Geographic Cline and Genetic Introgression Effects on Seed Morphology Variation and Germination Fitness in Two Closely Related Pine Species in Southeast Asia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Seed Morphology and Environmental Data Analysis
2.3. Seed Germination Experiment and Data Analysis
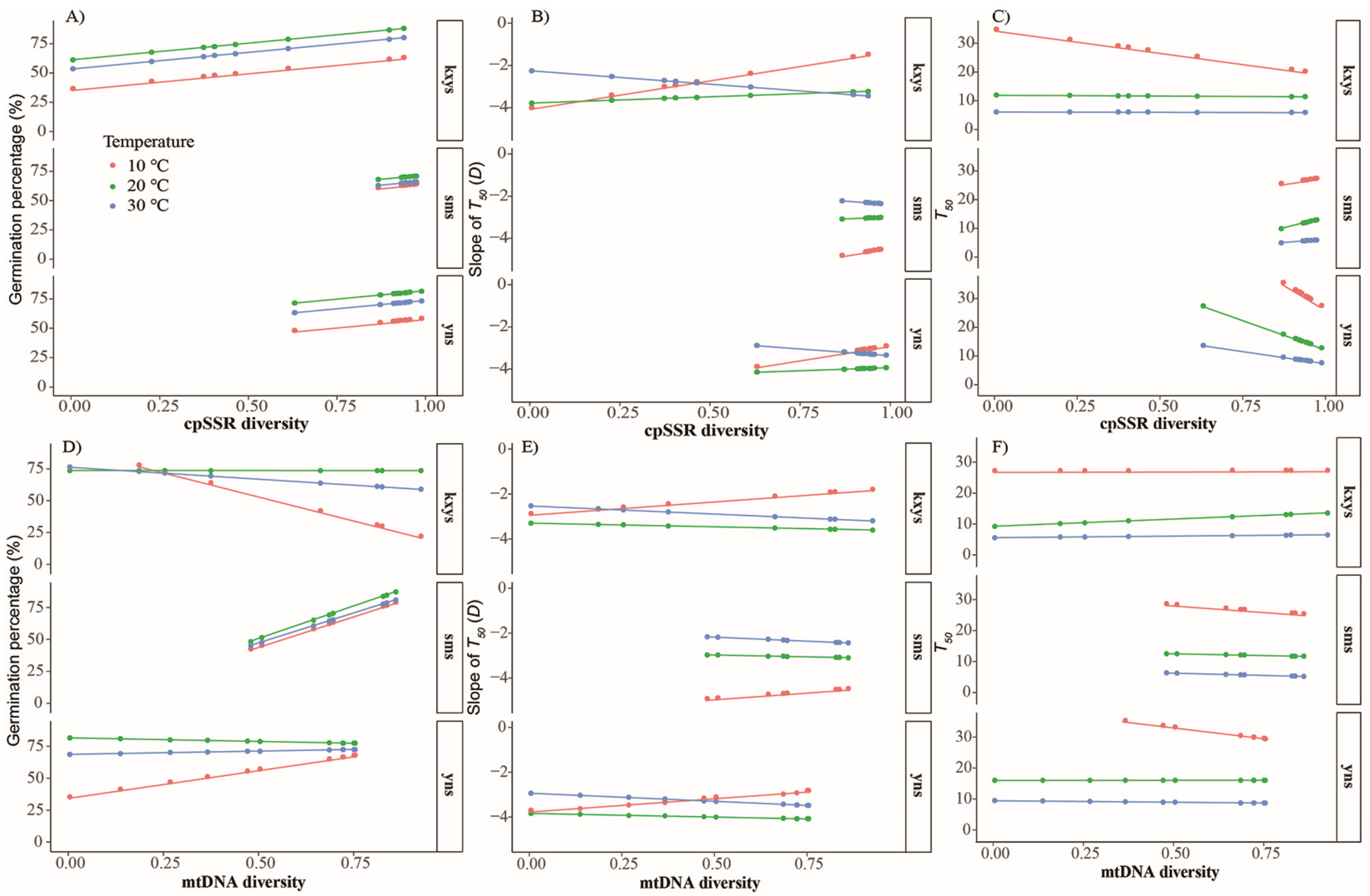
3. Results
3.1. Patterns of Variation in Seed Morphology
3.2. Seed Germination Tests
4. Discussion
4.1. Seed Morphology Variation and Its Association with Environmental Variables
4.2. Seed Fitness of Populations from the Hybrid Zone and the Parental Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Statements
References
- Ji, M.F.; Deng, J.M.; Yao, B.Q.; Chen, R.F.; Fan, Z.X.; Guan, J.W.; Li, X.W.; Wu, F.; Niklas, K.J. Ecogeographical variation of 12 morphological traits within Pinus tabulaeformis: The effects of environmental factors and demographic histories. J. Plant. Ecol. 2017, 10, 386–396. [Google Scholar]
- Ma, Y.Z.; Wang, J.; Hu, Q.J.; Li, J.L.; Sun, Y.S.; Zhang, L.; Abbott, R.J.; Liu, J.Q.; Mao, K.S. Ancient introgression drives adaptation to cooler and drier mountain habitats in a cypress species complex. Commun. Biol. 2019, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Scotti, I.; Gonzalez-Martinez, S.C.; Budde, K.B.; Lalague, H. Fifty years of genetic studies: What to make of the large amounts of variation found within populations? Ann. For. Sci. 2016, 73, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Diaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwaizumi, M.G.; Matsunaga, K.; Iki, T.; Yamanobe, T.; Hirao, T.; Watanabe, A. Geographical cline and inter-seaside difference in cone characteristics related to climatic conditions of old planted Pinus thunbergii populations throughout Japan. Plant Species Biol. 2020, 36, 218–229. [Google Scholar] [CrossRef]
- Iwaizumi, M.G.; Ohtani, M.; Takahashi, M. Geographic cline and climatic effects on cone characteristics of natural populations of Pinus densiflora throughout the Japanese archipelago. J. For. Res. 2019, 24, 187–196. [Google Scholar] [CrossRef]
- Leal-Saenz, A.; Waring, K.M.; Menon, M.; Cushman, S.A.; Eckert, A.; Flores-Renteria, L.; Hernandez-Diaz, J.C.; Lopez-Sanchez, C.A.; Martinez-Guerrero, J.H.; Wehenkel, C. Morphological differences in Pinus strobiformis across latitudinal and elevational gradients. Front. Plant Sci. 2020, 11, 1600. [Google Scholar] [CrossRef]
- Souza, M.L.; Duarte, A.A.; Lovato, M.B.; Fagundes, M.; Valladares, F.; Lemos, J.P. Climatic factors shaping intraspecific leaf trait variation of a neotropical tree along a rainfall gradient. PLoS ONE 2018, 13, e0208512. [Google Scholar]
- Hanusova, K.; Ekrt, L.; Vit, P.; Kolar, F.; Urfus, T. Continuous morphological variation correlated with genome size indicates frequent introgressive hybridization among Diphasiastrum species (Lycopodiaceae) in central Europe. PLoS ONE 2014, 9, e99552. [Google Scholar]
- Kobayashi, N.; Handa, T.; Yoshimura, K.; Tsumura, Y.; Arisumi, K.; Takayanagi, K. Evidence for introgressive hybridization based on chloroplast DNA polymorphisms and morphological variation in wild evergreen azalea populations of the Kirishima mountains, Japan. Edinb. J. Bot. 2000, 57, 209–219. [Google Scholar] [CrossRef]
- Mimura, M.; Suga, M. Ambiguous species boundaries: Hybridization and morphological variation in two closely related Rubus species along altitudinal gradients. Ecol. Evol. 2020, 10, 7476–7486. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, V.L.; Knutson, V.L.; Lee, M.; Zieba, J.; Edmands, S. Fitness and morphological outcomes of many generations of hybridization in the copepod Tigriopus californicus. J. Evol. Biol. 2013, 26, 416–433. [Google Scholar] [CrossRef] [PubMed]
- Tobler, M.; Carson, E.W. Environmental variation, hybridization, and phenotypic diversification in Cuatro Ciénegas pupfishes. J. Evol. Biol. 2010, 23, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, B.M.; Johnson, J.R.; Kump, D.K.; Smith, J.J.; Voss, S.R.; Shaffer, H.B. Rapid spread of invasive genes into a threatened native species. Proc. Natl. Acad. Sci. USA 2010, 107, 3606–3610. [Google Scholar] [CrossRef] [Green Version]
- Rhymer, J.M.; Simberloff, D. Extinction by hybridization and introgression. Annu. Rev. Ecol. Syst. 1996, 27, 83–109. [Google Scholar] [CrossRef]
- Todesco, M.; Pascual, M.A.; Owens, G.L.; Ostevik, K.L.; Moyers, B.T.; Hübner, S.; Heredia, S.M.; Hahn, M.A.; Caseys, C.; Bock, D.G. Hybridization and extinction. Evol. Appl. 2016, 9, 892–908. [Google Scholar] [CrossRef]
- Arnold, M.L.; Ballerini, E.S.; Brothers, A.N. Hybrid fitness, adaptation and evolutionary diversification: Lessons learned from Louisiana Irises. Heredity 2012, 108, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Pfennig, K.S.; Kelly, A.L.; Pierce, A.A. Hybridization as a facilitator of species range expansion. Proc. Royal Soc. B. 2016, 283, 1329. [Google Scholar] [CrossRef] [Green Version]
- Merila, J.; Hendry, A.P. Climate change, adaptation, and phenotypic plasticity: The problem and the evidence. Evol. Appl. 2014, 7, 1–14. [Google Scholar] [CrossRef]
- Tabas-Madrid, D.; Mendez-Vigo, B.; Arteaga, N.; Marcer, A.; Pascual-Montano, A.; Weigel, D.; Pico, F.X.; Alonso-Blanco, C. Genome-wide signatures of flowering adaptation to climate temperature: Regional analyses in a highly diverse native range of Arabidopsis thaliana. Plant Cell Environ. 2018, 41, 1806–1820. [Google Scholar] [CrossRef]
- Aitken, S.N.; Whitlock, M.C. Assisted gene flow to facilitate local adaptation to climate change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 367–388. [Google Scholar] [CrossRef]
- Neale, D.B.; Wheeler, N.C. The Conifers. In The Conifers: Genomes, Variation and Evolution; Neale, D.B., Wheeler, N.C., Eds.; Springer: Cham, Switzerland, 2019; Chapter 1; pp. 1–21. [Google Scholar]
- Prunier, J.; Verta, J.P.; MacKay, J.J. Conifer genomics and adaptation: At the crossroads of genetic diversity and genome function. New Phytol. 2016, 209, 44–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, F.B.; Burley, J. Pinus kesiya; Tropical Forestry Paper 9; Commonwealth Forestry Institute: Oxford, UK, 1980. [Google Scholar]
- Mirov, N.T. The Genus Pinus; Ronald Press Company: New York, NY, USA, 1967. [Google Scholar]
- Wu, C.L. The taxonomic revision and phytogeographical study of Chinese pines. J. Syst. Evol. 1956, 5, 131–163. [Google Scholar]
- Jin, W.T.; Gernandt, D.S.; Wehenkel, C.; Xia, X.M.; Wei, X.X.; Wang, X.Q. Phylogenomic and ecological analyses reveal the spatiotemporal evolution of global pines. Proc. Natl. Acad. Sci. USA 2021, 118, e2022302118. [Google Scholar] [CrossRef]
- Gao, J.; Tomlinson, K.W.; Zhao, W.; Wang, B.S.; Lapuz, R.S.; Liu, J.X.; Pasion, B.O.; Hai, B.T.; Chanthayod, S.; Peng, Y.Q.; et al. Phylogeography and Introgression between Pinus kesiya and P. yunnanensis in Tropical Southeast Asia. yunnanensis in Tropical Southeast Asia. J. Syst. Evol. 2022, submitted.
- Douglas, D.A. The balance between vegetative and sexual reproduction of Mimulus primuloides (Scrophulariaceae) at different altitudes in California. J. Ecol. 1981, 69, 295–310. [Google Scholar] [CrossRef]
- Liu, F.L.; Jensen, C.R.; Andersen, M.N. A review of drought adaptation in crop plants: Changes in vegetative and reproductive physiology induced by ABA-based chemical signals. Aust. J. Agric. Res. 2005, 56, 1245–1252. [Google Scholar] [CrossRef]
- He, J.D.; Xue, J.Y.; Gao, J.; Wang, J.N.; Wu, Y. Adaptations of the floral characteristics and biomass allocation patterns of Gentiana hexaphylla to the altitudinal gradient of the eastern Qinghai-Tibet Plateau. J. Mt. Sci. 2017, 14, 1563–1576. [Google Scholar] [CrossRef]
- Dobzhansky, T. A review of some fundamental concepts and problems of population genetics. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1955; Volume 20, pp. 1–15. [Google Scholar]
- Donohue, K.; de Casas, R.R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Jimenez-Alfaro, B.; Silveira, F.A.O.; Fidelis, A.; Poschlod, P.; Commander, L.E. Seed germination traits can contribute better to plant community ecology. J. Veg. Sci. 2016, 27, 637–645. [Google Scholar] [CrossRef]
- Hamaala, T.; Mattila, T.M.; Leinonen, P.H.; Kuittinen, H.; Savolainen, O. Role of seed germination in adaptation and reproductive isolation in Arabidopsis lyrata. Mol. Ecol. 2017, 26, 3484–3496. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Van Den Wollenberg, A.L. Redundancy analysis an alternative for canonical correlation analysis. Psychometrika 1977, 42, 207–219. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G.D. How and why to measure the germination process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.K.; Zhang, S.B.; Lan, Z.Q.; Lan, Q.Y. Possible causes for the differentiation of Pinus yunnanensis and P. kesiya var. Langbianensis in Yunnan, China: Evidence from seed germination. For. Ecol. Manag. 2021, 494, 119321. [Google Scholar]
- Jensen, S.M.; Andreasen, C.; Streibig, J.C.; Keshtkar, E.; Ritz, C. A note on the analysis of germination data from complex experimental designs. Seed Sci. Res. 2017, 27, 321–327. [Google Scholar] [CrossRef]
- Ritz, C.; Pipper, C.; Yndgaard, F.; Fredlund, K.; Steinrucken, G. Modelling flowering of plants using time-to-event methods. Eur. J. Agron. 2010, 32, 155–161. [Google Scholar] [CrossRef]
- Ritz, C.; Pipper, C.B.; Streibig, J.C. Analysis of germination data from agricultural experiments. Eur. J. Agron. 2013, 45, 1–6. [Google Scholar] [CrossRef]
- Jiang, X.Q.; Kopp-Schneider, A. Summarizing EC50 estimates from multiple dose-response experiments: A comparison of a meta- analysis strategy to a mixed- effects model approach. Biom. J. 2014, 56, 493–512. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Major, J.E.; Mosseler, A.; Barsi, D.C.; Campbell, M.; Rajora, O.P. Morphometric, allometric, and developmentally adaptive traits in red spruce and black spruce. II. Seedling and mature tree assessment of controlled intra- and inter-specific hybrids. Can. J. For. Res. 2003, 33, 897–909. [Google Scholar] [CrossRef]
- Monteleone, I.; Ferrazzini, D.; Belletti, P. Effectiveness of neutral RAPD markers to detect genetic Divergence between the subspecies uncinata and mugo of Pinus mugo Turra. Silva Fenn. 2006, 40, 391–406. [Google Scholar] [CrossRef] [Green Version]
- Goroshkevich, S.; Popov, A.; Vasilieva, G. Ecological and morphological studies in the hybrid zone between Pinus sibirica and Pinus pumila. Ann. For. Res. 2007, 51, 43–52. [Google Scholar]
- Roe, A.D.; MacQuarrie, C.J.K.; Gros-Louis, M.C.; Simpson, J.D.; Lamarche, J.; Beardmore, T.; Thompson, S.L.; Tanguay, P.; Isabel, N. Fitness dynamics within a poplar hybrid zone: I. Prezygotic and postzygotic barriers impacting a native poplar hybrid stand. Ecol. Evol. 2014, 4, 1629–1647. [Google Scholar] [CrossRef]
- Mao, J.F.; Li, Y.; Wang, X.R. Empirical assessment of the reproductive fitness components of the hybrid pine Pinus densata on the Tibetan Plateau. Evol. Ecol. 2009, 23, 447–462. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.D.; Tweddle, J.C.; Dickie, J.B.; Smith, R.; Leishman, M.R.; Mayfield, M.M.; Pitman, A.; Wood, J.T.; Westoby, M. Global patterns in seed size. Global Ecol. Biogeogr. 2007, 16, 109–116. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, V.P. A review of drought concepts. J. Hydrol. 2010, 391, 204–216. [Google Scholar]
- Williams, A.P.; Allen, C.D.; Millar, C.I.; Swetnam, T.W.; Michaelsen, J.; Still, C.J.; Leavitt, S.W. Forest responses to increasing aridity and warmth in the southwestern United States. Proc. Natl. Acad. Sci. USA 2010, 107, 21289–21294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restaino, C.M.; Peterson, D.L.; Littell, J. Increased water deficit decreases Douglas fir growth throughout western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 9557–9562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.L. Natural Hybridization and Evolution; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Johnston, J.A.; Arnold, M.L.; Donovan, L.A. High hybrid fitness at seed and seedling life history stages in Louisiana irises. J. Ecol. 2003, 91, 438–446. [Google Scholar] [CrossRef]
- Campbell, D.R.; Waser, N.M. Genotype-by-environment interaction and the fitness of plant hybrids in the wild. Evolution 2001, 55, 669–676. [Google Scholar] [CrossRef]
- Arnold, M.L.; Martin, N.H. Hybrid fitness across time and habitats. Trends Ecol. Evol. 2010, 25, 530–536. [Google Scholar] [CrossRef]
- Zhao, W.; Meng, J.; Wang, B.; Zhang, L.; Xu, Y.; Zeng, Q.Y.; Li, Y.; Mao, J.F.; Wang, X.R. Weak crossability barrier but strong juvenile selection supports ecological speciation of the hybrid pine Pinus densata on the Tibetan plateau. Evolution 2014, 68, 3120–3133. [Google Scholar] [CrossRef] [Green Version]
- Lammi, A.; Siikamaki, P.; Mustajarvi, K. Genetic diversity, population size, and fitness in central and peripheral populations of a rare plant Lychnis viscaria. Conserv. Biol. 1999, 13, 1069–1078. [Google Scholar] [CrossRef]
- Chen, Z.H.; Peng, J.F.; Zhang, D.M.; Zhao, J.G. Seed germination and storage of woody species in the lower subtropical forest. J. Integr. Plant Biol. 2002, 44, 1469–1476. [Google Scholar]
- Soriano, D.; Orozco-Segovia, A.; Marquez-Guzman, J.; Kitajima, K.; Gamboa-de Buen, A.; Huante, P. Seed reserve composition in 19 tree species of a tropical deciduous forest in Mexico and its relationship to seed germination and seedling growth. Ann. Bot. 2011, 107, 939–951. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Inbreeding depression and the cost of inbreeding on seed germination. Seed Sci. Res. 2015, 25, 355–385. [Google Scholar] [CrossRef]
- Leimu, R.; Mutikainen, P.; Koricheva, J.; Fischer, M. How general are positive relationships between plant population size, fitness and genetic variation? J. Ecol. 2006, 94, 942–952. [Google Scholar] [CrossRef]
- Markert, J.A.; Champlin, D.M.; Gutjahr-Gobell, R.; Grear, J.S.; Kuhn, A.; McGreevy, T.J.; Roth, A.; Bagley, M.J.; Nacci, D.E. Population genetic diversity and fitness in multiple environments. BMC Evol. Biol. 2010, 10, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neale, D.B.; Sederoff, R.R. Paternal inheritance of chloroplast DNA and maternal inheritance of mitochondrial-DNA in loblolly-pine. Theor. Appl. Genet. 1989, 77, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Szmidt, A.E.; Lu, M.Z. Genetic evidence for the presence of cytoplasmic DNA in pollen and megagametophytes and maternal inheritance of mitochondrial DNA in Pinus. For. Genet. 1996, 3, 37–44. [Google Scholar]

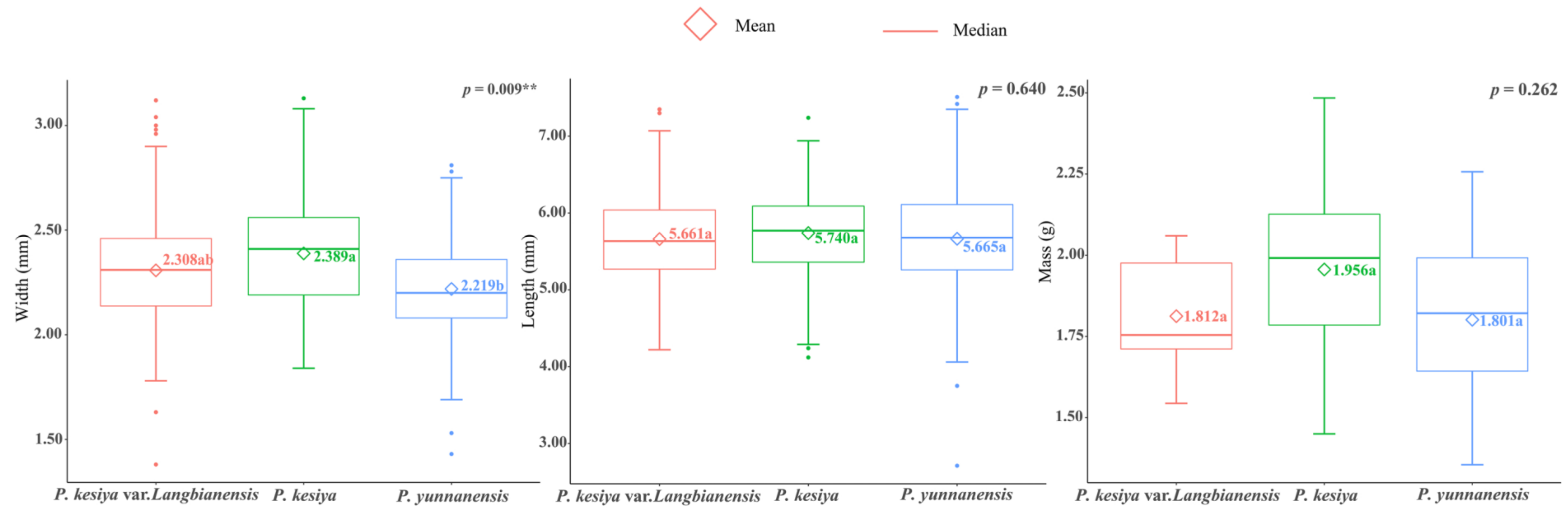
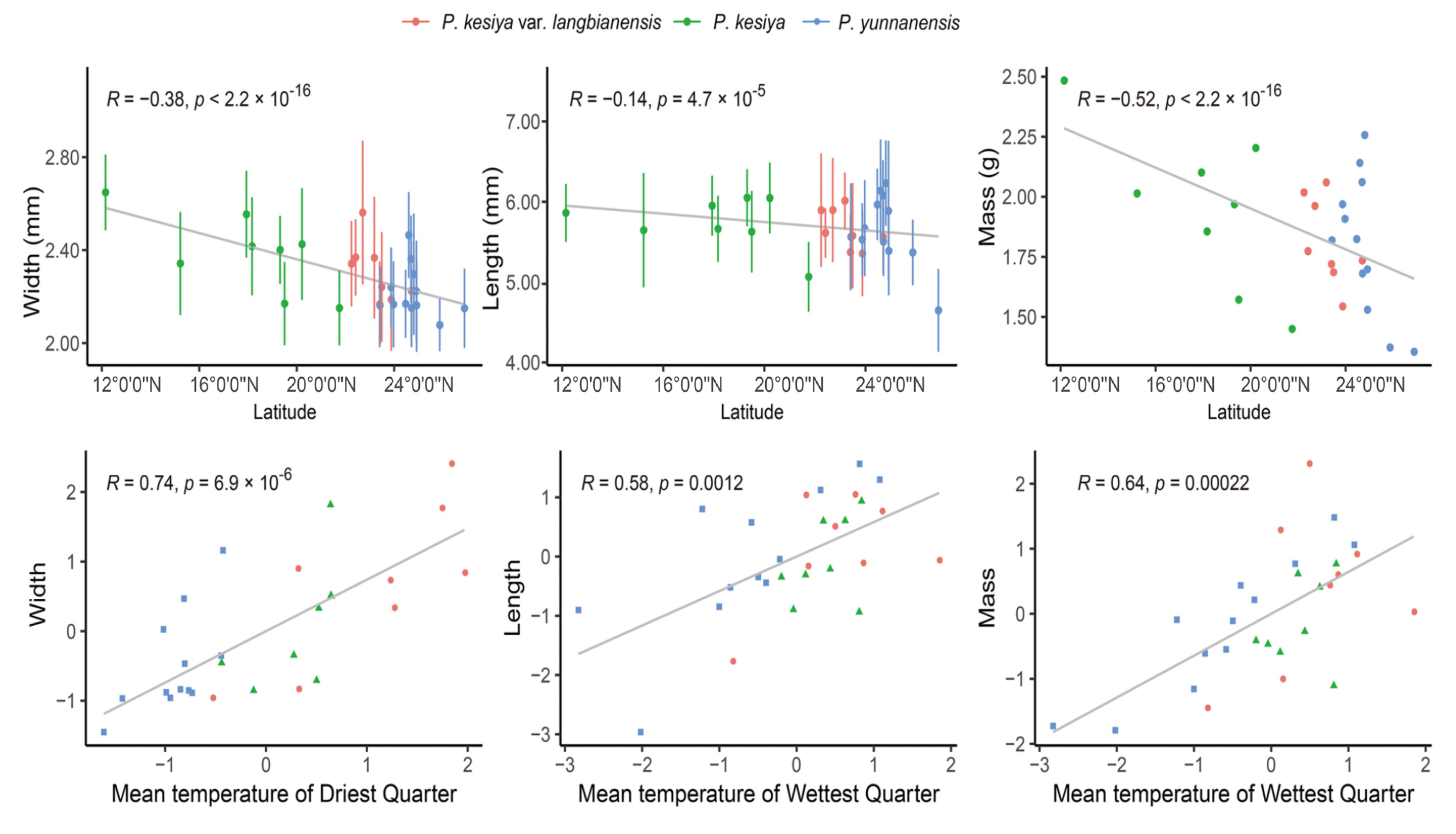
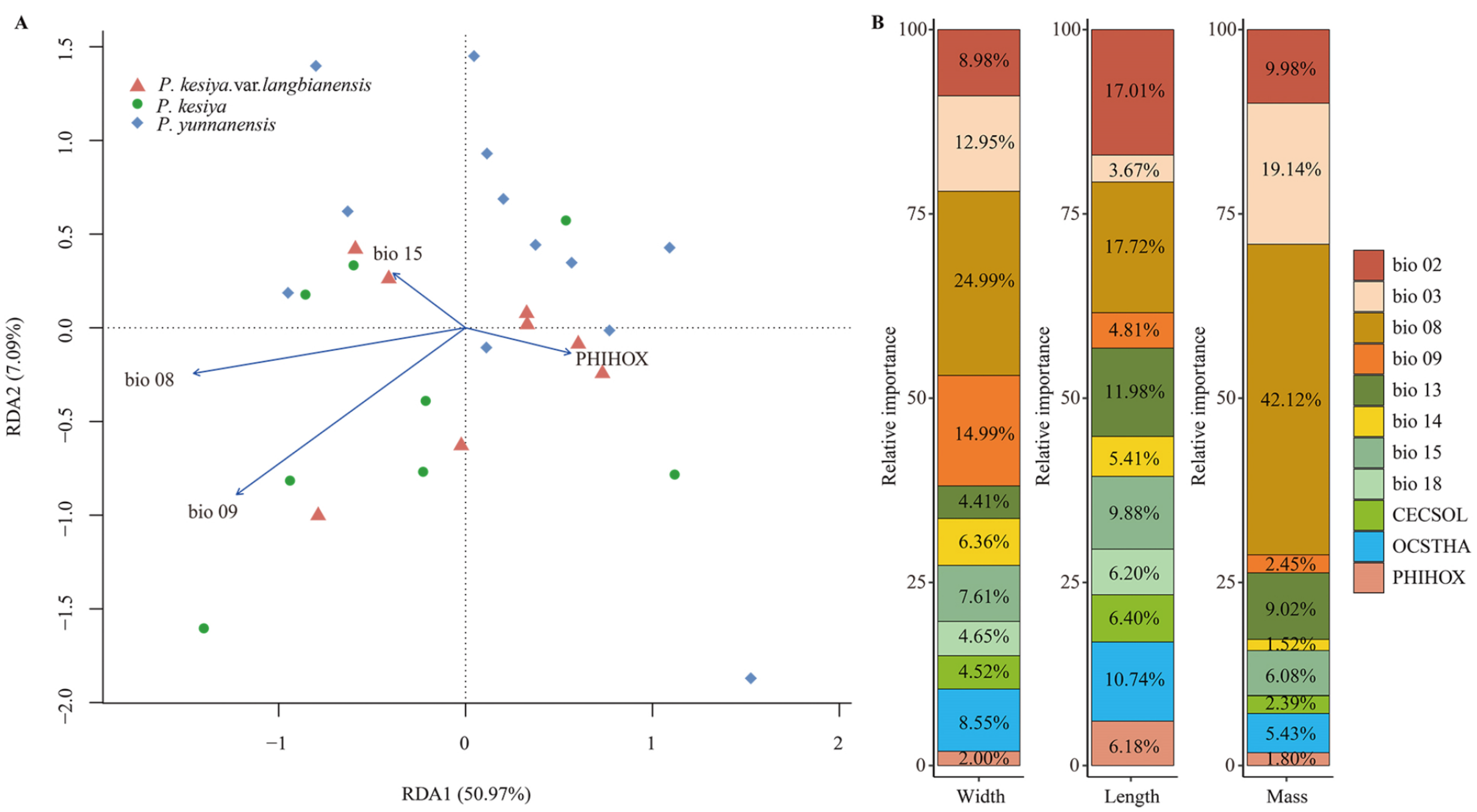
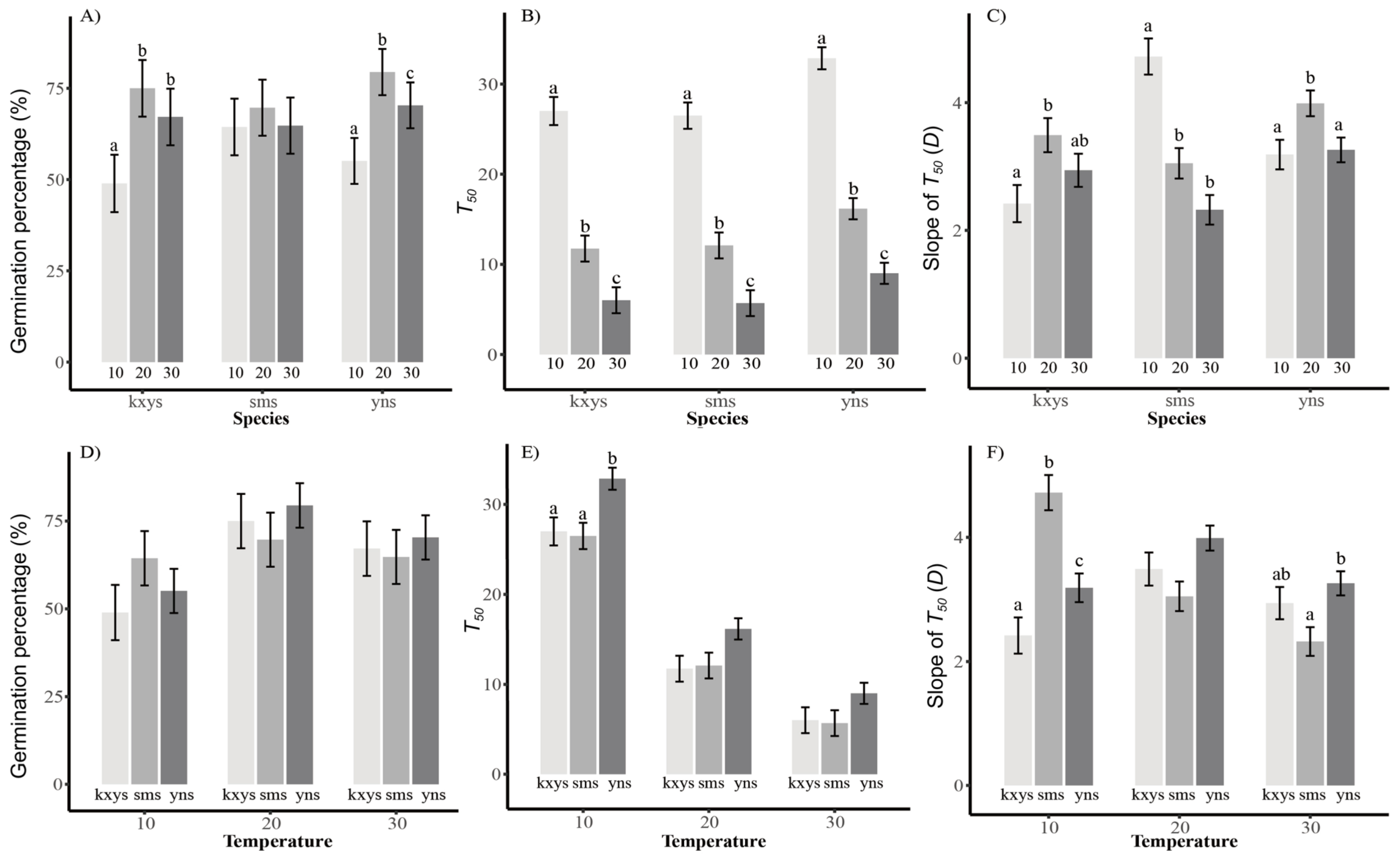
| Environmental Variable and Seed Morphology | Units | F-Statistic | df | adjR2 | P.yunnanensis | P. kesiya | P.kesiya. var. langbianensis |
|---|---|---|---|---|---|---|---|
| p-Value | |||||||
| Bio02: Mean diurnal temperature range | (°C × 10) | 0.193 | 26 | 0.028 | 8.681a | 9.108a | 8.714a |
| Bio03: Isothermality (Bio2/Bio7) (×100) | (%) | 0.000 *** | 26 | 0.582 | 39.450b | 53.635a | 44.586b |
| Bio08: Mean temperature of wettest quarter | (°C × 10) | 0.005 ** | 26 | 0.241 | 20.794b | 22.998a | 22.784ab |
| Bio09: Mean temperature of driest quarter | (°C × 10) | 0.000 *** | 26 | 0.674 | 9.272b | 17.299a | 14.852a |
| Bio13: Precipitation of wettest month | (mm) | 0.016 * | 26 | 0.174 | 210.077b | 327.625a | 284.714ab |
| Bio14: Precipitation of driest month | (mm) | 0.000 *** | 26 | 0.516 | 13.539a | 3.875b | 15.286a |
| Bio15: Precipitation seasonality | (CV3) | 0.024 * | 26 | 0.150 | 79.253a | 86.933a | 86.169a |
| Bio18: Precipitation of warmest quarter | (mm) | 0.258 | 26 | 0.012 | 586.539a | 700.750a | 761.429a |
| PHIHOX: Soil PH × 10 in H2O | PH × 10 | 0.007 ** | 26 | 0.217 | 59.615a | 54.375b | 56.286ab |
| OCSTHA: Soil organic carbon stock | Ton/hectare | 0.049 * | 26 | 0.108 | 26.385a | 21.375a | 19.857a |
| CECSOL: Cation exchange capacity of soil | cmolc/kg | 0.569 | 26 | −0.025 | 20.308a | 21.500a | 18.714a |
| Traits | Between Species | Between Populations | Between Individuals | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Coefficient of Variation (%) | Mean | Standard Deviation | Coefficient of Variation (%) | Mean | Standard Deviation | Coefficient of Variation (%) | |
| Width | 2.31 | 0.24 | 10.25 | 2.29 | 0.19 | 8.48 | 2.29 | 0.24 | 10.63 |
| Length | 5.69 | 0.6 | 10.63 | 5.69 | 0.51 | 8.97 | 5.69 | 0.62 | 10.92 |
| Mass | 1.86 | 0.25 | 14.56 | 1.85 | 0.27 | 14.64 | 1.85 | - | - |
| Seed Traits | Intercept | bio03 1 | bio08 2 | bio09 3 | bio14 4 | PHIHOX 5 | CECSOL 6 | OCSTHA 7 |
|---|---|---|---|---|---|---|---|---|
| Mass | −2.415 × 10−16 | 0.6443 | ||||||
| Width | −2.563 × 10−16 | 0.7248 | −0.2285 | |||||
| Length | −4.141 × 10−16 | 1.591 | 2.321 | −2.836 | 0.2987 | −0.7044 | 0.2151 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-R.; Li, W.-Y.; Dong, Y.-Y.; Liu, J.-X.; Lan, Q.-Y.; Yang, X.; Xin, P.-Y.; Gao, J. Geographic Cline and Genetic Introgression Effects on Seed Morphology Variation and Germination Fitness in Two Closely Related Pine Species in Southeast Asia. Forests 2022, 13, 374. https://doi.org/10.3390/f13030374
Zhang Z-R, Li W-Y, Dong Y-Y, Liu J-X, Lan Q-Y, Yang X, Xin P-Y, Gao J. Geographic Cline and Genetic Introgression Effects on Seed Morphology Variation and Germination Fitness in Two Closely Related Pine Species in Southeast Asia. Forests. 2022; 13(3):374. https://doi.org/10.3390/f13030374
Chicago/Turabian StyleZhang, Zheng-Ren, Wei-Ying Li, Yi-Yi Dong, Jing-Xin Liu, Qin-Ying Lan, Xue Yang, Pei-Yao Xin, and Jie Gao. 2022. "Geographic Cline and Genetic Introgression Effects on Seed Morphology Variation and Germination Fitness in Two Closely Related Pine Species in Southeast Asia" Forests 13, no. 3: 374. https://doi.org/10.3390/f13030374







