Abstract
During a routine survey conducted in July 2021, several dead and dying Ulmus macrocarpa trees were observed in an urban forest located in the Gyeonggi Province of South Korea. The trees had symptoms of wilt with yellowing and browning of leaves, and, in most cases, the trunks of dying trees were infested by bark beetles. Isolations were made from small pieces of wood taken from dying trees, and beetles were collected from the infested stems. Fungal isolates and the beetles were identified using DNA sequence-based phylogenies and morphology, respectively. The results revealed that the fungus was Ophiostoma novo-ulmi, the causal agent of Dutch elm disease, and the associated bark beetle was Scolytus jacobsoni. This study provides the first record of Dutch elm disease in South Korea and suggests that a nationwide survey for the disease should be undertaken.
1. Introduction
Some of the most devastating tree diseases are attributed to beetle–fungus associations, which have been recognized as major drivers of biotic damage to trees in natural and planted forests [1,2]. A relevant example in Korea is the oak wilt disease caused by Raffaelea quercus-mongolicae and its coevolved wood-boring ambrosia beetle vector Platypus koryoensis (Coleoptera; Curculionidae; Platypodidae) that led to the mass mortality of Korean oak trees, especially Quercus mongolica, in the country [3].
Dutch elm disease (DED) is one of the most destructive tree diseases in the Northern Hemisphere [4]. Native elm trees in Europe and North America have been devastated by two pandemics of DED caused by the pathogens Ophiostoma ulmi and O. novo-ulmi, respectively. The first of these pandemics was caused by O. ulmi, which spread across Europe, North America, and Southwest and Central Asia during the early 1900s. The second pandemic was caused by O. novo-ulmi in both Europe and North America in the 1970s [4,5]. In areas where DED currently occurs, O. ulmi has been replaced by O. novo-ulmi, which is more aggressive, leading to more destructive epidemics [6,7]. It is also relevant that O. novo-ulmi has two distinct subspecies known as O. novo-ulmi subsp. novo-ulmi and O. novo-ulmi subsp. americana, respectively [4,5].
The causal agents of DED are vectored by scolytine bark beetles (Curculionidae; Scolytinae) residing in the genera Scolytus and Hylurgopinus [8,9,10]. In addition to vector-related transmission, the DED fungi can move from infected to healthy trees by means of root grafts [11,12]. Initial symptoms of DED include wilting and discoloration of leaves on individual branches. As the disease progresses, leaves turn yellow to brown, shrivel and curl, leading to defoliation, and, eventually, entire trees die within a few weeks or a few years from the time of first infection [12,13].
During a routine disease survey conducted in an urban forest located in Gyeonggi Province, the Republic of Korea, in July 2021, elm trees with obvious symptoms of rapid wilt were observed. Symptomatic trees were infested with bark beetles, leading to their rapid death. Although a few diseases on elm have been reported in South Korea, including leaf spot and powdery mildew [14], there have been no major health problems on elm in the country. The fact that DED had not been reported in South Korea and that elm trees were displaying wilt symptoms raised the question as to whether DED had emerged in the country. The aim of this study was, thus, to identify a possible causal agent of the disease and to consider its pathogenicity on closely related tree species.
2. Materials and Methods
2.1. Disease Symptoms, Distribution, and Identification of the Insect Vector
The distribution of dead and dying large-fruited elm (Ulmus macrocarpa) trees in an urban forest located in Dongducheon, a city in the Gyeonggi Province of South Korea (37°53′38.2″ N 127°03′19.7″ E), is currently concentrated within a narrow area approximately 700 m long. Eight dead trees and several dying trees with symptoms, including wilting, yellowing, and browning of leaves, were observed (Figure 1A,D,E). Although the dead and dying trees were relatively young, estimated at between 25 and 35 years, the disease seriously affected urban planning structures, including parks and gardens in the city.

Figure 1.
Symptoms associated with DED on Ulmus macrocarpa in South Korea: (A) dead trees; (B) bark beetle infestation on trunk of the tree and breeding galleries developed under the bark flap; (C) insect vector Scolytus jacobsoni in the breeding galleries; (D) dieback with yellowing and browning of leaves; (E) close-up view of dying trees.
In most cases, the trunks of dying trees were infested by bark beetles (Figure 1C), leading to beetle-breeding galleries being frequently observed (Figure 1B). Bark beetles were collected and identified based on morphology. This was achieved using a Leica dissection microscope M205C (Leica microsystems) and keys for the genus Scolytus [15].
2.2. Isolation and Identification of Fungi from Bark Beetles and Wood
Tissue samples, including cambium and bark flaps, were removed from the trunks of eight dying trees for isolation. Five beetles infesting the trunks of trees were collected using an aspirator and transported to the laboratory in 1.5 mL Eppendorf tubes, where they were stored at 4 °C.
Small pieces of cambium from each of eight trees were plated on 2% malt extract agar (MEA: 20 g Difco malt extract and 20 g Difco agar) supplemented with 100 mg/L streptomycin sulfate (Sigma-Aldrich, Steinheim, Germany) after being surface sterilized. Emerging cultures were purified by transferring single hyphal tips taken from the edges of cultures to 2% MEA. The plates were incubated at 25 °C for 4 weeks in the dark and examined periodically.
Isolation from the five bark beetles was achieved by allowing them to crawl over the surface of MEA (identical to the agar used for fungal isolation). The plates were incubated at 25 °C for 2 days in the dark. The resulting cultures were purified using single hyphal tip transfers.
To check the morphology of the pathogen on medium, including synnematal production, the pure cultures were then transferred to 2% MEA in Petri dishes, to which freshly excised and subsequently 7 × 0.3 cm U. macrocarpa twigs sterilized by autoclaving had been placed on the agar surface. The plates were incubated following the same procedure as the isolation for cambial tissue.
2.3. DNA Isolation and PCR Amplifications
Two representative isolates (CDH058 and CDH059) recovered respectively from the wood sample and the beetles were cultured on 2% MEA and incubated for 2 weeks to allow sufficient mycelial growth. Mycelium was scraped from the surface of the agar with sterilized surgical scalpel blades and transferred to 1.5 mL Eppendorf tubes. Genomic DNA was then extracted using ZR Fungal/Bacterial DNA MiniPrep kit (Zymo Research, Irvine, CA, USA) following the manufacturer’s instructions. The quantity and quality of DNA extracted were evaluated with a spectrophotometer (ND-1000; NanoDrop) to calibrate the concentration and purity of DNA as PCR templates.
The PCR amplifications were conducted on a T-100 thermal cycler (Bio-Rad, Hercules, CA, USA) to amplify the internal transcribed spacer (ITS) regions of rDNA using the primer set, ITS1F/ITS4 [16,17]. The total volume of each PCR reaction mixture was 15 µL, containing 1 µL of genomic DNA, 0.5 µL (10 pM) of each primer (forward and reverse), 0.5 µL of MyTaq PCR buffer (Bioline), and 0.5 µL of MyTaq DNA polymerase (Bioline). The PCR cycling profile consisted of an initial denaturation stage at 95 °C for 5 min; 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 1 min; and a final extension at 72 °C for 7 min. The resulting PCR products were then submitted to Cosmogenetech (Seoul, Korea) for forward and reverse sequencing reactions.
2.4. Pathogenicity Test
To confirm pathogenicity, a conidial suspension was prepared from the isolate obtained from wood (CDH058). The suspension was diluted to a concentration of 1 × 105 conidia per ml and used for an inoculation trial. Three- to four-year-old saplings of U. macrocarpa were wounded with a 3 mm drill bit to a depth of approximately 15 mm at the root–collar region, and 10 μL of conidial suspension was placed in the wounds. In total, 19 trees were inoculated, and 8 trees served as controls (i.e., water was used instead of conidial suspension). To prevent desiccation of the inoculum and inoculation points and to reduce contamination, all inoculation points were covered with masking tape. The saplings were placed in a greenhouse located in the National Institute of Forest Science, Seoul, South Korea, for 8 weeks to allow for disease development.
3. Results
3.1. Identification of the Insect Vector
Following the description of Park [15], the five xylophagous beetles collected from either dead or dying elm trees were confirmed to be Scolytus jacobsoni (Figure 2).

Figure 2.
The insect vector, Scolytus jacobsoni (scale bar = 1 mm).
3.2. Isolation and Identification of Fungi from Bark Beetles and Wood
A total of nine isolates of an Ophiostoma species with profuse synnematal production were recovered from the MEA where twigs of U. macrocarpa had been added. Five of these were from tissue samples, and the others were from beetles. All the isolates recovered in this study were deposited in the Culture Collection (CDH) of the National Institute of Forest Science, Seoul, South Korea, with accession numbers CDH058–066. Sequences of the two representative isolates, CDH058 and CDH063, that were subjected to DNA sequencing were deposited to GenBank with accession numbers ON209351–352.
A BLAST search in GenBank revealed that the sequences showed high similarity to O. novo-ulmi (MH055667, [18]). In addition to the BLAST result, the phylogenetic tree (Figure 3) revealed that the isolates CDH058 and CDH059 were O. novo-ulmi.
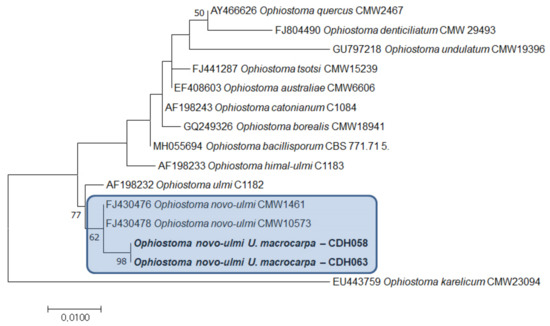
Figure 3.
Phylogenetic tree based on maximum likelihood (ML) analysis. Scale bar indicates 0.01 changes.
3.3. Pathogenicity Test
Symptoms, including yellowing and browning of leaves identical to those on naturally infected trees, were observed on all inoculated saplings after 8 weeks. Distinct lesions developed from the inoculated areas and the inoculated fungus were successfully reisolated from all of the dying saplings. The eight saplings inoculated with water to serve as controls remained healthy.
4. Discussion
This study represents the first record of the tree pathogen Ophiostoma novo-ulmi, causing a vascular wilt disease on U. macrocarpa in South Korea. The disease was detected in a relatively small area, and the pathogen was identified based on DNA sequence comparisons. The bark beetle associated with the disease was identified as Scolytus jacobsoni.
Scolytus jacobsoni is native to Asia and is known to occur in an area stretching from southeastern Russia to northeastern China and Japan [15,19]. The beetle was first recorded in the northern Korean peninsula in 1964 [20] and later in Daegu-si, Mt. Palgong, southeastern Korean peninsula [21], suggesting that it is endemic in Korea. Whether the association of S. jacobsoni and O. novo-ulmi, as found in this study, is novel or whether beetles carrying the fungus might have recently been introduced into Korea is unknown. This important question requires further investigation.
Large-scale dieback or mortality of elm species due to DED has recently been reported in countries neighboring South Korea, including Russia [22] and Japan [23]. These are areas relatively distant from Korea, but it remains possible that these are the likely origin of the disease found in the present study. Another possible pathway of the introduction of O. novo-ulmi could be via introductions of infested plant material into a U.S. military camp at Donghucheon. This camp is only three kilometers away from the area where the disease described in this study occurred. However, further evidence is needed to support or reject the military pathway hypothesis and to better understand its potential for introduction within South Korea. In addition, no disease control strategies, including chemical control for DED in South Korea, have been established yet. To effectively control DED in the country, disease occurrences must be further rigorously surveyed at a nationwide scale.
Author Contributions
Investigation, D.-H.L., Y.N. and H.P.; resources, D.-H.L.; data curation, D.-H.L.; writing—original draft preparation, D.-H.L. and Y.N.; writing—review and editing, M.J.W.; funding acquisition, D.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a project, Investigation and characterization on occurrence of emerging forest insect pests and fungal diseases (Project No. FE0702-2021-01), from the National Institute of Forest Science, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Sang-Wook Park for assistance in identifying the bark beetle Scolytus jacobsoni.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Wingfield, M.J.; Slippers, B.; Wingfield, B.D. Novel associations between pathogens, insects and tree species threaten world forests. N. Zeal. J. For. Sci. 2010, 40, S95–S104. [Google Scholar]
- Wingfield, M.J.; Garnas, J.R.; Hajek, A.; Hurley, B.P.; de Beer, Z.W.; Taerum, S.J. Novel and co-evolved associations between insects and microorganisms as drivers of forest pestilence. Biol. Invasions 2016, 18, 1045–1056. [Google Scholar] [CrossRef]
- Lee, D.H.; Jung, J.M.; Seo, S.T. Population genetic structure of Raffaelea quercus-mongolicae indicates a recent fungal introduction event to Jeju Island from inland areas of South Korea. Plant Pathol. 2021, 70, 1871–1882. [Google Scholar] [CrossRef]
- Brasier, C.M. Ophiostoma novo-ulmi sp. nov., causative agent of current Dutch elm disease pandemics. Mycopathologia 1991, 115, 151–161. [Google Scholar] [CrossRef]
- Brasier, C.M.; Kirk, S.A. Designation of the EAN and NAN races of Ophiostoma novo-ulmi as subspecies. Mycol. Res. 2001, 105, 547–554. [Google Scholar] [CrossRef]
- Brasier, C.M. International spread and continuing evolution of the Dutch elm disesase pathogens. In The Elms: Breeding, Conservation and Disease Management; Dunne, C.P., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 2000; pp. 61–72. [Google Scholar]
- Brasier, C.M.; Kirk, S.A. Rapid emergence of hybrids between the two subspecies of Ophiostoma novo-ulmi with a high level of pathogenic fitness. Plant Pathol. 2010, 59, 186–199. [Google Scholar] [CrossRef]
- Webber, J.F. Relative effectiveness of Scolytus scolytus, S. multistriatus and S. kirschi as vectors of Dutch elm disease. Eur. J. Plant Pathol. 1990, 20, 184–192. [Google Scholar] [CrossRef]
- Webber, J.F. Experimental studies on factors influencing the transmission of Dutch elm disease. For. Syst. 2004, 13, 197–205. [Google Scholar]
- Jacobi, W.R.; Koski, R.D.; Negron, J.F. Dutch elm disease pathogen transmission by the banded elm bark beetle Scolytus schevyrewi. For. Pathol. 2013, 43, 232–237. [Google Scholar] [CrossRef]
- Verrall, A.F.; Graham, T.W. The transmission of Ceratostomella ulmi through root grafts. Phytopathology 1935, 25, 1039–1050. [Google Scholar]
- Stipes, R.J.; Campana, R.J. Compendium of Elm Diseases; American Phytopathological Society: St. Paul, MN, USA, 1981; p. 96. [Google Scholar]
- Kirisits, T. Dutch elm Disease and Other Ophiostoma Diseases; CAB International: Wallingford, UK, 2013; pp. 256–282. [Google Scholar]
- NIFoS. Report of Forest Researches (I); National Institute of Forest Science: Seoul, Korea, 1967; p. 702. [Google Scholar]
- Park, S.W. Taxonomic review of Scolytinae and Platypodinae (Coeloptera: Curculionidae) in Korea. Ph.D. Thesis, Seoul National University, Seoul, Korea, 2016. [Google Scholar]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Method and Applications; Innis, M., Gefland, D., Sninsky, J., White, T., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Aas, T.; Solheim, H.; Jankowiak, R.; Bilański, P.; Hausner, G. Four new Ophiostoma species associated with hardwood-infesting bark beetles in Norway and Poland. Fungal. Biol. 2018, 122, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.L.; Bright, D.E. A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic index, volumes A and B. Great Basin Nat. Mem. 1992, 13, 1–1553. [Google Scholar]
- Ju, D.R. Biogeographical distribution of the scolytid beetles of Korea. Biology 1964, 3, 5–14. [Google Scholar]
- Paek, M.K.; Hwang, J.M.; Jung, K.S.; Kim, T.W.; Kim, M.C.; Lee, Y.J.; Cho, Y.B.; Park, S.W.; Lee, H.S.; Ku, D.S.; et al. Checklist of Korean Insects; Nature & Ecology, Academic Series 2; Nature & Ecology: Seoul, Korea, 2010; pp. 1–598. [Google Scholar]
- Jürisoo, L.; Selikhovkin, A.V.; Padari, A.; Shevchenko, S.V.; Shcherbakova, L.N.; Popovichev, B.G.; Drenkhan, R. The extensive damage to elms by Dutch elm disease agents and their hybrids in northwestern Russia. Urban. For. Urban. Green 2021, 63, 127214. [Google Scholar] [CrossRef]
- Miyamoto, T.; Masuya, H.; Koizumi, A.; Yamaguchi, T.; Ishihara, M.; Yamaoka, Y.; Okane, I.; Shizuki, M.; Ohara, M. A report of dieback and mortality of elm trees suspected of Dutch elm disease in Hokkaido, Japan. J. For. Res. 2019, 24, 396–400. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).