Mixed Forest of Larix principis-rupprechtii and Betula platyphylla Modulating Soil Fauna Diversity and Improving Faunal Effect on Litter Decomposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Statistical Analyses
3. Results
3.1. Soil Fauna Community Characteristics
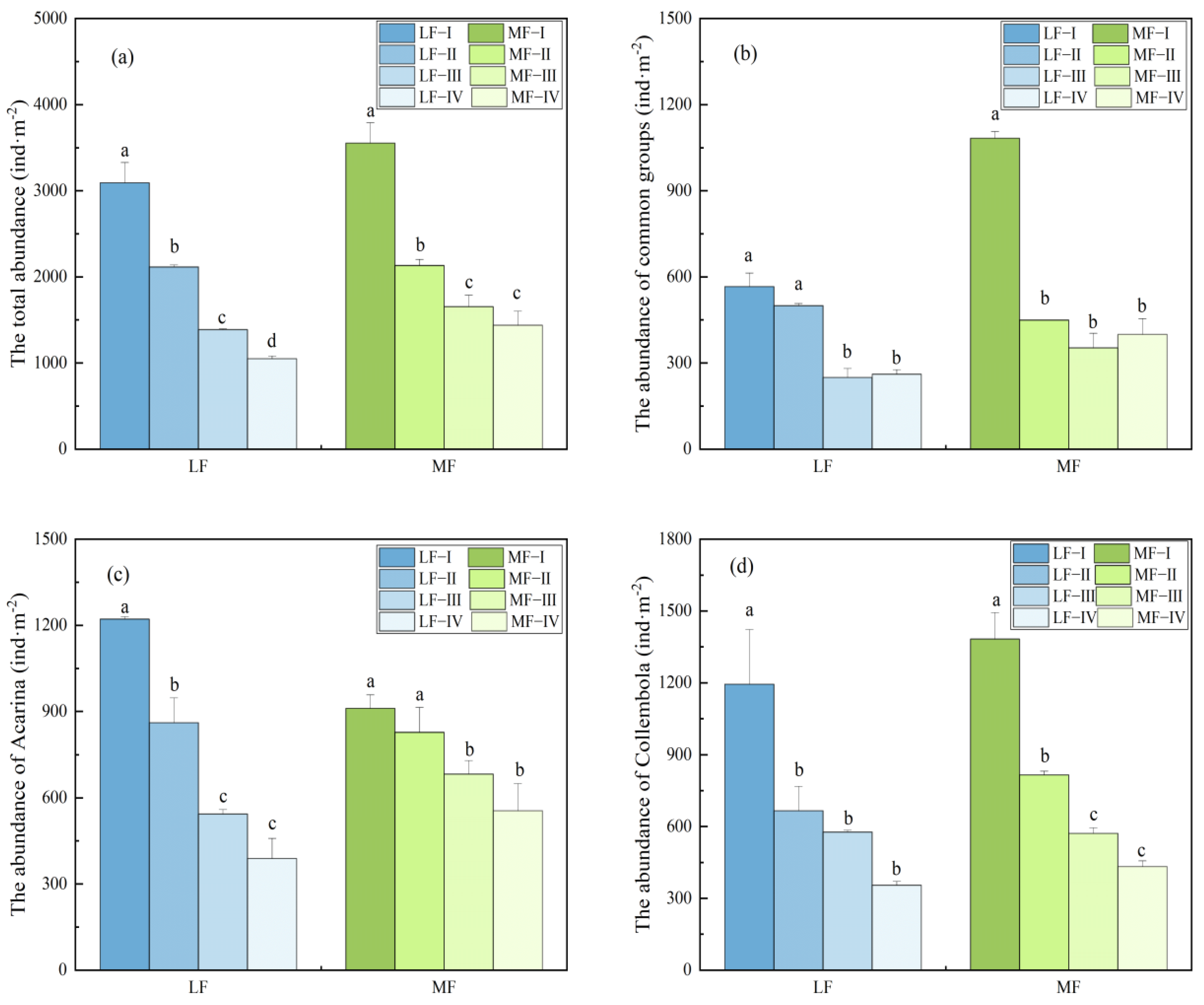
3.1.1. Temporal and Vertical Distribution of Soil Fauna Community
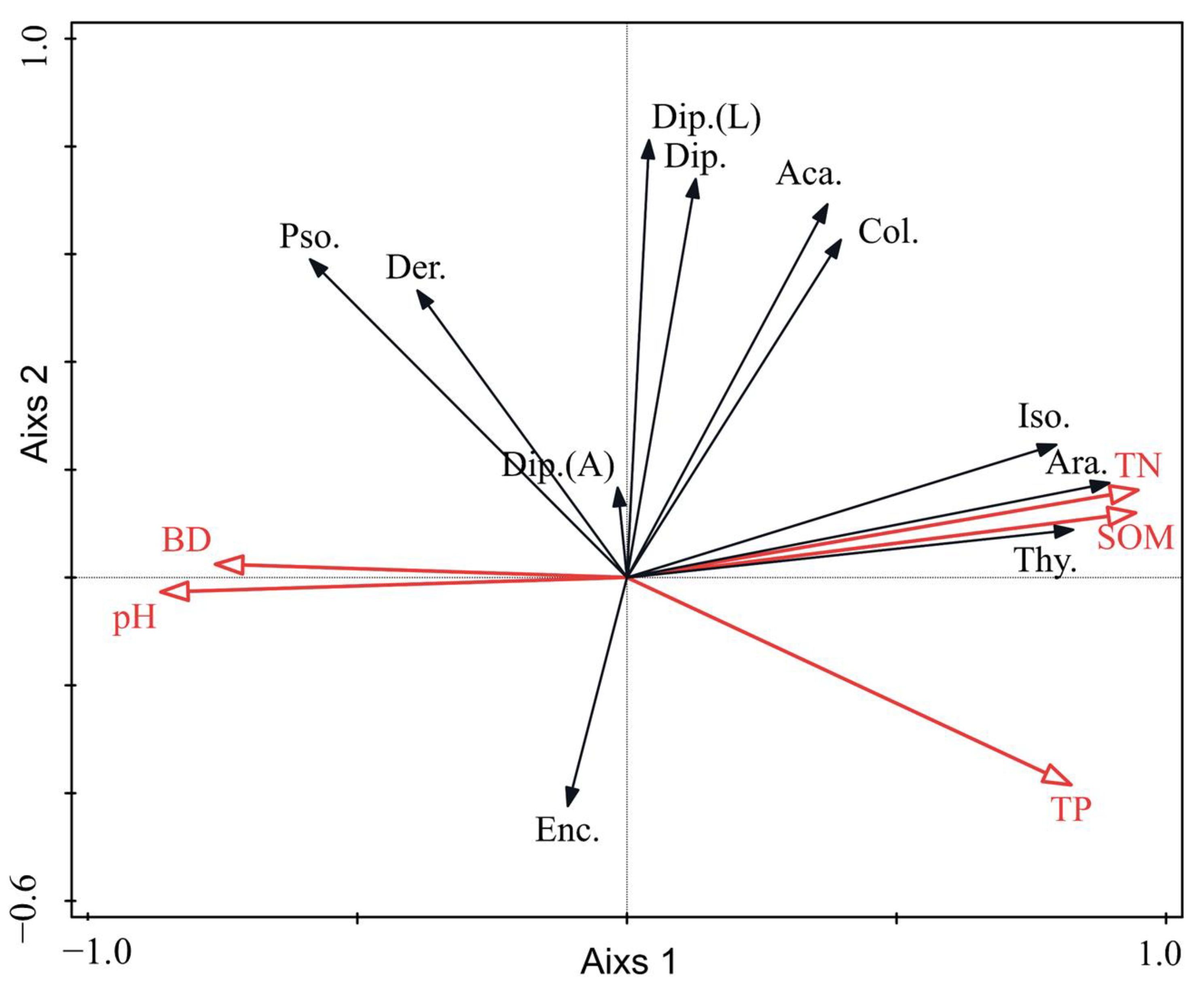
3.1.2. Correlation Analysis between the Soil Fauna and Soil Properties
3.2. Effects of Soil Fauna on Litter Decomposition
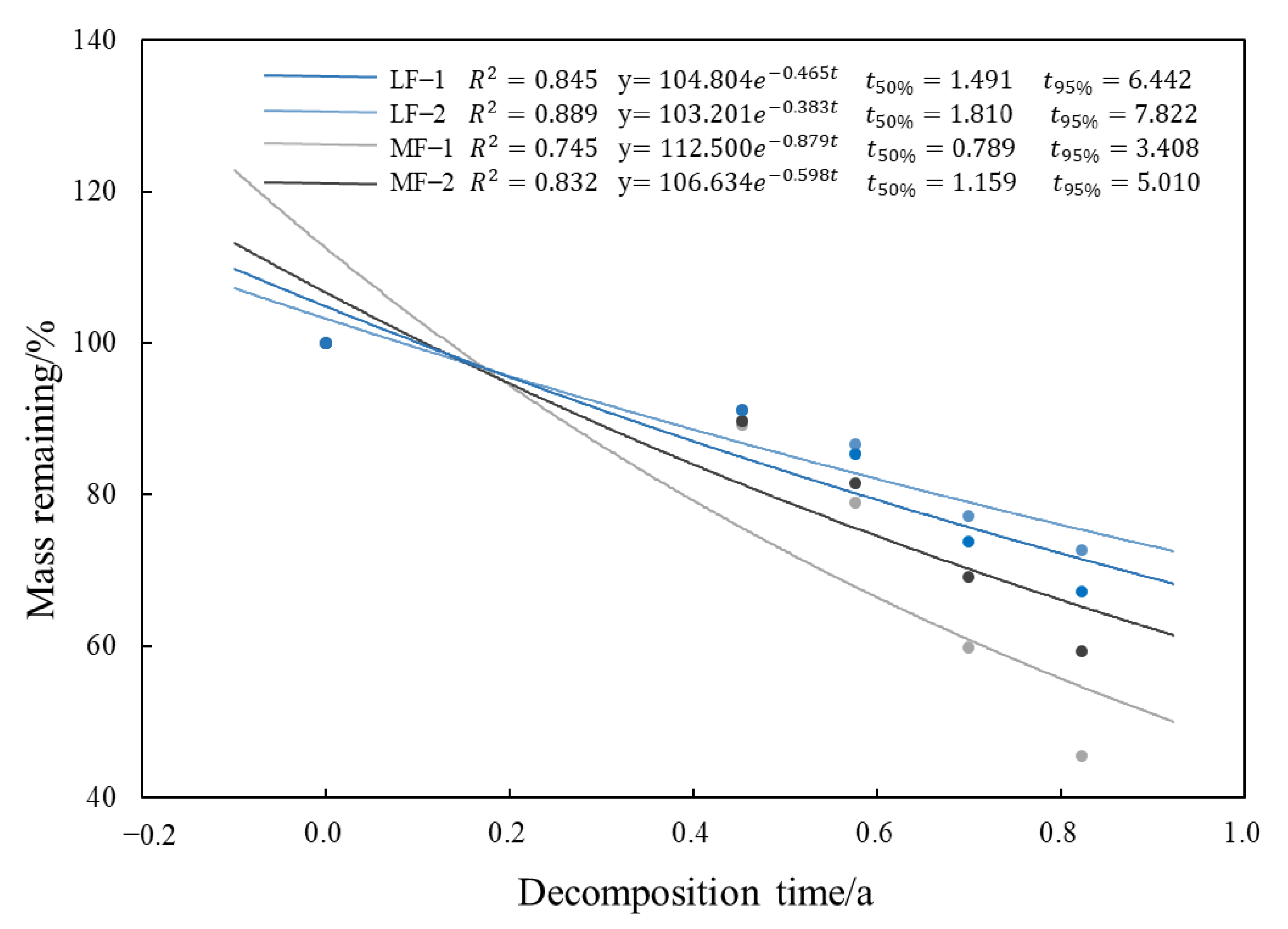
3.2.1. Dynamic Characteristics of Litter Decomposition
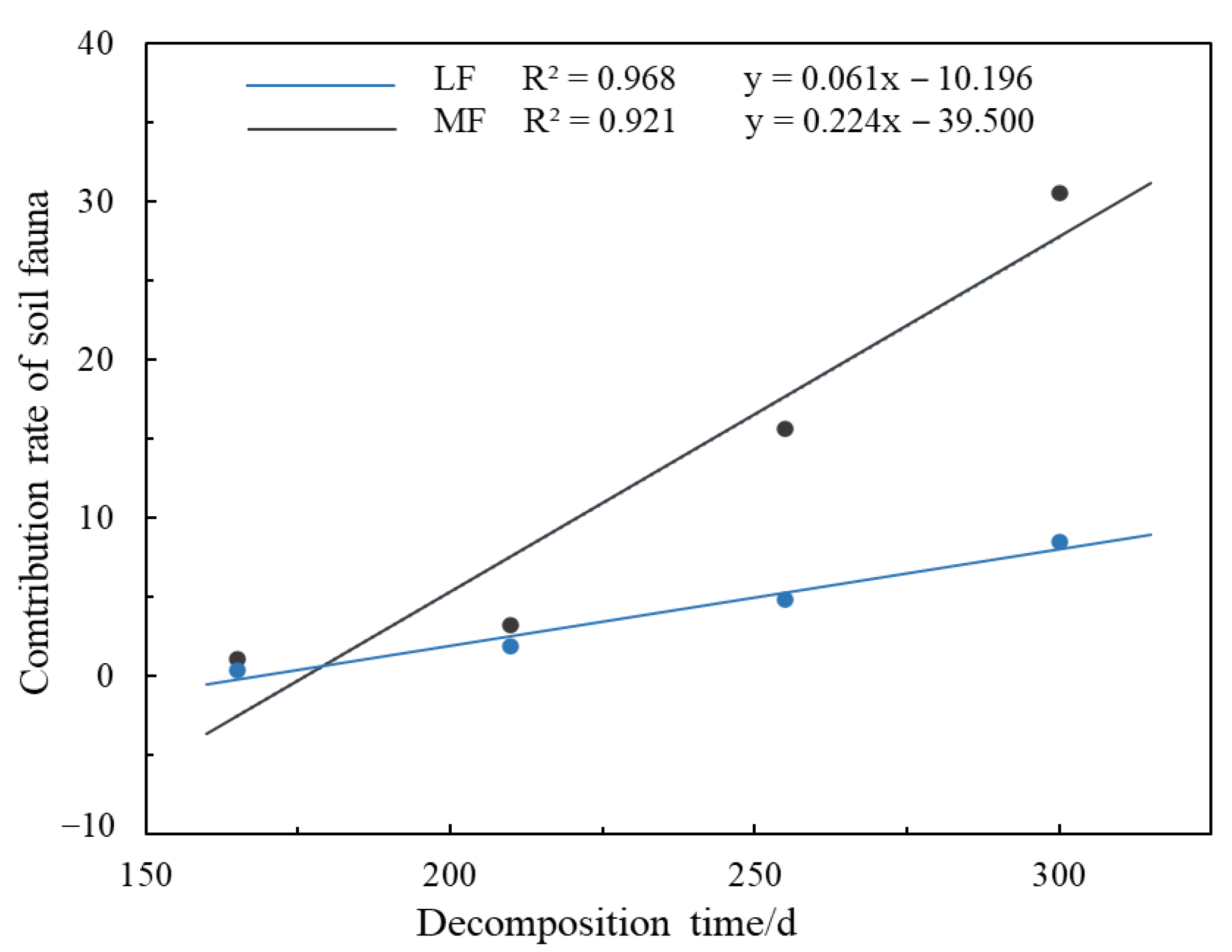
3.2.2. Contribution of the Soil Fauna Community to Litter Decomposition
4. Discussion
4.1. Soil Fauna Community Characteristics
4.2. Effects of Soil Fauna on Litter Decomposition
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yin, X.; Song, B.; Dong, W.; Xin, W.; Wang, Y. A Review on the Eco-Geography of Soil Fauna in China. J. Geogr. Sci. 2010, 20, 333–346. [Google Scholar] [CrossRef]
- García-Palacios, P.; Maestre, F.T.; Kattge, J.; Wall, D.H. Climate and Litter Quality Differently Modulate the Effects of Soil Fauna on Litter Decomposition across Biomes. Ecol. Lett. 2013, 16, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo, Y.; Ball, B.A.; Bradford, M.A.; Jordan, C.F.; Molina, M. Soil Fauna Alter the Effects of Litter Composition on Nitrogen Cycling in a Mineral Soil. Soil Biol. Biochem. 2011, 43, 1440–1449. [Google Scholar] [CrossRef]
- Gong, X.; Wang, S.; Wang, Z.; Jiang, Y.; Hu, Z.; Zheng, Y.; Chen, X.; Li, H.; Hu, F.; Liu, M.; et al. Earthworms Modify Soil Bacterial and Fungal Communities through Enhancing Aggregation and Buffering PH. Geoderma 2019, 347, 59–69. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Chan, K.F. Experimental Evidence That Soil Fauna Enhance Nutrient Mineralization and Plant Nutrient Uptake in Montane Grassland Ecosystems. Soil Biol. Biochem. 1999, 31, 1007–1014. [Google Scholar] [CrossRef]
- Yin, X.; Qiu, L.; Jiang, Y.; Wang, Y. Diversity and Spatial-Temporal Distribution of Soil Macrofauna Communities Along Elevation in the Changbai Mountain, China. Environ. Entomol. 2017, 46, 454–459. [Google Scholar] [CrossRef]
- Xu, J.; Lie, G.; Xue, L. Effects of Gap Size on Diversity of Soil Fauna in a Cunninghamia Lanceolata Stand Damaged by an Ice Storm in Southern China. J. For. Res. 2016, 27, 1427–1434. [Google Scholar] [CrossRef]
- Xu, H.; Yu, L.; Huang, X. Biodiversity of Macro Ground-Dwelling Arthropods in Secondary Forests and Plantationforests of Montane Region of Eastern Liaoning Province. Chin. J. Ecol. 2015, 34, 727–735. (In Chinese) [Google Scholar]
- dos Santos, R.N.; Cabreira, W.V.; Pereira, M.G.; de Souza, R.C.; de Lima, S.S.; Louzada, M.A.P.; dos Santos, G.L.; Silva, A.C.R. da Community Ecology of Soil Fauna Under Periodically Flooded Forest and Anthropic Fields. Floresta Ambient 2020, 28, e20200052. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, W.; Xu, H.; Wang, S.; Xu, X.; Fan, H.; Chen, H.Y.H.; Ruan, H. Effects of Soil Fauna on Leaf Litter Decomposition under Different Land Uses in Eastern Coast of China. J. For. Res. 2018, 29, 973–982. [Google Scholar] [CrossRef]
- Tan, B.; Yin, R.; Zhang, J.; Xu, Z.; Liu, Y.; He, S.; Zhang, L.; Li, H.; Wang, L.; Liu, S.; et al. Temperature and Moisture Modulate the Contribution of Soil Fauna to Litter Decomposition via Different Pathways. Ecosystems 2021, 24, 1142–1156. [Google Scholar] [CrossRef]
- Njoroge, D.M.; Chen, S.-C.; Zuo, J.; Dossa, G.G.O.; Cornelissen, J.H.C. Soil Fauna Accelerate Litter Mixture Decomposition Globally, Especially in Dry Environments. J. Ecol. 2022, 110, 659–672. [Google Scholar] [CrossRef]
- Hu, M.M.; Tian, L.; Wu, Y.N. Influences of Thinning and Mixed Transformation of Larix Principis-Rupprechtii Plantations on the Community Structure of Soil Macro Faunal in Saihanba Area. Sci. Silvae Sin. 2019, 55, 153–162. (In Chinese) [Google Scholar]
- Yang, X.; Shao, M.; Li, T.; Gan, M.; Chen, M. Community Characteristics and Distribution Patterns of Soil Fauna after Vegetation Restoration in the Northern Loess Plateau. Ecol. Indic. 2021, 122, 107236. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, Y.; Gao, B. Influence of Enviroment of Forest-Steppe Ecotone on Soil Arthropods Community in Northern Hebei, China. Procedia Environ. Sci. 2011, 10, 1862–1867. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.Y. Pictorial Keys to Soil Animals of China; Science Press: Beijing, China, 1998. [Google Scholar]
- Swift, M.J.; Andrén, O.; Brussaard, L.; Briones, M.; Couteaux, M.-M.; Ekschmitt, K.; Kjoller, A.; Loiseau, P.; Smith, P. Global Change, Soil Biodiversity, and Nitrogen Cycling in Terrestrial Ecosystems: Three Case Studies. Glob. Change Biol. 1998, 4, 729–743. [Google Scholar] [CrossRef]
- Crutsinger, G.M.; Sanders, N.J.; Classen, A.T. Comparing Intra- and Inter-Specific Effects on Litter Decomposition in an Old-Field Ecosystem. Basic Appl. Ecol. 2009, 10, 535–543. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Kenney, D.R. Methods of Soil Analysis Part 2 (Agronomy Monographs 9); American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Dick, W.A.; Cheng, L.; Wang, P. Soil Acid and Alkaline Phosphatase Activity as PH Adjustment Indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Pielou, E.C. The Measurement of Diversity in Different Types of Biological Collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Gao, J.; Kang, F.; Li, T.; Song, X.; Zhao, W.; Yu, X.; Han, H. Assessing the Effect of Leaf Litter Diversity on the Decomposition and Associated Diversity of Fungal Assemblages. Forests 2015, 6, 2371–2386. [Google Scholar] [CrossRef] [Green Version]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Seastedt, T.R. The Role of Microarthropods in Decomposition and Mineralization Processes. Annu. Rev. Entomol. 1984, 29, 25–46. [Google Scholar] [CrossRef]
- Hemerik, L.; Brussaard, L. Diversity of Soil Macro-Invertebrates in Grasslands under Restoration Succession. Eur. J. Soil Biol. 2002, 38, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Caruso, T.; Melecis, V.; Kagainis, U.; Bolger, T. Population Asynchrony Alone Does Not Explain Stability in Species-Rich Soil Animal Assemblages: The Stabilizing Role of Forest Age on Oribatid Mite Communities. J. Anim. Ecol. 2020, 89, 1520–1531. [Google Scholar] [CrossRef]
- Wei, Q.; Yin, R.; Huang, J.; Vogler, A.P.; Li, Y.; Miao, X.; Kardol, P. The Diversity of Soil Mesofauna Declines after Bamboo Invasion in Subtropical China. Sci. Total Environ. 2021, 789, 147982. [Google Scholar] [CrossRef]
- Peng, Y.; Holmstrup, M.; Schmidt, I.K.; De Schrijver, A.; Schelfhout, S.; Heděnec, P.; Zheng, H.; Bachega, L.R.; Yue, K.; Vesterdal, L. Litter Quality, Mycorrhizal Association, and Soil Properties Regulate Effects of Tree Species on the Soil Fauna Community. Geoderma 2022, 407, 115570. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Bretscher, D. Isopod Effects on Decomposition of Litter Produced under Elevated CO2, N Deposition and Different Soil Types. Glob. Change Biol. 2001, 7, 565–579. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Gasser, P. Soil Animals Alter Plant Litter Diversity Effects on Decomposition. Proc. Natl. Acad. Sci. USA 2005, 102, 1519–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santonja, M.; Fernandez, C.; Proffit, M.; Gers, C.; Gauquelin, T.; Reiter, I.M.; Cramer, W.; Baldy, V. Plant Litter Mixture Partly Mitigates the Negative Effects of Extended Drought on Soil Biota and Litter Decomposition in a Mediterranean Oak Forest. J. Ecol. 2017, 105, 801–815. [Google Scholar] [CrossRef] [Green Version]
- Vos, V.C.A.; van Ruijven, J.; Berg, M.P.; Peeters, E.T.H.M.; Berendse, F. Macro-Detritivore Identity Drives Leaf Litter Diversity Effects. Oikos 2011, 120, 1092–1098. [Google Scholar] [CrossRef]
- Chauvat, M.; Titsch, D.; Zaytsev, A.S.; Wolters, V. Changes in Soil Faunal Assemblages during Conversion from Pure to Mixed Forest Stands. For. Ecol. Manag. 2011, 262, 317–324. [Google Scholar] [CrossRef]
- Lavelle, P. Faunal Activities and Soil Processes: Adaptive Strategies That Determine Ecosystem Function. In Advances in Ecological Research; Begon, M., Fitter, A.H., Eds.; Academic Press: Cambridge, MA, USA, 1997; Volume 27, pp. 93–132. [Google Scholar]
- Moore, J.C.; de Ruiter, P.C.; Hunt, H.W.; Coleman, D.C.; Freckman, D.W. Microcosms and Soil Ecology: Critical Linkages between Fields Studies and Modelling Food Webs. Ecology 1996, 77, 694–705. [Google Scholar] [CrossRef]
- Brussaard, L. Soil Fauna, Guilds, Functional Groups and Ecosystem Processes. Appl. Soil Ecol. 1998, 9, 123–135. [Google Scholar] [CrossRef]
- Brussaard, L.; Pulleman, M.M.; Ouédraogo, É.; Mando, A.; Six, J. Soil Fauna and Soil Function in the Fabric of the Food Web. Pedobiologia 2007, 50, 447–462. [Google Scholar] [CrossRef]
- Ferris, H.; Matute, M.M. Structural and Functional Succession in the Nematode Fauna of a Soil Food Web. Appl. Soil Ecol. 2003, 23, 93–110. [Google Scholar] [CrossRef]
- Inkotte, J.; Bomfim, B.; da Silva, S.C.; Valadão, M.B.X.; da Rosa, M.G.; Viana, R.B.; D’Ângelo Rios, P.; Gatto, A.; Pereira, R.S. Linking Soil Biodiversity and Ecosystem Function in a Neotropical Savanna. Appl. Soil Ecol. 2022, 169, 104209. [Google Scholar] [CrossRef]
- Korboulewsky, N.; Perez, G.; Chauvat, M. How Tree Diversity Affects Soil Fauna Diversity: A Review. Soil Biol. Biochem. 2016, 94, 94–106. [Google Scholar] [CrossRef]
- Scheu, S. Linkages Between Tree Diversity, Soil Fauna and Ecosystem Processes. In Forest Diversity and Function: Temperate and Boreal Systems; Scherer-Lorenzen, M., Körner, C., Schulze, E.-D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 211–233. ISBN 978-3-540-26599-3. [Google Scholar]
- Wardle, D.A. The Influence of Biotic Interactions on Soil Biodiversity. Ecol. Lett. 2006, 9, 870–886. [Google Scholar] [CrossRef] [PubMed]
- Huuskonen, S.; Domisch, T.; Finér, L.; Hantula, J.; Hynynen, J.; Matala, J.; Miina, J.; Neuvonen, S.; Nevalainen, S.; Niemistö, P.; et al. What Is the Potential for Replacing Monocultures with Mixed-Species Stands to Enhance Ecosystem Services in Boreal Forests in Fennoscandia? For. Ecol. Manag. 2021, 479, 118558. [Google Scholar] [CrossRef]
- Ammer, C. Diversity and Forest Productivity in a Changing Climate. New Phytol. 2019, 221, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Río, M.; Pretzsch, H.; Alberdi, I.; Bielak, K.; Bravo, F.; Brunner, A.; Condés, S.; Ducey, M.J.; Fonseca, T.; von Lüpke, N.; et al. Characterization of the Structure, Dynamics, and Productivity of Mixed-Species Stands: Review and Perspectives. Eur. J. Forest Res. 2016, 135, 23–49. [Google Scholar] [CrossRef]
- Pretzsch, H.; del Río, M.; Ammer, C.; Avdagic, A.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; Dirnberger, G.; Drössler, L.; et al. Growth and Yield of Mixed versus Pure Stands of Scots Pine (Pinus Sylvestris L.) and European Beech (Fagus Sylvatica L.) Analysed along a Productivity Gradient through Europe. Eur. J. Forest Res. 2015, 134, 927–947. [Google Scholar] [CrossRef] [Green Version]
- Jacob, M.; Weland, N.; Platner, C.; Schaefer, M.; Leuschner, C.; Thomas, F.M. Nutrient Release from Decomposing Leaf Litter of Temperate Deciduous Forest Trees along a Gradient of Increasing Tree Species Diversity. Soil Biol. Biochem. 2009, 41, 2122–2130. [Google Scholar] [CrossRef]
- Szanser, M.; Ilieva-Makulec, K.; Kajak, A.; Górska, E.; Kusińska, A.; Kisiel, M.; Olejniczak, I.; Russel, S.; Sieminiak, D.; Wojewoda, D. Impact of Litter Species Diversity on Decomposition Processes and Communities of Soil Organisms. Soil Biol. Biochem. 2011, 43, 9–19. [Google Scholar] [CrossRef]
- Chang, L.; Wu, H.; Wu, D.; Sun, X. Effect of Tillage and Farming Management on Collembola in Marsh Soils. Appl. Soil Ecol. 2013, 64, 112–117. [Google Scholar] [CrossRef]
- Tang, T.W.; Feng, Y.; Wu, X.G. Characteristics of Soil Macrofauna Community under Urban Forests in Chengdu in Winter. Chin. J. Appl. Environ. Biol. 2020, 26, 1207–1217. (In Chinese) [Google Scholar]
- Chen, D.; Lan, Z.; Bai, X.; Grace, J.B.; Bai, Y. Evidence That Acidification-Induced Declines in Plant Diversity and Productivity Are Mediated by Changes in below-Ground Communities and Soil Properties in a Semi-Arid Steppe. J. Ecol. 2013, 101, 1322–1334. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, M.; Bilela, S.; Simon, J.; Dannenmann, M.; Liu, X.; Alfarraj, S.; Hou, L.; Chen, H.; Zhang, S.; et al. Nitrogen Nutrition of Native and Introduced Forest Tree Species in N-Limited Ecosystems of the Qinling Mountains, China. Trees 2017, 31, 1189–1202. [Google Scholar] [CrossRef]
- Lu, P.; Tan, Y.; Dai, N.; Di, M.; Weng, X.; Zhan, Y.; Xu, D. Composition and Structure of Soil Fauna Communities and Their Relationships with Environmental Factors in Copper Mine Waste Rock after Re-Vegetation. Glob. Ecol. Conserv. 2021, 32, e01889. [Google Scholar] [CrossRef]
- Bartz, M.L.C.; Pasini, A.; Brown, G.G. Earthworms as Soil Quality Indicators in Brazilian No-Tillage Systems. Appl. Soil Ecol. 2013, 69, 39–48. [Google Scholar] [CrossRef]
- Hong, J.; Lu, X.; Ma, X.; Xiaodan, W. Five-Year Study on the Effects of Warming and Plant Litter Quality on Litter Decomposition Rate in a Tibetan Alpine Grassland. Sci. Total Environ. 2021, 750, 142306. [Google Scholar] [CrossRef]
- Zhou, S.; Butenschoen, O.; Barantal, S.; Handa, I.T.; Makkonen, M.; Vos, V.; Aerts, R.; Berg, M.P.; McKie, B.; Van Ruijven, J.; et al. Decomposition of Leaf Litter Mixtures across Biomes: The Role of Litter Identity, Diversity and Soil Fauna. J. Ecol. 2020, 108, 2283–2297. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J. Plant Litter Quality Influences the Contribution of Soil Fauna to Litter Decomposition in Humid Tropical Forests, Southwestern China. Soil Biol. Biochem. 2009, 41, 910–918. [Google Scholar] [CrossRef]
- Xin, W.D.; Yin, X.Q.; Song, B. Contribution of Soil Fauna to Litter Decomposition in Songnen Sandy Lands in Northeastern China. J. Arid. Environ. 2012, 77, 90–95. [Google Scholar] [CrossRef]
- Zan, P.; Sun, T.; Mao, Z. Effects of Soil Fauna on Litter Decomposition Using FIeld Microcosms across 16 Co-Occurring Temperate Tree Species. Aust. For. 2021, 84, 33–38. [Google Scholar] [CrossRef]
- Lin, D.; Wang, F.; Fanin, N.; Pang, M.; Dou, P.; Wang, H.; Qian, S.; Zhao, L.; Yang, Y.; Mi, X.; et al. Soil Fauna Promote Litter Decomposition but Do Not Alter the Relationship between Leaf Economics Spectrum and Litter Decomposability. Soil Biol. Biochem. 2019, 136, 107519. [Google Scholar] [CrossRef]
- Anderson, J.M.; Ineson, P.; Huish, S.A. Nitrogen and Cation Mobilization by Soil Fauna Feeding on Leaf Litter and Soil Organic Matter from Deciduous Woodlands. Soil Biol. Biochem. 1983, 15, 463–467. [Google Scholar] [CrossRef]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M.; et al. Consequences of Biodiversity Loss for Litter Decomposition across Biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hättenschwiler, S.; Jørgensen, H.B. Carbon Quality Rather than Stoichiometry Controls Litter Decomposition in a Tropical Rain Forest. J. Ecol. 2010, 98, 754–763. [Google Scholar] [CrossRef]
- Makkonen, M.; Berg, M.P.; Handa, I.T.; Hättenschwiler, S.; van Ruijven, J.; van Bodegom, P.M.; Aerts, R. Highly Consistent Effects of Plant Litter Identity and Functional Traits on Decomposition across a Latitudinal Gradient. Ecol. Lett. 2012, 15, 1033–1041. [Google Scholar] [CrossRef]
- Wall, D.H.; Bradford, M.A.; St. John, M.G.; Trofymow, J.A.; Behan-Pelletier, V.; Bignell, D.E.; Dangerfield, J.M.; Parton, W.J.; Rusek, J.; Voigt, W.; et al. Global Decomposition Experiment Shows Soil Animal Impacts on Decomposition Are Climate-Dependent. Glob. Change Biol. 2008, 14, 2661–2677. [Google Scholar] [CrossRef] [Green Version]
- García-Palacios, P.; Prieto, I.; Ourcival, J.-M.; Hättenschwiler, S. Disentangling the Litter Quality and Soil Microbial Contribution to Leaf and Fine Root Litter Decomposition Responses to Reduced Rainfall. Ecosystems 2016, 19, 490–503. [Google Scholar] [CrossRef]
- Sanaullah, M.; Rumpel, C.; Charrier, X.; Chabbi, A. How Does Drought Stress Influence the Decomposition of Plant Litter with Contrasting Quality in a Grassland Ecosystem? Plant Soil 2012, 352, 277–288. [Google Scholar] [CrossRef]
- Walter, J.; Hein, R.; Beierkuhnlein, C.; Hammerl, V.; Jentsch, A.; Schädler, M.; Schuerings, J.; Kreyling, J. Combined Effects of Multifactor Climate Change and Land-Use on Decomposition in Temperate Grassland. Soil Biol. Biochem. 2013, 60, 10–18. [Google Scholar] [CrossRef]
- Rustad, L.E.; Cronan, C.S. Element Loss and Retention during Litter Decay in a Red Spruce Stand in Maine. Can. J. For. Res. 1988, 18, 947–953. [Google Scholar] [CrossRef]
- Staaf, H.; Staaf, H. Influence of Chemical Composition, Addition of Raspberry Leaves, and Nitrogen Supply on Decomposition Rate and Dynamics of Nitrogen and Phosphorus in Beech Leaf Litter. Oikos 1980, 35, 55. [Google Scholar] [CrossRef]
- Taylor, B.R.; Parsons, W.F.J.; Parkinson, D. Decomposition of Populustremuloides Leaf Litter Accelerated by Addition of Alnuscrispa Litter. Can. J. For. Res. 1989, 19, 674–679. [Google Scholar] [CrossRef]
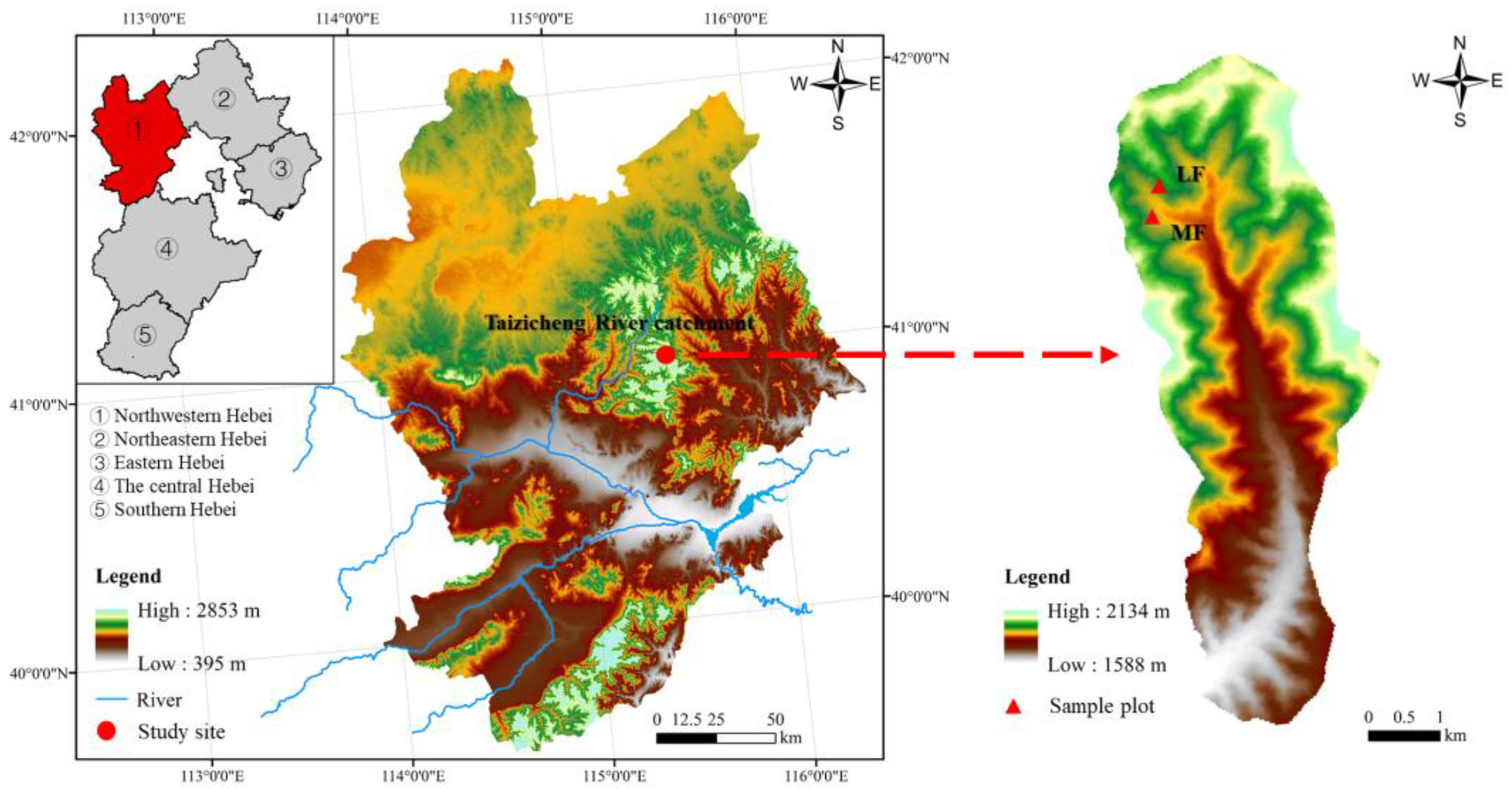




| Stands | Slope Aspect | Longitude and Latitude | Altitude m·a·s·l a | Stand Density (Trees/ha) | Average Height of Trees (m) | Diameter at Breast Height (cm) | Major Understory Vegetation |
|---|---|---|---|---|---|---|---|
| Larix principis-rupprechtii forest (LF) | Southwest | 115°26′58′′ E 40°58′53′′ N | 1985 | 1138 | 7.6 | 10.29 | Potentilla fruticose, Rubus swinhoei Hance, Malus baccata, etc. |
| Mixed Larix principis-rupprechtii and Betula platyphylla forest (MF) | Northeast | 115°27′51′′ E 40°58′37′′ N | 1904 | 2201 | 9.8 | 10.57 | Rubus swinhoei Hance, Crataegus pinnatifida Bunge, Rosa bella, Ribes rubrum, Malus baccata, etc. |
| BD (g·cm−3) | pH | SOM (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | ||
|---|---|---|---|---|---|---|
| Larix principis-rupprechtii forest | ||||||
| 0–10 cm | upslope | 0.96 ± 0.00 | 6.61 ± 0.02 | 57.28 ± 0.26 | 2.94 ± 0.06 | 0.54 ± 0.01 |
| mesoslope | 1.33 ± 0.00 | 6.98 ± 0.03 | 48.72 ± 0.94 | 2.66 ± 0.04 | 0.64 ± 0.01 | |
| downslope | 0.99 ± 0.00 | 6.90 ± 0.04 | 55.05 ± 0.39 | 2.96 ± 0.05 | 0.76 ± 0.03 | |
| 10–20 cm | upslope | 1.35 ± 0.00 | 6.80 ± 0.01 | 43.59 ± 0.74 | 2.36 ± 0.05 | 0.59 ± 0.00 |
| mesoslope | 0.96 ± 0.00 | 7.10 ± 0.01 | 46.84 ± 0.54 | 2.41 ± 0.04 | 0.67 ± 0.02 | |
| downslope | 1.06 ± 0.00 | 7.09 ± 0.03 | 51.40 ± 0.41 | 2.83 ± 0.03 | 0.81 ± 0.01 | |
| Mixed Larix principis-rupprechtii and Betula platyphylla forest | ||||||
| 0–10 cm | upslope | 0.83 ± 0.00 | 6.19 ± 0.05 | 94.87 ± 2.28 | 4.80 ± 0.04 | 0.97 ± 0.01 |
| mesoslope | 0.80 ± 0.00 | 6.29 ± 0.02 | 74.11 ± 0.88 | 3.88 ± 0.05 | 0.94 ± 0.01 | |
| downslope | 0.73 ± 0.00 | 6.72 ± 0.01 | 88.87 ± 1.92 | 4.39 ± 0.04 | 0.83 ± 0.02 | |
| 10–20 cm | upslope | 0.82 ± 0.00 | 6.82 ± 0.02 | 75.85 ± 1.39 | 3.85 ± 0.04 | 0.95 ± 0.01 |
| mesoslope | 0.88 ± 0.00 | 6.38 ± 0.04 | 68.32 ± 1.73 | 3.49 ± 0.06 | 0.94 ± 0.01 | |
| downslope | 0.90 ± 0.00 | 6.71 ± 0.02 | 60.50 ± 1.69 | 2.91 ± 0.06 | 0.78 ± 0.02 | |
| Groups | Larix principis-rupprechtii Forest | Mixed Larix principis-rupprechtii and Betula platyphylla Forest | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May. | Jun. | Aug. | Sep. | Nov. | Total | May. | Jun. | Aug. | Sep. | Nov. | Total | |||||
| Individuals | Individuals | Percentage | Abundance | Individuals | Individuals | Percentage | Abundance | |||||||||
| Acarina | 50 c | 104 b | 145 a | 134 a | 110 b | 543 | 39.43 | +++ | 30 d | 54 c | 173 a | 194 a | 85 b | 536 | 33.90 | +++ |
| Collembola | 34 c | 101 ab | 157 a | 117 ab | 94 b | 503 | 36.53 | +++ | 28 d | 54 c | 218 a | 212 a | 65 b | 577 | 36.50 | +++ |
| Hemiptera | 0 | 0 | 4 | 0 | 0 | 4 | 0.29 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | – |
| Isoptera | 4 | 7 | 16 | 9 | 3 | 39 | 2.83 | ++ | 5 | 12 | 74 | 20 | 6 | 117 | 7.40 | ++ |
| Orthoptera | 1 | 4 | 0 | 0 | 0 | 5 | 0.36 | + | 0 | 0 | 5 | 0 | 0 | 5 | 0.32 | + |
| Thysanoptera | 0 | 3 | 8 | 1 | 0 | 12 | 0.87 | + | 0 | 3 | 51 | 11 | 11 | 76 | 4.81 | ++ |
| Dermaptera | 3 | 0 | 0 | 0 | 0 | 3 | 0.22 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | – |
| Coleoptera (adult) | 5 | 3 | 3 | 2 | 0 | 13 | 0.94 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | – |
| Coleoptera (larvae) | 0 | 2 | 3 | 3 | 0 | 8 | 0.58 | + | 0 | 0 | 0 | 2 | 2 | 4 | 0.25 | + |
| Diptera (adult) | 1 | 4 | 10 | 2 | 0 | 17 | 1.23 | ++ | 0 | 0 | 13 | 0 | 4 | 17 | 1.08 | ++ |
| Diptera (larvae) | 47 | 23 | 21 | 10 | 8 | 109 | 7.92 | ++ | 4 | 6 | 42 | 20 | 10 | 82 | 5.19 | ++ |
| Lepidoptera (larvae) | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | – | 0 | 1 | 1 | 1 | 0 | 3 | 0.19 | + |
| Formicidae | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | – | 2 | 0 | 0 | 1 | 0 | 3 | 0.19 | + |
| Gastropoda | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | – | 0 | 0 | 3 | 0 | 0 | 3 | 0.19 | + |
| Diplopoda | 0 | 1 | 0 | 4 | 0 | 5 | 0.36 | + | 0 | 2 | 1 | 0 | 0 | 3 | 0.19 | + |
| Protura | 0 | 0 | 0 | 5 | 0 | 5 | 0.36 | + | 0 | 0 | 0 | 0 | 1 | 1 | 0.06 | + |
| Isopoda | 0 | 9 | 5 | 3 | 0 | 17 | 1.23 | ++ | 0 | 6 | 0 | 0 | 0 | 6 | 0.38 | + |
| Psocoptera | 0 | 0 | 30 | 12 | 0 | 42 | 3.05 | ++ | 0 | 1 | 3 | 3 | 1 | 8 | 0.51 | + |
| Enchytraeidae | 1 | 0 | 19 | 2 | 3 | 25 | 1.82 | ++ | 1 | 7 | 6 | 18 | 2 | 34 | 2.15 | ++ |
| Araneae | 2 | 1 | 0 | 1 | 19 | 23 | 1.67 | ++ | 5 | 4 | 16 | 47 | 2 | 74 | 4.68 | ++ |
| Geophilomorpha | 1 | 1 | 1 | 0 | 0 | 3 | 0.22 | + | 6 | 13 | 5 | 2 | 3 | 29 | 1.83 | ++ |
| Symphyla | 0 | 0 | 1 | 0 | 0 | 1 | 0.07 | + | 2 | 0 | 0 | 1 | 0 | 3 | 0.19 | + |
| Number of groups | 10 | 13 | 14 | 13 | 6 | 19 | 9 | 11 | 13 | 13 | 12 | 19 | ||||
| Sum | 149 d | 263 bc | 423 a | 305 b | 237 c | 1377 | 86 e | 163 d | 611 a | 532 b | 192 c | 1581 | ||||
| Saprozoite | Phytophage | Predator | Omnivore | |
|---|---|---|---|---|
| Abundance (inds.) | ||||
| LF | 721 ± 70 | 57 ± 4 | 53 ± 0 | 546 ± 33 |
| MF | 840 ± 30 | 16 ± 3 | 186 ± 13 | 539 ± 14 |
| Modulated ratio (%) | 16.86 ± 7.34 | −71.67 ± 7.07 | 250.94 ± 24.01 | −1.18 ± 3.30 |
| Proportion (%) | ||||
| LF | 52.31 ± 3.52 | 4.14 ± 0.18 | 3.85 ± 0.12 | 39.71 ± 3.59 |
| MF | 53.12 ± 1.02 | 1.01 ± 0.20 | 11.76 ± 0.62 | 34.13 ± 1.44 |
| Modulated ratio (%) | 1.73 ± 4.90 | −75.37 ± 5.80 | 205.78 ± 25.41 | 13.92 ± 4.14 |
| Parameters | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
|---|---|---|---|---|
| Eigenvalues | 0.3562 | 0.1876 | 0.0402 | 0.0195 |
| Explained variation (%) (cumulative) | 35.62 | 54.38 | 58.4 | 60.35 |
| Pseudo–canonical correlation | 0.916 | 0.9323 | 0.6247 | 0.4654 |
| Explained fitted variation (%) (cumulative) | 59.02 | 90.1 | 96.76 | 100 |
| Monte Carlo test after 499 permutations | ||||
| Text on the first axis | pseudo–F = 3.9, p = 0.024 | |||
| Text on all axes | pseudo–F = 2.7, p = 0.006 | |||
| LF | MF | |
|---|---|---|
| Total abundance | 0.995 ** | 0.894 ** |
| The number of groups | 0.671 | 0.877 ** |
| Shannon–Wiener index (H) | 0.893 ** | 0.654 |
| Pielou evenness index (J) | 0.285 | 0.061 |
| Simpson dominance index (C) | –0.880 ** | –0.586 |
| Collembola | 0.991 ** | 0.866 ** |
| Acarina | 0.846 * | 0.746 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Han, G.; Huang, T.; Feng, Y.; Tian, W.; Wu, X. Mixed Forest of Larix principis-rupprechtii and Betula platyphylla Modulating Soil Fauna Diversity and Improving Faunal Effect on Litter Decomposition. Forests 2022, 13, 703. https://doi.org/10.3390/f13050703
Zhang H, Han G, Huang T, Feng Y, Tian W, Wu X. Mixed Forest of Larix principis-rupprechtii and Betula platyphylla Modulating Soil Fauna Diversity and Improving Faunal Effect on Litter Decomposition. Forests. 2022; 13(5):703. https://doi.org/10.3390/f13050703
Chicago/Turabian StyleZhang, Huayong, Guixuan Han, Tousheng Huang, Yu Feng, Wang Tian, and Xiaochang Wu. 2022. "Mixed Forest of Larix principis-rupprechtii and Betula platyphylla Modulating Soil Fauna Diversity and Improving Faunal Effect on Litter Decomposition" Forests 13, no. 5: 703. https://doi.org/10.3390/f13050703






