Drought Changes the Trade-Off Strategy of Root and Arbuscular Mycorrhizal Fungi Growth in a Subtropical Chinese Fir Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of Sample Plots and Experimental Design
2.2. Sample Collection
2.3. Determination of Index Properties
2.4. Statistical Analysis
3. Results
3.1. Effects of Precipitation Exclusion on Soil Physical and Chemical Properties
3.2. Effects of Precipitation Exclusion on Root Characteristics of Chinese Fir
3.3. Effects of Precipitation Exclusion on the Arbuscular Mycorrhizal Fungi of the Chinese Fir
3.4. Factors Controlling the Response of Fine Root and Arbuscular Mycorrhizal Fungi to Drought
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. How Does Arbuscular Mycorrhizal Symbiosis Regulate Root Hydraulic Properties and Plasma Membrane Aquaporins in Phaseolus Vulgaris under Drought, Cold or Salinity Stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Mathimaran, N.; Sharma, M.P.; Mohan Raju, B.; Bagyaraj, D. Arbuscular Mycorrhizal Symbiosis and Drought Tolerance in Crop Plants. Mycosphere 2017, 8, 361–376. [Google Scholar] [CrossRef]
- Nikolova, P.S.; Bauerle, T.L.; Haeberle, K.-H.; Blaschke, H.; Brunner, I.; Matyssek, R. Fine-Root Traits Reveal Contrasting Ecological Strategies in European Beech and Norway Spruce During Extreme Drought. Front. Plant Sci. 2020, 11, 1211. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zhang, P.; Wang, P.; Wang, X.; Ji, B.; Mu, J. Environmental Factors Affect the Arbuscular Mycorrhizal Fungal Community through the Status of Host Plants in Three Patterns of Chinese Fir in Southern China. Glob. Ecol. Conserv. 2022, 36, e02121. [Google Scholar] [CrossRef]
- Cao, J.; Xie, L.; Zheng, Y.; Yang, Y. Drought Intensify the Effects of Warming on Root-Colonizing Arbuscular Mycorrhizal Fungal Community in Subtropical Chinese Fir Plantation. Forest Ecol. Manag. 2020, 464, 118078. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, F.A.; Smith, S.E. Structural Diversity in (Vesicular)-Arbuscular Mycorrhizal Symbioses. New Phytol. 1997, 137, 373–388. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; McCormack, M.L.; Chen, F.; Wang, H.; Ma, Z.; Guo, D. Different Responses of Absorptive Roots and Arbuscular Mycorrhizal Fungi to Fertilization Provide Diverse Nutrient Acquisition Strategies in Chinese Fir. Forest Ecol. Manag. 2019, 433, 64–72. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Ai, Z.; Zhang, J.; Zhang, C.; Xue, S.; Liu, G. Effects of the Interaction between Temperature and Revegetation on the Microbial Degradation of Soil Dissolved Organic Matter (DOM)—A DOM Incubation Experiment. Geoderma 2019, 337, 812–824. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide ‘Fast–Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Jia, L.; Chen, G.; Zhang, L.; Chen, T.; Jiang, Q.; Chen, Y.; Fan, A.; Wang, X. Plastic responses of fine root morphology and architecture traits to nitrogen addition in ectomycorrhizal and arbuscular mycorrhizal tree species in an evergreen broadleaved forest. Chin. J. Appl. Ecol. 2021, 2, 529–537. [Google Scholar]
- Valenzuela-Estrada, L.R.; Vera-Caraballo, V.; Ruth, L.E.; Eissenstat, D.M. Root Anatomy, Morphology, and Longevity among Root Orders in Vaccinium Corymbosum (Ericaceae). Am J Bot. 2008, 95, 1506–1514. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Qiu, Y.; Zhang, K.; Yang, F.; Chen, M.; Luo, X.; Yan, X.; Wang, P.; Zhang, Y.; Chen, H.; et al. Climate Warming Promotes Deterministic Assembly of Arbuscular Mycorrhizal Fungal Communities. Global Change Biol. 2021, 28, 1147–1161. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a Multidimensional Root Trait Framework: A Tree Root Review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [Green Version]
- Liese, R.; Leuschner, C.; Meier, I.C. The Effect of Drought and Season on Root Life Span in Temperate Arbuscular Mycorrhizal and Ectomycorrhizal Tree Species. J. Ecol. 2019, 107, 2226–2239. [Google Scholar] [CrossRef]
- Mrak, T.; Štraus, I.; Grebenc, T.; Gričar, J.; Hoshika, Y.; Carriero, G.; Paoletti, E.; Kraigher, H. Different Belowground Responses to Elevated Ozone and Soil Water Deficit in Three European Oak Species (Quercus Ilex, Q. Pubescens and Q. Robur). Sci. Total Environ. 2019, 651, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, W.H.; Zhou, J.Y.; Ma, C.; Han, W.J. Effects of drought stress on growth, physiological and biochemical parameters in fine roots of Quercusvariabilis Bl. seedlings. Acta Ecol. Sin. 2014, 34, 4223–4233. [Google Scholar]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Drought Accentuates the Role of Mycorrhiza in Phosphorus Uptake. Soil Biol. Biochem. 2021, 157, 108243. [Google Scholar] [CrossRef]
- Castaño, C.; Alday, J.G.; Parladé, J.; Pera, J.; Martínez de Aragón, J.; Bonet, J.A. Seasonal dynamics of the ectomycorrhizal fungus Lactarius vinosusare altered by changes in soil moisture and temperature. Soil Biol. Biochem. 2017, 115, 253–260. [Google Scholar] [CrossRef]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis. Mol Plant. 2017, 10, 1147–1158. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Mycorrhizal Stimulation of Leaf Gas Exchange in Relation to Root Colonization, Shoot Size, Leaf Phosphorus and Nitrogen: A Quantitative Analysis of the Literature Using Meta-Regression. Front. Plant Sci. 2016, 7, 1084. [Google Scholar] [CrossRef]
- Smith, S.E.; Facelli, E.; Pope, S.; Andrew Smith, F. Plant Performance in Stressful Environments: Interpreting New and Established Knowledge of the Roles of Arbuscular Mycorrhizas. Plant Soil 2010, 326, 3–20. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Yanai, R.D. The ecology of root Lifespan. Adv. Ecol. Res. 1997, 27, 1–60. [Google Scholar]
- Davison, J.; Moora, M.; Semchenko, M.; Adenan, S.B.; Ahmed, T.; Akhmetzhanova, A.A.; Alatalo, J.M.; Al-Quraishy, S.; Andriyanova, E.; Anslan, S.; et al. Temperature and Ph Define the Realised Niche Space of Arbuscular Mycorrhizal Fungi. New Phytol. 2021, 231, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Ryser, P. The Limits to Leaf and Root Plasticity: What Is So Special About Specific Root Length? New Phytol. 2015, 206, 1188–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, N.C. Resource Stoichiometry Elucidates the Structure and Function of Arbuscular Mycorrhizas across Scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Avio, L.; Pellegrino, E.; Bonari, E.; Giovannetti, M. Functional Diversity of Arbuscular Mycorrhizal Fungal Isolates in Relation to Extraradical Mycelial Networks. New Phytol. 2006, 172, 347–357. [Google Scholar] [CrossRef]
- Lambers, H.; Albornoz, F.; Kotula, L.; Laliberté, E.; Ranathunge, K.; Teste, F.P.; Zemunik, G. How Belowground Interactions Contribute to the Coexistence of Mycorrhizal and Non-Mycorrhizal Species in Severely Phosphorus-Impoverished Hyperdiverse Ecosystems. Plant Soil 2018, 424, 11–33. [Google Scholar] [CrossRef] [Green Version]
- Teste, F.P.; Laliberté, E.; Lambers, H.; Auer, Y.; Kramer, S.; Kandeler, E. Mycorrhizal Fungal Biomass and Scavenging Declines in Phosphorus-Impoverished Soils During Ecosystem Retrogression. Soil Biol. Biochem. 2016, 92, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Deepika, S.; Kothamasi, D. Soil Moisture—A Regulator of Arbuscular Mycorrhizal Fungal Community Assembly and Symbiotic Phosphorus Uptake. Mycorrhiza 2015, 25, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Deveautour, C.; Donn, S.; Power, S.A.; Bennett, A.E.; Powell, J.R. Experimentally Altered Rainfall Regimes and Host Root Traits Affect Grassland Arbuscular Mycorrhizal Fungal Communities. Mol. Ecol. 2018, 27, 2152–2163. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; He, X.H. Differences of Hyphal and Soil Phosphatase Activities in Drought-Stressed Mycorrhizal Trifoliate Orange (Poncirus Trifoliata) Seedlings. Sci. Hortic. 2011, 129, 294–298. [Google Scholar] [CrossRef]
- Staddon, P.L.; Thompson, K.; Jakobsen, I.; Grime, J.P.; Askew, A.P.; Fitter, A.H. Mycorrhizal Fungal Abundance Is Affected by Long-Term Climatic Manipulations in the Field. Global Change Biol. 2003, 9, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Augé, R.M. Water Relations, Drought and Vesicular-Arbuscular Mycorrhizal Symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Y.; Gao, C.; He, X.; Ding, Q.; Kim, Y.; Rui, Y.; Wang, S.; Guo, L.-D. The Arbuscular Mycorrhizal Fungal Community Response to Warming and Grazing Differs between Soil and Roots on the Qinghai-Tibetan Plateau. PLoS ONE 2013, 8, e76447. [Google Scholar] [CrossRef]
- Chen, C.; Park, T.; Wang, X.; Piao, S.; Xu, B.; Chaturvedi, R.K.; Fuchs, R.; Brovkin, V.; Ciais, P.; Fensholt, R.; et al. China and India Lead in Greening of the World through Land-Use Management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2005: Progress Towards Sustainable Forest Management; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Lyu, M.; Li, X.; Xie, J.; Homyak, P.M.; Ukonmaanaho, L.; Yang, Z.; Liu, X.; Ruan, C.; Yang, Y. Root–Microbial Interaction Accelerates Soil Nitrogen Depletion but Not Soil Carbon after Increasing Litter Inputs to a Coniferous Forest. Plant Soil 2019, 444, 153–164. [Google Scholar] [CrossRef]
- Wehner, M.F.; Reed, K.A.; Loring, B.; Stone, D.; Krishnan, H. Changes in tropical cyclones under stabilized 1.5 °C and 2.0 °C global warming scenarios as simulated by the Community Atmospheric Model under the HAPPI protocols. Earth Syst. Dynam. 2017, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Al-Karaki, G.; Mcmichael, B.; Zak, J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mychorrhiza 2004, 14, 263–269. [Google Scholar] [CrossRef]
- Zhou, G.; Wei, X.; Wu, Y.; Liu, S.; Huang, Y.; Yan, J.; Zhang, D.; Zhang, Q.; Liu, J.; Meng, Z.; et al. Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China. Glob. Change Biol. 2011, 17, 3736–3746. [Google Scholar] [CrossRef]
- Soil Survey Staff. Natural Resources Conservation Service 2014 National Soil Survey Handbook; Title 430-VI; U.S. Government Printing Office: Washington, DC, USA, 2014; Sec 602. [Google Scholar]
- State Soil Survey Service of China. China Soil; China Agricultural Press: Beijing, China, 1998. [Google Scholar]
- Sheteiwy, M.S.; El-Sawah, A.M.; Korany, S.M.; Alsherif, E.A.; Mowafy, A.M.; Chen, J.; Jośko, I.; Selim, S.; Abd Elgawad, H. Arbuscular Mycorrhizal Fungus “Rhizophagus Irregularis” Impacts on Physiological and Biochemical Responses of Ryegrass and Chickpea Plants under Beryllium Stress. Environ. Pollut. 2022, 315, 120356. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Sawah, A.M. The Mode of Integration between Azotobacter and Rhizobium Affect Plant Growth, Yield, and Physiological Responses of Pea (Pisum Sativum L.). J Soil Sci Plant Nut. 2022, 22, 1238–1251. [Google Scholar] [CrossRef]
- Kormanik, P.P.; Bryan, W.C.; Schultz, R.C. Procedures and equipment for staining large numbers of plant root samples for endomycorrhizal assay. Can. J. Microbiol. 1980, 26, 536–538. [Google Scholar] [CrossRef]
- Ianson, D.C.; Allen, M.F. The effects of soil texture on extraction of vesicular arbuscular mycorrhizal fungal spores from arid sites. Mycologia 1986, 1, 164–168. [Google Scholar] [CrossRef]
- An, Z.; Hendrix, J.; Hershman, D.; Henson, G. Evaluation of the "Most Probable Number" (Mpn) and Wet-Sieving Methods for Determining Soil-Borne Populations of Endogonaceous Mycorrhizal Fungi. Mycologia 1990, 82, 576. [Google Scholar] [CrossRef]
- Janos, D.P.; Garamszegi, S.; Beltran, B. Glomalin Extraction and Measurement. Soil Biol. Biochem. 2008, 40, 728–739. [Google Scholar] [CrossRef]
- Wright, S.F.; Franke-Snyder, M.; Morton, J.B.; Upadhyaya, A. Time-Course Study and Partial Characterization of a Protein on Hyphae of Arbuscular Mycorrhizal Fungi During Active Colonization of Roots. Plant Soil. 1996, 181, 193–203. [Google Scholar] [CrossRef]
- Mariotte, P.; Canarini, A.; Dijkstra, F.A. Stoichiometric N:P Flexibility and Mycorrhizal Symbiosis Favour Plant Resistance against Drought. J. Ecol. 2017, 105, 958–967. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading Dimensions in Absorptive Root Trait Variation across 96 Subtropical Forest Species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef]
- Solly, E.F.; Brunner, I.; Helmisaari, H.-S.; Herzog, C.; Leppälammi-Kujansuu, J.; Schöning, I.; Schrumpf, M.; Schweingruber, F.H.; Trumbore, S.E.; Hagedorn, F. Unravelling the Age of Fine Roots of Temperate and Boreal Forests. Nat. Commun. 2018, 9, 3006. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, G.; Wang, N.; Wang, Z.; Gu, J. Effects of Morphology and Stand Structure on Root Biomass and Length Differed between Absorptive and Transport Roots in Temperate Trees. Plant Soil. 2019, 442, 355–367. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, L.; Xu, X.; Zhang, L.; Zhang, K.; Chen, M.; Zhao, Y.; Burkey, K.O.; Shew, H.D.; Zobel, R.W.; et al. Warming and Elevated Ozone Induce Tradeoffs between Fine Roots and Mycorrhizal Fungi and Stimulate Organic Carbon Decomposition. Sci Adv. 2021, 7, eabe9256. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in Nutrient Foraging Strategies of Absorptive Fine Roots and Arbuscular Mycorrhizal Fungi across 14 Coexisting Subtropical Tree Species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef]
- Bergmann, J.; Weigelt, A.; van der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M.; et al. The Fungal Collaboration Gradient Dominates the Root Economics Space in Plants. Sci Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef]
- Kubisch, P.; Hertel, D.; Leuschner, C. Fine Root Productivity and Turnover of Ectomycorrhizal and Arbuscular Mycorrhizal Tree Species in a Temperate Broad-Leaved Mixed Forest. Front. Plant Sci. 2016, 7, 1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weemstra, M.; Sterck, F.J.; Visser, E.J.W.; Kuyper, T.W.; Goudzwaard, L.; Mommer, L. Fine-Root Trait Plasticity of Beech (Fagus Sylvatica) and Spruce (Picea Abies) Forests on Two Contrasting Soils. Plant Soil. 2017, 415, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How Tree Roots Respond to Drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [Green Version]
- Valverde-Barrantes, O.J.; Smemo, K.A.; Blackwood, C.B. Fine Root Morphology Is Phylogenetically Structured, but Nitrogen Is Related to the Plant Economics Spectrum in Temperate Trees. Funct. Ecol. 2015, 29, 796–807. [Google Scholar] [CrossRef]
- Wang, P.; Huang, K.; Hu, S. Distinct Fine-Root Responses to Precipitation Changes in Herbaceous and Woody Plants: A Meta-Analysis. New Phytol. 2020, 225, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Kim, K.; Krishnamoorthy, R.; Walitang, D.; Sundaram, S.; Joe, M.M.; Selvakumar, G.; Hu, S.; Oh, S.-H.; Sa, T. Mycorrhizal Symbiotic Efficiency on C3 and C4 Plants under Salinity Stress—A Meta-Analysis. Front. Microbiol. 2016, 7, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Morales, R.; Gavito, M.E.; Carrillo-Saucedo, S.M. Morphological and Physiological Responses of the External Mycelium of Rhizophagus Intraradices to Water Stress. Mycorrhiza 2019, 29, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, I.; Abbott, L.K.; Robson, A.D. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L.1.Spread of hyphea and phosphorus inflow into root. New Phytol. 1992, 120, 371–380. [Google Scholar] [CrossRef]


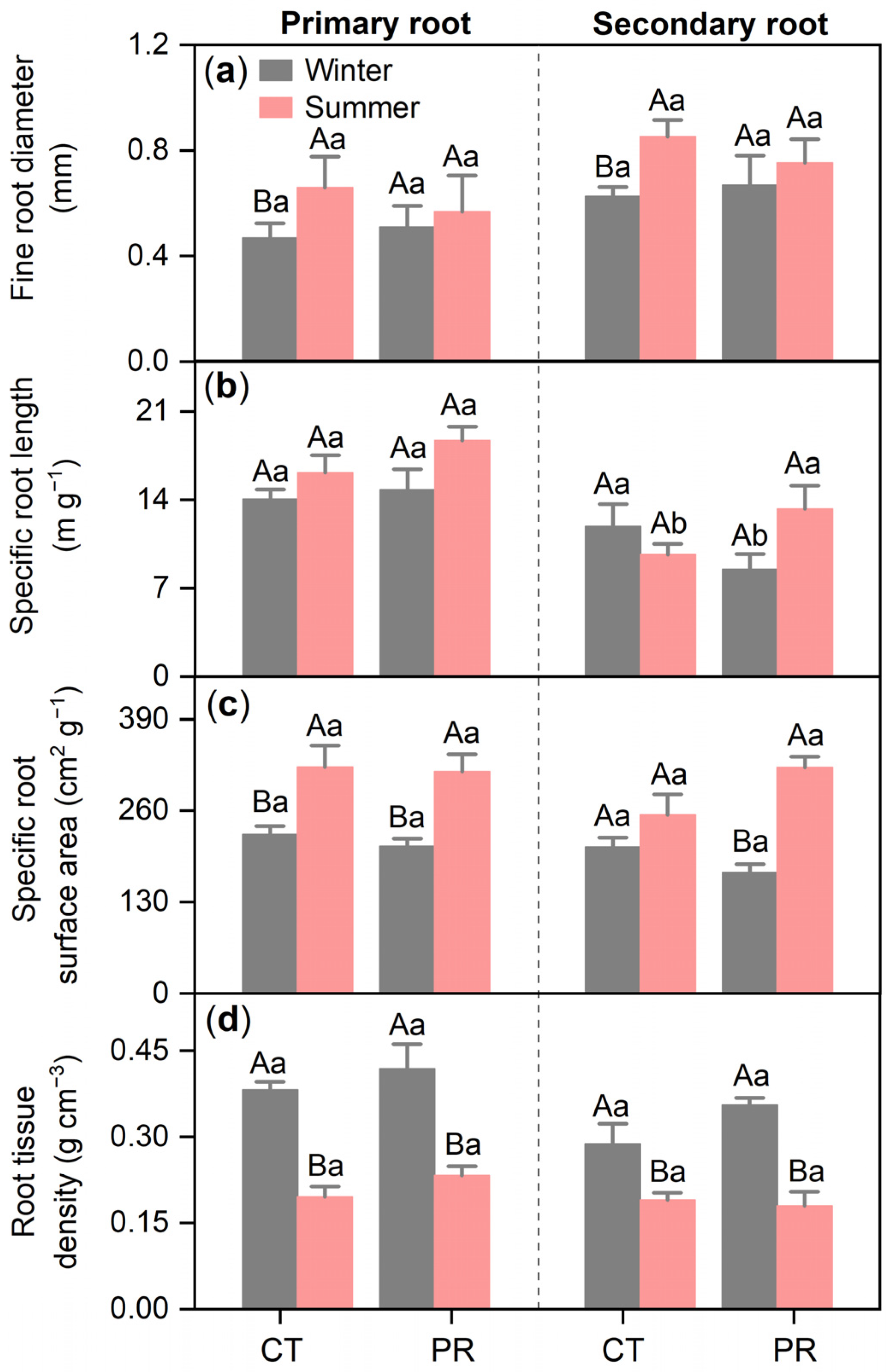

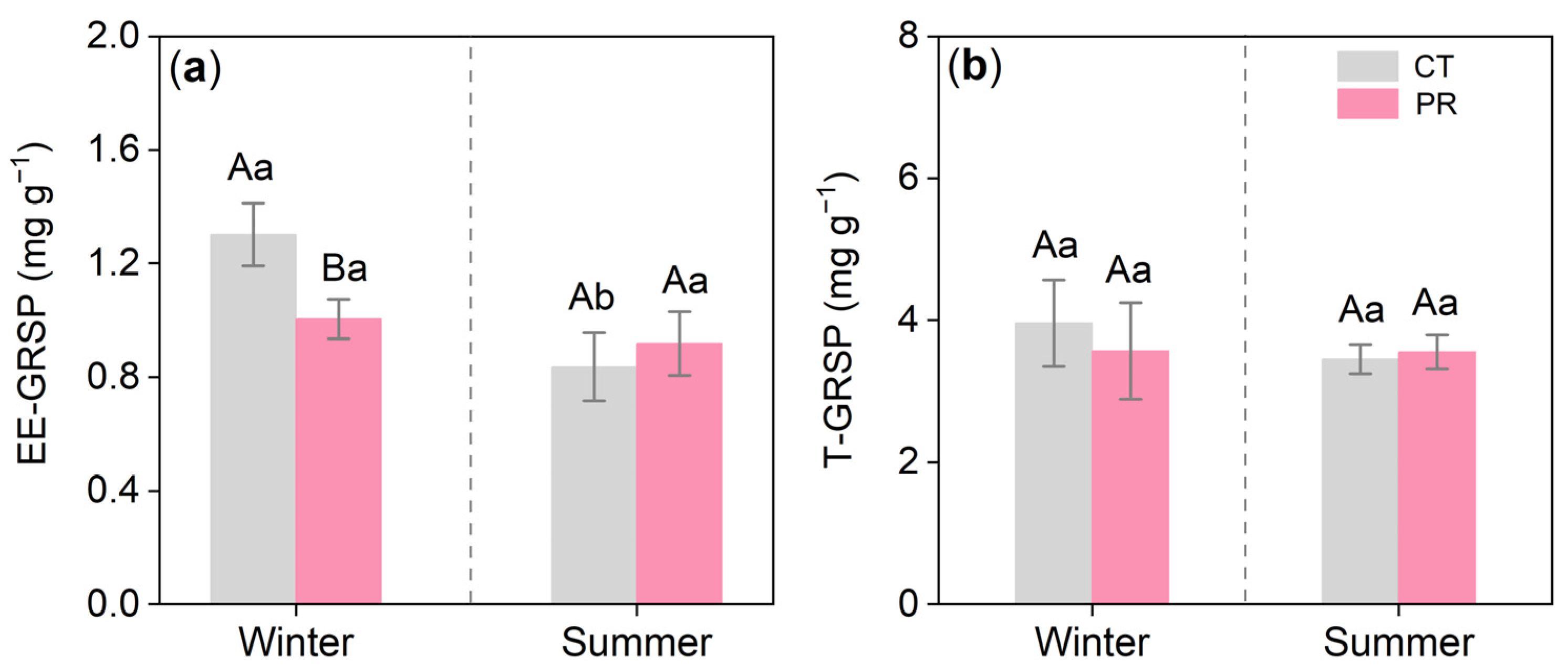
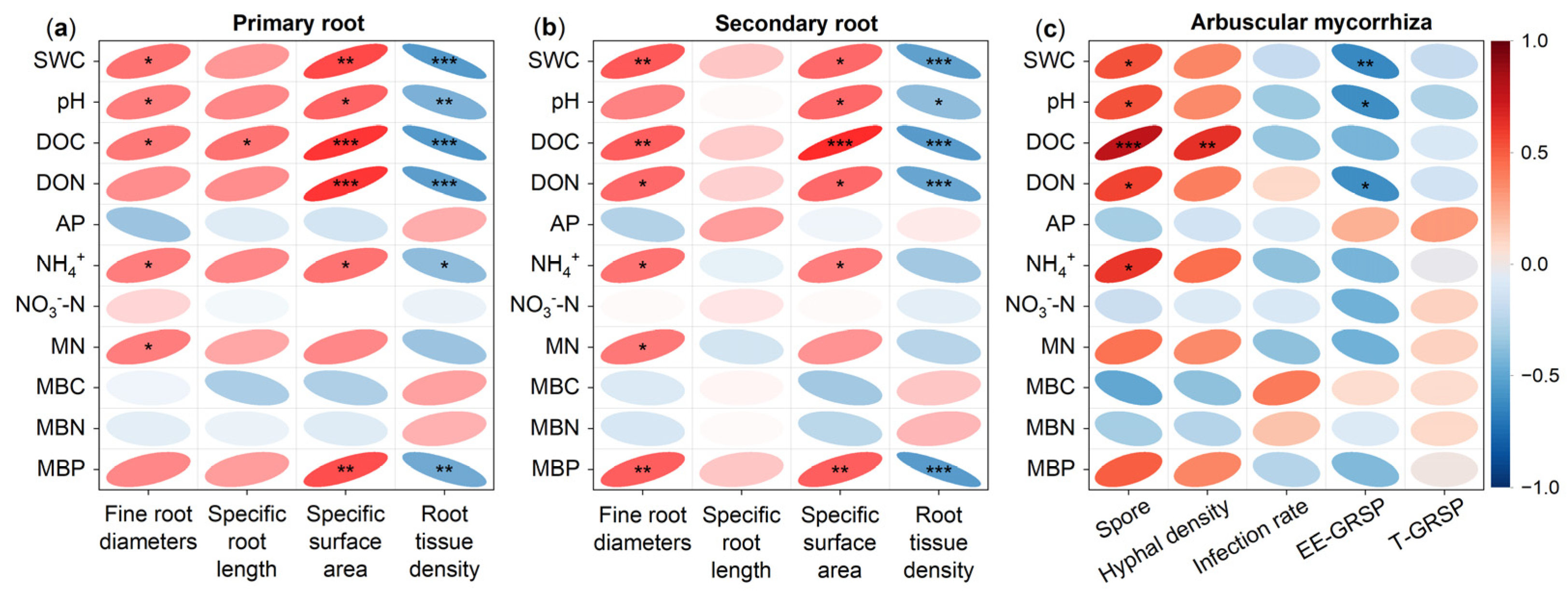
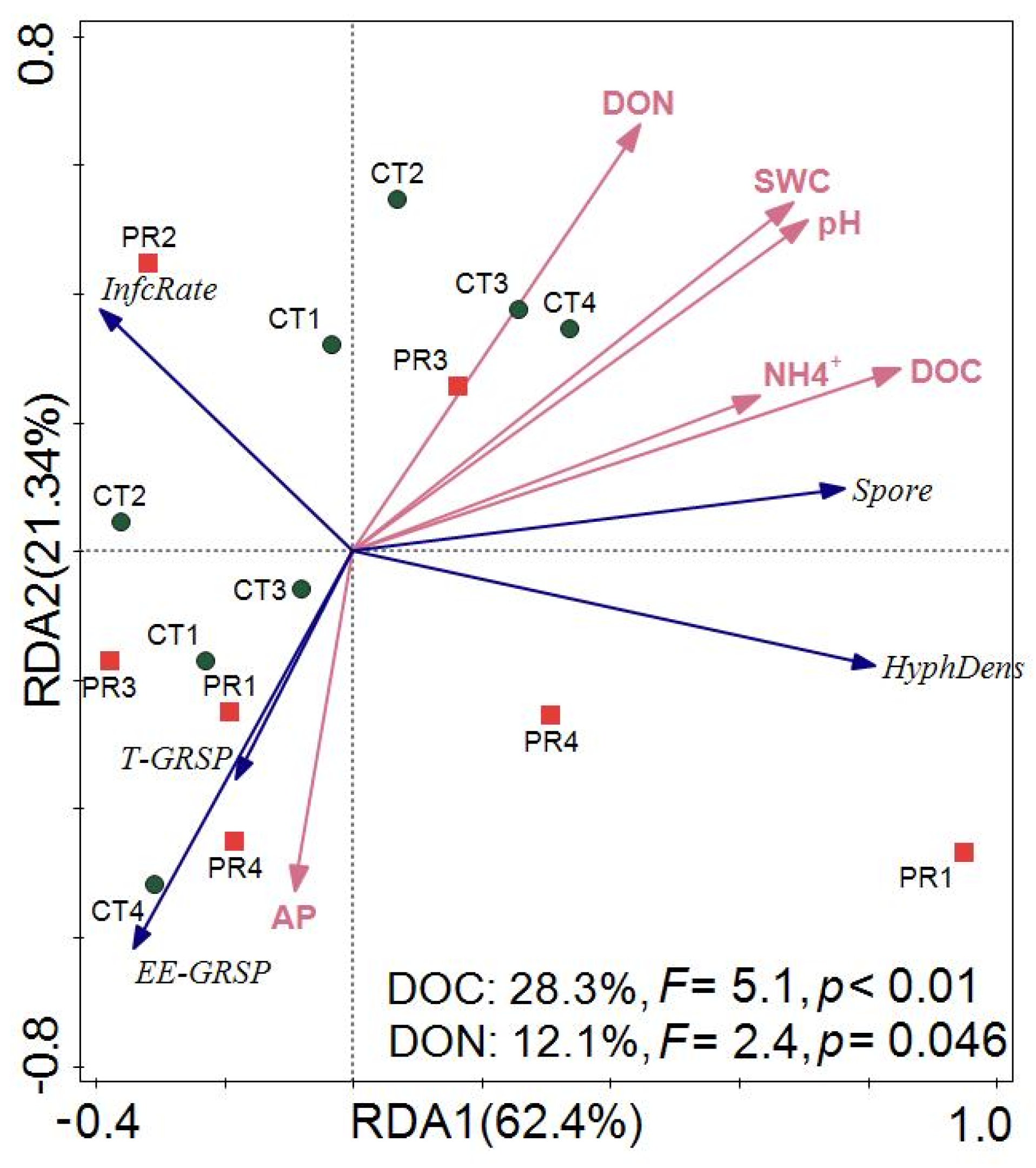
| Winter | Summer | Treatment | Season | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT | PR | CT | PR | F | P | F | P | F | P | |
| Moisture | 11.08 ± 1.86 Ab | 9.69 ± 0.91 Ab | 20.07 ± 1.98 Aa | 17.47 ± 1.75 Aa | 5.66 | 0.035 | 99.88 | 0.000 | 0.52 | 0.486 |
| pH | 4.25 ± 0.05 Ab | 4.30 ± 0.06 Aa | 4.36 ± 0.05 Aa | 4.37 ± 0.03 Aa | 1.41 | 0.259 | 14.84 | 0.002 | 0.62 | 0.445 |
| DOC (mg kg−1) | 20.91 ± 1.15 Ab | 22.06 ± 1.43 Ab | 38.43 ± 4.97 Aa | 40.08 ± 5.16 Aa | 0.57 | 0.465 | 92.30 | 0.000 | 0.02 | 0.896 |
| DON (mg kg−1) | 0.98 ± 0.13 Ab | 0.89 ± 0.09 Ab | 4.20 ± 0.39 Aa | 2.90 ± 1.17 Ba | 4.99 | 0.045 | 70.58 | 0.000 | 3.82 | 0.013 |
| AP (mg kg−1) | 1.31 ± 0.19 Aa | 1.02 ± 0.21 Aa | 0.88 ± 0.1 Ab | 1.17 ± 0.26 Aa | 0.002 | 0.967 | 1.94 | 0.189 | 8.49 | 0.075 |
| NH4+ (mg kg−1) | 3.00 ± 0.92 Bb | 4.64 ± 0.86 Aa | 5.96 ± 0.4 Aa | 5.88 ± 0.68 Aa | 4.38 | 0.058 | 31.81 | 0.000 | 5.32 | 0.040 |
| NO3− (mg kg−1) | 3.79 ± 0.53 Aa | 3.83 ± 0.38 Aa | 3.97 ± 0.48 Aa | 3.90 ± 0.63 Aa | 0.01 | 0.940 | 0.22 | 0.647 | 0.04 | 0.837 |
| MN (mg kg−1) | 6.24 ± 1.55 Bb | 8.47 ± 1.05 Aa | 9.93 ± 0.82 Aa | 9.78 ± 0.38 Aa | 3.97 | 0.070 | 23.07 | 0.000 | 5.23 | 0.041 |
| MBC (mg kg−1) | 464.51 ± 60.23 Aa | 451.33 ± 66.31 Aa | 445.45 ± 46.33 Aa | 383.0 ± 19.47 Bb | 2.17 | 0.167 | 2.90 | 0.115 | 0.92 | 0.356 |
| MBN (mg kg−1) | 44.38 ± 3.56 Aa | 50.77 ± 9.87 Aa | 46.25 ± 6.96 Aa | 41.31 ± 5.54 Aa | 0.04 | 0.837 | 1.22 | 0.292 | 2.72 | 0.125 |
| MBP (mg kg−1) | 13.20 ± 1.75 Ab | 12.25 ± 1.89 Ab | 18.27 ± 0.84 Aa | 16.76 ± 1.40 Aa | 2.59 | 0.133 | 39.42 | 0.000 | 0.14 | 0.718 |
| Root Ordinal | Treatment | Fine Root Production |
|---|---|---|
| Primary root | CT | 1.28 ± 0.25 |
| PR | 1.37 ± 0.38 | |
| Secondary root | CT | 1.25 ± 0.58 |
| PR | 1.03 ± 0.9 |
| Fine Root Diameter | Specific Root Length | Specific Root Surface Area | Root Density | ||
|---|---|---|---|---|---|
| Treatment (T) | F-value | 0.662 | 0.814 | 0.020 | 3.489 |
| p-value | 0.424 | 0.376 | 0.888 | 0.074 | |
| Diameter class (D) | F-value | 27.946 | 27.452 | 4.140 | 9.551 |
| p-value | <0.001 | <0.001 | 0.053 | 0.005 | |
| Season (S) | F-value | 18.226 | 4.766 | 48.201 | 85.990 |
| p-value | <0.001 | 0.039 | <0.001 | <0.001 | |
| T × D | F-value | 0.003 | 0.616 | 0.927 | 0.055 |
| p-value | 0.958 | 0.440 | 0.345 | 0.816 | |
| T × S | F-value | 4.330 | 5.175 | 3.981 | 1.203 |
| p-value | 0.048 | 0.032 | 0.057 | 0.284 | |
| D × S | F-value | 0.247 | 0.785 | 0.016 | 2.036 |
| p-value | 0.624 | 0.384 | 0.899 | 0.166 | |
| T × D × S | F-value | 0.004 | 1.828 | 2.663 | 1.254 |
| p-value | 0.950 | 0.189 | 0.116 | 0.274 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Jiang, Y.; Lyu, M.; Cao, C.; Li, X.; Xiong, X.; Lin, W.; Yang, Z.; Chen, G.; Yang, Y.; et al. Drought Changes the Trade-Off Strategy of Root and Arbuscular Mycorrhizal Fungi Growth in a Subtropical Chinese Fir Plantation. Forests 2023, 14, 114. https://doi.org/10.3390/f14010114
Dong J, Jiang Y, Lyu M, Cao C, Li X, Xiong X, Lin W, Yang Z, Chen G, Yang Y, et al. Drought Changes the Trade-Off Strategy of Root and Arbuscular Mycorrhizal Fungi Growth in a Subtropical Chinese Fir Plantation. Forests. 2023; 14(1):114. https://doi.org/10.3390/f14010114
Chicago/Turabian StyleDong, Jie, Yongmeng Jiang, Maokui Lyu, Cong Cao, Xiaojie Li, Xiaoling Xiong, Weisheng Lin, Zhijie Yang, Guangshui Chen, Yusheng Yang, and et al. 2023. "Drought Changes the Trade-Off Strategy of Root and Arbuscular Mycorrhizal Fungi Growth in a Subtropical Chinese Fir Plantation" Forests 14, no. 1: 114. https://doi.org/10.3390/f14010114
APA StyleDong, J., Jiang, Y., Lyu, M., Cao, C., Li, X., Xiong, X., Lin, W., Yang, Z., Chen, G., Yang, Y., & Xie, J. (2023). Drought Changes the Trade-Off Strategy of Root and Arbuscular Mycorrhizal Fungi Growth in a Subtropical Chinese Fir Plantation. Forests, 14(1), 114. https://doi.org/10.3390/f14010114






