Abstract
Invasive alien insects cause serious ecological and economical losses around the world. Here, we review the bionomics, modern ranges (and their dynamics), distribution pathways, monitoring, and control measures of 14 insect species known to be important invasive and emerging tree pests in forest and urban ecosystems of Russia: Leptoglossus occidentalis (Hemiptera: Heteroptera: Coreidae), Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae), Corythucha arcuata (Hemiptera: Heteroptera: Tingidae), Agrilus fleischeri, A. mali, A. planipennis, Lamprodila (Palmar) festiva (Coleoptera: Buprestidae), Ips amitinus, Polygraphus proximus (Coleoptera: Curculionidae: Scolytinae), Cydalima perspectalis (Lepidoptera: Crambidae), Acrocercops brongniardella, Cameraria ohridella, Phyllonorycter issikii, and P. populifoliella (Lepidoptera: Gracillariidae). We identified three major scenarios of tree pest invasions in the country and beyond: (1) a naturally conditioned range expansion, which results in the arrival of a pest to a new territory and its further naturalization in a recipient region; (2) a human-mediated, long-distance transfer of a pest to a new territory and its further naturalization; and (3) a widening of the pest’s trophic niche and shift to new host plant(s) (commonly human-introduced) within the native pest’s range frequently followed by invasion to new regions.
1. Introduction
Insect invasions are a widely recognized problem. Indeed, many invasive insect species pose a serious threat to biodiversity and ecosystem functioning worldwide, affecting the well-being of modern human society [1,2,3,4]. With increasing intercontinental trade (including the trade of living plants and plant origin products) and global changes happening in the environment (e.g., climate change, deforestation, ecosystem degradations, etc.), the impact of invasive species will continue to grow [5,6,7].
Invasive insect species are often not pests in their natural range, where they are well-controlled by biotic and abiotic factors [8]. Furthermore, some of these species become known to science only after their invasions [9,10]. On one hand, alien pests that are often released from their native enemies can switch on non-coevolved hosts, resulting in successful invasion, with notable damage to the invaded ecosystem [11]. On the other hand, alien plants introduced into a new region can be a target to native pests that can benefit from the susceptibility of the novel hosts [12]. The ability of invaders to form novel trophic associations may present a threat to plants in their native range in cases of a pest accidental introduction. Our knowledge of the biology of novel pests, their invasive potential and impacts, as well as possible methods of monitoring and control is usually limited [13]. Often, such information is unavailable or scarcely published on a national language of a country from where a pest is originally known and is thus not accessible to an international audience.
Russia occupies a huge territory in Europe and Asia with different climatic zones, and the country is actively involved in international trade. Thus, it is not surprising that harmful organisms, arriving to the country via international trade (e.g., airfreight or shipping containers carrying crops, ornamental plants, wood, etc.) or through the movement of people (e.g., global tourism), are regularly detected here [14], and those that succeed in becoming established cause significant economic losses to Russia [15]. The opposite is also true. Pests are regularly found on plants and plant material in vehicles driving from or through the territory of Russia and, as a result, are recorded in new regions of the country or beyond, in foreign countries [8]. Information about the biology, monitoring, and control of alien invasive species that actively expand their ranges and have recently arrived in Russia or, in contrast, native species that are able to spread from Russia to other countries, is of a high importance for international specialists dealing with pest quarantine and forest protection in their homelands.
In this review, we analyzed the biology, distribution, pathways, monitoring, and control approaches of 14 tree insect species, which are considered highly important invasive or emerging insect pests of forests and urban woody plants in Russia [8,15]. It should be understood that they are not the only actual or potential invasive or emerging insect pests in Russia deserving attention. However, in our opinion, they can be considered as representative models of insect pest invasions at a national level.
2. Materials and Methods
In order to provide up-to-date knowledge on insect pests of forests and urban woody plants in Russia, we reviewed numerous studies, mostly published in a national language, and the references are given in each species’ essay below. We have included the latest papers delivered at the All-Russia biennial conference “Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems (The Kataev Memorial Readings)”, which serves as the main platform for discussing the most important issues on forest entomology, pathology, plant protection, and quarantine in the country [15]. We also examined “grey” and not easily accessible national literature (e.g., proceedings, conference notes, papers in regional editions, etc.). As a result, we compiled 14 brief essays on important invaders in Russia (mainly within its European part). Most of these pests (both alien invasive species which penetrated Russia and native national pests) can potentially further expand their ranges and pose a threat to woody plants in other countries.
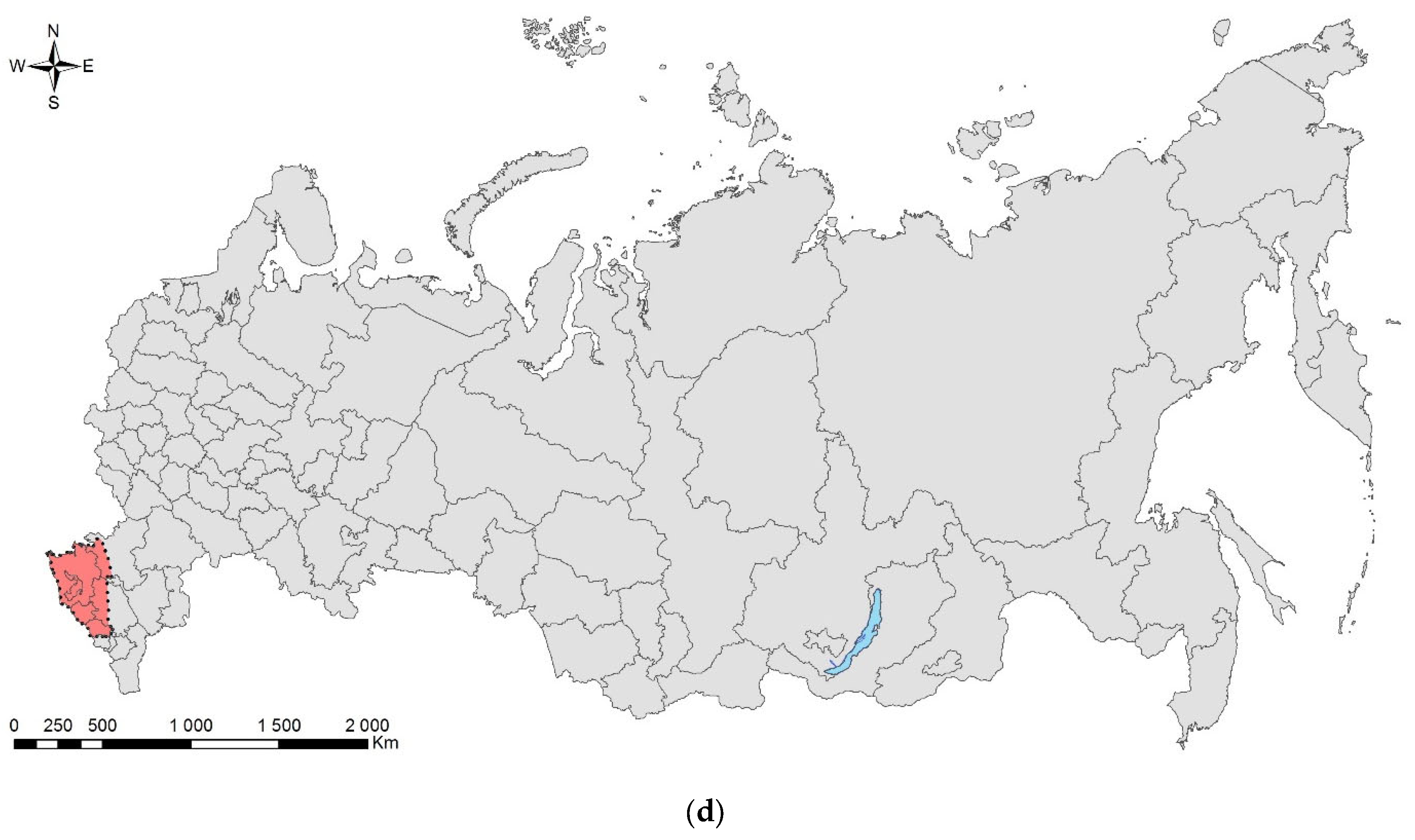
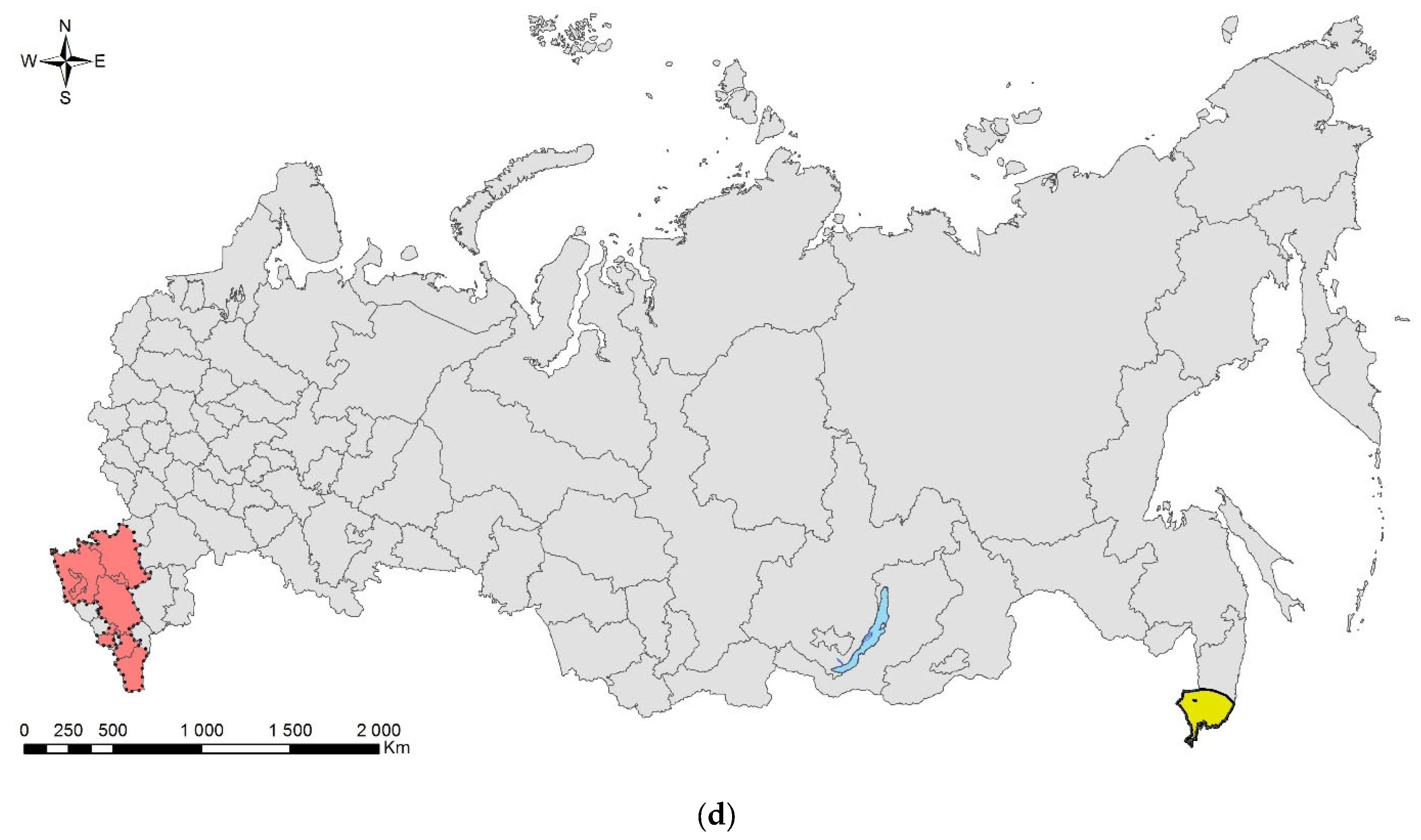
The essays are arranged in taxonomic order. They briefly overview history of invasion, biology, basic monitoring and control measures, and are accompanied with photographs of pests and damage symptoms. A schematic map of the natural range (colored in yellow) and invasive range (colored in red) in Russia is also provided. In most cases, we outlined the entire administrative region/unit for published records, except for the cases when the area of the record is relatively small, whereas the region is large. The current ranges were mapped using ArcGIS 9.3 [16].
As there are discrepancies in the English naming of Russian administrative units and settlements, we applied the most commonly used names. To designate administrative units, the following terms were used: “Oblast” for Province and “Krai” for Administrative Territory.
3. Results
3.1. The Western Coniferous Seed Bug, Leptoglossus occidentalis Heidemann, 1910 (Hemiptera: Heteroptera: Coreidae)
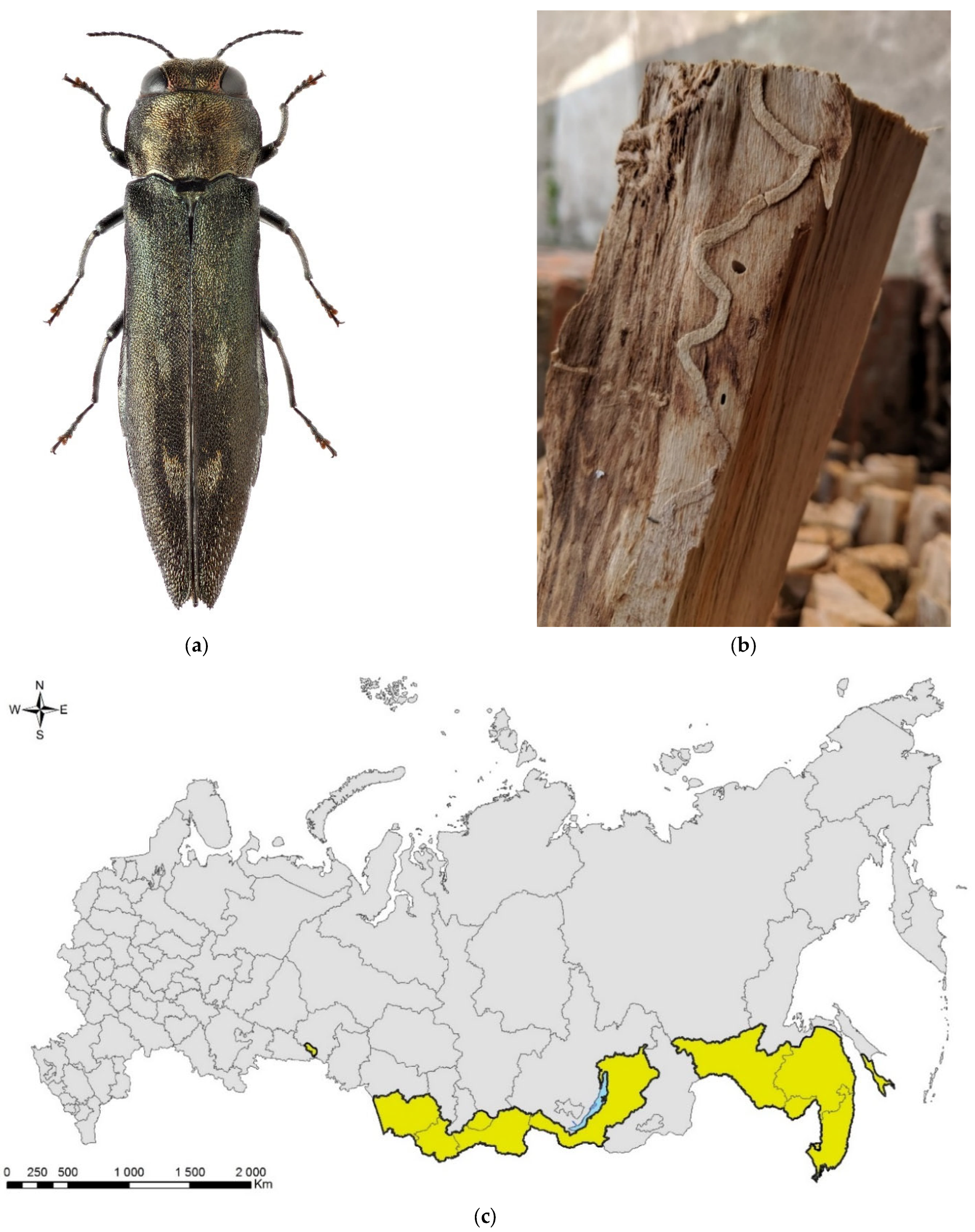
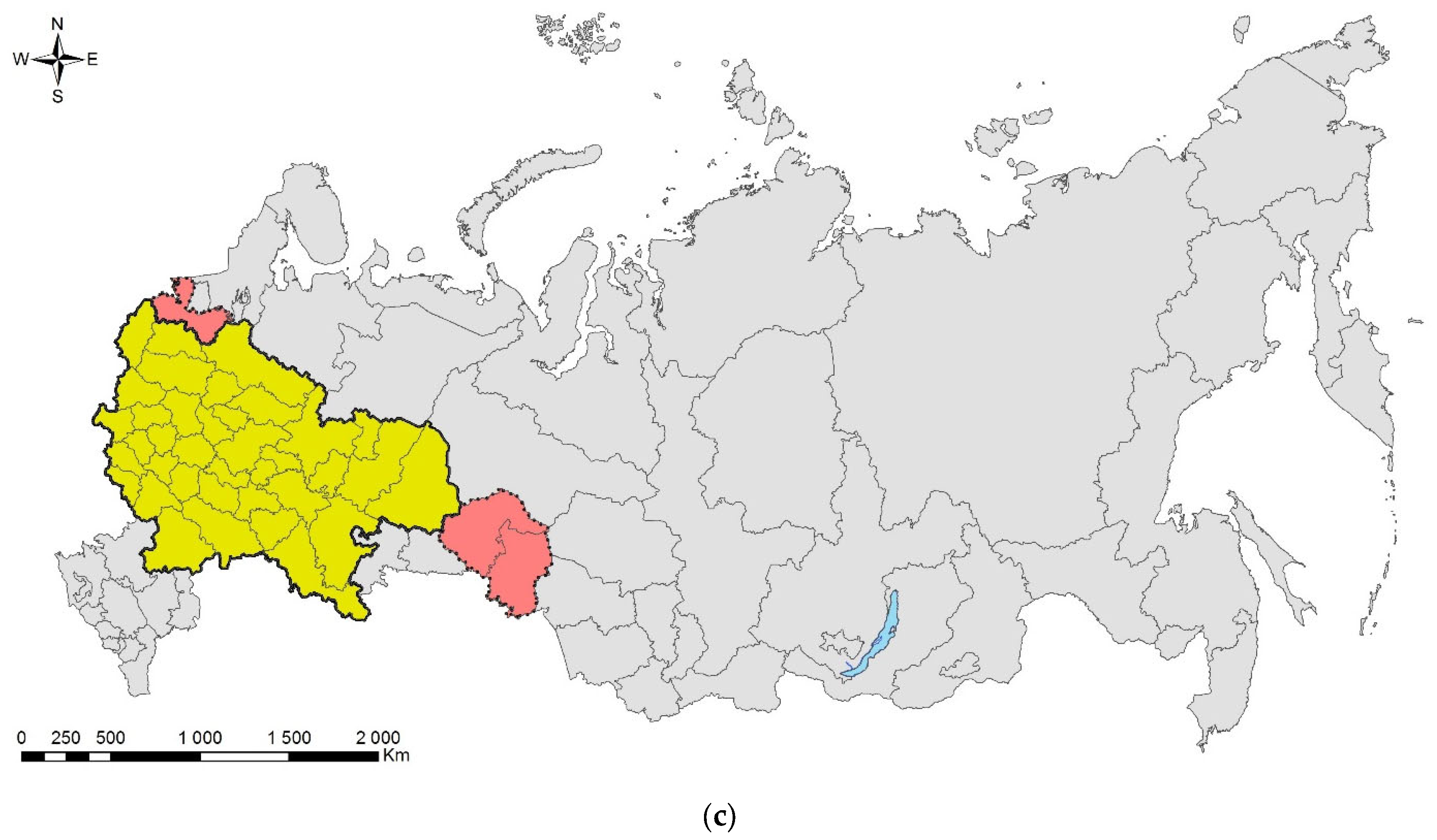
Leptoglossus occidentalis (Figure 1) is a North American species that invaded Eurasia, North Africa, and Central America. The native range of the species covers the west of North America, from the south of Canada to Mexico [17]. In Europe, it was first recorded in 1999 in northern Italy [18], where it was accidently introduced from North America, probably by plane [19]. Over the next 10 years, the bug spread from Italy across Europe northward (to Norway) and eastward (to Russia and Turkey) (Supplimentary Table S1). At present, the invasive range of L. occidentalis in Eurasia covers significant parts of the Palaearctic. In Asia, it is known from Turkey, Israel, Armenia, Kazakhstan, Northeast China, Japan, and South Korea [17,20,21,22,23,24,25]. In Africa, it is recorded in Tunisia [26], Morocco [27], and South Africa [28]. It is also recorded in Chile [29], Brazil [28], Argentina [30], and Uruguay [31]. The first records in European Russia refer to Rostov-on-Don (but also to the neighboring Crimean Peninsula) [17]. Up to now, it has been recorded also in the Smolensk Oblast [32], Voronezh City [33], Volgograd City [34], Stavropol Krai [27], Krasnodar Krai, and North Ossetia (central Causasus) (Figure 1c) [19,27,35]. The species has spread along the entire Black Sea coast of the Caucasus (Krasnodar Krai) to the border with neighboring region of Abkhazia [19,35], from which it is also known [27]. In addition, it is documented from Transcaucasia: the neighboring region of South Ossetia [27], Georgia [36], and Armenia [25].
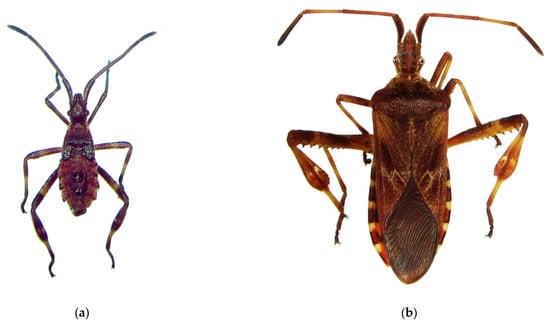
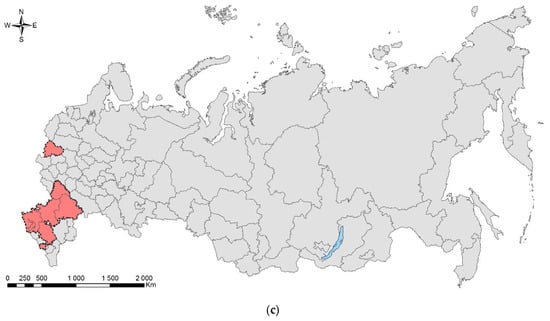
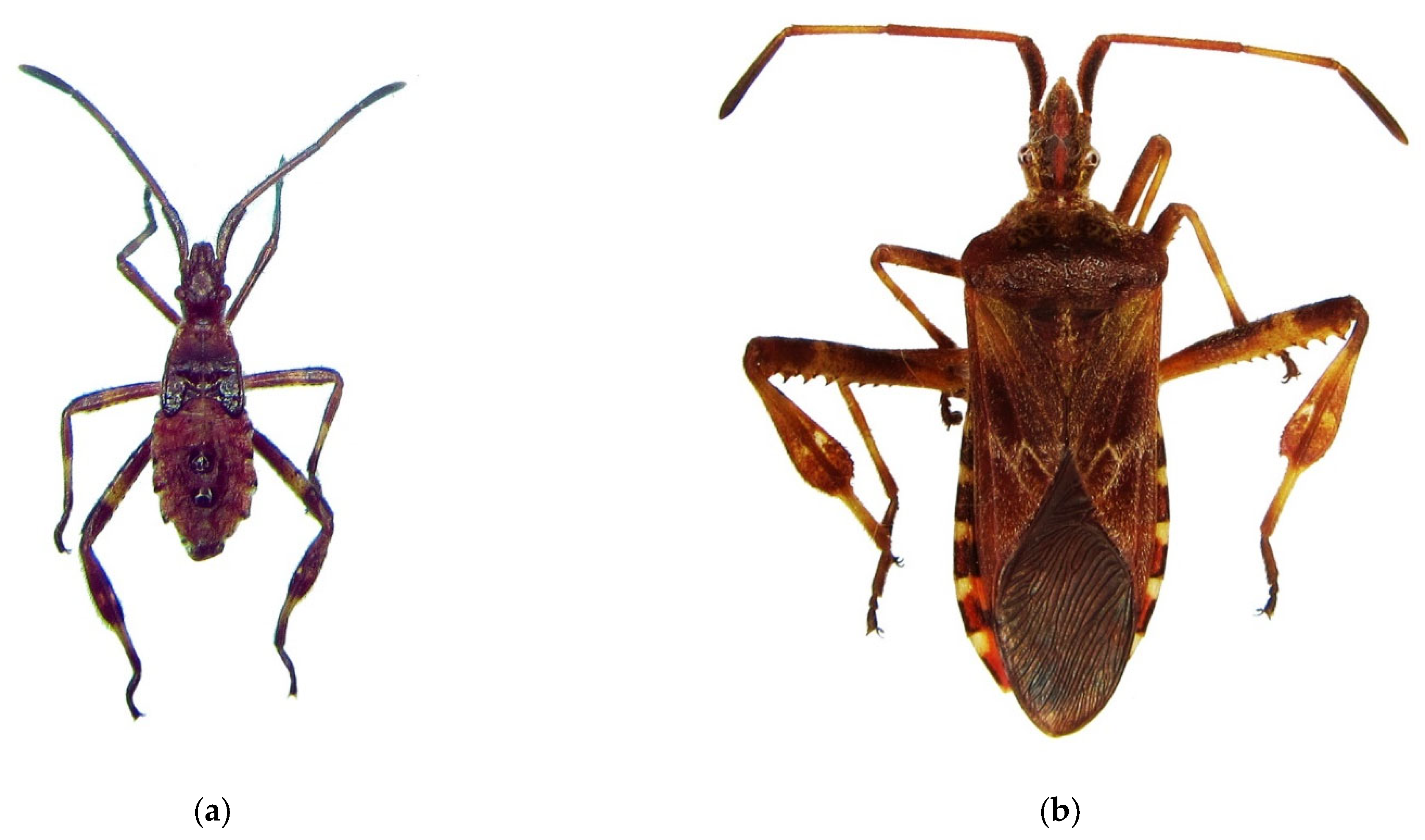
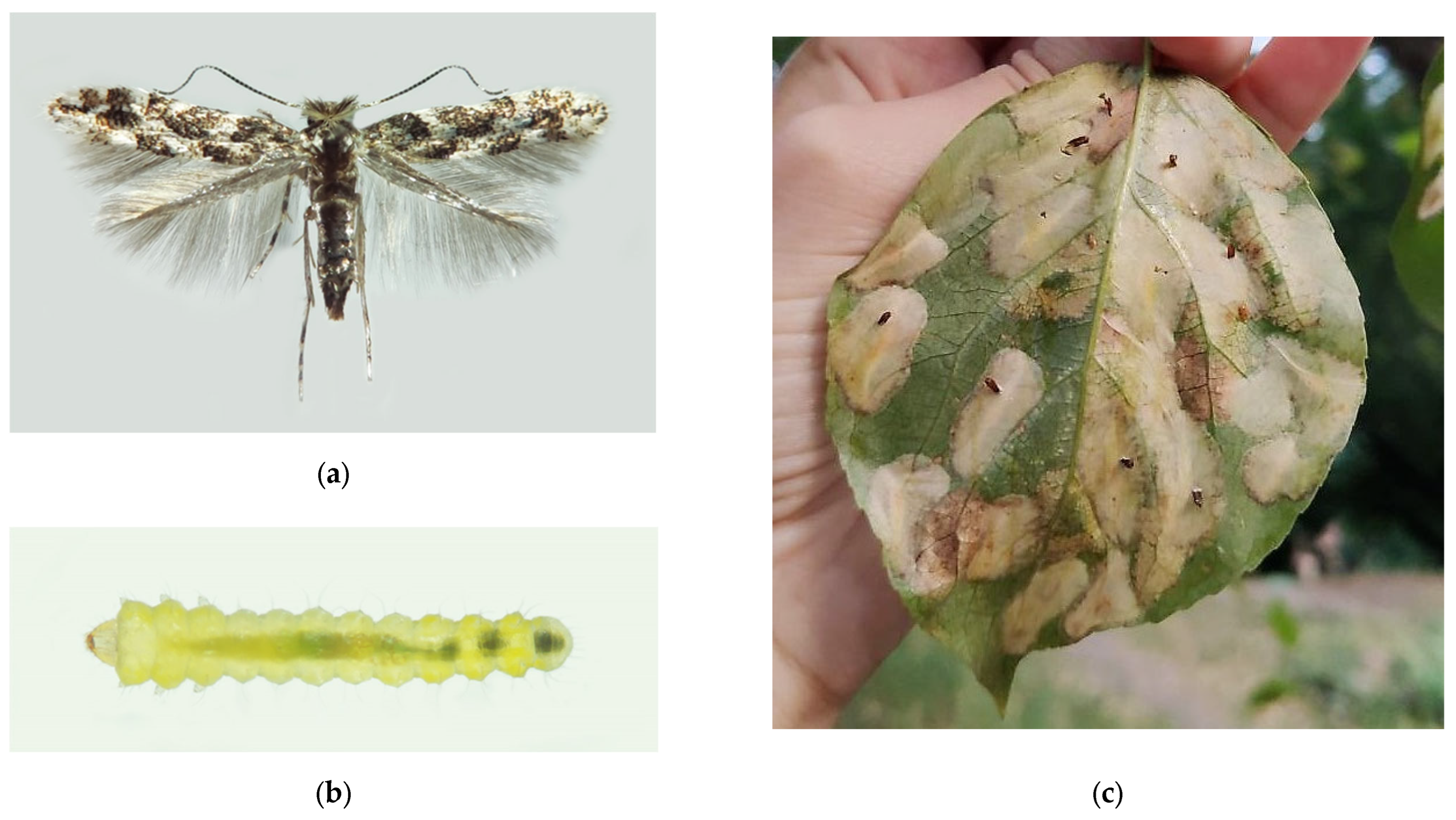
Figure 1.
The Western coniferous seed bug, Leptoglossus occidentalis: (a) a nymph of IV instar; (b) an adult, Voronezh City, September 2020; (c) the current invasive range in Russia (colored in red). Photo by E.V. Aksenenko.
The routes of introduction into Russia are not known. Adults of L. occidentalis have good flying abilities and, thus, they can fly for long distances over regions having suitable habitats and host plants [17]. The bugs could have also been accidently introduced with coniferous plants, either with plants for planting or Christmas trees (whole or cut branches) [17,37]. The rate of spreading of L. occidentalis from the place of its first detection in Europe is approximately the same in all directions of its expansion and is estimated to be about 200 km per year [19]. Most likely, in addition to active distribution, the invader is moved passively with various types of transport [19].
Based on the record of the species in Voronezh City (the forest-steppe zone; approx. 500 km from Rostov-on-Don) and in the Smolensk Oblast (the forest zone; approx. 1000 km northward from Rostov-on-Don), a significant expansion of the invaded range northward in the European part of Russia should be expected. Successful establishment of the species in the forest-steppe zone is now evident at least in urban conditions, keeping in mind that average temperatures are higher in cities than in the surrounding environment [33]. The probability of spreading and establishment further eastward remains unknown.
The species is trophically associated with a wide range of plants from Pinaceae (e.g., Abies, Cedrus, Picea, Pinus, Pseudotsuga, and Tsuga), Cupressaceae (e.g., Calocedrus, Cupressus, and Juniperus) [17,20,35,38], and exceptionally documented on Anacardiaceae (Pistacia vera) [39]. In Russia, feeding on spruce Picea glauca, P. pungens, and fir Abies cocolor has been recorded [19].
The bug overwinters as adults or nymphs of the last (V) instar. For overwintering, they aggregate in cracks in the bark and other similar microhabitats in forests and can also penetrate dwellings, cars, etc. They leave overwintering shelters in April to May and start feeding on young microstrobili and megastrobili, causing growth impairment, deformation, and reducing the production of microspores [17]. Then, the adults mate and the females lay eggs. Females lay about 30 eggs (up to 80 maximal) in clusters along the needles [40]. Hatched nymphs and adults feed on maturing and ripe seeds and also suck sap from the apical shoots throughout summer. Adults emerge close to the end of August.
Within its native range in northern California, L. occidentalis produces one generation per year [37,41]. In Italy, it has two or three generations on the plain, and only one generation in the highlands [42]. In the south of Krasnodar Krai, two generations per year were recorded [19]. In Dagestan (North Causasus), at least one generation per year is possible, and in the area between Anapa and Novorossiysk cities, two generations can occur [19]. Further northward, thermal conditions are not favorable for this species.
The damage caused by L. occidentalis has not yet been fully evaluated in Europe. In North America, it destroys up to 26% of the seeds of Pinus monticola and up to 41% of the seeds of different Pseudotsuga species [41]. In West Europe, the species has not caused any significant damage so far [43], probably because: (1) its abundance has not yet reached the critical level in the newly colonized territories, and (2) causing damage only to seeds, the bug practically does not harm the trees themselves. In Russia, a 3-year study of the influence of L. occidentalis on seed germination of the Crimean pine and Scots pine in the Rostov Oblast demonstrated the high harmfulness of the seed bug [19].
The bug carries spores of the pathogenic fungus Sphaeropsis sapinea (=Diplodia pinea), which causes diplodia tip blight: a necrosis of the needles and shoot bark, as well as the withering of seedlings and young plants [44,45]. Thus, the cumulative damage to pines can be highly significant. In its native range, L. occidentalis is parasitized by Trichopoda pennipes (Diptera: Tachinidae) [46] and hymenopteran egg parasitoids, Gryon pennsylvanicum (Scelionidae), Anastatus pearsalli (Eupelmidae), and Ooencyrtus spp. (Encyrtidae) [47]. In Italy, the hymenopterans, Anastatus bifasciatus (Eupelmidae), Tetrastichus servadeii (Eulophidae), and Ooencyrtus pityocampae (Encyrtidae) parasitize the eggs of L. occidentalis. In Russia, parasitoids that able to control the bug are not known, except Ectophasia crassipennis (Diptera: Tachinidae) [35].
Leptoglossus occidentalis can be effectively controlled using pyrethroid preparations in the years when the pine yield is low and the bug‘s population density is high; however, this and other chemical control measures against this pest, as well as biological control focusing on its natural enemies, should be developed in Russia [35].
3.2. The Brown Marmorated Stink Bug, Halyomorpha halys (Stål, 1855) (Hemiptera: Heteroptera: Pentatomidae)
The native range of Halyomorpha halys (Figure 2), covers Southeast Asia: China, the Korean Peninsula, Japan, Taiwan, and Vietnam [48,49]. The history of the pest invasion began in North America, where the species was first recorded in 1996, and since then, it has actively spread throughout the country. By 2015, the bug had been reported in 41 states [50,51,52]. In Canada, H. halys was documented in 2010 in Ontario and Quebec [53]. In Central and South America, H. halys has not yet been recorded, except for the only detection in the Chilean border zone in 2017 [54]. In Europe, H. halys was first detected in 2004 in Liechtenstein [55] and spread quickly to at least 30 countries (Supplimentary Table S2). The species expansion in Europe was recently analyzed based on the distribution of different haplotypes [56]. In 2010, live specimens of the bug were intercepted in England and New Zealand in the luggage of passengers traveling by air. In New Zealand, it was also found in used cars brought from Japan, but the species did not spread across the countries [57,58].
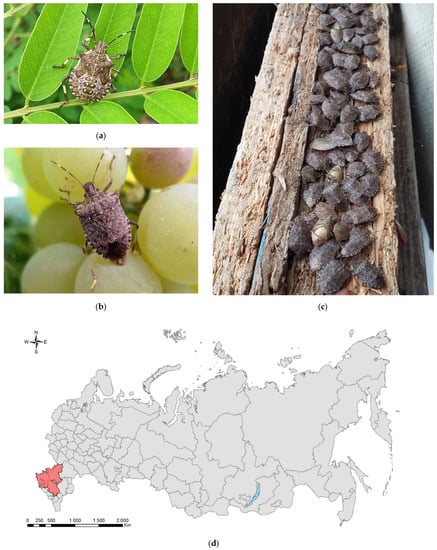
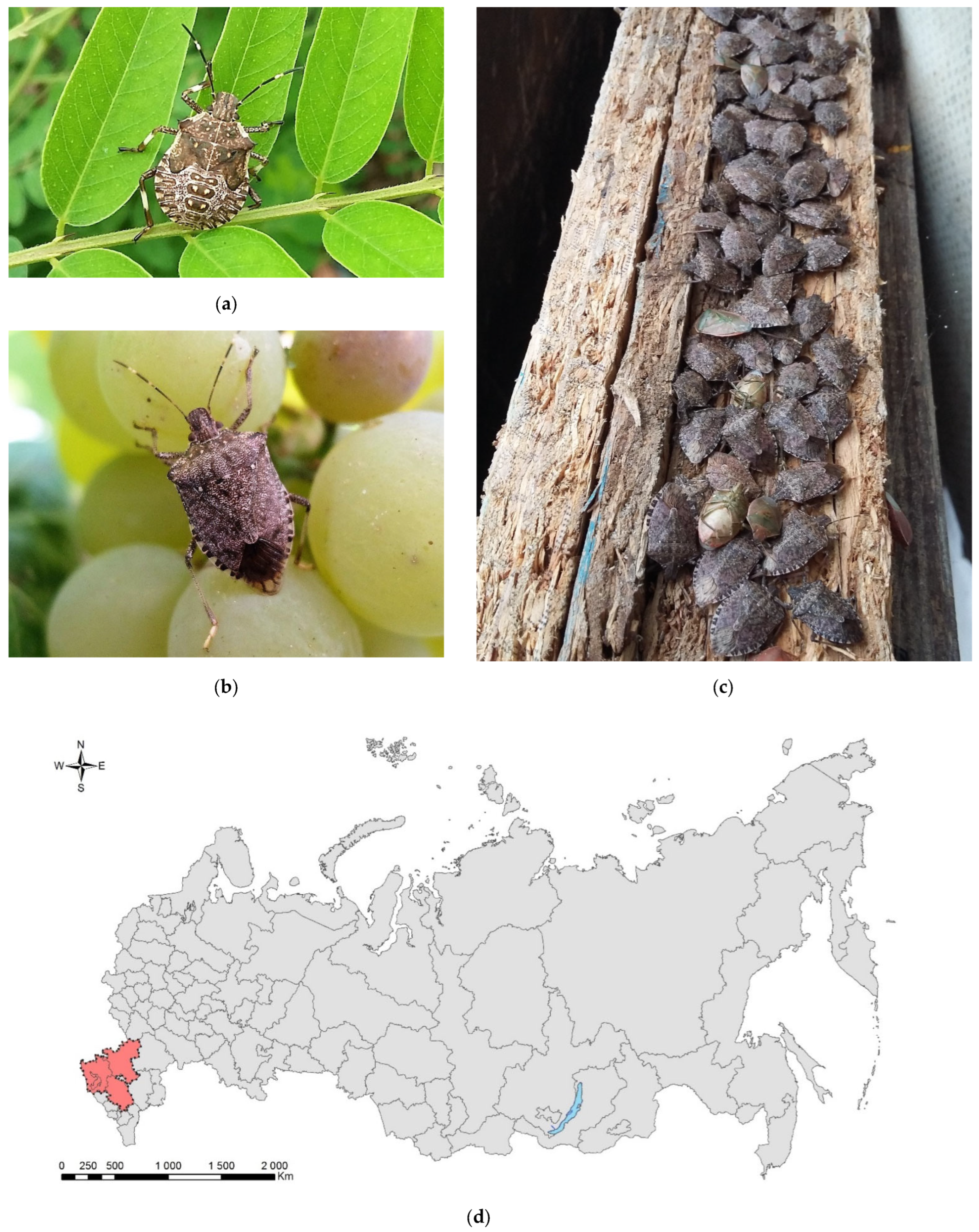
Figure 2.
The brown marmorated stink bug, Halyomorpha halys: (a) a nymph of the final (V) instar, Sochi, August 2017; (b) an adult on grape, Sochi, September 2017; (c) an overwintering aggregation in warehouse, Sochi, January 2018; (d) the current invasive range in Russia (colored in red). Photo: (a) by V.Ye. Zakharchenko and (b) and (c) by N.N. Karpun.
In Russia (Figure 2), H. halys was detected for the first time in 2014 [59] in two parks in Sochi, and already in 2015, an outbreak was observed in the humid subtropics of Russia and neighboring region of Abkhazia [60]. The species occurs on the Black Sea coast of the Caucasus at altitudes up to 800 m a.s.l. [61]. In the fall of 2016, the bug was noted in Krasnodar City, and during 2017, it invaded the territory of Krasnodar Krai and Adygea (West Causasus) [62,63,64]. In 2018, H. halys was detected in the Stavropol Krai and in neighboring Crimean Peninsula (in Simferopol, Sevastopol as well as in the nearby villages of Inkerman, Balaklava, on Cape Fiolent) [65,66]. Since 2019, the species adults were regularly caught in the City of Rostov-on-Don and in the Rostov Oblast [67,68]. In 2020, nymphs and adults were intercepted in the Stavropol City [69] and in Dagestan (North Caucasus, Samur Forest) [70]. It is likely that goods of non-plant origin transported during the winter are the major vector of H. halys distribution in Russia [63].
The brown marmorated stink bug is a polyphagous pest damaging 300+ species of plants [50,71,72]. In southern Russia, nymphs and adults feed on 107 species of cultivated and wild plants, and during the year, the species migrates from one crop to another. It locally migrates to forest and forest plantations at the end of August or in September, concentrating in the crowns of beech, linden, hazel, and ash [73]. In 2016–2017, H. halys caused severe losses in the yield of fruit, subtropical, and vegetable crops [60,63,74]. In woody species, the pest destructs fruits. Thus, in hazel, kernels fail to properly form, while in Rosaceae, fruits are deformed and do not ripen [73]. On the Black Sea coast of Russia, two generations develop per year, and only one is likely to develop further north [74,75]. The monitoring of H. halys is possible by regular visual inspections of plantations during the summer and overwintering sites in the autumn and winter, as well as by pheromone trapping [62]. The traps are placed in crowns of woody plants or on poles in fields or other open areas. The composition of the aggregation pheromone of H. halys was identified as a mixture of two stereoisomers: (3S, 6S, 7R, 10S)-10,11-epoxy-1-bisabolene-3-ol and (3R, 6S, 7R, 10S)-10,11-epoxy-1-bisabolene-3-ol, in a ratio of 3.5: 1, respectively [76]. Among the pheromones produced in Russia, the most effective is a mixture consisting of an aggregation pheromone obtained from citronelal racemate and methyl (E, E, Z)-2,4,6-decatrienoate [77].
In the south of Russia, no effective predators and parasitoids of H. halys have been found so far. As biological control measures, the use of preparations based on entomopathogenic fungi (strains) of the following species is considered promising: Beauveria bassiana, Metarhizium anisopliae, Isaria fumosorosea, and Ophiocordyceps nutans [74,78,79,80,81,82]. Pheromone traps catch more than 100 individuals per week and can be effectively used in autumn [77,83]. To control nymphs and adults, neonicotinoids (based on imidacloprid, acetamiprid, thiacloprid, and thiamethoxam) and pyrethroids (based on bifenthrine, cypermethrin, lambda-cyhalothrin, alphacypermethrin, etc.) are effective [48,74,84,85,86,87,88,89,90].
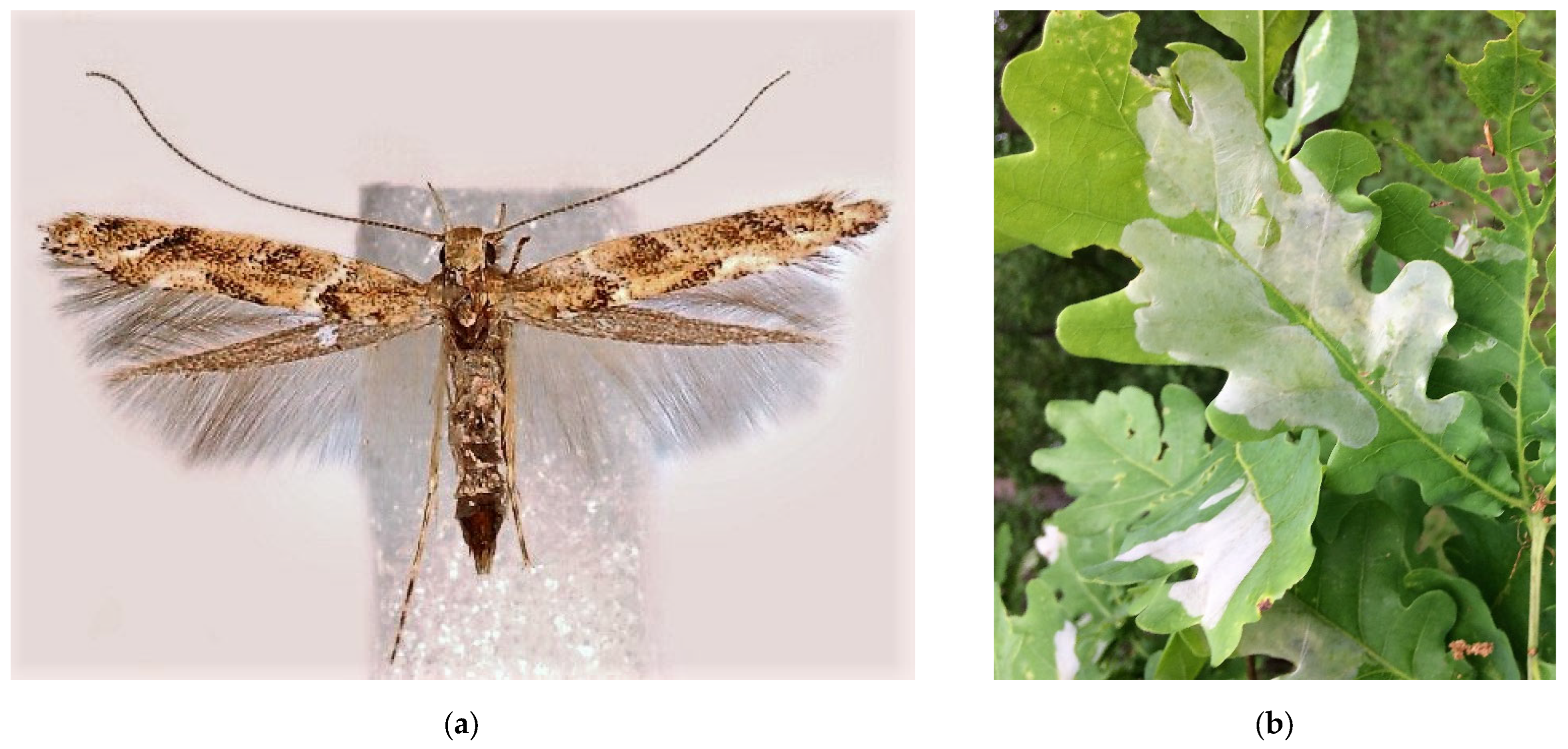
3.3. The Oak Lace Bug, Corythucha arcuata (Say, 1832) (Hemiptera: Heteroptera: Tingidae)
Corythucha arcuata (Figure 3) is a North American species that is naturally distributed in the USA and the southern regions of Canada [91,92]. In Europe, the oak lace bug was first recorded in 2000 in Italy [93], and by now, it has quickly spread in almost 20 countries (Supplimentary Table S3). In Asia, it was found in 2002 (2003) in Northwest Turkey, and by 2008, it had spread about 600 km eastward [94,95]. In 2005, a specimen of the bug was caught in the north of Iran at the latitude of 1370 m a.s.l. in the vicinity of Urmia (Urmiech) [96]; however, no data on further acclimatization of the species in Iran are available.
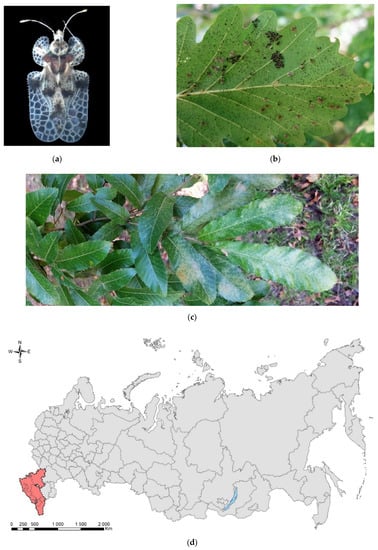
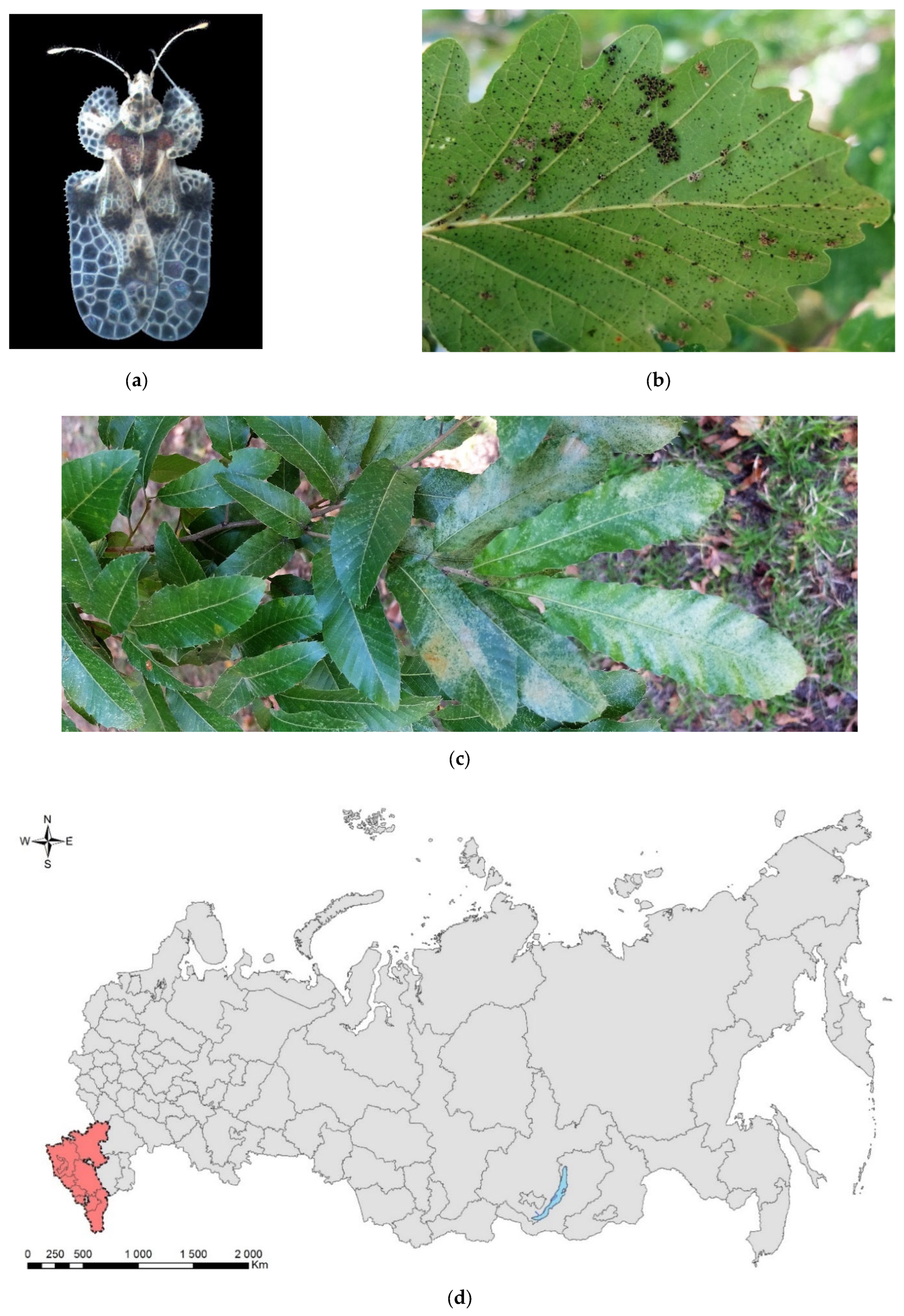
Figure 3.
The oak lace bug, Corythucha arcuata: (a) an adult, Krasnodar Krai, June 2016; (b) eggs, nymphs, and adults on a leaf of Quercus hartwissiana, Sochi, September 2017; (c) heavy discoloration of Q. variabilis, Sochi, September 2017; (d) the current invasive range in Russia (colored in red). Photo: (a) by V.A. Soboleva, with permission, (b) and (c) by V.Ye. Zakharchenko.
In Russia (Figure 3d), the first local outbreak of this pest was recorded in the summer of 2015 in Krasnodar City. By the autumn 2016, the species had spread across the Krasnodar Krai and was found in Adygea [90,97,98]. In September 2017, C. arcuata was found in the subtropical zone of the Black Sea coast of the Caucasus [99] and in the neighboring Crimean Peninsula [100], where it was later repeatedly documented [101,102]. In 2018, the bug was recorded in the Rostov Oblast and many regions of the North Caucasus (Stavropol Krai, Karachay-Cherkessia, Kabardino-Balkaria, North Ossetia, Chechnya, and Dagestan) [103]. Further pest expansion outside Russia to natural forests of Central Asia and the countries of the Caucasus is likely.
Corythucha arcuata spreads over long distances mainly due to human-mediated transportation (land, air, and water vehicles); the distribution on air currents and transportation with seedlings of plants and other plant material are not ruled out [104]. Within its natural range, it damages many oaks (Quercus spp.; Fagaceae) by sucking juice from leaves. Additionally, it has been noted feeding on toothed chestnut Castanea dentata (Fagaceae), willows (Salix spp.; Salicaceae), and Cercis canadensis (Fabaceae) [92,105]. In southern Russia, it damages various species of oak, as well as a number of other woody plants (e.g., Castanea sativa, Corylus avellana, Acer platanoides, Alnus glutinosa, A. incana, etc.) [106,107].
In North America, the oak lace bug produces two or three generations per year [108]. Under favorable conditions of the North Caucasus, Transcaucasia, and the Crimean Peninsula, up to three generations can develop in April to November (sometimes a part of its population can produce the fourth generation) [109].
Nymphs and adults suck juices from leaves, resulting in a pronounced discoloration, which is first seen as a marbled diffuse yellowing of foliage, and by the middle of summer, as complete discoloration due to chlorophyll loss [106,108]. Despite growing population densities, the bug impact on oak forests in Russia has not been estimated yet. Significant destruction of foliage in tree crowns during several years can greatly weaken the oaks, causing their gradual decline [103,109]. In addition, the decorative quality of trees massively infested by the oak lace bugs is significantly reduced, which may be critically important in urbanized environments [63].
The oak lace bug should be monitored through regular visual ground surveys in forest and urban plantations [103,106], and remote sensing should be developed [110], since the damage caused to trees is clearly visible. Sweeping with classical entomological net in tree crowns and shrubs can also be used for surveys [102].
In Russia, predators and parasitoids of the oak lace bug have not yet been studied [111]. Nevertheless, it is believed that entomopathogenic fungi (e.g., Beauveria spp., Metarhizium spp., and Cordyceps spp.) can be potentially used for the biological control of C. arcuata [112,113]. Of the chemical preparations, avermectins, pyrethroids, and neonicotinoids are considered effective against this pest [114,115,116].
3.4. The Spotted Poplar Borer, Agrilus fleischeri Obenberger, 1925 (Coleoptera: Buprestidae)
Agrilus fleischeri (Figure 4), is a North Asian pest of poplars (Populus spp.) and, to a lesser extent, willows (Salix spp.), which produced outbreaks and caused tree mortality in poplar plantations in China (see below in this essay). Because of these circumstances, it is considered a potentially invasive pest of poplars in Europe and North America [117,118,119] and included in the European and Mediterranean Plant Protection Organization (EPPO) A2 List of pests recommended for regulation as quarantine pests [120].
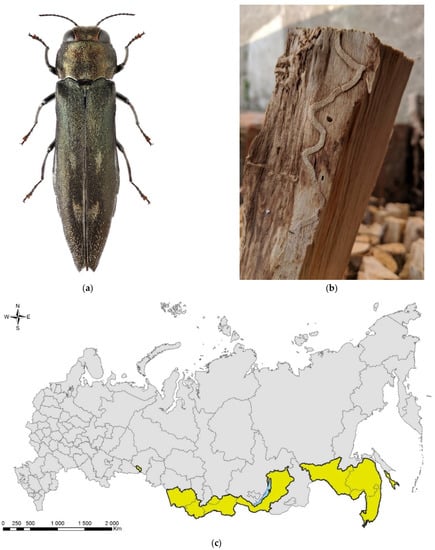
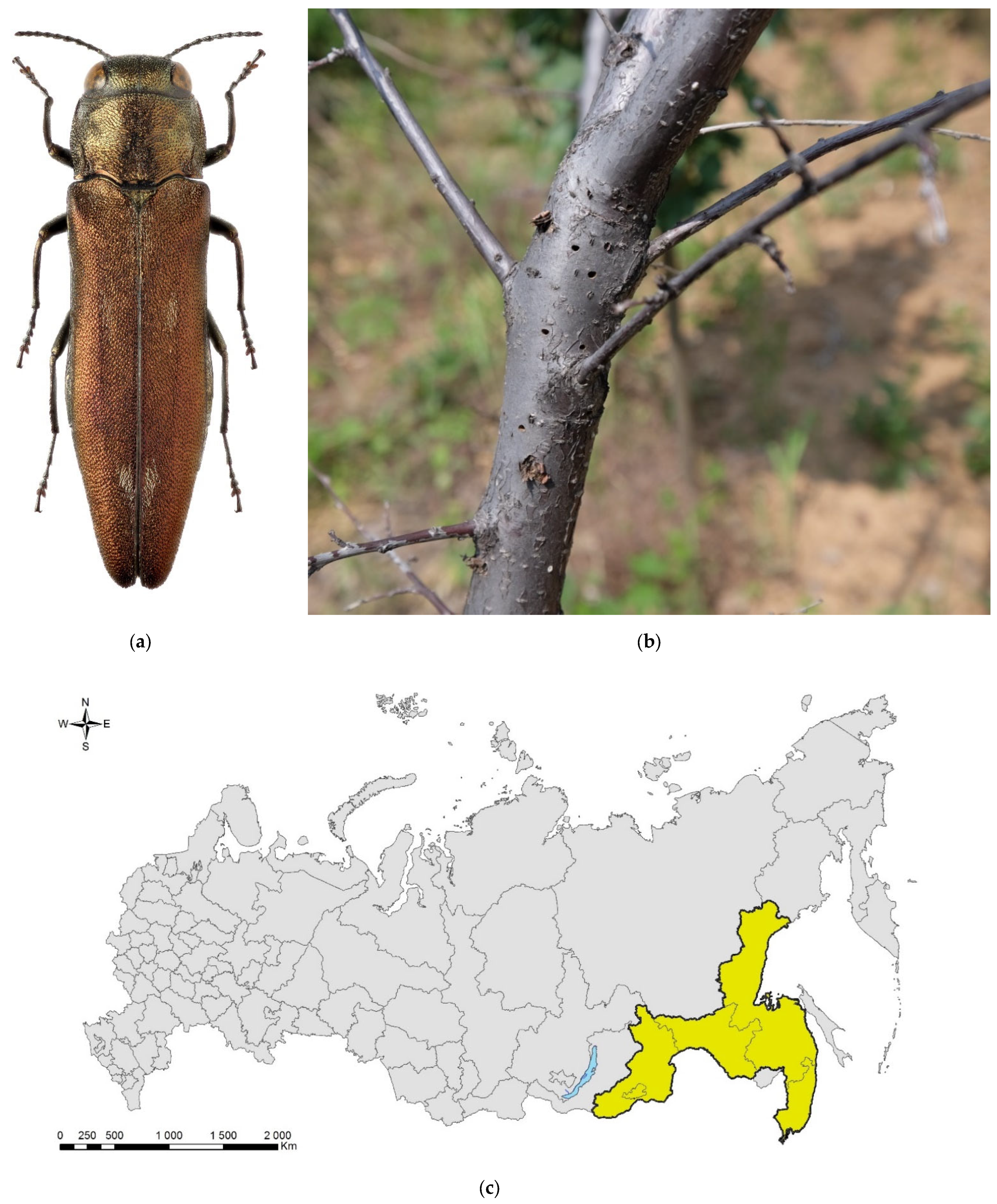
Figure 4.
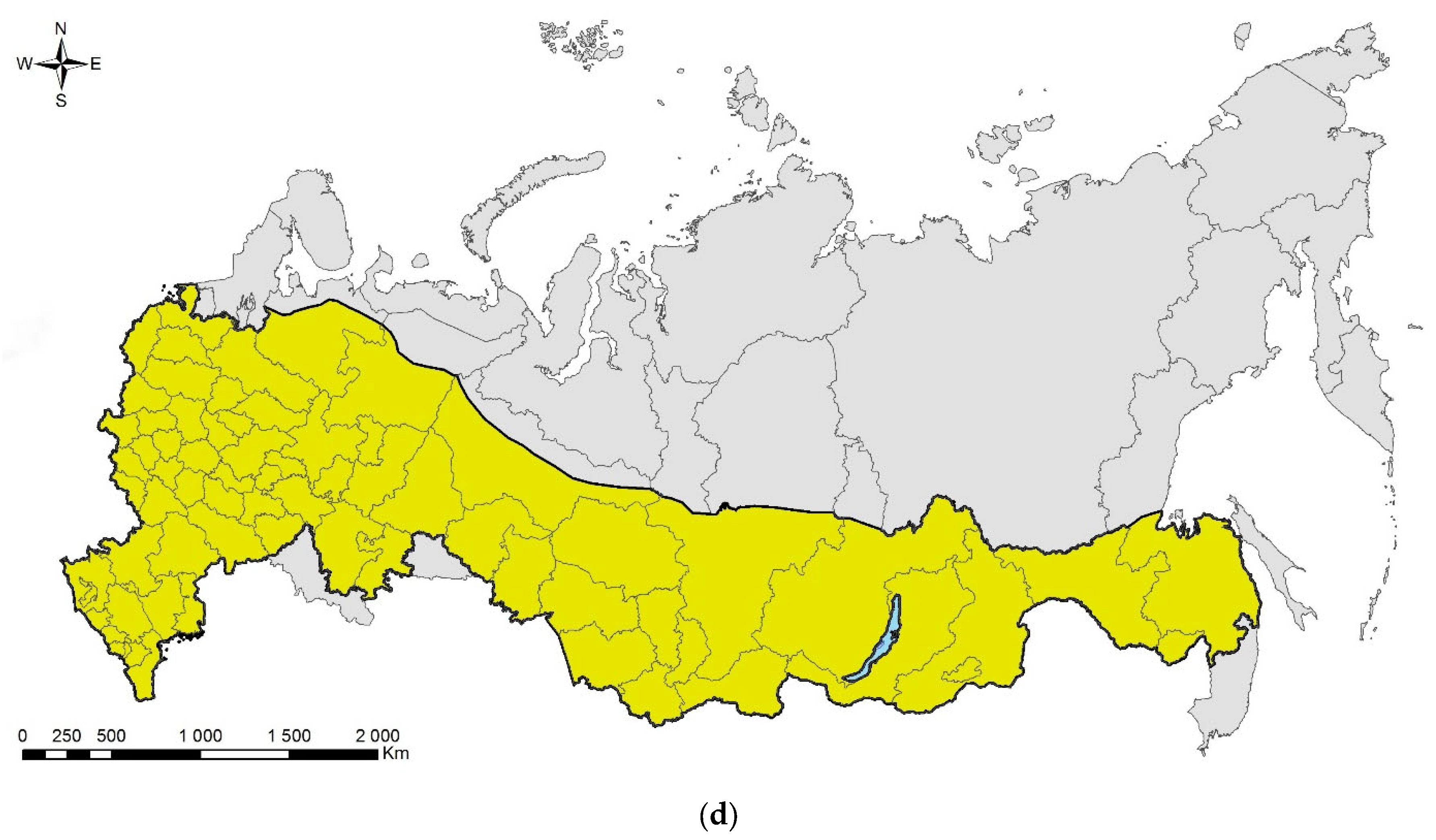
The spotted poplar borer, Agrilus fleischeri: (a) a female; (b) a fragment of a larval gallery and an exit hole on a piece of firewood (Populus sp.), Jilin Province, China; (c) the native range in Russia (colored in yellow). Photo: (a) by A.V. Kovalev, after [119]; (b) by E. Jendek, both with permission.
The species was described from East Siberia (Transbaikalia: Berezovka settlement) [121]. In Russia, it is distributed across its Asian part: in West Siberia (Tyumen Oblast, Krasnoyarsk and Altai Krais, the Altai Republic), East Siberia (Tyva and Buryatia), and Far East (Primorskii and Khabarovsk Krais, Sakhalin, and Amur region) (Figure 4c).
In Asia, it is also known from Kazakhstan (eastern and, probably, northern parts; report from Karaganda Oblast on the east needs confirmation), Mongolia (Töv Aimag), China (Northeast and Central China: Heilongjiang, Liaoning, Beijing, Sichuan, and Shaanxi Provinces), North Korea, South Korea, and Japan (Hokkaido, Honshu) [119,122]. In the westernmost part, the range of A. fleischeri reaches, but does not overlap with, the range of closely related European Agrilus ater [119].
The spotted poplar borer is considered a potentially invasive species. Although it has never been reported beyond its native range, it is known to outbreak on planted exotic poplars [117,118,119]. Outbreaks of A. fleischeri on introduced Populus nigra var. italica resulting in tree mortality were first observed in China (Liaoning Province) in 2013 to 2015 [123].
The species may potentially spread to Europe and North America, where poplars are native and where cultivated poplar species from China, Asian Russia, or East Kazakhstan are planted. In Canada, adults of A. fleischeri have been intercepted on two occasions being transported with wood packaging material and wood dunnage [120]. The pest can be accidently introduced with poplar plants for planting, round and sawn wood, wood residues, and wood packaging material. Natural spread (e.g., from Asian Russia via European Russia to EU) or hitchhiking with transport are considered unlikely but possible pathways [120].
In China (Liaoning Province), in addition to feeding on the indigenous species of poplars and willows, A. fleischeri can also severely damage introduced Populus nigra var. italica [123]. The current situation with A. fleischeri is reminiscent of the early stages of the invasion of the emerald ash borer, A. planipennis, which switched from the indigenous ash species to the cultivated American ash species, triggering its outbreaks in Eastern China. Then, the pest was accidentally imported to North America and European Russia [119] (see also Section 3.6 below).
Currently, no special control measures have been developed in Russia. In China, sanitary felling of infested, dead, and dying trees is performed [120]. The dimethoate is considered an effective insecticide against adults during the flight period of different Agrilus species in China [120,124]. Some hymenopteran parasitoids (i.e., Oobius saimaensis, O. fleischeri [Encyrtidae]; Polystenus rugosus, Spathius sp. [Braconidae]; Paramblynotus sp. [Liopteridae]; and Euderus fleischeri [Eulophidae]) and entomopathogenic fungi Beauveria bassiana (Ascomycota: Cordycipitaceae), which were found infesting A. fleischeri eggs and larvae in China, can be considered potential agents for the biological control of the pest [120].
3.5. The Apple Buprestid, Agrilus mali Matsumura, 1924 (Coleoptera: Buprestidae)
Agrilus mali (Figure 5), originally described as a destructive pest of apple plantations in Korea, is currently regarded a dangerous quarantine pest of cultivated and wild apple trees in Russian East Siberia and the Far East, East China, and both Koreas (see below in detail). Shortly before 1993, A. mali was unintentionally introduced in the Ili River valley (Xinjiang Province, China) from Shandong and later caused extensive tree mortality in the wild apple, Malus sieversii, forests in the Tien Shan Mountains [125,126,127,128,129,130]. Thereby, A. mali is considered a potentially invasive pest threatening cultivated apple trees outside its native range, wild apple trees and, in particular, M. sieversii, which is an important tree of mountain deciduous forests in Northwestern China, Kazakhstan, and adjacent countries of the Middle Asia [118,119] and a key ancestor of the domestic apple tree [127,128,129,130]. Agrilus mali is a quarantine species in the Russian Federation, the Eurasian Economic Union [131], and the EPPO region [132].
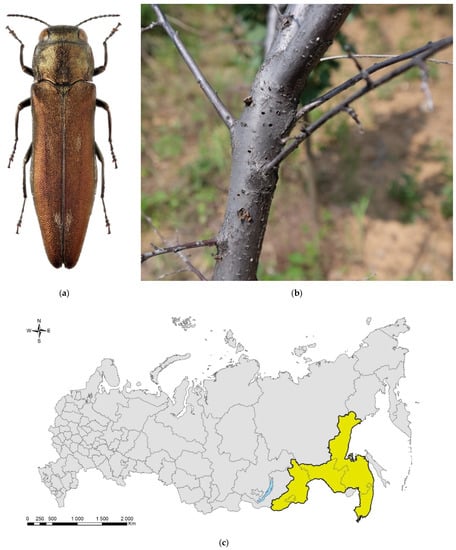
Figure 5.
The apple buprestid, Agrilus mali: (a) a male; (b) exit holes of A. mali on a dead Malus sp. tree, Beijing District, China; (c) the native range in Russia (colored in yellow). Photo (a) by A.V. Kovalev, after [119]; (b) by E. Jendek, both with permission.
Currently, A. mali is distributed in Asian Russia—East Siberia (Chita Oblast, Zabaikalskii Krai) and the Far East (Amur Oblast, Khabarovsk and Primorskii Krais) (Figure 5c)—Mongolia (Dornod and Tuv Aimags), China (Gansu, Guangxi, Hebei, Heilongjiang, Henan, Hubei, Nei Mongol, Qinghai, Sichuan, Shandong, Xinjiang, and Xizang Provinces), North Korea (Pyongyang), and South Korea (Gyeonggi-do, Inchon, Seoul, Taegu) [119,122,133], but some authors consider the Koreas and the Xinjiang Province of China as invasive range [126,127,128,129,130,134].
It is supposed that A. mali, was introduced to Korea from East China (Liaoning Province) [134], while the population in Xinjiang Province presumably originated from Shandong [127,128]. Imported apple tree seedlings are univocally indicated as a vector in both regions. It is believed that the potential invasive range of A. mali can cover the entire apple orchards’ cultivation area [135]. Based on the first findings in Korea in 1915 [134], the invasion of A. mali to that country could have happen in the early 1900s and the invasion to Xinjiang Province probably happened at the end of the 1980s to the early 1990s because the first outbreak took place there in 1993 [125,126,127,128,129,130]. Thus, the massive foci of A. mali on M. sieversii in Xinjiang Province may represent a newly forming invasive range. The proximity of these sites to the wild apple stands and cultivated apple plantations in the Kazakhstan’s part of Tien-Shan Mountains is a direct threat to Kazakhstan and other Central Asian countries [118,119].
Agrilus mali was reported to damage apple trees (i.e., Malus spp., M. asiatica var. rinki, M. baccata, M. domestica, M. prunifolia, M. pumila, M. sieversii, and M. spectabilis; Figure 5b), and it was also recorded to feed on other Rosaceae (i.e., Cydonia oblonga, Prunus spp., and Pyrus spp.) [127,128,133,136,137,138]. The record on Emmenopterys henryi (Rubiaceae) needs verification, and references to Juglans (Juglandaceae) and Salix (Salicaceae) are erroneous [133], while a report on Sorbus (Rosaceae) can refer to A. mendax [119].
An instance of large-scale damage by A. mali to cultivated apple trees was documented in Korea [134]. In Russia, it was also reported as a dangerous pest of apple trees in East Siberia and the Far East [135,139,140]. In the latter, the infested area covered about 320 ha [135]. Since the establishment in Xinjiang Province in 1993, A. mali has killed millions of wild apple trees and has infested about 95% of the total area of wild apple forests in the Ili valley [130].
Russia has a ban on import of apple seedlings from the areas infested by A. mali [135]. Agro-technical measures, mainly pruning and the destruction of infested branches and trees, as well as chemical treatments, are suggested as the control measures [139,140]. A parasitoid of emerald ash borer, Sclerodermus pupariae (Hymenoptera: Bethylidae), has been experimentally released in Xinjiang (China) and showed some positive results against A. mali [130]. This parasitoid, together with braconids (i.e., Atanycolus ivanowi, Doryctes undulatus, Pareucorystes varinervis, Polystenus rugosus, Spathius sinicus, and S. brevicaudis; Hymenoptera: Braconidae) parasitizing on A. mali were suggested as potential agents of biological control [141]. Aerial insecticide sprays during the beetle flight period can also be effective [128,130]. The combination of biocontrol and spraying/pruning turned out to be the most effective management approaches [130].
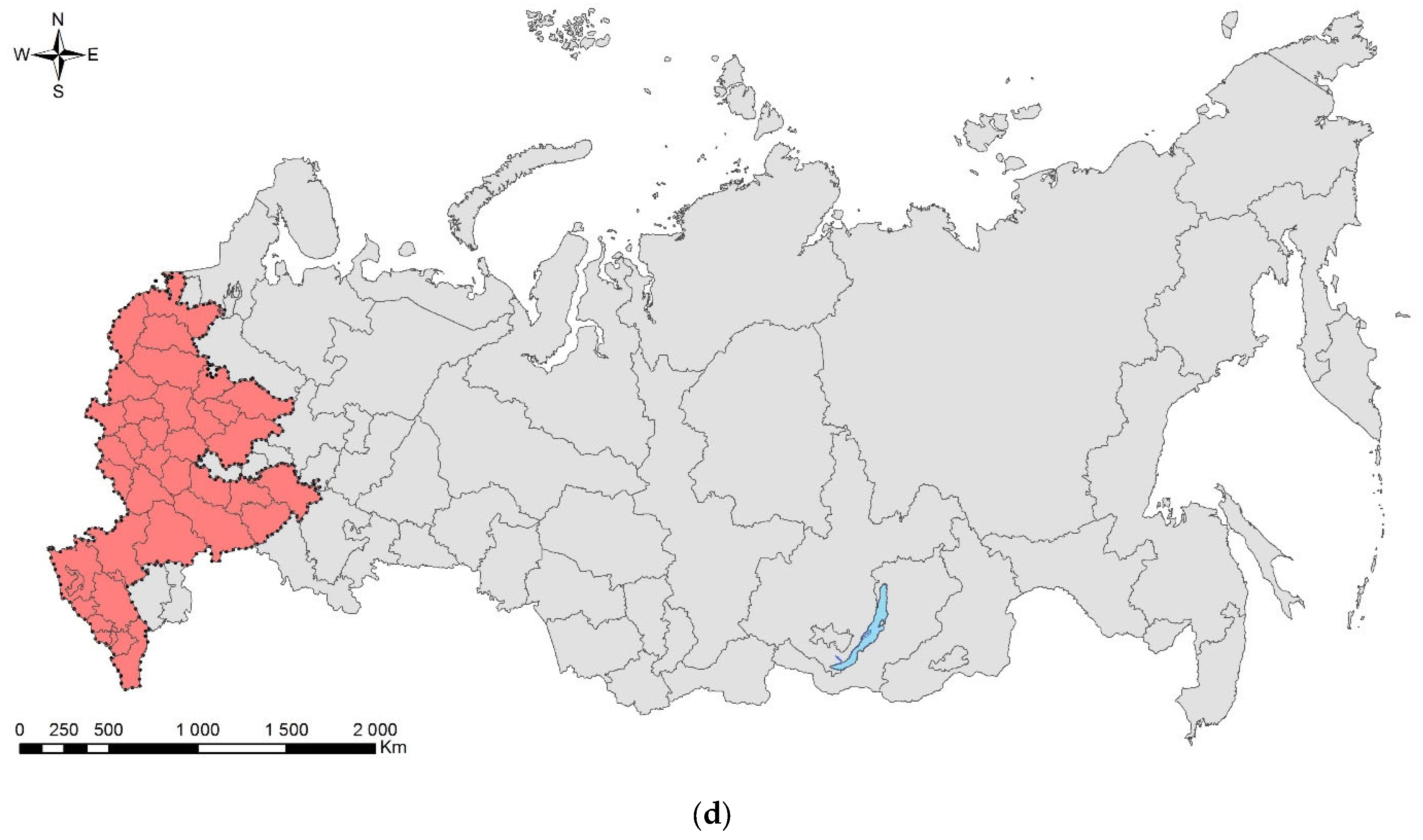
3.6. The Emerald Ash Borer, Agrilus planipennis Fairmaire, 1888 (Coleoptera: Buprestidae)
Agrilus planipennis (Figure 6) is described from China (Beijing) [142]. Since the beginning of the XXI century, the emerald ash borer has turned from a little-known East Asian species into one of the most devastating pests of ash trees in the world [143]. It has killed millions of trees in the forests and urban plantings in North America, European Russia, and East Ukraine [144,145,146,147,148].
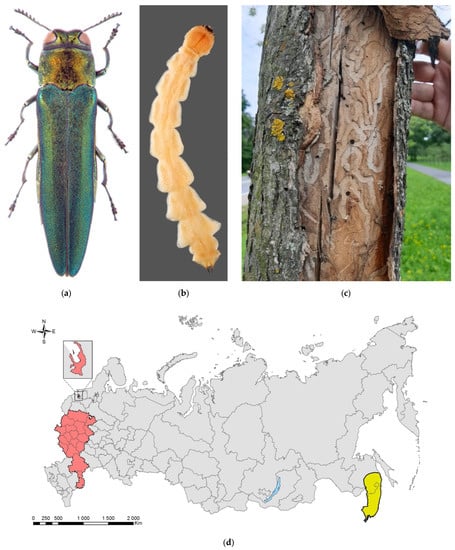
Figure 6.
The emerald ash borer, Agrilus planipennis: (a) an adult; (b) a mature larva; (c) larval galleries and exit holes in young tree, Saint Petersburg, September 2020; (d) the current distribution in Russia: the native range (colored in yellow) and invasive range (colored in red). Photo: (a) by K.V. Makarov, (b) by A.V. Kovalev, after [149], both with permission, (c) by M.G. Volkovitsh.
The species native range is confined to East Asia: Russian Far East (Khabarovsk and Primorskii Krais), China (Beijing, Hebei, Heilongjang, Jilin, Liaoning, Nei Mongol, Shandong, Sichuan, Taiwan (syn. A. feretrius), Tianjin, Xinjiang Provinces), Mongolia (syn. A. marcopoli), North Korea, South Korea, and Japan (Hokkaido, Honshu, Kyushu, Shikoku) (syn. A. marcopoli ulmi) [146]. There are some uncertainties regarding the native range and synonymy. As such, the records for Nei Mongol and Sichuan need confirmation, whereas the records for North Korea are still lacking, though A. planipennis occurs in all adjacent countries. In addition, the taxonomic state of A. feretrius and A. marcopoli ulmi needs verification, and the type locality of A. marcopoli (Mongolia or China) is still unclear.
Outside its native range, A. planipennis was first recorded in the USA (Michigan) in 2002 [150] and in the European part of Russia—in Moscow City in 2003 [144,145,151,152]. By early 2022, the emerald ash borer was recorded in 5 provinces of Canada and 35 states of the USA [153]; 18 provinces of European Russia, Moscow City, St. Petersburg City (Figure 6d); and two provinces of Ukraine (Luhansk and Kharkiv) [147,148,154,155,156,157].
The pest could be introduced to European Russia unintentionally in the late 1980s to early 1990s with wooden crafts, ash seedlings, or packaging material [158]. Taking into account that its outbreaks in North America and Moscow began almost simultaneously, an introduction from a common source (most probably from China) seems the most likely.
Under natural conditions, the emerald ash borer feeds on the East Asian ash tree species without causing significant damage to them due to the resistance of local ash species to the pest. However, in the areas of invasion, it completely destroys mature ash trees within a few years, depending largely on tree size and local A. planipennis population density [143]. Not all ash species are equally susceptible to A. planipennis. The examination of the ash collection in the N.V. Tsitsin Main Botanical Garden of the Russian Academy of Sciences (Moscow) revealed that only two Asian species (i.e., Chinese ash, F. chinensis, and Manchurian ash, F. mandshurica) were resistant to the emerald ash borer, whereas the pest killed the trees of both North American (i.e., F. pennsylvanica and F. americana) and European origins (i.e., F. excelsior, F. angustifolia, and F. ornus) [159].
The emerald ash borer can spread due to active flight, the human-mediated movement of infested wood (in North America, particularly, firewood), or the occasional transportation by vehicles and ships. Presumably, the beetles can also be transported over long distances by wind [143,158].
Since the first detection in Moscow in 2003, A. planipennis significantly expanded its invasive range in European Russia. In 2006, it was found in the Moscow Oblast, whereas by 2013, it was detected already in 11 oblasts of European Russia [160] and by early 2021, in 18 oblasts and in Saint Petersburg City [148,154,161]. The most recent detected localities are Saint Petersburg City and the Astrakhan Oblast [148,156,157].
The emerald ash borer has killed over tens of millions of healthy ash trees at a cost of billions of US dollars since its occurrence in North America [143]. In Europe, the potential losses can reach USD 1.81 billion. By this indicator, the species ranks fourth among the most “costly” invasive pests [162]. The actual losses (observed costs) from A. planipennis invasion in European Russia are estimated at as much as USD 258.9 million during 2011–2016 [163,164]. The impact might be even higher in the regions where the invasive range of A. planipennis overlaps with the invasive range of the devastating ash dieback fungus, Hymenoscyphus fraxineus (Ascomycota) [145].
Infestation usually begins in the canopy, with subsequent attacks lower along the trunk, while in small-diameter trees, infestation often starts along the main trunk. Canopy drying, epicormic branching along the lower trunk, bark crevices, and D-shaped exit holes on the bark are the most common symptoms of infestation [143,158,160].
Since its initial detections in Moscow City and the Moscow Oblast, monitoring in European Russia has been performed and, at the beginning, mostly by volunteers and enthusiasts. Initially, the monitoring was limited to the search for new foci and heavily infested ash trees in the urban environments. By now, seven quarantine phytosanitary zones in five oblasts of European Russia are declared by the National Plant Protection Organization (Rosselkhoznadzor) [148]. The pest is included in A Unified List of Quarantine Pests of the Eurasian Economic Union [131], and it is also included in the priority pest lists of the EPPO [120] and the European Food Safety Authority (EFSA) [165].
A recent analysis suggests that A. planipennis can potentially invade most European countries; however, it would probably not be able to establish in some regions of Norway, Sweden, Finland, Ireland, and Great Britain because of low annual heat accumulation in these regions [166].
Control measures include the cutting of infested trees (mostly detected by dry branches and typical D-shaped exit holes on the bark), with a clear-cut zone, replacement of North American and European ash trees with more resistant Asian ash species (first of all, F. chinensis and F. mandshurica) or possibly hybrids, and chemical and biological control [145,167]. Currently, the biological control agents are intensively looked for within the native and invasive ranges [145,168,169,170].
3.7. The Cypress Jewel Beetle, Lamprodila (Palmar) festiva (Linnaeus, 1767) (Coleoptera: Buprestidae)
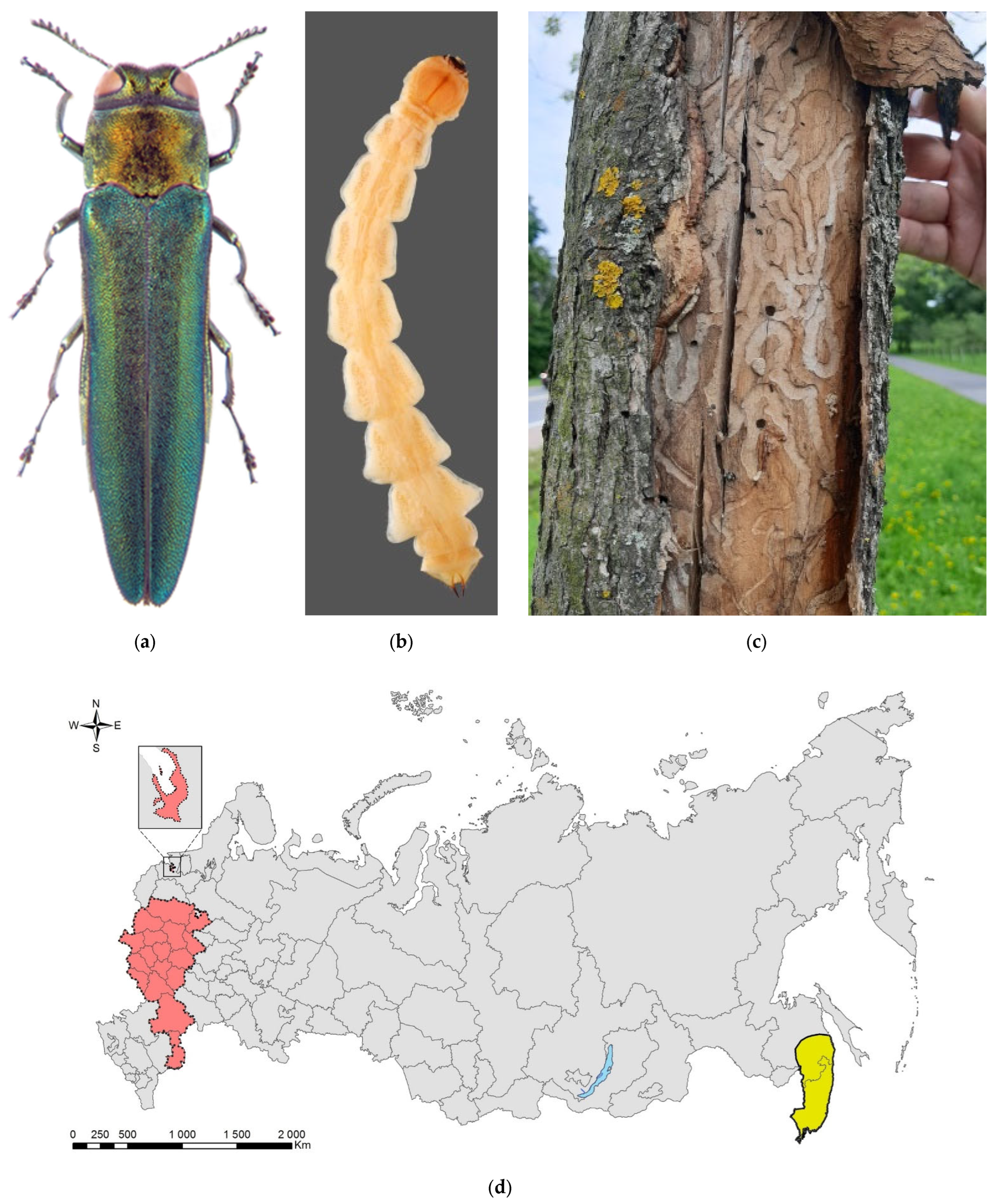
Lamprodila festiva (Figure 7) is originally a relatively rare Mediterranean species, which, about 20 years ago, suddenly became a devastating pest of ornamental cypress trees (Cupressaceae) in urban plantings and nurseries all over central and East Europe, including south of European Russia and Eastern Ukraine [160,171] (Supplimentary Table S4). In addition to the Mediterranean, the native range of L. festiva covers North Africa, South Europe, the southern part of central Europe (L. f. festiva, and L. f. cretica) and Southwest Asia (L. f. holzschuhi) [160,171,172].
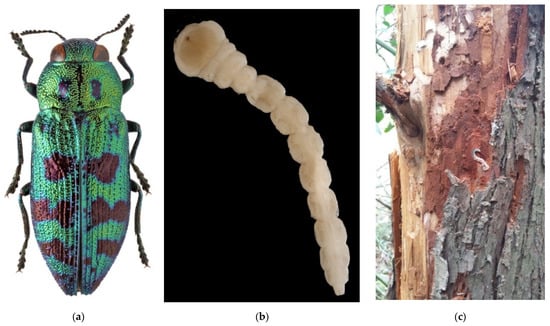
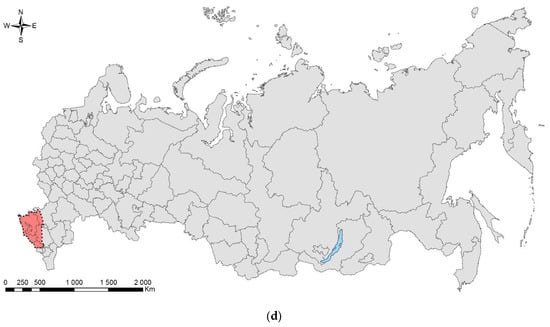
Figure 7.
The cypress jewel beetle, Lamprodila (Palmar) festiva: (a) an adult; (b) a larva; (c) larval tunnels with larvae on the trunk of Thuja plicata, Sochi City, 2016; (d) the invasive range in Russia (colored in red). Photos: (a) and (b) by A.V. Kovalev, after [171], with permission; (c) by N.N. Karpun, after [172].
Until recently, L. festiva has never been recorded in Russia or the former USSR. The first symptoms of infestation were detected in the Black Sea coast (Greater Sochi) in 2013, and the first larvae and adults were collected in 2016 [172,173]. Most probably, the pest was unintentionally introduced together with cypress planting stock from the European nurseries (likely from Italy, Spain, or Montenegro) in 2011 to 2013, during intense landscaping in Sochi before the XXII Winter Olympic Games in 2014 [172,174]. Presently in Russia, the cypress jewel beetle is distributed along the Black Sea coast from Sochi to Anapa, in North Caucasus (Dombai, Krasnodar, Nalchik, Rostov, Stavropol, Nevinnomyssk, and Cherkessk Cities), and the south coast of the neighbouring Crimean Peninsula, from Sevastopol to Gurzuf (Figure 7d) [107,160,171,172,175,176,177,178].
During 2013–2021, L. festiva expanded its invasive range as much as approx. 500 km north (Donetsk, Ukraine), about 240 km northeast (Stavropol), and 310 km east (Nalchik). It is distributed along the Black Sea coast for about 360 km from Vityazevo settlement at the north to Sukhum City in the south, with the maximal distance from the initial record locality (Sochi) of 250 km (Vityazevo). Actually, the first reported findings outside Sochi were from Gelendzhik (170 km northwest from Sochi) in 2017 [177]. These records indicated a high rate of distribution of the pest, most likely associated with the spread of infested planting stock.
Until recently, the cypress jewel beetle was regarded as a secondary pest of wild and some introduced Cupressaceae (Thuja spp.), without significant damage [172]. Currently, the beetle is recorded on a wide range of cultivated cypress species from Callitris, Chamaecyparis, Cupressus, Juniperus, Platycladus, Tetraclinis, and Thuja, including their hybrids and cultivars [171]. Recently, L. festiva was reported to attack Sequoia sempervirens, weakened by fungal decease in the Nikitsky Botanical Garden (Crimean Peninsula) [176]. Thus, now, L. festiva is considered a dangerous pest of ornamental cypress trees in many European countries [160,172,179,180,181,182,183,184]. There are still no quantitative data available on the economic losses caused by this pest to urban plantings; however, the estimated damage to ornamental plants is considered to be significant [160,174,181,183].
The early symptoms of tree infestation with Cypress jewel beetle are brown drying needles, the death of damaged branches, and, in case of heavy infestation, drying and death of a whole plant. The secondary symptoms are fissures, resin leakage, swellings, bark detachment, presence of frass on the trunks and on the soil under the plants, exit holes, and larval galleries under the bark (Figure 7c) [172].
Monitoring of L. festiva in Russia is performed locally since the first discovery of damaged cypress trees in the Black Sea coast [172,173,174,176,177,185,186]. The monitoring is mainly restricted to the search for new foci. The pest control has not been elaborated so far.
The parasitoids of the cypress jewel beetle, such as Pyemotes sp. (Acarina: Pyemotidae) and Metacolus unifasciatus (Hymenoptera: Pteromalidae), are reported from Bulgaria [184]. On the Black Sea coast, chemical control is carried out during the L. festiva adult flight period (preventive treatments of cypress trees with insecticides from the pyrethroid and organophosphorus compounds to prevent colonization of new plants) [107].
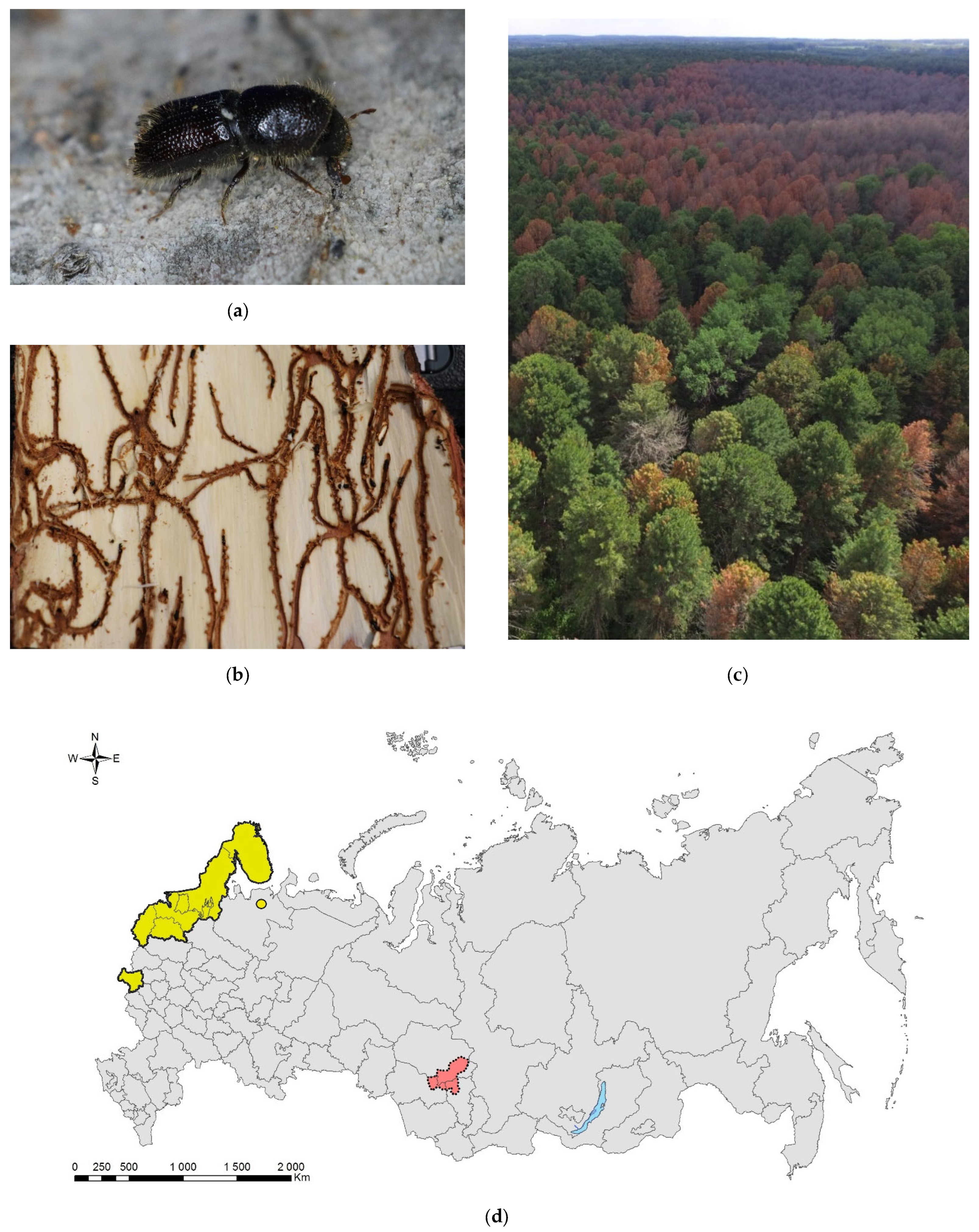
3.8. The Small Spruce Bark Beetle, Ips amitinus (Eichhoff, 1872) (Coleoptera: Curculionidae: Scolytinae)
Ips amitinus (Figure 8) is a European species which has been considered a secondary pest associated with dying conifers, including different spruce species (e.g., Picea abies and P. omorica) and several species of pines (e.g., Pinus cembra, P. mugo, P. peuce, and P. sylvestris) [187]. Its native range spreads from The Netherlands to Turkey [188]. In the XX and early XXI centuries, the range of I. amitinus rapidly expanded into Northern Europe [189,190,191,192]. The first recorded specimens of I. amitinus from Estonia are dated by 1900 [193,194,195]. In the following decades, the species spread northward, and it had occupied the whole of Estonia by the end of the 1930s [194,196,197] and was recorded from southern Finland (Tuusula Administrative Region) in 1951 [198,199]. The species spread rapidly northward in Finland [200], so that in 1968, it was already recorded in Northeast Finland at the boundary with the modern Paanajärvi National Park [201], where it was most probably absent in 1960 [202]. The small spruce bark beetle invaded northern Sweden, apparently from Finland, in 2012 and continues to move southward [203].
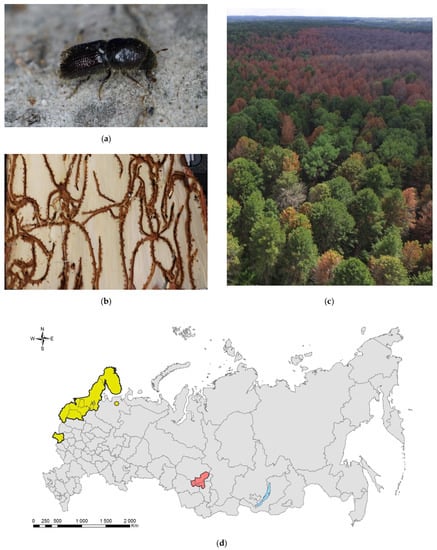
Figure 8.
The small spruce bark beetle, Ips amitinus: (a) an adult on a branch of Pinus sibirica, Tomsk Oblast, 2021; (b) galleries on P. sibirica, Tomsk Oblast, 2021; (c) I. amitinus foci in the Siberian pine forest near settlement Luchanovo, Tomsk Oblast, June 2020; (d) the current distribution in Russia: the native range (colored in yellow) and invasive range (colored in red). Photo by I.A. Kerchev.
The first record in Russia refers to the Briansk Oblast and is dated back to 1920 [204,205]. The “newly” inhabited territories of I. amitinus in Russia includes the Leningrad Oblast (Zelenogorsk in the Karelian Isthmus, 1976), though its appereance here was expected much earlier [205,206]. In the 1980s, the species was already widely distributed in Russian Karelia. Investigation of Titova [207] did not reveal I. amitinus in Karelia, but Yakovlev and coauthors [208] reported the species in southern Olonets and Loimola, as well as the central part of Karelia (Voloma). In 1989, it spread up to the Murmansk Oblast, Kandalaksha Nature Reserve [206,209]. In 2011, I. amitinus was recorded on pines nearby the northern range of a conifer forest in Pasvik Nature Reserve (Melkefoss; on the border between Russia and Norway) [210].
The easternmost record of I. amitinus in Europe known to date is the Pinega settlement in the Arkhangelsk Oblast, where the species was collected in 2013 on pine logs, but no infestations on spruces were recorded [189,211]. In 2000, the beetle was found in Pudozh, east to Onega Lake [206]. Thus, I. amitinus’ range spreading continues not only northward but also eastward. Natural barriers (such as Ladoga Lake) provide limitations to the species spreading and only in 2007 was I. amitinus trapped on Valamo Island and only in a wood harbor on the logs imported from the mainland [205,212].
The species invaded Western Siberia relatively recently (presumably, in 2005–2010), attacking a new host tree, the Siberian pine (Pinus sibirica), and, at least in the Tomsk Oblast, it was absent until 2012 [213]. The first symptoms of tree damage caused by the small spruce bark beetle were noted in 2014 in the Kemerovo Oblast on the borderline with the Tomsk Oblast [213,214,215]. A rapid growth of the I. amitinus population in 2016–2017 was provoked by the outbreak of the Siberian moth, Dendrolimus sibiricus, and favorable weather conditions, which altogether resulted in a considerable number of heavily defoliated and snow-broken trees [213,216]. By the time the invasion was registered in 2019, I. amitinus had already switched to healthy trees and started to breed on the tree stands nearby undamaged by the Siberian moth [217]. By 2019, the area of the outbreak foci had reached 237 ha in the Tomsk Oblast and 1033 ha in the Kemerovo Oblast. By the end of 2020, it was already 1207.5 and 1232.4 ha, respectively. The invasive range currently covers Tomsk, Kemerovo, and the Novosibirsk Oblasts, having a total area of 31.2 mln ha [218].
The most probable pathway of I. amitinus invasion is natural range expansion in the European part of Russia and Fennoscandia, and transportation with wood by railroads from European part of Russia to Western Siberia. This is confirmed by a number of proofs, such as a long distance between its native and invasive ranges, the proximity of the first recorded outbreak foci in Siberia to the Trans-Siberian Railway, and the rates of invasive range expansion observed at the moment [217]. In Western Siberia, the median density of I. amitinus’ nuptial chambers on P. sibirica was 2.7 (up to 20.0) per dm2 [216], i.e., 10 times higher than on trap spruce trees in the Czech Republic [219].
The strategy to control this new pest has not been developed in Russia yet. The application of a complex of classical forest protection measures are hampered by a ban on sanitary felling in specially protected natural areas. The first attempts to control I. amitinus carried out in the Tomsk and Kemerovo Oblasts were inherently flawed because a species-specific attractant of I. typographus was used for the mass pheromone trapping. Moreover, this approach had a negative effect, as it attracted to traps a natural enemy, a checkered beetle Thanasimus femoralis (Coleoptera: Cleridae) [215].
The outbreak of I. amitinus can be slowed down by sanitary cutting of infested trees and by using trap trees, as well as by timely removal of wind-broken and snow-fallen trees; however, these measures have not been undertaken so far [215]. In the Tomsk Oblast, the death of trees has reached a catastrophic rate [220]. As the attacks mostly begin on the tree tops, drones carrying digital cameras can help to accurately assess the damage [220].
In 2020, I. amitinus caused the death of introduced mountain pine (Pinus mugo), Korean pine (P. koraiensis), and some individuals of native conifers (e.g., P. sibirica, P. sylvestris, and Picea obovata) in the Arboretum “Kedr” of the Institute of Monitoring of Climatic and Ecological Systems, Siberian Branch of the Russian Academy of Sciences (Tomsk Oblast). The noted trophic preferences of the bark beetle indicate that it strongly prefers the five-needle pines, a trait that has likely developed in the course of coevolution with P. cembrae and P. peuce in its native range [217].
We believe that further expansion of I. amitinus range will continue eastward rather than northward, and the pest will be able to attack eastern pines, for instance, P. koraiensis in the Russian Far East [217]. In Western Russia, the species’ arrival is also expected up to the Ural Mountains.
Keeping in mind that the species is highly aggressive to Siberian pine and has a potential to outbreak in spruce stands in Europe, its populations should be monitored both in European and Asian parts of Russia. Dramatic rates of Siberian pine stands decline caused by the small spruce bark beetle in West Siberia has shown that the species invasion potential had been underestimated.
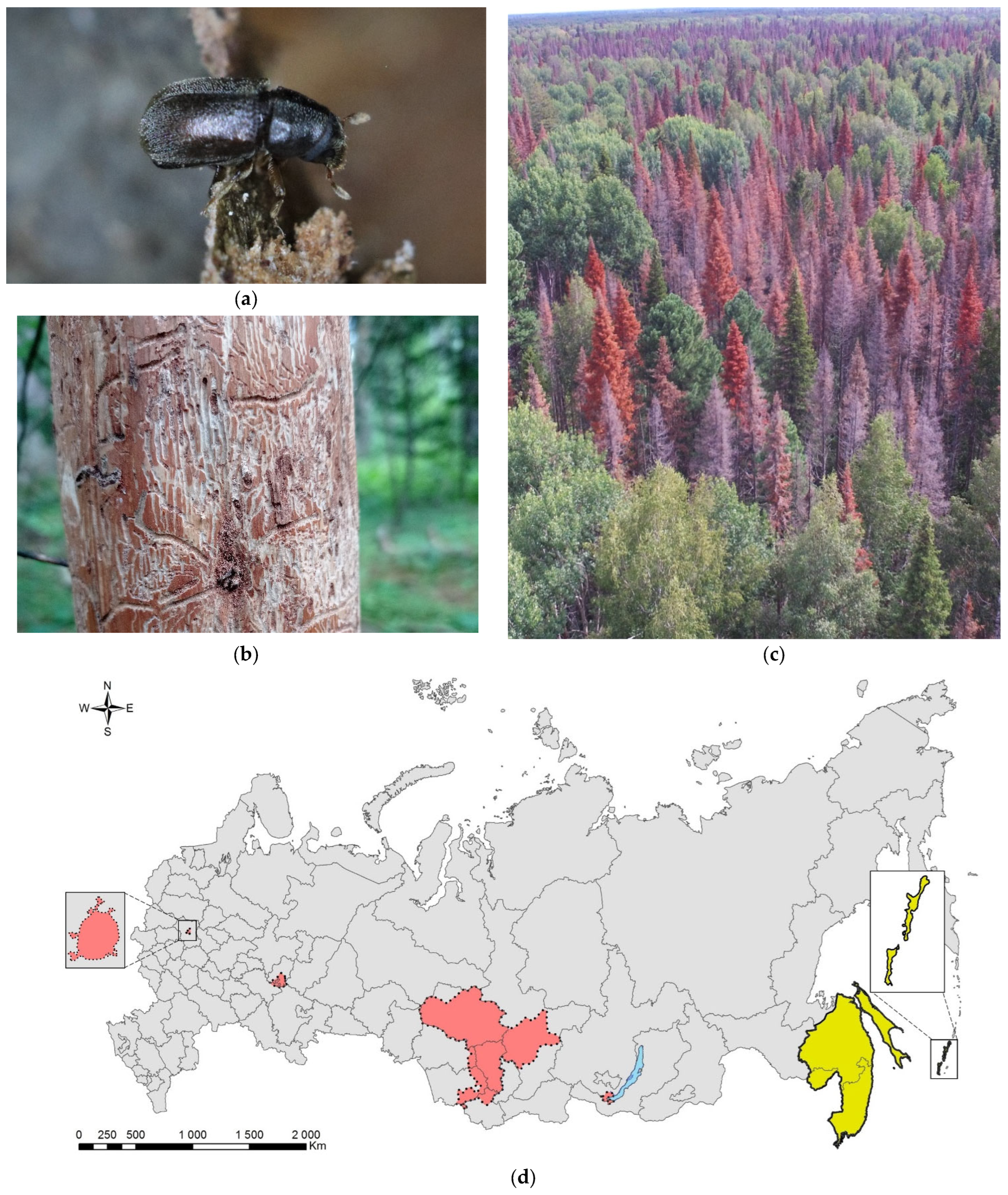
3.9. The Four-Eyed Fir Bark Beetle, Polygraphus proximus Blandford, 1894 (Coleoptera: Curculionidae: Scolytinae)
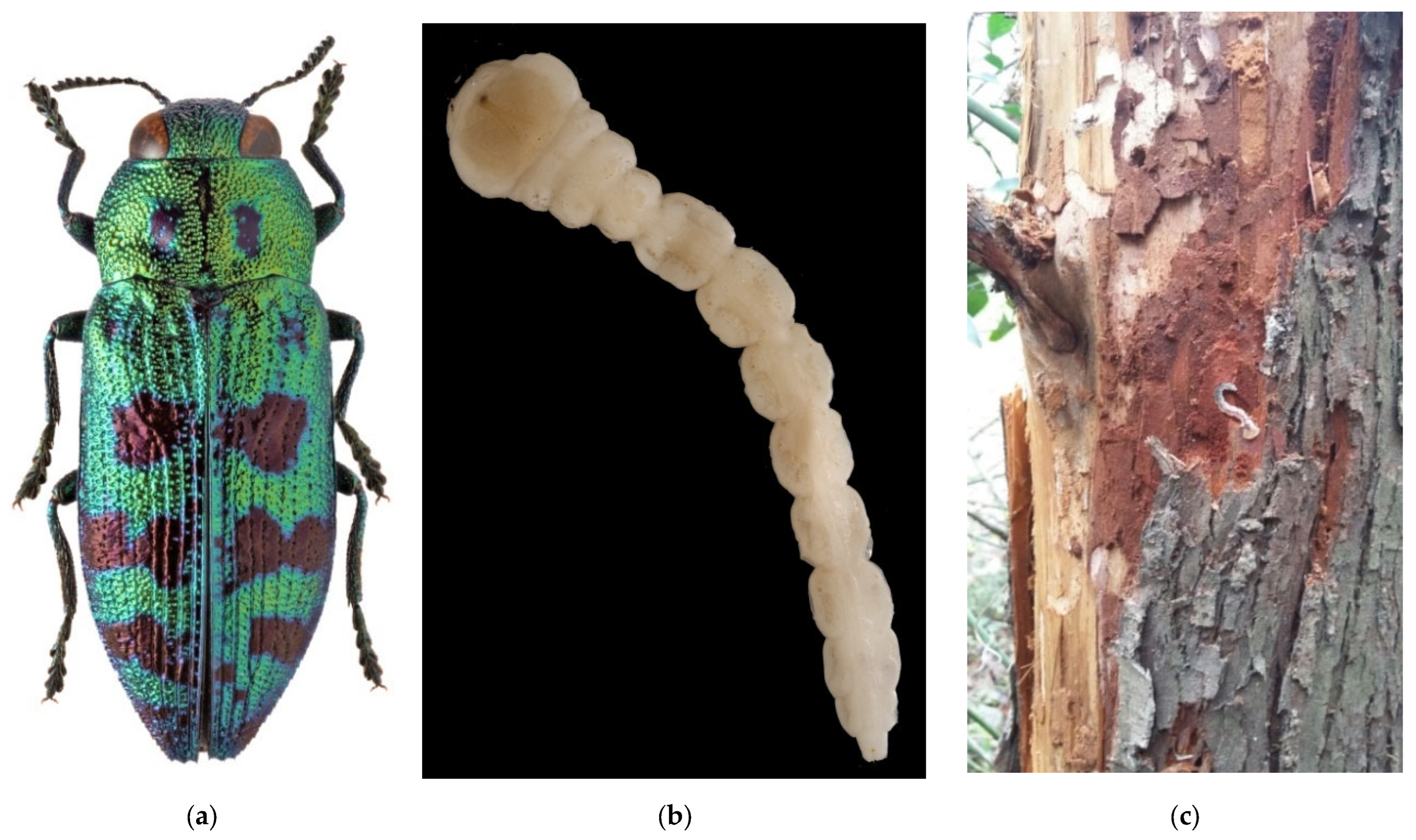
Polygraphus proximus is a conifer pest species of East Asian origin, which, in the last few decades spread in Russia westwards [221,222]. It naturally occurs in Japan, North and South Koreas, Northwest China (Heilongjiang and Jilin Provinces) [188], and in the south of the Russian Far East (Khabarovsk and Primorskii Krais, Sakhalin Island, and Southern Kurils) (Figure 9). In its native range, it develops on firs (i.e., Abies nephrolepis, A. sachalinensis, A. holophylla, and A. mayriana; Pinaceae) [223,224]. According to the list of the fir bark beetle’s host plants from the native range [225] and analysis of the trees colonized by the beetle in artificial plantations [226,227], it was concluded that firs from the sections Balsamea and Grandis are most preferred, while those from the section Abies are least preferred by this bark beetle [225]. Less common P. proximus can be found on other genera of Pinaceae: Picea (P. jezoensis), Pinus (including Korean pine, P. koraiensis), Larix, and Tsuga [224,225].
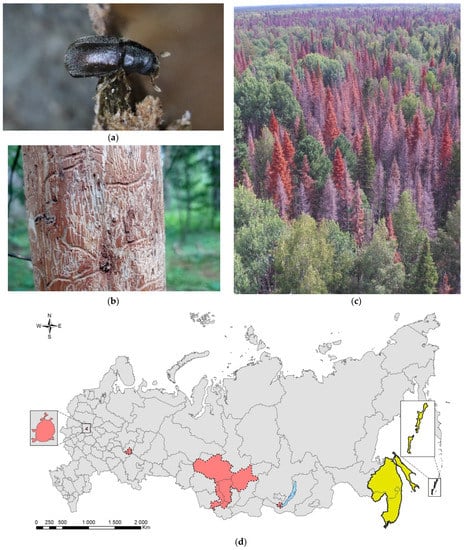
Figure 9.
The four-eyed fir bark beetle, Polygraphus proximus: (a) an adult on Abies sibirica, Tomsk Oblast, 2021; (b) maternal and larval galleries with pupal chambers on a stem of A. sibirica, Tomsk Oblast, 2021; (c) P. proximus foci in the dark coniferous forest near settlement Barbig, Tomsk Oblast, July 2017; (d) the current distribution in Russia: the native range (colored in yellow) and invasive range (colored in red). Photo by I.A. Kerchev.
The first record outside of the species’ native range is dated back to 1999, to the Leningrad Oblast [228]. As the species was never documented in this region again, we consider this record to be doubtful [190]. The species was unintentionally introduced to Moscow City [226], where it was recorded in 2006 on artificial stands of Abies sibirica and A. balsamea along Kurkino highway in the Khimki District. It was also discovered in 2006–2007 on Norway spruce (P. abies) and A. sibirica in Pushkin, Odintsovo, and Podolsk Districts of the Moscow Oblast [226]. In the East European Plain, fir bark beetle’s outbreaks have been registered in the Agryz region of Tatarstan, in the Sarapulsky, Kiyasovsky, Zavyalovsky, and Malopurginsky regions of the Udmurt Republic [229,230].
The species invaded Siberia, from where it was recorded for the first time in 2008, and the beetles were found in pheromone traps for Ips sexdentatus in P. sibrica stands nearby Tomsk City [231]. In 2009, the presence of P. proximus was confirmed in Krasnoyarsk Krai, and it then became clear that this species (and not Xylechinus pilosus as it had been though before) was the real cause of Siberian fir stands decline [221,222,223,224,225,226,227,228,229,230,231,232,233,234]. The most probable pathway of P. proximus invasion is the transportation of unbarked timber or packaging materials from the Far East. Presently in Siberia, the four-eyed fir bark beetle is found in Tomsk (except the north part), the Kemerovo, Novosibirsk, and Irkutsk Oblasts, Altai, and Khakassia (Figure 9d) [221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236]. It was recorded at an altitude of 100–200 m a.s.l., and in mountainous areas, it inhabits the belt of 300 to 1490 m a.s.l. [225].
The dendrochronological analysis revealed that the beetle killed the first trees in Krasnoyarsk Krai in about 1976 [237] and in the Tomsk Oblast before 2020 [238]. It was suggested that the pest could have been introduced to the Siberian fir range much earlier, e.g., in the 1960s when fir lumber from the Russian Far East was brought to the Kemerovo Oblast for construction of mines [229,237].
The four-eyed fir bark beetle was introduced into the Tomsk Oblast and the Udmurtia at approximately the same time, in the mid-1990s, since the beetles taken from both localities belong to the same haplogroup, as suggested by the cytochrome oxidase subunit 1 (COI) mitDNA analysis [239], whereas the specimens from the Krasnoyarsk Krai and Khakassia belong to a divergent group [239,240].
Since the first record in Siberia, mass infestations of P. proximus were recorded only on the Siberian fir, but not on other tree species, whereas laboratory tests demonstrated the pest’s ability to develop on other Pinaceae species (e.g., Pinus sibirica, Picea obovata, and Larix sibirica) [241]. Later, in the outbreak foci, infestation of wind-broken spruces, Scots pine (Pinus sylvestris) and Siberian pine (P. sibirica), by P. proximus was documented [225].
The beetle can vector an obligate symbitic fungus Grosmannia aoshimae (Ascomycota: Ophiostomataceae), and development of the fungus leads to rapid weakening of the host trees [242]. This fungus arrived at Siberia together with P. proximus [242,243]. Laboratory tests demonstrated that the fungus could colonize and develop on the same unusual host tree species as P. proximus [244]. This work revealed the possibility of a hidden existence of invasive complexes even on the hosts that are not favorable for reproduction of the pest.
Polygraphus proximus kills fir stands in Siberia causing significant changes in the structure of the ecosystem [245]. In declining forests, the microclimate changes, creating preconditions for future replacement of taiga with deciduous trees. Falling down of dead trees leads to a significant imbalance between the intensity of production and destruction processes in the ecosystems [246]. Significant changes are also observed in the vegetation cover and structure of the local insect fauna [233,247,248]. Natural enemies of P. proximus [225,249,250] and entomopathogens [251] are not able to control the pest in its invasive range [225]. One of the control methods that has shown high efficiency against the fir bark beetle is the traditional practice of keeping cut trees submerged in water [252]. An integrated approach including treatment with systemic insecticides, timely sanitary cutting and removal of colonized trees used in the N.V. Tsitsin Main Botanical Garden of the Russian Academy of Sciences (Moscow), showed high efficiency in suppression of an outbreak on a relatively small territory [253].
As mentioned above, firs from the sections Balsamea and Grandis are most preferred by the beetle [225]. Thus, possible accidental introduction of P. proximus to North America, where firs from these sections are widely distributed, would result in enormous damage. In Europe, firs are represented by Abies alba and A. nordmanniana from the section Abies [254], which is less preferred as a host by the beetle than representatives of sections Balsamea and Grandis [225]. Nevertheless, phytosanitary and monitoring measures aimed at preventing the invasion of this species and early detection should be prioritized. In the regions where P. proximus spreads widely, efforts should be put to minimize damage by conducting timely forest sanitary measures.
3.10. The Box Tree Moth, Cydalima perspectalis (Walker, 1859) (Lepidoptera: Crambidae)
Cydalima perspectalis (Figure 10) is an East Asian species naturally distributed in China, Japan, the Korean Peninsula, the Russian Far East, and India [255,256]. Outside of its native range, the pest was detected in Germany in 2006, where it was presumably imported with boxwood planting material from China [257]. Further invasion of the species in Europe proceeded rapidly (Supplimentary Table S5). In 2007, it was included in the EPPO Alert List, but already in 2011, it was excluded as a species widespread in the region [120]. In 2021, the boxwood moth was found in North America (USA: Connecticut, Michigan, and South Carolina) [258].

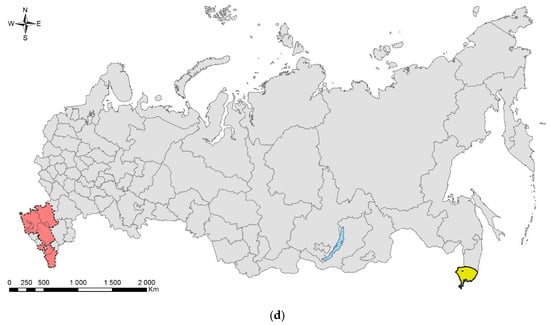
Figure 10.
Box tree moth, Cydalima perspectalis: (a) a larva of VI instar, Sochi, August 2013; (b) an adult moth, Sochi, September 2014; (c) severely damaged box tree, Buxus sempervirens, Sukhum District, Abkhazia, July 2015; (d) the current distribution in Russia: the native range (colored in yellow) and invasive range (colored in red). Photo: (a) and (c) by N.N. Karpun, (b) by E.N. Zhuravleva.
In Russia (Figure 10d), the larvae of C. perspectalis were first recorded on the planting stock of Buxus sempervirens in 2012, in the nursery with planting material imported for landscaping the territory of the Main Olympic Village in Sochi [259]. In 2013, the species gave an outbreak in the ornamental plantations in the city of Sochi [260] and was recorded in the plantations in the city of Grozny (Chechnya) [261], and by 2014, it had spread across the Krasnodar Krai and the neighboring region of Abkhazia [262]. In 2015, the species was recorded in the Crimean Peninsula, in Adygea (West Caucasus), in the Mineralnye Vody region (Stavropol Krai), and North Ossetia (North Caucasus) [97,263,264,265], while in 2016 and in 2017, it was recorded in Rostov-on-Don [266] and in the neighboring region of South Ossetia [267], respectively. In 2019, the boxwood moth was found in Dagestan (North Caucasus) [268] and in 2020 in the Kaliningrad Oblast (southeast of the Baltic Sea) [269]. The main pathway of C. perspectalis invasion was the introduction of infested boxwood planting material [63,257,259,270].
The pest causes dramatic defoliation of boxwood in both natural and ornamental plantations (Figure 10c). In 2015–2017, it had almost completely destroyed the natural plantations of boxwood in the North Caucasus and the Black Sea coast of Russia and further eastwards situated region of Abkhazia [63]. Caterpillars of the early (I–III) instars gnaw the lower part of the leaf without touching the upper epidermis, and matured caterpillars consume the whole leaf. At high population densities, they also eat the bark of branches and trunks.
Within the native range, C. perspectalis damages Buxus spp. (Buxaceae), Euonymus japonicus (Celastraceae), and Ilex purpurea (Aquifoliaceae). In Europe, it feeds on all native and cultivated species of boxwood, while in Russia, it feeds on 9 boxwood species and 13 cultivars [271].
In the humid subtropics of the Black Sea coast of Russia, the pest produces three generations per year and, under favourable conditions, four generations per year can develop. The species overwinter at different stages: egg, caterpillar, and pupa. As a result, different generations overlap [63].
The boxwood moth is monitored by regular visual inspections of plantations and by pheromone trapping. Standard glue deltoid traps are used and they are placed on the branches of boxwood or nearby. The pheromone combines unsaturated aldehydes: (Z)-11-hexadecenal (Z11-16:Ald), (E)-11-hexadecenal (E11-16:Ald), and (Z)-11-hexadecenol (Z11-16:OH) [272,273,274].
Within the invasive range in Southern Russia, only a few birds and parasitoid wasps (Hymenoptera) attack the alien pest. Despite the fact that in Europe, parasitoids, entomopathogenic fungi, and nematodes are considered ineffective in the control of this pest under natural conditions [275,276], in the humid subtropics of Russia, the early results of the application of entomopathogenic fungi are encouraging [277]. Bacillus thuringiensis-based preparations are effective against early-instar caterpillars [276,278,279], whereas avermectins, organophosphorus compounds, pyrethroids, and neonicotinoids are considered effective against older-instar caterpillars [279,280,281,282]. Further range expansion of C. perspectalis from Russia is likely into natural forests across the Caucasus (Transcaucasia) and to countries located further south.
3.11. The Leaf Blotch Miner Moth, Acrocercops brongniardella (Fabricius, 1798) (Lepidoptera: Gracillariidae)
Acrocercops brongniardella (Figure 11) is a micromoth species widely distributed in Europe and European Russia (except northern and southern regions) [283,284]. The presence of A. brongniardella in the Russian Far East is questionable. A single record of the species in Khabarovsk Krai, in fact, refers to Caloptilia infuscatus [285], a taxon which might represent a senior synonymy of the East Asian sister species, Acrocercops amurensis [286].
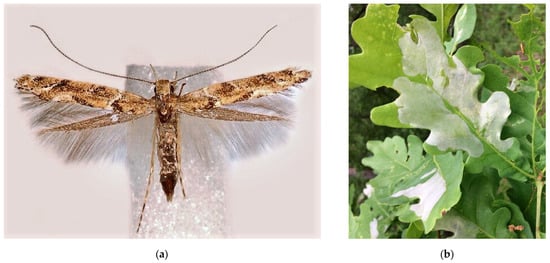
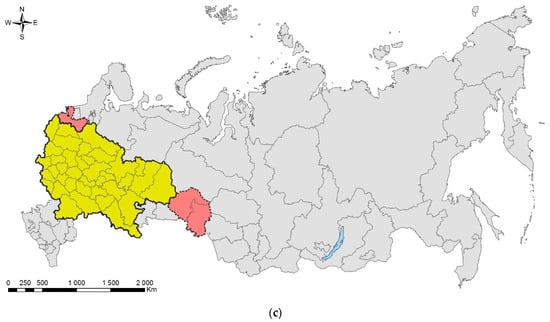
Figure 11.
The leaf blotch miner moth, Acrocercops brongniardella: (a) an adult moth; (b) leaves of Quercus robur with mines; (c) the current distribution in Russia: the native range (colored in yellow) and invasive range (colored in red). Photo: (a) by D. Lees; (b) by I.A. Utkina, both with permission.
In European Russia, A. brongniardella has been found to expand its native range to northern territories, in particular to the Leningrad Oblast and Saint Petersburg City, where it was detected for the first time in 2018 [287,288]. In the following years, the species was recorded south and north of Saint Petersburg, in Gatchina and Sestroretsk, respectively [289,290]. In addition, A. brongniardella was also documented in a few localities in Western Siberia, Omsk, and Tyumen Oblasts (Figure 11c) [291,292,293], where it is considered an alien species. In the city plantations of Omsk, it produces noticeable outbreaks [291].
The pest mines the leaves of oaks (Quercus spp.), including evergreen species (e.g., Q. ilex), whereas the most common host is the deciduous oak, Q. robur [284]; occasionally, the mines can be found on leaves of Castanea sativa (Fagaceae) [294]. The mines are big blotches on the upper sides of leaves, often covering a significant area of the leaf lamina (Figure 11b). Fully developed mines are easy to spot by the whitish color. Occasionally, up to 16 young mines can be found on a leaf, later on merging into a big blotch [291,295]. Leaf mining starts in May, then the larvae vacate the mines in July and pupate externally. Adult emergence is prolonged. Moths overwinter in bark crevices and dwellings [291,295]. In Ukraine, the species produces two generations per year [295,296], whereas in Russia, one generation per year was documented [291,297,298,299].
It is one of the most important folivore insect pests of oaks in Russia. The intense and prolonged outbreaks have been recorded in central parts of European Russia [299,300]. In a foci, often more than 75% of leaves carry mines and increased population density can be observed for several successive years [297,298]. Long-lasting outbreaks can lead to decrease of radial growth, drying of crowns, and death of oaks [301]. However, the impact of this species remains insufficiently well studied [297,298,299].
In the Leningrad Oblast, where Q. robur is a native tree and where the northern limit of its range in Russia is known [302], so far, the moth has been found in low density [287,289,290]. In contrast, in Western Siberia (in particular the Omsk Oblast), the moth shows a tendency to outbreak and harm Q. robur [291], an introduced tree in Western Siberia. The first planting of Q. robur in the Omsk Oblast is dated to 1898, whereas, in the city of Omsk, it has been planted since 1948. The moth could have arrived to the region with plants for planting, with vehicles or by itself, naturally expanding its range.
In the northeast of European Russia (Udmurtia), no outbreaks have been recorded in the last 35 years [303,304]. We hypothesize that insufficiently warm summers in the north of Russia could limit the moth population density in Saint Petersburg area. The observed climate warming in Saint Petersburg [288] and availability of the main host plant, Q. robur, in the city plantations may favor the pest outbreaks in the following years.
In cities, the control of A. brongniardella populations is complicated. Removal of leaf litter in order to reduce the population density will unlikely lead to desirable results as with the pest, its native enemies (in particular parasitoids) are killed. The use of insecticides is often impossible in urban areas. Injections of systemic insecticides into trees in high-value plantings and protected areas might be a perspective to keep in mind, given their effectiveness against other pestiferous invasive gracillariids [305,306,307]. However, so far, this approach has not been tested against this pest in Russia.
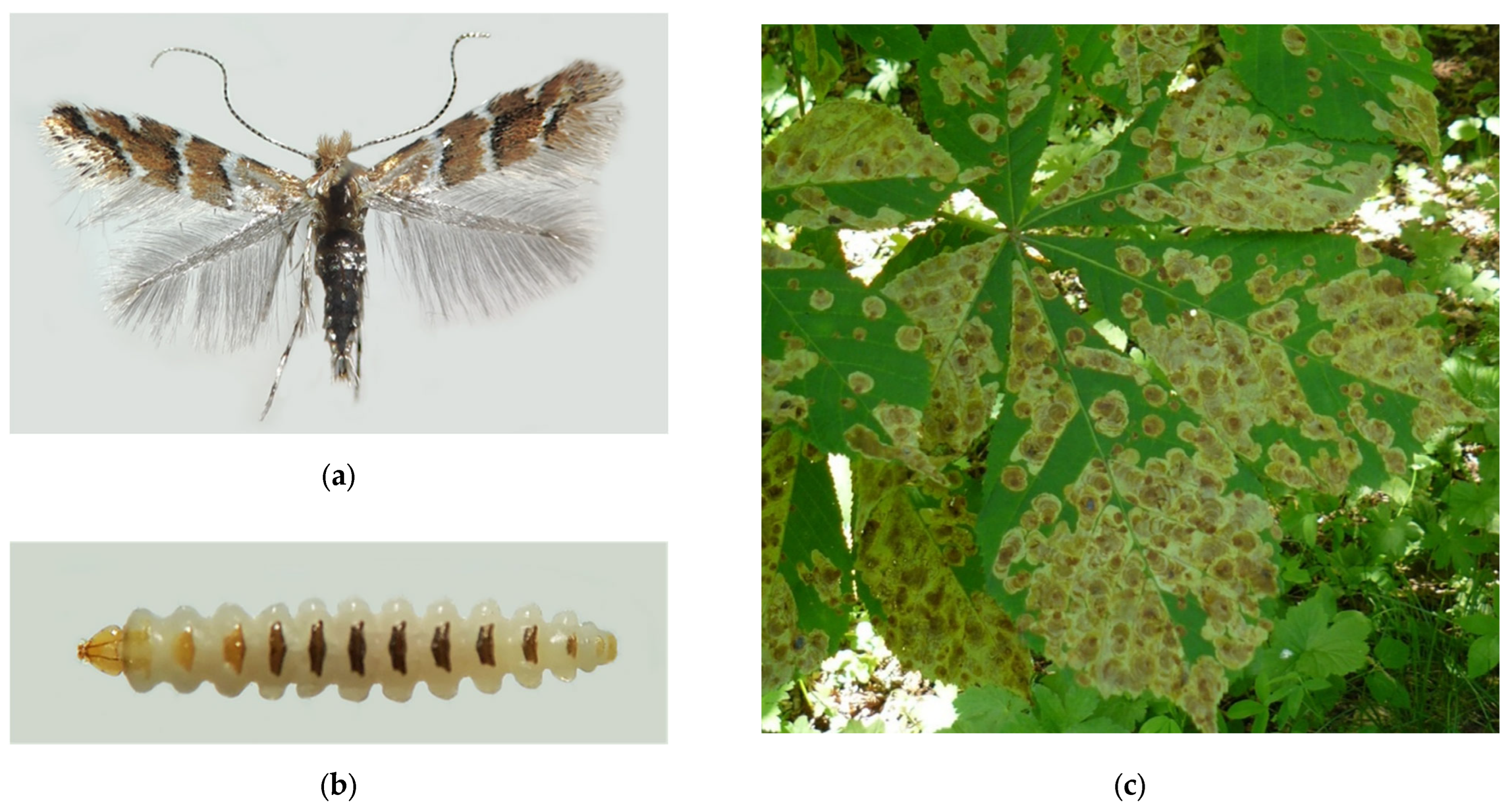
3.12. The Horse-Chestnut Leaf Miner, Cameraria ohridella Deschka et Dimić, 1986 (Lepidoptera: Gracillariidae)
Cameraria ohridella (Figure 12) is a tiny moth that became widely known due to its fast distribution across Europe and scenic damage to the horse chestnut, Aesculus hippocastanum (Sapindaceae) [308]. It is the only species of the genus Cameraria occurring in Europe, while the majority of the genus representatives (83 species) are distributed in North America, with a few species found in Asia and Africa [284]. Curiously, C. ohridella was not even known to science before it started invading Europe (Supplimentary Table S6). In the 1980s, it was discovered in high densities in North Macedonia near the Ohrid Lake, from where it was formally described [9]. In the following years, the moth was recorded in many European countries and, in approx. three decades, it occupied most of Europe [308,309].
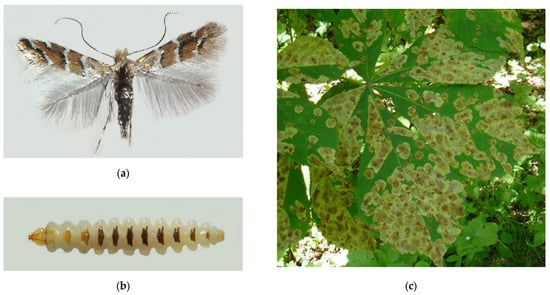
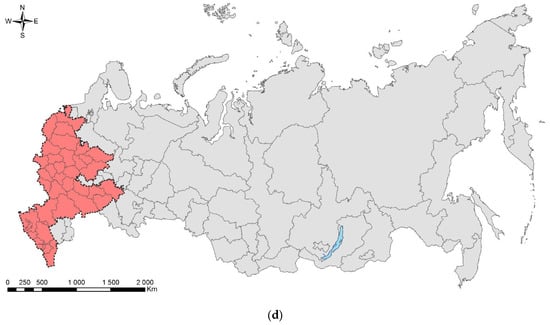
Figure 12.
The horse-chestnut leaf miner, Cameraria ohridella: (a) an adult moth; (b) a mature larva; (c) damaged leaves of Aesculus hippocastanum, Anapa City, 2021; (d) the invasive range in Russia (colored in red). Photo by N.I. Kirichenko.
For more than 20 years, the origin of the species was unclear. Some authors hypothesized it to be a relict species that, together with its host, survived the glaciation of the Tertiary Period [9]. Hellrigl [310] suggested that the species originated from southeastern Europe, where it could have shifted from maple (Acer spp.) to Ae. hippocastanum. Finally, others suggested the species to be alien for Europe and probably originating from North America or Asia [311]. A phylogeographic study showed that the most probable motherland of the species is the Balkans [312,313]. Furthermore, a survey of historical herbaria confirmed the long-term presence of the species in the Balkans, since the mines were found in pressed leaves sampled more than a century ago, that is, long before the moth was recorded in other parts of Europe [313].
Progressively spreading on the European continent, the moth arrived in European Russia [314,315]. In Russia, its first record is dated to 2003, and refers to Kaliningrad City (southeastern coast of the Baltic Sea) [315]. In 2005, the moth was documented in Moscow [316], about 1000 km east from Kaliningrad and about 450 km from the border with Ukraine and Belarus, where the pest was already present by that time [315,317]. In the following years, the species was found in several regions of European Russia, where the horse chestnut, an exotic plant for the country, is grown in ornamental plantings. By 2015, C. ohridella was recorded in 13 central regions of European Russia [318]. Shortly after that, the species occupied the south of European Russia, in particular the Black Sea coast, where it gives spectacular outbreaks in resort areas [319,320]. It also invaded the neighboring Crimean Peninsula (including Sevastopol City), Ciscaucasia [321,322], including North Caucasus [323]. On the north, C. ohridella was documented in Saint Petersburg in 2013 [287,324]. The easternmost region where the pest has been found in European Russia is the Volga region [325,326].
In European Russia, C. ohridella develops from early May to early October and produces two or three generations per year [306,318,321]. In Moscow and Pskov, it develops in two generations and the third generation is usually incomplete [306,327]. In the south, in particular in Stavropol City, it is able to complete four generations per year [321].
The up-to-date distribution map, compiled based on the literature and modern records, indicates that the species has already occupied a significant part of European Russia (Figure 12d). By now, C. ohridella was confirmed in 40 out of 58 (i.e., 69%) administrative units of European Russia, mostly in its central and southern parts. Since the horse chestnut is used ornamentally in nearly all regions of European Russia, further distribution of C. ohridella eastward up to the Urals, in our opinion, can be expected. Further expansion east of the Urals will not be possible, as Ae. hippocastanum is hardly used in plantings in Western Siberia due to the harsh climate [328].
The female lays eggs individually on the upper sides of leaves [306], and the average fecundity is about 40 eggs per female [329]. Larvae develop in blotches of reddish-brown mines between secondary veins (Figure 12c). Up to 250 mines per leaf have been reported from outbreaking populations [329]. Larvae live solitary in mines, but in heavily infested trees, individual mines merge into big ones [321]. Pupation occurs in the mine. The species hibernates as pupae in the mines of the fallen leaves [321], whereas, in Moscow, overwintering at the adult stage was also documented [306]. Multivoltinism, high fecundity, and easy distribution (that is largely associated with human activities) in short-to-long distances can explain the successful invasion and high population densities of C. ohridella in European Russia [306,329].
The main host plant is the horse chestnut, Ae. hippocastanum, a native species to the Balkans that started being widely planted in Europe in the early XVII century [330]. In European Russia, this tree has been actively used as an ornamental since the XIX century [328]. Other horse chestnut species, such as Japanese Ae. turbinate, American Ae. octandra (=flava), and Ae. Glabra, are also suitable hosts for the moth [331,332]. According to the recent observations in Kaliningrad City, Ae. carnea, Ae. glabra, and Ae. pavia are resistant to the pest [318], while in Moscow, the two latter species are the least damaged [333]. In Europe, the moth can also occasionally attack maples, Acer pseudoplatanus and A. platanoides (Sapindaceae, the same family as of the horse chestnut), when neighboring horse chestnut trees are infested [310,334]. Mines of C. ohridella were also found on A. platanoides in the N.V. Tsitsin Main Botanical Garden of the Russian Academy of Sciences (Moscow) in 2006–2009; however, the larvae did not succeed to pupate [306]. In Moscow, it can also successfully develop on Ae. glabra [306].
The moth spreads fast through natural dispersion and anthropogenic transportation [306]. In European countries, local dispersal involves both adult flight and the dissemination of infested leaves by the wind [335]. Over long distance, the dispersal of C. ohridella is possible by wind (as a part of the aerial plankton), but mostly it happens via passive transportation of infested leaves and adult moths as stowaway in/on cars, trucks, and other vehicles, as well as via the movement of infested seedlings [335,336]. Within Russia, the transportation of live plants of Ae. hippocastanum (with leaves that may already contain mines of the pest) undoubtedly facilitates the insect distribution. Furthermore, adults from outbreaking populations can be easily moved over significant distances with any plants and vehicles [306].
Similar to the European countries where C. ohridella causes severe aesthetic damage to horse chestnut trees [337], it is a highly important pest of Ae. hippocastanum in European Russia [306,329]. This species is listed among the Top 100 most dangerous invasive species in Russia [338]. Heavily infested trees start losing foliage already in July, resulting in pronounced aesthetic (i.e., social) impact in urban areas [318,327]. In addition to C. ohridella, since 2012–2013, in the Krasnodar Krai and Adygea (West Caucasus), the horse chestnut trees were affected by an invasive bacterium, Pseudomonas syringae pv. aesculi, which causes bleeding canker disease [339]. No link between the damage caused by the moth and the infestation by the bacteria was found, rejecting the hypothesis that C. ohridella can facilitate the distribution of the disease or increase its impact [340,341].
In European countries, at least 60 parasitoid species are known to attack C. ohridella [284]. However, parasitism rates remain generally low, usually within 10–25% in southeast Europe and generally lower than 10% in recently invaded countries of Europe [342,343,344,345]. The parasitoid Pediobius saulius (Walker) (Hymenoptera: Eulophidae), the most abundant species in the Balkans, gradually followed the invasion of its host, C. ohridella [346,347]. Exceptionally, on the south of European Russia, in particular in Krasnodar City, where C. ohridella was for the first time recorded in 2010, the parasitism level reached 33.6% already by 2013 [348]. The parasitoid complex in Krasnodar includes 44 parasitoid wasps from Braconidae, Eupelmidae, Ichneumonidae, Pteromalidae, and Eulophidae (Hymenoptera), with most of them having diverse trophic relations with local insects [348]. This parasitoid complex seemed to be effective in controlling C. ohridella in Krasnodar, dropping the population density significantly by 2014 [348]. In contrast, in another southern region (Stavropol Krai), no entomophages were recorded in a rather dense pest population, despite the following predators being found: three ladybirds (Adalia bipunctata, Adonia variegata, Coccinella septempunctata), the common earwig (Forficula auricularia), common orb-weaving spider (Araneus sp.), and tit (Parus sp.) [321]. However, this predator complex did not appear to be efficient in controlling the pest and stopping its outbreak [321].
Bearing in mind that the aesthetic damage caused to Ae. hippocastanum by the invasive moth can be severe, the strategies to control C. ohridella have been extensively explored in European Russia. Since the larvae of C. ohridella are endophagous, the use of chemicals with contact-intestinal action cannot provide reliable protection against the pest [306]. A high mortality rate of larvae can be reached by the application of systemic insecticides (e.g., new BI-58, Danadim, etc.); however, their use in settlements is impossible because of their toxicity [306]. Chemical control by aerial spraying is efficient but expensive and not adapted to the urban area [349], while in some European countries, such a measure has raised public concern [350]. Other pesticides with fewer non-target effects can be feasible [351], and in particular, stem injections can provide satisfactory results [352]. The 2-year experiment carried on the horse chestnut in Moscow, demonstrated the high efficiency of the stem injection with Abasol, already the next year after application [352].
In European Russia, the use of pheromone trapping utilizing the synthetic pheromone of C. ohridella developed in the Czech Republic [353] was intensively explored. Unitrap pheromone traps and Delta adhesive traps showed high efficiency in C. ohridella monitoring and could be also effective in its control [329,354]. Biocontrol approaches, such as the release of specialist parasitoids attacking the leafminer in its native range, have been studied but are still far from practical applications in European Russia [306].
The complete removal of leaf litter in which pupae hibernate can be an effective control measure [321]. However, bearing in mind that damaged leaves start shading early, foliage should be removed repeatedly starting already in mid-summer onward [306]. The majority of adults can be prevented from emerging when leaves are properly composted (by mulching damaged leaves with soil) [321]. However, such approach will likely adversely impact parasitoids, which might stay in C. ohridella mines [355].
Replacing horse chestnut with other species is one of the discussed measures [321], but evidently it will be economically very expensive for big cities. Furthermore, the feasibility of tree species chosen for replacement should be carefully assessed.
3.13. The Lime Leaf Miner, Phyllonorycter issikii (Kumata, 1963) (Lepidoptera: Gracillariidae)
Phyllonorycter issikii (Figure 13) is a tiny moth of East Asian origin that in the mid-1980s invaded the western part of the Palearctic, becoming a notable pest of limes Tilia spp. (Supplimentary Table S7; [288,356,357,358]). The moth is known to naturally occur in Japan [356], the Russian Far East (Primorskii Krai) [357], and South Korea [358]. A recent study based on the survey of historical herbaria and sequencing of the larvae and pupae found in the mines revealed a wide distribution of P. issikii in China [359,360].
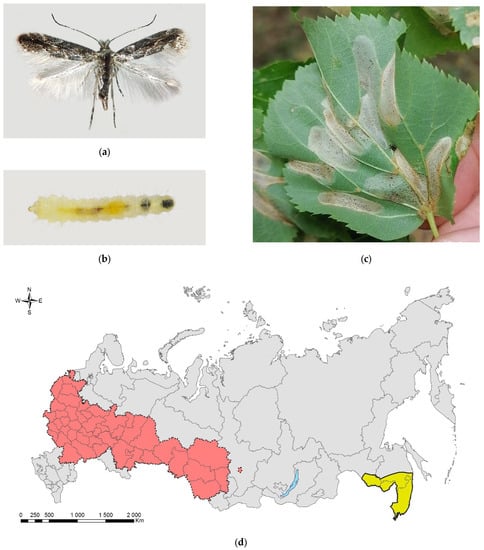
Figure 13.
The lime leaf miner, Phyllonorycter issikii: (a) an adult moth; (b) a mature larva; (c) damaged Tilia cordata in Central Siberian Botanical Garden of the Siberian Branch of the Russian Academy of Science, Novosibirsk. (d) the current distribution in Russia: the native range (colored in yellow) and invasive range (colored in red). Photo by N.I. Kirichenko.
The earliest record of P. issikii outside its native range is dated to 1985 and refers to the ornamental plantations of Moscow [361]. Two years later, the species was documented in Voronezh City, i.e., 500 km southwest of Moscow, already at a high population density [362]. During the next 20 years, it spread over the territory of Europe [363,364]. In Russia, the pest occupied most of the European part, except the southernmost and northernmost regions (Figure 13). In 2021, our surveys did not reveal the pest on limes in Sochi City and in settlements of Krasnodar and the Stavropol Krais. Remarkably, the species invaded Western Siberia, with the first record dated to 2006 in Tyumen City [365]. Presently, P. issikii is found in Siberia up to the river Yenisei, despite the fact that limes are rare elements of Siberian flora and are mostly used as ornamental plants in cities and smaller settlements [309]. Further expansion to Eastern Siberia is limited due to the absence of its host plants on the territory from the Krasnoyarsk Krai to the Amur Oblast [360].
A phylogeographic study of its currently known range discovered a high genetic diversity of P. issikii in Europe vs. East Asia, suggesting multiple pest introductions and its further dispersal [366]. Extensive surveys of 250-year-old herbaria confirmed the East Asian origin and the invasive status of the species in the western Palearctic, as well as highlighted the contribution of China to the species’ invasion westward [360]. Furthermore, the historical herbaria clarified the absence of the species from North America [360].
The pest larvae feed exclusively on limes, Tilia spp. (Malvaceae), and reference to Betula spp. as the host [356] should be regarded as an error. In its native range, the species develops on East Asian limes, among which T. amurensis and T. mandshurica are most regularly documented hosts [356,358,366]. In the invaded regions, P. issikii shifted to novel hosts, for example, T. cordata (a main host), T. platyphyllos, T. sibirica, and others [363,366,367]. Notably, P. issikii willingly attacks American lime (T. americana) planted in Russian botanical gardens, in particular in Moscow City [368]. It is a common plant in eastern USA [369], and in case of accidental P. issikii introduction, T. americana will likely fit as a favorable host plant for the pest.
In the invaded regions of Russia, the species develops in one or two generations per year, from May to September, depending on the region [364,368]. Larvae live in the lower side tentiform mines (Figure 13b,c), and occasionally, upper side mines occur in dense populations [360]. In exceptional cases, up to 29 mines per leaf were recorded in Novosibirsk on T. cordata [357]. The species hibernates at the adult stage.
Adults of P. issikii fly only over short distances [363]. Long-distance dispersal on air currents is possible, as well as the distribution of P. issikii by hitchhiking with various land and air transports, bearing in mind that adult moths hide themselves in crevices and other shelters and thus can be passively moved to remote territories [308,363,370].
The species is generally known as an ornamental pest causing aesthetic damage to limes in parks and gardens [363]. In addition, P. issikii was also reported as affecting natural stands in European Russia [371] and Western Siberia (Kemerovo Oblast) [366]. In high densities, the lime leaf miner negatively impacts lime growth; furthermore, the pest may adversely affect the sugar content in nectar, subsequently decreasing honey production, as documented in the Udmurtia (east of Volga River) [371]. In Western Siberia, the pest threatens vulnerable Tertiary relic limes groves [366]. Notably, so far, the species has been known by its exceptionally outbreaking character in the invaded regions of Russia [364,366]. Exploration of the lime herbarium specimens provided evidence of a population density increase in P. issikii in Primorskii Krai (Far East) in some years in the period of 1914 to 1958 [372].
The control methods have not been effectively developed against this pest. No data on pheromone monitoring and sex disruption are available in Russia. In European Russia, the parasitoid complex of P. issikii accounts for at least 43 species (Braconidae, Eulophidae, and Pteromalidae; Hymenoptera) vs. 13 parasitoid species known from its native range (East Asia), with only 6 species in common [373,374]. In European Russia and Siberia, the parasitism level varies within 1.4–37.0% but still remains low [368,375]; the highest value (up to 37.0%) was documented in the Volga Basin [376]. In the invaded regions of Russia, the commonly recorded hymenopteran parasitoids are Sympiesis gordius, Pnigalio soemius (both Eulophidae), and Minotetrastichus frontalis (Chalcidoidea) [373]. The latter species is also present in East Asia [374]. These are generalist parasitoids, with a wide host range among Gracillariidae [374]. Thus, their potential application against the invasive lime leaf miner is questionable, keeping in mind that they may also attack other native gracillariids, including rare species. Nevertheless, M. frontalis has been proposed as a biocontrol of P. issikii in Russia [377]. In East Asia, little data are available about parasitism rate in P. issikii populations [374], whilst these data would be of a high importance for the biocontrol of the pest.
3.14. The Poplar Leafminer, Phyllonorycter populifoliella (Treitschke, 1833) (Lepidoptera: Gracillariidae)
Phyllonorycter populifoliella (Figure 14), is a micromoth species widely distributed across the Palearctic [284]. In Russia, this native species occupies large territory from Kaliningrad City (southeastern coast of the Baltic Sea) to the Far East, except northern regions in the Asian part of the country (Figure 14d) [283,378,379]. Recently, the pest was detected beyond its known range, in northern India, in high densities on introduced poplars fueling the hypothesis about its accidental introduction [380].

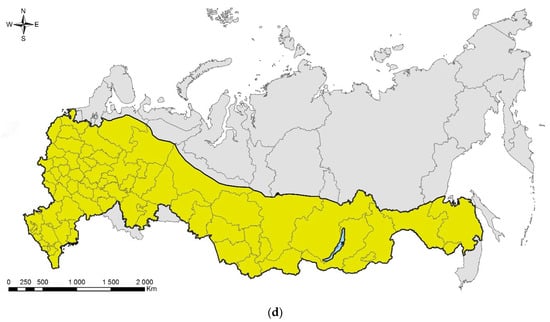
Figure 14.
The poplar leafminer, Phyllonorycter populifoliella: (a) an adult moth; (b) a mature larva; (c) a down side of a leaf of Populus × berolinensis heavily damaged by P. populifoliella, Saint Petersburg, August 2020; (d) the native range in Russia (colored in yellow). Photo: (a) and (b) by N.I. Kirichenko and (c) by N.A. Mamaev, with permission.
The moth develops on poplars, Populus spp., preferring black and balsam poplars (i.e., Aigeiros and Tacamahaca sections, respectively), to other poplars [379,381,382,383]. The larvae make distinctive blotch mines on the lower side of leaves, while in dense populations, upper side mines can also occur [384].
The poplar leafminer is known as a severe pest of balsam poplars and their hybrids with black poplars widely planted as ornamental in European Russia, in the Ural region and in Siberia [382,383,384,385,386,387,388]. In European Russia, the first outbreak of the pest was documented in the 1930s in Moscow [389]. Further east, in Siberia, the moth foci are known since the middle of the XX century in Irkutsk [385], and later, they were documented in other cities [382,386]. Interestingly, in some regions of European Russia where the poplar leaf miner was never known as an outbreaking pest, nowadays it provides noticeable damage to poplars. For instance, in Saint Petersburg, P. populifoliella was known as a rare species with the first record of the moth dating to 1936 [387,390], and the next finding was done only in 1974 [391]. In 1991, a sharp increase in population density was documented, with an outbreak in Saint Petersburg lasting from 1992 to 1999 [392,393]. For almost the next 20 years, the population density of this species was very low again, but since 2017, the new outbreak began in Saint Petersburg [387], where the pest showed the tendency to develop foci on new territories [289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390,394].
Severely damage poplars start losing their leaves already in late July to early August, which significantly reduces the aesthetic and ecological functioning of poplars in settlements and big cities. The pest can affect tree growth [395]. The mass death of poplars in Saint Petersburg in the early 2000s might have a link to the preceding pest outbreak [387]. Presently in Saint Petersburg, the P. populifoliella gives only one generation per year, since the larvae of the second generation are not able to complete their development and die not reaching the pupal stage. Climate warming will favor the second generation, thus increasing the pest population density [289,290]. Currently, there is no regular monitoring of the poplar leaf miner in Russia. Injection of poplars with systemic insecticides is a possible way to reduce the pest population densities, but this approach is not used due to its high economic and labor cost. The removal of fallen leaves (carrying mines with larvae and pupae of the pest) is not an effective approach as it can also affect survival of native enemies (parasitoids) that can be helpful in pest control [396]. The complexes of parasitoids attacking P. populfoliella in European Russia, includes overall 68 species [383], which in some years, can kill up to 90% of the second generation of the moth as found in Moscow [383,396].
The gradual replacement of poplars with other tree species could help decrease the pest’s impact. Black and balsam poplars commonly used as ornamental in Russian cities should be planted rather in small groups in order to avoid the formation of large foci of P. populifoliella [383,395].
Bearing in mind the P. populifoliella outbreaks on balsam poplars and their hybrids with black poplars in Russia, the accidental introduction of the moth to North America, where balsam and black poplars (e.g., Populus balsamifera, P. trichocarpa, P. deltoides, and Populus × canadensis) have a wide range, may potentially result in significant tree damage.
4. Discussion
4.1. Taxonomy
This review analyzes the most recent data on 14 species of insects from 3 orders: Hemiptera-Heteroptera (3 species from 3 families), Coleoptera (6 species from 2 families), and Lepidoptera (5 species from 2 families) that are known as highly invasive in Russia or native to the country but also pose a potential danger to woody plants elsewhere (Table 1). The very approximate estimations of the insect fauna of Russia suggest that it includes 80–100 thousand species [397], which is roughly close to the earlier assessments of the insect fauna of the former USSR (approximately the same, 81 and 119 thousand species, respectively [398]). Overall, 192 species of phytophagous alien insects from 48 families and 8 orders had been documented in the European part of Russia by 2011 [8]. Undoubtedly, their number has increased since that time, both because of the escalating invasions and the increased detection of alien species due to growing survey efforts. Looking at all these estimations, it is unjustified to conclude that the listed three orders and seven families of insects include all the most dangerous pests, and it is impossible to say that in other taxa there are no species which are already identified or potentially can be considered pestiferous for woody plants. Out of 14 species, 4 species belong to Gracillariidae, including Cameraria ohridella, which intensively invades urban environments providing scenic outbreaks. Four species, actual or potential invaders, represent the family Buprestidae. The question remains open whether or not there are specific family-level traits that drive the family representative to invade new territories. Taxonomic estimates are still very rough and cannot be used to estimate the invasive potential of any particular taxon. In the coming years, in our opinion, in addition Coleoptera and Lepidoptera, economically important invaders can also be well expected in Hemiptera-Homoptera, Hymenoptera, and Diptera.

Table 1.
Invasive and emerging insect pests of forest and urban woody plants in Russia: possibility of further invasions, damage, monitoring, and control (Summary).
4.2. Directions of Invasions
In terms of the directions of invasions, the pests included in this review can be clustered into four groups:
- (1)
- Invasions from Asia to West or central Europe and then (or directly) to European Russia: This is the most numerous group, which consists of five species—Cydalima perspectalis, Phyllonorycter issikii, Halyomorpha halys (likely first invaded North America and only then Europe and European Russia; see above), and two species, which actually skipped West or central Europe and arrived directly to European Russia, namely, Agrilus planipennis and Polygraphus proximus;
- (2)
- Invasions from North America to West or central Europe and then to European Russia: Leptoglossus occidentalis and Corythucha arcuata;
- (3)
- Invasions from Europe to Asia: the case of Ips amitinus;
- (4)
- Range expansions within Europe and invasions to European Russia: Lamprodila festiva and Cameraria ohridella.
Additionally, there is a group of potential invaders currently changing their ranges within European and/or Asian parts of Russia: As described above, two beetle species (Agrilus fleischeri and A. mali) have not yet noticeably expanded their native ranges in Asian Russia but demonstrate a potential of range expansion and/or host plant shift; somewhat similar could be said about Acrocercops brongniardella and Phyllonorycter populifoliella.
Thus, there is no common geographic pattern of invasion, and different invaders spread in very different directions.
4.3. Causes and Pathways of Invasions
Invasions of almost all discussed species were associated with human activity, except Ips amitinus (in North Europe) and Acrocercops brongniardella, which are believed to expand their ranges due to natural causes. Some species (e.g., Leptoglossus occidentalis, Halyomorpha halys, and Corythucha arcuata) at the beginning of their invasions were most likely transported by airplanes (from one continent to another) with goods of plant or non-plant origin. Agrilus planipennis was likely moved with wood products or packaging materials; Polygraphus proximus and Ips amitinus (in Siberia) with timber by railway roads; and Lamprodila festiva, Cydalima perspectalis, Cameraria ohridella, and Phyllonorycter issikii with plant materials and/or plants for planting. Very small insects such as Corythucha arcuata and Cameraria ohridella could use air flow for mass spreading. In other cases, the invasive processes (actively developing (e.g., in Agrilus planipennis and Lamprodila festiva) or just beginning at the early stages (e.g., in Agrilus fleischeri, A. mali, Acrocercops brongniardella, and Phyllonorycter populifoliella)) are probably more complicated and involve host plant shifts as a result of planting or introduction of susceptible host plant species or hybrids (see corresponding essays above).
4.4. Probability of Further Invasions to Neighboring and Distant Countries
All species reviewed are expected to spread further to (in) Europe, including countries of the Caucasus region (i.e., nine species: Leptoglossus occidentalis, Halyomorpha halys, Corythucha arcuata, Agrilus fleischeri, A. mali, A. planipennis, Lamprodila festiva, Polygraphus proximus, and Cydalima perspectalis) or to (in) Asia (i.e., nine species: Leptoglossus occidentalis, Halyomorpha halys, Corythucha arcuata, Agrilus fleischeri, A. mali, Ips amitinus, A. brongniardella, Cameraria ohridella, and Phyllonorycter populifoliella). Moreover, it is expected that nine species can potentially invade North America and/or other continents (i.e., Agrilus fleischeri, A. mali, Lamprodila festiva, Ips amitinus, Polygraphus proximus, Acrocercops brongniardella, Cameraria ohridella, Phyllonorycter issikii, and P. populifoliella) (Table 1).
4.5. Role of the Trophic Factor in Insect Pest Range Expansion
Analysis of invasion histories performed in the framework of this review, suggests that in some cases, invasions start with the widening of the pest’s trophic niche and shifts to new host plant(s) (commonly human-introduced) within the native pest’s range, frequently followed by invasion to new regions (e.g., Agrilus fleischeri, A. mali, Acrocercops planipennis, Lamprodila festiva, and Phyllonorycter populifoliella). The data reviewed above suggest that a few steps can be usually recognized within those five scenarios:
- (1)
- A shift in an insect species (often not even a pest) within its native range from its usual host plant(s) to introduced and cultivated host plant(s) (usually from the same or close genus of woody plants and often non-resistant because of lack of co-evolution) (e.g., a shift in Agrilus planipennis in Russian Far East and China, from local Asian ash species to introduced North American ash species; a shift in Phyllonorycter populifoliella in European Russia and Siberia, from local poplars to widely cultivated introduced North American balsam poplar and hybrids; a shift in Lamprodila festiva from wild Cupressaceae to introduced Thuja and other cultivated representatives of this family, including their hybrids and cultivars in the Mediterranean region; a shift in Agrilus fleischeri to introduced poplars in China);
- (2)
- A local niche expansion due to exploration of cultivated, recently introduced host plants in anthropogenic (urban or agricultural) landscapes; local population build-up and outbreaks (e.g., recorded earlier in Agrilus planipennis, A. mali, Phyllonorycter populifoliella, and Lamprodila festiva, and currently seen in A. fleischeri);
- (3)
- A range expansion outside the limits of the native range through anthropogenic (urban or agricultural) landscapes, i.e., beginning of invasion (e.g., Agrilus mali and Lamprodila festiva);
- (4)
- A distant invasion (e.g., invasions of Agrilus planipennis to European Russia or North America or Ips amitinus to Siberia);
- (5)
- A secondary host plant shift to the native (for the invaded region) food plant(s) (e.g., shift in Agrilus planipennis to Chionanthus virginicus in North America [399] and to Fraxinus excelsior in European Russia; shift in A. mali from cultivated apples to the wild apple, Malus sieversii in China).
Separately, we should mention Acrocercops brongniardella. This species does not shift to new host plants but simply follows range expansion of its usual host plant (Quercus robur) [360].
4.6. Monitoring and Control Measures
The monitoring methods recommend for the reviewed species are mainly limited by visual inspections and application of pheromone and color traps (Table 1). These methods might be quite effective when used appropriately and systematically. However, as this review demonstrates, in many cases, the monitoring system does not work properly, and many invasive pests rapidly increase their secondary ranges in Russia (e.g., Leptoglossus occidentalis, Halyomorpha halys, Agrilus planipennis, Cydalima perspectalis, Lamprodila festiva, and Cameraria ohridella). The lack of effective integration between organizations responsible for the monitoring and management of forests, urban, and suburban woody plantations in Russia (on both the national and local levels) creates serious difficulties for the practical and systematic use of these methods, especially keeping in mind the vast territory of the country. To increase the effectiveness of the monitoring, it is advisable to create a fast and flexible system of information exchange between the public and the organizations responsible for the monitoring and management of forests, urban, and suburban woody plantations, as well as to increase the level of effective involvement of scientists, university professionals, and citizens in monitoring of invasive insect pests of woody plants.
The control measures understandably differ between forests and urban woody plantations. To control the spread of the reviewed invasive pests in forests, we mostly suggest developing and use biological and chemical methods (Table 1). Special attention should be paid to a braconid Spathius polonicus Niezabitowski (Hymenoptera: Braconidae: Doryctinae), a parasitoid, which seems to have potential to effectively control Agrilus planipennis in European Russia, although it is likely that a few years are needed to build up a sufficient parasitoid population [145,169,400]. The effective use of sanitation fellings in the forests of the Russian Federation is currently much overcomplicated by the existing regulatory framework, which does not allow timely felling of trees infested by insect pests. The use of pheromone traps proposed as a measure to control some invaders (e.g., Halyomorpha halys, Corythucha arcuata, Ips amitinus, Polygraphus proximus, and Cydalima perspectalis; Table 1) is possible and potentially effective only when we deal with a limited, relatively small forest area.
The situation is different with the invasive pest control in urban and suburban woody tree plantations. The use of chemical control is significantly legally limited in such environments, whereas the application of stem injections is not always possible (taking into account its cost) but advisable, especially against insect pests that live inside the plant tissues (e.g., mining insects, gallers, wood-borers, etc.) in individual trees in urban areas. Within a relatively small woody tree plantation, it is possible to use biological control methods, pheromone traps, elimination of individual infested trees and other specific methods. It should be kept in mind that overall such control methods might be very expensive.
5. Conclusions
The majority of the reviewed insect pests have demonstrated their invasive behaviour in Russia during the last 10–30 years. In most cases, first reports of these invaders in Russia were unexpected for the stakeholders, who were supposed to provide forest and urban woody plants’ monitoring and management. These invasions led to significant ecological and economic losses and caused negative social consequences [15,63,338,360].
We suggest that there are three major scenarios of invasions of woody plant’s pests: (1) a naturally conditioned range expansion, which results in the arrival of a pest to a new territory and its further naturalization; (2) a human-mediated long-distance transfer of a pest to a new territory and its further naturalization; and (3) a widening of the pest’s trophic niche and shift to new host plant(s) (commonly human-introduced) within the native pest’s range, frequently followed by invasion to new regions.
Bearing in mind these and many other examples of devastating invasions of tree pests as well as unpredictable emergence of novel invaders, it is essential to stress the importance of insect pest monitoring (including the episodes of host plant shift) and more effective use of early detection programs (e.g., sentinel plantings) [7,401,402] and application of new and developing species identification techniques (e.g., DNA-barcoding) [403].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13040521/s1; Supplimentary Table S1: Timeline of the first records of the Western coniferous seed bug, Leptoglossus occidentalis in its invasive range in Europe; Supplimentary Table S2: Timeline of the first records of Halyomorpha halys in its invasive range in Europe; Supplimentary Table S3: Timeline of the first records of Corythucha arcuata in its invasive range in Europe; Supplimentary Table S4: Timeline of the first records of Lamprodila (Palmar) festiva in its invasive range in Europe; Supplimentary Table S5: Timeline of the first records of Cydalima perspectalis in its invasive range in Europe; Supplimentary Table S6: Timeline of the first records of Cameraria ohridella in its invasive range in Europe; Supplimentary Table S7: Timeline of the first records of Phyllonorycter issikii in its invasive range in Europe.
Author Contributions
Conceptualization, D.L.M., N.I.K., A.V.S. and R.V.; methodology, D.L.M., N.I.K. and A.V.S.; formal analysis, D.L.M., N.I.K., N.N.K., E.V.A., V.B.G., I.A.K., M.Y.M., R.V., M.G.V., E.N.Z. and A.V.S.; writing—original draft preparation, D.L.M., N.I.K., N.N.K., E.V.A., V.B.G., I.A.K., M.Y.M., M.G.V., E.N.Z. and A.V.S.; writing—review and editing, D.L.M., N.I.K., N.N.K., E.V.A., V.B.G., I.A.K., M.Y.M., R.V., M.G.V., E.N.Z. and A.V.S.; visualization, D.L.M., N.I.K., N.N.K., E.V.A., I.A.K., M.G.V. and E.N.Z.; supervision, D.L.M., R.V. and A.V.S.; project administration, D.L.M.; funding acquisition, R.V., A.V.S. and D.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Russian Science Foundation (data collection and analysis for Hemiptera-Heteroptera and Lepidoptera species in general, preparation of maps: project No. 21-16-00050, D.L.M.; data collection and analysis for Ips amitinus and Coleoptera species in general: project No. 21-16-00065, A.V.S.). N.I.K. was partially funded by the basic project of Sukachev Institute of Forest SB RAS, Russia (Project No. 0287-2021-0011) [review of Phyllonorycter issikii]. I.A.K. was partially supported by the Russian Foundation for Basic Research (project No. 20-04-00587) [review of Polygraphus proximus]. N.N.K. and E.N.Z. were partially supported by the State assignment of the Federal Research Centre of the Subtropical Scientific Centre RAS, project No. FGRW-2022-0006 [review of Cydalima perspectalis]. The study by M.G.V. [review of Lamprodila (Palmar) festiva, Agrilus fleischeri, A. planipennis, and A. mali] was performed in the frame of state research project No. 122031100272-3. The study by R.V. was funded by a grant from the Forest Damage Centre, Swedish University of Agricultural Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon email request to the authors.
Acknowledgments
We sincerely acknowledge V.A. Soboleva (Voronezh State University, Voronezh, Russia), E. Jendek (Czech University of Life Sciences, Prague, Czech Republic), K.V. Makarov (Moscow Pedagogical State University, Moscow, Russia), A.V. Kovalev (All-Russian Plant Protection Institute, Saint Petersburg, Russia), D. Lees (Museum of Natural History, London, UK), I.A. Utkina (Institute of Forest Science, Moscowskya Oblast, Russia), and N.A. Mamaev (Saint Petersburg State Forest Technical University, Saint Petersburg, Russia) for permissions to use their photographs of insects or damage symptoms and I.A. Mikhailova (Sukachev Institute of Forest SB RAS, Krasnoyarsk, Russia) for help with mapping. We thank Eddy Velez for language improvement and two annonimous reviwers for their useful comments and helpful criticism.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Pimentel, D.; McNair, S.; Janecka, J.; Wightman, J.; Simmonds, C.; O’Connell, C.; Won, E.; Russel, L.; Zern, J.; Aquino, T.; et al. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric. Ecosys. Environ. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- Fei, S.; Phillips, J.; Shouse, M. Biogeomorphic impacts of invasive species. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 69–87. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockerhoff, E.G.; Nunez, M.A. Biological invasions in forest ecosystems: A global problem requiring international and multidisciplinary integration. Biol. Invasions 2017, 19, 3073–3077. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; van Kleunen, M.; Winter, M.; et al. The global rise in emerging alien species results from increased accessibility of new source pools. Proc. Natl. Acad. Sci. USA 2018, 115, E2264–E2273. [Google Scholar] [CrossRef]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Paap, T.; Burgess, T.I.; Wingfield, M.J. Urban trees: Bridge-heads for forest pest invasions and sentinels for early detection. Biol. Invasions 2017, 19, 3515–3526. [Google Scholar] [CrossRef]
- Panzavolta, T.; Bracalini, M.; Benigno, A.; Moricca, S. Alien invasive pathogens and pests harming trees, forests, and plantations: Pathways, global consequences and management. Forests 2021, 12, 1364. [Google Scholar] [CrossRef]
- Maslyakov, V.Y.; Izhevsky, S.S. Alien Phytophagous Insects Invasions in the European Part of Russia; Institute of Geography ot Russian Academy of Sciences: Moscow, Russia, 2011; pp. 1–272. (In Russian) [Google Scholar]
- Deschka, G.; Dimić, N. Cameraria ohridella n. sp. (Lepidoptera, Lithocolletidae) aus Mazedonien, Jugoslawien. Acta Entomol. Jugosl. 1986, 22, 11–23. [Google Scholar]
- van Nieukerken, E.; Wagner, D.; Baldessari, M.; Mazzon, L.; Angeli, G.; Girolami, V.; Duso, C.; Doorenweerdet, C. Antispila oinophylla new species (Lepidoptera, Heliozelidae), a new North American grapevine leafminer invading Italian vineyards: Taxonomy, DNA barcodes and life cycle. ZooKeys 2012, 170, 29–77. [Google Scholar] [CrossRef]
- Mlynarek, J.J. Testing the enemy release hypothesis in a native insect species with an expanding range. PeerJ 2015, 3, e1415. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Wei, K.; Wang, X.; Duan, J.J.; Jennings, D.E.; Poland, T.M. Introduced plants induce outbreaks of a native pest and facilitate invasion in the plants’ native range: Evidence from the emerald ash borer. J. Ecol. 2022; in press. [Google Scholar] [CrossRef]
- Venette, R.C.; Hutchison, W.D. Invasive insect species: Global challenges, strategies & opportunities. Front. Insect Sci. 2021, 1, 1. [Google Scholar] [CrossRef]
- Akulov, E.N.; Ponomarenko, M.G.; Kirichenko, N.I. Exploring fauna of Microlepidoptera in South Siberia: Novel regional records and interception of quarantine species. J. Asia-Pac. Biodivers. 2019, 12, 597–612. [Google Scholar] [CrossRef]
- Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. In The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; Saint Petersburg State Forest Technical University: St. Petersburg, Russia, 2020; p. 452. Available online: http://spbftu.ru/wp-content/uploads/2020/10/Kataev-Readings-XI-2020.pdf (accessed on 20 January 2022).
- ESRI. ArcGIS Desktop: Release 9.3; Environmental Systems Research Institute: Redlands, CA, USA, 2008; Available online: http://www.esri.com/software/arcgis/eval-help/arcgis-93 (accessed on 20 January 2022).
- Gapon, D.A. First records of the western conifer seed bug Leptoglossus occidentalis Heid. (Heteroptera, Coreidae) from Russia and Ukraine, regularities in its distribution and possibilities of its range expansion in the Palaearctic region. Entomol. Rev. 2013, 93, 174–181. [Google Scholar] [CrossRef]
- Tescari, G. Leptoglossus occidentalis coreide nearctico rinveuto in Italia (Heteroptera, Coreidae). Lavor. Societa Veneziana Scie. Nat. 2001, 26, 3–5. [Google Scholar]
- Gninenko, Y.I.; Cheplyansky, I.Y.; Chernova, U.A.; Rakov, A.G.; Khegai, I.V.; Latyshova, N.S.; Gimranov, R.I. Methodical Recommendations for Protection against Pine Seed Bug (for Industrial Trial); VNIILM: Pushkino, Russia, 2019; p. 28. [Google Scholar]
- Fent, M.; Kment, P. First record of the invasive western conifer seed bug Leptoglossus occidentalis (Heteroptera: Coreidae) in Turkey. North-West. J. Zool. 2011, 7, 72–80. Available online: https://www.researchgate.net/publication/228493133_First_record_of_the_invasive_western_conifer_seed_bug_Leptoglossus_occidentalis_Heteroptera_Coreidae_in_Turkey (accessed on 20 January 2022).
- Ahn, S.J.; Son, D.; Choo, P.Y.; Parka, C.G. The first record on Leptoglossus occidentalis (Hemiptera: Coreidae) in Korea, a potential pest of the pinaceous tree species. J. Asia-Pac. Entomol. 2013, 16, 281–284. [Google Scholar] [CrossRef]
- Aukema, B.; Rieger, C.; Rabitsch, W. (Eds.) Catalogue of the Heteroptera of the Palaearctic Region. Vol. 6. Supplement; The Netherlands Entomological Society: Wageningen, The Netherlands, 2013; 629p. [Google Scholar]
- Barclay, M.; Nikolaeva, S. Arrival in Kazakhstan of Leptoglossus occidentalis (Hemiptera: Heteroptera: Coreidae); a North American invasive species expands 2500 kilometres to the east. Klapalekiana 2018, 54, 1–3. [Google Scholar]
- van der Heyden, T. Leptoglossus occidentalis Heidemann (Heteroptera: Coreidae: Coreinae: Anisoscelini) in Israel. Revista Chilena Entomol. 2019, 45, 435–437. [Google Scholar] [CrossRef]
- Kalashian, M.Y.; Ghrejyan, T.L.; Karagyan, G.H. First finding of Western conifer seed bug Leptoglossus occidentalis Heid. (Heteroptera, Coreidae) in Armenia. Russ. J. Biol. Invasions 2021, 12, 274–276. [Google Scholar] [CrossRef]
- Ben Jamâa, M.L.; Mejri, M.; Naves, P.; Sousa, E. Detection of Leptoglossus occidentalis Heidemann, 1910 (Heteroptera: Coreidae) in Tunisia. Afr. Entomol. 2013, 21, 165–167. [Google Scholar] [CrossRef]
- Gapon, D.A.; Busarova, N.V.; Komarov, Y.E. New records of the western conifer seed bug Leptoglossus occidentalis (Heteroptera: Coreidae) in Russia and in adjacent territories. Cauc. Entomol. Bull. 2016, 12, 221–222. (In Russian) [Google Scholar] [CrossRef]
- van der Heyden, T.; Faúndez, E.I. First records of Leptoglossus occidentalis Heidemann, 1910 (Hemiptera: Heteroptera: Coreidae) in Brazil and South Africa. Boletín Mus. Nac. Hist. Nat. Parag. 2020, 24, 28–30. Available online: https://www.researchgate.net/publication/341832223_First_records_of_Leptoglossus_occidentalis_Heidemann_1910_Hemiptera_Heteroptera_Coreidae_in_Brazil_and_South_Africa (accessed on 20 January 2022).
- Faúndez, E.I.; Rocca, J.; Villablanca, J. Detection of the invasive western conifer seed bug Leptoglossus occidentalis Heidemann, 1910 (Heteroptera: Coreidae: Coreinae) in Chile. Arq. Entomol. 2017, 17, 317–320. Available online: https://www.researchgate.net/publication/316277401_Detection_of_the_invasive_western_conifer_seed_bug_Leptoglossus_occidentalis_Heidemann_1910_Heteroptera_Coreidae_Coreinae_in_Chile (accessed on 20 January 2022).
- Carpintero, D.L.; Farina, J.L.; De Biase, S. Reporte para la provincia de Buenos Aires de tres especies de Heteroptera (Hemiptera) introducidas en Argentina. Hist. Nat. Terc. Ser. 2019, 9, 63–70. [Google Scholar]
- Faúndez, E.I.; Silvera, M. Sobre la presencia de la chinche de las coníferas occidental Leptoglossus occidentalis Heidemann (Heteroptera: Coreidae) en Uruguay. Rev. Chil. Entomol. 2019, 45, 549–551. [Google Scholar] [CrossRef]
- Gildenkov, M.Y. Invasive bug species Leptoglossus occidentalis Heidemann, 1910 (Heteroptera: Coreidae) in ecosystems of the Smolensk area. In Modern Scientific Research: Trends and Prospects, Proceedings of the 2nd All-Russia Conference with International Participation, Chistopol, Tatarstan, Russia, 26 February 2021; Nazarov, E.A., Ed.; Astor i Ya Publisher: Kazan, Russia, 2021; pp. 5–8. (In Russian) [Google Scholar]
- Golub, V.B.; Aksenenko, E.V.; Soboleva, V.A.; Kornev, I.I. New data on the distribution of the tropical bed bug Cimex hemipterus and the western conifer seed bug Leptoglossus occidentalis (Heteroptera: Cimicidae, Coreidae) in the European part of Russia. Russ. J. Biol. Invasions 2020, 11, 97–100. [Google Scholar] [CrossRef]
- Golub, V.B. Personal Communication; Voronezh State University: Voronezh, Russia, 2021. [Google Scholar]
- Gninenko, Y.I.; Gapon, D.A.; Shchurov, V.I.; Bondarenko, A.S. A western conifer seed bug Leptoglossus occidentalis (Heteroptera, Coreidae) emerged in Russia. Plant Prot. Quar. 2014, 6, 38–40. Available online: https://www.elibrary.ru/download/elibrary_21561963_71479165.pdf (accessed on 20 January 2022). (In Russian).
- van der Heyden, T. New data on the distribution of Leptoglossus occidentalis Heidemann (Heteroptera: Coreidae: Coreinae: Anisoscelini), including the first record of the species in Georgia. Rev. Chil. Entomol. 2018, 44, 433–435. [Google Scholar]
- Koerber, T.W. Leptoglossus occidentalis (Hemiptera, Coreidae), a newly discovered pest of coniferous seed. Ann. Entomol. Soc. Am. 1963, 56, 229–234. [Google Scholar] [CrossRef]
- Werner, D.J. Die amerikanische Koniferen-Samen-Wanze Leptoglossus occidentalis (Heteroptera: Coreidae) als Neozoon in Europa und in Deutschland: Ausbreitung und Biologie. Entomol. Heute 2011, 23, 31–68. Available online: https://www.zobodat.at/pdf/Entomologie-heute_23_0031-0068.pdf (accessed on 20 January 2022).
- Rice, R.E.; Uyemoto, J.K.; Ogawa, J.M.; Pemberton, W.M. New findings on pistachio problems. Calif. Agric. 1985, 39, 15–18. [Google Scholar]
- Barta, M. Biology and temperature requirements of the invasive seed bug Leptoglossus occidentalis (Heteroptera: Coreidae) in Europe. J. Pest. Sci. 2016, 89, 31–44. [Google Scholar] [CrossRef]
- Hedlin, A.F.; Yates, H.O., III; Tovar, D.C.; Ebel, B.H.; Koerber, T.W.; Merkel, E.P. Cone and Seed Insects of North American Conifers; Environment Canada, Forestry Service, Pacific Forestry Centre: Victoria, BC, Canada; The United States Forest Service: Wahington, DC, USA; Secretaría de Agricultura y Recursos Hidráulicos: Tulla de Allende, Mexico, 1981; pp. 1–122. Available online: https://cfs.nrcan.gc.ca/publications?id=2026 (accessed on 2 January 2022).
- Bernardinelli, I.; Rovato, M.; Zandigiacomo, P. Life history and laboratory rearing of Leptoglossus occidentalis. In IUFRO Working Party 7.III.10, Proceedings of the Workshop, Gmunden, Austria, 11–14 September 2006; Federal Research and Training Centre for Forests, Natural Hazards and Landscape Forest Training Centre Ort: Gmunden, Austria, 2006; p. 225. [Google Scholar]
- Rabitsch, W. Alien true bugs of Europe (Insecta: Hemiptera: Heteroptera). Zootaxa 2008, 1827, 1–44. [Google Scholar] [CrossRef]
- Luchi, N.; Mancini, V.; Feducci, M.; Santini, A.; Capretti, P. Leptoglossus occidentalis and Diplodia pinea: A new insect-fungus association in Mediterranean forests. For. Pathol. 2012, 42, 246–251. [Google Scholar] [CrossRef]
- Tamburini, M.; Maresi, G.; Salvadori, C.; Battisti, A.; Zottele, F.; Pedrazzoli, F. Adaptation of the invasive western conifer seed bug Leptoglossus occidentalis to Trentino, an alpine region (Italy). Bull. Insectol. 2012, 65, 161–170. Available online: https://www.researchgate.net/publication/233100158_Adaptation_of_the_invasive_western_conifer_seed_bug_Leptoglossus_occidentalis_to_Trentino_an_alpine_region_Italy (accessed on 20 January 2022).
- Cargnus, E.; Buian, F.M.; Zandigiacomo, P. Presenza di Trichopoda pennipes (Diptera, Tachinidae) nell’Italia nord-orientale. Boll. Soc. Nat. Silvia Zenari 2011, 35, 123–130. [Google Scholar]
- Maltese, M.; Caleca, V.; Guerrieri, E.; Strong, W.B. Parasitoids of Leptoglossus occidentalis Heidemann (Heteroptera: Coreidae) recovered in western North America and first record of its egg parasitoid Gryon pennsylvanicum (Ashmead) (Hymenoptera: Platygastridae) in California. Pan-Pac. Entomol. 2012, 88, 347–355. [Google Scholar] [CrossRef]
- Lee, D.-H.; Short, B.D.; Joseph, S.V.; Bergh, J.C.; Leskey, T.C. Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ. Entomol. 2013, 42, 627–641. [Google Scholar] [CrossRef]
- Haye, T.; Gariepy, T.; Hoelmer, K.; Rossi, J.-P.; Streito, J.-C.; Tassus, X.; Desneux, N. Range expansion of the invasive brown marmorated stink bug, Halyomorpha halys: An increasing threat to field, fruit and vegetable crops worldwide. J. Pest Sci. 2015, 88, 665–673. [Google Scholar] [CrossRef]
- Hoebeke, E.R.; Carter, M.E. Halyomorpha halys (Stål) (Heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in North America. Proc. Entomol. Soc. Wash. 2003, 105, 225–237. Available online: https://www.researchgate.net/publication/279897494_Halyomorpha_halys_Stal_Heteroptera_Pentatomidae_A_polyphagous_plant_pest_from_Asia_newly_detected_in_North_America (accessed on 20 January 2022).
- Lee, D.-H. Current status of research progress on the biology and management of Halyomorpha halys (Hemiptera: Pentatomidae) as an invasive species. Appl. Entomol. Zool. 2015, 50, 277–290. [Google Scholar] [CrossRef]
- Hamilton, G.C.; Ahn, J.J.; Bu, W.; Leskey, T.C.; Nielsen, A.L.; Park, Y.-L.; Rabitsch, W.; Hoelmer, K.A. Halyomorpha halys (Stål). In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 243–292. [Google Scholar] [CrossRef]
- Fogain, R.; Graff, S. First records of the invasive pest, Halyomorpha halys (Hemiptera: Pentatomidae), in Ontario and Quebec. J. Entomol. Soc. Ont. 2011, 142, 45–48. [Google Scholar]
- Faúndez, E.I.; Rider, D.A. The brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae) in Chile. Arquivos Entomol. 2017, 17, 305–307. Available online: https://www.researchgate.net/publication/316277383_The_brown_marmorated_stink_bug_Halyomorpha_halys_Stal_1855_Heteroptera_Pentatomidae_in_Chile (accessed on 12 December 2021).
- Arnold, K. Halyomorpha halys (Stål, 1855), eine für die europäische Fauna neu nachgewiesene Wanzenart (Insecta: Heteroptera: Pentatomidae: Cappaeini). Mitt. Des. Thüring. Entomol. 2009, 16, 19. [Google Scholar]
- Gariepy, T.D.; Musolin, D.L.; Konjević, A.; Karpun, N.N.; Zakharchenko, V.Y.; Zhuravleva, E.N.; Tavella, L.; Bruin, A.; Haye, T. Diversity and distribution of cytochrome oxidase I (COI) haplotypes of the brown marmorated stink bug, Halyomorpha halys Stål (Hemiptera, Pentatomidae), along the eastern front of its invasive range in Eurasia. NeoBiota 2021, 68, 53–77. [Google Scholar] [CrossRef]
- Harris, A.C. Halyomorpha halys (Hemiptera: Pentatomidae) and Protaetia brevitaris (Coleoptera: Scarabaeidae: Cetoniinae) intercepted in Dunedin. Weta 2010, 40, 42–44. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.694.5502&rep=rep1&type=pdf (accessed on 20 January 2022).
- Malumphy, C. Second interception of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) in Britain. Het News 2014, 21, 4–5. [Google Scholar]
- Mityushev, I.M. First record of Halyomorpha halys in Russia. Plant Prot. Quar. 2016, 3, 48. Available online: https://www.elibrary.ru/download/elibrary_25606587_18888986.pdf (accessed on 2 December 2021). (In Russian).
- Musolin, D.L.; Konjević, A.; Karpun, N.N.; Protsenko, V.Y.; Ayba, L.Y.; Saulich, A.K. Invasive brown marmorated stink bug Halyomorpha halys (Stål) (Heteroptera: Pentatomidae) in Russia, Abkhazia, and Serbia: Range expansion, early stages of establishment and first records of damage to local crops. Arthropod-Plant Interact. 2018, 12, 517–529. [Google Scholar] [CrossRef]
- Borisov, B.A.; Karpun, N.N.; Protsenko, V.Y. New data on trophic relationships of the invasive brown marmorated stink bug Halyomorpha halys Stål (Heteroptera: Pentatomidae) in the subtropical zone of the Black Sea coast of the Caucasus. In Monitoring and Biological Control Methods of Woody Plant Pests and Pathogens: From Theory to Practice, Proceedings of the 2nd International Conference, Moscow, Russia, 22–26 April 2019; Baranchikov, Y.N., Ed.; SIF SN RASc.: Krasnoyarsk, Russia, 2019; pp. 33–35. (In Russian) [Google Scholar]
- Karpun, N.N.; Grebennikov, K.A.; Protsenko, V.E.; Ayba, L.Y.; Borisov, B.A.; Mityushev, I.M.; Zhimerkin, V.N.; Ponomaryov, V.L.; Chekmaryov, P.A.; Dolzhenko, V.I.; et al. Brown marmorated stink bug monitoring and identification methods Halyomorpha halys Stål, 1855. Plant Quar. Sci. Pract. 2018, 2, 7–11. Available online: https://elibrary.ru/download/elibrary_35209732_42561776.pdf (accessed on 18 January 2022). (In Russian).
- Karpun, N.N. The Structure of Complexes of Harmful Organisms of Woody Plants in the Humid Subtropics of Russia and the Biological Basis of Protection Measures. Ph.D. Thesis, Russian State Agricultural University, Moscow, Russia, 2018. Available online: http://www.old.timacad.ru/catalog/disser/dd/karpun/disser.pdf (accessed on 20 January 2022). (In Russian).
- Zamotajlov, A.S.; Belyi, A.I.; Esipenko, L.P. Harmfulness of herbivorous bugs of the family Pentatomidae (Insecta, Heteroptera) on tomatoes. In Results of Research Work for 2017, Proceedings of the 73th Research Conference of the Kuban’ State Agricultural University, Krasnodar, Russia, 14 March 2018; Koshaev, A.G., Ed.; Kuban State Agricultural University: Krasnodar, Russia, 2018; pp. 43–44. (In Russian) [Google Scholar]
- Stryukova, N.M.; Stryukov, A.A. The first detection of the brown marmorated stink bug in Crimea. In Sustainable Neospheric Development, Proceedings of the Scientific Inter-university Conference Devoted to the 156th Anniversary of V.I. Vernadsky, Simferopol, Russia, 15 March 2019; Bashta, A.I., Ed.; IP Zueva, T.V. Publisher: Simferopol, Russia, 2019; pp. 68–70. (In Russian) [Google Scholar]
- Zhuravleva, E.N.; Karpun, N.N. On the discovery of the brown marmorated stink bug Halyomorpha halys Stål (Heteroptera: Pentatomidae) in Sevastopol. In Monitoring and Biological Control Methods of Woody Plant Pests and Pathogens: From Theory to Practice, Proceedings of the 2nd International Conference, Moscow, Russia, 22–26 April 2019; Baranchikov, Y.N., Ed.; SIF SN RASc.: Moscow, Russia; Krasnoyarsk, Russia, 2019; pp. 74–75. (In Russian) [Google Scholar]
- Gapon, D.A. The brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae): Expansion of its range in the European part of Russia, description of the imago and larvae, and the diagnostics of the species. Cauc. Entomol. Bull. 2019, 15, 241–247. [Google Scholar] [CrossRef]
- Bulgakov, T.S. (Federal Research Centre the Subtropical Scientific Centre of the Russian Academy of Sciences, Sochi, Russia). Personal communication, 2020.
- Chenikalova, E.V. Marmorated stink bug Halyomorpha halys Stål in Stavropol. Proc. Stavropol Branch Russ. Entomol. Soc. 2020, 16, 46–49. [Google Scholar]
- Petrov, A.V. (Institute of Forest Science of Russian Academy of Science, Moscow, Russia). Personal communication, 2020.
- Watanabe, K. Specific characteristics of damage to cherry caused by Lycogoris (Apolygus) lucorum (Meyer-Dür) (Heteroptera: Miridae) and Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). Annu. Rep. Plant Prot. Soc. N. Jpn. 1996, 1996, 143–144. [Google Scholar]
- Bergmann, E.; Bernhard, K.M.; Bernon, G.; Bickerton, M.; Gill, S.; Gonzales, C.; Hamilton, G.C.; Hedstrom, C.; Kamminga, K.; Koplinka-Loehr, C.; et al. Host Plants of the Brown Marmorated Stink Bug in the U.S. Available online: https://www.stopbmsb.org/where-is-bmsb/host-plants (accessed on 2 January 2022).
- Zakharchenko, V.; Karpun, N.; Borisov, B. Trophic connections of the brown marmorated stink bug Halyomorpha halys Stål in the conditions of the invasive area on the Black Sea coast of the Caucasus. BIO Web. Conf. 2020, 21, 7. [Google Scholar] [CrossRef]
- Zakharchenko, V.Y. Bioecological Features of the Brown Marmorated Stink Bug (Halyomorpha halys Stål) in the Humid Subtropics of Russia and Control Measures. Ph.D. Thesis, Russian State Agricultural University, Moscow, Russia, 2021. Available online: http://www.old.timacad.ru/catalog/disser/kd/zaharchenko/zaharchenko_disser.pdf (accessed on 10 January 2022). (In Russian).
- Musolin, D.L.; Dolgovskaya, M.Y.; Protsenko, V.Y.; Karpun, N.N.; Reznik, S.Y.; Saulich, A.K. Photoperiodic and temperature control of nymphal growth and adult diapause induction in the invasive Caucasian population of the Brown marmorated stink bug, Halyomorpha halys. J. Pest Sci. 2019, 92, 621–631. [Google Scholar] [CrossRef]
- Khrimian, A.; Zhang, A.; Weber, D.C.; Ho, H.-Y.; Aldrich, J.R.; Vermillion, K.E.; Siegler, M.A.; Shirali, S.; Guzma, F.; Leskey, T.C. Discovery of the aggregation pheromone of the Brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1-bisabolen-3-ols. J. Nat. Prod. 2014, 77, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Sinitsyna, E.V.; Protsenko, V.E.; Karpun, N.N.; Mityushev, I.M.; Lobur, A.Y.; Todorov, N.G. First field trials of Russia-produced pheromone preparations for monitoring and control of the Brown marmorated stink bug, Halyomorpha halys Stål. Bull. Timiryazev Agricult. Acad. 2019, 3, 60–79. [Google Scholar] [CrossRef]
- Gouli, V.; Gouli, S.; Skinner, M.; Hamilton, G.; Kim, J.S.; Parker, B.L. Virulence of select entomopathogenic fungi to the brown marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). Pest Manag. Sci. 2012, 68, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.L.; Skinner, M.; Gouli, S.; Gouli, V.; Kim, J.S. Virulence of BotaniGard® to second instar brown marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). Insects 2015, 6, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Gorgadze, O.; Bakhtadze, G.; Kereselidze, M.; Lortkhipanidze, M. The efficacy of entomopathogenic agents against Halyomorpha halys (Hemiptera: Pentatomidae). Int. J. Current Res. 2017, 9, 62177–62180. Available online: https://www.journalcra.com/sites/default/files/issue-pdf/27484.pdf (accessed on 10 January 2022).
- Borisov, B.A.; Protsenko, V.Y.; Karpun, N.N. Potential use of entomotrophic bacteria and fungi to contain the brown marmorated stink bug Halyomorpha halys Stål (Heteroptera: Pentatomidae). In Biological Plant Protection as a Basis for Sustainable Agroecosystems, Proceedings of the X International Scientific and Practical Conference, Krasnodar, Russia, 11–13 September 2018; All-Russia Research Institute of Biological Plant Protection: Krasnodar, Russia, 2018; pp. 165–168. (In Russian) [Google Scholar]
- Protsenko, V.Y.; Borisov, B.A.; Karpun, N.N. The results of evaluating the insecticidal action of some species and strains of entomoparasitic fungi on the marmorated bug (Halyomorpha halys). In New and Unconventional Plants and Prospects of their Application, Proceedings of the XIII International Conference; Sochi, Russia, 4–8 June 2018; RUDN University Publishing: Moscow, Russia, 2018; pp. 591–594. (In Russian) [Google Scholar]
- Kulava, L.D.; Karpun, N.N.; Zhuravleva, E.N.; Shoshina, E.I.; Ayba, L.Y. The effectiveness of pheromones of a brown marmorated stink bug and traps of various designs in mandarin agrocenoses in Abkhazia. Subtrop. Ornam. Hortic. 2021, 77, 161–169. [Google Scholar] [CrossRef]
- Fujisawa, T. Damage and control of the brown marmorated stink bug in apple orchards. Jpn. Agric. Technol. 2001, 45, 42–47. [Google Scholar]
- Willrich, M.M.; Leonard, B.R.; Cook, D.R. Arthropod management laboratory and field evaluations of insecticide toxicity to stink bugs (Heteroptera: Pentatomidae). J. Cotton Sci. 2003, 7, 156–163. Available online: https://www.cotton.org/journal/2003-07/4/upload/jcs07-156.pdf (accessed on 10 January 2022).
- Nielsen, A.L.; Shearer, P.W.; Hamilton, G.C. Toxicity of insecticides to Halyomorpha halys (Hemiptera: Pentatomidae) using glass vial bioassays. J. Econ. Entomol. 2008, 101, 1439–1442. [Google Scholar] [CrossRef]
- Leskey, T.C.; Lee, D.-H.; Short, B.D.; Wright, S.E. Impact of insecticides on the invasive Halyomorpha halys (Hemiptera: Pentatomidae): Analysis of insecticide lethality. J. Econ. Entomol. 2012, 105, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Funayama, K. Control effect on the brown-marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae), by combined spraying of pyrethroid and neonicotinoid insecticides in apple orchards in northern Japan. Appl. Entomol. Zool. 2012, 47, 75–78. [Google Scholar] [CrossRef]
- Mooneyham, K.L.; Aigner, J.D., Jr.; Kuhar, T.P. Control of brown marmorated stink bug with insecticide-treated window screens. Arthropod Manag. Tests 2016, 41, tsw021. [Google Scholar] [CrossRef][Green Version]
- Kuhar, T.P.; Kamminga, K.L. Review of the chemical control research on Halyomorpha halys in the USA. J Pest. Sci. 2017, 90, 1021–1031. [Google Scholar] [CrossRef]
- Osborn, H.; Drake, C.J. Notes on American Tingidae with descriptions of new species. Ohio J. Sci. 1917, 17, 295–307. [Google Scholar]
- Torres-Miller, L. Additions to the West Virginia tingid fauna (Hemiptera: Heteroptera: Tingidae). Insecta Mundi 1995, 9, 281–282. [Google Scholar]
- Bernardinelli, I.; Zandigiacomo, P. First record of the oak lace bug Corythucha arcuata (Say) (Heteroptera, Tingidae) in Europa. Inf. Fitopatol. 2000, 50, 47–49. [Google Scholar]
- Mutun, S. First report of the oak lace bug, Corythucha arcuata (Say, 1832) (Heteroptera: Tingidae) from Bolu, Turkey. Isr. J. Zool. 2003, 49, 323–324. [Google Scholar]
- Mutun, S.; Ceyhan, Z.; Sözen, C. Invasion by the oak lace bug, Corythucha arcuata (Say) (Heteroptera: Tingidae), in Turkey. Turk. J. Zool. 2009, 33, 263–268. [Google Scholar] [CrossRef]
- Samin, N.; Linnavuori, R.E. A contribution to the Tingidae (Heteroptera) from north and northwestern Iran. Entomofauna. Z. Entomol. 2011, 32, 373–380. [Google Scholar]
- Shchurov, V.I.; Bondarenko, A.S.; Skvortsov, M.M.; Shchurova, A.V. Alien forest insect pests revealed in the Northwest Caucasus in 2010–2016 and consequences of their uncontrolled dispersal. Izv. St.-Peterbg. Lesoteh. Akad. 2017, 220, 212–228. (In Russian) [Google Scholar] [CrossRef]
- Neimorovets, V.V.; Shchurov, V.I.; Bondarenko, A.S.; Skvortsov, M.M.; Konstantinov, F.V. First documented outbreak and new data on the distribution of Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) in Russia. Acta Zool. Bulg. 2017, 9, 139–142. Available online: https://www.researchgate.net/publication/321496929_First_Documented_Outbreak_and_New_Data_on_the_Distribution_of_Corythucha_arcuata_Say_1832_Hemiptera_Tingidae_in_Russia (accessed on 10 January 2022).
- Karpun, N.N.; Protsenko, V.Y.; Borisov, B.A.; Shiryaeva, N.V. Discovery of oak lace bug Corythucha arcuata (Say) (Heteroptera: Tingidae) in the subtropical zone of the Black Sea coast of the Caucasus. Eurasian Entomol. J. 2018, 17, 113–119. [Google Scholar] [CrossRef]
- Stryukova, N.M.; Omel’yanenko, T.Z.; Golub, V.B. Oak lace bug in the Republic of Crimea. Plant Prot. Quar. 2019, 9, 43–44. Available online: https://www.elibrary.ru/download/elibrary_39322104_97303903.pdf (accessed on 10 January 2022). (In Russian).
- Martynov, V.V.; Nikulina, T.V. Oak lace bug Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae)—A new invasive pest in the forests of the southwestern part of mountain Crimea. Subtrop. Ornam. Hortic. 2020, 72, 124–138. (In Russian) [Google Scholar] [CrossRef]
- Golub, V.B.; Golub, N.V.; Soboleva, V.A. Distribution and trophic relations of the oak lace bug Corythucha arcuata (Say) (Heteroptera: Tingidae) in Crimea. Field Biol. J. 2020, 2, 179–184. Available online: https://www.elibrary.ru/download/elibrary_44184062_33929269.pdf (accessed on 10 January 2022). (In Russian).
- Gninenko, Y.I.; Chernova, U.A.; Gimranov, R.I.; Rakov, A.G.; Khegay, I.V. Oak lace bug expanses its area on the territory of Russia. Plant Prot. Quar. 2020, 10, 37–38. Available online: https://www.elibrary.ru/download/elibrary_43992021_73556954.pdf (accessed on 10 January 2022). (In Russian).
- Blyummer, A.G. Invasive species of not-Arctic lace bugs of the genus Corythucha (Heteroptera, Tingidae) in Eurasia: Features of distribution and injuriousness. In Ecological and Economic Consequences of Invasions of Dendrophilous Insects, Proceedings of the All-Russian Conference with International Participation, Krasnoyarsk, Russia, 25–27 September 2012; Baranchikov, Y.N., Ed.; Forest Institute SB RAS: Krasnoyarsk, Russia, 2012; pp. 139–143. (In Russian) [Google Scholar]
- Drake, C.J.; Ruhoff, F.A. Lacebugs of the World: A Catalog (Hemiptera: Tingidae). Bull. U.S. Natl. Mus. 1965, 243, 1–634. [Google Scholar] [CrossRef]
- Borisov, B.A.; Karpun, N.N.; Bibin, A.R.; Grabenko, Y.A.; Shiryaeva, N.V.; Lyanguzov, M.Y. New data on trophic relations of the invasive oak lace bug Corythucha arcuata (Heteroptera: Tingidae) in the Krasnodar region and in the Republic of Adygea based on the research findings for the year 2018. Subtrop. Ornam. Hortic. 2018, 67, 188–203. (In Russian) [Google Scholar] [CrossRef]
- Karpun, N.N. (Russian Research Institute of Floriculture and Subtropical Crops, Sochi, Russia). Personal communication, 2021.
- Paulin, M.; Hirka, A.; Eötvös, C.B.; Gáspár, C.; Fürjes-Mikó, Á.; Csóka, G. Known and predicted impacts of the invasive oak lace bug (Corythucha arcuata) in European oak ecosystems—A review. Folia Oecologica 2020, 47, 131–139. [Google Scholar] [CrossRef]
- Shchurov, V.I.; Zamotajlov, A.S.; Shchurova, A.V. Assessment of climatic conditions for the expansion of Corythucha arcuata (Say, 1832) (Heteroptera: Tingidae) in the European part of Russia. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 379–380. (In Russian) [Google Scholar]
- Shchurov, V.I.; Zamotajlov, A.S.; Bondarenko, A.S.; Shchurova, A.V.; Skvortsov, M.M.; Glushchenko, L.S. The oak lace bug Corythucha arcuata (Say, 1832) (Heteroptera: Tingidae) in the Northwestern Caucasus: Phenology, biology, monitoring of the territorial expansion, and harmfulness. Izv. St.-Peterbg. Lesoteh. Akad. 2019, 228, 58–87. (In Russian) [Google Scholar] [CrossRef]
- Gninenko, Y.I.; Chernova, U.A.; Nalepin, V.P. Oak lace bug: Stages of formation of the secondary range in Russia. In Innovations in the Conservation and Sustainable Development of Forest Ecosystems, Proceedings of International and Practical Conference Dedicated to the 20th Anniversary of State National Park “Burabay” Creation, Burabay, Kazakhstan, 2–5 September 2020; Bykov, S.V., Ed.; State National Park “Burabay”: Burabay, Kazakhstan, 2020; pp. 66–68. [Google Scholar]
- Kovač, M.; Gorczak, M.; Wrzosek, M.; Tkaczuk, C.; Pernek, M. Identification of entomopathogenic fungi as naturally occurring enemies of the invasive oak lace bug, Corythucha arcuata (Say) (Hemiptera: Tingidae). Insects 2020, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Borisov, B.A. (AgroBioTechnology Ltd., Moscow, Russia). Personal communication, 2021.
- Chernova, U.A.; Rakov, A.G.; Khegay, I.V. Mixed insecticide against oak lace bug. Plant Prot. Quar. 2019, 11, 51. Available online: https://www.elibrary.ru/download/elibrary_41239385_98490426.pdf (accessed on 10 January 2022). (In Russian).
- Bălăcenoiu, F.; Nețoiu, C.; Tomescu, R.; Simon, D.C.; Buzatu, A.; Toma, D.; Petrițan, I.C. Chemical control of Corythucha arcuata (Say, 1832), an invasive alien species, in oak forests. Forests 2021, 12, 770. [Google Scholar] [CrossRef]
- Besedina, E.N.; Ismailov, V.Y.; Nastasiy, A.S. Field evaluation of the effectiveness of biological and biorational insecticides against oak lace bug Corythucha arcuata Say (Hemiptera, Tingidae). Agrochemistry 2021, 3, 45–50. (In Russian) [Google Scholar] [CrossRef]
- EPPO. Pest Risk Analysis for Agrilus fleischeri. 2019. Available online: https://gd.eppo.int/taxon/AGRLFL/documents (accessed on 2 January 2022).
- Volkovitsh, M.G. On the invasive potential of buprestid beetles (Coleoptera: Buprestidae) damaging woody plants. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 113–116, (In Russian and English). [Google Scholar]
- Volkovitsh, M.G.; Kovalev, A.V.; Orlova-Bienkowskaja, M.J. Current distribution and diagnostic features of two potentially invasive Asian buprestid species: Agrilus mali Matsumura and A. fleischeri Obenberger (Coleoptera: Buprestidae). Insects 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- EPPO. A2 List of Pests Recommended for Regulation as Quarantine Pests (Version 2019-09). Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list (accessed on 15 August 2021).
- Obenberger, J. De novis Buprestidarum regionis palaearcticae speciebus V. Cas. Ceskoslovenske Spoiecnosti Entomol. 1925, 22, 30–34. [Google Scholar]
- Kubáň, V.; Jendek, E.; Kalashian, M.Y.; Volkovitsh, M.G. Superfamily Buprestoidea Leach, 1815. In Catalogue of Palaearctic Coleoptera. Scarabaeoidea, Scirtoidea, Dascilloidea, Buprestoidea and Byrrhoidea; Löbl, I., Löbl, D., Eds.; BRILL: Leiden, The Netherlands, 2016; Volume 3, pp. 19–32, 432–574. [Google Scholar]
- Zang, K.; Wang, X.-Y.; Yang, Z.-Q.; Wei, K.; Duan, J.J. Biology and natural enemies of Agrilus fleischeri (Coleoptera: Buprestidae), a newly emerging destructive buprestid pest in Northeast China. J. Asia-Pac. Entomol. 2017, 20, 47–52. [Google Scholar] [CrossRef]
- Wang, J.S.; Shang, P.H.; Li, F. Preliminary observations on the life history of Agrilus sp. Shaanxi For. Sci. Technol. 1995, 3, 40–42. [Google Scholar]
- Ji, Y.; Ji, R.; Huang, R.-X. Invasive species—Agrilus mali Matsumura and damage in Xinjiang. Xinjiang Agric. Sci. 2004, 41, 31–33. [Google Scholar]
- Cui, X.N.; Liu, D.G.; Li, A.H. Research progress in integrated management of Agrilus mali. Plant Prot. 2015, 41, 16–23. [Google Scholar]
- Bozorov, T.A.; Luo, Z.; Li, X.; Zhang, D. Agrilus mali Matsumura (Coleoptera: Buprestidae), a new invasive pest of wild apple in western China: DNA barcoding and life cycle. Ecol. Evol. 2018, 9, 1160–1172. [Google Scholar] [CrossRef]
- Cui, Z.-J.; Zhang, Y.-L.; Zhang, X.; Luo, Z.-H.; Zhang, P.; Golec, J.; Poland, T.M.; Zalucki, M.P.; Han, P.; Lu, Z.-Z. Life history and mortality factors of Agrilus mali Matsumura (Coleoptera: Buprestidae) in wild apples in Northwestern China. Agric. For. Entomol. 2019, 21, 309–317. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, Z.; Xu, H.; Ali, A.; Zhang, X.; Liu, X.; Zhang, Y.; Zhou, X.; Lu, Z. Thirst or malnutrition: The impacts of invasive insect Agrilus mali on the physiological status of wild apple trees. Insects 2020, 11, 440. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.-L.; Zhang, P.; Cui, Z.-J.; Han, P.; Gao, G.-Z.; Poland, T.M.; Zalucki, M.P.; Lu, Z.-Z. Agrilus mali Matsumura (Coleoptera: Buprestidae) density and damage in wild apple Malus sieversii (Rosales: Rosaceae) forests in Central Eurasia under four different management strategies. Entomol. Gen. 2021, 41, 257–266. [Google Scholar] [CrossRef]
- A Unified List of Quarantine Pests of the Eurasian Economic Union. (As Amended by 8 August 2019.) (Decision of the Council of the Eurasian Economic Commission of 8 August 2019 No. 74). Available online: https://vniikr.ru/edinyij-perechen-karantinnyix-obektov-evrazijskogo-ekonomicheskogo-soyuza (accessed on 22 August 2021).
- Agrilus mali. EPPO Global Database. 2021. Available online: https://gd.eppo.int/taxon/AGRLMA (accessed on 22 August 2021).
- Jendek, E.; Grebennikov, V. Agrilus (Coleoptera, Buprestidae) of East Asia; Jan Farkač: Prague, Czech Republic, 2011; p. 362. [Google Scholar]
- Matsumura, S. Life history of Agrilus mali Mats. (Buprestidae). In Bionomics on Apple Trees in China and Korea; Muramatsu, S., Ed.; Korea Agricultural Experiment Station: Seoul, Korea, 1924; pp. 1–21. (In Japanese) [Google Scholar]
- Sokolov, E.A.; Atanov, N.M.; Zhimerikin, V.N.; Gura, N.A.; Komarova, G.F.; Nikritin, L.M.; Shakhramanov, I.K. Quarantine pests with limited distribution on the territory of the Russian Federation. Plant Prot. 1995, 5, 37–41. (In Russian) [Google Scholar]
- Jendek, E.; Poláková, J. Host Plants of World Agrilus (Coleoptera, Buprestidae). A Critical Review; Springer: New York, NY, USA, 2014; pp. 1–706. [Google Scholar]
- Liu, Z.Q.; Chen, W.M.; Xu, Z.; Liang, Q.L. Malus sieversii forest distribution and Agrilus mali Matsumura status of damage in the west part of Tianshan Mountains. North. Hortic. 2014, 17, 121–124. [Google Scholar]
- Li, M.L.; Zhang, Z.Q. Discussion on biology and life history associated with Agrilus mali Matsumura. J. Northwest For. Univ. 2017, 32, 139–146. [Google Scholar]
- Nikritin, L.M.; Shutova, N.N. Apple buprestid. Plant Prot. 1985, 1, 44. (In Russian) [Google Scholar]
- Nikritin, L.M. Apple buprestid. Plant Prot. 1994, 3, 46. (In Russian) [Google Scholar]
- Cao, L.M.; Zhang, Y.L.; van Achterberg, C.; Wang, Z.Y.; Wang, X.Y.; Zhao, W.X.; Yang, Z.Q. Notes on braconid wasps (Hymenoptera, Braconidae) parasitising on Agrilus mali Matsumura (Coleoptera, Buprestidae) in China. ZooKeys 2019, 867, 97–121. [Google Scholar] [CrossRef]
- Fairmaire, L. Notes sur les Coléoptères des environs de Pékin (2e Partie). Revue Ent. Caen 1888, 7, 111–160. [Google Scholar]
- Chamorro, M.L.; Jendek, E.; Haack, R.A.; Petrice, T.R.; Woodley, N.E.; Konstantinov, A.S.; Volkovitsh, M.G.; Yang, X.K.; Grebennikov, V.V. Illustrated Guide to the Emerald Ash Borer, Agrilus planipennis Fairmaire and Related Species (Coleoptera, Buprestidae); Pensoft Publishers: Sofia, Bulgaria; Moscow, Russia, 2015; pp. 1–198. [Google Scholar]
- Baranchikov, Y.; Mozolevskaya, E.; Yurchenko, G.; Kenis, M. Occurrence of the emerald ash borer, Agrilus planipennis in Russia and its potential impact on European forestry. EPPO Bull. 2008, 38, 233–238. [Google Scholar] [CrossRef]
- Musolin, D.L.; Selikhovkin, A.V.; Shabunin, D.A.; Zviagintsev, V.B.; Baranchikov, Y.N. Between ash dieback and emerald ash borer: Two Asian invaders in Russia and the future of ash in Europe. Balt. For. 2017, 23, 316–333. [Google Scholar]
- Orlova-Bienkowskaja, M.J.; Volkovitsh, M.G. Are native ranges of the most destructive invasive pests well known? A case study of the native range of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Biol. Invasions 2018, 20, 1275–1286. [Google Scholar] [CrossRef]
- Meshkova, V.L.; Kucheryavenko, T.V.; Zinchenko, O.V.; Borysenko, A.I. Beginning of the spread of Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) on the territory of Ukraine. Izv. St.-Peterbg. Lesoteh. Akad. 2021, 236, 163–184. (In Russian) [Google Scholar] [CrossRef]
- Volkovitsh, M.G.; Bieńkowski, A.O.; Orlova-Bienkowskaja, M.J. Emerald ash borer approaches the borders of the European Union and Kazakhstan and is confirmed to infest European ash. Forests 2021, 12, 691. [Google Scholar] [CrossRef]
- Volkovitsh, M.G.; Orlova-Bienkowskaja, M.J.; Kovalev, A.V.; Bieńkowski, A.O. An illustrated guide to distinguish emerald ash borer (Agrilus planipennis) from its congeners in Europe. Forestry 2020, 93, 316–325. [Google Scholar] [CrossRef]
- Haack, R.A.; Jendek, E.; Liu, H.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The emerald ash borer: A new exotic pest in North America. Newsl. Mich. Entomol. Soc. 2002, 47, 1–5. Available online: https://www.researchgate.net/publication/228544207_The_Emerald_Ash_Borer_A_New_Exotic_Pest_in_North_America (accessed on 10 January 2022).
- Izhevskii, S.S. Threatening Findings of the Emerald Ash Borer Agrilus planipennis in the Moscow Region. 2007. Available online: http://www.zin.ru/Animalia/Coleoptera/rus/agrplaiz.htm (accessed on 6 July 2021). (In Russian).
- Volkovitsh, M.G. Emerald Ash Borer Agrilus planipennis—New Extremely Dangerous Pest of Ash in the European Part of Russia. 2007. Available online: http://www.zin.ru/Animalia/Coleoptera/rus/eab_2007.htm (accessed on 7 July 2021).
- Emerald Ash Borer Informative Network. Available online: http://www.emeraldashborer.info/ (accessed on 6 July 2021).
- Orlova-Bienkowskaja, M.J.; Drogvalenko, A.N.; Zabaluev, I.A.; Sazhnev, A.S.; Peregudova, E.Y.; Mazurov, S.G.; Komarov, E.V.; Struchaev, V.V.; Martynov, V.V.; Nikulina, T.V.; et al. Current range of Agrilus planipennis Fairmaire, an alien pest of ash trees, in European Russia and Ukraine. Ann. For. Sci. 2020, 77, 1–14. [Google Scholar] [CrossRef]
- Meshkova, V.L.; Skrylnik, Y.Y.; Terekhova, V.V.; Kucheryavenko, T.V. Emerald ash borer (Agrilus planipennis) in Kharkiv region. In Modern Problems of Forestry and Ecology and Ways of Their Solution, Proceedings of the International Scientific and Practical Conference “Faculty of Forestry and Ecology—20 years”, Zhytomyr, Ukraine, 7–8 October 2021; Skidan, O.V., Romanchuk, L.D., Vishnevskiy, A.V., Siruk, Y.V., Kratiuk, O.L., Zhitova, O.P., Andreeva, O.Y., Shvets, M.V., Ishuk, O.V., Eds.; Poliskiy National University: Zhytomyr, Ukraine, 2021; pp. 125–126. [Google Scholar]
- Musolin, D.L.; Selikhovkin, A.V.; Peregudova, E.Y.; Popovichev, B.G.; Mandelshtam, M.Y.; Baranchikov, Y.N.; Vasaitis, R. North-westward expansion of the invasive range of Emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) towards the EU: From Moscow to Saint Petersburg. Forests 2021, 12, 502. [Google Scholar] [CrossRef]
- Selikhovkin, A.V.; Musolin, D.L.; Popovichev, B.G.; Merkuryev, S.A.; Volkovitsh, M.G.; Vasaitis, R. Invasive populations of the Emerald ash borer Agrilus planipennis Fairmaire, 1888 (Coleoptera: Buprestidae) in Saint Petersburg, Russia: A hitchhiker? Insects 2022, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Volkovitsh, M.G.; Mozolevskaya, E.G. The tenth «anniversary» of the invasion of emerald ash borer Agrilus planipennis Fairm. (Coleoptera: Buprestidae) in Russia: Results and prospects. Izv. St.-Peterbg. Lesoteh. Akad. 2014, 207, 8–19. (In Russian) [Google Scholar]
- Baranchikov, Y.N.; Seraya, L.G.; Grinash, M.N. All European ash species are susceptible to emerald ash borer Agrilus planipennis Fairmaire (Coleoptera: Buprestidae)—A Far Eastern invader. Siberian Forest J. 2014, 6, 80–85. Available online: https://www.researchgate.net/publication/272822249_Baranchikov_Yu_N_Seraya_L_G_Grinash_M_N_All_European_ash_species_are_susceptible_to_emerald_ash_borer_Agrilus_planipennis_Fairmaire_Coleoptera_Buprestidae_-_a_Far_Eastern_invader_Sibirskiy_Lesnoy_Zurn (accessed on 10 January 2022). (In Russian).
- Volkovitsh, M.G. Buprestidae. In Inventory of Alien Beetles of European Russia; Orlova-Bienkowskaja, M.J., Ed.; Mukhametov, G.V. Publisher: Livny, Russia, 2019; pp. 84–95. (In Russian) [Google Scholar]
- Volkovitsh, M.G.; Suslov, D.V. The first record of the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), in St. Petersburg signals a real threat to the palace and park ensembles of Peterhof and Oranienbaum. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 119–122, (In Russian and English). [Google Scholar]
- Haubrock, P.J.; Turbelin, A.J.; Cuthbert, R.N.; Novoa, A.; Taylor, N.G.; Angulo, E.; Ballesteros-Mejia, L.; Bodey, T.W.; Capinha, C.; Diagne, C.; et al. Economic costs of invasive alien species across Europe. NeoBiota 2021, 67, 153–190. [Google Scholar] [CrossRef]
- Gninenko, Y.I.; Kliukin, M.S.; Khegai, I.V. Emerald ash borer: Catastrophe postponed? Plant Health Res. Pract. 2016, 3, 38–45. Available online: https://elibrary.ru/download/elibrary_28379702_35791451.pdf (accessed on 10 January 2022). (In Russian).
- Kirichenko, N.; Haubrock, P.J.; Cuthbert, R.N.; Akulov, E.; Karimova, E.; Shneider, Y.; Liu, C.; Angulo, E.; Diagne, C.; Courchamp, F. Economic costs of biological invasions in terrestrial ecosystems in Russia. NeoBiota 2021, 67, 103–130. [Google Scholar] [CrossRef]
- Commission Delegated Regulation (EU) 2019/1702 of 1 August 2019 Supplementing Regulation (EU) 2016/2031 of the European Parliament and of the Council by Establishing the List of Priority Pests. C/2019/5637. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1570788497660&uri=CELEX:32019R1702 (accessed on 16 January 2022).
- Orlova-Bienkowskaja, M.J.; Bienkowski, A.O. Low heat availability could limit the potential spread of the emerald ash borer to Northern Europe (Prognosis based on growing degree days per year). Insects 2022, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- EPPO. PM 9/14 (1) Agrilus planipennis: Procedures for official control. Bull. OEPP/EPPO Bull. 2013, 43, 499–509. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Strazanac, J.S.; Marsh, P.M.; Van Achterberg, C.; Choi, W.Y. First recorded parasitoid from China of Agrilus planipennis: A new species of Spathius (Hymenoptera: Braconidae: Doryctinae). Ann. Entomol. Soc. Am. 2005, 98, 636–642. [Google Scholar] [CrossRef]
- Orlova-Bienkowskaja, M.J.; Belokobylskij, S.A. Discovery of the first European parasitoid of the emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Eur. J. Entomol. 2014, 111, 594–596. [Google Scholar] [CrossRef]
- Bauer, L.S.; Duan, J.J.; Gould, J.R. Emerald ash borer (Agrilus planipennis Fairmaire) (Coleoptera: Buprestidae). In The Use of Classical Biological Control to Preserve Forests in North America; Van Driesche, R., Reardon, R., Eds.; FHTET-2013-02; U.S. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2014; pp. 189–209. Available online: https://www.fs.usda.gov/treesearch/pubs/48051 (accessed on 16 January 2022).
- Volkovitsh, M.G.; Zykov, I.E.; Karpun, N.N.; Zakharchenko, V.Y.; and Kovalev, A.V. A description of the larva of the cypress jewel beetle, Lamprodila (Palmar) festiva (L.), with notes on the larval characters of Poecilonotini and Dicercini (Coleoptera, Buprestidae). Entomol. Rev. 2020, 99, 1304–1317. [Google Scholar] [CrossRef]
- Volkovitsh, M.G.; Karpun, N.N. A new invasive species of Buprestid beetles in the Russian fauna: Lamprodila (Palmar) festiva (L.) (Coleoptera, Buprestidae), a pest of Cupressaceae. Entomol. Rev. 2017, 97, 425–437. [Google Scholar] [CrossRef]
- Karpun, N.N.; Gnezdilov, A.A. Why do Arborvitae and False Cypresses Dry Up on the Black Sea Coast? 2016. Available online: http://www.vniisubtrop.ru/novosti/731-pochemu-usykhayut-tui-i-kiparisoviki-na-cheromorskompoberezhe.html (accessed on 3 August 2021). (In Russian).
- Karpun, N.N.; Zhuravleva, Y.N.; Volkovitsh, M.G.; Procenko, V.Y.; Musolin, D.L. To the fauna and biology of new alien insect pest species of woody plants in humid subtropics of Russia. Izv. St.-Peterbg. Lesoteh. Akad. 2017, 220, 169–185. (In Russian) [Google Scholar] [CrossRef]
- Gubin, A.I.; Martynov, V.V.; Nikulina, T.V. The first record of Cypress jewel beetle Lamprodila (Palmar) festiva (Linnaeus, 1767) (Coleoptera, Buprestidae) in the Donbass. Subtrop. Ornam. Hortic. 2020, 75, 96–107. [Google Scholar] [CrossRef]
- Sinelnikov, K.Y. Cypress jewel beetle Lamprodila festiva (L.) on the Southern Coast of the Crimea (2019). Available online: https://vitusltd.ru/blog/lesozaschita/17147 (accessed on 3 August 2021). (In Russian).
- Skvortsov, M.M. The Cypress Jewel Beetle is a New Object of State Forest Pathology Monitoring in Krasnodar Territory. 2017. Available online: http://www.czl23.ru/print.php?news.221 (accessed on 3 August 2021). (In Russian).
- Zhuravleva, Y.N. (Research Centre the Subtropical Scientific Centre of RAS, Sochi, Russia). Personal communication, 2021.
- Wermelinger, B. Der Grüne Wacholder-Prachtkäfer. G’plus Gart.-Fachz. 2011, 3, 30. [Google Scholar]
- Razinger, J.; Zerjav, M.; Modic, S. Thuja occidentalis L. is commonly a host for Cypress jewel beetle (Ovalisia festiva L.) in Slovenia. In Zbornik Predavanj in Referatov 11. Slovenskega Posvetovanja o Varstvu Rastlin z Mednarodno Udeleibo, Proceedings of Conference, Bled, Ljubljana, Slovenia, 5–6 March 2013; Društvo za varstvo rastlin Slovenije: Ljubljana, Slovenia, 2013; pp. 359–365. Available online: http://www.dvrs.bf.uni-lj.si/spvr/2013/61Razinger.pdf (accessed on 18 January 2022).
- Schmidt, G.; Diószegi, M.S.; Szabó, V.; Hrotkó, K. Cypress borer (Lamprodila festiva), a new urban pest in Hungary. In Plants in Urban Areas and Landscape, Proceedings of International Symposium, Nitra, Slovakia, 14–15 May 2014; Slovak University of Agriculture: Nitra, Slovakia, 2014; pp. 32–34. [Google Scholar]
- Thoma, J.; Eickermann, M. Erstauftreten des Wacholderprachtkafers Ovalisia festiva (Linnaeus, 1767) in Luxemburg. Bull. Soc. Nat. Luxemb. 2014, 115, 227–229. [Google Scholar]
- Nitzu, E.; Dobrin, I.; Dumbravă, M.; Gutue, M. The range expansion of Ovalisia festiva (Linnaeus, 1767) (Coleoptera: Buprestidae) in Eastern Europe and its damaging potential for Cupressaceae. Trav. Muséum Natl. Hist. Nat. 2016, 58, 51–57. [Google Scholar] [CrossRef]
- Ruseva, S.; Todorov, I.; Pencheva, A. New data on Ovalisia (Palmar) festiva (Linnaeus) (Coleoptera: Buprestidae) and its natural enemies reported from Bulgaria. Ecol. Montenegrina 2020, 28, 53–60. Available online: https://www.researchgate.net/publication/339539961_New_data_on_Ovalisia_Palmar_festiva_Linnaeus_Coleoptera_Buprestidae_and_its_natural_enemies_reported_from_Bulgaria (accessed on 18 January 2022). [CrossRef]
- Karpun, N.N.; Volkovitsh, M.G. Cypress jewel-beetle Lamprodila (Palmar) festiva (L.) (Coleoptera: Buprestidae)—A new invasive pest on the Black Sea coast of the Caucasus. In The Kataev Memorial Readings–IX. Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems, Proceedings of the International Conference, St. Petersburg, Russia, 23–25 November 2016; Musolin, D.L., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2016; pp. 45–46. Available online: https://www.researchgate.net/publication/310832354_Cypress_jewel-beetle_Lamprodila_Palmar_festiva_L_Coleoptera_Buprestidae_-_a_new_invasive_pest_on_the_Black_Sea_Coast_of_the_Caucasus_in_Russian (accessed on 18 January 2022). (In Russian)
- Volkovitsh, M.G.; Karpun, N.N. Lamprodila (Palmar) festiva (L.), a New Invasive Pest of Cupressaceae in the Russian Fauna (Coleoptera: Buprestidae: Poecilonotini). 2016. Available online: http://www.zin.ru/animalia/coleoptera/pdf/Volkovitsh_Karpun_2016_Lamprodila_festiva.pdf (accessed on 5 August 2021). (In Russian).
- Pfeffer, A. Zentral- und Westpaläarktishe Borken- und Kernkäfer (Coleoptera: Scolytidae, Platypodidae). Entomol. Basiliensia 1994, 17, 5–310. [Google Scholar]
- Knížek, M. Curculionidae: Scolytinae, In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2011; Volume 7, Curculionoidea I; pp. 86–88, 204–251. [Google Scholar]
- Økland, B.; Flø, D.; Schroeder, M.; Zach, P.; Cocos, D.; Martikainen, P.; Siitonen, J.; Mandelshtam, M.Y.; Musolin, D.L.; Neuvonen, S.; et al. Range expansion of the small spruce bark beetle Ips amitinus: A newcomer in northern Europe. Agric. For. Entomol. 2019, 21, 286–298. [Google Scholar] [CrossRef]
- Mandelshtam, M.Y.; Selikhovkin, A.V. Bark and ambrosia beetles (Coleoptera, Curculionidae: Scolytinae) of Northwest Russia: History of the study, composition and genesis of the fauna. Entomol. Rev. 2020, 100, 800–826. [Google Scholar] [CrossRef]
- Mandelshtam, M.Y.; Selikhovkin, A.V. Comparative characteristics of species richness of the bark beetles (Coleoptera: Curculionidae, Scolytinae) fauna in north-west subregions of Russia. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 214–215. (In Russian) [Google Scholar]
- Mandelshtam, M.Y.; Musolin, D.L.; Økland, B.; Flø, D.; Schroeder, M.; Zach, P.; Cocos, D.; Martikainen, P.; Siitonen, J.; Neuvonen, S.; et al. Range expansion of the small spruce bark beetle Ips amitinus (Coleoptera, Curculionidae: Scolytinae) in Northern Europe and Western Siberia. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 211–212. (In Russian) [Google Scholar]
- Mikutowicz, J.M. Zur Koleopterenfauna der Ostseeprovinzen Russlands. Korresp.-Bl. Des Nat.-Ver. Zu Riga 1905, 48, 73–92. [Google Scholar]
- Leius, K. Taiendavaid andmeid kodumaa urasklaste (Ipidae) fauna kohta. Erganzende Angaben uber die Borkenkafer-Fauna (Ipidae) in Estland (Zusammenfassung). Eest. Metsanduse Aastaraam. (Estländisches Forstwirtsch. Jahrb. Tartu) 1939, 9, 317–328. (In Estonian) [Google Scholar]
- Voolma, K. (Estonian University of Life Sciences, Tartu, Estonia). Personal communication, 2021.
- Zolk, K. Kodumaa ürasklased (Ipidae) ühes lühikese ülevaatega nende bionoomiast ja levimisest Eestis [Die Borkenkäfer (Ipidae) Estlands mit kurzer Berücksichtigung ihrer Bionomie und Verbreitung]. Eest. Metsanduse Aastaraam. (Estländisches Forstwirtsch. Jahrb. Tartu) 1932, 6, 127–176. (In Estonian) [Google Scholar]
- Zolk, K. Metsakahjurite esinemine Eestis 1934 [Das Vorkommen der Forstschädlinge in Estland im Jahre 1934]. Eest. Metsanduse Aastaraam. (Estländisches Forstwirtsch. Jahrb. Tartu) 1935, 7, 614–638. (In Estonian) [Google Scholar]
- Nuorteva, M. Fennoskandialle uusi kaarnakuoriaislaji Ips amitinus Eichh. tavattu Suomesta [Ips amitinus Eichh. (Col., Scolytidae) neu für Fennoscandien]. Ann. Entomol. Fenn. 1955, 21, 30–32. [Google Scholar]
- Koponen, M. Distribution of Ips amitinus Eichh. (Coleoptera, Scolytidae) in Finland in 1950–1973. Ann. Entomol. Fenn. 1975, 41, 65–69. [Google Scholar]
- Koponen, M. Distribution of Ips amitinus Eichh. (Coleoptera, Scolytidae) in Finland in 1974–1979. Not. Entomol. 1980, 60, 223–225. [Google Scholar]
- Muona, J.; Viramo, J. The Coleoptera of the Koillismaa area (Ks), North-East Finland. Oulanka Rep. 1986, 6, 3–50. [Google Scholar]
- Hansen, V. Catalogus Coleopterorum Fennoscandiae et Daniae; Lindroth, C.H., Ed.; Entomologiska Sällskapet i Lund: Lund, Sweden, 1960; pp. 1–476. [Google Scholar]
- Lindelöw, Å. Väntad barkborre funnen i Sverige-fynd av Ips amitinus (Coleoptera, Scolytinae) [Ips amitinus (Coleoptera, Scolytinae) expected and found in Sweden]. Entomol. Tidskr. 2013, 134, 203–206. [Google Scholar]
- Stark, V.N. Materials to knowledge of bark beetles of Briansk Gouvernement. Zashchita Rasteniy [Plant Protection]. 1926, III, 330–339. (In Russian) [Google Scholar]
- Mandelshtam, M. Notes on the current status of Ips amitinus Eichh. (Coleoptera, Scolytidae) in North-West Russia. Entomol. Fenn. 1999, 10, 29–34. [Google Scholar] [CrossRef][Green Version]
- Voolma, K.; Mandelshtam, M.Y.; Shcherbakov, A.N.; Yakovlev, E.B.; Õunap, H.; Süda, I.; Popovichev, B.G.; Sharapa, T.V.; Galasjeva, T.V.; Khairetdinov, R.R.; et al. Distribution and spread of bark beetles (Coleoptera: Scolytidae) around the Gulf of Finland: A comparative study with notes on rare species of Estonia, Finland and North-Western Russia. Entomol. Fenn. 2004, 15, 198–210. [Google Scholar] [CrossRef]
- Titova, E.V. Bark beetles associated with coniferous coppice in forest clearings of Karelia. In Studies of Forest Regeneration in Karelia, Proceedings of the Karelian Branch of the USSR Academy of Sciences, Petrozavodsk, Russia, 1959; Shiperovich, V.Y., Kishenko, T.I., Lisenkov, A.F., Eds.; Karelian Branch of the USSR Academy of Sciences: Petrozavodsk, Russia, 1959; Volume 16, pp. 110–126. (In Russian) [Google Scholar]
- Yakovlev, E.B.; Shorokhov, V.V.; Gorbunova, V.N. Materials on the fauna of xylophagous beetles of Karelia. In The Fauna and Ecology of Arthropods in Karelia; Yakovlev, E.B., Uzenbaev, S.D., Eds.; Forest Institute: Petrozavodsk, Russia, 1986; pp. 40–43. (In Russian) [Google Scholar]
- Mozolevskaya, E.G.; Sharapa, T.V. Species composition of xylophagous insects of Murmansk Province. Entomol. Obozr. 1996, 75, 558–566. (In Russian) [Google Scholar]
- Shcherbakov, A.N.; Nikitsky, N.B.; Polevoi, A.V.; Humala, A.E. On the fauna of beetles (Insecta, Coleoptera) of Pasvik Nature Reserve. Lesn. Vestn. 2013, 6, 16–21. (In Russian) [Google Scholar]
- Mandelshtam, M.Y.; Musolin, D.L. Bark beetle Ips amitinus (Eichhoff, 1872) (Coleoptera: Curculionidae: Scolytinae) continues to expand its range in north-western and northern Russia. In Monitoring and Biological Control Methods of Woody Plant Pests and Pathogens: From Theory to Practice, Proceedings of International Conference, Moscow, Russia, 18–22 April 2016; Baranchikov, Y.N., Ed.; SIF SN RASc.: Moscow, Russia; Krasnoyarsk, Russia, 2016; pp. 129–130. (In Russian) [Google Scholar]
- Mandelshtam, M.Y.; Khairetdinov, R.R. Additions to the check-list of bark beetles (Coleoptera, Curculionidae: Scolytinae) from Leningrad province, Russia. Entomol. Rev. 2017, 97, 893–899. [Google Scholar] [CrossRef]
- Kerchev, I.A.; Mandelshtam, M.Y.; Krivets, S.A.; Ilinsky, Y.Y. Small spruce bark beetle Ips amitinus (Eichhoff, 1872) (Coleoptera, Curculionidae: Scolytinae): A new alien species in Western Siberia. Entomol. Rev. 2019, 99, 639–644. [Google Scholar] [CrossRef]
- Skorokhodov, S.N. Save the neighboring stone pine woods. Yashkinsky Vestn. 2017, 37, 8564. (In Russian) [Google Scholar]
- Krivets, S.A. Problems of protection of Siberian stone pine forests from the small spruce bark beetle Ips amitinus (Coleoptera: Curculionidae, Scolytinae). In Dendrobiotic Invertebrates and Fungi and Their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 183–184. (In Russian) [Google Scholar]
- Kerchev, I.A.; Krivets, S.A.; Smirnov, N.A. Analysis of the population characteristics of the small spruce bark beetle Ips amitinus (Coleoptera: Curculionidae: Scolytinae) in the areas of invasion in Western Siberia. In Dendrobiotic Invertebrates and Fungi and Their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 170–171. (In Russian) [Google Scholar]
- Kerchev, I.A.; Krivets, S.A. An attack of Ips amitinus (Coleoptera: Curculionidae: Scolytinae) on arboretum in West Siberia: New host of invasive bark beetle among exotic conifers. J. Asia-Pac. Entomol. 2021, 24, 148–152. [Google Scholar] [CrossRef]
- Kerchev, I.A.; Krivets, S.A.; Bisirova, E.M.; Smirnov, N.A. Distribution of the small spruce bark beetle Ips amitinus (Eichhoff, 1872) in the Western Siberia. Russ. J. Biol. Invasions 2021, 4, 77–84. (In Russian) [Google Scholar] [CrossRef]
- Holuša, J.; Lukasova, K.; Grodzki, W.; Kula, E.; Matousek, P. Is Ips amitinus (Coleoptera: Curculionidae) abundant in wide range of altitudes? Acta Zool. Bulg. 2012, 64, 219–228. Available online: https://www.researchgate.net/publication/236260984_Is_Ips_amitinus_Coleoptera_Curculionidae_Abundant_in_Wide_Range_of_Altitudes (accessed on 18 January 2022).
- Bisirova, E.M.; Kerchev, I.A. Assessment of the state of the Siberian stone pine Pinus sibirica in the outbreak foci of the small spruce bark beetle Ips amitinus (Coleoptera: Curculionidae, Scolytinae) a new invasive pest in Siberia. In Dendrobiotic Invertebrates and Fungi and Their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 82–83. (In Russian) [Google Scholar]
- Baranchikov, Y.N.; Krivets, S.A. About professional skills in determination of insects: How the occurrence of new aggressive invasive fir pest in Siberia was missed. Ecol. South. Sib. Adjac. Territ. 2010, 1, 50–52. (In Russian) [Google Scholar]
- Baranchikov, Y.N.; Pet’ko, V.M.; Astapenko, S.A.; Akulov, E.N.; Krivets, S.A. Four-eyed fir bark beetle as a new aggressive pest of fir in Siberia. Lesn. Vestn. 2011, 80, 78–81. (In Russian) [Google Scholar]
- Kurentsov, A.I. The Bark Beetles of the USSR Far East; Academy of Sciences of the USSR: Moscow, Russia; St. Petersburg, Russia, 1941; pp. 1–234. (In Russian) [Google Scholar]
- Krivolutskaya, G.O. Fam. Scolytidae—The bark beetles. In Key for Identification of Insects of the Russian Far East; Ler, P.A., Ed.; Dal’nauka: Vladivostok, Russia, 1996; Volume 3, Part 3. Coleoptera, pp. 312–373. (In Russian) [Google Scholar]
- Kerchev, I.A. Ecology of four-eyed fir bark beetle Polygraphus proximus Blandford (Coleoptera: Curculionidae, Scolytinae) in the West Siberian region of invasion. Russ. J. Biol. Invasion 2014, 5, 176–185. [Google Scholar] [CrossRef]
- Chilakhsaeva, E.A. First record of Polygraphus proximus (Coleoptera: Scolytidae) in Moscow Province. Bull. Mosc. Soc. Nat. Biol. 2008, 113, 39–42. (In Russian) [Google Scholar]
- Baranchikov, Y.N.; Seraya, L.G. For whom the bell tolls: Evaluation of potential invasive organisms and their role in regional biota. In Monitoring and Biological Control Methods of Woody Plant Pests and Pathogens: From Theory to Practice. Proceedings of International Conference, Moscow, Russia, 18–22 April 2016; Baranchikov, Y.N., Ed.; SIF SN RASc.: Moscow, Russia; Krasnoyarsk, Russia, 2016; pp. 25–26. (In Russian) [Google Scholar]
- Mandelshtam, M.Y.; Popovichev, B.G. Annotated list of bark beetles (Coleoptera, Scolytidae) of Leningrad Province. Entomol. Rev. 2000, 80, 887–903. [Google Scholar]
- Baranchikov, Y.N.; Efremenko, A.A.; Demidko, D.A.; Titova, V.V. Four-eyed fir bark beetle Polygraphus proximus Blandford (Coleoptera: Curculionidae, Scolytinae) in the western piedmont of the Ural Mountains: Where, whence and when? In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 74–75. (In Russian) [Google Scholar]
- Dedyukhin, S.V.; Titova, V.V. Finding of the bark beetle Polygraphus proximus Blandford, 1894 (Coleoptera, Curculionidae: Scolytinae) in Udmurtia. Russ. J. Biol. Invasions 2021, 12, 258–263. [Google Scholar] [CrossRef]
- Krivets, S.A.; Kerchev, I.A.; Kizeev, Y.M.; Kozhurin, M.A.; Kozak, R.G.; Filimonov, M.N.; Chemodanov, A.V.; Chugin, B.S. Four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera, Scolytidae) in fir forests of Tomsk Oblast. In Diseases and Pests in the Forests of Russia: XXI Century, Proceedings of the All-Russian Conference and V Annual Kataev Memorial Reading, Yekaterinburg, Russia, 20–25 September 2011; Selikhovkin, A.V., Shavnin, S.A., Zalesov, S.V., Onuchin, A.A., Isaev, A.S., Mozolevskaya, E.G., Ponomarev, V.I., Baranchikov, Y.N., Petrova, I.V., Meshkova, V.L., et al., Eds.; Institute of Forest, SB RAS: Krasnoyarsk, Russia, 2011; pp. 53–55. (In Russian) [Google Scholar]
- Krivets, S.A.; Bisirova, E.M.; Kerchev, I.A.; Pashenova, N.V.; Demidko, D.A.; Petko, V.M.; Baranchikov, Y.N. Four-eyed Fir Bark Beetle in Siberian Forests (Distribution, Biology, Ecology, Detection and Survey of Damaged Stands). Manual; Umium Publisher: Tomsk, Russia; Krasnoyarsk, Russia, 2015; pp. 1–48. (In Russian) [Google Scholar]
- Krivets, S.A.; Bisirova, E.M.; Kerchev, I.A.; Pats, E.N.; Chernova, N.A. Transformation of taiga ecosystems in the Western Siberian invasion focus of four-eyed fir bark beetle Polygraphus proximus Blandford (Coleoptera: Curculionidae, Scolytinae). Russ. J. Biol. Invasions 2015, 6, 94–108. [Google Scholar] [CrossRef]
- Krivets, S.A.; Kerchev, I.A.; Bisirova, E.M.; Demidko, D.A.; Pet’ko, V.M.; Baranchikov, Y.N. Distribution of the four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera, Curculionidae: Scolytinae) in Siberia. Izv. St.-Peterbg. Lesoteh. Akad. 2015, 211, 33–45. (In Russian) [Google Scholar]
- Krivets, S.A.; Kerchev, I.A.; Bisirova, E.M.; Debkov, N.M. Current distribution and forecasted invasive area expansion of the four-eyed fir bark beetle Polygraphus proximus Blandford, 1894 in Tomsakaya Oblast’ (Western Siberia). Eurasian Entomol. J. 2018, 17, 53–60. (In Russian) [Google Scholar]
- Bystrov, S.O.; Antonov, I.A. First record of the four-eyed beetle Polygraphus proximus Blandford, 1894 (Coleoptera: Curculionidae, Scolytinae) from Irkutsk Province, Russia. Entomol. Rev. 2019, 98, 54–55. [Google Scholar] [CrossRef]
- Baranchikov, Y.N.; Demidko, D.A.; Laptev, A.B.; Pet’ko, V.M. The dynamics of Siberian fir death in the outbreak focus of four-eyed fir bark beetle. Lesn. Vestn. 2014, 18, 78–81. (In Russian) [Google Scholar]
- Demidko, D.A. The dates of invasion of four-eyed fir bark beetle Polygraphus proximus Blandford (Coleoptera: Curculionidae, Scolytinae) in Tomsk Oblast. Izv. St.-Peterbg. Lesoteh. Akad. 2014, 207, 225–234. Available online: https://www.researchgate.net/publication/273321170_Dating_of_Four-Eyed_Fir_Bark_Beetle_Polygraphus_proximus_Blandford_Coleoptera_Curculionidae_Scolytinae_Invasion_into_Tomsk_Region (accessed on 18 January 2022). (In Russian).
- Bykov, R.; Kerchev, I.; Demenkova, M.; Ryabinin, A.; Ilinsky, Y. Sex-specific Woldbachia infection patterns in populations of Polygraphus proximus Blandford (Coleoptera: Curculionidae: Scolytinae). Insects 2020, 11, 547. [Google Scholar] [CrossRef]
- Kononov, A.; Ustyantsev, K.; Blinov, A.; Fet, V.; Baranchikov, Y.N. Genetic diversity of aboriginal and invasive populations of four-eyed fir bark beetle Polygraphus proximus Blandford (Coleoptera, Curculionidae, Scolytinae). Agric. For. Entomol. 2016, 18, 294–301. [Google Scholar] [CrossRef]
- Kerchev, I.A. Experimental study of the possibility of the emergence of new trophic connections of four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera: Curculionidae, Scolytinae) in the West Siberian region. Vestn. Tomsk. Gos. Univ. Biol. (Tomsk State Univ. J. Biol.) 2012, 3, 169–177. (In Russian) [Google Scholar]
- Pashenova, N.V.; Kononov, A.V.; Ustyantsev, K.V.; Blinov, A.G.; Pertsovaya, A.A.; Baranchikov, Y.N. Ophiostomatoid fungi associated with the four-eyed fir bark beetle on the territory of Russia. Russ. J. Biol. Invasion 2018, 9, 63–74. [Google Scholar] [CrossRef]
- Pashenova, N.V.; Petko, V.M.; Babichev, N.S.; Kerchev, I.A. Transfer of ophiostomatoid fungi by a four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera, Scolytidae) in Siberia. Izv. St.-Peterbg. Lesoteh. Akad. 2012, 210, 114–120. Available online: https://www.researchgate.net/publication/286452586_Transfer_of_Ophiostomatales_fungi_by_Polygraphus_proximus_Blandford_Coleoptera_Sco_lytidae_in_Sibera (accessed on 20 January 2022). (In Russian).
- Pashenova, N.V.; Demidko, D.A.; Pertsovaya, A.A.; Baranchikov, Y.N. Phloem sensibility of Siberian conifers towards Grosmannia aoshimae (Ohtaka, Masuya & Yamaoka) Masuya & Yamaoka, a symbiotic fungus of the four-eyed fir bark beetle. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 247–248. (In Russian) [Google Scholar]
- Bisirova, E.M.; Krivets, S.A. Dynamics of the state of Siberian fir tree stands damaged by the four-eyed fir bark beetle Polygraphus proximus Blandf. in Tomsk Oblast. Vestn. Tomsk. Gos. Univ. Biol. (Tomsk State Univ. J. Biol.) 2018, 44, 118–140. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Mukhortova, L.V.; Sergeeva, O.V.; Demidko, D.A.; Krivobokov, L.V.; Baranchikov, Y.N. Dynamics of coarse woody debris stocks in the fir forests damaged by the bark beetle Polygraphus proximus Blandf. (Coleoptera: Curculionidae, Scolytinae). In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 231–232. (In Russian) [Google Scholar]
- Kerchev, I.A.; Krivets, S.A. Composition and number of xylophagous consort’s members of Siberian fir associated with four-eyed fit bark beetle in Tomsk Oblast. In Environmental and Economic Consequences of Invasions of Dendrophilous Insects, Proceedings of All-Russian Conference with International Participation, Krasnoyarsk, Russia, 25–27 September 2012; Baranchikov, Y.N., Ed.; Institute of Forest, SB RAS: Krasnoyarsk, Russia, 2012; pp. 57–59. (In Russian) [Google Scholar]
- Bisirova, E.M.; Kerchev, I.A.; Krivets, S.A.; Pats, E.N. The state of fir forests of Tashtagol District of Kemerovo Province, Russia, damaged by the four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera: Curculionidae, Scolytinae). In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 84–85. (In Russian) [Google Scholar]
- Krivets, S.A.; Kerchev, I.A. Insects inhabiting the galleries of the four-eyed fir bark beetle Polygraphus proximus Blandf. (Coleoptera, Curculionidae: Scolytinae) in Siberia. Entomol. Rev. 2016, 96, 545–558. [Google Scholar] [CrossRef]
- Krivosheina, M.G.; Krivosheina, N.P.; Kerchev, I.A. Flies (Diptera) associated with Polygraphus proximus Blandford, 1894 (Coleoptera, Curculionidae) in Siberia and the Russian Far East. Entomol. Rev. 2018, 98, 156–164. [Google Scholar] [CrossRef]
- Kerchev, I.A.; Kryukov, V.Y.; Yaroslavtseva, O.N.; Polovinko, G.P.; Tokarev, Y.S.; Glupov, V.V. The first data on fungal pathogens (Ascomycota, Hypocreales) in the invasive populations of four-eyed fir bark beetle Polygraphus proximus Blandf. Russ. J. Biol. Invasions 2017, 8, 34–40. [Google Scholar] [CrossRef]
- Efremenko, A.A.; Demidko, D.A.; Baranchikov, Y.N. An old remedy for a new problem: Submersion of logs to prevent adult emergence of the invasive bark beetle Polygraphus proximus Blandford (Coleoptera: Curculionidae, Scolytinae). In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; p. 159. (In Russian) [Google Scholar]
- Seraya, L.G.; Pashenova, N.V.; Demidko, D.A.; Kozhenkova, A.A.; Efremenko, A.A.; Gninenko, Y.I.; Baranchikov, Y.N. Attempts of chemical control of invasive populations of the bark beetle Polygraphus proximus (Coleoptera: Curculionidae). In The Kataev Memorial Readings—X, Proceedings of the International Conference, St. Petersburg, Russia, 22–25 October 2018; Musolin, D.L., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2018; Volume 1, pp. 97–98. (In Russian) [Google Scholar]
- Xiang, Q.P.; Wei, R.; Shao, Y.Z.; Yang, Z.Y.; Wang, X.Q.; Zhang, X.C. Phylogenetic relationships, possible ancient hybridization, and biogeographic history of Abies (Pinaceae) based on data from nuclear, plastid, and mitochondrial genomes. Mol. Phylogenet. Evol. 2015, 82, 1–14. [Google Scholar] [CrossRef]
- Kirpichnikova, V.A. Pyralidae. In Key for Identification of Insects of the Russian Far East; Ler, P.A., Ed.; Dal’nauka: Vladivostok, Russia, 2005; Volume 5, Part 2, pp. 526–539. (In Russian) [Google Scholar]
- Mally, R.; Nuss, M. Phylogeny and nomenclature of the box tree moth, Cydalima perspectalis (Walker, 1859) comb. n., which was recently introduced into Europe (Lepidoptera: Pyraloidea: Crambidae: Spilomelinae). Eur. J. Entomol. 2010, 107, 393–400. [Google Scholar] [CrossRef]
- Kruger, E.O. Glyphodes perspectalis (Walker, 1859)—Neu für die Fauna Europas (Lepidoptera: Crambidae). Entomol. Z. 2008, 118, 81–83. [Google Scholar]
- USDA Confirms Box Tree Moth and Takes Action to Contain and Eradicate the Pest. 2021. Available online: https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2021/sa-05/box-tree-moth (accessed on 2 January 2022).
- Gninenko, Y.I.; Shiryaeva, N.V.; Shchurov, V.I. Box tree moth—A new invasive organism in the forests of the Russian Caucasus. Plant Quar. Sci. Prac. 2014, 7, 32–36. Available online: https://elibrary.ru/download/elibrary_25051637_65094735.pdf (accessed on 18 January 2022).
- Karpun, N.N.; Ignatova, Y.A. The first report about Cydalima perspectalis Walker on Black Sea Coast of Russia. In Zprávy Vědecké Ideje—2013 (19), Proceedings of the IX International Scientific and Practical Conference, Prague, Czech Republic, 27 October—5 November 2013; Publishing House “Education and Science” s.r.o.: Prague, Czech Republic, 2013; pp. 29–32. Available online: http://www.rusnauka.com/31_NNM_2013/Biologia/7_146134.doc.htm (accessed on 18 January 2022).
- Proklov, V.V.; Karayeva, S.Z. New and interesting Lepidoptera records from Chechen Republic (Russia). Cauc. Entomol. Bull. 2013, 9, 281–282. Available online: https://www.ssc-ras.ru/files/files/11_%20Proklov.pdf (accessed on 18 January 2022). [CrossRef]
- Karpun, N.N.; Ignatova, Y.A.; Zhuravleva, E.N. New species of harmful entomofauna on ornamental woody plants in humid subtropics of Krasnodar region. Izv. St.-Peterbg. Lesoteh. Akad. 2015, 211, 187–203. Available online: https://elibrary.ru/download/elibrary_23815865_37414681.pdf (accessed on 18 January 2022). (In Russian).
- Shchurov, V.I.; Kuchmistaya, E.V.; Vibe, E.N.; Bondarenko, A.S.; Skvortsov, M.M. The box-tree moth Cydalima perspectalis (Walker, 1859)—The real threat to biological diversity of natural forests of the Northwest Caucasus. Proc. Kuban State Agrar. Univ. 2015, 53, 178–198. (In Russian) [Google Scholar]
- Balykina, E.B.; Trikoz, N.N. Changes in the taxonomic structure of the ornamental plants phytophages complex in Crimean parks. Veles 2017, 43, 59–63. [Google Scholar]
- Dobronosov, V.V. New Data on the Box Tree Moth Cydalima perspectalis (Walker, 1859) in the Central Caucasus. 2017. Available online: http://aeconomy.ru/news/agro/novye-dannye-o-samshitovoy-ognevke-.html (accessed on 2 January 2022). (In Russian).
- Poltavskiy, A.N. Annotated catalog of insect pests of introduced plants in the Botanical Garden of the Southern Federal University. Proc. Bot. Gard. South. Fed. Univ. 2017, 2, 120–138. (In Russian) [Google Scholar]
- Tuniyev, B.S.; Aliev, K.U.; Timukhin, I.N. Colchis boxwood and the new pest Cydalima perspectalis Walker, 1859 (Lepidoptera, Crambidae) in South Ossetia. Bot. Her. North Cauc. 2018, 1, 30–36. Available online: http://gorbotsad.ru/files/Tuniev_2018_1.pdf (accessed on 18 January 2022). (In Russian).
- Il’ina, E.V.; Gasanova, N.M.-S. New Information about Invasive Insects in the Republic of Dagestan. Plant Prot. Quar. 2020, 4, 39–40. Available online: https://elibrary.ru/download/elibrary_42673425_76973702.pdf (accessed on 18 January 2022). (In Russian).
- Rozhina, V.I.; Volodina, L.S.; Drotikova, A.V. First detection of Cydalima perspectalis (Walker, 1859) (Lepidoptera: Crambidae: Pyraustinae) in Kaliningrad region. In ChemBioSeasons, Proceedings of the Abstracts of the Forum of Young Researchers, Kaliningrad, Russia, 23 April 2021; Baltic Federal University Named after I. Kant: Kaliningrad, Russia, 2021; pp. 67–68. Available online: https://elibrary.ru/item.asp?id=46359179 (accessed on 4 January 2022).
- Cydalima perspectalis. EPPO Global Database. 2021. Available online: https://gd.eppo.int/taxon/DPHNPE (accessed on 1 July 2021).
- Karpun, N.N.; Trochov, E.S.; Ignatova, Y.A.; Zhuravleva, E.N.; Kaurova, Z.G. Analysis of the food specialization of the box tree moth (Cydalima perspectalis Walker). Regul. Issues Vet. Med. 2015, 4, 173–176. Available online: https://elibrary.ru/item.asp?id=24825731 (accessed on 18 January 2022).
- Kawazu, K.; Honda, H.; Nakamura, S.; Adati, T. Identification of sex pheromone components of the box tree pyralid, Glyphodes perspectalis. J. Chem. Ecol. 2007, 33, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, I.-K. Female sex pheromone components of the box tree pyralid, Glyphodes perspectalis, in Korea: Field test and development of film-type lure. J. Asia-Pacific Entomol. 2013, 16, 473–477. [Google Scholar] [CrossRef]
- Nesterenkova, A.E.; Ponomarev, V.L.; Karpun, N.N.; Protsenko, V.E.; Glebov, V.E.; Danilenko, E.A.; Rastegaeva, V.M. Field testing of biological activity of pheromone box tree moth Cydalima perspectalis Walker. Lesn. Vestn. 2017, 21, 70–77. (In Russian) [Google Scholar] [CrossRef]
- Göttig, S.; Herz, A. The box tree pyralid Cydalima perspectalis: New results of the use of biological control agents and pheromone traps in the field. J. Plant Dis. Prot. 2014, 121, 98–99. [Google Scholar]
- Göttig, S.; Herz, A. Susceptibility of the box tree pyralid Cydalima perspectalis Walker (Lepidoptera: Crambidae) to potential biological control agents Neem (NeemAzal®-T/S) and entomopathogenic nematodes (Nemastar®) assessed in laboratory bioassays and field trials. J. Plant Dis. Prot. 2018, 125, 365–375. [Google Scholar] [CrossRef]
- Borisov, B.A.; Karpun, N.N.; Zhuravleva, E.N.; Borisova, I.P. Evaluation of the possibility of biological control of box tree moth (Cydalima perspectalis) by entomoparasitic fungi. In Monitoring and Biological Control Methods of Woody Plant Pests and Pathogens: From Theory to Practice, Proceedings of International Conference, Moscow, Russia, 18–22 April 2016; Baranchikov, Y.N., Ed.; SIF SN RASc.: Moscow, Russia; Krasnoyarsk, Russia, 2016; pp. 41–43. (In Russian) [Google Scholar]
- Kenis, M.; Nacambo, S.; Leuthardt, F.L.G.; Di Domenico, F.; Haye, T. The box tree moth, Cydalima perspectalis, in Europe: Horticultural pest or environmental disaster? Aliens 2013, 33, 38–41. Available online: https://www.researchgate.net/publication/271483672_The_box_tree_moth_Cydalima_perspectalis_in_Europe_horticultural_pest_or_environmental_disaster (accessed on 18 January 2022).
- Agasieva, I.S.; Ismailov, V.Y.; Fedorenko, E.V.; Nefedova, M.V. Biological control of box tree moth. Plant Prot. Quar. 2017, 8, 21–23. Available online: https://elibrary.ru/download/elibrary_29879952_31932504.pdf (accessed on 18 January 2022).
- Fora, C.G.; Sasu, L.; Poşta, D.; Berar, C. Chemical possibilities of Cydalima perspectalis Walk. (Lepidoptera: Crambidae) control. J. Hortic. For. Biotechnol. 2016, 20, 31–34. Available online: https://journal-hfb.usab-tm.ro/romana/2016/Lucrari%20PDF/Vol%2020(3)%20PDF/7Fora%20George.pdf (accessed on 18 January 2022).
- Somsai, A.P.; Lukacs, L.; Florian, T.; Sestraş, A.F.; Bunea, C.I.; Hoble, A.; Vlaşin, H. Chemical control of box tree moth Cydalima perspectalis (Walker, 1859) (Lepidoptera: Crambidae) in North-Western Romania. Agricultura 2019, 111, 210–216. [Google Scholar] [CrossRef]
- Plugutar, Y.V.; Sharmagy, A.K.; Shishkin, V.A. Effectiveness of bioinsecticides against the caterpillar of Cydalima perspectalis (Walker, 1859) on the Southern Coast of the Crimea. Bull. State Nikitsk. Bot. Gard. 2020, 137, 7–15. (In Russian) [Google Scholar] [CrossRef]
- Baryshnikova, S.V. Gracillariidae. In Catalogue of the Lepidoptera of Russia, 2nd ed.; Sinev, S.Y., Ed.; Zoological Institute RAS: St. Petersburg, Russia, 2019; pp. 36–43. (In Russian) [Google Scholar]
- De Prins, J.; De Prins, W. Global Taxonomic Database of Gracillariidae (Lepidoptera). World Wide Web Electronic Publication. 2006–2021. Available online: http://www.gracillariidae.net) (accessed on 10 November 2021).
- Caradja, A. Beitrag zur Kenntnis der geographischen Verbreitungder Mikrolepidopteren des palaearktischen Faunengebietes nebst Beschreibung neuer Formen. Dt. Ent. Z. Ires 1920, 34, 75–179. [Google Scholar]
- Baryshnikova, S.V.; Dubatolov, V.V. To the knowledge of small moths (Microlepidoptera) of the Bolshekhekhtsirskii Nature Reserve (Khabarovsk District). 2nd report. Bucculatricidae, Gracillariidae, Lyonetiidae]. Anim. World Far East 2007, 6, 47–50. Available online: https://elibrary.ru/download/elibrary_20453720_27411630.pdf (accessed on 18 January 2022). (In Russian).
- Selikhovkin, A.V.; Drenkhan, R.; Mandelstam, M.Y.; Musolin, D.L. Invasions of insect pests and fungal pathogens of woody plants into the North-Western part of the European Russia. Vestn. St. Petersburg Univ. Earth Sci. 2020, 65, 263–283. (In Russian) [Google Scholar] [CrossRef]
- Selikhovkin, A.; Merkuriev, S.; Khodachek, A. Native and alien tree insect pests: Climate change impact and economic losses in Northwestern Russia. In Proceedings of the 1st International Electronic Conference on Entomology, Basel, Switzerland, 1–15 July 2021; Available online: https://sciforum.net/paper/view/10412 (accessed on 4 January 2021).
- Buy, D.D.; Baryshnikova, S.V.; Denisova, N.V.; Mamaev, N.A.; Musolin, D.L.; Selikhovkin, A.V. Changes in the complex of dendrobiotic phyllophagous insects in Saint Petersburg. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. In The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 98–99. (In Russian) [Google Scholar]
- Buy, D.D.; Baryshnikova, S.V.; Denisova, N.V.; Shevchenko, S.V.; Selikhovkin, A.V. Actual changes in the species composition and population density of phyllophagous insects in St. Petersburg. Izv. St.-Peterbg. Lesoteh. Akad. 2020, 230, 73–99. (In Russian) [Google Scholar] [CrossRef]
- Chursina, V.A.; Vokhtantseva, K.V.; Gayvas, A.A. The main pest of English oak on the territory of the city of Omsk is the leaf blotch miner moth. In Innovative Technologies in Agriculture, Proceedings of the II International Scientific Conference. St. Petersburg, Russia, 20–23 July 2012; Akhmetov, I.G., Akhmetova, M.N., Ivanova, Y.V., Kalenskiy, A.V., Kutashov, V.A., Laktionova, K.S., Saraeva, N.M., Abdrasilov, T.K., Avdejuk, O.A., Aidarov, O.T., et al., Eds.; Svoye Izdatel’stvo Publisher: St. Petersburg, Russia, 2016; pp. 21–25. (In Russian) [Google Scholar]
- Baryshnikova, S.V. (Zoological Institute RAS, Saint Petersburg, Russia). Personal communication, 2021.
- Galich, D.E. (Siberian Forest Experiment Station, Tyumen, Russia). Personal communication, 2021.
- Heckford, R.J. Previously unrecorded foodplants of three species of Microlepidoptera. Entomol. Rec. J. Var. 1993, 105, 93. [Google Scholar]
- Nikitenko, H.M.; Fursov, V.M.; Hershenzon, Z.S.; Svyrydov, S.V. Oak broad-leaved moths and other mining insects on the oak. 2. Morphobiological and ecological characteristics of oak widespread moth and other mining pests of oak. Vestn. Zool. 2004, 38, 53–61. (In Ukrainian) [Google Scholar]
- Apostolov, L.G. Harmful Entomofauna of Forest Biogeocenoses in the Vicinity of the Central Dnieper; Vishcha Shkola Publisher: Kiyev, Ukraine, 1981; pp. 1–232. (In Russian) [Google Scholar]
- Utkina, I.A.; Rubtsov, V.V. The leaf blotch miner moth Acrocercops brongniardella F. (Lepidoptera: Gracillariidae) in Tellerman oakwood. In The Kataev Memorial Readings—X, Proceedings of the International Conference, St. Petersburg, Russia, 22–25 October 2018; Musolin, D.L., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2018; Volume 1. Insects and Other Invertebrates, pp. 104–105. Available online: https://elibrary.ru/download/elibrary_36373591_75292794.pdf (accessed on 18 January 2022). (In Russian)
- Utkina, I.A.; Rubtsov, V.V. The leaf blotch miner moth—Long known, but still little studied species. Izv. St.-Peterbg. Lesoteh. Akad. 2019, 228, 42–56. (In Russian) [Google Scholar]
- Golub, V.B.; Soboleva, V.A.; Aksenenko, E.V. Damage of oak by the leaf blotch miner moth Acrocercops brongniardella F. (Lepidoptera: Gracillariidae) in the Usman’ Forest (Voronezh Province, Russia) 10 years after the fire. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 136–137. (In Russian) [Google Scholar]
- Utkina, I.A.; Rubtsov, V.V. Relationship of different species of oak and phyllophages as an object of biogeocenotic research. Lesovedenie 2021, 5, 547–554. (In Russian) [Google Scholar] [CrossRef]
- Golub, V.B.; Berezhnova, O.N.; Kornev, I.I. Outbreak of the leaf blotch miner moth (Acrocercops brongniardella F., Lepidoptera, Gracillariidae) in the Voronezh Region. Izv. St.-Peterbg. Lesoteh. Akad. 2009, 187, 96–102. Available online: https://elibrary.ru/download/elibrary_13020131_77590310.pdf (accessed on 18 January 2022). (In Russian).
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publisher Off. EU: Luxembourg, Belgium, 2016; pp. 160–163. [Google Scholar]
- Ermolaev, I.V.; Vasil’ev, A.A. Phytophagous insects associated with the pedunculate oak (Quercus robur L.) in the Siva River valley. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 155–156. (In Russian) [Google Scholar]
- Ermolaev, I.V.; Ponomarev, V.I.; Vasil’ev, A.A.; Kumaeva, M.S. Phytophagous insects of the pedunculate oak (Quercus robur) in the northeast of its distribution area. Zool. J. 2021, 100, 640–651. (In Russian) [Google Scholar] [CrossRef]
- Marion, D.F.; Larew, H.G.; Knodel, J.J.; Natoli., W. Systemic activity of neem extract against birch leafminer. J. Arboric. 1990, 16, 12–16. [Google Scholar]
- Golosova, M.A.; Gninenko, Y.I.; Golosova, E.I. Ohrid Leaf-Miner Cameraria ohridella is a Dangerous Quarantine Pest on Objects of Urban Ggreening; VNIILM: Moscow, Russia, 2008; pp. 1–26. (In Russian) [Google Scholar]
- Gous, S.; Richardson, B. Stem injection of insecticides to control herbivorous insects on Eucalyptus niten. New Zealand Plant Prot. 2008, 61, 174–178. [Google Scholar] [CrossRef]
- Šefrová, H. Invasions of Lithocolletinae species in Europe—Causes, kinds, limits and ecological impact (Lepidoptera, Gracillariidae). Ekológia 2003, 22, 132–142. Available online: https://www.researchgate.net/publication/297685678_Invasions_of_Lithocolletinae_species_in_Europe_-_Causes_kinds_limits_and_ecological_impact_Lepidoptera_Gracillariidae (accessed on 18 January 2022).
- Kirichenko, N.I.; Triberti, P.; Akulov, E.N.; Ponomarenko, M.G.; Lopez-Vaamonde, C. Novel data on the taxonomic diversity, distribution, and host plants of leafmining moths of the family Gracillariidae (Lepidoptera) in Siberia, based on DNA barcoding. Entomol. Rev. 2019, 99, 796–819. [Google Scholar] [CrossRef]
- Hellrigl, K. Neue Erkenntnisse und Untersuchungen über die Roßkastanien-Miniermotte Cameraria ohridella Deschka & Dimić, 1986 (Lepidoptera, Gracillariidae). Gredleriana 2001, 1, 9–81. [Google Scholar]
- Grabenweger, G.; Avtzis, N.; Girardoz, S.; Hrasovec, B.; Tomov, R.; Kenis, M. Parasitism of Cameraria ohridella (Lepidoptera, Gracillariidae) in natural and artificial horse-chestnut stands in the Balkans. Agric. For. Entomol. 2005, 7, 291–296. [Google Scholar] [CrossRef]
- Valade, R.; Kenis, M.; Hernandez-Lopez, A.; Augustin, S.; Mari Mena, N.; Magnoux, E.; Rougerie, R.; Lakatos, F.; Roques, A.; Lopez-Vaamonde, C. Mitochondrial and microsatellite DNA markers reveal a Balkan origin for the highly invasive horse-chestnut leaf miner Cameraria ohridella (Lepidoptera, Gracillariidae). Mol. Ecol. 2009, 18, 3458–3470. [Google Scholar] [CrossRef] [PubMed]
- Lees, D.C.; Lack, H.W.; Rougerie, R.; Hernandez-Lopez, A.; Raus, T.; Avtzis, N.; Augustin, S.; Lopez-Vaamonde, C. Tracking origins of invasive herbivores using herbaria and archival DNA: The case of the horse-chestnut leafminer. Front. Ecol. Environ. 2011, 9, 322–328. [Google Scholar] [CrossRef]
- Gninenko, Y.I.; Shepelev, S.V. New phytophages and diseases of tree species. Lesn. Khozyaistvo 2004, 3, 48. (In Russian) [Google Scholar]
- Gninenko, Y.I.; Orlinski, A.D. New insect pests of forest plantations. Plant Prot. Quar. 2004, 4, 33. (In Russian) [Google Scholar]
- Golosova, M.A.; Gninenko, Y.I. The appearance of the horse-chestnut leafminer on horse chestnut in Moscow. Lesn. Vestn. 2006, 2, 43–46. Available online: https://elibrary.ru/download/elibrary_9273118_72290780.pdf (accessed on 18 January 2022). (In Russian).
- Akimov, I.A.; Zerova, M.D.; Gershenson, Z.S.; Narolsky, N.B.; Kochanez, O.M.; Sviridov, S.V. First record of the horse-chestnut leafminer Cameraria ohridella (Lepidoptera, Gracillariidae) on Aesculus hippocastanum (Hippocastanaceae) in Ukraine. Vestn. Zool. 2003, 37, 3–12. (In Russian) [Google Scholar]
- Rakov, A.G. The Horse-chestnut Leafminer and other Invasive Phyllophagous Species under the Conditions of Formation of their Ranges in the European Part of Russia. Ph.D. Thesis, Russian State Agricultural University, Moscow, Russia, 2015. (In Russian). [Google Scholar]
- Zhuravleva, E.N. The first appearance of the Ohrid leaf miner Cameraria ohridella (Lepidoptera: Gracillariidae) on the horse chestnut in the Greater Sochi. In The Kataev Memorial Readings—VIII, Proceedings of the International Conference, St. Petersburg, Russia, 18–20 November 2014; Musolin, D.L., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2014; p. 32. (In Russian) [Google Scholar]
- Karpun, N.N. Features of formation of dendrophagous invasive pest fauna in the humid subtropics of Russia at the beginning of the XXI century. Izv. St.-Peterbg. Lesoteh. Akad. 2019, 228, 104–119. (In Russian) [Google Scholar] [CrossRef]
- Chenikalova, E.V. Balkan leafminer moth—A threat to the horse chestnut in the Stavropol Region. Plant Prot. Quar. 2018, 11, 38–39. Available online: https://elibrary.ru/download/elibrary_36498157_20089242.pdf (accessed on 18 January 2022). (In Russian).
- Chenikalova, E.V. The chestnut moth Cameraria ohridella (Deschka et Dimić, 1986) and boxwood moth Cydalima perspectalis (Walker, 1859) in the tree stands of Stavropol and its environs. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 347–348. (In Russian) [Google Scholar]
- Martynov, V.V.; Nikulina, T.V.; Shokhin, I.V.; Terskov, E.N. Contributions to the fauna of invasive insects of Ciscaucasia. Field Biol. J. 2020, 2, 99–122. (In Russian) [Google Scholar] [CrossRef]
- Selikhovkin, A.V.; Popovichev, B.G.; Denisova, N.V.; Timofeeva, Y.A. Invasive mining moths (Lepidoptera: Gracillariidae) of woody plantations in St. Petersburg. In XV Congress of the Russian Entomological Society, Proceedings of the Congress, Novosibirsk, Russia, 31 July—7 August 2017; Azarkina, G.N., Baranchikov, Y.N., Barkalov, A.V., Belokobilskiy, S.A., Glupov, V.V., Grichanov, I.Y., Danilov, Y.N., Dubatolov, V.V., Dudko, R.Y., Kireychuk, A.G., et al., Eds.; Garamond Publisher: Novosibirsk, Russia, 2017; pp. 446–447. (In Russian) [Google Scholar]
- Anikin, V.V. Present day bio-invasions in the Volga-Ural Region: From the South to the North or from the East to the West? Cameraria ohridella (Lepidoptera: Gracillariidae) in the Lower and Middle Volga. Zootaxa 2019, 4624, 583–588. [Google Scholar] [CrossRef]
- Anikin, V.V.; Zolotuhin, V.V.; Polumordvinov, O.A. Mass damage of horse chestnut’s leaves (Aesculus hippocastanum) by Ohrid leafminer (Cameraria ohridella) on the territory of Penza in 2019. Bull. Botanic Garden Saratov State Univ. 2019, 17, 235–241. (In Russian) [Google Scholar] [CrossRef]
- Kryukova, A.V.; Nikolaeva, Z.V. Harmfulness of chestnut mining moth in Pskov region. Bull. Velikoluk. State Agric. Acad. 2018, 1, 1–7. Available online: https://elibrary.ru/download/elibrary_35034180_72824990.pdf (accessed on 18 January 2022). (In Russian).
- Grigoriev, M.A.; Grigoriev, A.I. Ecological and biological characteristic of horse chestnut (Aesculus hippocastanum L.) in the conditions of the south of Western Siberia. Bull. Omsk State Agric. Univ. 2017, 3, 61–68. Available online: https://elibrary.ru/download/elibrary_30467968_44356667.pdf (accessed on 18 January 2022). (In Russian).
- Triebel, S.A.; Gamanova, O.N. Monitoring chestnut miner moth. Plant Prot. Quar. 2009, 2, 45–47. [Google Scholar]
- Lack, H.W. The discovery and rediscovery of the horse chestnut. Arnoldia 2002, 61, 15–19. [Google Scholar] [CrossRef]
- Kenis, M.; Tomov, R.; Svatos, A.; Schlinsog, P.; Lopez-Vaamonde, C.; Heitland, W.; Grabenweger, G.; Girardoz, S.; Freise, J.; Avtzis, N. The horse-chestnut leaf miner in Europe—Prospects and constraints for biological control. In Proceedings of the Second International Symposium on Biological Control of Arthropods, Davos, Switzerland, 12–16 September 2005; Hoddle, M., Ed.; Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2005; pp. 77–90. [Google Scholar]
- D’Costa, L.; Koricheva, J.; Straw, N.; Simmonds, M.S.J. Oviposition patterns and larval damage by the invasive horse-chestnut leaf miner Cameraria ohridella on different species of Aesculus. Ecol. Entomol. 2013, 38, 456–462. [Google Scholar] [CrossRef]
- Davydova, I.A.; Gradusov, V.M.; Rybinceva, A.L. Cameraria ohridella Deschka et Dimić (Lepidoptera: Gracillariidae) on different species of horse chestnut in Moscow landscaping. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November, 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 141–142. (In Russian) [Google Scholar]
- Pschorn-Walcher, H. Freiland-Biologie der eingeschleppten Roßkastanien-Miniermotte Cameraria ohridella Deschka and Dimić (Lep., Gracillariidae) im Wienerwald. Linz. Biol. Beiträge 1994, 26, 633–642. [Google Scholar]
- Gilbert, M.; Grégoire, J.-C.; Freise, J.F.; Heitland, W. Long-distance dispersal and human population density allow the prediction of invasive patterns in the horse chestnut leafminer Cameraria ohridella. J. Anim. Ecol. 2004, 73, 459–468. [Google Scholar] [CrossRef]
- Augustin, S.; Reynaud, P. Un nouveau ravageur pour le marronnier: Cameraria ohridella. PHM Rev. Hortic. 2000, 418, 44–45. [Google Scholar]
- Kirichenko, N.; Augustin, S.; Kenis, M. Invasive leafminers on woody plants: A global review of pathways, impact and management. J. Pest Sci. 2019, 92, 93–106. [Google Scholar] [CrossRef]
- The Most Dangerous Invasive Species in Russia (TOP-100); Dgebuadze, Y.Y., Petrosyan, V.G., Khlyap, L.A., Eds.; KMK Scientific Press Ltd.: Moscow, Russia, 2018; pp. 1–688. (In Russian) [Google Scholar]
- Cherpakov, V.V. About bacterial damage caused by Pseudomonas syringae pv. aesculi on horse chestnut in the Russian Federation. Actual Probl. For. Complex 2013, 36, 72–80. Available online: https://elibrary.ru/item.asp?id=21267711 (accessed on 20 January 2022). (In Russian).
- Straw, N.A.; Williams, D.T. Impact of the leaf miner Cameraria ohridella (Lepidoptera: Gracillariidae) and bleeding canker disease on horse-chestnut: Direct effects and interaction. Agric. For. Entomol. 2013, 15, 321–333. [Google Scholar] [CrossRef]
- Koskella, B.; Meaden, S.; Crowther, W.J.; Leimu, R.; Metcalf, C.J.E. A signature of tree health? Shifts in the microbiome and the ecological drivers of horse chestnut bleeding canker disease. New Phytol. 2017, 215, 737–746. [Google Scholar] [CrossRef]
- Freise, J.; Heitland, W.; Tosevski, I. Parasitism of the horse chestnut leafminer, Cameraria ohridella Deschka and Dimić (Lep., Gracillariidae), in Serbia and Macedonia. Anz. Schädlingskunde 2002, 75, 152–157. [Google Scholar] [CrossRef]
- Bhatti, I.; Shaw, O.; Shaw, P. Parasitoids and parasitism rates of the horse chestnut leaf miner Cameraria ohridella Deschka and Dimić [Lepidoptera: Gracillariidae] across four sites in south-west London. Arboricultural J. 2013, 35, 147–159. [Google Scholar] [CrossRef]
- Volter, L.; Kenis, M. Parasitoid complex and parasitism rates of the horse chestnut leafminer, Cameraria ohridella (Lepidoptera: Gracillariidae) in the Czech Republic, Slovakia and Slovenia. Eur. J. Entomol. 2006, 103, 365–370. [Google Scholar] [CrossRef]
- Girardoz, S.; Volter, L.; Tomov, R.; Quicke, D.L.J.; Kenis, M. Variations in parasitism in sympatric populations of three invasive leaf miners. J. Appl. Entomol. 2007, 131, 603–612. [Google Scholar] [CrossRef]
- Grabenweger, G.; Kehrli, P.; Zweimuller, I.; Augustin, S.; Avtzis, N.; Bacher, S.; Freise, J.; Girardoz, S.; Guichard, S.; Heitland, W.; et al. Temporal and spatial variations in the parasitoid complex of the horse chestnut leafminer during its invasion of Europe. Biol. Invasions 2010, 12, 2797–2813. [Google Scholar] [CrossRef]
- Hernandez-Lopez, A.; Rougerie, R.; Augustin, S.; Lees, D.C.; Tomov, R.; Kenis, M.; Cota, E.; Kullaj, E.; Hansson, C.; Grabenweger, G.; et al. Host tracking or cryptic adaptation? Phylogeography of Pediobius saulius (Hymenoptera, Eulophidae), a parasitoid of the highly invasive horse-chestnut leafminer. Evol. Appl. 2012, 5, 256–269. [Google Scholar] [CrossRef]
- Kostyukov, V.V.; Kosheleva, O.V.; Nakonechnaya, I.V.; Gunasheva, Z.M. First report on parasitoids of the horse chestnut moth in Russia. Plant Prot. Quar. 2014, 9, 41–42. Available online: https://elibrary.ru/download/elibrary_21841455_76822640.pdf (accessed on 18 January 2022). (In Russian).
- Měsić, A.; Barčić, J.; Barčić, J.I.; Miličević, T.; Duralija, B.; Čuljak, T.G. A low environmental impact method to control horse chestnut leaf miner Cameraria ohridella (Deschka & Dimic). J. Food Agric. Environ. 2008, 6, 421–427. Available online: https://www.researchgate.net/publication/231167581_A_low_environmental_impact_method_to_control_horse_chestnut_leaf_miner_Cameraria_ohridella_Deschka_Dimic (accessed on 18 January 2022).
- De Prins, J.; De Prins, W. The occurrence of Cameraria ohridella in Belgium (Lepidoptera: Gracillariidae). Phegea 2001, 29, 81–88. Available online: https://www.researchgate.net/publication/250928533_The_occurrence_of_Cameraria_ohridella_in_Belgium_Lepidoptera_Gracillariidae (accessed on 18 January 2022).
- Jagiełło, R.; Walczak, U.; Iszkuło, G.; Karolewski, P.; Baraniak, E.; Giertych, M.J. Impact of Cameraria ohridella on Aesculus hippocastanum growth and long-term effects of trunk injection with pesticides. Int. J. Pest Manag. 2019, 65, 33–43. [Google Scholar] [CrossRef]
- Larina, G.E.; Seraya, L.G.; Kashtanova, O.A.; Dymovich, A.M.; Baranchikov, Y.N. The effectiveness of endotherapy of trunks in protection of horse-chestnut Aesculus hippocastanum L. against mining insects. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 187–188. (In Russian) [Google Scholar]
- Svatos, A.; Kalinová, B.; Hoskovec, M.; Kindl, J.; Hrdy, I. Chemical communication in horse-chestnut leafminer Cameraria ohridella Deschka & Dimic. Plant. Prot. Sci. 1999, 35, 10–13. [Google Scholar]
- Atanov, N.M.; Kuzina, N.P.; Kuzin, A.A.; Chirskaya, M.V. Impact of modifiers on the attractiveness of the horse-chestnut leaf-miner’s pheromone Cameraria ohridella Deschka et Dimič, 1986. Plant Health Res. Pract. 2016, 4, 47–49. Available online: http://www.rsn-msk.ru/files/karantin-18-2016.pdf (accessed on 18 January 2022).
- Kehrli, P.; Lehmann, M.; Bacher, S. Mass-emergence devices: A biocontrol technique for conservation and augmentation of parasitoids. Biol. Control 2005, 32, 191–199. [Google Scholar] [CrossRef]
- Kumata, T. Taxonomic studies on the Lithocolletinae of Japan. Insecta Matsumurana 1963, 25, 53–90. [Google Scholar]
- Kirichenko, N.; Ryazanova, M.; Akulov, E.; Ponomarenko, M. Can native parasitoids control the invasive lime leaf-miner Phyllonorycter issikii (Lepidoptera: Gracillariidae) in Western Siberia? In Proceedings of the 1st International Electronic Conference on Entomology, Basel, Switzerland, 1–15 July 2021; Available online: https://sciforum.net/paper/view/10537 (accessed on 4 January 2021).
- Kumata, T.; Kuroko, H.; Park, K. Some Korean species of the subfamily Lithocolletinae Gracillariidae, Lepidoptera. Korean J. Plant Prot. 1983, 22, 213–227. [Google Scholar]
- Kirichenko, N.I.; Akulov, E.N.; Babichev, N.S.; Mikhailova, I.A.; Ponomarenko, M.G.; Lopez-Vaamonde, C. Past distribution of Tilia-feeding Phyllonorycter micromoth (Lepidoptera: Gracillariidae) in the Russian Far East based on survey of historical herbarium. Far Eastern Entomol. 2019, 390, 19–32. [Google Scholar] [CrossRef]
- Kirichenko, N.I. Trophic Associations and Invasion Patterns of the Dendrophilous Gracillariid Moths (Lepidoptera: Gracillariidae) in the Asian Part of Russia. Ph.D. Thesis, Siberian Federal University, Krasnoyarsk, Russia, 2020. Available online: https://research.sfu-kras.ru/sites/research.sfu-kras.ru/files/Dissertaciya_Kirichenko.pdf (accessed on 18 January 2022). (In Russian).
- Bednova, O.V.; Belov, D.A. Lime leaf miner (Lepidoptera, Gracillariidae) in green spaces of Moscow and Moscow Region. Lesn. Vestn. 1999, 2, 172–177. (In Russian) [Google Scholar]
- Kozlov, M.V. Leaf-mining moth is a pest of limes. Plant Prot. Quar. 1991, 4, 46. (In Russian) [Google Scholar]
- Šefrová, H. Phyllonorycter issikii (Kumata, 1963)—Bionomics, ecological impact and spread in Europe (Lepidoptera, Gracillariidae). Acta Univ. Agric. Silvic. Mendel. Brun. 2002, 50, 99–104. [Google Scholar]
- Ermolaev, I.V.; Rubleva, E.A. History, rate and factors of invasion of lime leafminer Phyllonorycter issikii (Kumata, 1963) (Lepidoptera, Gracillariidae) in Eurasia. Russ. J. Biol. Invasions 2017, 1, 2–19. (In Russian) [Google Scholar] [CrossRef]
- Gninenko, Y.I.; Kozlova, E.I. Lime leaf miner in Russia and problems of biological control. In Proceedings of the International Organization for Biological Control of Harmful Animals and Plants, East Palaearctic Regional Section, Abstracts, Poznan, Poland, 15–19 May 2006; IOBC: Poznan, Poland, 2006; p. 16. [Google Scholar]
- Kirichenko, N.; Triberti, P.; Ohshima, I.; Haran, J.; Byun, B.-K.; Li, H.; Augustin, S.; Roques, A.; Lopez-Vaamonde, C. From east to west across the Palearctic: Phylogeography of the invasive lime leaf miner Phyllonorycter issikii (Lepidoptera: Gracillariidae) and discovery of a putative new cryptic species in East Asia. PLoS ONE 2017, 12, e0171104. [Google Scholar] [CrossRef]
- Ermolaev, I.V.; Rubleva, E.A.; Rysin, S.L.; Ermolaeva, M.V. Forage plants of lime leafminer Phyllonorycter issikii (Kumata, 1963) (Lepidoptera, Gracillariidae). Russ. J. Biol. Invasions 2018, 2, 2–13. Available online: https://elibrary.ru/download/elibrary_35166252_58174722.pdf (accessed on 18 January 2022). (In Russian).
- Kirichenko, N. The lime leafminer Phyllonorycter issikii in Western Siberia: Some ecological characteristics of the population of the recent invader. Contemp. Probl. Ecol. 2014, 7, 114–121. [Google Scholar] [CrossRef]
- Pigott, C.D. Lime-trees and Basswoods. A Biological Monograph of the Genus Tilia; Cambridge University Press: New York, NY, USA, 2012; pp. 1–395. [Google Scholar]
- Zolotukhin, V.V. About some invasive arthropods on the territory of the Ulyanovsk region. Nat. Simbirsk Volga Reg. 2002, 2, 200–203. [Google Scholar]
- Ermolaev, I.V.; Zorin, D.A. Ecological consequences of invasion of Phyllonorycter issikii (Lepidoptera, Gracillariidae) in lime forests in Udmurtia. Entomol. Rev. 2011, 91, 592–598. [Google Scholar] [CrossRef]
- Kirichenko, N.I.; Zakharov, E.V.; Lopez-Vaamonde, C. Revealing the invasion history of the lime leafminer Phyllonorycter issikii (Lepidoptera: Gracillariidae) using historical herbaria and next generation sequencing. In Dendrobiotic Invertebrates and Fungi and their Role in Forest Ecosystems. The Kataev Memorial Readings—XI, Proceedings of the All-Russia Conference with International Participation, St. Petersburg, Russia, 24–27 November 2020; Musolin, D.L., Kirichenko, N.I., Selikhovkin, A.V., Eds.; SPbFTU: St. Petersburg, Russia, 2020; pp. 172–173, 175. (In Russian) [Google Scholar]
- Ermolaev, I.V.; Yefremova, Z.A.; Izhboldina, N.V. Parasitoids as a mortality factor for the lime leafminer (Phyllonorycter issikii, Lepidoptera, Gracillariidae). Entomol. Rev. 2011, 91, 326–334. [Google Scholar] [CrossRef]
- Szőcs, L.; George, M.; Thuróczy, C.; Csóka, G. Parasitoids of the lime leaf miner Phyllonorycter issikii (Lepidoptera: Gracillariidae) recorded throughout the area it recently colonized. Eur. J. Entomol. 2015, 112, 591–598. [Google Scholar] [CrossRef]
- Ermolaev, I.V.; Yefremova, Z.A.; Gerasimova, N.A.; Koryoleva, E.A.; Lushnikov, N.N.; Petrov, A.I.; Pchel’nikov, A.A. Parasitoids (Hymenoptera) of the lime leafminer (Phyllonorycter issikii, Lepidoptera, Gracillariidae) in different cities of the Russian Federation and their role in the mortality of the invasive pest. Entomol. Rev. 2019, 99, 485–493. [Google Scholar] [CrossRef]
- Yefremova, Z.A.; Mishchenko, A.V. The parasitoid complex (Hymenoptera, Eulophidae) of the leafminer Phyllonorycter issikii (Kumata) (Lepidoptera, Gracillariidae) from the Middle Volga Basin. Entomol. Rev. 2008, 88, 178–185. [Google Scholar] [CrossRef]
- Mishchenko, A.V. Method of mass rearing ichneumons Minotetrastichus frontalis (Nees). Bull. Russ. Fed. Fed. Serv. Intellect. Property 2015, 10, 1–8. [Google Scholar]
- Baryshnikova, S.V.; Bolshakov, L.V. Microlepidoptera of Tula Province. 15. Families Bucculatricidae, Gracillariidae, and Lyonetiidae (Hexapoda: Lepidoptera). In Biodiversity of Tula Region at the Turn of Centuries; Bolshakov, L.V., Ed.; Grif & K: Tula, Russia, 2004; Volume 4, pp. 31–37. (In Russian) [Google Scholar]
- Ermolaev, I.V.; Rubleva, E.A.; Rysin, S.L.; Kozhenkova, A.A.; Ermolaeva, M.V. Trophic specialization of the poplar leafminer Phyllonorycter populifoliella (Treitschke, 1833) (Lepidoptera, Gracillariidae). Entomol. Rev. 2020, 100, 287–300. [Google Scholar] [CrossRef]
- Shashank, P.R.; Singh, N.; Harshana, A.; Sinha, T.; Kirichenko, N. First report of the poplar leaf miner, Phyllonorycter populifoliella (Treitschke) (Lepidoptera: Gracillariidae) from India. Zootaxa 2021, 4915, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Kozhanchikov, I.V.; Danilevskiy, A.S.; Djakonov, A.M. Order Lepidoptera. In Pests of Forest (Reference Book); Shtakelberg, A.A., Ed.; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia; St. Petersburg, Russia, 1955; Volume 1, pp. 35–287. (In Russian) [Google Scholar]
- Tarasova, O.V.; Kovalev, A.V.; Sukhovolsky, V.G.; Khlebopros, R.G. Phylophagous Insects of Green Plantations of Cities: Species Compositions and Population Dynamics; Nauka: Novosibirsk, Russia, 2004; pp. 1–180. (In Russian) [Google Scholar]
- Ermolaev, I.V. Ecological mechanisms of nonperiodical population wave: A case study of the poplar leafminer—Phyllonorycter populifoliella (Lepidoptera, Gracillariidae). J. General Biol. 2019, 80, 451–476. [Google Scholar]
- Polezhaev, V.G. Struggle for existence in the poplar leafminer Lithocolletis populifoliella Tr. Zool. Zhurnal. 1934, 13, 67–75. (In Russian) [Google Scholar]
- Frolov, D.N. The poplar leafminer as a landscaping pest in Irkutsk. Proceedings Irkutsk State Univ. Ser. Biol. 1948, 3, 19. (In Russian) [Google Scholar]
- Selikhovkin, A.V. Population dynamics of Microlepidoptera under the conditions of industrial air pollution: A follow-up. Izv. St.-Peterbg. Lesoteh. Akad. 1996, 162, 26–38. (In Russian) [Google Scholar]
- Selikhovkin, A.V.; Baryshnikova, S.V.; Denisova, N.V.; Timofeeva, Y.A. Species composition and population dynamics of dominant dendrophagous moths (Lepidoptera) in St. Petersburg and its environs. Entomol. Rev. 2018, 98, 963–978. [Google Scholar] [CrossRef]
- Selikhovkin, A.V.; Egorov, A.A.; Sitnikova, D.D.; Mamaev, N.A. Occurrence of leaf miners Phyllonorycter populifoliella (Treitschke) and Ph. pastorella (Zeller) (Lepidoptera, Gracillariidae) on different species of poplars. Entomol. Rev. 2020, 100, 301–306. [Google Scholar] [CrossRef]
- Rumyantsev, P.D. Biology of the poplar leaf miner Lithocolletis populifoliella in the conditions of Moscow. Zool. Zhurnal. 1934, 13, 257–279. (In Russian) [Google Scholar]
- Mamaev, N.A.; Buy, D.D.; Selikhovkin, A.V. The second outbreak of the poplar leafminer Phyllonorycter populifoliella in St. Petersburg. Izv. St.-Peterbg. Lesoteh. Akad. 2020, 233, 81–94. (In Russian) [Google Scholar] [CrossRef]
- Lvovsky, A.L. Lepidoptera (Insecta) within the city limits of St. Petersburg. Proceedings Kharkiv Entomol. Soc. 1994, 2, 4–48. (In Russian) [Google Scholar]
- Bondarenko, E.A. An outbreak of the poplar leafminer Phyllonorycter populifoliella Tr. (Lepidoptera, Gracillariidae) in St. Petersburg. Izv. St.-Peterbg. Lesoteh. Akad. 2008, 182, 45–55. (In Russian) [Google Scholar]
- Selikhovkin, A.V. Peculiarities of the population dynamics of the poplar mining moth Phyllonorycter populifoliella Tr. (Gracillariidae). Izv. St.-Peterbg. Lesoteh. Akad. 2010, 192, 220–235. Available online: https://elibrary.ru/download/elibrary_17047133_73905770.pdf (accessed on 18 January 2022). (In Russian).
- Ermolaev, V.P. Ecologo-faunistic review of leafmining moths (Lepidoptera, Gracillariidae) of southern Primorye. Tr. Zool. Inst. Akad. Nauk SSSR 1977, 70, 98–116. (In Russian) [Google Scholar]
- Ðức, B.Đ.; Leont’ev, L.L.; Baryshnikova, S.V.; Selikhovkin, A.V. Consequences of the outbreaks of the poplar leaf miner and other mining Microlepidoptera in St. Petersburg. Contemp. Probl. Ecol. 2021, 14, 822–826. [Google Scholar] [CrossRef]
- Sulkhanov, A.V. Ecology of urban populations of the poplar moth Lithocolletis populifoliella Tr. In Dendrobiont Insects of Trees and Shrubs of Moscow; Krivosheina, N.P., Striganova, B.R., Eds.; Nauka Publisher House: Moscow, Russia, 1992; pp. 70–97. (In Russian) [Google Scholar]
- Chaika, S.Y. Insects. In Great Russian Encyclopedia; Electronic Version; 2017; Available online: https://bigenc.ru/biology/text/2250439 (accessed on 2 November 2021).
- Kerzhner, I.M. An approach and perspective of study of Insecta in the territory of the former USSR. In Biological Diversity: The Level of Taxonomic Study; Sokolov, V.E., Reshetnikov, Y.S., Eds.; Nauka Publisher House: Moscow, Russia, 1994; pp. 65–69. (In Russian) [Google Scholar]
- Cippolini, D. White fringetree as a novel larval host for emerald ash borer. J. Econ. Entomol. 2015, 108, 370–375. [Google Scholar] [CrossRef]
- Orlova-Bienkowskaja, M.J. Cascading ecological effects caused by the establishment of the emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae) in European Russia. Eur. J. Entomol. 2015, 112, 778–789. [Google Scholar] [CrossRef]
- Eschen, R.; O’Hanlon, R.; Santini, A.; Vannini, A.; Roques, A.; Kirichenko, N.; Kenis, M. Safeguarding global plant health: The rise of sentinels. J. Pest Sci. 2018, 62, 29–36. [Google Scholar] [CrossRef]
- Morales-Rodríguez, C.; Anslan, S.; Auger-Rozenberg, M.-A.; Augustin, S.; Baranchikov, Y.; Bellahirech, A.; Burokienė, D.; Čepukoit, D.; Çota, E.; Davydenko, K.; et al. Forewarned is forearmed: Preventive detection of potentially invasive pests and pathogens in sentinel plantings. NeoBiota 2019, 47, 95–123. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).