Is the Invasiveness of Pittosporum undulatum in Eucalypt Forests Explained by the Wide Ranging Effects of Its Secondary Metabolites?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Germination Experiment
- −
- Germination Percentage (GP), a numeric parameter representing the top horizontal asymptote of the S-shape curve, signifying the total germination obtained, where 0 corresponds to no germination and 1 is the full germination (Asym in R);
- −
- t50, a numeric parameter representing the day of the inflection point of the curve, i.e., where the germination is half of the total final germination (Xmid in R);
- −
- t75, a numeric scale parameter obtained from the number of days between 3/4 of GP and t50, representing the growth rate during the exponential phase (Scal in R).
2.3. Analyses of Total Saponins and Total Condensed Tannins in Leaf Litter Leachates
2.4. HPLC Analysis of Polyphenols in Leaves and Leaf Litter
2.5. Collection and Analysis of BVOCs
2.6. Statistical Analyses
- i.
- A Nonlinear Mixed-Effects Models analysis was used on each daily cumulate count (for each combination of substrate and watering treatments) given their non-linear trends over time. The starting estimates of the S-shape curves were estimated through the SSlogis() function (nlme library) and the model space was investigated by comparing marginal models [41] to select the most parsimonious model. Two non-linear models were fitted on the data: one model for Eucalyptus ovata seeds and another for Pittosporum undulatum seeds.
- ii.
- Each index (i.e., t0, tf, tf − t0) was calculated at the end of the germination experiment for every single species. Counts data were fitted using a General Linear Model (glm(), requiring the lattice and faraway packages [50]) using a Poisson distribution family with log-link function (glm (INDEX ~ Substrate × Treatment, family = poisson (link = “log”))), and the significance was calculated on the exponents and not on the values of the indices. The effect of different substrates (Substrate) and leachates (Treatment), their interaction on germination, and seedling development was observed. The models were carried out with the Petri dishes characterized by filter paper and distilled water as reference (Intercept). Finally, the models were chosen after checking for overdispersion.
- iii.
- A two-way Analysis of Variance (ANOVA) was conducted for the continuous data obtained from the Vigor Index (VI), observing the interaction substrate × treatment. Before carrying out the ANOVA, the assumption of normality and homoscedasticity were checked using Shapiro and Levene’s tests, respectively [51,52]. Finally, a Tukey post-hoc test was conducted.
- iv.
- For continuous variables (TSC, TcTC, TTC, TFC and THC), a one-way non-parametric analysis of variance (Kruskal–Wallis Test) was conducted. This test was carried out, since the ANOVA’s assumptions of normality tested with Shapiro’s were not met, while the heteroscedasticity tested with Levene’s test was met. After that, Dunn’s Multiple Comparison post-hoc test was carried out.
- v.
- For BVOCs compounds, in order to test differences between the two studied areas (I and R), we calculated the relative amount of each monoterpene (MT) and sesquiterpene (SQT) identified, expressed as a percentage of total terpenes peak areas obtained by GC-MS (TMTs + TSQTs) for both areas. The mean percentages of each terpene were analysed by a one-way analysis of variance.
3. Results
3.1. Leachates and Substrates Effects on the Germination of P. undulatum or E. ovata
3.2. Total Saponins and Total Condensed Tannins Content
3.3. HPLC-DAD Analyses of Polyphenol Content
3.4. BVOC Analysis
4. Discussion
4.1. Does Pittosporum undulatum Inhibit Germination of Eucalypts?
4.2. Is the Invasiveness of P. undulatum due to the Storing and Emission of Secondary Metabolites?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kijowska-Oberc, J.; Staszak, A.M.; Kamiński, J.; Ratajczak, E. Adaptation of Forest Trees to Rapidly Changing Climate. Forests 2020, 11, 123. [Google Scholar] [CrossRef] [Green Version]
- Myers, N. The Biodiversity Challenge: Expanded Hot-Spots Analysis. Environmentalist 1990, 10, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagacherie, P.; Álvaro-Fuentes, J.; Annabi, M.; Bernoux, M.; Bouarfa, S.; Douaoui, A.; Grünberger, O.; Hammani, A.; Montanarella, L.; Mrabet, R.; et al. Managing Mediterranean Soil Resources under Global Change: Expected Trends and Mitigation Strategies. Reg. Environ. Chang. 2018, 18, 663–675. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Hydraulic Redistribution by Plants and Nutrient Stoichiometry: Shifts under Global Change: Plants and Availability and Cycling of Water and Nutrients. Ecohydrology 2014, 7, 1–20. [Google Scholar] [CrossRef]
- Rocha, J.; Carvalho-Santos, C.; Diogo, P.; Beça, P.; Keizer, J.J.; Nunes, J.P. Impacts of Climate Change on Reservoir Water Availability, Quality and Irrigation Needs in a Water Scarce Mediterranean Region (Southern Portugal). Sci. Total Environ. 2020, 736, 139477. [Google Scholar] [CrossRef]
- Prietzel, J.; Falk, W.; Reger, B.; Uhl, E.; Pretzsch, H.; Zimmermann, L. Half a Century of Scots Pine Forest Ecosystem Monitoring Reveals Long-term Effects of Atmospheric Deposition and Climate Change. Glob. Chang. Biol. 2020, 26, 5796–5815. [Google Scholar] [CrossRef]
- Simberloff, D. How Common Are Invasion-Induced Ecosystem Impacts? Biol. Invasions 2011, 13, 1255–1268. [Google Scholar] [CrossRef]
- Adair, R.J.; Groves, R.H. Impact of Environmental Weeds on Biodiversity: A Review and Development of a Methodology; Environment Australia: Camberra, Australia, 1998; ISBN 0-642-21412-3. [Google Scholar]
- Klepeis, P.; Gill, N.; Chisholm, L. Emerging Amenity Landscapes: Invasive Weeds and Land Subdivision in Rural Australia. Land Use Policy 2009, 26, 380–392. [Google Scholar] [CrossRef]
- O’Leary, B.; Burd, M.; Venn, S.E.; Gleadow, R. Integrating the Passenger-Driver Hypothesis and Plant Community Functional Traits to the Restoration of Lands Degraded by Invasive Trees. For. Ecol. Manag. 2018, 408, 112–120. [Google Scholar] [CrossRef]
- Gris, D.; Boaretto, A.G.; Marques, M.R.; Damasceno-Junior, G.A.; Carollo, C.A. Secondary Metabolites That Could Contribute to the Monodominance of Erythrina Fusca in the Brazilian Pantanal. Ecotoxicology 2019, 28, 1232–1240. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Dudley, T.M.; Mack, M. Disturbance and Biological Invasions: Direct Effects and Feedbacks. In Ecosystem of Disturbed Ground; Elsevier: New York, NY, USA, 1999; pp. 413–452. [Google Scholar]
- MacDougall, A.S.; McCann, K.S.; Gellner, G.; Turkington, R. Diversity Loss with Persistent Human Disturbance Increases Vulnerability to Ecosystem Collapse. Nature 2013, 494, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Gleadow, R.M.; Ashton, D.H. Invasion by Pittosporum undulatum of the Forests of Central Victoria. I. Invasion Patterns and Plant Morphology. Aust. J. Bot. 1981, 29, 705–720. [Google Scholar] [CrossRef]
- Lunt, I.D. Two Hundred Years of Land Use and Vegetation Change in a Remnant Coastal Woodland in Southern Australia. Aust. J. Bot. 1998, 46, 629. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Tanner, E.V.J.; Martin, P.H.; Healey, J.R.; Burge, O.R. Endemic Trees in a Tropical Biodiversity Hotspot Imperilled by an Invasive Tree. Biol. Conserv. 2018, 217, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Head, L.; Muir, P. Nativeness, Invasiveness, and Nation in Australian Plants. Geogr. Rev. 2004, 94, 199–217. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Narayan, I. Temperature Thresholds for Germination and Survival of Pittosporum undulatum: Implications for Management by Fire. Acta Oecologica 2007, 31, 151–157. [Google Scholar] [CrossRef]
- Nunes, H.; Falé, P.; Duarte, M.F. Pittosporum undulatum and Hedychium gardnerianum Nutritive Value and Secondary Metabolites on Cattle Reproductive Performances. Int. J. Pure Appl. Sci. Technol. 2014, 22, 1–9. [Google Scholar]
- Goodland, T.; Healey, J. The Effect of Pittosporum undulatum on the Native Vegetation of the Blue Mountains of Jamaica; University of Wales Bangor: Bangor, UK, 1997. [Google Scholar]
- Gleadow, R.M.; Rowan, K.S. Invasion by Pittosporum undulatum of the Forests of Central Victoria. III Effects of Temperature and Light on Growth and Drought Resistance. Aust. J. Bot. 1982, 30, 347–357. [Google Scholar] [CrossRef]
- Negrelle, R.R.B.; Mielke, E.C.; Cuquel, F.L.; Pulido, E.E. Pittosporum undulatum Vent.: Subsidies to the Control and Management. Ornam. Hortic. 2018, 24, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Gleadow, R.M. Invasion by Pittosporum undulatum of the Forests of Central Victoria. II Dispersal, Germination and Establishment. Aust. J. Bot. 1982, 30, 185–198. [Google Scholar] [CrossRef]
- O’Leary, B.A.; Burd, M.; Venn, S.E.; Gleadow, R.M. Bird Community Recovery Following Removal of an Invasive Tree. Ecol. Solut. Evid. 2021, 2, e12080. [Google Scholar] [CrossRef]
- Goodger, J.Q.D.; Ades, P.K.; Woodrow, I.E. Cyanogenesis in Eucalyptus polyanthemos Seedlings: Heritability, Ontogeny and Effect of Soil Nitrogen. Tree Physiol. 2004, 24, 681–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleadow, R.; Rowan, K.; Ashton, D. Invasion by Pittosporum undulatum of the Forests of Central Victoria. IV. Shade Tolerance. Aust. J. Bot. 1983, 31, 151. [Google Scholar] [CrossRef]
- Cooper, R.P. Birds Feeding on Pittosporum Seeds. Emu-Austral Ornithol. 1959, 59, 60–61. [Google Scholar] [CrossRef]
- Mullett, T.L. Ecological aspects of sweet pittosporum (Pittosporum undulatum Vent.): Implications for control and management. In Proceedings of the Eleventh Australian Weeds Conference, Melbourne, Australia, 30 September–3 October 1996; pp. 489–492. [Google Scholar]
- Carpanezzi, F.B.; Perez, S.C.J. Alelopatia de extratos aquosos foliares da exótica invasora Pittosporum undulatum na germinação e crescimento do capim-arroz. Pesqui. Florest. Bras. 2014, 34, 173. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, A.; Ray, S. Allelopathic Potential of Tannic Acid and Its Equivalent Phenolics Extracted from Aerial Parts of Ampelocissus latifolia (Roxb.) Planch. IOSR J. Agric. Vet. Sci. 2016, 9, 90–100. [Google Scholar]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [Green Version]
- Faizal, A.; Geelen, D. Saponins and Their Role in Biological Processes in Plants. Phytochem. Rev. 2013, 12, 877–893. [Google Scholar] [CrossRef]
- Mundim, F.M.; Pringle, E.G. Whole-Plant Metabolic Allocation Under Water Stress. Front. Plant Sci. 2018, 9, 852. [Google Scholar] [CrossRef]
- Murray, K.D.; Janes, J.K.; Jones, A.; Bothwell, H.M.; Andrew, R.L.; Borevitz, J.O. Landscape Drivers of Genomic Diversity and Divergence in Woodland Eucalyptus. Mol. Ecol. 2019, 28, 5232–5247. [Google Scholar] [CrossRef] [Green Version]
- Santonja, M.; Bousquet-Mélou, A.; Greff, S.; Ormeño, E.; Fernandez, C. Allelopathic Effects of Volatile Organic Compounds Released from Pinus Halepensis Needles and Roots. Ecol. Evol. 2019, 9, 8201–8213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelmigid, H.M.; Morsi, M.M. Cytotoxic and Molecular Impacts of Allelopathic Effects of Leaf Residues of Eucalyptus globulus on Soybean (Glycine max). J. Genet. Eng. Biotechnol. 2017, 15, 297–302. [Google Scholar] [CrossRef]
- Dorning, M.; Cipollini, D. Leaf and Root Extracts of the Invasive Shrub, Lonicera Maackii, Inhibit Seed Germination of Three Herbs with No Autotoxic Effects. Plant Ecol. 2006, 184, 287–296. [Google Scholar] [CrossRef]
- Boland, D.J.; Brooker, M.I.H.; Turnbull, J.W. Eucalyptus Seed; CSIRO: Camberra, Australia, 1980. [Google Scholar]
- Close, D.C.; Wilson, S.J. Provenance Effects on Pre-Germination Treatments for Eucalyptus regnans and E. delegatensis Seed. For. Ecol. Manag. 2002, 170, 299–305. [Google Scholar] [CrossRef]
- Battaglia, M. Modelling Seed Germination and Seedling Survival of Eucalyptus delegatensis R.T. Baker to Facilitate Optimal Reafforestation. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 1993; p. 295. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Nonlinear Mixed-Effects Models: Basic Concepts and Motivating Examples. In Mixed-Effects Models in S and S-PLUS; Statistics and Computing; Springer: New York, NY, USA, 2000; pp. 285–304. ISBN 0-387-98957-9. [Google Scholar]
- Aravind, J.; Vimala Devi, S.; Radhamani, J.; Jacob, S.R.; Kalyani, S. The Germinationmetrics Package: A Brief Introduction. ICAR-Natl. Bur. Plant Genet. Resour. 2021, 1–62. [Google Scholar]
- Kader, M.A. A Comparison of Seed Germination Calculation Formulae and the Associated Interpretation of Resulting Data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar]
- Vashisth, A.; Nagarajan, S. Effect on Germination and Early Growth Characteristics in Sunflower (Helianthus annuus) Seeds Exposed to Static Magnetic Field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef]
- Le, A.V.; Parks, S.E.; Nguyen, C.H.; Roach, P.D. Improving the Vanillin-Sulphuric Acid Method for Quantifying Total Saponins. Technologies 2018, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- St-Pierre, A.; Blondeau, D.; Boivin, M.; Beaupré, V.; Boucher, N.; Desgagné-Penix, I. Study of Antioxidant Properties of Thylakoids and Application in UV Protection and Repair of UV-induced Damage. J. Cosmet. Dermatol. 2019, 18, 1980–1991. [Google Scholar] [CrossRef]
- Pasquini, D.; Gori, A.; Ferrini, F.; Brunetti, C. An Improvement of SPME-Based Sampling Technique to Collect Volatile Organic Compounds from Quercus Ilex at the Environmental Level. Metabolites 2021, 11, 388. [Google Scholar] [CrossRef]
- Noe, S.M.; Hüve, K.; Niinemets, Ü.; Copolovici, L. Seasonal Variation in Vertical Volatile Compounds Air Concentrations within a Remote Hemiboreal Mixed Forest. Atmos. Chem. Phys. 2012, 12, 3909–3926. [Google Scholar] [CrossRef] [Green Version]
- Vezzola, L.C.; Michelozzi, M.; Calamai, L.; Gonthier, P.; Giordano, L.; Cherubini, P.; Pelfini, M. Tree-Ring Volatile Terpenes Show Potential to Indicate Fungal Infection in Asymptomatic Mature Norway Spruce Trees in the Alps. For. Int. J. For. Res. 2019, 92, 149–156. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R. Generalized Linera, Mixed Effects and Nonparametric Regression Models; Chapman & Hall/CRC: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2006. [Google Scholar]
- Nordstokke, D.W.; Zumbo, B.D. A New Nonparametric Levene Test for Equal Variances. Psicologica 2010, 31, 401–430. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Oxf. J. 1965, 52, 591–611. [Google Scholar]
- Zhang, C.; Fu, S. Allelopathic Effects of Eucalyptus and the Establishment of Mixed Stands of Eucalyptus and Native Species. For. Ecol. Manag. 2009, 258, 1391–1396. [Google Scholar] [CrossRef]
- Cheng, H.H. Characterization of the Mechanisms of Allelopathy: Modeling and Experimental Approaches. In Allelopathy: Organisms, Processes, and Applications; American Chemical Society: Washington, DC, USA, 1995; pp. 132–141. [Google Scholar]
- Inderjit Plant Phenolics in Allelopathy. Bot. Rev. 1996, 62, 186–202. [CrossRef]
- Tunbridge, A.; Simmons, D.; Adams, R. Allelopathic Effects of Sweet Pittosporum (Pittosporum undulatum Vent.) on the Germination of Selected Native Plant Species. Vic. Nat. 2000, 117, 44–50. [Google Scholar]
- Richardson, D.M.; Brink, P. Notes on Pittosporum undulatum in the South-Western Cape. Veld Flora 1985, 71, 75–77. [Google Scholar]
- Ahmed, R.; Hoque, A.T.M.R.; Hossain, M.K. Allelopathic Effects of Leaf Litters of Eucalyptus camaldulensis on Some Forest and Agricultural Crops. J. For. Res. 2008, 19, 19–24. [Google Scholar] [CrossRef]
- Bayle, G. Ecological and Social Impacts of Eucalyptus Tree Plantation on the Environment. J. Biodivers. Conserv. Bioresour. Manag. 2019, 5, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Investigation of Phytochemicals and Antioxidant Capacity of Selected Eucalyptus Species Using Conventional Extraction. Chem. Pap. 2015, 70, 567–575. [Google Scholar] [CrossRef]
- Scognamiglio, M.; D’Abrosca, B.; Fiumano, V.; Chambery, A.; Severino, V.; Tsafantakis, N.; Pacifico, S.; Esposito, A.; Fiorentino, A. Oleanane Saponins from Bellis sylvestris Cyr. and Evaluation of Their Phytotoxicity on Aegilops geniculata Roth. Phytochemistry 2012, 84, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Constabel, C.P. Tannins in Plant–Herbivore Interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holopainen, J.K.; Himanen, S.J.; Yuan, J.S.; Chen, F.; Stewart, C.N. Ecological Functions of Terpenoids in Changing Climates. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2913–2940. ISBN 978-3-642-22143-9. [Google Scholar]
- Muller, W.H.; Lorber, P.; Haley, B. Volatile Growth Inhibitors Produced by Salvia leucophylla: Effect on Seedling Growth and Respiration. Bull. Torrey Bot. Club 1968, 95, 415. [Google Scholar] [CrossRef]
- Langenheim, J.H. Higher Plant Terpenoids: A Phytocentric Overview of Their Ecological Roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef]
- Schulz, M.; Kussmann, P.; Knop, M.; Kriegs, B.; Gresens, F.; Eichert, T.; Ulbrich, A.; Marx, F.; Fabricius, H.; Goldbach, H.; et al. Allelopathic Monoterpenes Interfere with Arabidopsis thaliana Cuticular Waxes and Enhance Transpiration. Plant Signal. Behav. 2007, 2, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Rassaeifar, M.; Hosseini, N.; Asl, N.H.H.; Zandi, P.; Aghdam, A.M. Allelopathic Effect of Eucalyptus globulus Essential Oil on Seed Germination and Seedling Establishment of Amaranthus blitoides and Cyndon dactylon. Trakia J. Sci. 2013, 11, 73–81. [Google Scholar]
- Duke, S.O.; Oliva, A. Mode of Action of Phytotoxic Terpenoids. In Allelopathy, Chemistry and Mode of Action of Allelochemicals; CRC Press: Boca Raton, FL, USA, 2004; pp. 201–216. [Google Scholar]
- Macías, F.A.; Torres, A.; Molinllo, J.M.G.; Varela, R.M.; Castellano, D. Potential Allelopathic Sesquiterpene Lactones from Sunflower Leaves. Phytochemistry 1996, 43, 1205–1215. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Cui, K.; Lu, Q.; Wang, C.; Wu, H.; Yang, Z.; Ding, W.; Shao, S.; Wang, H.; et al. Molecular Mechanisms of Tannin Accumulation in Rhus Galls and Genes Involved in Plant-Insect Interactions. Sci. Rep. 2018, 8, 9841. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Sultanbawa, Y. Profiling Ellagic Acid Content: The Importance of Form and Ascorbic Acid Levels. Food Res. Int. 2014, 66, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; uz Zaman, Q.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective Roles and Mechanisms of Caffeic Acid in Counter Plant Stress: A Mini Review. Pak. J. Agric. Res. 2018, 32, 8–19. [Google Scholar] [CrossRef]
- Klein, A.; Keyster, M.; Ludidi, N. Response of Soybean Nodules to Exogenously Applied Caffeic Acid during NaCl-Induced Salinity. South Afr. J. Bot. 2015, 96, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the Composition of Phenolic Compounds and Antioxidant Properties of Grapevine Roots and Leaves (Vitis vinifera L.) under Continuous of Long-Term Drought Stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory Mechanism against Oxidative Stress of Caffeic Acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Llusià, J.; Peñuelas, J.; Asensio, D.; Munne-Bosch, S. Airborne Limonene Confers Limited Thermotolerance to Quercus Ilex. Physiol. Plant. 2005, 123, 40–48. [Google Scholar] [CrossRef]
- Tian, Z.; Luo, Q.; Li, Y.; Zuo, Z. Terpinene and β-Pinene Acting as Signaling Molecules to Improve Cinnamomum camphora Thermotolerance. Ind. Crops Prod. 2020, 154, 112641. [Google Scholar] [CrossRef]
- Bertin, N.; Staudt, M. Effect of Water Stress on Monoterpene Emissions from Young Potted Holm Oak (Quercus Ilex L.) Trees. Oecologia 1996, 107, 456–462. [Google Scholar] [CrossRef]
- Sánchez-Osorio, I.; López-Pantoja, G.; Tapias, R.; Pareja-Sánchez, E.; Domínguez, L. Monoterpene Emission of Quercus suber L. Highly Infested by Cerambyx Welensii Küster. Ann. For. Sci. 2019, 76, 98. [Google Scholar] [CrossRef]
- McFrederick, Q.S.; Kathilankal, J.C.; Fuentes, J.D. Air Pollution Modifies Floral Scent Trails. Atmos. Environ. 2008, 42, 2336–2348. [Google Scholar] [CrossRef]
- Keszei, A.; Brubaker, C.L.; Carter, R.; Köllner, T.; Degenhardt, J.; Foley, W.J. Functional and Evolutionary Relationships between Terpene Synthases from Australian Myrtaceae. Phytochemistry 2010, 71, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Copolovici, L.O.; Filella, I.; Llusià, J.; Niinemets, Ü.; Peñuelas, J. The Capacity for Thermal Protection of Photosynthetic Electron Transport Varies for Different Monoterpenes in Quercus Ilex. Plant Physiol. 2005, 139, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef] [PubMed]
- Elaissi, A.; Salah, K.H.; Mabrouk, S.; Larbi, K.M.; Chemli, R.; Harzallah-Skhiri, F. Antibacterial Activity and Chemical Composition of 20 Eucalyptus Species’ Essential Oils. Food Chem. 2011, 129, 1427–1434. [Google Scholar] [CrossRef]
- Su, V.; King, D.K.; Woodrow, I.E.; McFadden, G.; Gleadow, R.M. Plasmodium falciparum Growth Is Arrested by Monoterpenes from Eucalyptus Oil. Flavour Fragr. J. 2008, 23, 315–318. [Google Scholar] [CrossRef]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes. In Biochemistry of Plant Secondary Metabolism; Wink, M., Ed.; Wiley-Blackwell: Oxford, UK, 2010; pp. 258–303. ISBN 978-1-4443-2050-3. [Google Scholar]
- Köllner, T.G.; Held, M.; Lenk, C.; Hiltpold, I.; Turlings, T.C.J.; Gershenzon, J.; Degenhardt, J. A Maize (E)-β-Caryophyllene Synthase Implicated in Indirect Defense Responses against Herbivores Is Not Expressed in Most American Maize Varieties. Plant Cell 2008, 20, 482–494. [Google Scholar] [CrossRef] [Green Version]
- Emmerson, K.M.; Galbally, I.E.; Guenther, A.B.; Paton-Walsh, C.; Guerette, E.-A.; Cope, M.E.; Keywood, M.D.; Lawson, S.J.; Molloy, S.B.; Dunne, E.; et al. Current Estimates of Biogenic Emissions from Eucalypts uncertain for Southeast Australia. Atmos. Chem. Phys. 2016, 16, 6997–7011. [Google Scholar] [CrossRef] [Green Version]
- Papiez, M.R.; Potosnak, M.J.; Goliff, W.S.; Guenther, A.B.; Matsunaga, S.N.; Stockwell, W.R. The Impacts of Reactive Terpene Emissions from Plants on Air Quality in Las Vegas, Nevada. Atmos. Environ. 2009, 43, 4109–4123. [Google Scholar] [CrossRef] [Green Version]
- Kirstine, W.; Galbally, I.; Ye, Y.; Hooper, M. Emissions of Volatile Organic Compounds (Primarily Oxygenated Species) from Pasture. J. Geophys. Res. 1998, 103, 10605–10619. [Google Scholar] [CrossRef]
- Leff, J.W.; Fierer, N. Volatile Organic Compound (VOC) Emissions from Soil and Litter Samples. Soil Biol. Biochem. 2008, 40, 1629–1636. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Fávero, O.A.; Romoff, P. Microclimatic Factors and Phenology Influences in the Chemical Composition of the Essential Oils from Pittosporum undulatum Vent. Leaves. J. Braz. Chem. Soc. 2006, 17, 1334–1338. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Ma, Y.; Tian, Z.; Luo, Q.; Zheng, T.; Wang, B.; Zuo, Z. Monoterpene Emissions and Their Protection Effects on Adult Cinnamomum Camphora against High Temperature. Trees 2022, 36, 711–721. [Google Scholar] [CrossRef]

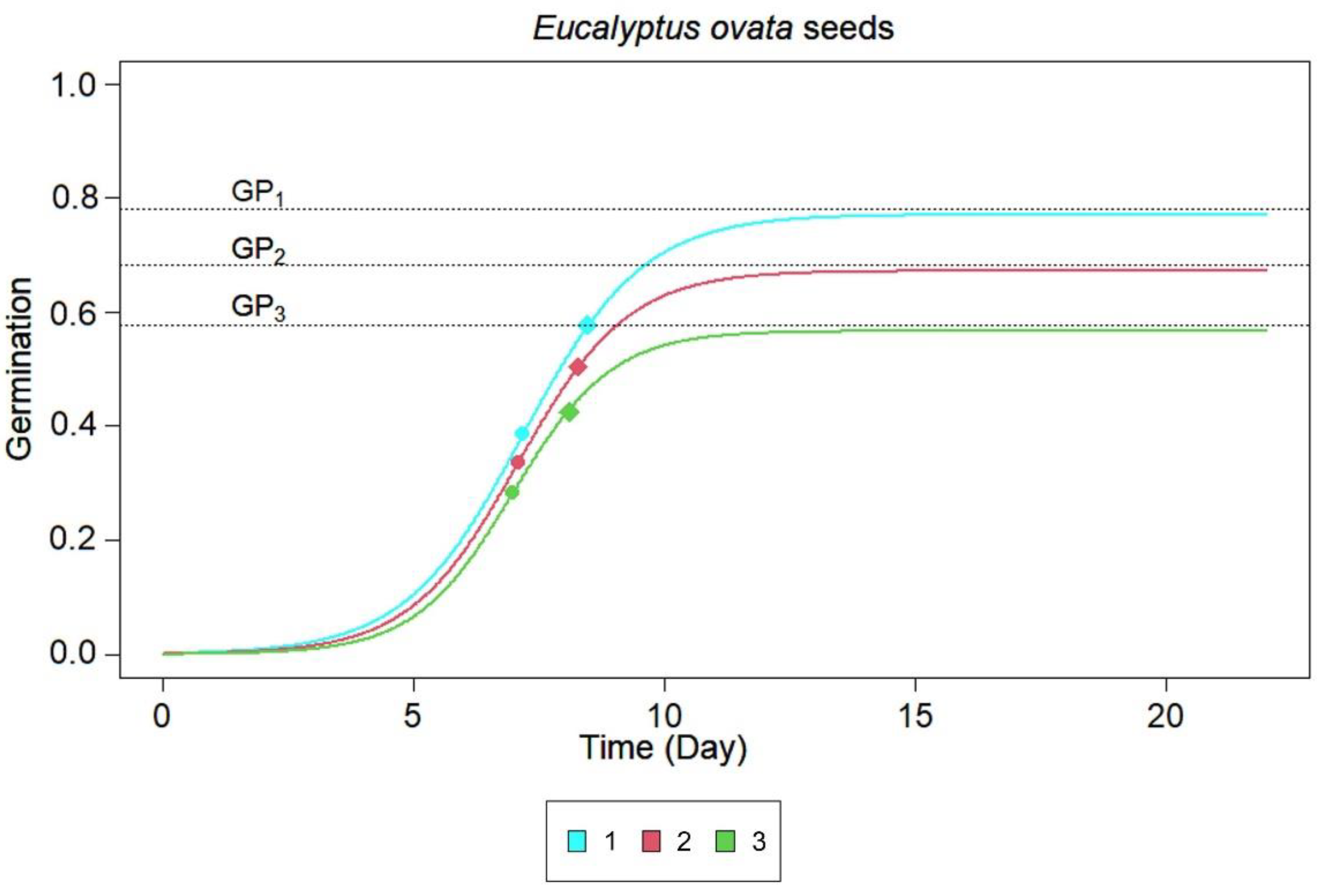

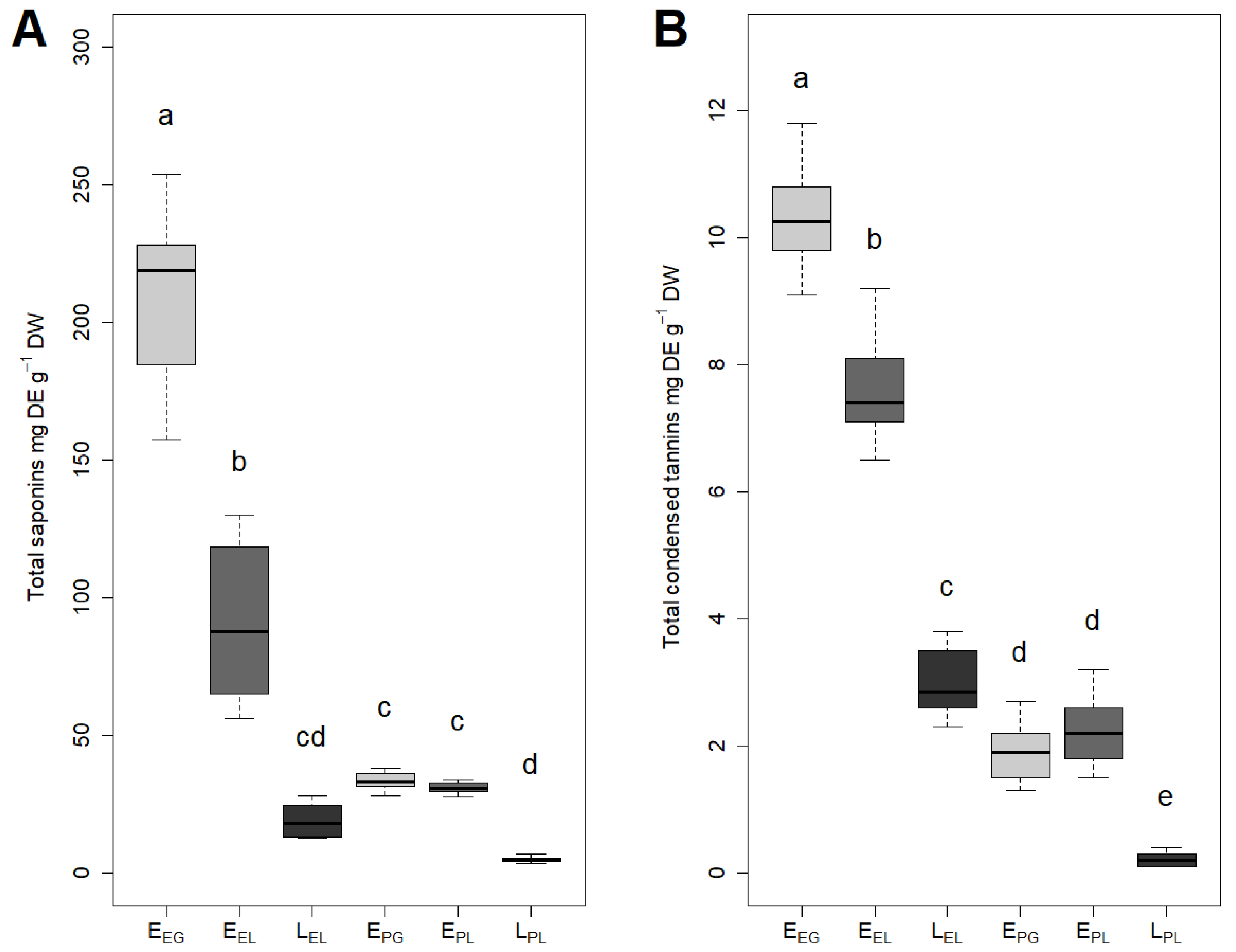
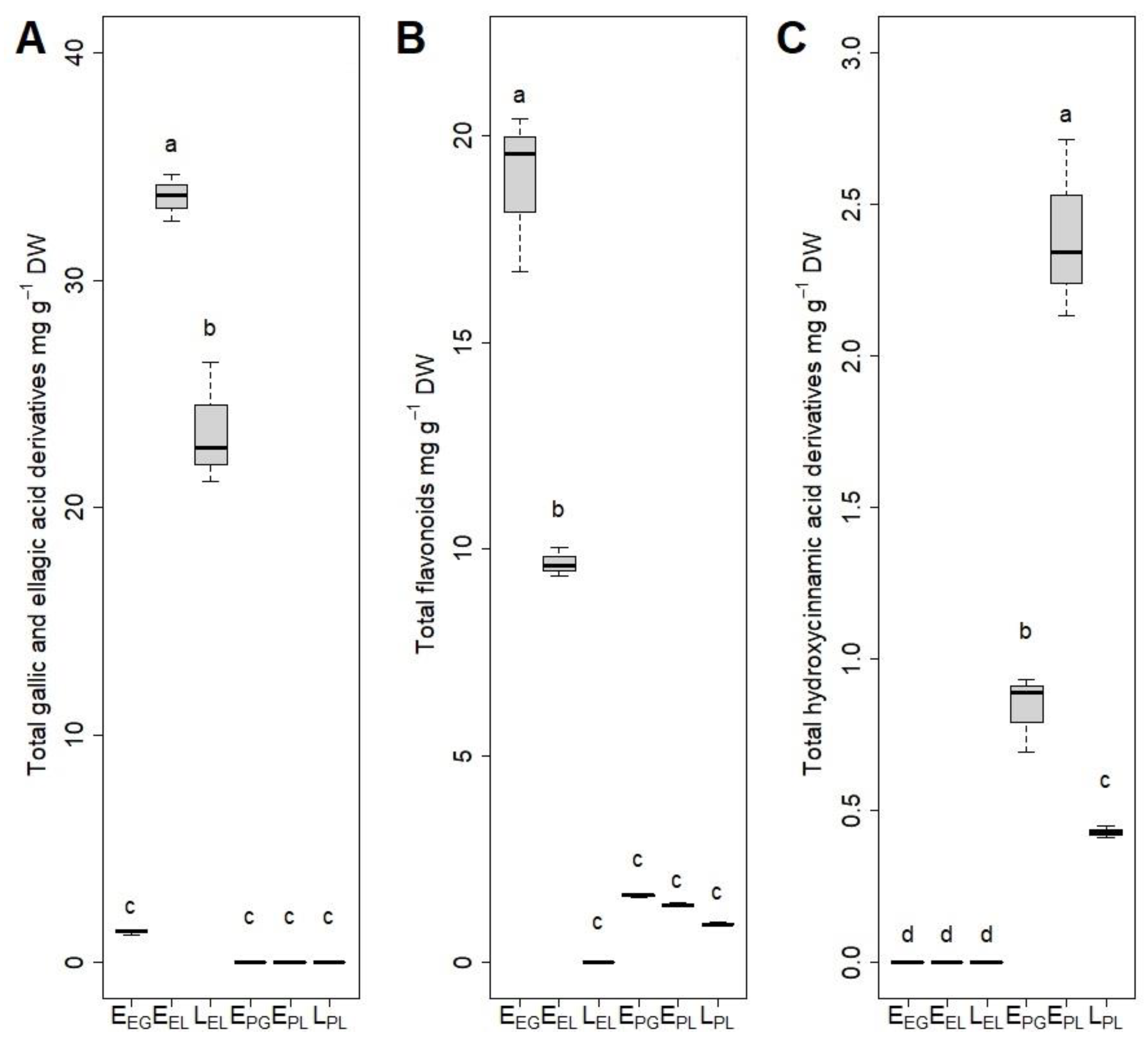
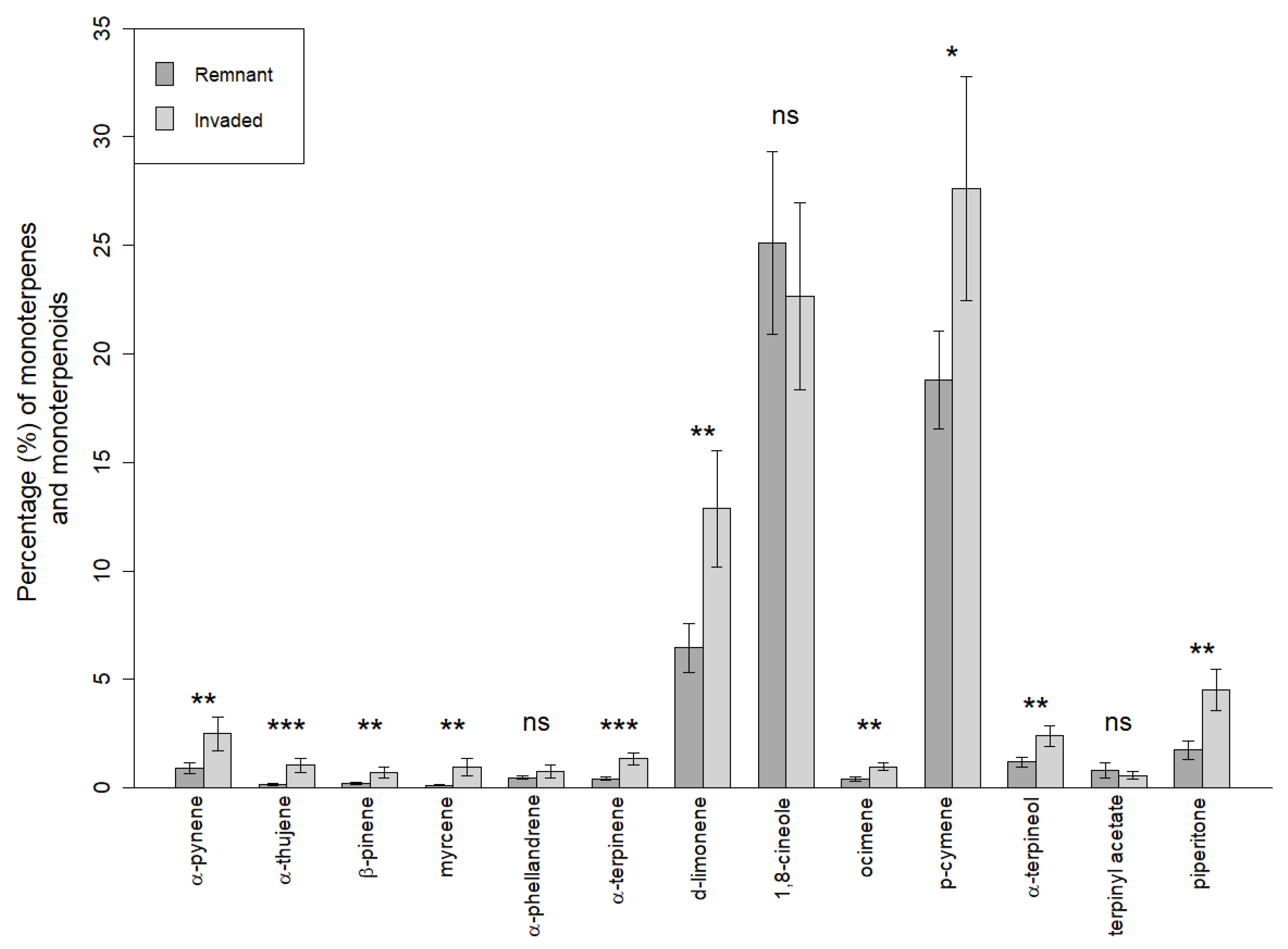

| Substrate | GP | t50 | t75 |
|---|---|---|---|
| (%) | (Day) | (Day) | |
| Filter paper (Intercept) | 0.77 | 7.18 | 1.19 |
| P. undulatum soil | 0.67 * | 6.26 *** | 0.80 *** |
| Eucalyptus soil | 0.57 *** | 6.19 *** | 1.04 ns |
| Substrate | Watering Treatment | GP | t50 | t75 |
|---|---|---|---|---|
| (%) | (Day) | (Day) | ||
| Filter paper | Water | 0.85 | 25.36 | 1.37 |
| P. undulatum soil | Water | 0.66 ** | 26.50 ns | 1.96 * |
| Eucalyptus soil | Water | 0.88ns | 25.28 ns | 2.65 *** |
| Filter paper | LEL | 0.27 *** | 23.63 * | 2.36 ** |
| P. undulatum soil | LEL | 0.73 *** | 28.14 *** | 1.85 * |
| Eucalyptus soil | LEL | 0.71 *** | 25.44 * | 1.84 *** |
| Filter paper | LPG | 0.35 *** | 31.00 *** | 0.92* |
| P. undulatum soil | LPG | 0.66 *** | 27.05 *** | 1.74 ns |
| Eucalyptus soil | LPG | 0.86 *** | 24.34 *** | 1.45 * |
| Filter paper | LPL | 0.51 *** | 30.37 *** | 1.40 ns |
| P. undulatum soil | LPL | 0.71 *** | 26.50 *** | 1.88 ns |
| Eucalyptus soil | LPL | 0.86 *** | 26.33 *** | 2.01 ns |
| Eucalyptus ovata SEEDS | Pittosporum undulatum Seeds | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Substrate | Watering | t0 | tf | tf − t0 | VI | t0 | tf | tf − t0 | VI |
| Treatment | (Day) | (Day) | (Day) | (% * mm) | (Day) | (Day) | (Day) | (% * mm) | |
| Filter paper | Water | 5.0 | 14.0 | 9.0 | 1390.5 ± 164.4 a | 21.3 | 31.0 | 9.7 | 4494.3 ± 126.7 ab |
| P. undulatum soil | Water | 4.7 ns | 11.7 ns | 7.0 ns | 1310.0 ± 217.3 ab | 22.7 ns | 32.0 ns | 9.3 ns | 3272.6 ± 488.1 b |
| Eucalyptus soil | Water | 4.0 ns | 11.3 ns | 7.3 ns | 876.3 ± 278.9 bcde | 20.0 ns | 32.0 ns | 12.0 ns | 5424.3 ± 581.2 a |
| Filter paper | LEL | 4.7ns | 14.3 ns | 9.7 ns | 550.9 ± 140.6 de | 19.7 ns | 28.7 ns | 9.0 ns | 1260.3 ± 285.1 c |
| P. undulatum soil | LEL | 5.3 ns | 15.7 ns | 10.3 ns | 1044.2 ± 93.8 abc | 22.3 ns | 32.3 ns | 10.0 ns | 3376.4 ± 312.1 b |
| Eucalyptus soil | LEL | 4.0 ns | 12.3 ns | 8.3 ns | 1134.1 ± 99.8 abc | 19.3 ns | 31.0 ns | 11.7 ns | 3794.9 ± 203.7 ac |
| Filter paper | LPG | 4.7 ns | 15.6 ns | 11.0 ns | 446.0 ± 54.9 e | 27.3 ns | 33.0 ns | 5.7 ns | 493.3 ± 238.5 c |
| P. undulatum soil | LPG | 4.7 ns | 11.3 ns | 6.7 ns | 1414.6 ± 18.1 a | 22.7 ns | 32.7 ns | 10.0 ns | 3829.2 ± 985.2 ab |
| Eucalyptus soil | LPG | 4.0 ns | 13.0 ns | 9.0 ns | 723.6 ± 78.1 cde | 20.0 ns | 31.3 ns | 11.3 ns | 4525.3 ± 428.9 ab |
| Filter paper | LPL | 5.0 ns | 15.0 ns | 10.0 ns | 1179.1 ± 189.4 ab | 26.7 ns | 32.7 ns | 6.0 ns | 1282.5 ± 289.1 c |
| P. undulatum soil | LPL | 4.3 ns | 14.0 ns | 9.7 ns | 927.2 ± 108.8 bcd | 22.0 ns | 32.0 ns | 10.0 ns | 3511.6 ± 728.7 ab |
| Eucalyptus soil | LPL | 4.3 ns | 15.7 ns | 11.3 ns | 1036.9 ± 160.0 abc | 21.0 ns | 31.0 ns | 10.0 ns | 3508.9 ± 1622.4 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquini, D.; dos Santos Nascimento, L.B.; Brunetti, C.; Ferrini, F.; Gleadow, R.M. Is the Invasiveness of Pittosporum undulatum in Eucalypt Forests Explained by the Wide Ranging Effects of Its Secondary Metabolites? Forests 2023, 14, 39. https://doi.org/10.3390/f14010039
Pasquini D, dos Santos Nascimento LB, Brunetti C, Ferrini F, Gleadow RM. Is the Invasiveness of Pittosporum undulatum in Eucalypt Forests Explained by the Wide Ranging Effects of Its Secondary Metabolites? Forests. 2023; 14(1):39. https://doi.org/10.3390/f14010039
Chicago/Turabian StylePasquini, Dalila, Luana Beatriz dos Santos Nascimento, Cecilia Brunetti, Francesco Ferrini, and Roslyn M. Gleadow. 2023. "Is the Invasiveness of Pittosporum undulatum in Eucalypt Forests Explained by the Wide Ranging Effects of Its Secondary Metabolites?" Forests 14, no. 1: 39. https://doi.org/10.3390/f14010039
APA StylePasquini, D., dos Santos Nascimento, L. B., Brunetti, C., Ferrini, F., & Gleadow, R. M. (2023). Is the Invasiveness of Pittosporum undulatum in Eucalypt Forests Explained by the Wide Ranging Effects of Its Secondary Metabolites? Forests, 14(1), 39. https://doi.org/10.3390/f14010039











