Temporal Variability in Soil Greenhouse Gas Fluxes and Influencing Factors of a Primary Forest on the Eastern Qinghai-Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Greenhouse Gas Flux Measurements
2.3. Soil Microbial Community and Extracellular Enzyme Activity
2.4. Statistical Analysis
3. Results
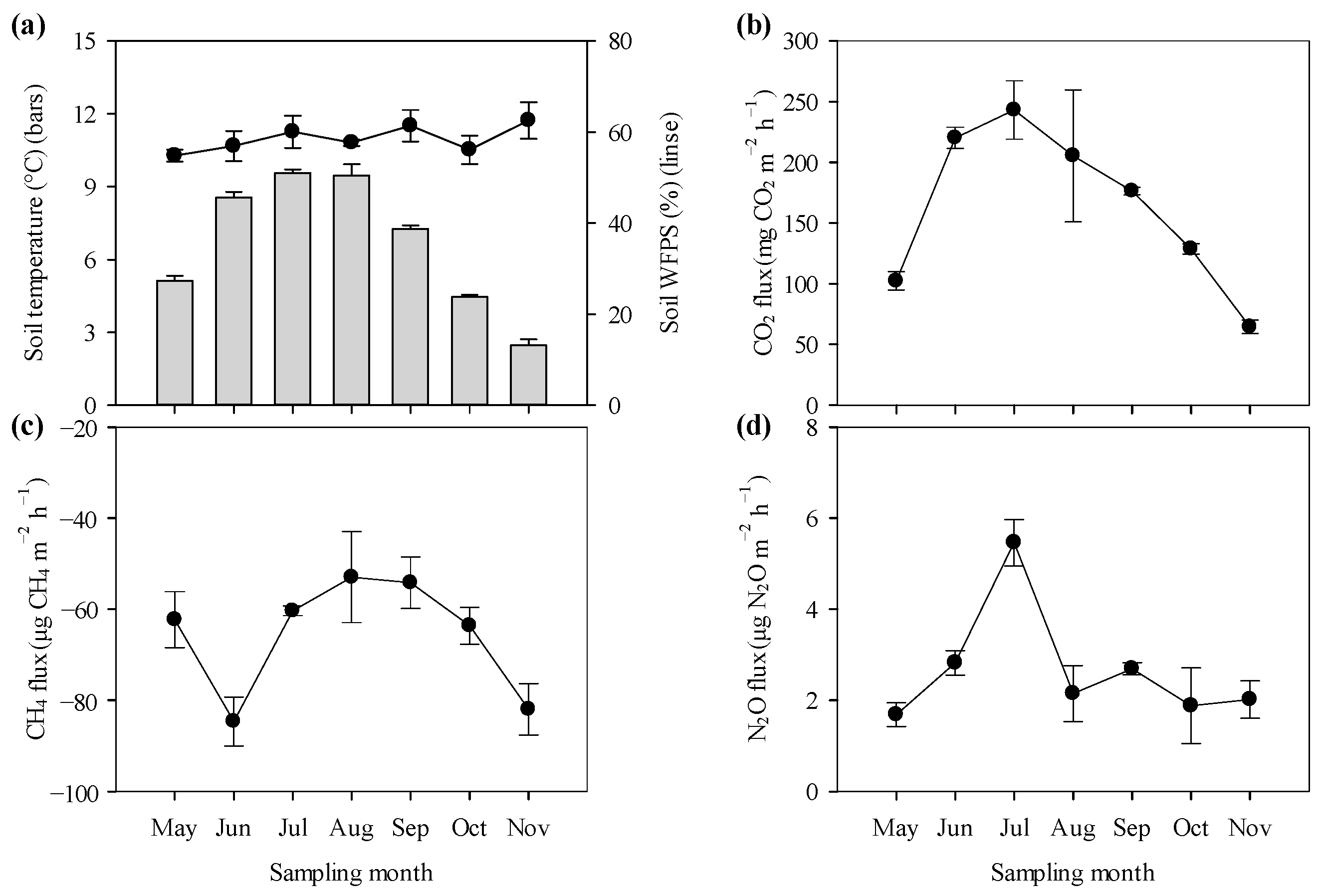
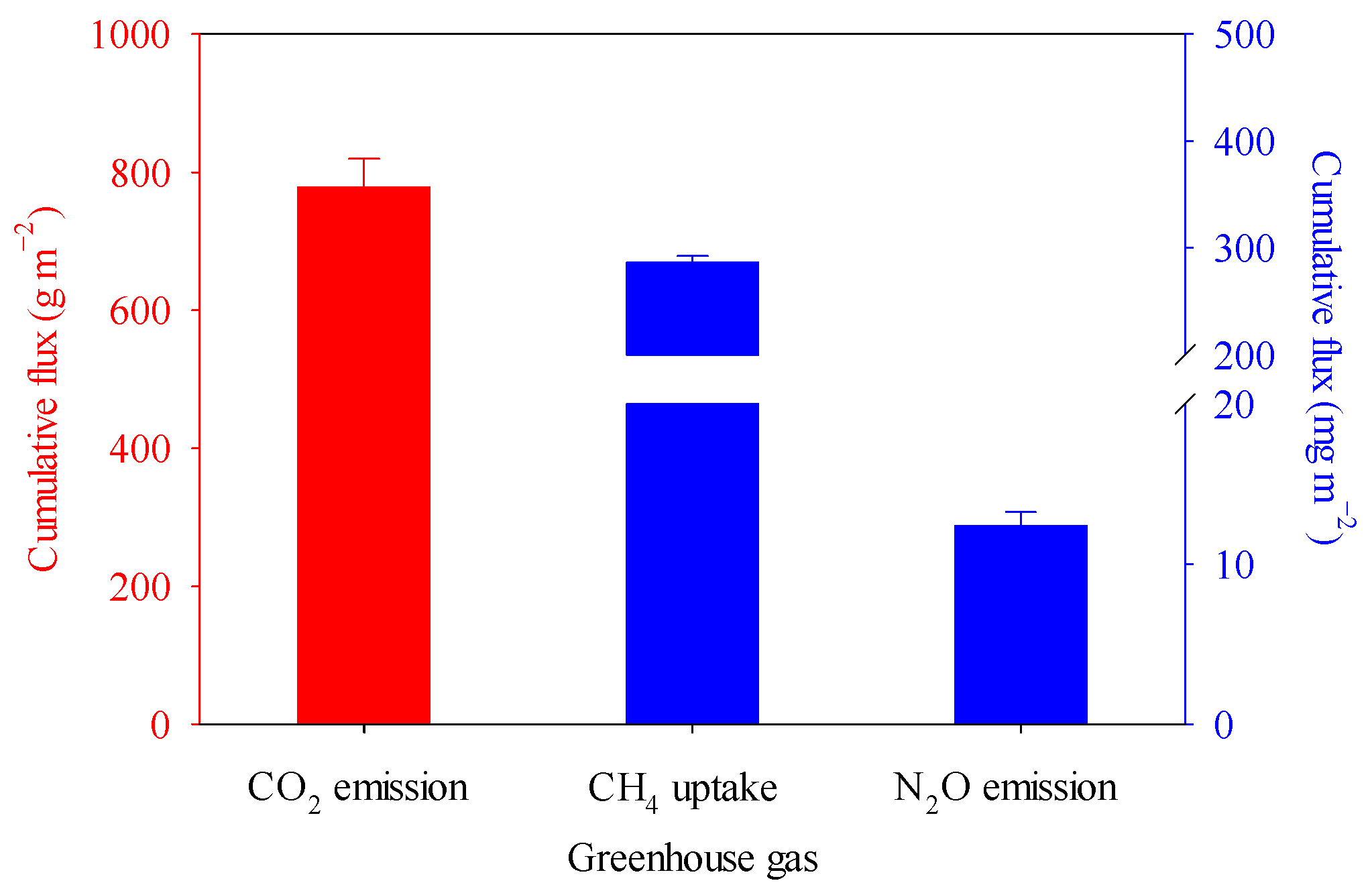
3.1. Soil Microclimate and Greenhouse Gas Fluxes
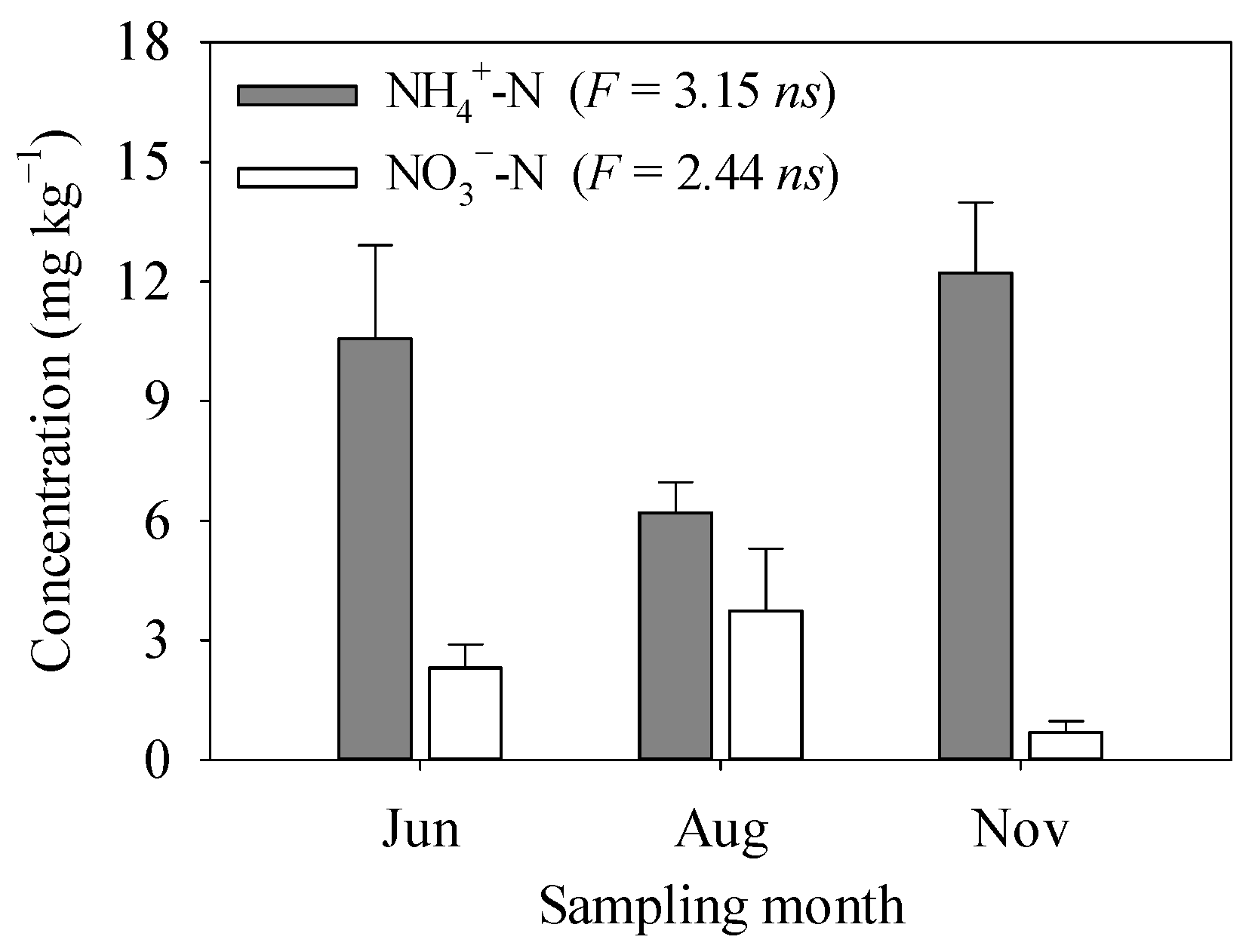
3.2. Soil N Availability
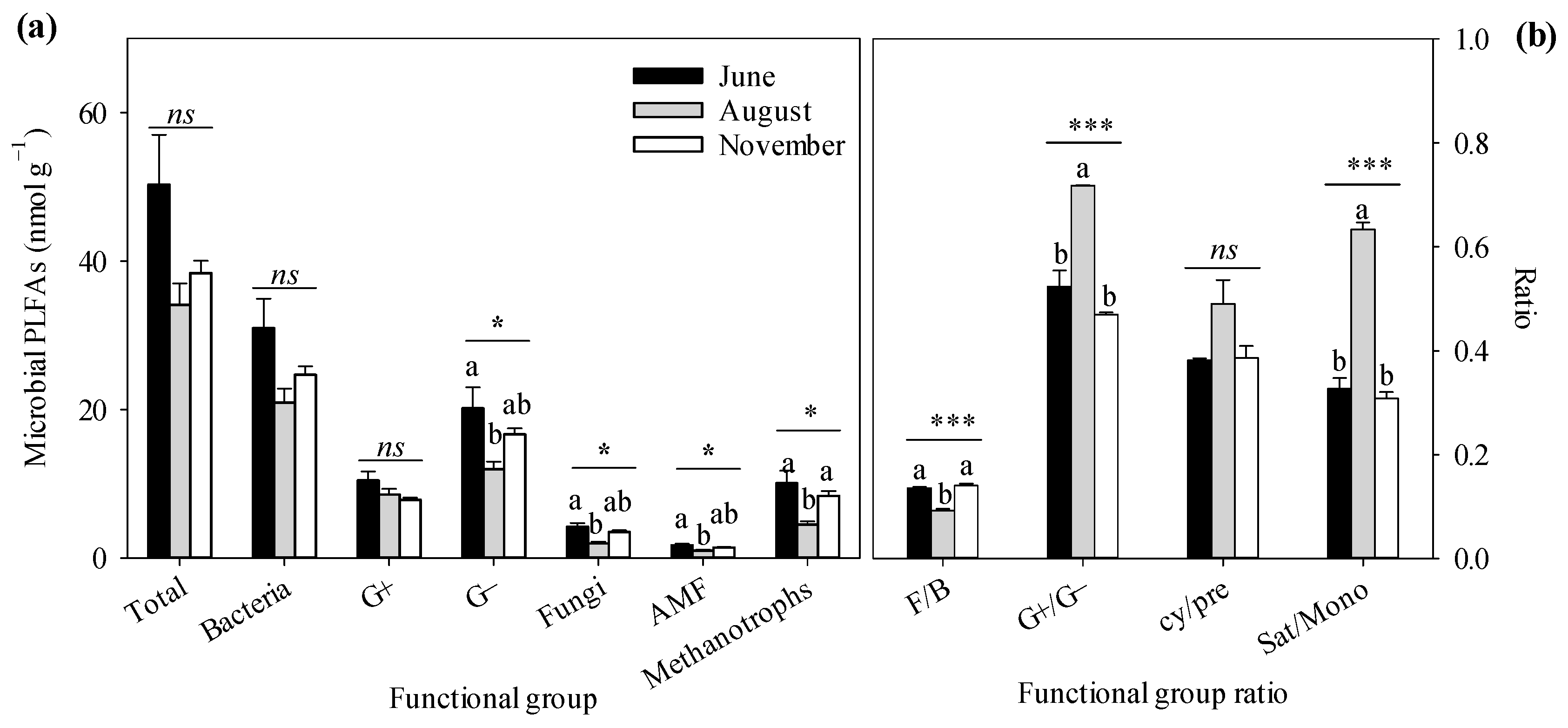
3.3. Soil Microbial Community
3.4. Soil Enzyme Activity
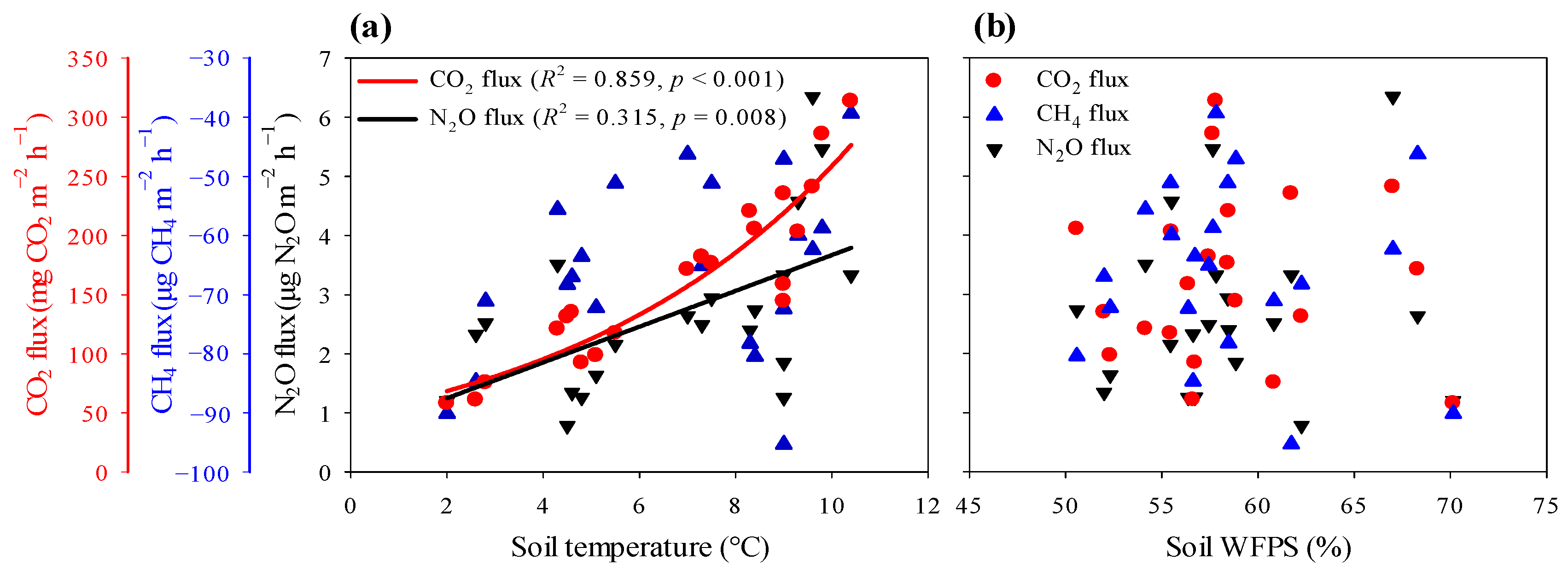
3.5. Key Factors Affecting Soil GHG Fluxes
4. Discussion
4.1. The Direction and Magnitude of GHG Fluxes
4.2. Environmental Controls on the Temporal Variability of GHG Fluxes
4.3. Temporal Dependence of Soil GHG Fluxes on Soil Microbial Attributes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dellasala, D.A.; Kormos, C.F.; Keith, H.; Mackey, B.; Young, V.; Rogers, B.; Mittermeier, R.A. Primary Forests Are Undervalued in the Climate Emergency. BioScience 2020, 70, 445. [Google Scholar] [CrossRef]
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Thom, D.; Golivets, M.; Edling, L.; Meigs, G.W.; Gourevitch, J.D.; Sonter, L.J.; Galford, G.L.; Keeton, W.S. The climate sensitivity of carbon, timber, and species richness covaries with forest age in boreal–temperate North America. Glob. Change Biol. 2019, 25, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Lennox, G.D.; Gardner, T.A.; Thomson, J.R.; Ferreira, J.; Berenguer, E.; Lees, A.C.; Mac Nally, R.; Aragão, L.E.O.C.; Ferraz, S.F.B.; Louzada, J.; et al. Second rate or a second chance? Assessing biomass and biodiversity recovery in regenerating Amazonian forests. Glob. Change Biol. 2018, 24, 5680–5694. [Google Scholar] [CrossRef] [PubMed]
- Luyssaert, S.; Schulze, E.D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Hadden, D.; Grelle, A. Net CO2 emissions from a primary boreo-nemoral forest over a 10 year period. For. Ecol. Manag. 2017, 398, 164–173. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Y.; Yu, G.; Zhou, G.; Zhang, L.; Li, K.; Tan, Z.; Sha, L. Seasonal and inter-annual variations in net ecosystem exchange of two old-growth forests in southern China. Agric. For. Meteorol. 2013, 182–183, 257–265. [Google Scholar] [CrossRef]
- Mazza, G.; Agnelli, A.E.; Cantiani, P.; Chiavetta, U.; Doukalianou, F.; Kitikidou, K.; Milios, E.; Orfanoudakis, M.; Radoglou, K.; Lagomarsino, A. Short-term effects of thinning on soil CO2, N2O and CH4 fluxes in Mediterranean forest ecosystems. Sci. Total Environ. 2018, 651, 713–724. [Google Scholar] [CrossRef]
- Hibbard, K.A.; Law, B.E.; Reichstein, M.; Sulzman, J. An analysis of soil respiration across northern hemisphere temperate ecosystems. Biogeochemistry 2005, 73, 29–70. [Google Scholar] [CrossRef]
- Stielstra, C.M.; Lohse, K.A.; Chorover, J.; McIntosh, J.C.; Barron-Gafford, G.A.; Perdrial, J.N.; Litvak, M.; Barnard, H.R.; Brooks, P.D. Climatic and landscape influences on soil moisture are primary determinants of soil carbon fluxes in seasonally snow-covered forest ecosystems. Biogeochemistry 2015, 123, 447–465. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Scott, R.L.; Jenerette, G.D.; Huxman, T.E. The relative controls of temperature, soil moisture, and plant functional group on soil CO2 efflux at diel, seasonal, and annual scales. J. Geophys. Res. Biogeosci. 2011, 116, G01023. [Google Scholar] [CrossRef]
- Han, M.; Zhu, B. Changes in soil greenhouse gas fluxes by land use change from primary forest. Glob. Change Biol. 2020, 26, 2656–2667. [Google Scholar] [CrossRef] [PubMed]
- Myhre, G.; Shindell, D.; Breon, F.; Collins, W.; Fuglesttvedt, J.; Huang, J.; Koch, D.; Lamarque, J.; Lee, D.; Mendoza, B.; et al. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T., Plattner, G.-K., Tignor, M., Allen, S., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 659–740. [Google Scholar]
- Perez-Quezada, J.F.; Urrutia, P.; Olivares-Rojas, J.; Meijide, A.; Sánchez-Cañete, E.P.; Gaxiola, A. Long term effects of fire on the soil greenhouse gas balance of an old-growth temperate rainforest. Sci. Total Environ. 2020, 755, 142442. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.C.; Allen, D.E. Greenhouse gas fluxes from natural ecosystems. Aust. J. Bot. 2008, 56, 369–407. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A.; Ullah, S.; Moore, T.R. Carbon dioxide, methane, and nitrous oxide exchanges in an age-sequence of temperate pine forests. Glob. Change Biol. 2010, 16, 2198–2212. [Google Scholar] [CrossRef]
- Stefaner, K.; Ghosh, S.; Yusof, M.L.M.; Ibrahim, H.; Leitgeb, E.; Schindlbacher, A.; Kitzler, B. Soil greenhouse gas fluxes from a humid tropical forest and differently managed urban parkland in Singapore. Sci. Total Environ. 2021, 786, 147305. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; Chotte, J.-L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Change Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Wanyama, I.; Pelster, D.E.; Butterbach-Bahl, K.; Verchot, L.V.; Martius, C.; Rufino, M.C. Soil carbon dioxide and methane fluxes from forests and other land use types in an African tropical montane region. Biogeochemistry 2019, 143, 171–190. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, J.; Ji, H.; Bai, X.; Lin, Y.; Zhang, Y.; Sha, L.; Liu, Y.; Song, Q.; Dörsch, P.; et al. Topography-related controls on N2O emission and CH4 uptake in a tropical rainforest catchment. Sci. Total Environ. 2021, 775, 145616. [Google Scholar] [CrossRef]
- Duan, M.; Li, A.; Wu, Y.; Zhao, Z.; Peng, C.; DeLuca, T.H.; Sun, S. Differences of soil CO2 flux in two contrasting subalpine ecosystems on the eastern edge of the Qinghai-Tibetan Plateau: A four-year study. Atmos. Environ. 2019, 198, 166–174. [Google Scholar] [CrossRef]
- McCalmont, J.P.; Rowe, R.; Elias, D.; Whitaker, J.; McNamara, N.P.; Donnison, I.S. Soil nitrous oxide flux following land-use reversion from Miscanthus and SRC willow to perennial ryegrass. GCB Bioenergy 2018, 10, 914–929. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Peng, C.; Chen, H.; Wang, H.; Zhu, Q.; Yang, Y.; Zhang, J.; Yang, W. Nitrous oxide emissions from three temperate forest types in the Qinling Mountains, China. J. For. Res. 2019, 30, 1417–1427. [Google Scholar] [CrossRef]
- Rowlings, D.W.; Grace, P.R.; Kiese, R.; Weier, K.L. Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest. Glob. Change Biol. 2012, 18, 726–738. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Han, M.; Wang, W.; Peng, C.; Jin, J.; Song, X.; Yu, S. A review of the mechanisms and controlling factors of methane dynamics in forest ecosystems. For. Ecol. Manag. 2020, 455, 117702. [Google Scholar] [CrossRef]
- Cao, R.; Yang, W.; Chang, C.; Wang, Z.; Wang, Q.; Li, H.; Tan, B. Differential seasonal changes in soil enzyme activity along an altitudinal gradient in an alpine-gorge region. Appl. Soil Ecol. 2021, 166, 104078. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.; Zhu, W.; Gundersen, P.; Fang, Y.; Li, D.; Wang, H. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Change Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Sun, J.; Xia, Z.; He, T.; Dai, W.; Peng, B.; Liu, J.; Gao, D.; Jiang, P.; Han, S.; Bai, E. Ten years of elevated CO2 affects soil greenhouse gas fluxes in an open top chamber experiment. Plant Soil 2017, 420, 435–450. [Google Scholar] [CrossRef]
- Liu, X.P.; Zhang, W.J.; Hu, C.S.; Tang, X.G. Soil greenhouse gas fluxes from different tree species on Taihang Mountain, North China. Biogeosciences 2014, 11, 1649–1666. [Google Scholar] [CrossRef]
- Yamulki, S.; Forster, J.; Xenakis, G.; Ash, A.; Brunt, J.; Perks, M.; Morison, J.I.L. Effects of clear-fell harvesting on soil CO2, CH4, and N2O fluxes in an upland Sitka spruce stand in England. Biogeosciences 2021, 18, 4227–4241. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M.; Gunapala, N.; Graham, K.J. Determinants of Soil Microbial Communities: Effects of Agricultural Management, Season, and Soil Type on Phospholipid Fatty Acid Profiles. Microb. Ecol. 1998, 36, 1–12. [Google Scholar] [CrossRef]
- Hackl, E.; Pfeffer, M.; Donat, C.; Bachmann, G.; Zechmeisterboltenstern, S. Composition of the microbial communities in the mineral soil under different types of natural forest. Soil Biol. Biochem. 2005, 37, 661–671. [Google Scholar] [CrossRef]
- Yang, J.; Blondeel, H.; Meeussen, C.; Govaert, S.; Vangansbeke, P.; Boeckx, P.; Lenoir, J.; Orczewska, A.; Ponette, Q.; Hedwall, P.-O.; et al. Forest density and edge effects on soil microbial communities in deciduous forests across Europe. Appl. Soil Ecol. 2022, 179, 104586. [Google Scholar] [CrossRef]
- Smith, A.P.; Marín-Spiotta, E.; Balser, T. Successional and seasonal variations in soil and litter microbial community structure and function during tropical postagricultural forest regeneration: A multiyear study. Glob. Change Biol. 2015, 21, 3532–3547. [Google Scholar] [CrossRef] [PubMed]
- Pollierer, M.M.; Ferlian, O.; Scheu, S. Temporal dynamics and variation with forest type of phospholipid fatty acids in litter and soil of temperate forests across regions. Soil Biol. Biochem. 2015, 91, 248–257. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Raiesi, F.; Salek-Gilani, S. The potential activity of soil extracellular enzymes as an indicator for ecological restoration of rangeland soils after agricultural abandonment. Appl. Soil Ecol. 2018, 126, 140–147. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, S.; Du, T.; Yu, X.; Chen, L. A study on forest soil respiration in Chinese fir plantation. Acta Agric. Univ. Jiangxiensis 2005, 27, 580–584. [Google Scholar]
- Fang, J.Y.; Liu, G.H.; Zhu, B.; Wang, X.K.; Liu, S.H. Carbon budgets of three temperate forest ecosystems in Dongling Mt., Beijing, China. Sci. China Ser. D Earth Sci. 2007, 50, 92–101. [Google Scholar] [CrossRef]
- Wei, W.; Weile, C.; Shaopeng, W. Forest soil respiration and its heterotrophic and autotrophic components: Global patterns and responses to temperature and precipitation. Soil Biol. Biochem. 2010, 42, 1236–1244. [Google Scholar] [CrossRef]
- Tan, Z.-H.; Zhang, Y.-P.; Liang, N.; Song, Q.-H.; Liu, Y.-H.; You, G.-Y.; Li, L.-H.; Yu, L.; Wu, C.-S.; Lu, Z.-Y.; et al. Soil respiration in an old-growth subtropical forest: Patterns, components, and controls. J. Geophys. Res. Atmos. 2013, 118, 2981–2990. [Google Scholar] [CrossRef]
- Chen, B.; Liu, S.; Ge, J.; Chu, J. Annual and seasonal variations of Q10 soil respiration in the sub-alpine forests of the Eastern Qinghai-Tibet Plateau, China. Soil Biol. Biochem. 2010, 42, 1735–1742. [Google Scholar] [CrossRef]
- Monson, R.K.; Burns, S.P.; Williams, M.W.; Delany, A.C.; Weintraub, M.; Lipson, D.A. The contribution of beneath-snow soil respiration to total ecosystem respiration in a high-elevation, subalpine forest. Glob. Biogeochem. Cycles 2006, 20, GB3030. [Google Scholar] [CrossRef]
- Liu, L.; Estiarte, M.; Peñuelas, J. Soil moisture as the key factor of atmospheric CH4 uptake in forest soils under environmental change. Geoderma 2019, 355, 113920. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Gatica, G.; Fernández, M.E.; Juliarena, M.P.; Gyenge, J. Environmental and anthropogenic drivers of soil methane fluxes in forests: Global patterns and among-biomes differences. Glob. Change Biol. 2020, 26, 6604–6615. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Yang, G.; Zhu, D.; Tian, J.; Tian, L.; Kang, X.; et al. Soil methane uptake by grasslands and forests in China. Soil Biol. Biochem. 2014, 74, 70–81. [Google Scholar] [CrossRef]
- Fang, H.J.; Yu, G.R.; Cheng, S.L.; Zhu, T.H.; Wang, Y.S.; Yan, J.H.; Wang, M.; Cao, M.; Zhou, M. Effects of multiple environmental factors on CO2 emission and CH4 uptake from old-growth forest soils. Biogeosciences 2010, 7, 395–407. [Google Scholar] [CrossRef]
- MacDonald, J.A.; Skiba, U.; Sheppard, L.J.; Ball, B.; Roberts, J.D.; Smith, K.A.; Fowler, D. The effect of nitrogen deposition and seasonal variability on methane oxidation and nitrous oxide emission rates in an upland spruce plantation and moorland. Atmos. Environ. 1997, 31, 3693–3706. [Google Scholar] [CrossRef]
- Erickson, H.E.; Perakis, S.S. Soil fluxes of methane, nitrous oxide, and nitric oxide from aggrading forests in coastal Oregon. Soil Biol. Biochem. 2014, 76, 268–277. [Google Scholar] [CrossRef]
- Morishita, T.; Sakata, T.; Takahashi, M.; Ishizuka, S.; Mizoguchi, T.; Inagaki, Y.; Terazawa, K.; Sawata, S.; Igarashi, M.; Yasuda, H.; et al. Methane uptake and nitrous oxide emission in Japanese forest soils and their relationship to soil and vegetation types. Soil Sci. Plant Nutr. 2007, 53, 678–691. [Google Scholar] [CrossRef]
- Ishizuka, S.; Tsuruta, H.; Murdiyarso, D. An intensive field study on CO2, CH4, and N2O emissions from soils at four land-use types in Sumatra, Indonesia. Glob. Biogeochem. Cycles 2002, 16, 1049. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.; Tian, X.; Wang, Y.; Cong, J.; Cui, Z. Evaluation of variation in background nitrous oxide emissions: A new global synthesis integrating the impacts of climate, soil, and management conditions. Glob. Change Biol. 2021, 28, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, D.; Cheng, R.; Yang, H.; Wu, J.; Shi, Z. Soil-atmosphere exchange of greenhouse gases from typical subalpine forests on the eastern Qinghai-Tibetan Plateau: Effects of forest regeneration patterns. Land Degrad. Dev. 2020, 31, 2019–2032. [Google Scholar] [CrossRef]
- Bowden, R.D.; Castro, M.S.; Melillo, J.M.; Steudler, P.A.; Aber, J.D. Fluxes of greenhouse gases between soils and the atmosphere in a temperate forest following a simulated hurricane blowdown. Biogeochemistry 1993, 21, 61–71. [Google Scholar] [CrossRef]
- Barrena, I.; Menéndez, S.; Duñabeitia, M.; Merino, P.; Stange, C.F.; Spott, O.; González-Murua, C.; Estavillo, J.M. Greenhouse gas fluxes (CO2, N2O and CH4) from forest soils in the Basque Country: Comparison of different tree species and growth stages. For. Ecol. Manag. 2013, 310, 600–611. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Mo, J.; Zhang, T. Soil-atmosphere exchange of greenhouse gases in subtropical plantations of indigenous tree species. Plant Soil 2010, 335, 213–227. [Google Scholar] [CrossRef]
- Martins, C.S.C.; Nazaries, L.; Delgado-Baquerizo, M.; Macdonald, C.A.; Anderson, I.C.; Hobbie, S.E.; Venterea, R.T.; Reich, P.B.; Singh, B.K. Identifying environmental drivers of greenhouse gas emissions under warming and reduced rainfall in boreal–temperate forests. Funct. Ecol. 2017, 31, 2356–2368. [Google Scholar] [CrossRef]
- Chen, Q.; Long, C.; Chen, J.; Cheng, X. Differential response of soil CO2, CH4, and N2O emissions to edaphic properties and microbial attributes following afforestation in central China. Glob. Change Biol. 2021, 27, 5657–5669. [Google Scholar] [CrossRef]
- Wu, Q.; Lian, R.; Bai, M.; Bao, J.; Liu, Y.; Li, S.; Liang, C.; Qin, H.; Chen, J.; Xu, Q. Biochar co-application mitigated the stimulation of organic amendments on soil respiration by decreasing microbial activities in an infertile soil. Biol. Fertil. Soils 2021, 57, 793–807. [Google Scholar] [CrossRef]
- Ali, R.S.; Poll, C.; Kandeler, E. Dynamics of soil respiration and microbial communities: Interactive controls of temperature and substrate quality. Soil Biol. Biochem. 2018, 127, 60–70. [Google Scholar] [CrossRef]
- Maithani, K.; Tripathi, R.S.; Arunachalam, A.; Pandey, H.N. Seasonal dynamics of microbial biomass C, N and P during regrowth of a disturbed subtropical humid forest in north-east India. Appl. Soil Ecol. 1996, 4, 31–37. [Google Scholar] [CrossRef]
- Tietema, A.; van der Lee, G.E.M.; Bouten, W.; Rappoldt, C.; Verstraten, J.M. The production of N2O in Douglas fir litter as affected by anoxic conditions within litter particles and pores. Soil Biol. Biochem. 2007, 39, 239–248. [Google Scholar] [CrossRef]
- Stange, C.F.; Spott, O.; Arriaga, H.; Menéndez, S.; Estavillo, J.M.; Merino, P. Use of the inverse abundance approach to identify the sources of NO and N2O release from Spanish forest soils under oxic and hypoxic conditions. Soil Biol. Biochem. 2013, 57, 451–458. [Google Scholar] [CrossRef]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003, 54, 779–791. [Google Scholar] [CrossRef]
- Duan, B.; Cai, T.; Man, X.; Xiao, R.; Gao, M.; Ge, Z.; Mencuccini, M. Different variations in soil CO2, CH4, and N2O fluxes and their responses to edaphic factors along a boreal secondary forest successional trajectory. Sci. Total Environ. 2022, 838, 155983. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Duan, C.; Wu, H.; Luo, X.; Han, L. Effects of changes in throughfall on soil GHG fluxes under a mature temperate forest, northeastern China. J. Environ. Manag. 2021, 294, 112950. [Google Scholar] [CrossRef]
- Lavoie, M.; Kellman, L.; Risk, D. The effects of clear-cutting on soil CO2, CH4, and N2O flux, storage and concentration in two Atlantic temperate forests in Nova Scotia, Canada. For. Ecol. Manag. 2013, 304, 355–369. [Google Scholar] [CrossRef]
- Kim, Y.; Ueyama, M.; Nakagawa, F.; Tsunogai, U.; Harazono, Y.; Tanaka, N. Assessment of winter fluxes of CO2 and CH4 in boreal forest soils of central Alaska estimated by the profile method and the chamber method: A diagnosis of methane emission and implications for the regional carbon budget. Tellus B Chem. Phys. Meteorol. 2007, 59, 223–233. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, F.; Yin, H.; Liu, Q. Winter soil CO2 efflux in two contrasting forest ecosystems on the eastern Tibetan Plateau, China. J. For. Res. 2015, 26, 679–686. [Google Scholar] [CrossRef][Green Version]
- Peng, B.; Sun, J.; Liu, J.; Dai, W.; Sun, L.; Pei, G.; Gao, D.; Wang, C.; Jiang, P.; Bai, E. N2O emission from a temperate forest soil during the freeze-thaw period: A mesocosm study. Sci. Total Environ. 2019, 648, 350–357. [Google Scholar] [CrossRef]
- Wu, Y.F.; Whitaker, J.; Toet, S.; Bradley, A.; Davies, C.A.; McNamara, N.P. Diurnal variability in soil nitrous oxide emissions is a widespread phenomenon. Glob. Change Biol. 2021, 27, 4950–4966. [Google Scholar] [CrossRef]
- Wang, Q.; He, T.; Wang, S.; Liu, L. Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agric. For. Meteorol. 2013, 178–179, 152–160. [Google Scholar] [CrossRef]

| Sampling Month | AG | BG | NAG | LAP | AP | GMea |
|---|---|---|---|---|---|---|
| June | 11.20 ± 1.04 | 244.34 ± 60.48 | 114.91 ± 12.59 | 89.96 ± 3.40a | 883.65 ± 87.63 | 118.84 ± 18.09 |
| August | 10.45 ± 1.46 | 269.12 ± 40.48 | 146.97 ± 33.15 | 51.22 ± 4.57b | 1098.54 ± 157.62 | 116.24 ± 18.29 |
| November | 9.44 ± 1.68 | 201.49 ± 7.00 | 81.40 ± 6.57 | 64.69 ± 5.84b | 673.83 ± 53.14 | 91.76 ± 8.74 |
| One-way ANOVA | ||||||
| F-value | 0.39 ns | 0.66 ns | 2.48 ns | 17.44 ** | 3.83 ns | 2.72 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Luo, D.; Xu, G.; Wu, J.; Feng, Q.; Shi, Z. Temporal Variability in Soil Greenhouse Gas Fluxes and Influencing Factors of a Primary Forest on the Eastern Qinghai-Tibetan Plateau. Forests 2023, 14, 2255. https://doi.org/10.3390/f14112255
Liu S, Luo D, Xu G, Wu J, Feng Q, Shi Z. Temporal Variability in Soil Greenhouse Gas Fluxes and Influencing Factors of a Primary Forest on the Eastern Qinghai-Tibetan Plateau. Forests. 2023; 14(11):2255. https://doi.org/10.3390/f14112255
Chicago/Turabian StyleLiu, Shun, Da Luo, Gexi Xu, Jiamei Wu, Qiuhong Feng, and Zuomin Shi. 2023. "Temporal Variability in Soil Greenhouse Gas Fluxes and Influencing Factors of a Primary Forest on the Eastern Qinghai-Tibetan Plateau" Forests 14, no. 11: 2255. https://doi.org/10.3390/f14112255
APA StyleLiu, S., Luo, D., Xu, G., Wu, J., Feng, Q., & Shi, Z. (2023). Temporal Variability in Soil Greenhouse Gas Fluxes and Influencing Factors of a Primary Forest on the Eastern Qinghai-Tibetan Plateau. Forests, 14(11), 2255. https://doi.org/10.3390/f14112255






