Comparison of Soil Microbial Community between Managed and Natural Vegetation Restoration along a Climatic Gradient in Karst Regions
Abstract
:1. Introduction
2. Methods and Materials
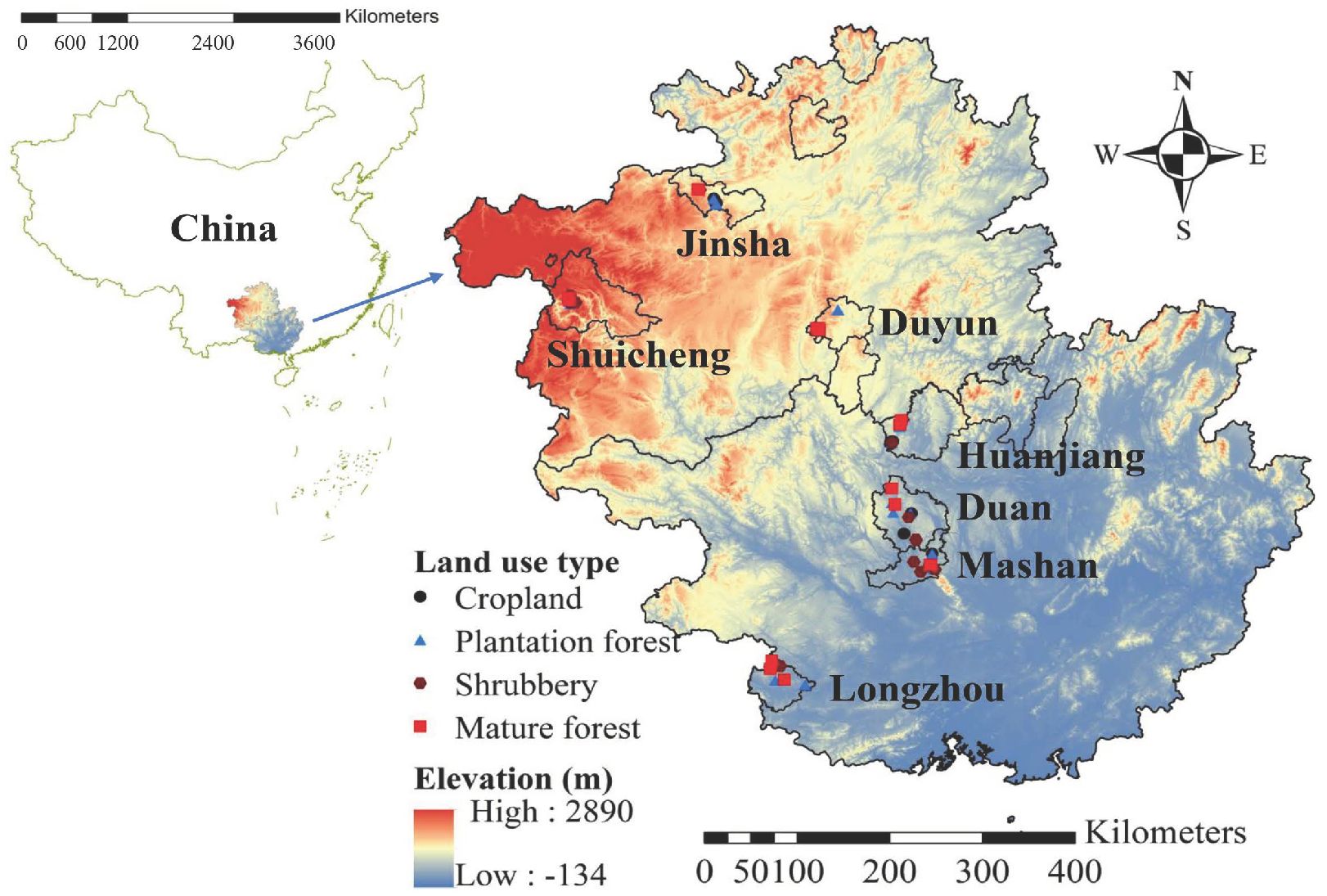
2.1. Study Region
2.2. Experimental Design
2.3. PLFA Analysis
2.4. Soil Physicochemical Analyses
2.5. Statistical Analysis
3. Results
3.1. Soil Properties
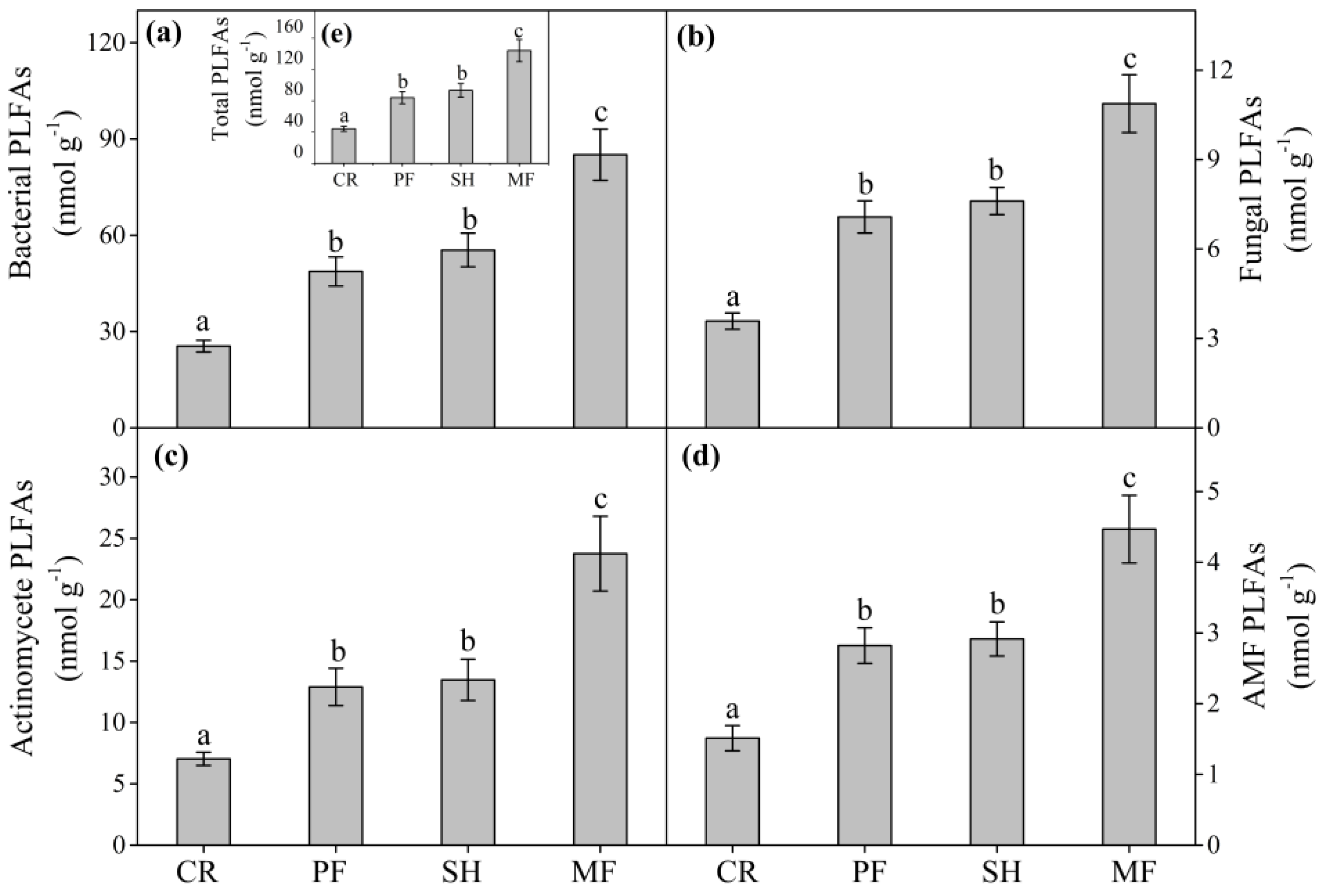
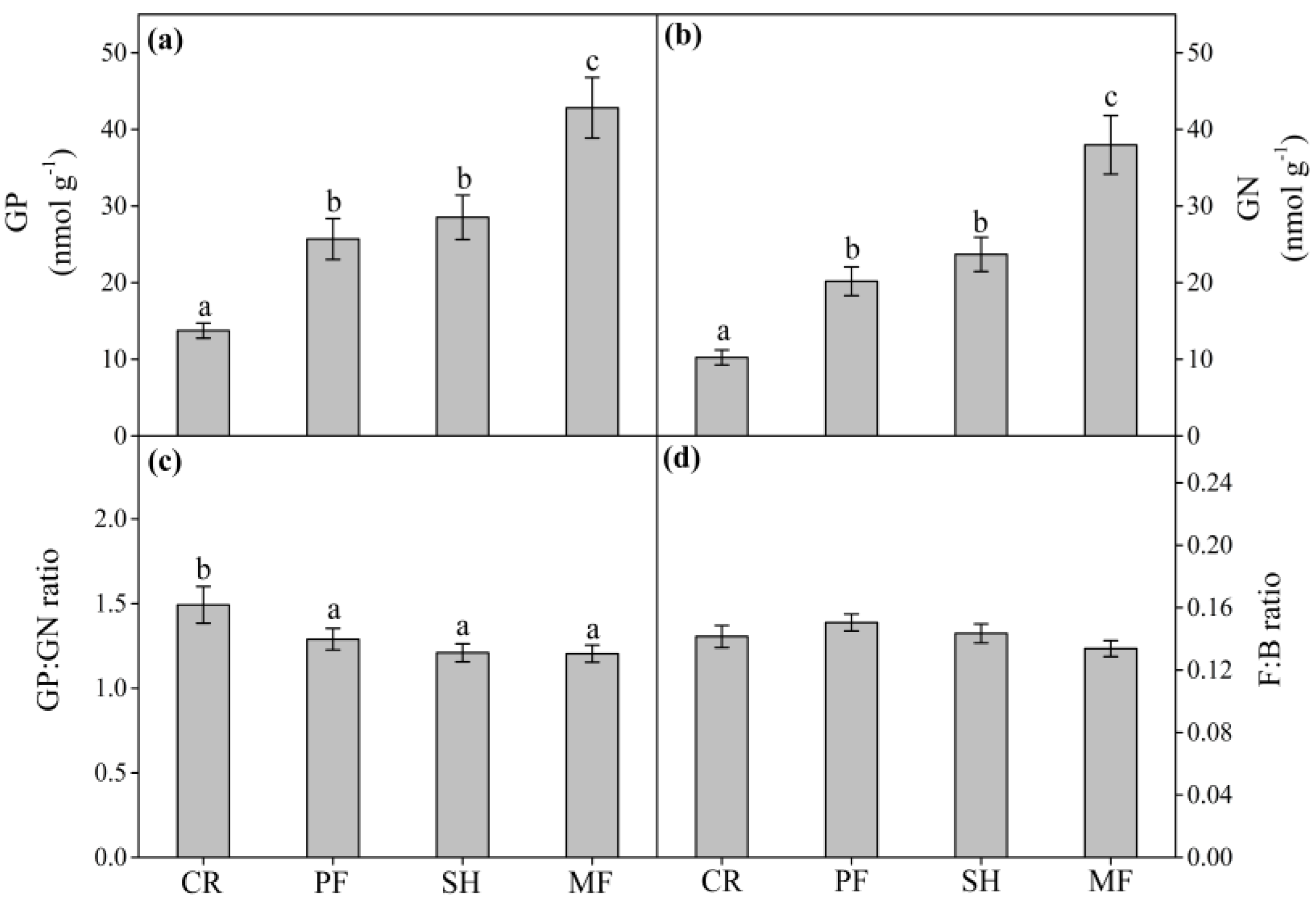
3.2. Soil Microbial Community Abundance, Structure, and Composition
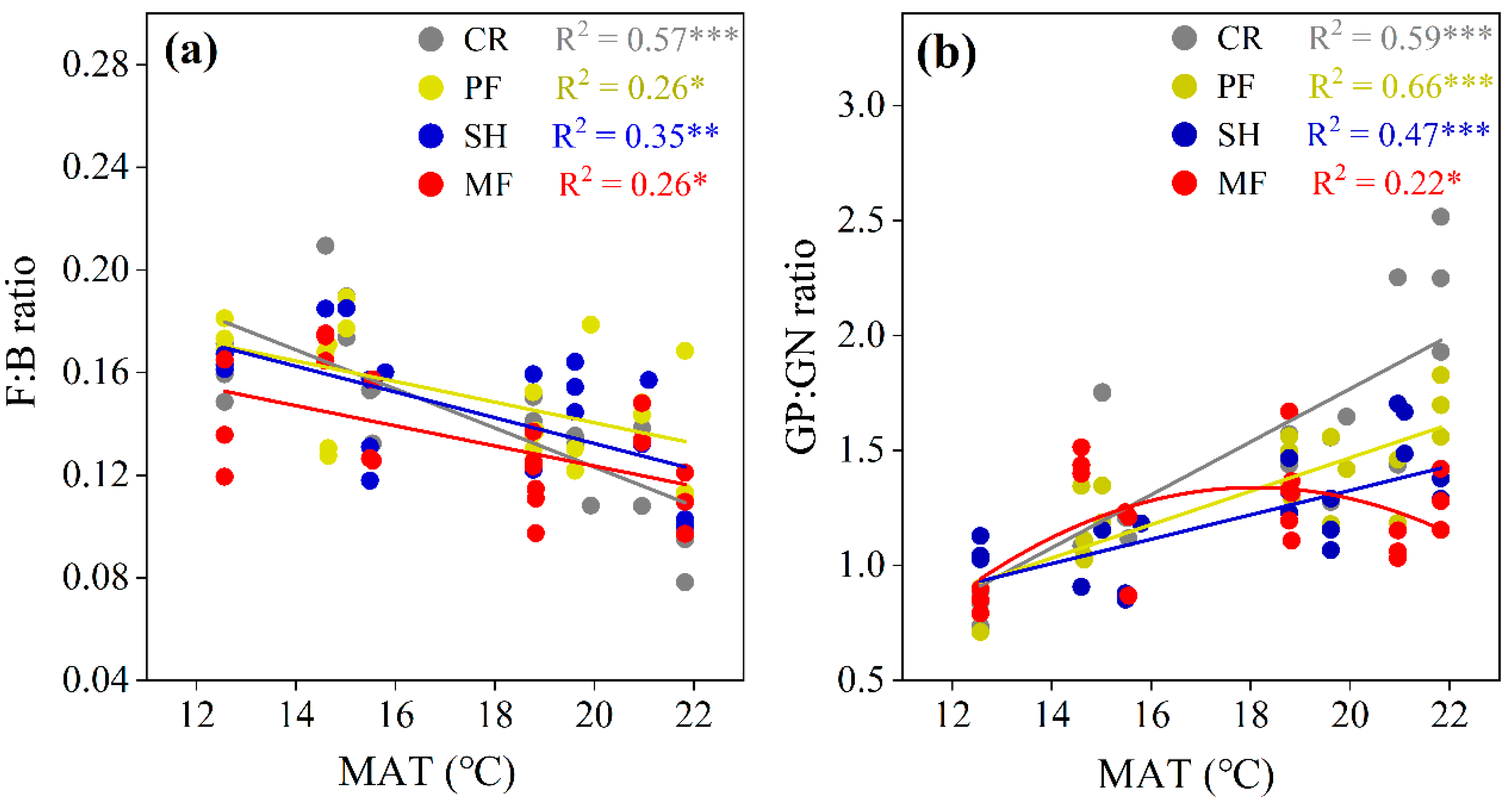
3.3. Soil Microbial Community along Precipitation and Temperature Gradients
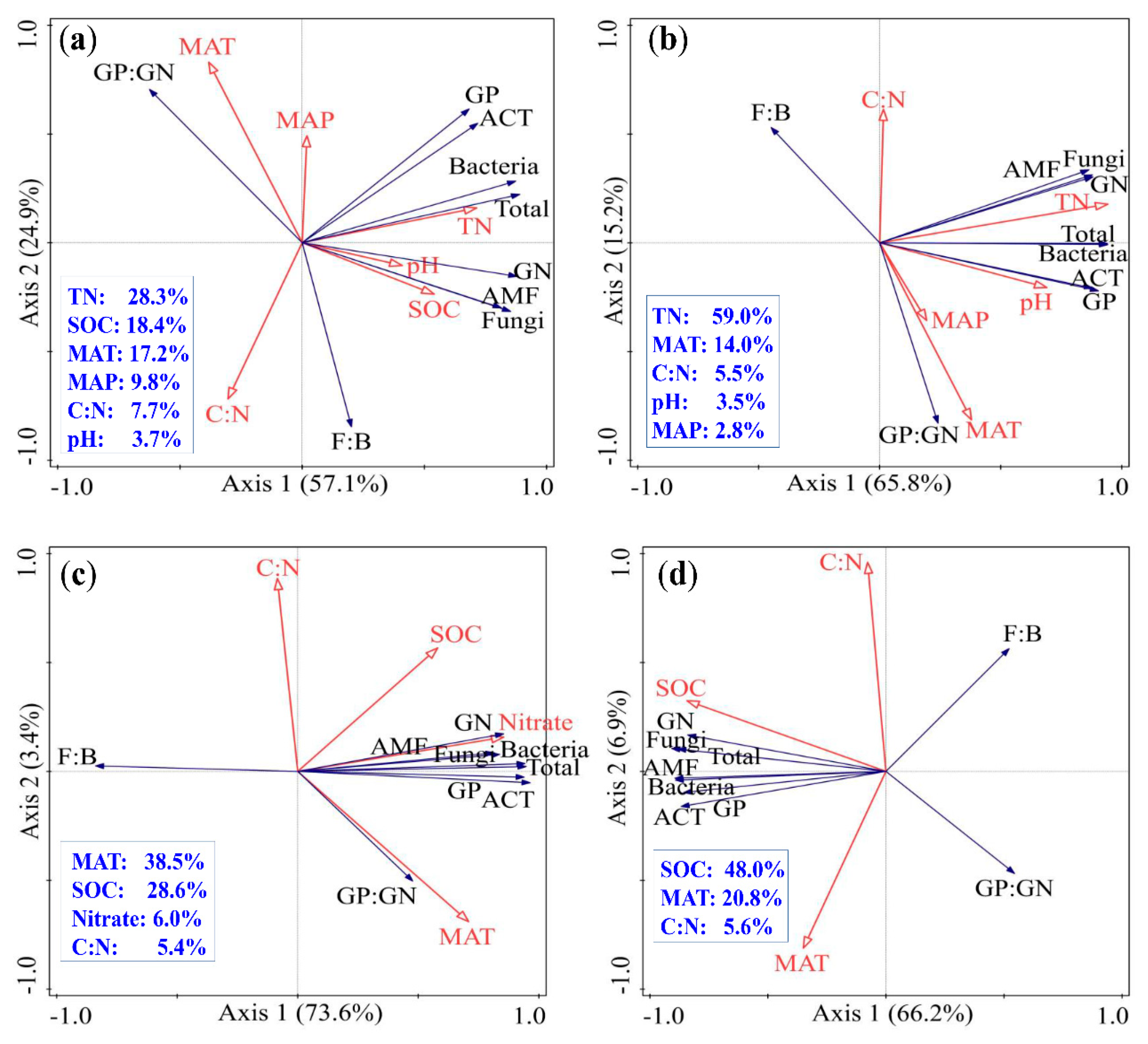
3.4. Factors Affecting the Variation of Soil Microbial Community
4. Discussion
4.1. Effect of Vegetation Restoration on Soil Microbial Community
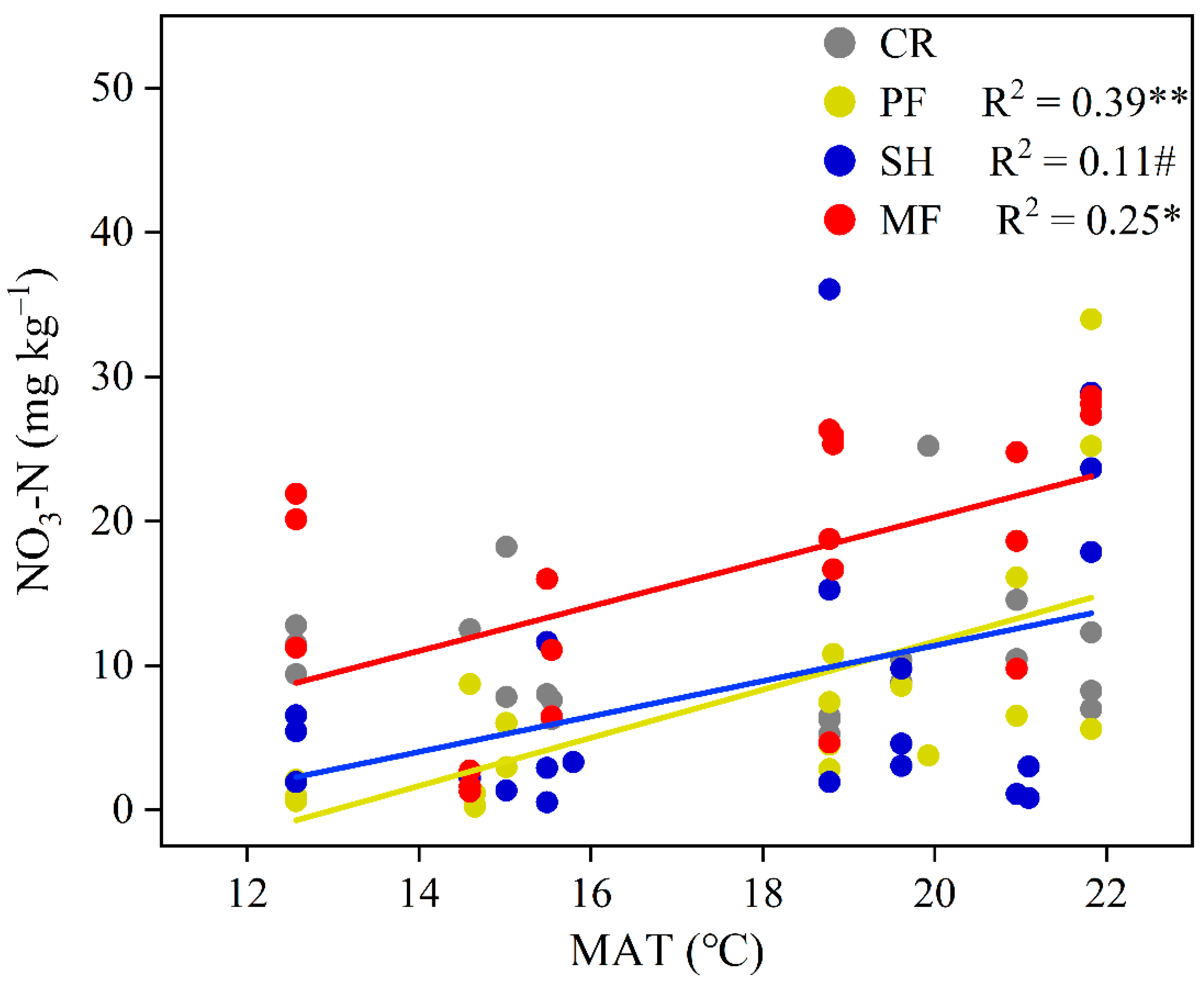
4.2. Influence of Climatic Factors on Soil Microbial Community
4.3. Implications for Vegetation Restoration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jacobs, D.; Oliet, J.; Aronson, J.; Bolte, A.; Bullock, J.; Donoso, P.; Landhäusser, S.; Madsen, P.; Peng, S.; Rey-Benayas, J.; et al. Restoring Forests: What Constitutes Success in the Twenty-First Century? New For. 2015, 46, 601–614. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Aichi Biodiversity Targets; Convention on Biological Diversity: Montreal, QC, Canada, 2010. [Google Scholar]
- Chen, C.; Park, T.; Wang, X.; Piao, S.; Xu, B.; Chaturvedi, R.K.; Fuchs, R.; Brovkin, V.; Ciais, P.; Fensholt, R.; et al. China and India Lead in Greening of the World through Land-Use Management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar] [CrossRef]
- Angelini, C.; Altieri, A.H.; Silliman, B.R.; Bertness, M.D. Interactions among Foundation Species and Their Consequences for Community Organization, Biodiversity, and Conservation. BioScience 2011, 61, 782–789. [Google Scholar] [CrossRef]
- Li, D.; Niu, S.; Luo, Y. Global Patterns of the Dynamics of Soil Carbon and Nitrogen Stocks Following Afforestation: A meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Delgado-Baquerizo, M.; Anderson, I.C.; Singh, B.K. Response of Soil Properties and Microbial Communities to Agriculture: Implications for primary productivity and soil health indicators. Front. Plant Sci. 2016, 7, 990. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.N.; Winding, A.; Binnerup, S.; Hansen, B. Microorganisms as Indicators of Soil Health; NERI Technical Report No. 388; National Environmental Research Institute: Roskilde, Denmark, 2002; 82p. [Google Scholar]
- Coban, O.; De Deyn, G.B.; van der Ploeg, M. Soil Microbiota as Game-Changers in Restoration of Degraded Lands. Science 2022, 375, abe0725. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Rousk, J.; Brookes, P.C.; Bååth, E. Bacterial PH-Optima for Growth Track Soil pH, but are Higher than Expected at Low pH. Soil Biol. Biochem. 2011, 43, 1569–1575. [Google Scholar] [CrossRef]
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global Patterns in Belowground Communities. Ecol. Lett. 2009, 12, 1238–1249. [Google Scholar] [CrossRef]
- Ma, L.; Guo, C.; Lü, X.; Yuan, S.; Wang, R. Soil Moisture and Land Use Are Major Determinants of Soil Microbial Community Composition and Biomass at a Regional Scale in Northeastern China. Biogeosciences 2015, 12, 2585–2596. [Google Scholar] [CrossRef]
- Feng, X.; Simpson, M.J. Temperature and Substrate Controls on Microbial Phospholipid Fatty Acid Composition during Incubation of Grassland Soils Contrasting in Organic Matter Quality. Soil Biol. Biochem. 2009, 41, 804–812. [Google Scholar] [CrossRef]
- De Vries, F.T.; Manning, P.; Tallowin, J.R.B.; Mortimer, S.R.; Pilgrim, E.S.; Harrison, K.A.; Hobbs, P.J.; Quirk, H.; Shipley, B.; Cornelissen, J.H.C.; et al. Abiotic Drivers and Plant Traits Explain Landscape-Scale Patterns in Soil Microbial Communities. Ecol. Lett. 2012, 15, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, S.; Wang, H.; Hu, Z.; Li, Z.; You, Y. Changes of Soil Microbial Biomass Carbon and Community Composition through Mixing Nitrogen-Fixing Species with Eucalyptus urophylla in Subtropical China. Soil Biol. Biochem. 2014, 73, 42–48. [Google Scholar] [CrossRef]
- Deng, Q.; Cheng, X.; Hui, D.; Zhang, Q.; Li, M.; Zhang, Q. Soil Microbial Community and Its Interaction with Soil Carbon and Nitrogen Dynamics Following Afforestation in Central China. Sci. Total Environ. 2016, 541, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, J.; Yang, F.; Lei, Y.; Zhang, Q.; Cheng, X. Alterations in Soil Microbial Community Composition and Biomass Following Agricultural Land Use Change. Sci. Rep. 2016, 6, 36587. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.S.; Callaham, M.A.; Gardiner, E.S.; Stanturf, J.A.; Leff, J.W.; Fierer, N.; Bradford, M.A. Response of Soil Microbial Community Composition and Function to a Bottomland Forest Restoration Intensity Gradient. Appl. Soil Ecol. 2017, 119, 317–326. [Google Scholar] [CrossRef]
- Li, D.; Wen, L.; Jiang, S.; Song, T.; Wang, K. Responses of Soil Nutrients and Microbial Communities to Three Restoration Strategies in a Karst Area, Southwest China. J. Environ. Manag. 2018, 207, 456–464. [Google Scholar] [CrossRef]
- Bonner, M.T.L.; Herbohn, J.; Gregorio, N.; Pasa, A.; Avela, M.S.; Solano, C.; Moreno, M.O.M.; Almendras-Ferraren, A.; Wills, J.; Shoo, L.P.; et al. Soil Organic Carbon Recovery in Tropical Tree Plantations May Depend on Restoration of Soil Microbial Composition and Function. Geoderma 2019, 353, 70–80. [Google Scholar] [CrossRef]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Scow, K.M.; Arcenegui, V.; Mataix-Beneyto, J. Changes in Soil Microbial Community Structure Following the Abandonment of Agricultural Terraces in Mountainous Areas of Eastern Spain. Appl. Soil Ecol. 2009, 42, 315–323. [Google Scholar] [CrossRef]
- Del Galdo, I.; Six, J.; Peressotti, A.; Francesca Cotrufo, M. Assessing the Impact of Land-Use Change on Soil C Sequestration in Agricultural Soils by Means of Organic Matter Fractionation and Stable C Isotopes. Glob. Chang. Biol. 2003, 9, 1204–1213. [Google Scholar] [CrossRef]
- Laganiere, J.; Angers, D.A.; Para, D. Carbon Accumulation in Agricultural Soils after Afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, W.; Zhang, P.; Yu, Y.; Ding, F. A Synthesis of Change in Deep Soil Organic Carbon Stores with Afforestation of Agricultural Soils. Forest Ecol. Manag. 2013, 296, 53–63. [Google Scholar] [CrossRef]
- Solly, E.; Schöning, I.; Boch, S.; Kandeler, E.; Marhan, S.; Michalzik, B.; Müller, J.; Zscheischler, J.; Trumbore, S.; Schrumpf, M. Factors Controlling Decomposition Rates of Fine Root Litter in Temperate Forests and Grasslands. Plant Soil 2014, 382, 203–218. [Google Scholar] [CrossRef]
- Chen, Y.L.; Ding, J.Z.; Peng, Y.F.; Li, F.; Yang, G.B.; Liu, L.; Qin, S.Q.; Fang, K.; Yang, Y.H. Patterns and Drivers of Soil Microbial Communities in Tibetan Alpine and Global Terrestrial Ecosystems. J. Biogeogr. 2016, 43, 2027–2039. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground Biodiversity and Ecosystem Functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Asmelash, F.; Bekele, T.; Birhane, E. The Potential Role of Arbuscular Mycorrhizal Fungi in the Restoration of Degraded Lands. Front. Microbiol. 2016, 7, 1095. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ball, B.A. Impacts of Altered Precipitation Regimes on Soil Communities and Biogeochemistry in Arid and Semi-Arid Ecosystems. Glob. Chang. Biol. 2015, 21, 1407–1421. [Google Scholar] [CrossRef]
- Gombert, P. Role of Karstic Dissolution in Global Carbon Cycle. Glob. Planet Chang. 2002, 33, 177–184. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky Desertification in Southwest China: Impacts, causes, and restoration. Earth Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Tong, X.; Brandt, M.; Yue, Y.; Horion, S.; Wang, K.; Keersmaecker, W.; Tian, F.; Schurgers, G.; Xiao, X.; Luo, Y.; et al. Increased Vegetation Growth and Carbon Stock in China Karst via Ecological Engineering. Nat. Sustain. 2018, 1, 44–50. [Google Scholar] [CrossRef]
- Hu, P.; Xiao, J.; Zhang, W.; Xiao, L.; Yang, R.; Xiao, D.; Zhao, J.; Wang, K. Response of Soil Microbial Communities to Natural and Managed Vegetation Restoration in a Subtropical Karst Region. Catena 2020, 195, 104849. [Google Scholar] [CrossRef]
- Liu, M.; Xu, X.; Sun, A.Y.; Wang, K.; Liu, W.; Zhang, X. Is Southwestern China Experiencing More Frequent Precipitation Extremes? Environ. Res. Lett. 2014, 9, 064002. [Google Scholar] [CrossRef]
- Lozano-García, B.; Parras-Alcántara, L.; Brevik, E.C. Impact of Topographic Aspect and Vegetation (Native and Reforested Areas) on Soil Organic Carbon and Nitrogen Budgets in Mediterranean Natural Areas. Sci. Total Environ. 2016, 544, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Liu, S.; Ye, Y.; Zhang, W.; Wang, K.; Su, Y. Effects of Environmental Factors on Soil Organic Carbon under Natural or Managed Vegetation Restoration. Land Degrad. Dev. 2018, 29, 387–397. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Chen, H.; Li, D.; Zhao, Y.; Zhao, J.; Xiao, J.; Wu, F.; He, X.; Luo, Y.; et al. Soil Carbon Accumulation with Increasing Temperature under Both Managed and Natural Vegetation Restoration in Calcareous Soils. Sci. Total Environ. 2021, 767, 145298. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of Carbon and Flooding on Soil Microbial Communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Chung, H.; Zak, D.R.; Reich, P.B.; Ellsworth, D.S. Plant Species Richness, Elevated CO2, and Atmospheric Nitrogen Deposition Alter Soil Microbial Community Composition and Function. Glob. Chang. Biol. 2007, 13, 980–989. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, Z.; He, X.; Chen, H.; Wang, K. Effects of Monoculture and Mixed Culture of Grass and Legume Forage Species on Soil Microbial Community Structure under Different Levels of Nitrogen Fertilization. Eur. J. Soil Biol. 2015, 68, 61–68. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature Fatty Acids Provide Tools for Determination of the Distribution and Interactions of Mycorrhizal Fungi in Soil. Fems Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E.; Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E.; et al. Total carbon, organic carbon, and organic matter. Methods Soil Anal. 1996, 9, 961–1010. [Google Scholar]
- Xiao, L.; Liu, G.; Zhang, J.; Xue, S. Long-Term Effects of Vegetational Restoration on Soil Microbial Communities on the Loess Plateau of China: Revegetation on soil microbial communities. Restor. Ecol. 2016, 24, 794–804. [Google Scholar] [CrossRef]
- Hamonts, K.; Bissett, A.; Macdonald, B.C.T.; Barton, P.S.; Manning, A.D.; Young, A. Effects of Ecological Restoration on Soil Microbial Diversity in a Temperate Grassy Woodland. Appl. Soil Ecol. 2017, 117, 117–128. [Google Scholar] [CrossRef]
- Moscatelli, M.C.; Di Tizio, A.; Marinari, S.; Grego, S. Microbial Indicators Related to Soil Carbon in Mediterranean Land Use Systems. Soil Till. Res. 2007, 97, 51–59. [Google Scholar] [CrossRef]
- Hu, Y.; Xiang, D.; Veresoglou, S.D.; Chen, F.; Chen, Y.; Hao, Z.; Zhang, X.; Chen, B. Soil Organic Carbon and Soil Structure Are Driving Microbial Abundance and Community Composition across the Arid and Semi-Arid Grasslands in Northern China. Soil Biol. Biochem. 2014, 77, 51–57. [Google Scholar] [CrossRef]
- Stevenson, B.A.; Sarmah, A.K.; Smernik, R.; Hunter, D.W.F.; Fraser, S. Soil Carbon Characterization and Nutrient Ratios across Land Uses on Two Contrasting Soils: Their Relationships to Microbial Biomass and Function. Soil Biol. Biochem. 2016, 97, 50–62. [Google Scholar] [CrossRef]
- Ren, C.; Wang, T.; Xu, Y.; Deng, J.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Differential Soil Microbial Community Responses to the Linkage of Soil Organic Carbon Fractions with Respiration across Land-Use Changes. Forest Ecol. Manag. 2018, 409, 170–178. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Steenwerth, K.L.; Jackson, L.E.; Scow, K.M. Land Use and Climatic Factors Structure Regional Patterns in Soil Microbial Communities: Biogeography of Soil Microbial Communities. Glob. Ecol. Biogeogr. 2010, 19, 27–39. [Google Scholar] [CrossRef]
- Zhang, W.; Parker, K.M.; Luo, Y.; Wan, S.; Wallace, L.L.; Hu, S. Soil Microbial Responses to Experimental Warming and Clipping in a Tallgrass Prairie. Glob. Chang. Biol. 2005, 11, 266–277. [Google Scholar] [CrossRef]
- Stromberger, M.; Shah, Z.; Westfall, D. Soil Microbial Communities of No-till Dryland Agroecosystems across an Evapotranspiration Gradient. Appl. Soil Ecol. 2007, 35, 94–106. [Google Scholar] [CrossRef]
- Eclesia, R.P.; Jobbagy, E.G.; Jackson, R.B.; Rizzotto, M.; Piñeiro, G. Stabilization of New Carbon Inputs Rather than Old Carbon Decomposition Determines Soil Organic Carbon Shifts Following Woody or Herbaceous Vegetation Transitions. Plant Soil 2016, 409, 99–116. [Google Scholar] [CrossRef]
- Chen, D.; Mi, J.; Chu, P.; Cheng, J.; Zhang, L.; Pan, Q.; Xie, Y.; Bai, Y. Patterns and Drivers of Soil Microbial Communities along a Precipitation Gradient on the Mongolian Plateau. Landsc. Ecol. 2015, 30, 1669–1682. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, Y.; Liu, S.; Zhang, X.; Wang, Q.; Liu, K.; Yin, Y.; Deng, J. Interactive Effects of Soil Warming, Throughfall Reduction, and Root Exclusion on Soil Microbial Community and Residues in Warm-Temperate Oak Forests. Appl. Soil Ecol. 2019, 142, 52–58. [Google Scholar] [CrossRef]
- Bailey, V.L.; Smith, J.L.; Bolton, H. Fungal-to-Bacterial Ratios in Soils Investigated for Enhanced C Sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- Waring, B.G.; Averill, C.; Hawkes, C.V. Differences in Fungal and Bacterial Physiology Alter Soil Carbon and Nitrogen Cycling: Insights from meta-analysis and theoretical models. Ecol. Lett. 2013, 16, 887–894. [Google Scholar] [CrossRef]
- Börjesson, G.; Menichetti, L.; Kirchmann, H.; Kätterer, T. Soil Microbial Community Structure Affected by 53 Years of Nitrogen Fertilisation and Different Organic Amendments. Biol. Fertil. Soils 2012, 48, 245–257. [Google Scholar] [CrossRef]
- Wen, L.; Li, D.; Chen, H.; Wang, K. Dynamics of Soil Organic Carbon in Density Fractions during Post-Agricultural Succession over Two Lithology Types, Southwest China. J. Environ. Manag. 2017, 201, 199–206. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization Mechanisms of Soil organic Matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]


| County | Land Use Types | N | MAT (°C) | MAP (mm) | Altitude (m) |
|---|---|---|---|---|---|
| Shuicheng | CR | 3 | 12.6 | 1208 | 2195–2233 |
| PF | 3 | 12.6 | 1208 | 2118–2241 | |
| SH | 3 | 12.6 | 1208 | 2186–2264 | |
| MF | 3 | 12.6 | 1208 | 2001–2146 | |
| Jinsha | CR | 3 | 14.6 | 1013 | 1119–1188 |
| PF | 3 | 14.6 | 1013 | 1150–1181 | |
| SH | 3 | 14.6 | 1013 | 1006–1127 | |
| MF | 3 | 14.6 | 1013 | 1030–1041 | |
| Duyun | CR | 3 | 15.5 | 1248 | 1028–1067 |
| PF | 3 | 14.7 | 1220 | 1016–1098 | |
| SH | 3 | 15.5 | 1273 | 1098–1127 | |
| MF | 3 | 15.5 | 1248 | 1035–1077 | |
| Huanjiang | CR | 3 | 18.8 | 1371 | 305–392 |
| PF | 3 | 18.8 | 1371 | 288–377 | |
| SH | 3 | 18.8 | 1371 | 388–428 | |
| MF | 3 | 18.8 | 1371 | 349–496 | |
| Duan | CR | 3 | 19.6 | 1607 | 293–303 |
| PF | 3 | 19.6 | 1607 | 350–392 | |
| SH | 3 | 19.6 | 1607 | 402–428 | |
| MF | 3 | 18.8 | 1516 | 367–422 | |
| Mashan | CR | 3 | 21.0 | 1594 | 276–331 |
| PF | 3 | 21.0 | 1594 | 292–367 | |
| SH | 3 | 21.0 | 1594 | 245–375 | |
| MF | 3 | 21.0 | 1594 | 558–598 | |
| Longzhou | CR | 3 | 21.8 | 1296 | 347–362 |
| PF | 3 | 21.8 | 1296 | 252–378 | |
| SH | 3 | 21.8 | 1296 | 395–413 | |
| MF | 3 | 21.8 | 1296 | 359–436 |
| CR | PF | SH | MF | |
|---|---|---|---|---|
| pH | 6.51(0.20) | 6.63(0.16) | 6.42(0.15) | 6.47(0.17) |
| SOC (g kg–1) | 20.13(1.72) a | 29.85(2.76) b | 32.85(2.64) b | 50.96(4.60) c |
| TN (g kg–1) | 2.14(0.19) a | 2.85(0.22) b | 3.04(0.25) b | 4.65(0.39) c |
| C:N | 9.57(0.31) a | 10.45(0.38) ab | 10.84(0.435) b | 11.01(0.33) b |
| NH4+ (mg kg–1) | 4.47(0.42) a | 8.11(0.95) b | 8.67(0.84) b | 7.65(0.77) b |
| NO3– (mg kg–1) | 10.45(1.06) b | 7.53(1.85) a | 8.66(2.21) a | 16.54(2.05) b |
| Climatic Variable | Edaphic Variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MAP | MAT | pH | SOC | TN | C:N | NH4+ | NO3– | ||
| CR | Bacteria | 0.13 | −0.11 | 0.29 | 0.42 | 0.68 ** | −0.46 * | 0.43 | 0.12 |
| Fungi | −0.20 | −0.60 ** | 0.37 | 0.55 * | 0.58 ** | −0.03 | 0.46 * | 0.08 | |
| ACT | 0.28 | 0.22 | 0.37 | 0.19 | 0.56 ** | −0.67 ** | 0.19 | 0.24 | |
| AMF | −0.02 | −0.56 * | 0.37 | 0.58 ** | 0.57 ** | −0.02 | 0.40 | 0.04 | |
| Total | 0.11 | −0.16 | 0.33 | 0.43 | 0.68 ** | −0.43 | 0.42 | 0.14 | |
| GP | 0.26 | 0.26 | 0.24 | 0.16 | 0.54 * | −0.65 ** | 0.26 | 0.15 | |
| GN | −0.05 | −0.51 * | 0.28 | 0.61 ** | 0.67 ** | −0.13 | 0.51 * | 0.06 | |
| F:B | −0.58 ** | −0.76 ** | 0.28 | 0.24 | −0.01 | 0.50 * | 0.11 | 0.07 | |
| GP:GN | 0.22 | 0.78 ** | −0.35 | −0.41 | −0.24 | −0.32 | −0.26 | 0.01 | |
| PF | Bacteria | 0.16 | 0.35 | 0.63 ** | 0.80 ** | 0.89 ** | 0.03 | 0.36 | 0.57 ** |
| Fungi | 0.02 | 0.11 | 0.60 ** | 0.89 ** | 0.90 ** | 0.14 | 0.32 | 0.32 | |
| ACT | 0.21 | 0.50 * | 0.65 ** | 0.66 ** | 0.79 ** | −0.11 | 0.27 | 0.60 ** | |
| AMF | 0.14 | 0.12 | 0.60 ** | 0.87 ** | 0.87 ** | 0.16 | 0.22 | 0.39 | |
| Total | 0.17 | 0.36 | 0.65 ** | 0.80 ** | 0.89 ** | 0.01 | 0.34 | 0.56 ** | |
| GP | 0.20 | 0.51 * | 0.66 ** | 0.68 ** | 0.82 ** | −0.11 | 0.37 | 0.66 ** | |
| GN | 0.07 | 0.05 | 0.50 * | 0.88 ** | 0.87 ** | 0.24 | 0.29 | 0.36 | |
| F:B | −0.45 * | −0.55 * | −0.27 | −0.13 | −0.28 | 0.15 | −0.12 | −0.60 ** | |
| GP:GN | 0.28 | 0.83 ** | 0.47 * | −0.16 | 0.11 | −0.64 ** | 0.26 | 0.52 * | |
| SH | Bacteria | 0.20 | 0.56 ** | 0.34 | 0.58 ** | 0.64 ** | 0.01 | −0.23 | 0.82 ** |
| Fungi | 0.32 | 0.52 * | 0.45 * | 0.55 * | 0.55 ** | 0.08 | −0.17 | 0.62 ** | |
| ACT | 0.19 | 0.58 ** | 0.49 * | 0.57 ** | 0.68 ** | −0.14 | −0.37 | 0.90 ** | |
| AMF | 0.24 | 0.40 | 0.50 * | 0.65 ** | 0.68 ** | 0.03 | −0.36 | 0.69 ** | |
| Total | 0.22 | 0.57 ** | 0.39 | 0.58 ** | 0.65 ** | −0.02 | −0.26 | 0.83 ** | |
| GP | 0.26 | 0.64 ** | 0.39 | 0.55 * | 0.64 ** | −0.09 | −0.21 | 0.83 ** | |
| GN | 0.09 | 0.39 | 0.26 | 0.61 ** | 0.61 ** | 0.15 | −0.27 | 0.75 ** | |
| F:B | −0.30 | −0.63 ** | −0.15 | −0.42 | −0.50 * | 0.08 | 0.16 | −0.77 ** | |
| GP:GN | 0.51 * | 0.71 ** | 0.24 | −0.13 | 0.06 | −0.50 * | 0.29 | 0.19 | |
| MF | Bacteria | 0.53 * | 0.35 | 0.74 ** | 0.73 ** | 0.77 ** | 0.03 | −0.52 * | 0.82 ** |
| Fungi | 0.46 * | 0.20 | 0.66 ** | 0.73 ** | 0.69 ** | 0.25 | −0.31 | 0.64 ** | |
| ACT | 0.49 * | 0.41 | 0.80 ** | 0.67 ** | 0.72 ** | −0.01 | −0.55 * | 0.79 ** | |
| AMF | 0.52 * | 0.26 | 0.78 ** | 0.78 ** | 0.76 ** | 0.19 | −0.49 * | 0.75 ** | |
| Total | 0.52 * | 0.36 | 0.76 ** | 0.73 ** | 0.76 ** | 0.05 | −0.51 * | 0.81 ** | |
| GP | 0.56 ** | 0.46 * | 0.75 ** | 0.65 ** | 0.73 ** | −0.07 | −0.54 * | 0.85 ** | |
| GN | 0.47 * | 0.21 | 0.70 ** | 0.80 ** | 0.79 ** | 0.16 | −0.47 * | 0.75 ** | |
| F:B | −0.58 ** | −0.58 ** | −0.56 ** | −0.33 | −0.54 * | 0.56 ** | 0.84 ** | −0.81 ** | |
| GP:GN | −0.15 | 0.29 | −0.29 | −0.71 ** | −0.58 ** | −0.43 | 0.12 | −0.32 | |
| pH | SOC | TN | C:N | NH4+ | NO3– | ||
|---|---|---|---|---|---|---|---|
| CR | MAP | 0.33 | −0.49 * | −0.29 | −0.47 * | −0.57 ** | 0.13 |
| MAT | 0.07 | −0.67 ** | −0.37 | −0.54 * | −0.58 ** | 0.01 | |
| PF | MAP | 0.57 ** | −0.17 | 0.06 | −0.61 ** | −0.28 | 0.21 |
| MAT | 0.72 ** | −0.14 | 0.20 | −0.77 ** | 0.21 | 0.65 ** | |
| SH | MAP | 0.45 * | −0.08 | 0.07 | −0.38 | −0.05 | 0.02 |
| MAT | 0.48 * | −0.11 | 0.18 | −0.62 ** | 0.10 | 0.40 | |
| MF | MAP | 0.66 ** | 0.27 | 0.36 | −0.23 | −0.59 ** | 0.52 * |
| MAT | 0.58 ** | −0.17 | 0.07 | −0.63 ** | −0.61 ** | 0.54 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Hu, P.; Zhang, W.; Xiao, D.; Zou, D.; Ye, Y.; Wang, K. Comparison of Soil Microbial Community between Managed and Natural Vegetation Restoration along a Climatic Gradient in Karst Regions. Forests 2023, 14, 1980. https://doi.org/10.3390/f14101980
Sun Z, Hu P, Zhang W, Xiao D, Zou D, Ye Y, Wang K. Comparison of Soil Microbial Community between Managed and Natural Vegetation Restoration along a Climatic Gradient in Karst Regions. Forests. 2023; 14(10):1980. https://doi.org/10.3390/f14101980
Chicago/Turabian StyleSun, Zhuanzhuan, Peilei Hu, Wei Zhang, Dan Xiao, Dongsheng Zou, Yingying Ye, and Kelin Wang. 2023. "Comparison of Soil Microbial Community between Managed and Natural Vegetation Restoration along a Climatic Gradient in Karst Regions" Forests 14, no. 10: 1980. https://doi.org/10.3390/f14101980





