Dynamic Physiological Responses of Cinnamomum camphora with Monoterpene Protection under High Temperature Shock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and High Temperature Treatment
2.2. Measurement of H2O2 Content
2.3. Determination of TBARS Content
2.4. Determination of Ascorbic Acid Content
2.5. Assay of Chlorophyll Fluorescence Transient
2.6. Measurement of Gas Exchange
2.7. qRT-PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of Monoterpene Emission on H2O2 Content Variations under High Temperature
3.2. Effects of Monoterpene Emission on TBARS Content Changes under High Temperature
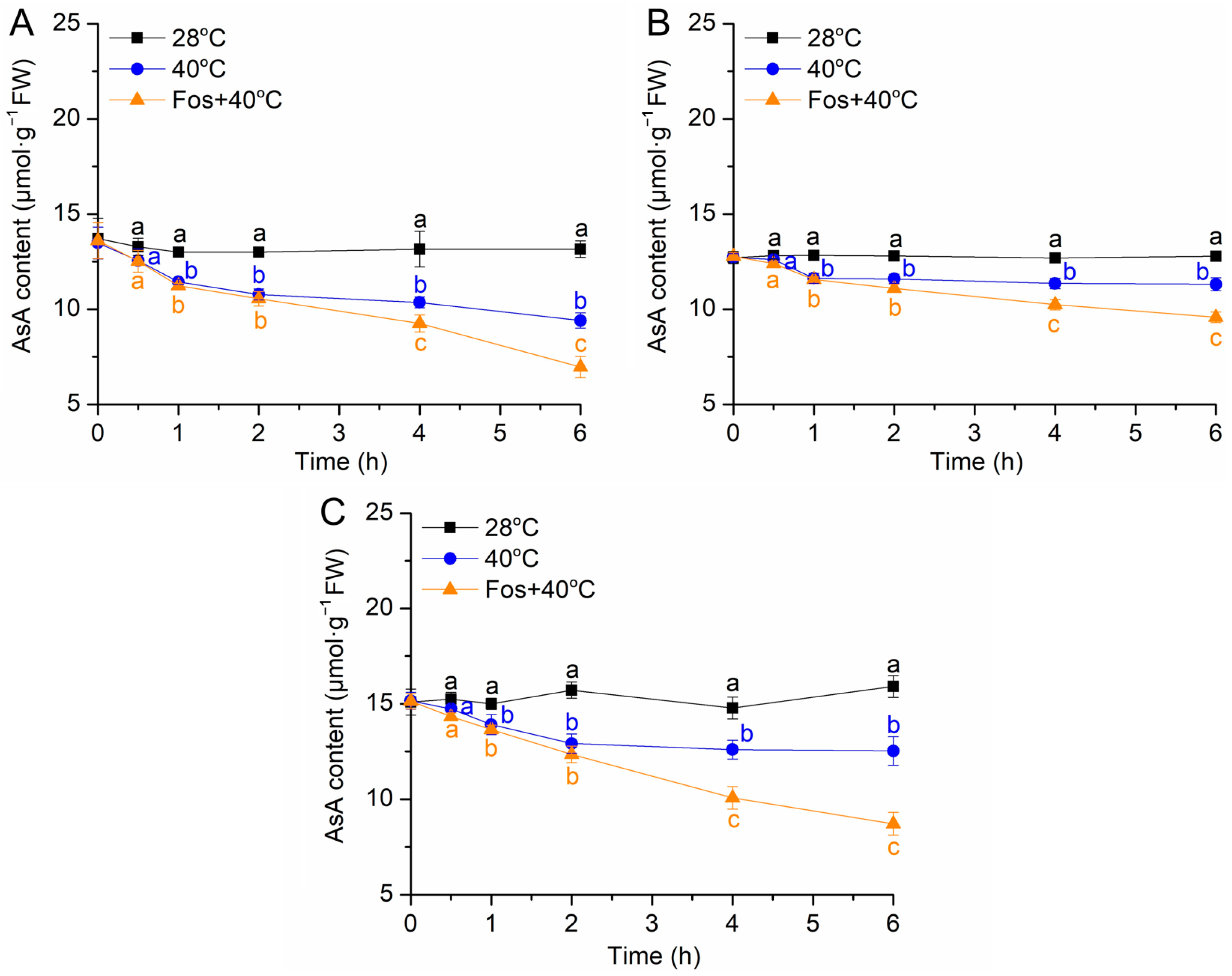
3.3. Effects of Monoterpene Emission on Ascorbic Acid Content Variations under High Temperature
3.4. Effects of Monoterpene Emission on PSII Efficiency Changes under High Temperature
3.5. Effects of Monoterpene Emission on Photosynthetic Rate Variations under High Temperature
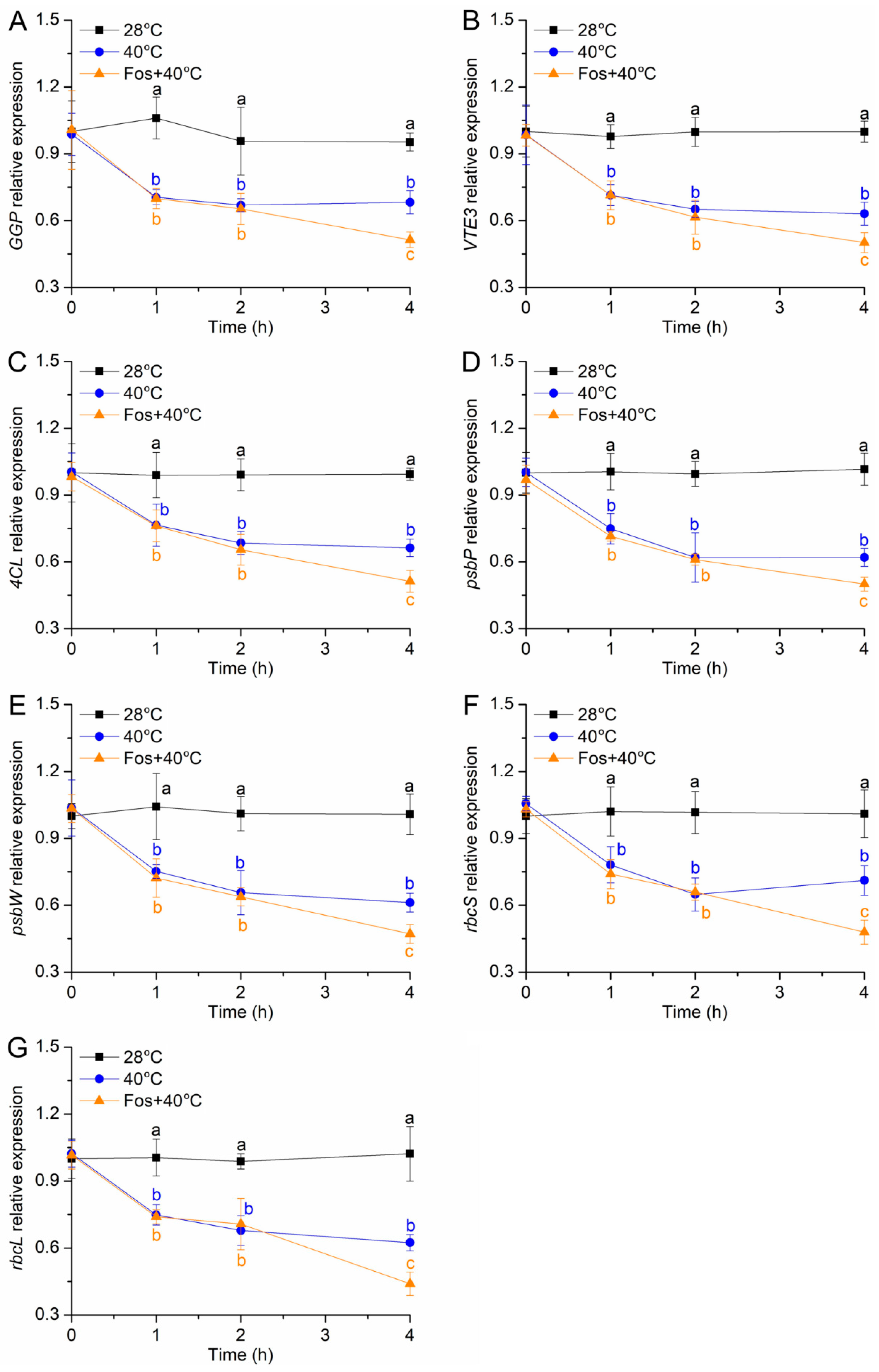
3.6. Effects of Monoterpene Emission on Gene Expression Alterations under High Temperature
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Katz, D.S.W.; Dzul, A.; Kendel, A.; Batterman, S.A. Effect of intra-urban temperature variation on tree flowering phenology, airborne pollen, and measurement error in epidemiological studies of allergenic pollen. Sci. Total Environ. 2019, 653, 1213–1222. [Google Scholar] [CrossRef]
- Tian, Z.; Luo, Q.; Li, Y.; Zuo, Z. Terpinene and β-pinene acting as signaling molecules to improve Cinnamomum camphora thermotolerance. Ind. Crop. Prod. 2020, 154, 112641. [Google Scholar] [CrossRef]
- Crous, K.Y.; Uddling, J.; De Kauwe, M.G. Temperature responses of photosynthesis and respiration in evergreen trees from boreal to tropical latitudes. New Phytol. 2022, 234, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, C.; Zheng, T.; Zuo, Z. Thermal protection function of camphor on Cinnamomum camphora cell membrane by acting as a signaling molecule. Plant Physiol. Biochem. 2023, 198, 107672. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, B.; Zhang, R.; Gao, Y.; Zhang, X.; Li, Y.; Zuo, Z. Initial simulated acid rain impacts reactive oxygen species metabolism and photosynthetic abilities in Cinnamonum camphora undergoing high temperature. Ind. Crop. Prod. 2019, 135, 352–361. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.; Luo, Q.; Ma, Y.; Zheng, T.; Wang, Y.; Cai, Y.; Zuo, Z. The uppermost monoterpenes improving Cinnamomum camphora thermotolerance by serving signaling functions. Front. Plant Sci. 2022, 13, 1072931. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Várkonyi, Z.; Szabó, M.; Maslenkova, L.; Nogues, I.; Kovács, L.; Peeva, V.; Busheva, M.; Garab, G.; Sharkey, T.D.; et al. Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol. 2011, 157, 905–916. [Google Scholar] [CrossRef]
- Taylor, T.C.; Smith, M.N.; Slot, M.; Feeley, K.J. The capacity to emit isoprene differentiates the photosynthetic temperature responses of tropical plant species. Plant Cell Environ. 2019, 42, 2448–2457. [Google Scholar] [CrossRef]
- Monson, R.K.; Weraduwage, S.M.; Rosenkranz, M.; Schnitzler, J.P.; Sharkey, T.D. Leaf isoprene emission as a trait that mediates the growth-defense tradeoff in the face of climate stress. Oecologia 2021, 197, 885–902. [Google Scholar] [CrossRef]
- Byron, J.; Kreuzwieser, J.; Purser, G.; van Haren, J.; Ladd, S.N.; Meredith, L.K.; Werner, C.; Williams, J. Chiral monoterpenes reveal forest emission mechanisms and drought responses. Nature 2022, 609, 307–312. [Google Scholar] [CrossRef]
- Zuo, Z. Why algae release volatile organic compounds—the emission and roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Palmer, P.I.; Marvin, M.R.; Siddans, R.; Kerridge, B.J.; Moore, D.P. Nocturnal survival of isoprene linked to formation of upper tropospheric organic aerosol. Science 2022, 375, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Lantz, A.T.; Allman, J.; Weraduwage, S.M.; Sharkey, T.D. Isoprene: New insights into the control of emission and mediation of stress tolerance by gene expression. Plant Cell Environ. 2019, 42, 2808–2826. [Google Scholar] [CrossRef]
- Zuo, Z. Emission of cyanobacterial volatile organic compounds and their roles in blooms. Front. Microbiol. 2023, 14, 1097712. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Jardine, A.B.; Holm, J.A.; Lombardozzi, D.L.; Negron-Juarez, R.I.; Martin, S.T.; Beller, H.R.; Gimenez, B.O.; Higuchi, N.; Chambers, J.Q. Monoterpene ‘thermometer’ of tropical forest-atmosphere response to climate warming. Plant Cell Environ. 2017, 40, 441–452. [Google Scholar] [CrossRef]
- Guidolotti, G.; Pallozzi, E.; Gavrichkova, O.; Scartazza, A.; Mattioni, M.; Loreto, F.; Calfapietra, C. Emission of constitutive isoprene, induced monoterpenes, and other volatiles under high temperatures in Eucalyptus camaldulensis: A 13C labelling study. Plant Cell Environ. 2019, 42, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Grausgruber-Gröger, S.; Schmiderer, C.; Steinborn, R.; Novak, J. Seasonal influence on gene expression of monoterpene synthases in Salvia officinalis (Lamiaceae). J. Plant Physiol. 2012, 169, 353–359. [Google Scholar] [CrossRef]
- Bertamini, M.; Faralli, M.; Varotto, C.; Grando, M.S.; Cappellin, L. Leaf monoterpene emission limits photosynthetic downregulation under heat stress in field-grown grapevine. Plants 2021, 10, 181. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Baker, C.R.; Walker, A.P.; McDowell, N.; Rogers, A.; Higuchi, N.; Chambers, J.Q.; Jardine, K.J. Stimulation of isoprene emissions and electron transport rates as key mechanisms of thermal tolerance in the tropical species Vismia guianensis. Glob. Chang. Biol. 2020, 26, 5928–5941. [Google Scholar] [CrossRef]
- Pollastri, S.; Jorba, I.; Hawkins, T.J.; Llusià, J.; Michelozzi, M.; Navajas, D.; Peñuelas, J.; Hussey, P.J.; Knight, M.R.; Loreto, F. Leaves of isoprene-emitting tobacco plants maintain PSII stability at high temperatures. New Phytol. 2019, 223, 1307–1318. [Google Scholar] [CrossRef]
- Zuo, Z.; Weraduwage, S.M.; Lantz, A.T.; Sanchez, L.M.; Weise, S.E.; Wang, J.; Childs, K.L.; Sharkey, T.D. Isoprene acts as a signaling molecule in gene networks important for stress responses and plant growth. Plant Physiol. 2019, 180, 124–152. [Google Scholar] [CrossRef] [PubMed]
- Behnke, K.; Ehlting, B.; Teuber, M.; Bauerfeind, M.; Louis, S.; Hänsch, R.; Polle, A.; Bohlmann, J.; Schnitzler, J.P. Transgenic, non-isoprene emitting poplars don’t like it hot. Plant J. 2007, 51, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Delfine, S.; Csiky, O.; Seufert, G.; Loreto, F. Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): Monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol. 2000, 146, 27–36. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. Linking photorespiration, monoterpenes and thermotolerance in Quercus. New Phytol. 2002, 155, 227–237. [Google Scholar] [CrossRef]
- Vickers, C.E.; Possell, M.; Cojocariu, C.I.; Velikova, V.B.; Laothawornkitkul, J.; Ryan, A.; Mullineaux, P.M.; Hewitt, C.N. Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ. 2009, 32, 520–531. [Google Scholar] [CrossRef]
- Pollastri, S.; Tsonev, T.; Loreto, F. Isoprene improves photochemical efficiency and enhances heat dissipation in plants at physiological temperatures. J. Exp. Bot. 2014, 65, 1565–1570. [Google Scholar] [CrossRef]
- Harvey, C.M.; Li, Z.; Tjellström, H.; Blanchard, G.J.; Sharkey, T.D. Concentration of isoprene in artificial and thylakoid membranes. J. Bioenergy Biomembr. 2015, 47, 419–429. [Google Scholar] [CrossRef]
- Xu, C.; Ma, Y.; Tian, Z.; Luo, Q.; Zheng, T.; Wang, B.; Zuo, Z. Monoterpene emissions and their protection effects on adult Cinnamomum camphora against high temperature. Trees Struct. Funct. 2022, 36, 711–721. [Google Scholar] [CrossRef]
- Luo, Q.; Xu, C.; Zheng, T.; Ma, Y.; Li, Y.; Zuo, Z. Leaf morphological and photosynthetic differences among four chemotypes of Cinnamomum camphora in different seasons. Ind. Crop. Prod. 2021, 169, 113651. [Google Scholar] [CrossRef]
- Tian, Z.; Luo, Q.; Zuo, Z. Seasonal emission of monoterpenes from four chemotypes of Cinnamomum camphora. Ind. Crop. Prod. 2021, 163, 113327. [Google Scholar] [CrossRef]
- Liu, J.; Hu, T.; Feng, P.; Yao, D.; Gao, F.; Hong, X. Effect of potassium fertilization during fruit development on tomato quality, potassium uptake, water and potassium use efficiency under deficit irrigation regime. Agr. Water Manag. 2021, 250, 106831. [Google Scholar] [CrossRef]
- Christen, D.; Schönmanna, S.; Jermini, M.; Strasser, R.J.; Défago, G. Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ. Exp. Bot. 2007, 60, 504–514. [Google Scholar] [CrossRef]
- Peng, X.; Wang, B.; Wang, X.; Ni, B.; Zuo, Z. Variations in aroma and specific flavor in strawberry under different colored light-quality selective plastic film. Flavour Fragr. J. 2020, 35, 350–359. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Boyle, D.L.; Welti, R.; Jagadish, S.V.K.; Prasad, P.V.V. Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. 2018, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, M.; Yang, L.; Wang, Y.; Wang, Y.; Meng, Y.; Liu, J.; Zuo, Z. Effects of high light and temperature on Microcystis aeruginosa cell growth and β-cyclocitral emission. Ecotox. Environ. Safe 2020, 192, 110313. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wang, B.; Ying, B.; Zhou, L.; Zhang, R. Monoterpene emissions contribute to thermotolerance in Cinnamomum camphora. Trees Struct. Funct. 2017, 31, 1759–1771. [Google Scholar] [CrossRef]
- Kirkan, B. Antioxidant potential, enzyme inhibition activity, and phenolic profile of extracts from Stachys cretica subsp. vacillans. Ind. Crop. Prod. 2019, 140, 111639. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ. Exp. Bot. 2015, 112, 44–54. [Google Scholar] [CrossRef]
- Liu, R.; Shu, B.; Wang, Y.; Yu, B.; Wang, Y.; Gan, Y.; Liang, Y.; Qiu, Z.; Yang, J.; Yan, S.; et al. Transcriptome analysis reveals key genes involved in the eggplant response to high-temperature stress. Environ. Exp. Bot. 2023, 211, 105369. [Google Scholar] [CrossRef]
- Liu, C.; Luo, S.; Zhao, Y.; Miao, Y.; Wang, Q.; Ye, L.; Gao, L.; Ahammed, G.J.; Cheng, Y. Multiomics analyses reveal high temperature-induced molecular regulation of ascorbic acid and capsaicin biosynthesis in pepper fruits. Environ. Exp. Bot. 2022, 201, 104941. [Google Scholar] [CrossRef]
- Bulley, S.; Laing, W. The regulation of ascorbate biosynthesis. Curr. Opin. Plant Biol. 2016, 33, 15–22. [Google Scholar] [CrossRef]
- Koukounaras, A.; Mellidou, I.; Patelou, E.; Kostas, S.; Shukla, V.; Engineer, C.; Papaefthimiou, D.; Amari, F.; Chatzopoulos, D.; Mattoo, A.K.; et al. Over-expression of GGP1 and GPP genes enhances ascorbate content and nutritional quality of tomato. Plant Physiol. Biochem. 2022, 193, 124–138. [Google Scholar] [CrossRef]
- Mène-Saffrané, L.; DellaPenna, D. Biosynthesis, regulation and functions of tocochromanols in plants. Plant Physiol. Biochem. 2010, 48, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, J.; Cai, Y.; Zhu, P.; Liu, C.; Zhao, A.; Lü, R.; Li, M.; Xu, F.; Yu, M. Characterization and functional analysis of 4-coumarate: CoA ligase genes in mulberry. PLoS ONE 2016, 11, e0155814. [Google Scholar]
- Zushi, K.; Kajiwara, S.; Matsuzoe, N. Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit. Sci. Hortic. 2012, 148, 39–46. [Google Scholar] [CrossRef]
- Jedmowski, C.; Brüggemann, W. Imaging of fast chlorophyll fluorescence induction curve (OJIP) parameters, applied in a screening study with wild barley (Hordeum spontaneum) genotypes under heat stress. J. Photoch. Photobio. B 2015, 151, 153–160. [Google Scholar] [CrossRef]
- Che, Y.; Wang, H.; Zhang, B.; Gao, S.; Wang, Z.; Wang, Y.; Zhang, H.; Sun, G. Elevated air temperature damage to photosynthetic apparatus alleviated by enhanced cyclic electron flow around photosystem I in tobacco leaves. Ecotox. Environ. Safe 2020, 204, 111136. [Google Scholar]
- Liang, M.H.; Dai, J.L.; Xie, S.R.; Wu, J.X.; Chen, H.H.; Jiang, J.G. Orange protein (DbOR) from the salt-tolerant green alga Dunaliella bardawil mediates photosynthesis against heat stress via interacting with DbPsbP1. Algal Res. 2023, 72, 103105. [Google Scholar] [CrossRef]
- Plöchinger, M.; Schwenkert, S.; von Sydow, L.; Schröder, W.P.; Meurer, J. Functional update of the auxiliary proteins PsbW, PsbY, HCF136, PsbN, TerC and ALB3 in maintenance and assembly of PSII. Front. Plant Sci. 2016, 7, 423. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Chen, J.; Wang, X.; Wang, R.; Peng, S.; Chen, L.; Ma, L.; Luo, J. Identification of Rubisco rbcL and rbcS in Camellia oleifera and their potential as molecular markers for selection of high tea oil cultivars. Front. Plant Sci. 2015, 6, 189. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Glauser, G.; Lou, Y.; Erb, M.; Hu, L. Molecular dissection of early defense signaling underlying volatile-mediated defense regulation and herbivore resistance in rice. Plant Cell 2019, 31, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Kutty, N.N.; Mishra, M. Dynamic distress calls: Volatile info chemicals induce and regulate defense responses during herbivory. Front. Plant Sci. 2023, 14, 1135000. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, A.; Higaki, T.; Koeduka, T.; Ishigami, K.; Hosokawa, S.; Watanabe, H.; Matsui, K.; Hasezawa, S.; Touhara, K. Transcriptional regulators involved in responses to volatile organic compounds in plants. J. Biol. Chem. 2019, 294, 2256–2266. [Google Scholar] [CrossRef]




| Gene | Protein | Forward (5′ to 3′) | Reverse (3′ to 5′) |
|---|---|---|---|
| 18s rRNA | 18s rRNA | ATTCGTGCCTTGCCTTAA | CCATGCTAATGTATCCAGAG |
| GGP | GDP-L-Galactose phosphorylase | GTCCTTGAATGCCTTCCCCA | GCGCTTGTTTCTCCGCATAG |
| VTE3 | 2-Methyl-6-phytyl-1,4-benzoquinone (MPBQ)/2-methyl-6-solanyl-1,4-benzoquinone (MSBQ) methyltransferase | GAGGATGTCAGCAAGCCTGT | ATATGTTGCCGCCATTGCAC |
| 4CL | 4-Coumarate-CoA ligase | GATCGCTCTCGCGAAGTACA | CTCCGCCCTTTGGACTTTCT |
| PsbP | PSII oxygen-evolving enhancer protein 2 | AGGAAAGCAAGCCTACTCCG | GTATGTTTGCCGTTGCCACA |
| PsbW | PSII PsbW protein | TGCCAACAGAGTACTGGCTC | TGCCCCCTTATGTACATGGC |
| rbcS | Ribulose-1,5-bisphosphate carboxylase/oxygenase small chain | CATGGGATGGGTTCCTTGCT | CTTGGCGGTTGTTGTCGAAG |
| rbcL | Ribulose-1,5-bisphosphate carboxylase/oxygenase large chain | CCAAAACTTTCCAAGGCCCG | TCCCAATAGGGGACGACCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qian, Q.; Xu, H.; Zuo, Z. Dynamic Physiological Responses of Cinnamomum camphora with Monoterpene Protection under High Temperature Shock. Forests 2023, 14, 2005. https://doi.org/10.3390/f14102005
Wang Y, Qian Q, Xu H, Zuo Z. Dynamic Physiological Responses of Cinnamomum camphora with Monoterpene Protection under High Temperature Shock. Forests. 2023; 14(10):2005. https://doi.org/10.3390/f14102005
Chicago/Turabian StyleWang, Yingying, Qixia Qian, Haozhe Xu, and Zhaojiang Zuo. 2023. "Dynamic Physiological Responses of Cinnamomum camphora with Monoterpene Protection under High Temperature Shock" Forests 14, no. 10: 2005. https://doi.org/10.3390/f14102005





