Carbon Addition Modified the Response of Heterotrophic Respiration to Soil Sieving in Ectomycorrhizal-Dominated Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Collection
2.2. Soil Chemical and Microbial Analysis
2.3. Soil Incubation
2.4. Data Calculation and Statistic Analysis
3. Results
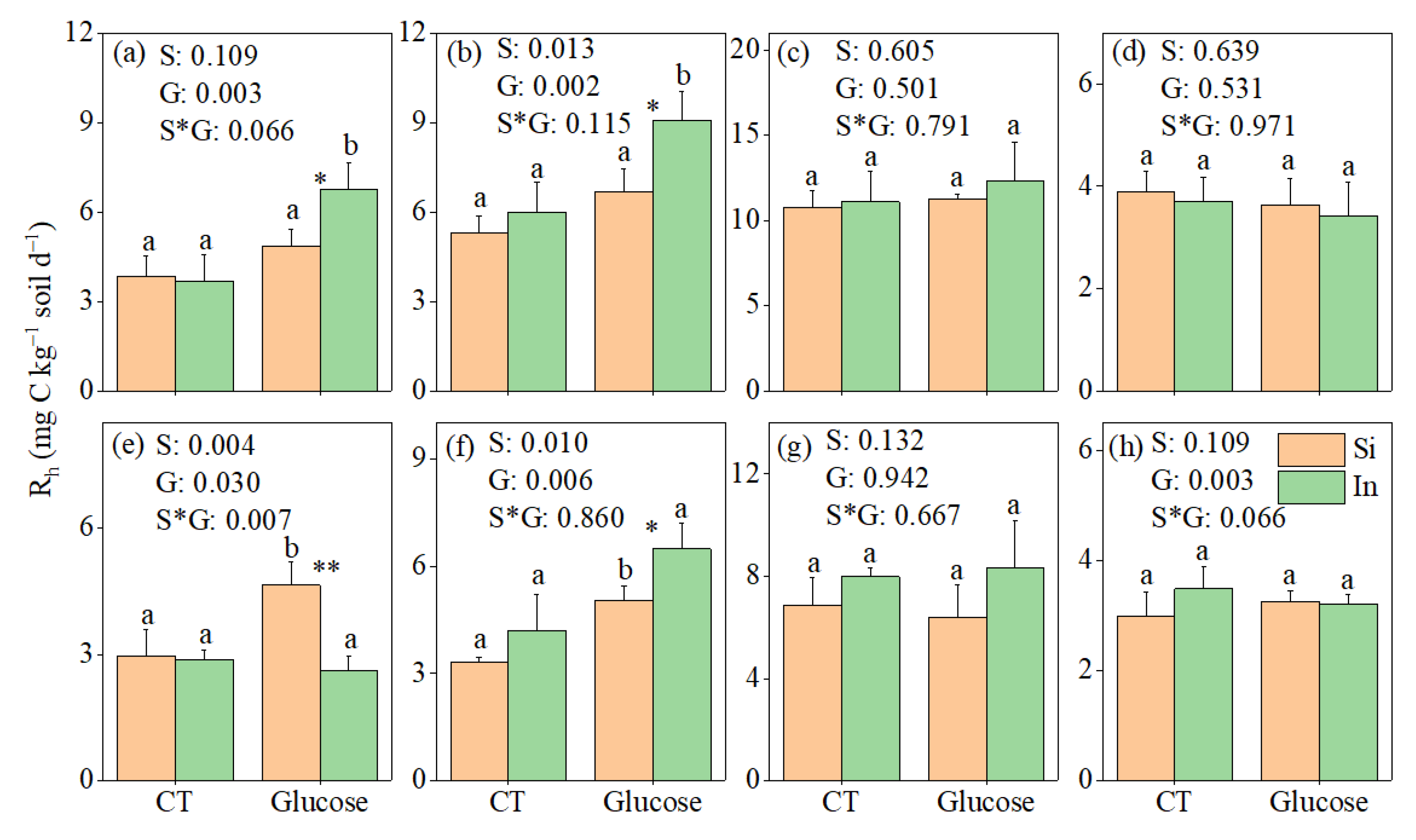
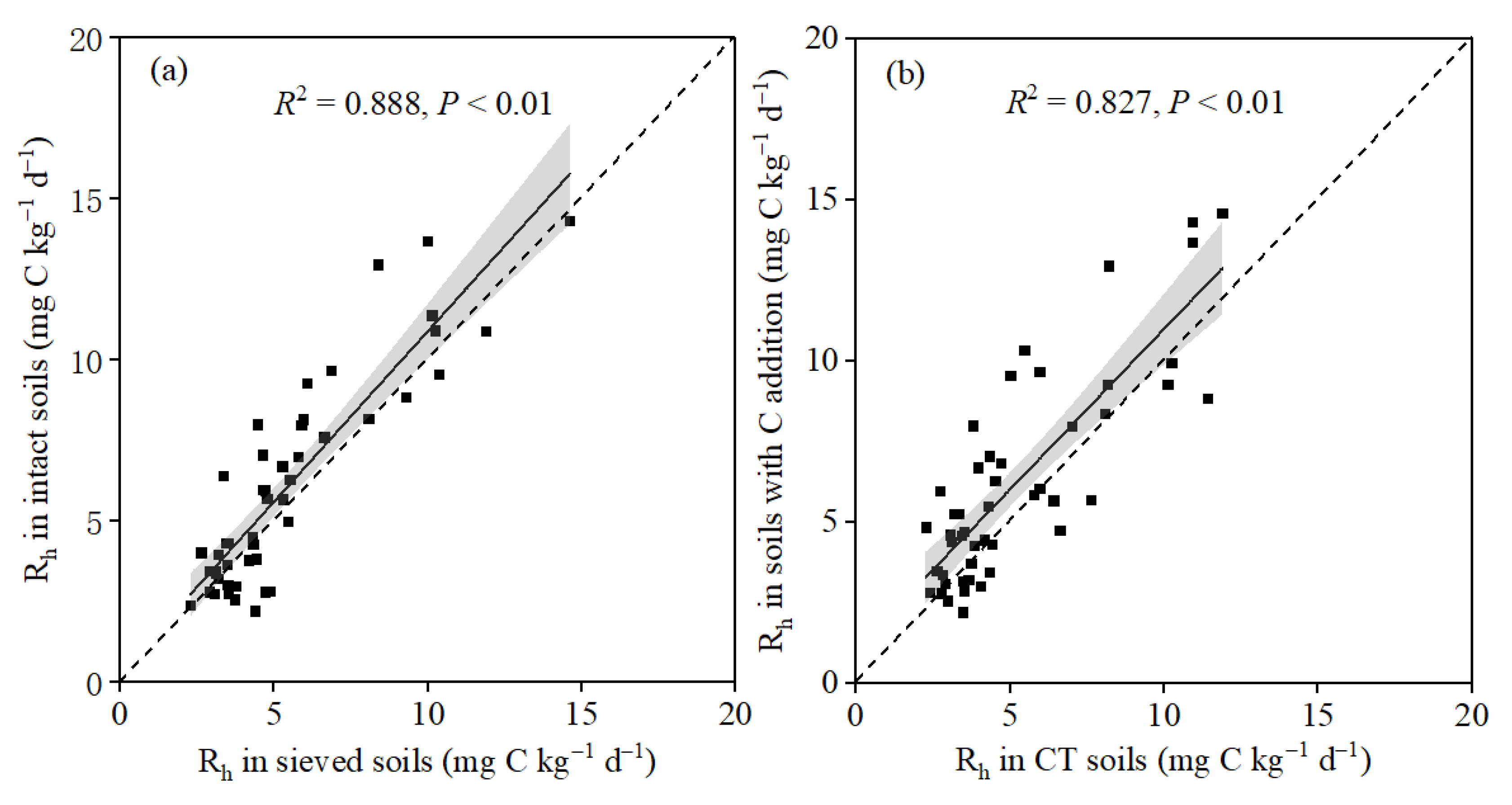
3.1. Responses of Rh to Soil Sieving and Glucose Addition
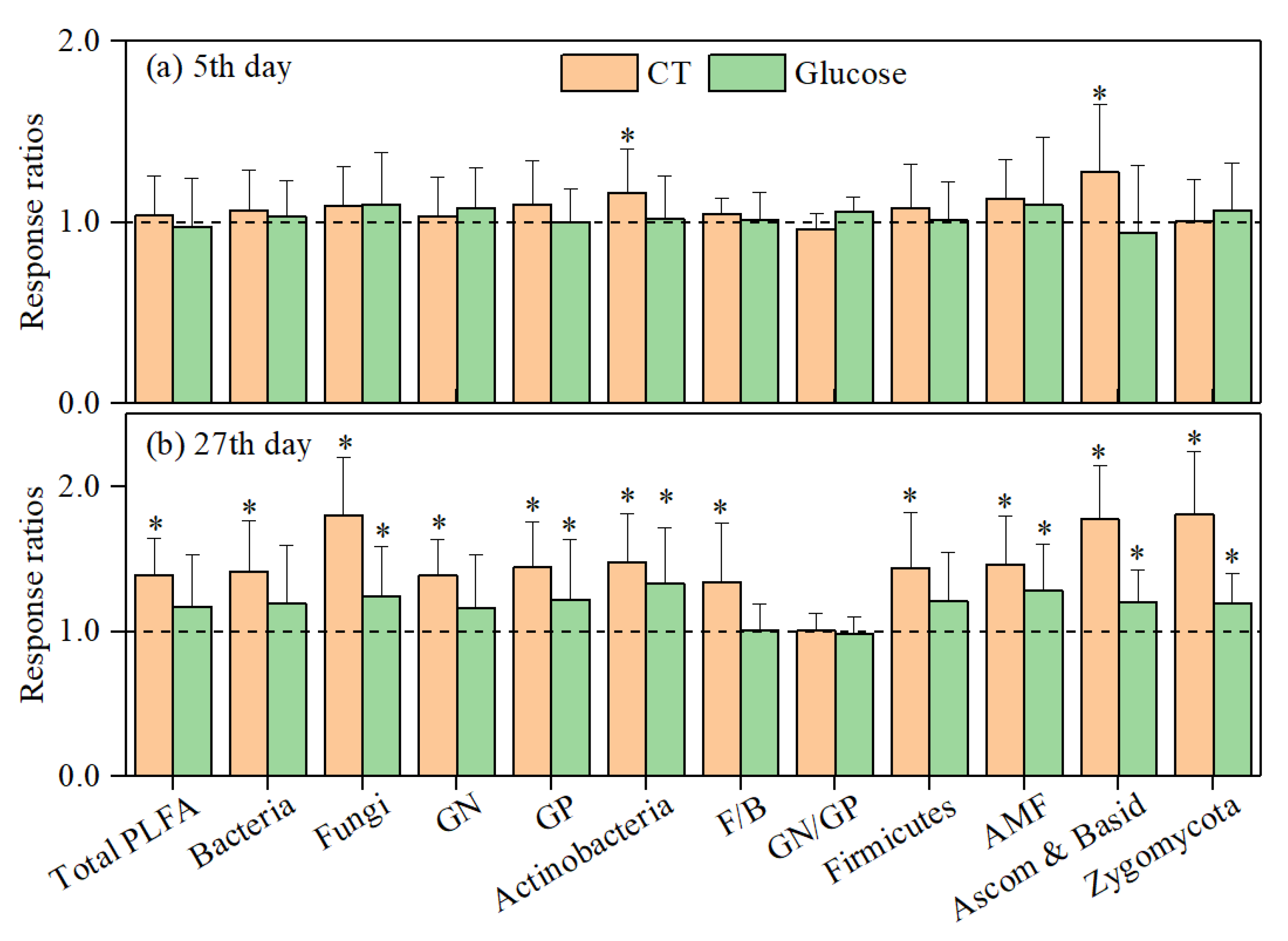
3.2. Soil Microbial Responses to Sieving and Glucose Addition
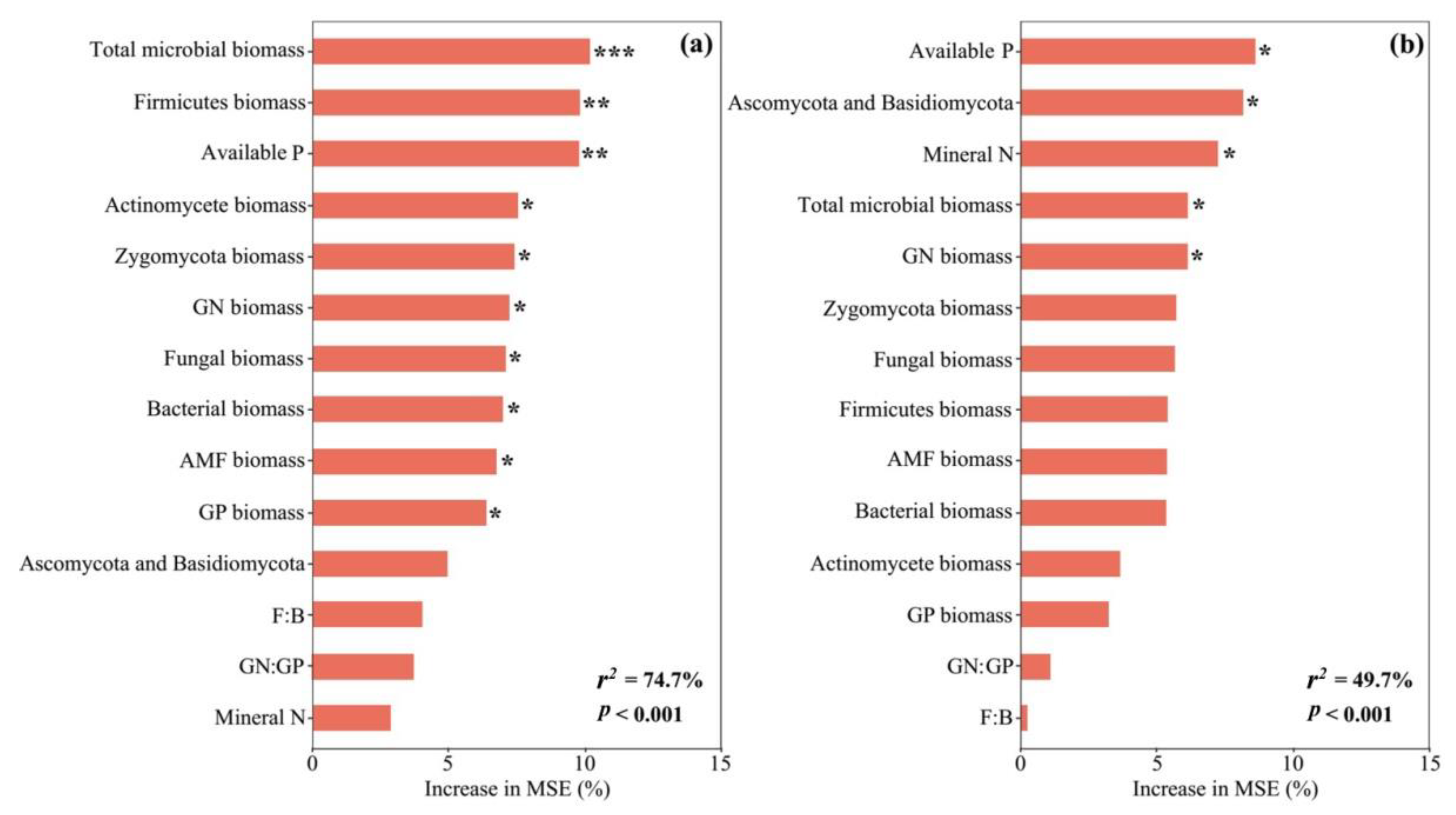
3.3. Mechanism of Regulating Soil Rh
4. Discussions
4.1. Response of Soil Heterotrophic Respiration to Sieving
4.2. Carbon Addition Mediating the Response of Soil Heterotrophic Respiration to Sieving
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond-Lamberty, B.; Wang, C.K.; Gower, S.T. A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob. Chang. Biol. 2004, 10, 1756–1766. [Google Scholar] [CrossRef]
- Gabriel, C.E.; Kellman, L. Examining moisture and temperature sensitivity of soil organic matter decomposition in a temperate coniferous forest soil. Biogeosci. Discuss. 2011, 8, 1369–1409. [Google Scholar]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Gutinas, M.E.; Gil-Sotres, F.; Leiros, M.C.; Trasar-Cepeda, C. Sensitivity of soil respiration to moisture and temperature. J. Soil Sci. Plant Nutr. 2013, 13, 445–461. [Google Scholar]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; He, T.X.; Liu, L.; Wu, J.B. Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol. Biochem. 2014, 71, 13–20. [Google Scholar] [CrossRef]
- Yan, D.; Li, J.Q.; Pei, J.M.; Cui, J.; Nie, M.; Fang, C.M. The temperature sensitivity of soil organic carbon decomposition is greater in subsoil than in topsoil during laboratory incubation. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Baveye, P.C.; Otten, W.; Kravchenko, A.; Balseiro-Romero, M.; Beckers, É.; Chalhoub, M.; Darnault, C.; Eickhorst, T.; Garnier, P.; Hapca, S.; et al. Emergent properties of microbial activity in heterogeneous soil microenvironments: Different research approaches are slowly converging, yet major challenges remain. Front. Microbiol. 2018, 9, 1929. [Google Scholar] [CrossRef]
- Curtin, D.; Beare, M.H.; Scott, C.L.; Hernandez-Ramirez, G.; Meenken, E.D. Mineralization of soil carbon and nitrogen following physical disturbance: A laboratory assessment. Soil Sci. Soc. Am. J. 2014, 78, 925–935. [Google Scholar] [CrossRef]
- Meyer, N.; Welp, G.; Amelung, W. Effect of sieving and sample storage on soil respiration and its temperature sensitivity (Q10) in mineral soils from Germany. Biol. Fertil. Soils 2019, 55, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Mo, F.; Zhang, Y.Y.; Liu, Y.; Liao, Y.C. Microbial carbon-use efficiency and straw-induced priming effect within soil aggregates are regulated by tillage history and balanced nutrient supply. Biol. Fertil. Soils 2021, 57, 409–420. [Google Scholar] [CrossRef]
- Lamparter, A.; Bachmann, J.; Goebel, M.-O.; Woche, S.K. Carbon mineralization in soil: Impact of wetting-drying, aggregation and water repellency. Geoderma 2009, 150, 324–333. [Google Scholar] [CrossRef]
- Gao, F.; Lin, W.; Cui, X.Y. Effects of sieving process on soil organic carbon mineralization for two forest types in Xiaoxing’an Mountains, Northeast China. J. Beijing For. Univ. 2017, 39, 30–39. [Google Scholar]
- Adkanmbi, A.A.; Shaw, L.J.; Sizmur, T. Effect of sieving on ex situ soil respiration of soils from three land use types. J. Soil Sci. Plant Nutr. 2020, 20, 912–916. [Google Scholar]
- Moinet, G.Y.K.; Millard, P. Temperature sensitivity of decomposition: Discrepancy between field and laboratory estimates is not due to sieving the soil. Geoderma 2020, 374, 114444. [Google Scholar] [CrossRef]
- Stenger, R.; Barkle, G.F.; Burgess, C.P. Mineralization of organic matter in intact versus sieved/refilled soil cores. Aust. J. Crop. Sci. 2002, 40, 149–160. [Google Scholar]
- Hinojosa, M.B.; Laudicina, V.A.; Parra, A.; Albert-Belda, E.; Moreno, J.M. Drought and its legacy modulate the post-fire recovery of soil functionality and microbial community structure in a Mediterranean shrubland. Glob. Chang. Biol. 2019, 25, 1409–1427. [Google Scholar] [CrossRef]
- Navarro-Garcia, F.; Casermeiro, M.A.; Schimel, J.P. When structure means conservation: Effect of aggregate structure in controlling microbial responses to rewetting events. Soil Biol. Biochem. 2012, 44, 1–8. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Dietze, M.C.; Jackson, R.B.; Phillips, R.P.; Rhoades, C.C.; Rustad, L.E.; Vose, J.M. Forest biogeochemistry in response to drought. Glob. Chang. Biol. 2016, 22, 2318–2328. [Google Scholar] [CrossRef]
- Choi, R.T.; Reed, S.C.; Tucker, C.L. Multiple resource limitation of dryland soil microbial carbon cycling on the Colorado Plateau. Ecology 2022, 106, e3671. [Google Scholar] [CrossRef]
- Liu, L.; Estiarte, M.; Bengtson, P.; Li, J.; Asensio, D.; Wallander, H.; Penuelas, J. Drought legacies on soil respiration and microbial community in a Mediterranean forest soil under different soil moisture and carbon inputs. Geoderma 2022, 405, 115425. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Jacobs, L.M.; Sulman, B.N.; Brzostek, E.R.; Feighery, J.J.; Phillips, R.P. Interactions among decaying leaf litter, root litter and soil organic matter vary with mycorrhizal type. J. Ecol. 2018, 106, 502–513. [Google Scholar] [CrossRef]
- Midgley, M.G.; Brzostek, E.; Phillips, R.P. Decay rates of leaf litters from arbuscular mycorrhizal trees are more sensitive to soil effects than litters from ectomycorrhizal trees. J. Ecol. 2015, 103, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Lu, R. Methods of Soil Agricultural Chemistry Analysis; Chinese Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- White, D.C.; Ringelberg, D.B. Signature lipid biomarker analysis. In Techniques in Microbial Ecology; Burlage, R.S., Atlas, R., Stahl, D., Geesey, G., Sayler, G., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 255–272. [Google Scholar]
- Joergensen, R.G. Phospholipid fatty acids in soil-drawbacks and future prospects. Biol. Fertil. Soils 2022, 58, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liu, Y.X.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.R.; Qin, Y.; Yan, P.X.; Zhang, X.N.; Guo, X.X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef]
- Persson, T.; Karlsson, P.S.; Seyferth, U.; Sjöberg, R.M.; Rudebeck, A. Carbon mineralisation in European forest soils. Ecol. Stud. 2000, 142, 257–275. [Google Scholar]
- Thomson, B.C.; Ostle, N.J.; McNamara, N.P.; Whiteley, A.S.; Griffiths, R.I. Effects of sieving, drying and rewetting upon soil bacterial community structure and respiration rates. J. Microbiol. Methods 2010, 83, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Vranová, V.; Pavelka, M.; Rejšek, K.; Formánek, P. Effect of soil sieving on respiration induced by low-molecular-weight substrates. Int. Agrophys. 2014, 28, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Černohlávková, J.; Jarkovský, J.; Nešporová, M.; Hofman, J. Variability of soil microbial properties: Effects of sampling, handling and storage. Ecotoxicol. Environ. Saf. 2009, 72, 2102–2108. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J.Plant Nutr. Soil Sci. 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Hassink, J. Effects of soil texture and structure on carbon and nitrogen mineralization in grassland soils. Biol. Fertil. Soils 1992, 14, 126–134. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Potential C and N mineralization and microbial biomass from intact and increasingly disturbed soils of varying texture. Soil Biol. Biochem. 1999, 31, 1083–1090. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.F.; Sayer, E.J.; Zhou, J.G.; Li, Y.W.; Li, Y.X.; Li, Z.A.; Wang, F.M. Long-term fertilization modifies the mineralization of soil organic matter in response to added substrate. Sci. Total Environ. 2021, 798, 149341. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Carrillo, Y.; Pendall, E.; Morgan, J.A. Rhizosphere priming: A nutrient perspective. Front. Microbiol. 2013, 4, 216. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Lange, M.; Koller-France, E.; Hildebrandt, A.; Oelmann, Y.; Wilcke, W.; Gleixner, G. How plant diversity impacts the coupled water, nutrient and carbon cycles. Adv. Ecol. Res. 2019, 61, 185–219. [Google Scholar]
- Liao, H.; Gao, S.H.; Hao, X.L.; Qin, F.; Ma, S.L.; Chen, W.L.; Huang, Q.Y. Soil aggregate isolation method affects interpretation of protistan community. Soil Biol. Biochem. 2021, 161, 108388. [Google Scholar] [CrossRef]
- Marinari, S.; Lagomarsino, A.; Moscatelli, M.C.; Di Tizio, A.; Campiglia, E. Soil carbon and nitrogen mineralization kinetics in organic and conventional three-year cropping systems. Soil Till. Res. 2010, 109, 161–168. [Google Scholar] [CrossRef]
- Zakharova, A.; Midwood, A.J.; Hunt, J.E.; Graham, S.L.; Artz, R.R.E.; Turnbull, M.H.; Whitehead, D.; Millard, P. Loss of labile carbon following soil disturbance determined by measurement of respired δ13CO2. Soil Biol. Biochem. 2014, 68, 125–132. [Google Scholar] [CrossRef]
- Phillips, R.P.; Finzi, A.C.; Bernhardt, E.S. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 2010, 14, 187–194. [Google Scholar] [CrossRef]


| Forest | SOC (g kg −1) | Total N (g kg−1) | C:N | NH4-N (mg kg−1) | NO3-N (mg kg−1) | AP (mg kg−1) | pH | BD (g cm3) |
|---|---|---|---|---|---|---|---|---|
| CM | 12.9 c | 1.33 b | 9.7 c | 29.5 c | 7.1 c | 9.5 b | 4.28 b | 1.28 a |
| PM | 36.5 a | 2.47 a | 14.5 b | 34.7 bc | 23.5 a | 10.3 b | 4.07 b | 1.02 c |
| SS | 34.7 a | 2.62 a | 23.3 a | 55.4 a | 6.8 c | 4.61 c | 4.27 b | 1.12 bc |
| CL | 20.9 b | 1.62 b | 12.9 b | 38.6 b | 17.0 b | 39.5 a | 4.70 a | 1.19 b |
| Day | Total PLFA | Bacteria | Fungi | GN | GP | Actinobacteria | F/B | GN/GP | Firmicutes | AMF | Ascomycota & Basidiomycota | Zygomycota | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5th | MFT | 0.233 | 0.094 | 0.318 | 0.083 | 0.137 | 0.101 | 0.689 | 0.266 | 0.229 | 0.731 | 0.839 | 0.192 |
| Glucose | 0.642 | 0.790 | 0.972 | 0.797 | 0.504 | 0.398 | 0.739 | 0.175 | 0.664 | 0.875 | 0.775 | 0.699 | |
| MFT × G | 0.011 | 0.033 | 0.034 | 0.042 | 0.033 | 0.273 | 0.355 | 0.944 | 0.019 | 0.237 | 0.973 | 0.033 | |
| 27th | MFT | 0.038 | 0.096 | 0.180 | 0.238 | 0.056 | 0.103 | 0.940 | 0.042 | 0.037 | 0.230 | 0.523 | 0.063 |
| Glucose | 0.345 | 0.418 | 0.293 | 0.410 | 0.428 | 0.800 | 0.436 | 0.772 | 0.364 | 0.666 | 0.713 | 0.168 | |
| MFT × G | 0.944 | 0.883 | 0.622 | 0.955 | 0.727 | 0.800 | 0.486 | 0.297 | 0.803 | 0.820 | 0.612 | 0.408 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Zhao, X.; Sun, Z.; Li, J.; Jing, Y.; Wang, Q. Carbon Addition Modified the Response of Heterotrophic Respiration to Soil Sieving in Ectomycorrhizal-Dominated Forests. Forests 2022, 13, 1263. https://doi.org/10.3390/f13081263
Zheng S, Zhao X, Sun Z, Li J, Jing Y, Wang Q. Carbon Addition Modified the Response of Heterotrophic Respiration to Soil Sieving in Ectomycorrhizal-Dominated Forests. Forests. 2022; 13(8):1263. https://doi.org/10.3390/f13081263
Chicago/Turabian StyleZheng, Sijia, Xuechao Zhao, Zhaolin Sun, Jing Li, Yanli Jing, and Qingkui Wang. 2022. "Carbon Addition Modified the Response of Heterotrophic Respiration to Soil Sieving in Ectomycorrhizal-Dominated Forests" Forests 13, no. 8: 1263. https://doi.org/10.3390/f13081263
APA StyleZheng, S., Zhao, X., Sun, Z., Li, J., Jing, Y., & Wang, Q. (2022). Carbon Addition Modified the Response of Heterotrophic Respiration to Soil Sieving in Ectomycorrhizal-Dominated Forests. Forests, 13(8), 1263. https://doi.org/10.3390/f13081263






