreased Soil N2O Emission and Increased Soil CH4 Uptake in a Subtropical Typical Bamboo Plantation. Forests 2022, 13, 2181. https://doi.org/10.3390/f13122181
Wang E, Yuan N, Lv S, Tang X, Wang G, Wu L, Zhou Y, Zhou G, Shi Y, Xu L. Biochar-Based Fertilizer Decreased Soil N2O Emission and Increased Soil CH4 Uptake in a Subtropical Typical Bamboo Plantation. Forests. 2022; 13(12):2181. https://doi.org/10.3390/f13122181
Chicago/Turabian StyleWang, Enhui, Ning Yuan, Shaofeng Lv, Xiaoping Tang, Gang Wang, Linlin Wu, Yufeng Zhou, Guomo Zhou, Yongjun Shi, and Lin Xu. 2022. "Biochar-Based Fertilizer Decreased Soil N2O Emission and Increased Soil CH4 Uptake in a Subtropical Typical Bamboo Plantation" Forests 13, no. 12: 2181. https://doi.org/10.3390/f13122181
APA StyleWang, E., Yuan, N., Lv, S., Tang, X., Wang, G., Wu, L., Zhou, Y., Zhou, G., Shi, Y., & Xu, L. (2022). Biochar-Based Fertilizer Decreased Soil N2O Emission and Increased Soil CH4 Uptake in a Subtropical Typical Bamboo Plantation. Forests, 13(12), 2181. https://doi.org/10.3390/f13122181






