Effects of Precipitation and Soil Moisture on the Characteristics of the Seedling Bank under Quercus acutissima Forest Plantation in Mount Tai, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Investigation of Q. acutissima Plantation
2.3. Measurement of Seedling Traits of Q. acutissima
2.4. Monitoring Environmental Parameters under Q. acutissima Plantation
2.5. Statistical Analysis
3. Results
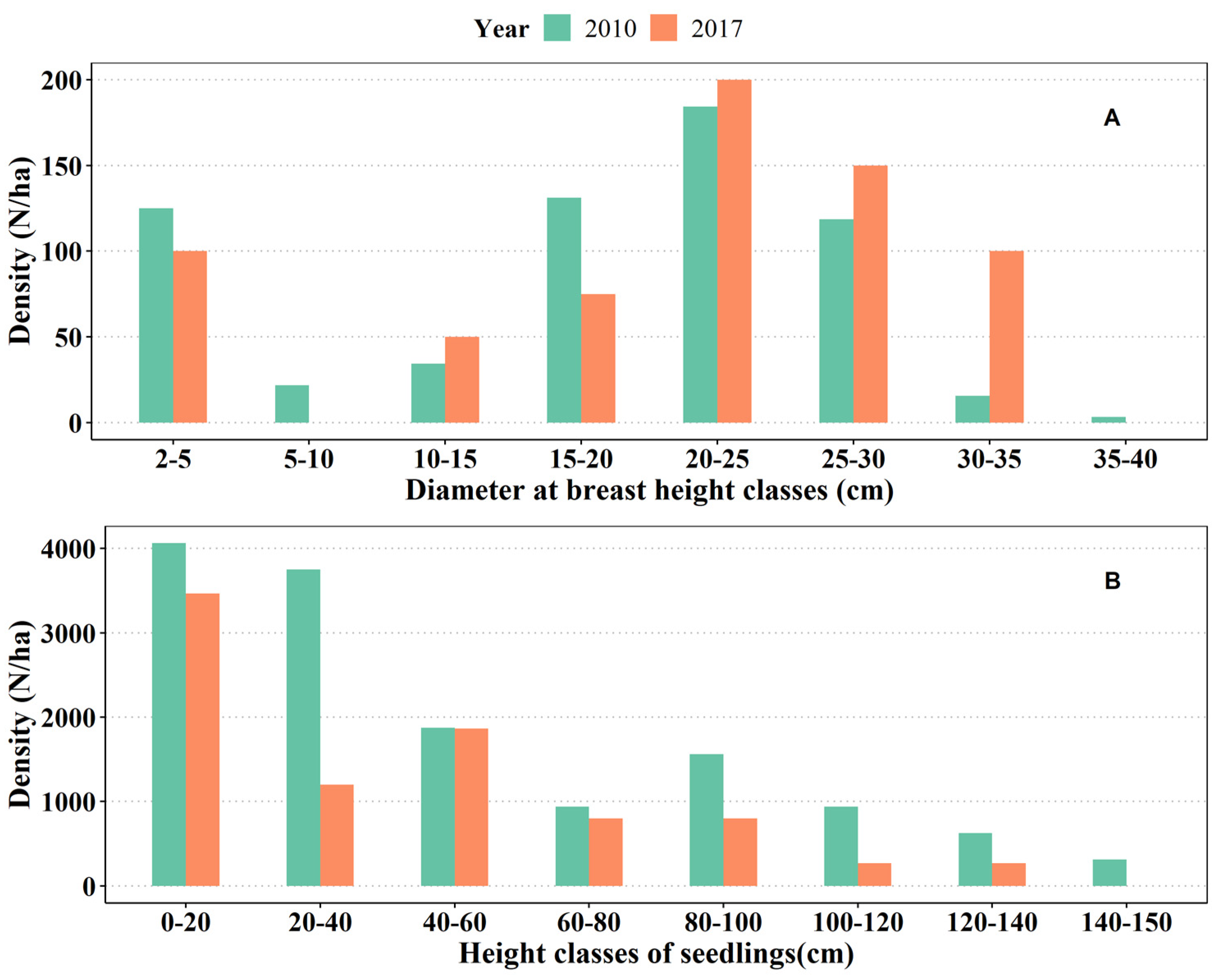
3.1. The Diameter Structure of Q. acutissima Plantation
3.2. Characteristics of Environmental Factors under Q. acutissima Plantation
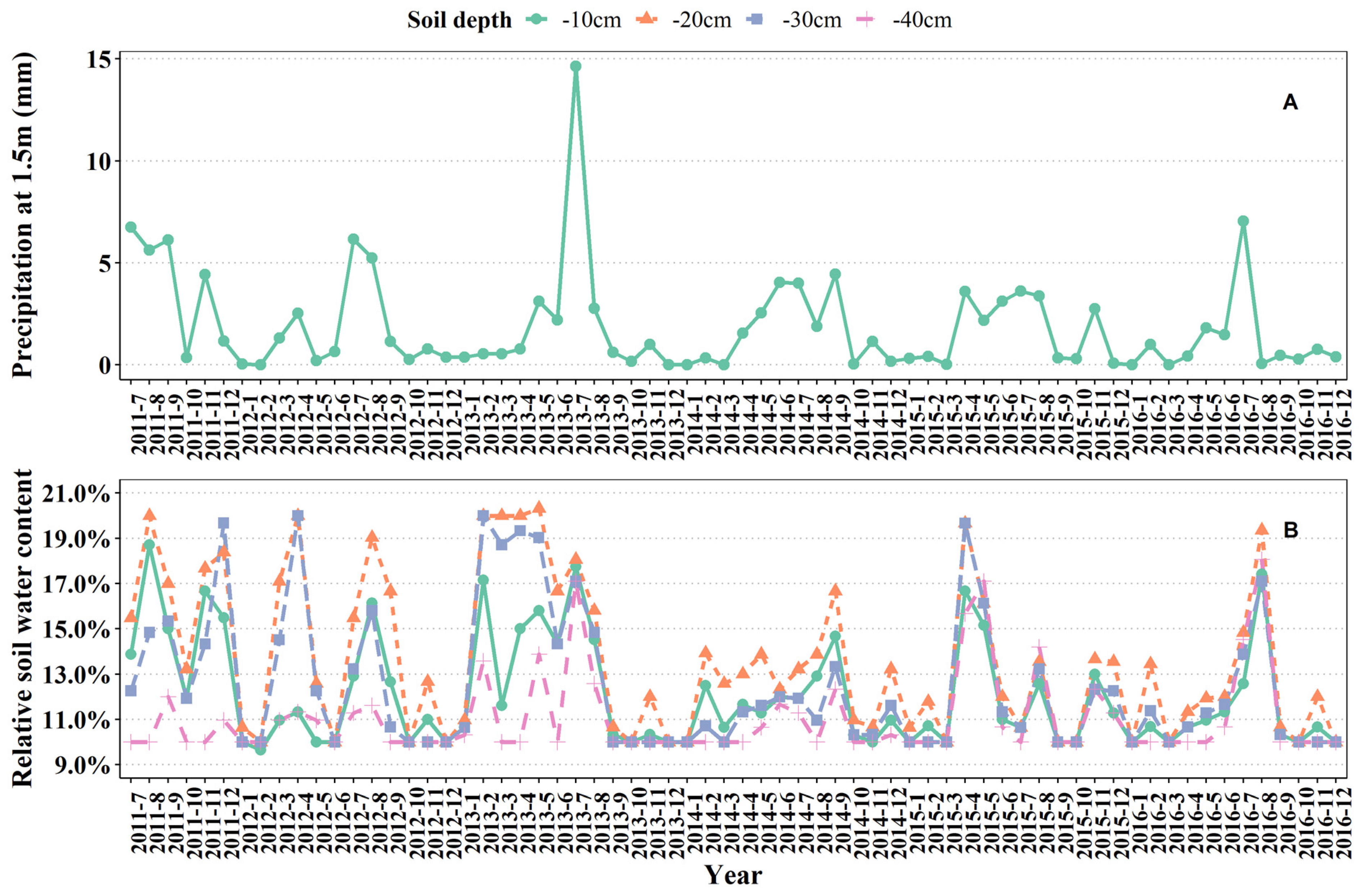
3.2.1. Precipitation
3.2.2. Soil Moisture
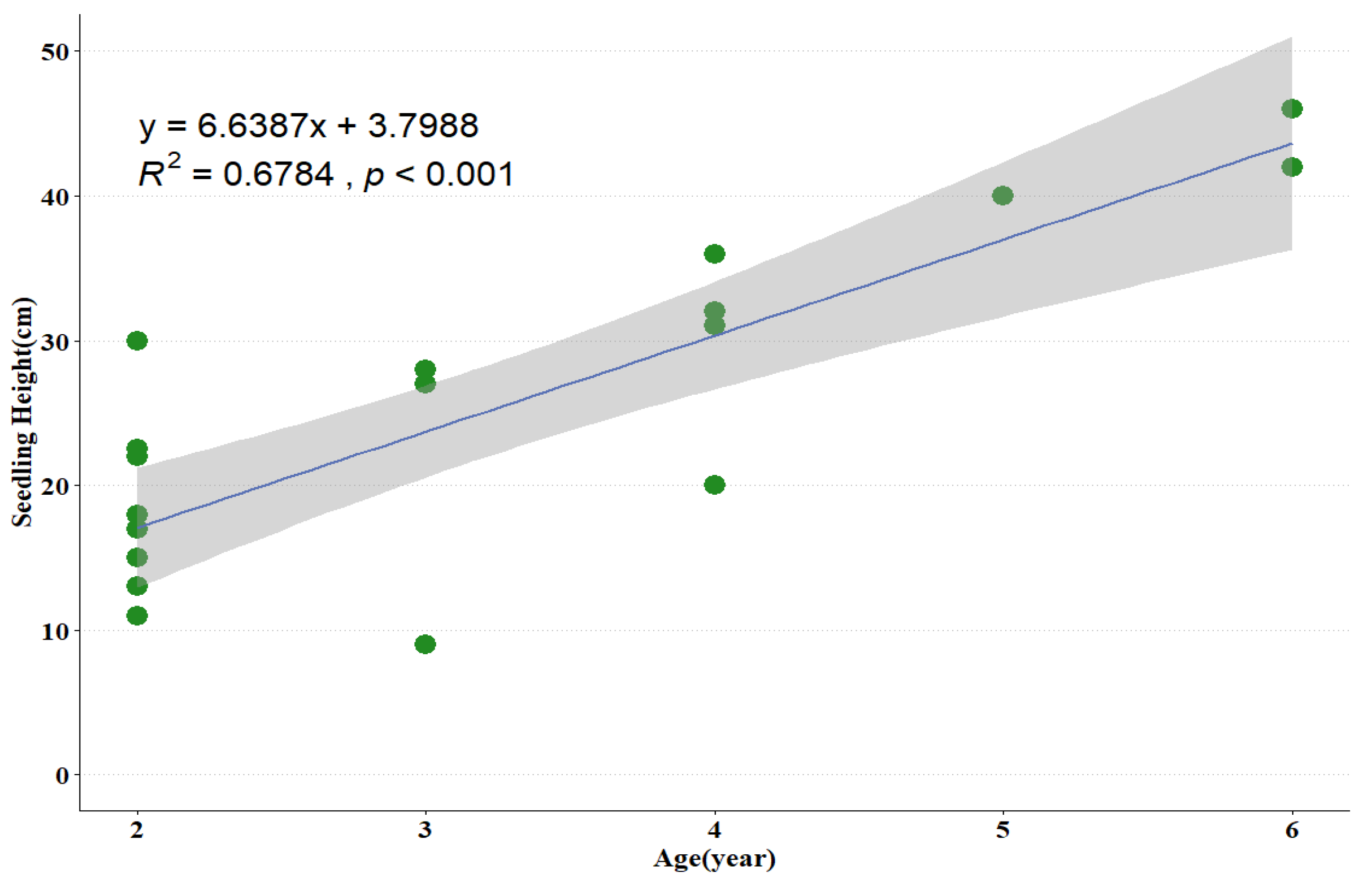
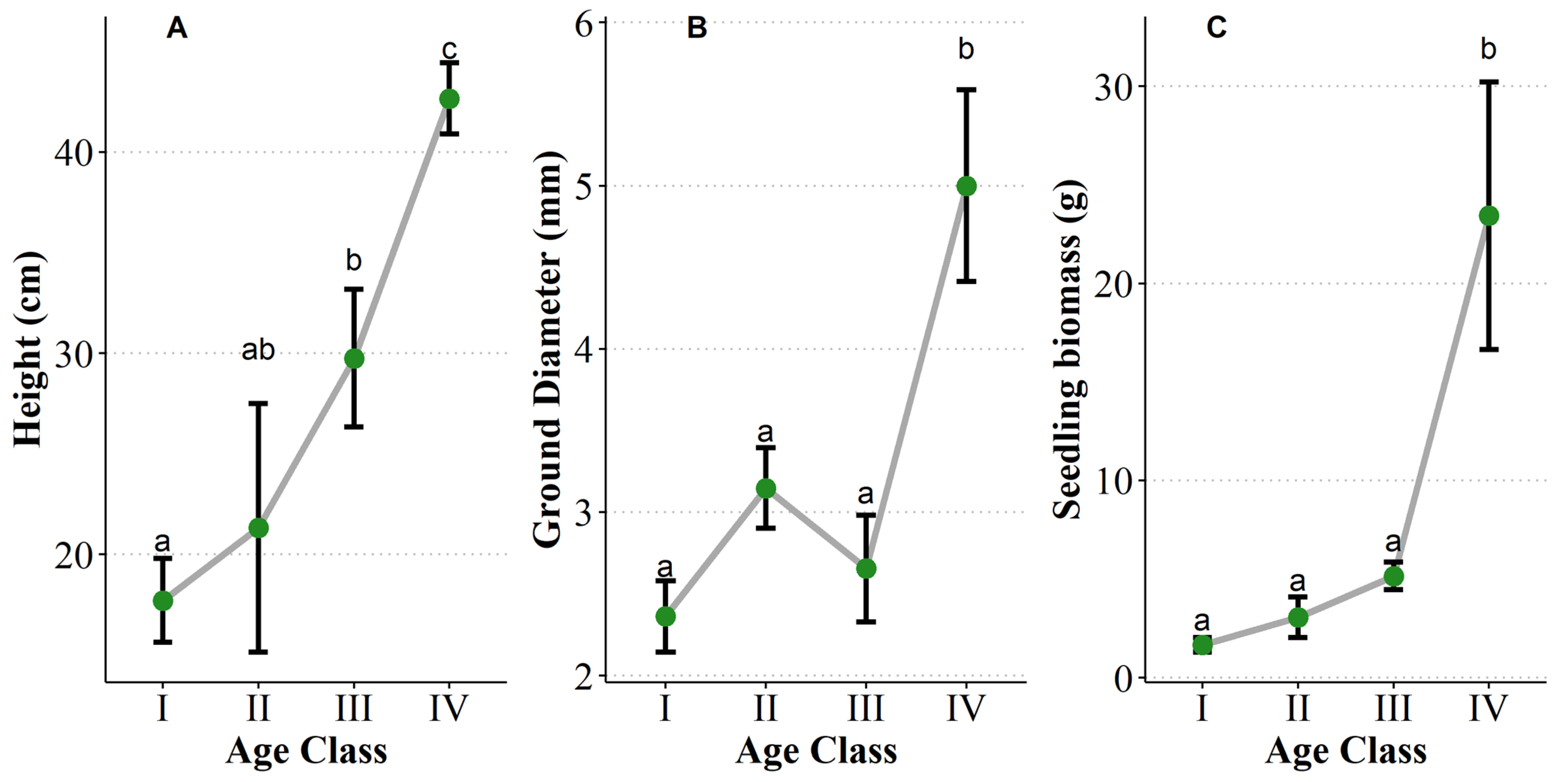
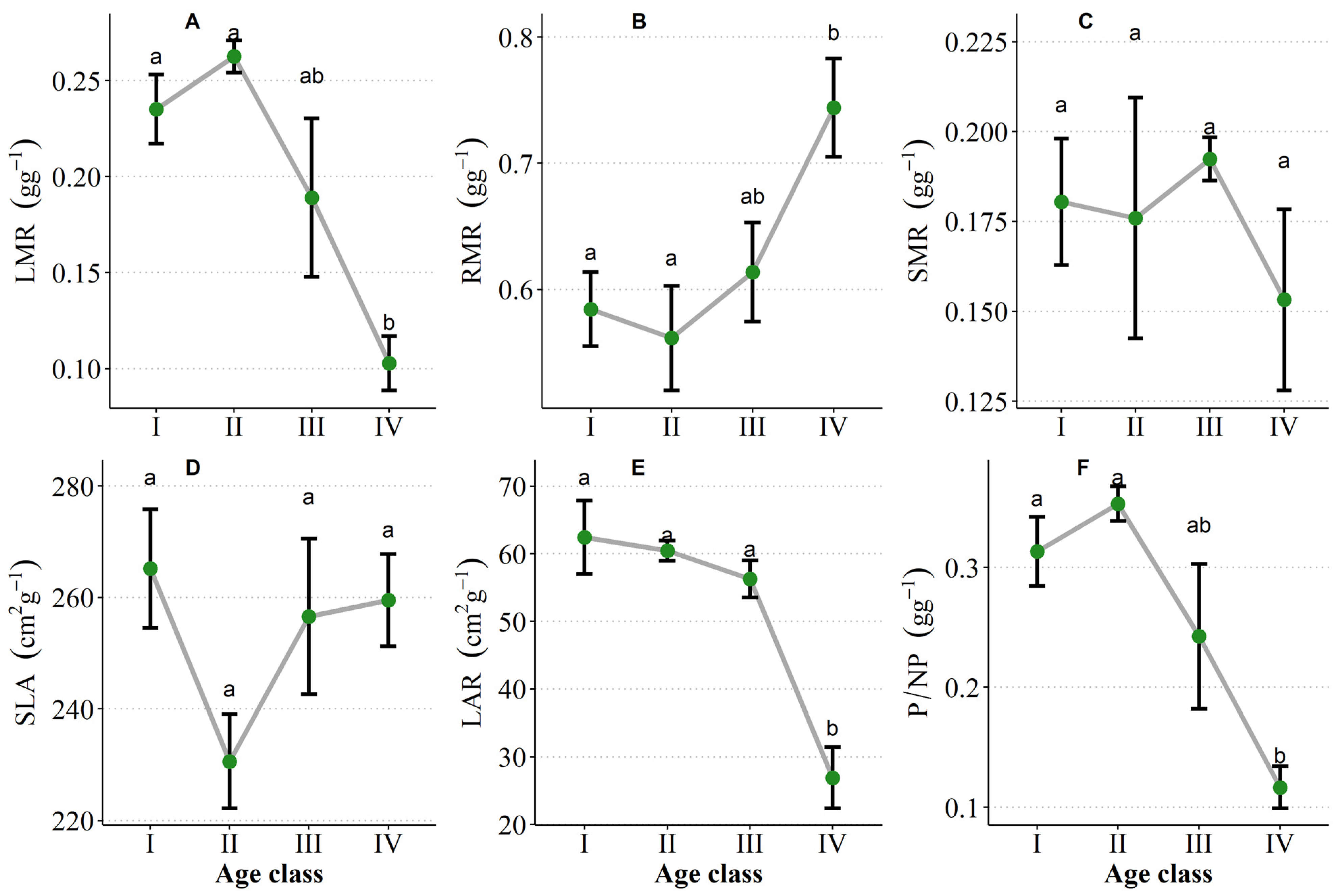
3.3. Seedling Size Traits with the Increase of Age
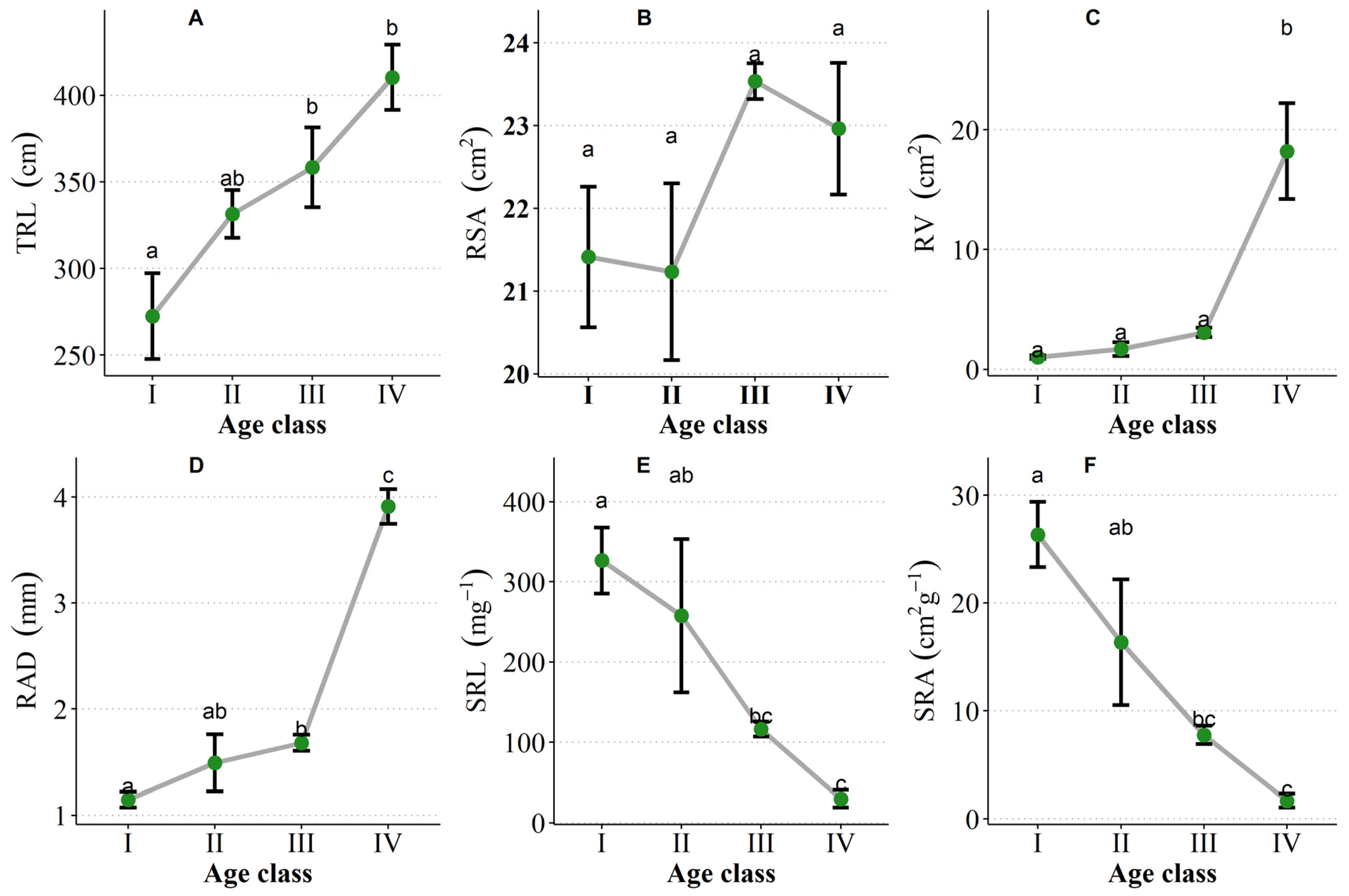
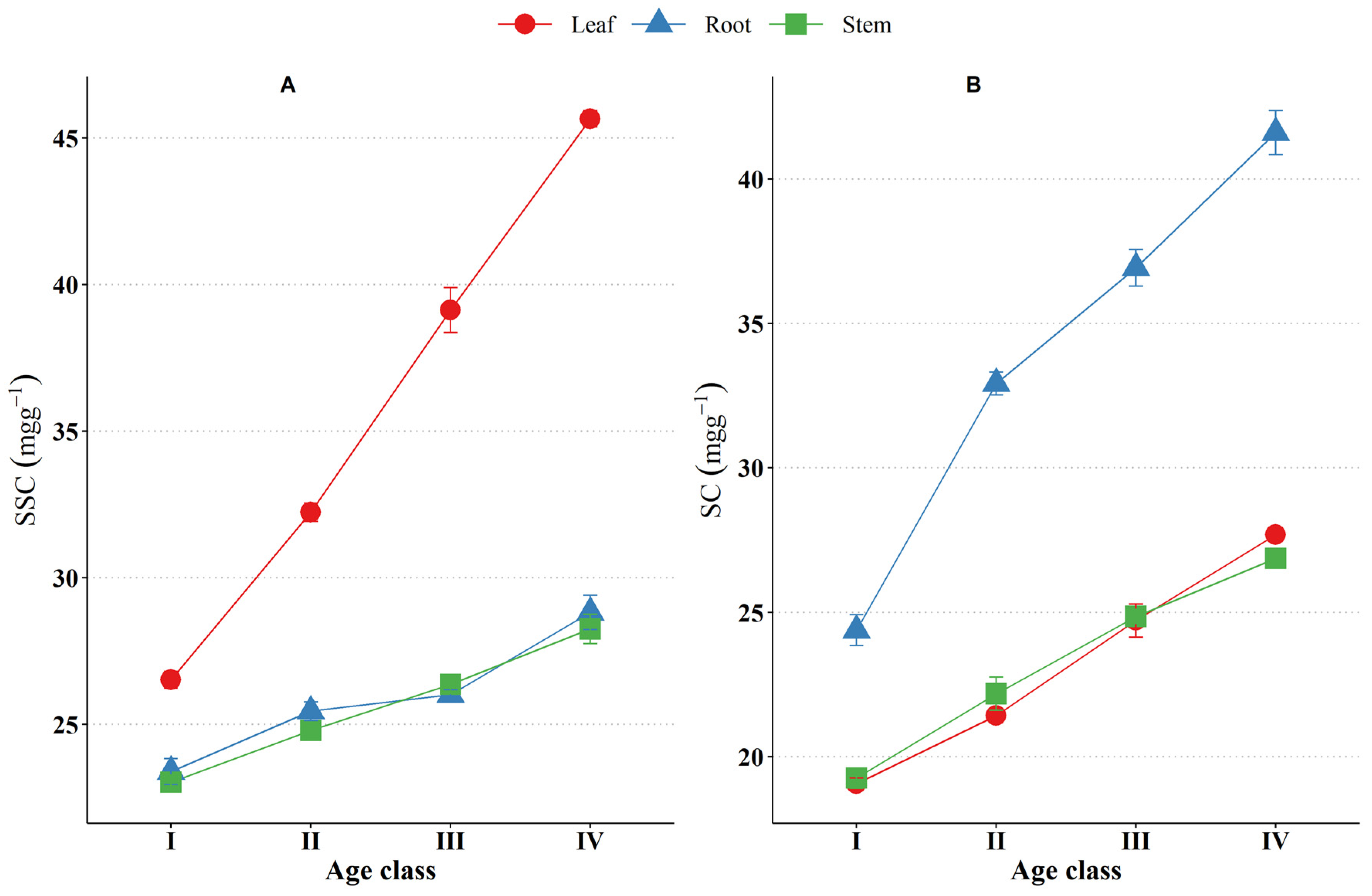
3.4. Adaptation Traits of Seedlings with Increasing Age
3.4.1. Biomass Allocation
3.4.2. Root Traits
3.4.3. Non-Structural Carbohydrate (NSC)
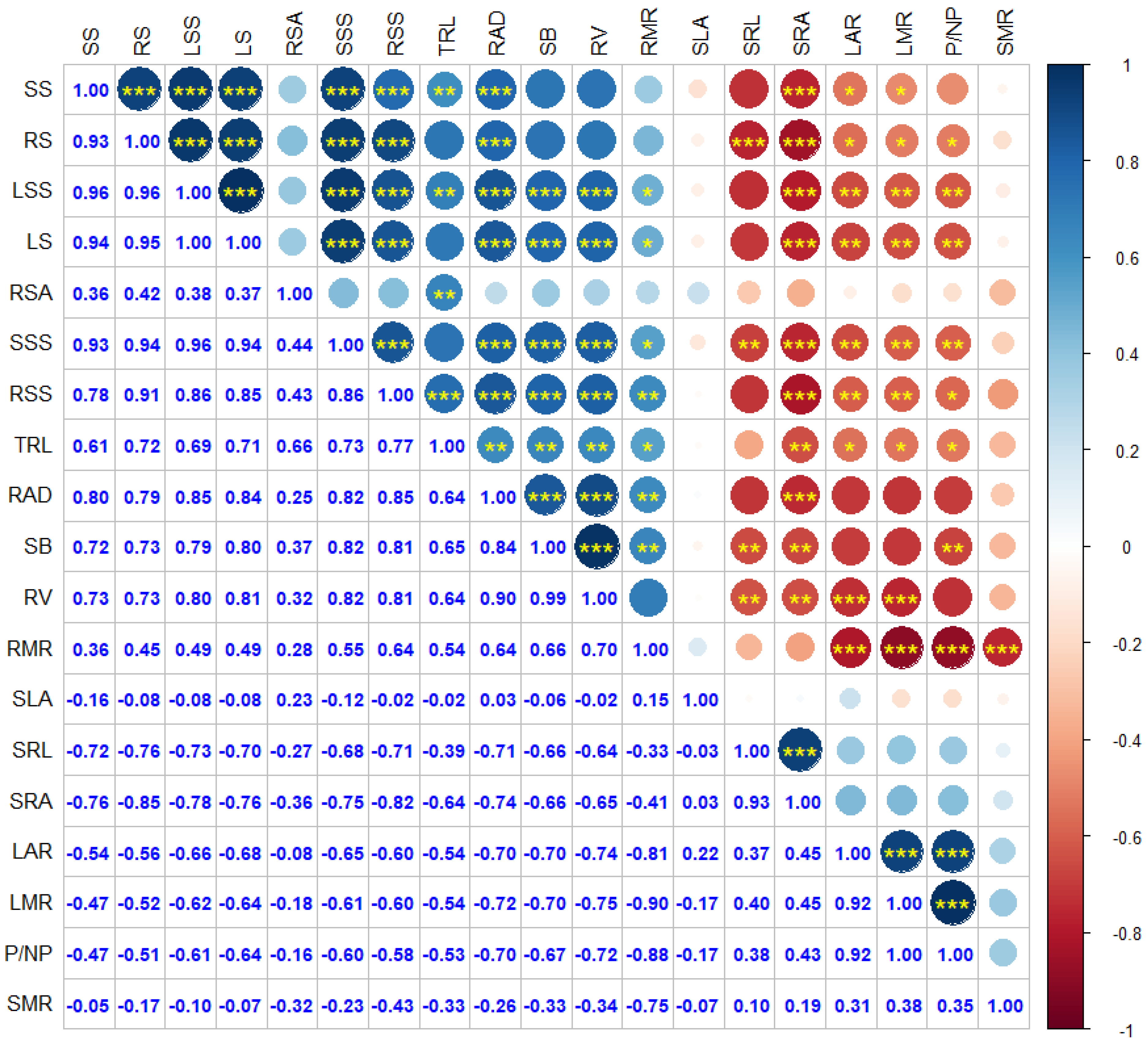
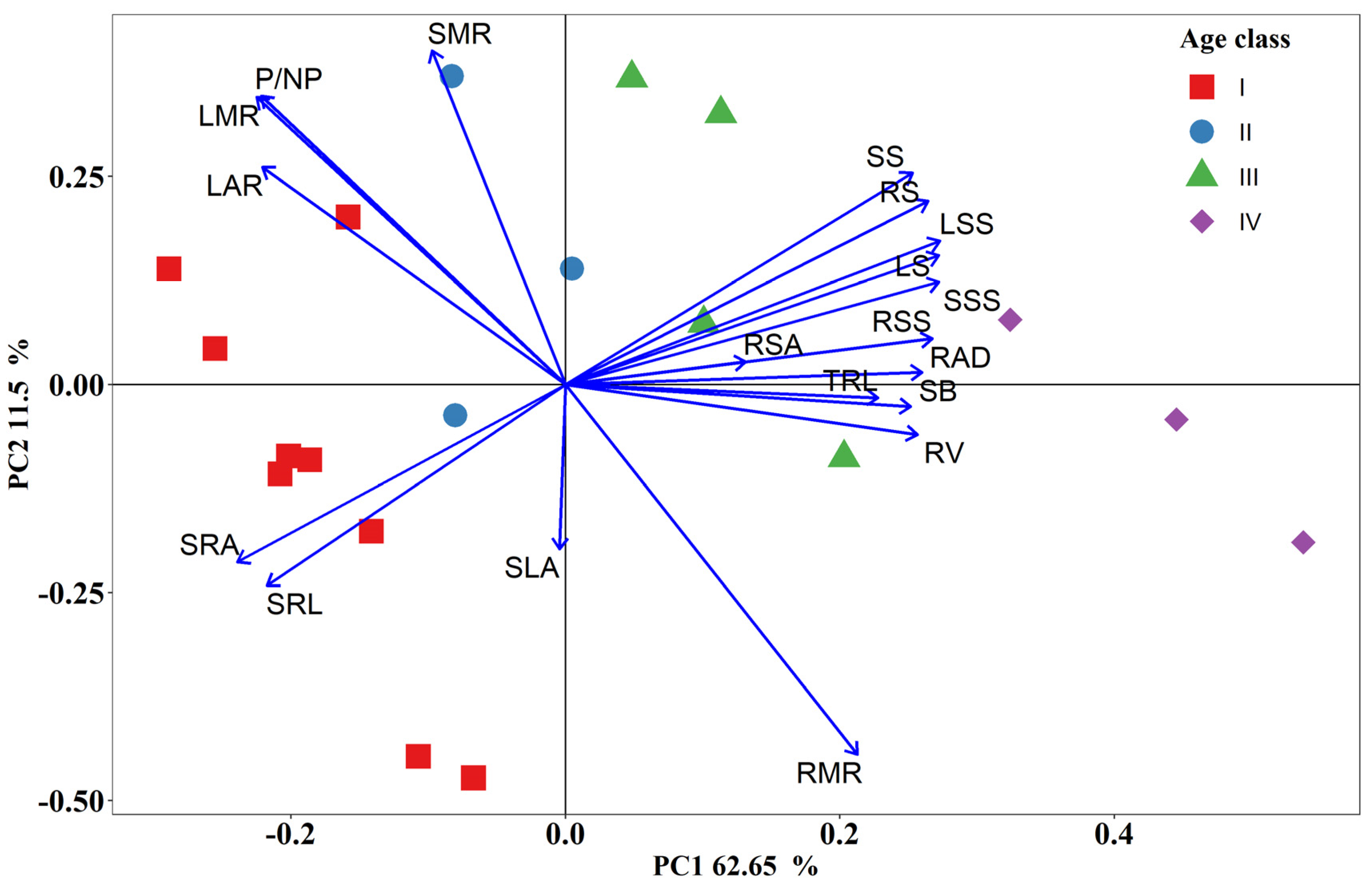
3.5. Correlation Analysis of Seedling Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yang, Y.; Wang, H. Development strategy and management countermeasures of planted forests in China: Transforming from timber-centered single objective management towards multi-purpose management for enhancing quality and benefits of ecosystem services. Acta Ecol. Sin. 2018, 3, 1–10. [Google Scholar] [CrossRef]
- Jaworski, A.; Podlaski, R. Structure and dynamics of selected stands of primeval character in the Pieniny National Park. Dendrobiology 2007, 58, 25–41. [Google Scholar]
- Szymura, T.H.; Dunajski, A.; Aman, I.; Makowski, M.; Szymura, M. The spatial pattern and microsites requirements of Abies alba natural regeneration in the Karkonosze Mountains. Dendrobiology 2007, 58, 51–57. [Google Scholar]
- Madsen, P.; Hahn, K. Natural regeneration in a beech-dominated forest managed by close-to-nature principles—A gap cutting based experiment. Can. J. For. Res. 2008, 38, 1716–1729. [Google Scholar] [CrossRef]
- Agestam, E.; Ekö, P.M.; Nilsson, U.; Welander, N.T. The effects of shelterwood density and site preparation on natural regeneration of Fagus sylvatica in southern Sweden. For. Ecol. Manag. 2003, 176, 61–73. [Google Scholar] [CrossRef]
- Petritan, A.M.; Biris, I.A.; Merce, O.; Turcu, D.O.; Petritan, I.C. Structure and diversity of a natural temperate sessile oak (Quercus petraea L.)—European Beech (Fagus sylvatica L.) forest. For. Ecol. Manag. 2012, 280, 140–149. [Google Scholar] [CrossRef]
- Pröll, G.; Darabant, A.; Gratzer, G.; Katzensteiner, K. Unfavourable microsites, competing vegetation and browsing restrict post-disturbance tree regeneration on extreme sites in the Northern Calcareous Alps. Eur. J. For. Res. 2015, 134, 293–308. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef] [Green Version]
- Jarčuška, B. Growth, survival, density, biomass partitioning and morphological adaptations of natural regeneration in Fagus sylvatica. A review. Dendrobiology 2009, 61, 3–11. [Google Scholar]
- Valladares, F.; Laanisto, L.; Niinemets, Ü.; Zavala, M.A. Shedding light on shade: Ecological perspectives of understorey plant life. Plant Ecol. Divers 2016, 9, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Guariguata, M.R. Seed and seedling ecology of tree species in neotropical secondary forests: Management implications. Ecol. Appl. 2000, 10, 145–154. [Google Scholar] [CrossRef]
- Barna, M.; Bosela, M. Tree species diversity change in natural regeneration of a beech forest under different management. For. Ecol. Manag. 2015, 342, 93–102. [Google Scholar] [CrossRef]
- Pardos, M.; Montes, F.; Aranda, I.; Cañellas, I. Influence of environmental conditions on germinant survival and diversity of Scots pine (Pinus sylvestris L.) in central Spain. Eur. J. For. Res. 2007, 126, 37–47. [Google Scholar] [CrossRef]
- Szewczyk, J.; Szwagrzyk, J. Spatial and temporal variability of natural regeneration in a temperate old-growth forest. Ann. For. Sci. 2010, 67, 202. [Google Scholar] [CrossRef]
- Dobrowolska, D. Effect of stand density on oak regeneration in flood plain forests in Lower Silesia, Poland. Forestry 2008, 81, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Sinz, A.; Gardiner, E.S.; Lockhart, B.R.; Souter, R.A. Morphological acclimation and growth of ash (Fraxinus pennsylvanica Marsh.) advance regeneration following overstory harvesting in a Mississippi River floodplain forest. For. Ecol. Manag. 2011, 261, 246–254. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M.; Rugani, T.; Diaci, J. Gap regeneration and replacement patterns in an old-growth Fagus-Abies forest of Bosnia-Herzegovina. Plant Ecol. 2010, 208, 307–318. [Google Scholar] [CrossRef]
- Príncipe, A.; Nunes, A.; Pinho, P.; do Rosário, L.; Correia, O.; Branquinho, C. Modeling the long-term natural regeneration potential of woodlands in semi-arid regions to guide restoration efforts. Eur. J. For. Res. 2014, 133, 757–767. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, E.; Gratzer, G.; Bravo, F. Climatic variability and other site factor influences on natural regeneration of Pinus pinaster Ait. in Mediterranean forests. Ann. For. Sci. 2011, 68, 811–823. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolska, D.; Bolibok, L. Is climate the key factor limiting the natural regeneration of silver fir beyond the northeastern border of its distribution range? For. Ecol. Manag. 2019, 439, 105–121. [Google Scholar] [CrossRef]
- Rodríguez-García, E.; Juez, L.; Bravo, F. Environmental influences on post-harvest natural regeneration of Pinus pinaster Ait. in Mediterranean forest stands submitted to the seed-tree selection method. Eur. J. For. Res. 2010, 129, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Szwagrzyk, J.; Szewczyk, J.; Bodziarczyk, J. Dynamics of seedling banks in beech forest: Results of a 10-year study on germination, growth and survival. For. Ecol. Manag. 2001, 141, 237–250. [Google Scholar] [CrossRef]
- Rey, P.J.; Alcántara, J.M. Recruitment dynamics of a fleshy-fruited plant (Olea europaea): Connecting patterns of seed dispersal to seedling establishment. J. Ecol. 2000, 88, 622–633. [Google Scholar] [CrossRef]
- Gaucher, C.; Gougeon, S.; Mauffette, Y.; Messier, C. Seasonal variation in biomass and carbohydrate partitioning of understory sugar maple (Acer saccharum) and yellow birch (Betula alleghaniensis) seedlings. Tree Physiol. 2005, 25, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Balandier, P.; Sinoquet, H.; Frak, E.; Giuliani, R.; Vandame, M.; Descamps, S.; Coll, L.; Adam, B.; Prevosto, B.; Curt, T. Six-year time course of light-use efficiency, carbon gain and growth of beech saplings (Fagus sylvatica) planted under a Scots pine (Pinus sylvestris) shelterwood. Tree Physiol. 2007, 27, 1073–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claveau, Y.; Messier, C.; Comeau, P.G. Interacting influence of light and size on aboveground biomass distribution in sub-boreal conifer saplings with contrasting shade tolerance. Tree Physiol. 2005, 25, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Lusk, C.H.; Piper, F.I. Seedling size influences relationships of shade tolerance with carbohydrate-storage patterns in a temperate rainforest. Funct. Ecol. 2007, 21, 78–86. [Google Scholar] [CrossRef]
- Sendall, K.M.; Reich, P.B.; Lusk, C.H. Size-related shifts in carbon gain and growth responses to light differ among rainforest evergreens of contrasting shade tolerance. Oecologia 2018, 187, 609–623. [Google Scholar] [CrossRef]
- Gasser, D.; Messier, C.; Beaudet, M.; Lechowicz, M.J. Sugar maple and yellow birch regeneration in response to canopy opening, liming and vegetation control in a temperate deciduous forest of Quebec. For. Ecol. Manag. 2010, 259, 2006–2014. [Google Scholar] [CrossRef] [Green Version]
- Soto, D.P.; Jacobs, D.F.; Salas, C.; Donoso, P.J.; Fuentes, C.; Puettmann, K.J. Light and nitrogen interact to influence regeneration in old-growth Nothofagus-dominated forests in south-central Chile. For. Ecol. Manag. 2017, 384, 303–313. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Lusk, C.H.; Falster, D.S.; Jara-Vergara, C.K.; Jimenez-Castillo, M.; Saldaña-Mendoza, A. Ontogenetic variation in light requirements of juvenile rainforest evergreens. Funct. Ecol. 2008, 22, 454–459. [Google Scholar] [CrossRef]
- Deng, X.; Xiao, W.; Shi, Z.; Zeng, L.; Lei, L. Combined effects of drought and shading on growth and non-structural carbohydrates in Pinus massoniana lamb. Seedlings. Forests 2020, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Duque, L.O.; Setter, T.L. Partitioning index and non-structural carbohydrate dynamics among contrasting cassava genotypes under early terminal water stress. Environ. Exp. Bot. 2019, 163, 24–35. [Google Scholar] [CrossRef]
- Gleason, S.M.; Ares, A. Photosynthesis, carbohydrate storage and survival of a native and an introduced tree species in relation to light and defoliation. Tree Physiol. 2004, 24, 1087–1097. [Google Scholar] [CrossRef] [Green Version]
- Veneklaas, E.J.; Den Ouden, F. Dynamics of non-structural carbohydrates in two Ficus species after transfer to deep shade. Environ. Exp. Bot. 2005, 54, 148–154. [Google Scholar] [CrossRef]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Galvez, D.A.; Landhäusser, S.M.; Tyree, M.T. Low root reserve accumulation during drought may lead to winter mortality in poplar seedlings. New Phytol. 2013, 198, 139–148. [Google Scholar] [CrossRef]
- Ivanov, Y.V.; Kartashov, A.V.; Zlobin, I.E.; Sarvin, B.; Stavrianidi, A.N.; Kuznetsov, V.V. Water deficit-dependent changes in non-structural carbohydrate profiles, growth and mortality of pine and spruce seedlings in hydroculture. Environ. Exp. Bot. 2019, 157, 151–160. [Google Scholar] [CrossRef]
- Tomasella, M.; Casolo, V.; Aichner, N.; Petruzzellis, F.; Savi, T.; Trifilò, P.; Nardini, A. Non-structural carbohydrate and hydraulic dynamics during drought and recovery in Fraxinus ornus and Ostrya carpinifolia saplings. Plant Physiol. Biochem. 2019, 145, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Imaji, A.; Seiwa, K. Carbon allocation to defense, storage, and growth in seedlings of two temperate broad-leaved tree species. Oecologia 2010, 162, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Piper, F.I.; Reyes-Díaz, M.; Corcuera, L.J.; Lusk, C.H. Carbohydrate storage, survival, and growth of two evergreen Nothofagus species in two contrasting light environments. Ecol. Res. 2009, 24, 1233–1241. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Liu, Y. The effect of low irradiance on growth, photosynthetic characteristics, and biomass allocation in two deciduous broad-leaved tree seedlings in southeast of Hubei Province. Acta Ecol. Sin. 2010, 30, 6082–6090. [Google Scholar]
- Xue, W.Y.; Yang, B.; Zhang, W.H.; Yu, S.C. Spatial pattern and spatial association of Quercus acutissima at different developmental stages in the Qiaoshan mountains. Acta Ecol. Sin. 2017, 37, 3375–3384. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Cao, B.; Dong, C.; Dong, X. Mount Taishan Forest ecosystem health assessment based on forest inventory data. Forests 2019, 10, 657. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Cao, B.; Mao, P.; Dong, C.; Cao, X.; Qi, L.; Wang, M.; Wu, Y. Tree species distribution change study in Mount Tai based on Landsat remote sensing image data. Forests 2020, 11, 130. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wu, D.; Yu, D.; Sui, C. Variations of nonstructural carbohydrate content in Betula ermanii at different elevations of Changbai Mountain, China. Chin. J. Plant Ecol. 2009, 33, 118–124. [Google Scholar] [CrossRef]
- Lai, H.R.; Craven, D.; Hall, J.S.; Hui, F.K.C.; van Breugel, M. Successional syndromes of saplings in tropical secondary forests emerge from environment-dependent trait–demography relationships. Ecol. Lett. 2021, 24, 1776–1787. [Google Scholar] [CrossRef]
- Dodson, E.K.; Root, H.T. Conifer regeneration following stand-replacing wildfire varies along an elevation gradient in a ponderosa pine forest, Oregon, USA. For. Ecol. Manag. 2013, 302, 163–170. [Google Scholar] [CrossRef]
- Silva, D.; Rezende Mazzella, P.; Legay, M.; Corcket, E.; Dupouey, J.L. Does natural regeneration determine the limit of European beech distribution under climatic stress? For. Ecol. Manag. 2012, 266, 263–272. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Schwalm, C.R.; Anderegg, W.R.L. Ghosts of the past: How drought legacy effects shape forest functioning and carbon cycling. Ecol. Lett. 2020, 23, 891–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammer, C.; Stimm, B.; Mosandl, R. Ontogenetic variation in the relative influence of light and belowground resources on European beech seedling growth. Tree Physiol. 2008, 28, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collet, C.; Lanter, O.; Pardos, M. Effects of canopy opening on the morphology and anatomy of naturally regenerated beech seedlings. Trees Struct. Funct. 2002, 16, 291–298. [Google Scholar] [CrossRef]
- Di Iorio, A.; Montagnoli, A.; Scippa, G.S.; Chiatante, D. Fine root growth of Quercus pubescens seedlings after drought stress and fire disturbance. Environ. Exp. Bot. 2011, 74, 272–279. [Google Scholar] [CrossRef]
- Zang, U.; Goisser, M.; Häberle, K.H.; Matyssek, R.; Matzner, E.; Borken, W. Effects of drought stress on photosynthesis, rhizosphere respiration, and fine-root characteristics of beech saplings: A rhizotron field study. J. Plant Nutr. Soil Sci. 2014, 177, 168–177. [Google Scholar] [CrossRef]
- Torreano, S.J.; Morris, L.A. Loblolly Pine Root Growth and Distribution under Water Stress. Soil Sci. Soc. Am. J. 1998, 62, 818–827. [Google Scholar] [CrossRef]
- Bengough, A.G.; Bransby, M.F.; Hans, J.; McKenna, S.J.; Roberts, T.J.; Valentine, T.A. Root responses to soil physical conditions; growth dynamics from field to cell. J. Exp. Bot. 2006, 57, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Gholz, H.L.; Cropper, W.P.J. Carbohydrate dynamics in mature pinus elliottii var. elliottii trees. Can. J. For. Res. 1991, 21, 1742–1747. [Google Scholar] [CrossRef]
- Kobe, R.K. Carbohydrate Allocation to Storage as a Basis of Interspecific Variation in Sapling Survivorship and Growth. Oikos 1997, 80, 226. [Google Scholar] [CrossRef]
- Walters, M.B.; Kruger, E.L.; Reich, P.B. Growth, biomass distribution and CO2 exchange of northern hardwood seedlings in high and low light: Relationships with successional status and shade tolerance. Oecologia 1993, 94, 7–16. [Google Scholar] [CrossRef] [PubMed]
| Seedling Characteristic Indices | Abbreviations | Units |
|---|---|---|
| Relative soil water content | RSWC | % |
| Seedling biomass (leaf + stem + root mass) | SB | g |
| Leaf mass ratio (leaf dry mass/total seedling dry mass) | LMR | g g−1 |
| Stem mass ratio (stem + petiole mass)/total plant mass | SMR | g g−1 |
| Root mass ratio (root dry mass/total seedling dry mass) | RMR | g g−1 |
| Specific leaf area (total leaf area/total leaf dry mass) | SLA | cm2 g−1 |
| Leaf area ratio (total leaf area/total seedling dry mass) | LAR | cm2 g−1 |
| Photosynthetic tissues/non-photosynthetic tissues (leaf mass/(stem + root mass)) | P/NP | g g−1 |
| Total Root length | TRL | cm |
| Root volume | RV | cm3 |
| Root average diameter | RAD | mm |
| Root total surface area | RSA | cm2 |
| Specific root length (root length/dry root mass) | SRL | cm g−1 |
| Specific root area (root surface area/ dry root mass) | SRA | cm2 g−1 |
| Root soluble sugar | RSS | mg g−1 |
| Stem soluble sugar | SSS | mg g−1 |
| Leaf soluble sugar | LSS | mg g−1 |
| Root starch | RS | mg g−1 |
| Stem starch | SS | mg g−1 |
| Leaf starch | LS | mg g−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Ni, R.; Kan, X.; Lin, Q.; Mao, P.; Cao, B.; Gao, P.; Dong, J.; Mi, W.; Zhao, B. Effects of Precipitation and Soil Moisture on the Characteristics of the Seedling Bank under Quercus acutissima Forest Plantation in Mount Tai, China. Forests 2022, 13, 545. https://doi.org/10.3390/f13040545
Guo L, Ni R, Kan X, Lin Q, Mao P, Cao B, Gao P, Dong J, Mi W, Zhao B. Effects of Precipitation and Soil Moisture on the Characteristics of the Seedling Bank under Quercus acutissima Forest Plantation in Mount Tai, China. Forests. 2022; 13(4):545. https://doi.org/10.3390/f13040545
Chicago/Turabian StyleGuo, Longmei, Ruiqiang Ni, Xiaoli Kan, Qingzhi Lin, Peili Mao, Banghua Cao, Peng Gao, Jinwei Dong, Wendong Mi, and Boping Zhao. 2022. "Effects of Precipitation and Soil Moisture on the Characteristics of the Seedling Bank under Quercus acutissima Forest Plantation in Mount Tai, China" Forests 13, no. 4: 545. https://doi.org/10.3390/f13040545






