Carbon Storage Expectations on Swamp Jelutung (Dyera polyphylla Miq. Steenis.) on Peatland for Tackling Climate Change
Abstract
:1. Introduction
2. Materials and Methods
- 1.
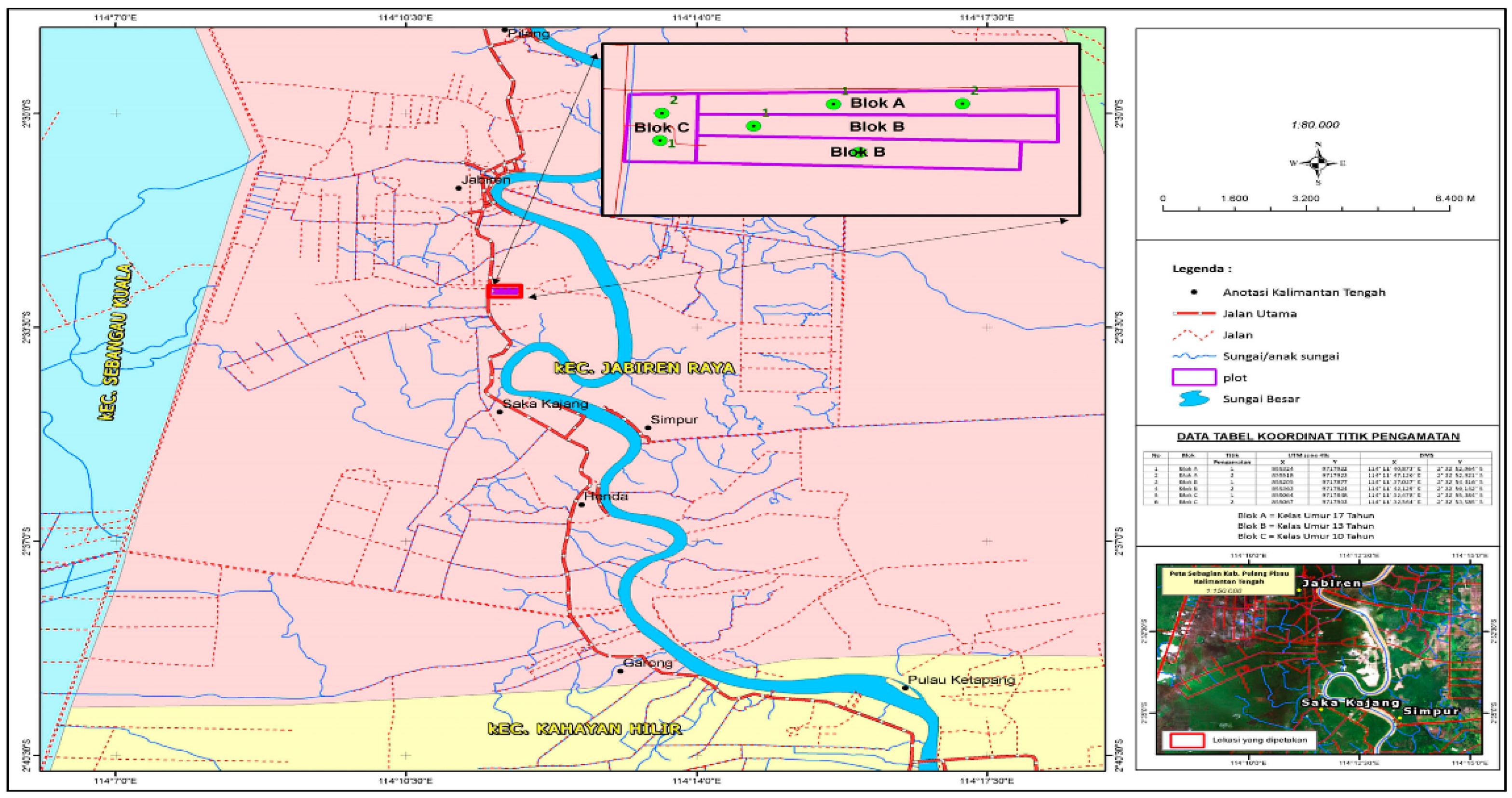
- Observation plots were determined based on the age classes of D. polyphylla plants by measuring and marking the boundary markers with ribbons to mark the boundaries of the plots. The plots were determined using a purposive sampling method with a size of 20 m × 5 m (area = 0.01 ha) for 2 (two) sample plots in each class of plant age.
- 2.
- We created an observation subplot measuring 1 m × 1 m (area 1 m2) in the observation plots for each plant age class to observe understory and litter in the plots. The observed subplots were determined randomly with 5 subplots, as shown in Figure 3.
- 3.
- The tree biomass calculation used the measurement of the diameters of living trees without damage in the measuring plots of 5 m × 20 m with two replications.
- 4.
- To measure the understory biomass, 10 plots of samples were made randomly using a 1 m × 1 m plot. The plots’ lower plants (shrubs, grass, and herbs) were obtained by cutting and then placed in a container. The wet weight was recorded after weighing, and 200 g of lower plants were taken. The samples were put in plastic bags and then labeled to be taken to the laboratory. This activity calculated the dry weight in an oven, and from a plot of 1 m × 1 m, a wet weight of understory <200 g was used as a sample.
- 5.
- Measurement of the wood necromass was carried out in a plot of 20 m × 5 m by measuring the diameter and length. The measurement of necromass was carried out in two forms, namely wood and dead trees. Deadwood was a fallen tree with a diameter of ≥2.5 cm and a length of ≥1 m. It consisted of 3 categories: good, moderate, and a degree of weathering. Dead trees could stand upright with a height of ≥1 m and a diameter of ≥2.5 cm [13]. They were categorized based on the level of the tree’s integrity, affecting the amount of carbon stored in this section.
- 6.
- Carbon storage in the litter necromass was dead biomass with a size larger than soil organic matter, as well as deadwood measuring <2.5 cm with a height <1 m that underwent a decay process on the surface or became soil organic minerals [14]. The plot for measuring carbon storage for litter types was a square with an area of 1 m2 (1 m × 1 m). Therefore, measurement plots of 10 units were randomly placed on the research subplot.
- 7.
- Data processing:
- (a)
- Measurement of tree biomass:We entered the tree diameter data into the allometric model developed by [15] with the following model equation:whereBAP: aboveground biomass in kgThe root biomass content was estimated using the root-to-shoot ratio (RSR) method. The allometric equation used in [13] was as follows:DBH: diameter at breast height in cmwhereBBP: belowground biomass in kgBAP: aboveground biomass in kg
- (b)
- The laboratory measured the dry weight of the understory biomass and litter necromass. The biomass estimation formula was used to calculate the amount of understory biomass and litter necromass stored in a 1 m × 1 m plot as follows:whereWKt: total dry biomass (g)WBt: total wet biomass (g)WBc: sample of wet biomass (g)WKc: sample of dry biomass (g)
- (c)
- Measurement of the dry weight of woody necromass:The calculation of the dry weight of the necromass using the allometric formula for living trees referred to the research of [13]. The allometric equation for estimating the total biomass of deadwood used that of living trees multiplied by a correction or decomposition factor. The following formula was used to calculate the biomass of D dead trees:whereNPM: dead tree necromass (kg)π: 22/7D1, D2: base and tip diameter, respectively (cm)T: tree height (m)The value of the correction factor in calculating the biomass of dead trees was based on SNI (Indonesian National Standard) measurements according to carbon stock:RKρ: mean squared density of deadwood (kg/m3)Dead Tree A: multiplied by the value of correction factor 0.9.Dead Tree B: multiplied by the value of correction factor 0.8.Dead Tree C: multiplied by the value of correction factor 0.7.
- (d)
- Calculation of the amount of C stored between fields:The calculation of the amount of carbon stored per plot followed the research procedure. Most laboratory results use dry weight and biomass parameters, not carbon units. In contrast, all estimates of biomass units use total carbon, and the content analysis requires special tools and methods [13]. The use of universal carbon estimates at the global level corresponds to a correction factor of 0.47 [14]. Therefore, all measurement sources in the field should calculate the total carbon in each subplot and convert it to tons/ha. The extrapolation process should pay attention to the area of the subplot. The total biomass calculation for the mathematical equation plot was as follows:whereBP plot: total tree biomass in the plot (ton/ha)BP123: total tree biomass in plots 1, 2, and 3, respectively (kg)Plot area123: width of plots 1, 2, and 3, respectively (m2)Necromass and other biomass could be calculated using the above formula.The following formula was used to calculate each biomass component in the land:whereB: average biomass and necromass in the land (ton/ha)∑ BP: total tree biomass from all the plots (ton/ha)∑ BA: total root biomass of all the plots (ton/ha)∑ BTb: total understory biomass from all the plots (ton/ha)∑ NS: total litter necromass of the entire plot (ton/ha)∑ TG: total peat soil biomass from all the plots (ton/ha)N plot: total plotsThe mathematical equation for calculating the conversion of biomass to carbon (C) was:whereC stock: amount of carbon stored on average (ton/ha)B: average biomass and necromass of land (ton/ha)CF: carbon fraction (0.47)The data measurements were tabulated and analyzed using a t-test analysis to determine the differences in biomass and the amount of carbon stored in each age class of D. polyphylla stand on peatlands. The calculation result was then compared with the t-table value for certain degrees of freedom, and there were two alternative hypotheses, as follows:There was a real difference when t count > t tabel.There was no real difference when t count < t tabel.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Istomo Komar, T.E.; Tata, M.H.L. Hutan Rawa Gambut di Indonesia; Indonesia’s Work Programme for 2008 ITTO CITES Project; Pusat Penelitian dan Pengembangan Hutan dan Konservasi Alam Badan Penelitian dan Pengembangan Kehutanan, Kementerian Kehutanan: Bogor, Indonesia, 2010; ISBN 9786029584233. [Google Scholar]
- Daryono, H. Lahan Rawa Gambut Secara Lestari (Potency, problems, policy and peatland management needed for sustainable peat swamp forest). J. Anal. Kebijak. Kehutan. 2009, 6, 71–101. [Google Scholar]
- Nurhayati, A.; Aryanti, E.; Saharjo, B. Kandungan Emisi Gas Rumah Kaca Pada Kebakaran Hutan Rawa Gambut Di Pelalawan Riau (Greenhouse Gases Emission From Peat Fire At Pelalawan Riau). J. Ilmu Pertan. Indones. 2010, 15, 78–82. [Google Scholar]
- Raditya, I.N. Sejarah Kebakaran Hutan & Lahan di Indonesia Terparah Tahun 1997. 2019. Available online: https://tirto.id/sejarah-kebakaran-hutan-lahan-di-indonesia-terparah-tahun-1997-eijN (accessed on 6 May 2021).
- Hiraishi, T.; Krug, T.; Tanabe, K.; Srivastava, N.; Baasansuren, J.; Fukuda, M.; Troxler, T.G. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands; IPCC: Geneva, Switzerland, 2014; ISBN 9789291691395. [Google Scholar]
- Apriando, T. Pakar: Kebakaran Hutan dan Lahan Percepat Perubahan Iklim. 2015. Available online: https://www.mongabay.co.id/2015/10/22/pakar-kebakaran-hutan-dan-lahan-percepat-perubahan-iklim/ (accessed on 17 August 2021).
- Tata, H.L.; Sofiyuddin, M.; Mulyoutami, E.; Perdana, A. Jelutung Rawa: Teknik Budidaya dan Prospek Ekonominya; World Agroforestry Centre (ICRAF) Southeast Asia Regional Program: Bogor, Indonesia, 2015; ISBN 9789793198781. [Google Scholar]
- Aminah Ervizal, A.M.; Zuhud, I.Z.S. Utilization of Jelutung (Dyera spp.) among Anak Dalam Tribe in Bukit Duabelas National Park). Media Konserv. 2016, 21, 168–173. [Google Scholar]
- Santosa, P.B.; Rachmanadi, D.W.H. Pengaruh Genangan Terhadap Tanaman Jelutung (Dyera polyphylla. (Miq.) V. Steenis); Lukmandaru, G., Pujiarti, R., Widyorini, R., Irawati, W.D.N.D., Listyanto, T.P., Eds.; Fakultas Kehutanan Universitas Gadjah Mada: Yogyakarta, Indonesia, 2014; pp. 258–264. [Google Scholar]
- Hani, A.; Siarudin, M.; Indrajaya, Y. Revegetation of Peatlands in West Kalimantan with Superior Commodities. In Proceedings of the 5th SATREPS Conference, Bogor, Indonesia, 17 November 2020; pp. 1–5. [Google Scholar]
- Rochmayanto, Y.; Priatna, D.; Wibowo, A.; Fentie, M.S.J.; Lestari, S.N.S.; Muttaqin, M.Z.; Samsoedin, I.; Ari Rosadi, D.S. Strategi dan Teknik Restorasi Ekosistem Hutan Rawa Gambut; Yanto Rochmayanto Dolly Priatna, M.Z.M., Ed.; IPB Press: Bogor, Indonesia, 2021; ISBN 9786232566927. [Google Scholar]
- Krisnawati, H.; Adinugroho, W.C.; Imanuddin, R.; dan Hutabarat, S. Indonesian National Carbon Accounting System (INCAS): Pendugaan Emisi Gas Rumah Kaca Tahunan dari Hutan dan Lahan Gambut di Kalimantan Tengah Kementerian Lingkungan Hidup dan Kehutanan; Siregar, C.A., Agus, F., Parsons, M., Fitzgerald, N., Karyanto, O., Boer, R., Waterworth, R., Sugardiman, R.A., Teddy Rusolono, T.H.d.Y.J., Eds.; Pusat Penelitian dan Pengembangan Konservasi dan Rehabilitasi: Bogor, Indonesia, 2015; ISBN 9786021681305. [Google Scholar]
- Manuri, S.; Putra, C.A.S.; Saputra, A.D. Tehnik Pendugaan Cadangan Karbon Hutan; Deutsche Gesellschaft für Internationalle Zusamenarbeit (GIZ) GmbH—German International Cooperation Merang REDD Pilot Project (MRPP): Palembang, Indonesia, 2011; ISBN 9786029949223. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, S., Buendia, L., Miwa, K., Todd Ngara, K.T., Eds.; IGES: Kanagawa, Japan, 2006; ISBN 4887880324. [Google Scholar]
- Manuri, S.; Brack, C.; Nugroho, N.P.; Hergoualc’h, K.; Novita, N.; Dotzauer, H.; Verchot, L.; Putra, C.A.S.; Widyasari, E. Tree biomass equations for tropical peat swamp forest ecosystems in Indonesia. For. Ecol. Manag. 2014, 334, 241–253. [Google Scholar] [CrossRef]
- Simon, H. Metode Inventore Hutan; Pustaka Pelajar: Yogyakarta, Indonesia, 2007; ISBN 9789791277174. [Google Scholar]
- Gunawan, H.; Mudiyarso, D.; Mizuno, K.; Forest, I. Taksiran Akumulasi Biomassa atas Permukaan pada Eksperimen Restorasi Lahan Gambut Bekas Terbakar, Area Transisi Cagar Biosfer Giam Siak Kecil-Bukit Batu, Riau, Sumatera Indonesia Pusat Studi Bencana Universitas Riau, Sumatera Center for Southeast Asia. J. Riau Biol. 2016, 1, 8–16. [Google Scholar]
- Krisnawati, H.; Adinugroho, W.C.; Imanuddin, R.; Hutabarat, S. Pendugaan Biomassa Hutan untuk Perhitungan Emisi CO2 di Kalimantan Tengah: Pendekatan Komprehensif Dalam Penentuan Faktor; Waterworth, R., Parsons, M., Susmianto, A., Eds.; Pusat Penelitian dan Pengembangan Konservasi dan Rehabilitasi, Badan Penelitian dan Pengembangan Kehutanan—Kementerian Kehutanan: Bogor, Indonesia, 2014; ISBN 9786021681091. [Google Scholar]
- Sutaryo, D. Penghitungan Biomassa: Sebuah Pengantar Untuk Studi Karbon dan Perdagangan Karbon; Wetlands International Indonesia Programme Jl: Bogor, Indonesia, 2009; ISBN 9789604742332. [Google Scholar]
- Windusari, Y.; Sari, N.A.; Yustian, I.; Zulkifli, H. Estimation of carbon biomass from the understorey and litter vegetation at tailings deposition area of PT Freeport Indonesia. Biospecies 2012, 5, 22–28. [Google Scholar]
- Agus, F.; Made, I.G. Lahan Gambut: Potensi untuk Pertanian dan Aspek Lingkungan; World Agroforestry Centre (ICRAF): Bogor, Indonesia, 2008; ISBN 9786028039109. [Google Scholar]
- De Oliveira Neto, S.N.; Salles, T.T.; Leite, H.G.; Ferreira, G.B.; Melido, R.C.N. Tree Modeling and Economic Evaluation of Agroforestry Systems in Southeastern Brazil. Silva Lusit. 2013, 21, 43–60. [Google Scholar]
- Irawan, U.S.; Purwanto, E. Pengukuran dan Pendugaan Cadangan Karbon pada Ekosistem Hutan Gambut dan Mineral, Studi Kasus di Hutan Rawa Gambut Pematang Gadung dan Hutan Lindung Sungai Lesan, Kalimantan; Yayasan Tropenbos Indonesia: Bogor, Indonesia, 2020. [Google Scholar]




| Plant Age Class | No. Plot | Tree Biomass (kg/100 m2) | Tree Biomass (ton/ha) |
|---|---|---|---|
| I (17 years) | 1 | 582.93 | 58.29 |
| 2 | 534.36 | 53.44 | |
| Total | 1,117.29 | 111.73 | |
| II (13 years) | 1 | 266.05 | 26.60 |
| 2 | 293.53 | 29.35 | |
| Total | 559.57 | 55.96 | |
| III (10 years) | 1 | 283.50 | 28.35 |
| 2 | 217.32 | 21.73 | |
| Total | 500.82 | 50.08 |
| Plant Age Class | No. Plot | Stored Biomass (kg/ha) | Stored Biomass (ton/ha) |
|---|---|---|---|
| I (17 years) | 1 | 488.69 | 0.49 |
| 2 | 661.89 | 0.66 | |
| Total | 1150.58 | 1.15 | |
| II (13 years) | 1 | 725.13 | 0.73 |
| 2 | 457.93 | 0.46 | |
| Total | 1183.06 | 1.18 | |
| III (10 years) | 1 | 483.14 | 0.49 |
| 2 | 507.82 | 0.51 | |
| Total | 990.96 | 1.00 |
| Plant Age Class | No. Plot | Litter Necromass (kg/ha) | Litter Necromass (ton/ha) |
|---|---|---|---|
| I (17 years) | 1 | 11,186.50 | 11.19 |
| 2 | 13,419.89 | 13.42 | |
| Total | 24,606.40 | 24.61 | |
| II (13 years) | 1 | 6993.81 | 6.99 |
| 2 | 7041.84 | 7.04 | |
| Total | 14,035.64 | 14.04 | |
| III (10 years) | 1 | 5814.91 | 5.81 |
| 2 | 4322.27 | 4.32 | |
| Total | 10,137.18 | 10.14 |
| Plant Age Class | No. Plot | Root Biomass (kg/ha) | Root Biomass (ton/ha) |
|---|---|---|---|
| I (17 years) | 1 | 948.19 | 9.48 |
| 2 | 887.60 | 8.88 | |
| Total | 1835.80 | 18.36 | |
| II (13 years) | 1 | 437.75 | 4.38 |
| 2 | 507.20 | 5.07 | |
| Total | 944.95 | 9.45 | |
| III (10 years) | 1 | 507.02 | 5.07 |
| 2 | 400.00 | 4.00 | |
| Total | 907.02 | 9.07 |
| Plant Age Class | No. Plot | Peat Maturity Rate | Peat Thickness (m) | Organic C (%) |
|---|---|---|---|---|
| I (17 years) | 1 | Hemic | 0.87 | 32.37 |
| 2 | Sapric | 0.96 | 30.26 | |
| Average | 0.92 | 31.32 | ||
| II (13 years) | 1 | Hemic | 0.92 | 32.48 |
| 2 | Hemic | 0.88 | 34.41 | |
| Average | 0.90 | 33,45 | ||
| III (10 years) | 1 | Hemic | 0.88 | 24.09 |
| 2 | Hemic | 0.84 | 28.18 | |
| Average | 0.86 | 26.14 |
| Composing Components | Source of Diversity | Sum of Squares | df | Mean Square | F-Statistic | Sig. |
|---|---|---|---|---|---|---|
| Tree | Treatment | 2686.14 | 2 | 1343.07 | 21.32 | 0.00 |
| Error | 3591.63 | 57 | 63.01 | |||
| Total | 6277.78 | 59 |
| Age Class | Mean C of Trees |
|---|---|
| 10 | 11.77 a |
| 13 | 12.38 a |
| 17 | 26.26 b |
| Composing Components | Source of Diversity | Sum of Squares | df | Mean Square | F-Statistics | Sig. |
|---|---|---|---|---|---|---|
| Tree | Treatment | 61.89 | 2 | 30.94 | 19.97 | 0.00 |
| Error | 41.83 | 27 | 1.55 | |||
| Total | 103.71 | 29 |
| Age Class | C Litter Average |
|---|---|
| 10 | 2.38 a |
| 13 | 2.30 a |
| 17 | 5.78 b |
| T | df | Sig. | |
|---|---|---|---|
| Ages of 17 and 13 years old | 10 | 0.401 | 0.251 |
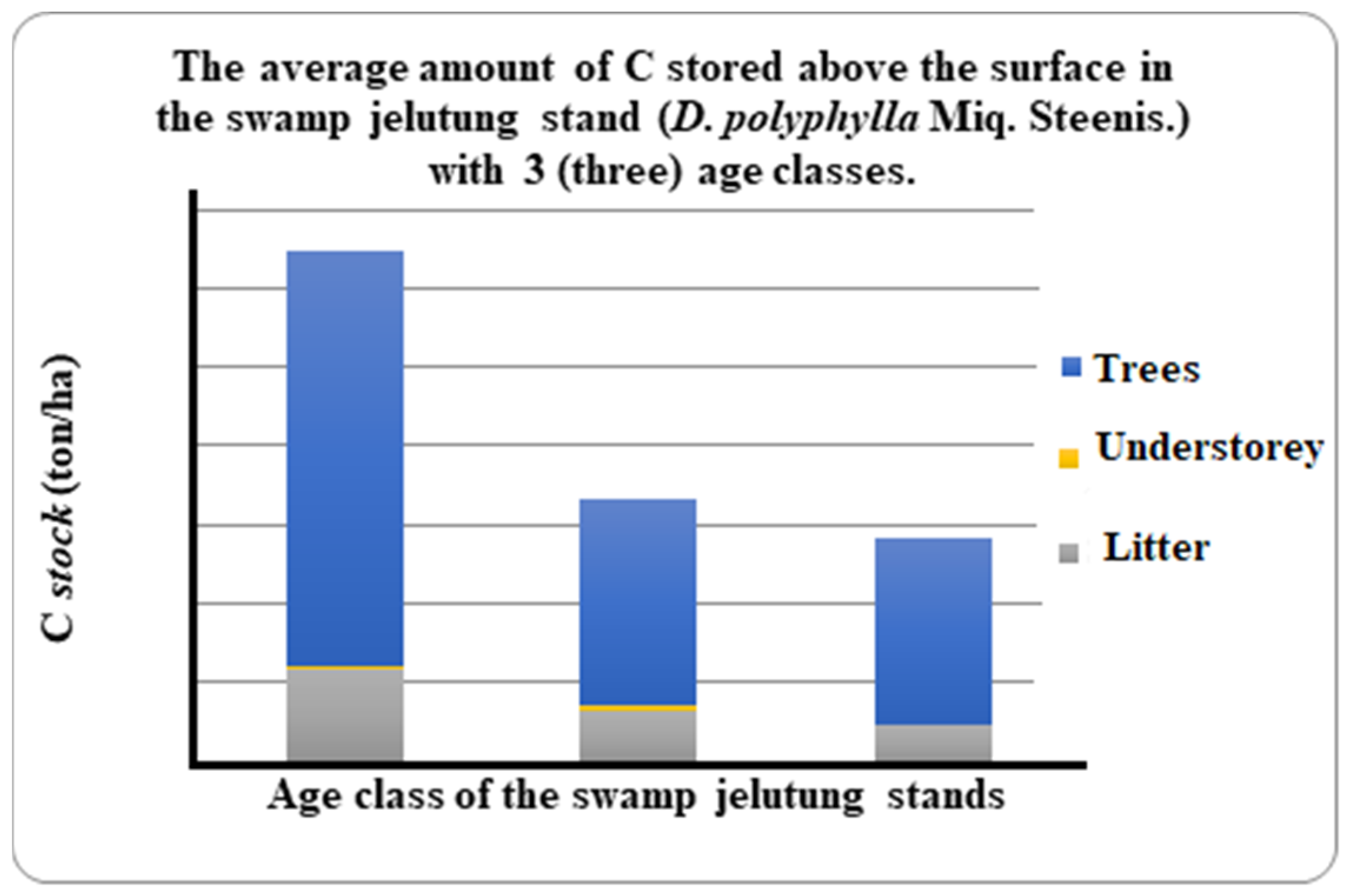
| Composing Components | Age Class I (ton/ha) | Age Class II (ton/ha) | Age Class III (ton/ha) |
|---|---|---|---|
| C stock aboveground: | |||
| Tree (∑ BP) | 26.26 | 13.12 | 11.77 |
| Bottom plants (∑ BTb) | 0.27 | 0.28 | 0.00 |
| Litter necromass (∑ NS) | 5.78 | 3.30 | 2.38 |
| Total C stock | 32.31 | 16.69 | 14.15 |
| C stock belowground: | |||
| Root of the tree (∑ BA) | 4.31 | 2.35 | 2.13 |
| Peat soil (∑ TG) | 715.14 | 706.81 | 517.25 |
| Total C stock | 719.46 | 709.15 | 519.38 |
| Total C stock | 751.77 | 725.85 | 533.53 |
| t | df | Sig. | |
|---|---|---|---|
| BAP and BBP | 1.933 | 65 | 0.058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sufrayogi, D.; Mardiatmoko, G. Carbon Storage Expectations on Swamp Jelutung (Dyera polyphylla Miq. Steenis.) on Peatland for Tackling Climate Change. Forests 2022, 13, 1297. https://doi.org/10.3390/f13081297
Sufrayogi D, Mardiatmoko G. Carbon Storage Expectations on Swamp Jelutung (Dyera polyphylla Miq. Steenis.) on Peatland for Tackling Climate Change. Forests. 2022; 13(8):1297. https://doi.org/10.3390/f13081297
Chicago/Turabian StyleSufrayogi, Dendi, and Gun Mardiatmoko. 2022. "Carbon Storage Expectations on Swamp Jelutung (Dyera polyphylla Miq. Steenis.) on Peatland for Tackling Climate Change" Forests 13, no. 8: 1297. https://doi.org/10.3390/f13081297
APA StyleSufrayogi, D., & Mardiatmoko, G. (2022). Carbon Storage Expectations on Swamp Jelutung (Dyera polyphylla Miq. Steenis.) on Peatland for Tackling Climate Change. Forests, 13(8), 1297. https://doi.org/10.3390/f13081297





