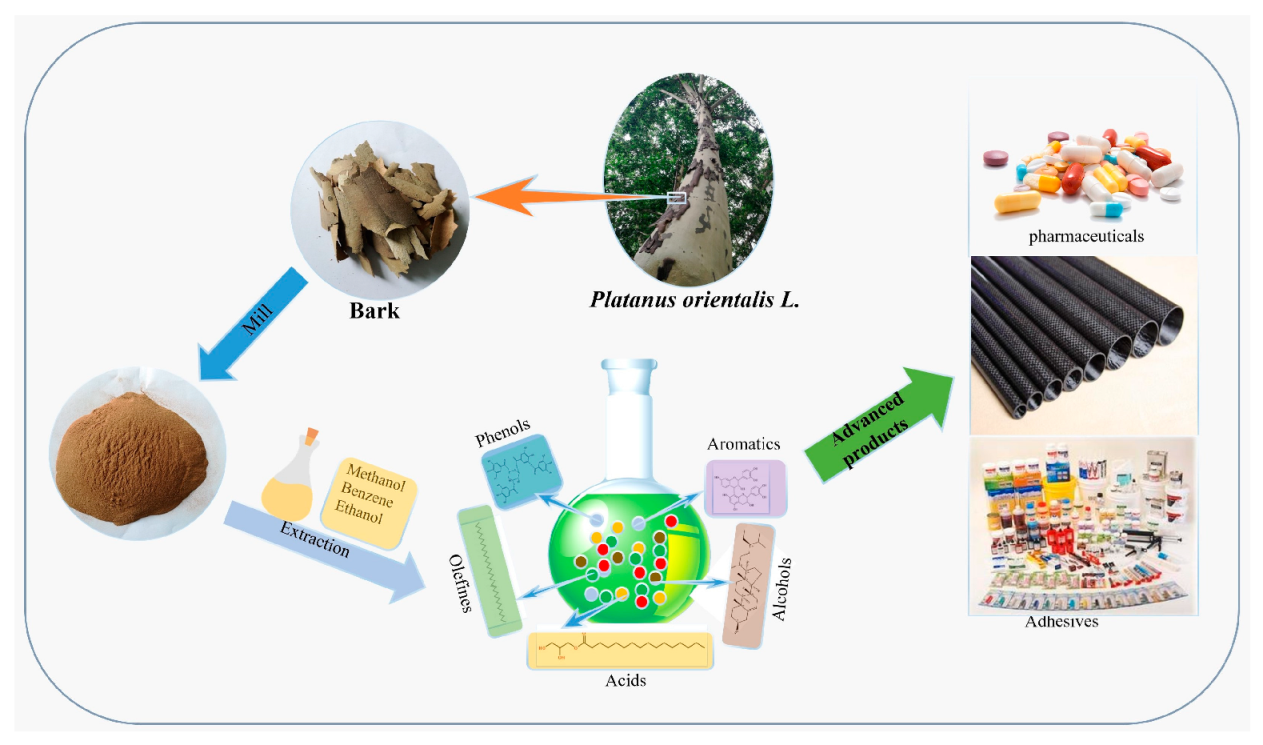
The Potential of Platanus orientalis L. Bark for High-Grade Resource Utilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. Characterizations
3. Results and Discussion
3.1. Characterization of POL Bark Extracts
3.1.1. Characterization of POL Bark Extracts using GC/MS
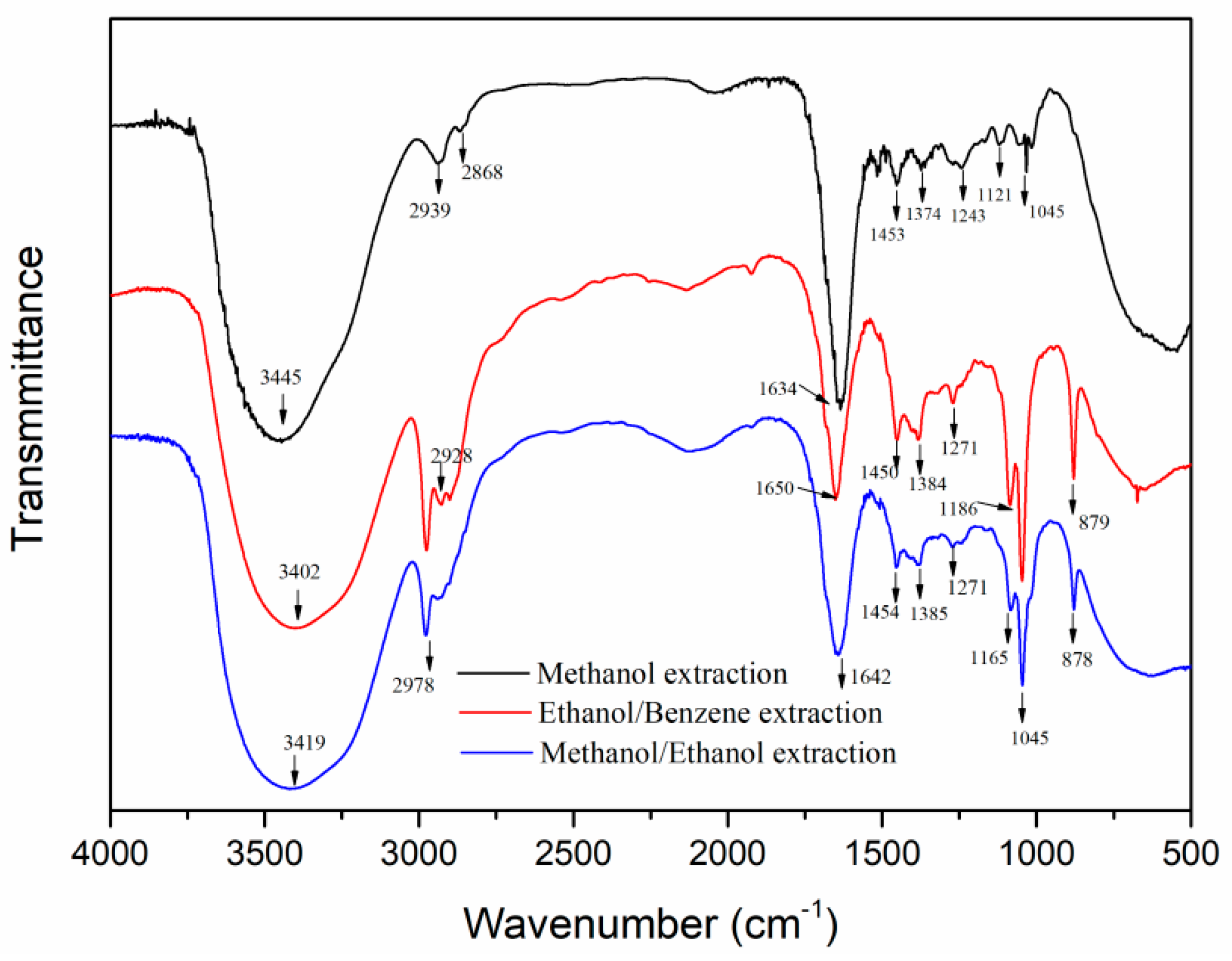
3.1.2. FTIR Analysis of POL Bark Extracts
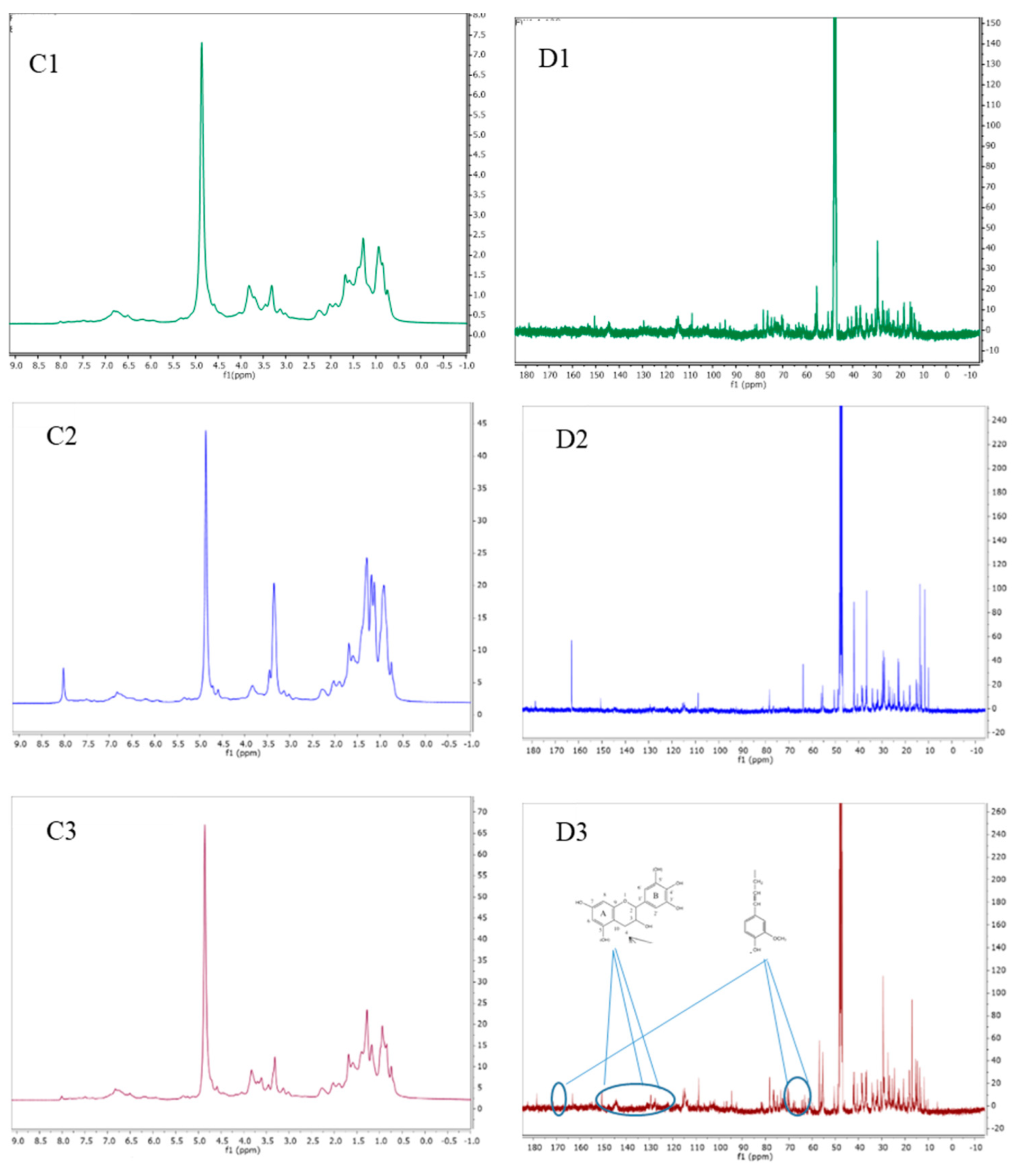
3.1.3. Characterization of POL Bark Extracts using IH NMR, 13C NMR, and 2D-HSQC NMR
3.1.4. Characterization of POL Bark Extracts using TOF-LC-MS
3.2. Effects of Catalysts on Pyrolysis Behavior of POL Bark
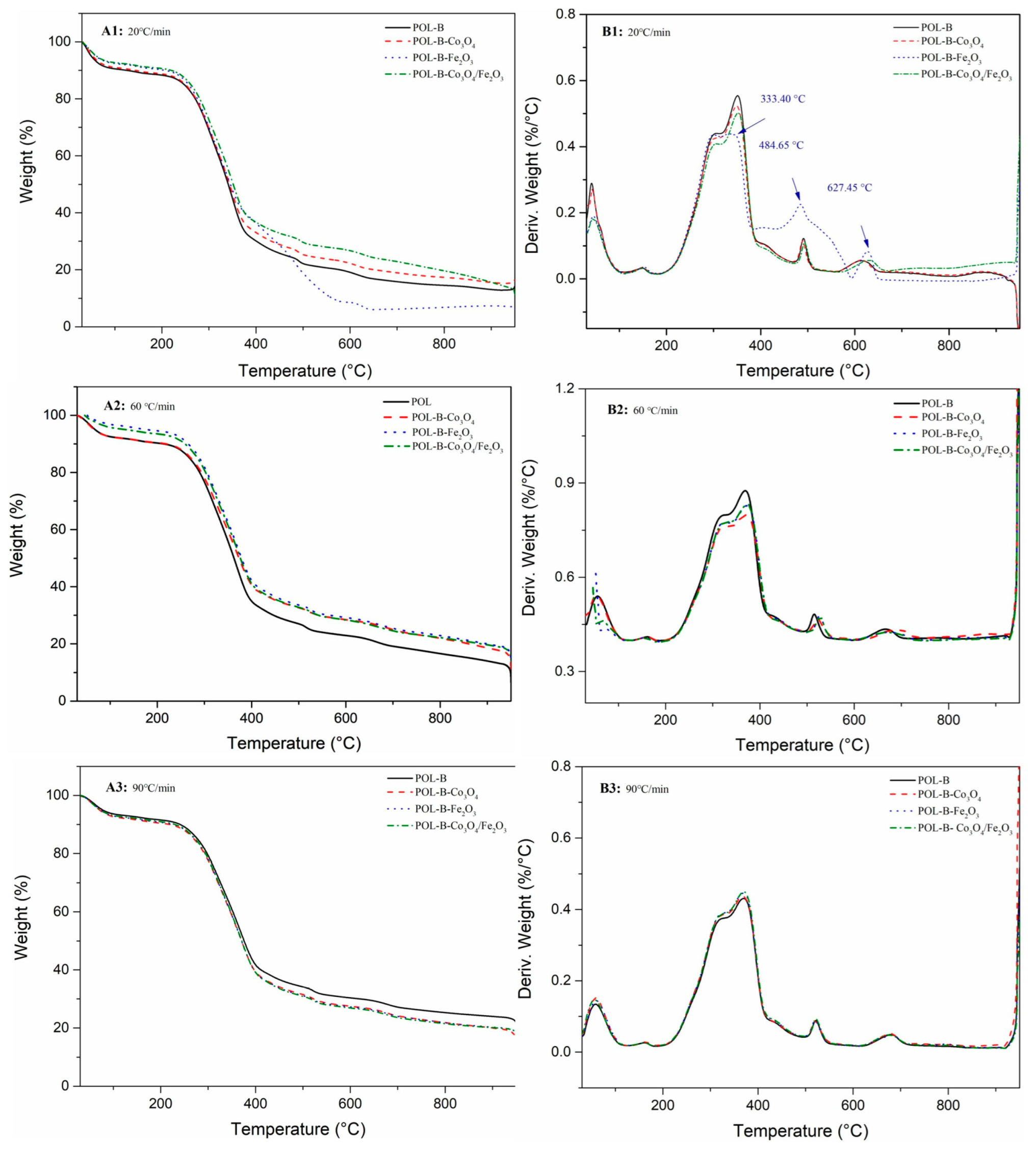
3.2.1. TG Analysis
3.2.2. TG-FTIR Analysis
3.2.3. Py-GC/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morales, M.; Ataman, M.; Badr, S.; Linster, S.; Kourlimpinis, I.; Papadokonstantakis, S.; Hatzimanikatis, V.; Hungerbühler, K. Sustainability assessment of succinic acid production technologies from biomass using metabolic engineering. Energy Environ. Sci. 2016, 9, 2794–2805. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Delucchi, M.A.; Bazouin, G.; Bauer, Z.A.F.; Heavey, C.C.; Fisher, E.; Morris, S.B.; Piekutowski, D.J.Y.; Vencill, T.A.; Yeskoo, T.W. 100% clean and renewable wind, water, and sunlight (WWS) all-sector energy roadmaps for the 50 United States. Energy Environ. Sci. 2015, 8, 2093–2117. [Google Scholar] [CrossRef]
- Philp, J. Balancing the bioeconomy: Supporting biofuels and bio-based materials in public policy. Energy Environ. Sci. 2015, 8, 3063–3068. [Google Scholar] [CrossRef]
- Pramanik, A.; Sinha, A.; Chaubey, K.K.; Hariharan, S.; Dayal, D.; Bachheti, R.K.; Bachheti, A.; Chandel, A.K. Second-Generation Bio-Fuels: Strategies for Employing Degraded Land for Climate Change Mitigation Meeting United Nation-Sustainable Development Goals. Sustainability 2023, 15, 7578. [Google Scholar] [CrossRef]
- Grams, J.; Niewiadomski, M.; Ruppert, A.M.; Kwapiński, W. Influence of Ni catalyst support on the product distribution of cellulose fast pyrolysis vapors upgrading. J. Anal. Appl. Pyrolysis 2015, 113, 557–563. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Novoselov, K.S.; Liang, F.; Meng, J.; Ho, S.H.; Zhao, T.; Zhou, H.; Ahmad, A.; Zhu, Y.; et al. Bioresource Upgrade for Sustainable Energy, Environment, and Biomedicine. Nanomicro Lett. 2023, 15, 35. [Google Scholar] [CrossRef]
- Wanxi Peng, L.W. Makoto Ohkoshi, Minglong Zhang, Separation of Hemicelluloses from Eucalyptus Species: Investigating the Residue after Alkaline Treatment. Cellul. Chem. Technol. 2013, 49, 757–764. [Google Scholar]
- Cai, H.; Wang, J.; Feng, Y.; Wang, M.; Qin, Z.; Dunn, J.B. Consideration of land use change-induced surface albedo effects in life-cycle analysis of biofuels. Energy Environ. Sci. 2016, 9, 2855–2867. [Google Scholar] [CrossRef]
- Margellou, A.G.; Pappa, C.P.; Psochia, E.A.; Petala, M.D.; Triantafyllidis, K.S. Mild isolation and characterization of surface lignin from hydrothermally pretreated lignocellulosic forestry and agro-industrial waste biomass. Sustain. Chem. Pharm. 2023, 33, 101056. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I. López Granados Model-Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Ge, Y.; Li, L. System-level energy consumption modeling and optimization for cellulosic biofuel production. Appl. Energy 2018, 226, 935–946. [Google Scholar] [CrossRef]
- Zhao, J.; Wilkins, M.R.; Wang, D. A review on strategies to reduce ionic liquid pretreatment costs for biofuel production. Bioresour. Technol. 2022, 364, 128045. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Jayakrishnan, U.; Shree, B.; Bhatt, P.; Eshkabilov, S.; Simsek, H. Biological pretreatment for algal biomass feedstock for biofuel production. J. Environ. Chem. Eng. 2023, 11, 109870. [Google Scholar] [CrossRef]
- Arias, K.S.; Climent, M.J.; Corma, A.; Iborra, S. Synthesis of high quality alkyl naphthenic kerosene by reacting an oil refinery with a biomass refinery stream. Energy Environ. Sci. 2015, 8, 317–331. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Stariha, L.; Kodali, M.; Gordon, J.; Babanova, S.; Bretschger, O.; Artyushkova, K.; Atanassov, P. Iron based catalysts from novel low-cost organic precursors for enhanced oxygen reduction reaction in neutral media microbial fuel cells. Energy Environ. Sci. 2016, 9, 2346–2353. [Google Scholar] [CrossRef]
- Guan, J.; Cao, X.; Yuan, Y.; Wang, C.; An, R.; Lu, P.; Lu, N. Synergic mechanisms of electricity generation and bisphenol A degradation in a novel photocatalytic-microbial fuel cell equipped with a TiO2-C-BiVO4 photo-anode and a biofilm-anode. Chem. Eng. J. 2023, 471, 144308. [Google Scholar] [CrossRef]
- Karnjanakom, S.; Guan, G.; Asep, B.; Hao, X.; Kongparakul, S.; Samart, C.; Abudula, A. Catalytic Upgrading of Bio-Oil over Cu/MCM-41 and Cu/KIT-6 Prepared by β-Cyclodextrin-Assisted Coimpregnation Method. J. Phys. Chem. C 2016, 120, 3396–3407. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Yang, J.; Xu, D.; Li, Y.; Li, J.; Zhang, Y. Hydrothermal liquefaction and gasification of biomass and model compounds: A review. Green Chem. 2020, 22, 8210–8232. [Google Scholar] [CrossRef]
- Wang, H.; Lee, S.-J.; Olarte, M.V.; Zacher, A.H. Bio-oil Stabilization by Hydrogenation over Reduced Metal Catalysts at Low Temperatures. ACS Sustain. Chem. Eng. 2016, 4, 5533–5545. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels 2004, 18, 590–598. [Google Scholar]
- Ge, S.; Liang, Y.; Zhou, C.; Sheng, Y.; Zhang, M.; Cai, L.; Zhou, Y.; Huang, Z.; Manzo, M.; Wu, C.; et al. The potential of Pinus armandii Franch for high-grade resource utilization. Biomass Bioenergy 2022, 158, 106345. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z. Catalytic Conversion of Biomass into Chemicals and Fuels over Magnetic Catalysts. ACS Catal. 2015, 6, 326–338. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Lin, X.X.C.-X.; Tong, D.-S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Khoja, A.H.; Ali, I.; Naqvi, M.; Noor, T.; Ahmad, A.; Luque, R.; Amin, N.A.S. Recent progress in catalytic deoxygenation of biomass pyrolysis oil using microporous zeolites for green fuels production. Fuel 2023, 333, 126268. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Xu, D.; Guan, Q. A review on recent advances in clean microalgal bio-oil production via catalytic hydrothermal deoxygenation. J. Clean. Prod. 2022, 366, 132978. [Google Scholar] [CrossRef]
- Jia, L.Y.; Raad, M.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Bettahar, M.M.; Mauviel, G.; Tarrighi, M.; Pinard, L.; Dufour, A. Catalytic fast pyrolysis of biomass--superior selectivity of hierarchical zeolite to aromatics. Green Chem. 2017, 19, 5442–5459. [Google Scholar] [CrossRef]
- Zhou, G.; Jensen, P.A.; Le, D.M.; Knudsen, N.O.; Jensen, A.D. Direct upgrading of fast pyrolysis lignin vapor over the HZSM-5 catalyst. Green Chem. 2015, 18, 1965–1975. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Production of high grade fuels and chemicals from catalytic pyrolysis of biomass. Catal. Today 1996, 29, 285–295. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Chen, L.; Zhao, B.; Yang, S.; Xie, X. Comparision of catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts. J. Anal. Appl. Pyrolysis 2016, 121, 342–346. [Google Scholar] [CrossRef]
- Li, Y.; Shaheen, S.M.; Rinklebe, J.; Ma, N.L.; Yang, Y. Muhammad Aqeel Ashraf, Xiangmeng Chen, Wan-Xi Peng. Pyrolysis of Aesculus chinensis Bunge Seed with Fe2O3/NiO as nanocatalysts for the production of bio-oil material. J. Hazard. Mater. 2021, 416, 126012. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gao, J.; Zhang, C.; Tsang, D.C.W.; Fan, J.; Clark, J.H.; Luo, G.; Zhu, X.; Zhang, S. Fast and Selective Production of Aromatics via Efficient Lignin Depolymerization: Critical Factors and Mechanism Studies. ACS Sustain. Chem. Eng. 2022, 10, 15273–15283. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Chang, C.; Kulsreshtha, M.; Gupta, P. Bio-separation of value-added products from Kraft lignin: A promising two-stage lignin biorefinery via microbial electrochemical technology. Int. J. Biol. Macromol. 2023, 227, 307–315. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.-F.; Dong, C.-Q.; Zhu, X.-F. Catalytic Upgrading of Biomass Fast Pyrolysis Vapors with Nano Metal Oxides: An Analytical Py-GC/MS Study. Energies 2010, 3, 1805–1820. [Google Scholar] [CrossRef]
- Khelfa, A.; Sharypov, V.; Finqueneisel, G.; Weber, J.V. Catalytic pyrolysis and gasification of Miscanthus Giganteus: Haematite (Fe2O3) a versatile catalyst. J. Anal. Appl. Pyrolysis 2009, 84, 84–88. [Google Scholar] [CrossRef]
- Sakthivel, R.; Ramesh, K.; Shameer, P.M.; Purnachandran, R. A Complete Analytical Characterization of Products Obtained from Pyrolysis of Wood Barks of Calophyllum inophyllum. Waste Biomass Valorization 2018, 10, 2319–2333. [Google Scholar] [CrossRef]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Tumbalam Gooty, A.; Li, D.; Briens, C.; Berruti, F. Fractional condensation of bio-oil vapors produced from birch bark pyrolysis. Sep. Purif. Technol. 2014, 124, 81–88. [Google Scholar] [CrossRef]
- Lauberts, M.; Pals, M. Antioxidant Activity of Different Extracts from Black Alder (Alnus glutinosa) Bark with Greener Extraction Alternative. Plants 2021, 10, 2531. [Google Scholar] [CrossRef]
- Peng, W.; Wu, F.; Wang, L.; Xu, Q. Crystal structure of 3-(4-bromophenyl)-4-(4-chloro-phenylamino)furan-2(5H)-one, C16H11BrClNO2. Z. Für Krist.-New Cryst. Struct. 2012, 227, 61–62. [Google Scholar] [CrossRef]
- Li, C.; Yue, X.C.; Yang, J.; Yang, Y.F.; Gu, H.P.; Peng, W.X. Catalytica fast pyrolyisis of forestry wood waste for bio-energy recovery using nano-catalysts. Energies 2019, 12, 3972. [Google Scholar] [CrossRef]
- Peng, W.; Lin, Z.; Chang, J.; Gu, F.; Zhu, X. Biomedical Molecular Characteristics of YBSJ Extractives from Illicium verum Fruit. Biotechnol. Biotechnol. Equip. 2014, 27, 4311–4316. [Google Scholar] [CrossRef]
- Peng, W.; Lin, Z.; Wang, L.; Chang, J.; Gu, F.; Zhu, X. Molecular characteristics of Illicium verum extractives to activate acquired immune response. Saudi J. Biol. Sci. 2016, 23, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Peng, W.; Li, D.; Mo, B.; Zhang, M.; Qin, D. Study on antibacterial molecular drugs in Eucalyptus granlla wood extractives by GC-MS. Pak. J. Pharm. Sci. 2015, 28, 1445–1448. [Google Scholar]
- Ouyang, H.; Hou, K.; Ge, S.; Deng, H.; Peng, W. Antimicrobial activities of flavonoids against bamboo-destroying fungi and molds. Toxicol. Environ. Chem. 2017, 99, 892–899. [Google Scholar] [CrossRef]
- Ban, S.E.; Lee, E.J.; Lim, D.J.; Kim, I.S.; Lee, J.W. Evaluation of sulfuric acid-pretreated biomass-derived biochar characteristics and its diazinon adsorption mechanism. Bioresour. Technol. 2022, 348, 126828. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-L.; Wang, L.-S.; Peng, W.-X.; Ge, S.-B.; Li, N.-C.; Furuta, Y. Chemical structure of hemicellulosic polymers isolated from bamboo bio-composite during mold pressing. Polym. Compos. 2017, 38, 2009–2015. [Google Scholar] [CrossRef]
- N’Guessan, J.L.L.; Niamké, B.F.; Yao, J.C.N.; Amusant, N. Wood Extractives: Main Families, Functional Properties, Fields of Application and Interest of Wood Waste. For. Prod. J. 2023, 73, 194–208. [Google Scholar] [CrossRef]
- Lall, N.; Weiganand, O.; Hussein, A.; Meyer, J. Antifungal activity of naphthoquinones and triterpenes isolated from the root bark of Euclea natalensis. S. Afr. J. Bot. 2006, 72, 579–583. [Google Scholar] [CrossRef]
- Mbambo, B.; Odhav, B.; Mohanlall, V. Antifungal activity of stigmasterol, sitosterol and ergosterol from Bulbine natalensis Baker (Asphodelaceae). J. Med. Plants Res. 2012, 6, 5135–5141. [Google Scholar]
- Ren, Y.; Zhang, D.; Yin, Y.; Ye, Z.; Yin, Z.; Tu, S.; Ye, L.; Chen, X.; Zhao, S. Mechanically strong, thermostable, and flame-retardant composites enabled by Brown paper made from bamboo. Compos. Sci. Technol. 2022, 226, 109544. [Google Scholar] [CrossRef]
- Abou Dib, M.; Hucher, N.; Gore, E.; Grisel, M. Original tools for xanthan hydrophobization in green media: Synthesis and characterization of surface activity. Carbohydr. Polym. 2022, 291, 119548. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.H.; de Melo, M.M.; Portugal, I.; Silva, C.M. Extraction of Eucalyptusleaves using solvents of distinct polarity. Cluster analysis and extracts characterization. J. Supercrit. Fluids 2018, 135, 263–274. [Google Scholar] [CrossRef]
- Ping, L.; Pizzi, A.; Guo, Z.D.; Brosse, N. Condensed tannins from grape pomace: Characterization by FTIR and MALDI TOF and production of environment friendly wood adhesive. Ind. Crops Prod. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Characterization of proanthocyanidin in hot water extract isolated from Pinus radiata bark. Wood Sci. Technol. 2006, 41, 235–247. [Google Scholar] [CrossRef]
- Grasel Fdos, S.; Ferrao, M.F.; Wolf, C.R. Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Buhlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochemistry 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, N.; Feng, M.W. Biobased Phenol Formaldehyde Resins Derived from Beetle-Infested Pine Barks—Structure and Composition. ACS Sustain. Chem. Eng. 2012, 1, 91–101. [Google Scholar] [CrossRef]
- Vázquez, G.; Pizzi, A.; Freire, M.S.; Santos, J.; Antorrena, G.; González-Álvarez, J. MALDI-TOF, HPLC-ESI-TOF and 13C-NMR characterization of chestnut (Castanea sativa) shell tannins for wood adhesives. Wood Sci. Technol. 2012, 47, 523–535. [Google Scholar] [CrossRef]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Teoh, S.L.; Das, S. Phytochemicals and their effective role in the treatment of diabetes mellitus: A short review. Phytochem. Rev. 2018, 17, 1111–1128. [Google Scholar] [CrossRef]
- Mu, W.; Ben, H.; Newalkar, G.; Ragauskas, A.; Qiu, D.; Deng, Y. Structure Analysis of Pine Bark-, Residue-, and Stem-Derived Light Oil and Its Hydrodeoxygenation Products. Ind. Eng. Chem. Res. 2014, 53, 11269–11275. [Google Scholar] [CrossRef]
- Chen, W.; McClelland, D.J.; Azarpira, A.; Ralph, J.; Luo, Z.; Huber, G.W. Low temperature hydrogenation of pyrolytic lignin over Ru-TiO2—2D HSQC and 13C NMR study of reactants and products. Green Chem. 2016, 17, 271–278. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, J.H.; Kim, E.M.; Shin, H.J.; Hwang, S.G.; Song, J.Y.; Um, H.D.; Park, J.K. beta-Apopicropodophyllin functions as a radiosensitizer targeting ER stress in non-small cell lung cancer. Biomed Pharm. 2019, 113, 108769. [Google Scholar] [CrossRef]
- Guo, Z.; Niu, X.; Xiao, T.; Lu, J.; Li, W.; Zhao, Y. Chemical profile and inhibition of α-glycosidase and protein tyrosine phosphatase 1B (PTP1B) activities by flavonoids from licorice (Glycyrrhiza uralensis Fisch). J. Funct. Foods 2015, 14, 324–336. [Google Scholar] [CrossRef]
- Breyer, S.; Effenberger-Neidnicht, K.; Schobert, R. Total synthesis and anticancer activities of (-)- and (+)-thespesone. J. Org. Chem. 2010, 75, 6214–6218. [Google Scholar] [CrossRef]
- Kok, M.V.; Özgür, E. Thermal analysis and kinetics of biomass samples. Fuel Process. Technol. 2013, 106, 739–743. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghari, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, E.; Chen, A. Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Kaewpengkrow, P.; Atong, D.; Sricharoenchaikul, V. Selective catalytic fast pyrolysis of Jatropha curcas residue with metal oxide impregnated activated carbon for upgrading bio-oil. Int. J. Hydrogen Energy 2017, 42, 18397–18409. [Google Scholar] [CrossRef]

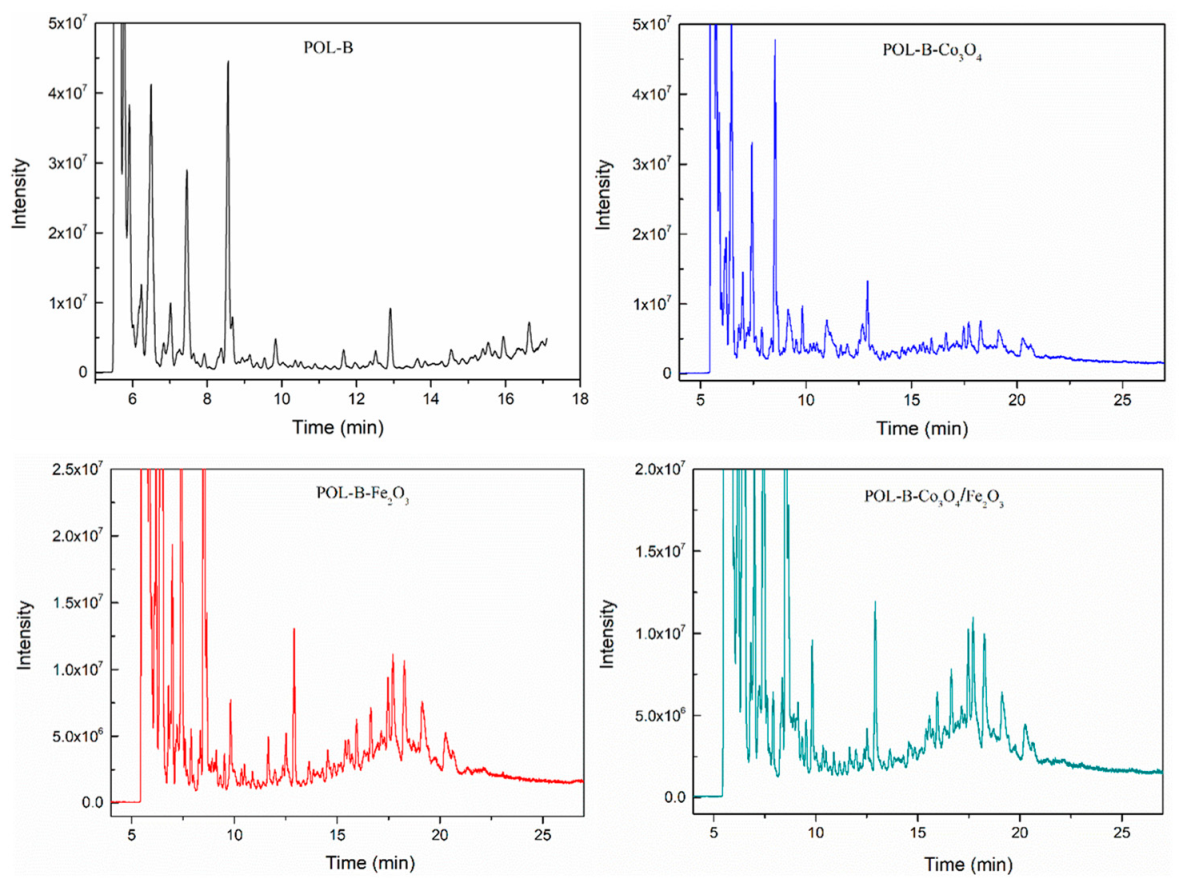
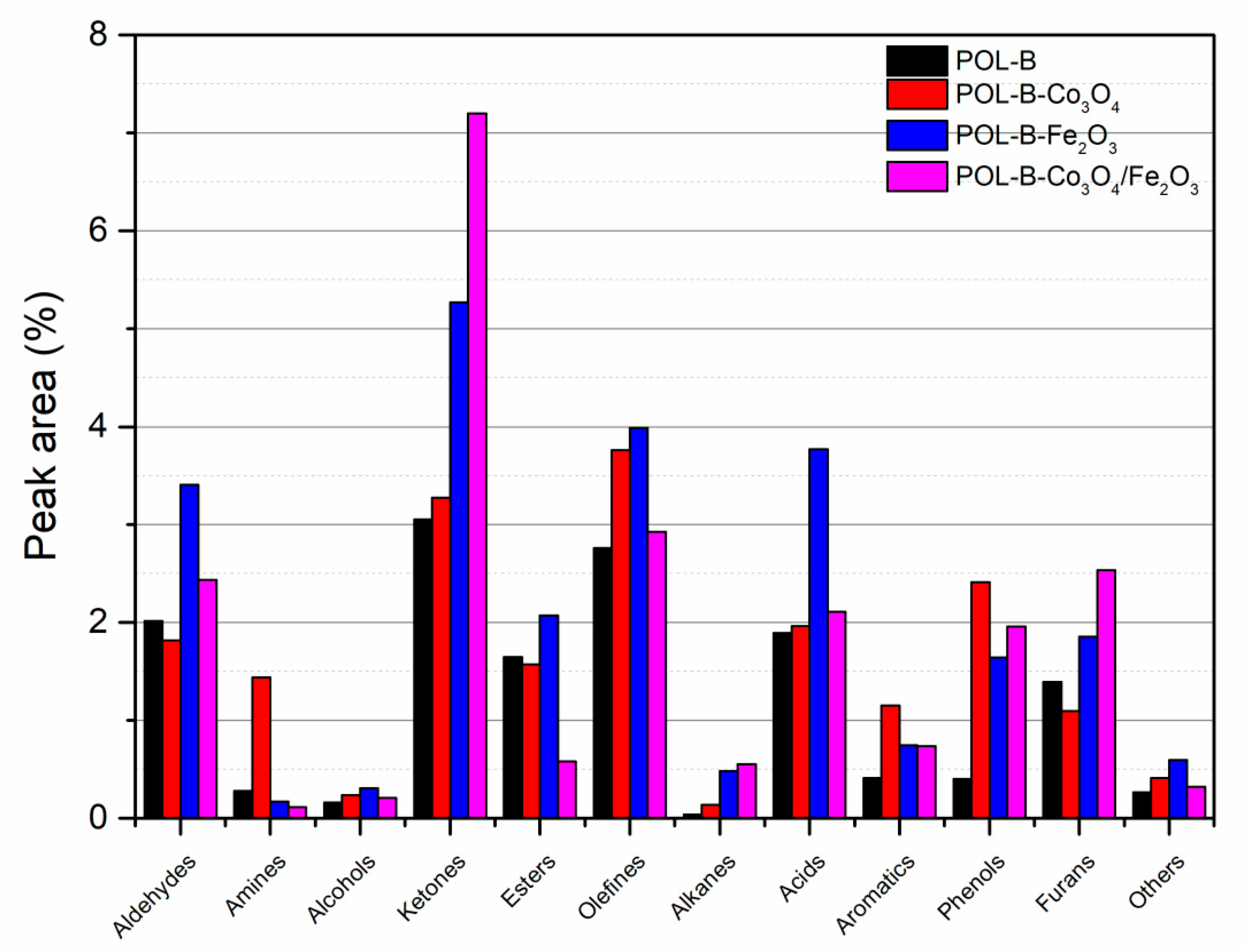
| Samples | Barks (g) | Co3O4 (g) | Fe2O3 (g) | Co3O4 + Fe2O3 (g) |
|---|---|---|---|---|
| POL-B | 20.00 | |||
| POL-B- Co3O4 | 20.00 | 0.20 | ||
| POL-B- Fe2O3 | 20.00 | 0.20 | ||
| POL-B-Co3O4/Fe2O3 | 20.00 | 0.10 + 0.10 |
| Compound | Concentration (wt.%) |
|---|---|
| Acid | 9.708 |
| 9,12-Octadecadienoic | 1.844 |
| 9-Hexadecenoic, acid | 3.549 |
| n-Hexadecanoic, acid | 2.176 |
| Oleic acid | 2.139 |
| Alcohols | 34.421 |
| 1-Hexanol, 2-ethyl- | 1.982 |
| 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | 1.196 |
| 1,30-Triacontanediol | 28.331 |
| Cryptomeridiol, | 1.606 |
| 7,8-Epoxylanostan-11-ol-3-acetoxy- | 1.306 |
| Amides | 2.391 |
| Formamide, N,N-diethyl- | 2.391 |
| Phenol | 1.600 |
| (E)-4-(3-Hydroxyprop-1-en-1-yl)-2-methoxyphenol, | 0.743 |
| Phenol, 3,4,5-trimethoxy- | 0.857 |
| Aromatics | 18.753 |
| 5H-Cyclopropa[3,4]benz[1,2-e]azulen-5-one, 9,9a-bis(acetyloxy)-3-[(acetyloxy)methyl]-1,1a,1b,2,3,4,4a,7a,7b,8,9,9a-dodecahydro-2,3,4a,7b-tetrahydroxy-1,1,6,8-tetramethyl-, | 0.687 |
| 1H-Cyclopropa[3,4]benz[1,2-e]azulene-4a,5,7b,9,9a(1aH)-pentol, 3-[(acetyloxy)methyl]-1b,4,5,7a,8,9-hexahydro-1,1,6,8-tetramethyl-, | 7.145 |
| 4H-Cyclopropa[5′,6′]benz[1′,2′:7,8]azuleno[5,6]oxiren-4-one, 8,8a-bis(acetyloxy)-2a-[(acetyloxy)methyl]-1,1a,1b,1c,2a,3,3a,6a,6b,7,8,8a-dodecahydro-6b-hydroxy-3a-methoxy-1,1,5,7-tetramethyl-, | 9.812 |
| Benzenemethanol, 3,4,5-trimethoxy- | 1.109 |
| Olefine | 33.125 |
| 17-Pentatriacontene | 33.125 |
| Compound | Concentration (wt.%) |
|---|---|
| Acid | 6.235 |
| Hexadecanoic acid,2-methyl- | 4.646 |
| elaidate | 1.589 |
| Alcohols | 55.821 |
| β-Sitosterol | 7.389 |
| γ-Sitosterol | 0.91 |
| 1-Hexanol,2-ethyl- | 15.582 |
| 1H-Cyclopropa[3,4]benz[1,2-e]azulene-5,7b,9,9a-tetrol, 1a,1b,4,4a,5,7a,8,9-octahydro-3-(hydroxymethyl)-1,1,6,8-tetramethyl-,5,9,9a-triacetate, | 2.601 |
| 1H-Cyclopropa[3,4]benz[1,2-e]azulene-4a,5,7b,9,9a(1aH)-pentol, 1b,4,5,7a,8,9-hexahydro-3-(hydroxymethyl)-1,1,6,8-tetramethyl-, | 19.811 |
| 1,22-Docosanediol | 9.528 |
| Esters | 11.585 |
| Heptadecanoic acid, 15-methyl-, methyl ester | 3.161 |
| i-Propyl 14-methyl-pentadecanoate | 2.432 |
| 1b,4,5,7a,8,9-hexahydro-3-(hydroxymethyl)-1,1,6,8-tetramethyl-, 9,9a-diacetate, | 2.322 |
| 11-Octadecenoic acid, methyl ester | 2.803 |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 0.867 |
| Saccharides | 0.939 |
| 1-Hydroxy-2-(2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl)-9H-xanthene-3,6,7-triyl triacetate | 0.939 |
| Amides | 18.141 |
| N,N-diethyl-formamide | 18.141 |
| Ketones | 3.787 |
| 17.β.-Acetoxy-1′,1′-dicarboethoxy-1.β.,2.β.-dihydrocycloprop[1,2]-5α-androst-1-en-3-one | 3.203 |
| 1,9-Dioxacyclohexadeca-4,13-diene-2-10-dione,7,8,15,16-tetramethyl- | 0.584 |
| Olefine | 1.471 |
| 2,5,5,8a-Tetramethyl-4-methylene-6,7,8,8a-tetrahydro-4H,5H-chromen-4a-yl hydroperoxide | 0.547 |
| 17-Pentatriacontene | 0.924 |
| Ethers | 2.021 |
| 2,5-dimethoxyphenylethylsulfide | 0.734 |
| (Z)-18-Octadec-9-enolide | 1.287 |
| Compound | Concentration (wt.%) |
|---|---|
| Acids | 5.538 |
| n-Hexadecanoic acid | 4.700 |
| Vanillic acid | 0.838 |
| Aldehydes | 0.796 |
| 3,5-Dimethoxy-4-hydroxycinnamaldehyde | 0.796 |
| Alcohols | 23.567 |
| γ-Sitosterol | 19.319 |
| 2-ethyl-1-Hexanol | 1.848 |
| 1-Heptatriacotanol | 0.968 |
| 7,8-Epoxylanostan-11-ol,3-acetoxy- | 1.432 |
| Esters | 17.133 |
| 9-Octadecenoic acid, methyl ester, (E)- | 4.100 |
| 2-Oxo-1-(3-oxo-butyl)-cyclohexanecarboxylic acid, ethyl ester | 0.781 |
| 7-Methyl-Z-tetradecen-1-ol acetate | 0.847 |
| Hexadecanoic acid, methyl ester | 5.827 |
| Methyl stearate | 5.578 |
| Saccharides | 1.691 |
| d-Mannose | 0.814 |
| β-D-Glucopyranose,.β.-D-Glucopyranose,4-O-.β.-D-galactopyranosyl- | 0.877 |
| Amides | 2.150 |
| N,N-diethyl-Formamide | 2.150 |
| Phenols | 7.048 |
| 3,4,5-trimethoxy-Phenol | 1.817 |
| Aromatics | 40.609 |
| 1H-Cyclopropa[3,4]benz[1,2-e]azulene-4a,5,7b,9,9a(1aH)-pentol,1b,4,5,7a,8,9-hexahydro-3-(hydroxymethyl)-1,1,6,8-tetramethyl-,9,9a-diacetate, | 5.231 |
| 4H-Cyclopropa[5′,6′]benz[1′,2′:7,8]azuleno [5,6-b]oxiren-4-one,8-(acetyloxy)-1,1a,1b,1c,2a,3,3a,6a,6b,7,8,8a-dodecahydro-3a,6b,8a-trihydroxy-2a-(hydroxymethyl)-1,1,5,7-tetramethyl-, | 28.084 |
| 5H-Cyclopropa[3,4]benz[1,2-e]azulen-5-one,9,9a-bis(acetyloxy)-3-[(acetyloxy)methyl]-2-chloro-1,1a,1b,2,3,4,4a,7a,7b,8,9,9a-dodecahydro-3,4a,7b-trihydroxy-1,1,6,8-tetramethyl-, | 5.429 |
| γ-Sitostenone, | 1.865 |
| Olefines | 4.318 |
| 3-Buten-2-ol,2-methyl-4-(1,3,3-trimethyl-7-oxabicyclo[4.1.0]hept-2-yl)- | 1.126 |
| 3-Buten-2-ol,3-Buten-2-ol,2-methyl-4-(1,3,3-trimethyl-7-oxabicyclo[4.1.0]hept-2-yl)- | 3.192 |
| Unidentified | 2.381 |
| Samples | Heating Rate (°C/min) | The First Stage: | The Second Stage: | The Third Stage: | Residues (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30–200 °C | 200–450 °C | 450–950 °C | ||||||||
| TP1 (°C) | Weight Loss (%) | TP2 (°C) | TP3 (°C) | Weight Loss (%) | TP4 (°C) | TP5 (°C) | Weight Loss (%) | |||
| POL-B | 20 | 149 | 11.55 | 306 | 351 | 61.77 | 491 | 613 | 12.66 | 14.02 |
| 60 | 161 | 9.66 | 324 | 369 | 60.35 | 515 | 666 | 16.86 | 13.13 | |
| 90 | 162 | 8.44 | 330 | 370 | 54.80 | 521 | 679 | 12.96 | 23.80 | |
| POL-B -Co3O4 | 20 | 149 | 11.23 | 307 | 349 | 59.90 | 489 | 615 | 11.98 | 16.89 |
| 60 | 161 | 9.57 | 326 | 368 | 55.05 | 523 | 691 | 17.49 | 17.89 | |
| 90 | 163 | 9.40 | 326 | 368 | 56.29 | 521 | 678 | 21.08 | 13.23 | |
| POL-B -Fe2O3 | 20 | 153 | 9.75 | 302 | 333 | 61.55 | 485 | 627 | 21.69 | 7.01 |
| 60 | 158 | 7.03 | 327 | 367 | 58.06 | 515 | 668 | 10.06 | 24.85 | |
| 90 | 163 | 9.13 | 331 | 369 | 56.91 | 520 | 680 | 13.85 | 20.11 | |
| POL-B- Co3O4/Fe2O3 | 20 | 150 | 9.29 | 308 | 352 | 57.38 | 493 | 631 | 17.90 | 15.43 |
| 60 | 160 | 8.49 | 338 | 373 | 57.09 | 528 | 664 | 15.43 | 18.99 | |
| 90 | 161 | 9.02 | 331 | 370 | 57.24 | 523 | 675 | 13.79 | 19.95 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zou, Y.; Liang, J.; Zhao, Z.; Zhou, N.; Gao, Y.; Yan, R.; Zhou, Q.; Li, C. The Potential of Platanus orientalis L. Bark for High-Grade Resource Utilization. Forests 2023, 14, 2002. https://doi.org/10.3390/f14102002
Li H, Zou Y, Liang J, Zhao Z, Zhou N, Gao Y, Yan R, Zhou Q, Li C. The Potential of Platanus orientalis L. Bark for High-Grade Resource Utilization. Forests. 2023; 14(10):2002. https://doi.org/10.3390/f14102002
Chicago/Turabian StyleLi, Hanyin, Yunming Zou, Jingyi Liang, Zijie Zhao, Na Zhou, Yan Gao, Ruohan Yan, Qiongqiong Zhou, and Cheng Li. 2023. "The Potential of Platanus orientalis L. Bark for High-Grade Resource Utilization" Forests 14, no. 10: 2002. https://doi.org/10.3390/f14102002
APA StyleLi, H., Zou, Y., Liang, J., Zhao, Z., Zhou, N., Gao, Y., Yan, R., Zhou, Q., & Li, C. (2023). The Potential of Platanus orientalis L. Bark for High-Grade Resource Utilization. Forests, 14(10), 2002. https://doi.org/10.3390/f14102002






