Influence of Chrysoporthe deuterocubensis Canker Disease on the Chemical Properties and Durability of Eucalyptus urograndis against Wood Rotting Fungi and Termite Infestation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of Chemical Properties
2.1.1. Wet Chemical Analysis
2.1.2. Fourier Transform Infrared (FTIR) Analysis
2.2. Evaluation of Biological Properties
2.2.1. Decay Resistance
2.2.2. Termite Resistance
2.3. Statistical Analysis
3. Results and Discussion
3.1. Chemical Properties of Healthy and Infected Wood
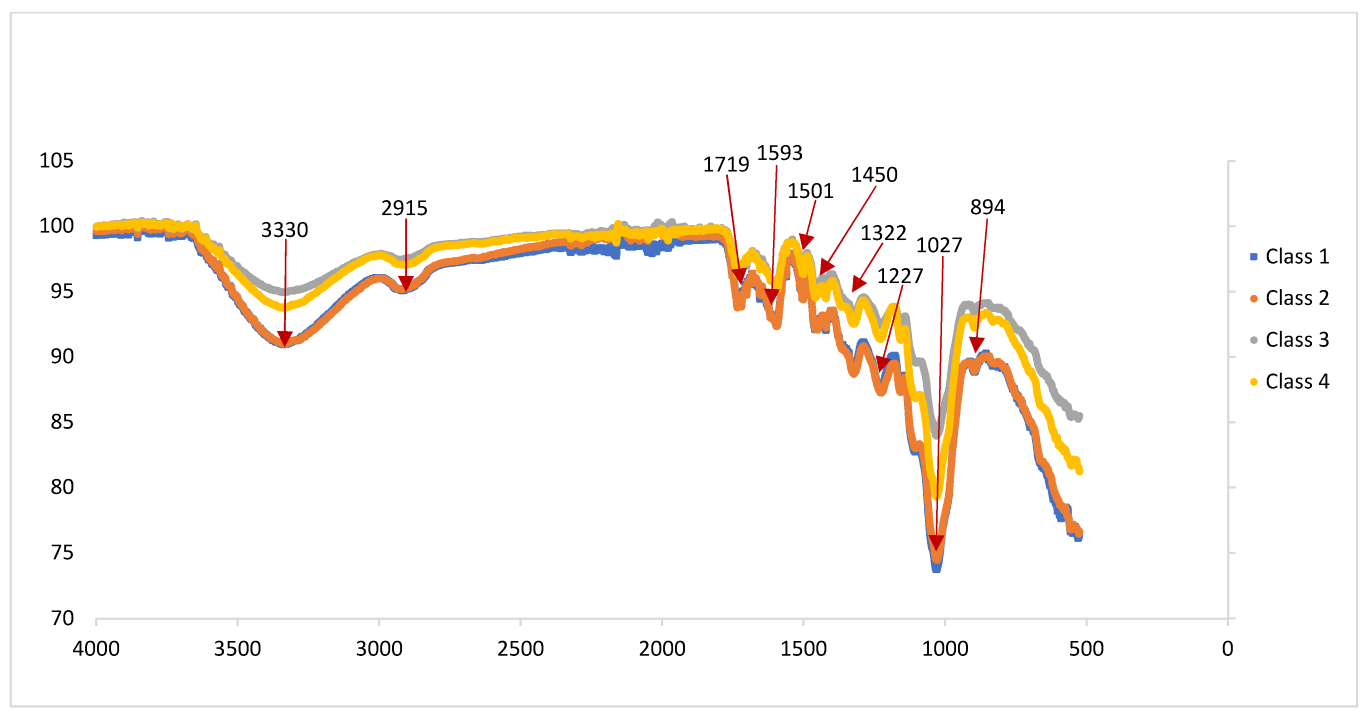
3.2. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.3. Durability
3.3.1. Fungal Decay
3.3.2. Termite Attack
3.4. Correlation between Infection Class and Weight Loss against Fungal Decay and Termite Attacks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, J.; Arnold, R.; Ren, S.; Jiang, Y.; Lu, W.; Peng, Y.; Xie, Y. Veneer grades, recoveries, and values from 5-year-old eucalypt clones. Ann. For. Sci. 2013, 70, 417–428. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Hishamuddin, M.S. Trees Diseases and Disorders in Urban Forests of Peninsular Malaysia. In Urban Forestry and Arboriculture in Malaysia “An Interdisciplinary Research Perspective”; Maruthaveeran, S., Chen, W.Y., Morgenroth, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; Volume 1, pp. 83–104. [Google Scholar]
- Alves, I.C.N.; Gomide, J.L.; Colodette, J.L.; Silva, H.D. Technological characterization of Eucalyptus benthamii wood for kraftpulp production. Cienc. Florestal. 2011, 21, 167–174. [Google Scholar] [CrossRef]
- Bassa, A.G.M.C.; Francides, G.D.S.J.; Sacon, V.M. Mixtures of Eucalyptus grandis x Eucalyptus urophylla and Pinus taeda woodchips for kraft pulp production by Lo-Solids process. Scientia Forestalis. 2007, 75, 19–30. [Google Scholar]
- Nakabonge, G.; Roux, J.; Gryzenhout, M.; Wingfield, M.J. Distribution of Chrysoporthe canker pathogens on Eucalyptus and Syzygium spp. in eastern and southern Africa. Plant Dis. 2006, 90, 734–740. [Google Scholar] [CrossRef]
- Leonardi, G.D.A.; Carlos, N.A.; Mazzafera, P.; Balbuena, T.S. Eucalyptus urograndis stem proteome is responsive to short-term cold stress. Genet. Mol. Biol. 2015, 38, 191–198. [Google Scholar] [CrossRef]
- Lee, S.H.; Lum, W.C.; Antov, P.; Kristak, L.; Paridah, M.T. Engineering Wood Products from Eucalyptus spp. Adv. Mater. Sci. Eng. 2022, 2022, 8000780. [Google Scholar]
- Carvalho, A.M. The valuation of Eucalyptus grandis x Eucalyptus urophylla hybrid wood through the production of small dimension sawn wood, pulpwood and fuelwood. Sci. Forestalis. 2000, 59, 61–76. [Google Scholar]
- Jiang, X.M.; Ye, K.L.; Lu, J.X.; Zhao, Y.K.; Yin, Y.F. Guide on Utilisation of Eucalyptus and Acacia Plantations in China for Solid Wood Products. The Final Technical Report for: Improved and Diversified Use of Tropical Plantation Timbers in China to Supplement Diminishing Supplies from Natural Forests “ITTO ProjectPD69/01 REV.2”; Science Press: Beijing, China, 2007; p. 181. [Google Scholar]
- Labate, C.A.; de Assis, T.F.; Oda, S.; Mello, E.J.; Mori, E.S.; de Moraes, M.L.T.; Barrueto, L.P.; Gonzalez, E.R.; Alfenas, A.C.; Edival, A.; et al. Eucalyptus. In Compendium of Transgenic Crop Plants: Transgenic Forest Tree Species; Cole, C., Hall, T.C., Eds.; Wiley: New York, NY, USA, 2008; Volume 9, pp. 35–99. ISBN 978-1-405-16924-0. [Google Scholar]
- Brigatti, R.A.; Ferreira, M.; Silva, A.P.; Freitas, M. Comparative study of the behavior of some Eucalyptus spp. hybrids. Silvicultura 1983, 32, 761–764. [Google Scholar]
- Ikemori, Y.K.; Campinhos, E. Eucalyptus urophylla x Eucalyptus grandis seed production by open pollination—Preliminaries results. Silvicultura 1983, 8, 306–308. [Google Scholar]
- Bertolucci, F.; Rezende, G.; Penchel, R. Production and use of eucalyptus hybrids. Silvicultura 1995, 51, 12–16. [Google Scholar]
- Sembiring, N.; Napitupulu, H.L.; Sembiring, M.T.; Ishak, A.; Gunawan, H.A. Fulfilling Eucalyptus raw materials for pulp and paper production plants. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 912, p. 012008. [Google Scholar] [CrossRef]
- Scanavaca, L., Jr.; Garcia, J.N. Yield in sawed wood of Eucalyptus urophylla. Sci. For. 2003, 63, 32–43. [Google Scholar]
- Ahmad, Z.Y. Planting of Eucalyptus in Malaysia. Acta Sci. Agric. 2020, 4, 139–140. [Google Scholar]
- Ahmad, Z.Y.; Hassan, N.H.; Loon, N.T.; Heng, L.H.; Zorkarnain, F.A. Comparing the early growth performance of plantation–grown Eucalyptus hybrid and Eucalyptus pellita, South Johore, Peninsular Malaysia. WJARR 2020, 6, 234–238. [Google Scholar]
- Muhammad, S.A.R. Isolation and Identification of Causal Disease of Eucalyptus pellita. Bachelor’s Thesis, Universiti of Malaysia, Sarawak, Kota Samarahan, Malaysia, 2012. [Google Scholar]
- Suzuki, H.; Marincowitz, S.; Wingfield, B.D.; Wingfield, M.J. Genetic diversity and population structure of Chrysoporthe deuterocubensis isolates from Melastoma and Eucalyptus in Malaysia and Indonesia. For. Pathol. 2022, 52, 12762. [Google Scholar] [CrossRef]
- Rauf, M.R.B.A.; McTaggart, A.R.; Marincowitz, S.; Barnes, I.; Japarudin, Y.; Wingfield, M.J. Pathogenicity of Chrysoporthe deuterocubensis and Myrtoporthe bodenii gen. et sp. nov. on Eucalyptus in Sabah, Malaysia. Australas. Plant Pathol. 2019, 49, 53–64. [Google Scholar] [CrossRef]
- Gezahgne, A. Main Diseases of Eucalyptus Species in Ethiopia. In Eucalyptus Species Management, History, Status and Trends in Ethiopia; Wubalem, G., Lopez, T., Eds.; Technical University of Madrid: Madrid, Spain, 2010; pp. 351–369. [Google Scholar]
- Lee, S.S. Observations on the successes and failures of acacia plantations in Sabah and Sarawak and the way forward. J. Trop. For. Sci. 2018, 30, 468–475. [Google Scholar] [CrossRef]
- Forestry Economics and Policy Division. Global Review of Forest Pests and Diseases; Food and Agriculture Organization (FAO): Rome, Italy, 2009; p. 235. ISBN 978-92-5-106208-1. [Google Scholar]
- Dahali, R.; Md Tahir, P.; Roseley, A.S.M.; Hua, L.S.; Bakar, E.S.; Ashaari, Z.; Abdul Rauf, M.R.; Zainuddin, N.A.; Mansoor, N.S. Influence of Chrysoporthe deuterocubensis Canker Disease on the Physical and Mechanical Properties of Eucalyptus urograndis. Forests 2021, 12, 639. [Google Scholar] [CrossRef]
- Dahali, R.; Lee, S.H.; Md Tahir, P.; Bakar, E.S.; Muhammad Roseley, A.S.; Ibrahim, S.A.; Mohd Yusof, N.; Mohammad Suffian James, R. Tahir, P.; Bakar, E.S.; Muhammad Roseley, A.S.; Ibrahim, S.A.; Mohd Yusof, N.; Mohammad Suffian James, R. Influence of Chrysoporthe deuterocubensis Canker Disease on the Machining Properties of Eucalyptus urograndis. Forests 2022, 13, 1366. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Goncalves, J.L.D.M.; Sparovek, G. Koppen’s climate classification map for Brazil. Meteorol. Z. 2014, 22, 711–728. [Google Scholar] [CrossRef]
- TAPPI Standard, T 257 cm-02; Sampling and Preparing Wood for Chemical Analysis. TAPPI: Atlanta, GA, USA, 2002.
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- ASTM Standard D2017; Standard Test Method of Accelerated Laboratory Test of Natural Decay Resistance of Woods. ASTM International: West Conshohocken, PA, USA, 2012.
- Bakar, E.S.; Hao, J.; Ashaari, Z. Durability of phenolic-resin-treated oil palm wood against subterranean termites a white-rot fungus. Int. Biodeterior. Biodegrad. 2013, 85, 126–130. [Google Scholar] [CrossRef]
- AWPA Standard E1-09; Standard Method for Laboratory Evaluation to Determine Resistance to Subterranean Termites. American Wood Protection Association: Birmingham, AL, USA, 2012.
- Arinana, A.; Philippines, I.; Koesmaryono, Y.; Sulaeha, S.; Maharani, Y.; Indarwatmi, M. The daytime indoor and outdoor temperatures of the subterranean termite Coptotermes curvignathus Holmgren (Isoptera: Rhinotermitidae) tunnel. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 807, p. 022027. [Google Scholar] [CrossRef]
- Kuswanto, E.; Ahmad, I.; Dungani, R. Threat of subterranean termites attack in the Asian countries and their control: A review. Asian J. Appl. Sci. 2015, 8, 227–239. [Google Scholar] [CrossRef]
- Dahali, R.; Hua, L.S.; Ashaari, Z.; Bakar, E.S.; Ariffin, H.; San, K.P.; Bawon, P.; Salleh, Q.N. Durability of superheated steam-treated light red meranti (Shorea spp.) and kedondong (Canarium spp.) wood against white rot fungus and subterranean termite. Sustainability 2020, 12, 4431. [Google Scholar] [CrossRef]
- Anantharaju, T.; Kaur, G.; Gajalakshmi, S.; Abbasi, S.A. Sampling and identification of termites in Northeastern. Puducherry J. Entomol. Zool. Stud. 2014, 2, 225–230. [Google Scholar]
- Rilatupa, J. 2006 Kondisi komponen konstruksi bangunan tinggi dan hubungannya dengan karakteristik serangan rayap. J. Sains Teknol. EMAS 2006, 16, 71–86. [Google Scholar]
- SNI Standard 01.7207; Standard Method for Test of Resistance Wood and Wood Products against Wood Destroying Organisms “Ujian ketahanan kayu dan produk kayu terhadap organisme perusak kayu”. Indonesian National Standard (Standar Nasional Indonesia): Jakarta, Indonesia, 2006.
- Terzi, E.; Kartal, S.N.; Muin, M.; Hassanin, A.H.; Hamouda, T.; Kıilic, A.; Candan, Z. Biological Performance of Novel Hybrid Green Composites Produced from Glass Fibers and Jute Fabric Skin by the VARTM Process. BioResources 2018, 13, 662–677. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef]
- Ferrari, R.; Gautier, V.; Silar, P. Lignin degradation by ascomycetes Wood Degradation and Ligninolytic Fungi. Adv. Bot. Res. 2021, 9, 77–113. [Google Scholar]
- Savory, J.G.; Pinion, L.C. Chemical aspects of decay of beech wood by Chaetomium globosum. Int. J. Biol. Chem. Phys. Technol. Wood 1958, 12, 99–103. [Google Scholar]
- Andlar, M.; Rezic, T.; Mardetko, N.; Kracher, D.; Ludwig, R.; Santek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, J.; Paszczyoski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [Green Version]
- Kirk, T.K.; Farrell, R.L. Enzymatic “combustion”: The microbial degradation of lignin. Annu. Rev. Microbiol. 1987, 41, 465–505. [Google Scholar] [CrossRef]
- Mafia, R.G.; Ferreira, M.A.; Zauza, E.A.V.; Silva, J.F.; Colodette, J.L.; Alfenas, A.C. Impact of Ceratocystis wilt on Eucalyptus tree growth and cellulose pulp yield. For. Pathol. 2013, 43, 379–385. [Google Scholar] [CrossRef]
- Foelkel, C.E.B.; Zvinakevicius, C.; Andrade, J.M. A qualidade do eucalipto. Silvicultura 1978, 2, 53–62. [Google Scholar]
- Pereira, B.L.; Carneiro, A.C.; Carvalho, A.M.; Colodette, J.L.; Oliveira, A.C.; Fontes, M.P. Influence of Chemical Composition of Eucalyptus Wood on Gravimetric Yield and Charcoal Properties. Bioresources 2013, 8, 4574–4592. [Google Scholar] [CrossRef]
- Rowell, R.M.; Pettersen, R.; Han, J.S.; Rowell, J.S.; Tshabalala, M.A. Cell wall chemistry, In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Shebani, A.N.; Van Reenen, A.J.; Meincken, M. The effect of wood extractives on the thermal stability of different wood species. Thermochim. Acta 2008, 471, 43–50. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood, Chemistry, Ultrastructure, Reactions; Walter de Gruyter: New York, NY, USA, 1984. [Google Scholar]
- Stenius, P. Paper Making Science and Technology: Forest Products Chemistry (Book 3); Fapet: Helsinki, Finland, 2000. [Google Scholar]
- Gunduz, G.; Oral, M.A.; Akyuz, M.; Aydemir, D.; Yaman, B.; Asik, N.; Bulbul, A.S.; Allahverdiyev, S. Physical, morphological properties and raman spectroscopy of chestnut blight diseased Castanea sativa Mill. wood. CERNE 2016, 22, 43–58. [Google Scholar] [CrossRef]
- Fernandes, B.V.; Zanuncio, A.J.V.; Furtado, E.L.; Andrade, H.B. Damage and Loss Due to Ceratocystis fimbriata in Eucalyptus Wood for Charcoal Production, “Eucalyptus fungal loss”. BioResources 2014, 9, 5473–5479. [Google Scholar] [CrossRef]
- Mafia, R.G.; Santos, P.C.; Demuner, B.J.; Massoquete, A.; Sarto rio, R.C. Eucalyptus wood decay: Effects on productivity and quality of cellulose. For. Pathol. 2012, 42, 321–329. [Google Scholar] [CrossRef]
- Souza, S.E.; Sansigolo, C.A.; Furtado, E.L.; de Jesus, W.C.; Oliveira, R.R. Influencia do cancro basal em Eucalyptus grandis nas propriedades da madeira e polpacao Kraft. Sci. Florest. 2010, 38, 447–457. [Google Scholar]
- Leśniewska, J.; Öhman, D.; Krzesłowska, M.; Kushwah, S.; Barciszewska-Pacak, M.; Kleczkowski, L.A.; Sundberg, B.; Moritz, T.; Mellerowicz, E.J. Defense responses in aspen with altered pectin methylesterase activity reveal the hormonal inducers of tyloses. Plant Physiol. 2017, 173, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.L.; Hammerschmid, T.R. Phenolic compounds and their role in disease resistance. Ann. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Vance, C.P.; Kirk, T.K.; Sherwood, R.T. Lignification as a mechanism of disease resistance. Ann. Rev. Phytopathol. 1980, 18, 259–288. [Google Scholar] [CrossRef]
- Kuc, J. Concepts and direction of induced systemic resistance in plants and its application. Eur. J. Plant Pathol. 2001, 107, 7–12. [Google Scholar] [CrossRef]
- Shigo, A.I.; Marx, H.G. 1977: Compartimentalization of decay in trees. USDA Agric. Inform. Bull. 1977, 405, 1–73. [Google Scholar]
- Elgersma, D.M. Tylose formation in elms after inoculation with Ceratocystis ulmi, a possible resistance mechanism. Eur. J. Forest Pathol. 1973, 79, 218–220. [Google Scholar] [CrossRef]
- El Modafar, C.; Clerivet, A.; Macheix, J.J. Flavan accumulation in stems of Platanus acerifolia seedlings inoculated with Ceratocystis fimbriata f. sp. platani, the canker stain disease agent. Can. J. Bot. 1996, 74, 1982–1987. [Google Scholar] [CrossRef]
- Broda, M.; Popescu, C.M.; Curling, S.F.; Timpu, D.I.; Ormondroyd, G.A. Effects of Biological and Chemical Degradation on the Properties of Scots Pine Wood-Part I: Chemical Composition and Microstructure of the Cell Wall. Materials 2022, 15, 2348. [Google Scholar] [CrossRef]
- Hua, L.S.; Ashaari, Z.; Ang, A.F.; Halip, J.A.; Lum, W.C.; Dahali, R.; Halis, R. Effects of two-step post heat-treatment in palm oil on the properties of oil palm trunk particleboard. Ind. Crop. Prod. 2018, 116, 249–258. [Google Scholar]
- Esteves, B.; Videira, R.; Pereira, H. Chemistry and ecotoxicity of heat-treated pine wood extractives. Wood Sci Technol. 2011, 45, 661–676. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar]
- Esteves, B.; Marques, A.V.; Domingos, I.; Pereira, H. Chemical changes of heat-treated pine and eucalypt wood monitored by FTIR. Maderas. Cienc. Y Tecnol. 2013, 15, 245–258. [Google Scholar] [CrossRef]
- Moharram, M.; Mahmoud, O. FTIR Spectroscopic Study of the Effect of Microwave Heating on the Transformation of Cellulose I into Cellulose II during Mercerization. J. Appl. Pol. Sci. 2008, 107, 30–36. [Google Scholar] [CrossRef]
- Spiridon, I.; Teaca, C.; Bodirlau, R. Structural changes evidenced by FTIR spectroscopy in cellulosic materials after pre-treatment with ionic liquid and enzymatic hydrolysis. Bioresources 2011, 6, 400–413. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Zhang, X. Comparative studies of heat degradation between larch lignin and Manchurian ash lignin. Polym. Degrad. Stab. 2002, 78, 279–285. [Google Scholar] [CrossRef]
- Kotilainen, R.; Toivannen, T.; Alen, R. FTIR monitoring of chemical changes in softwood during heating. J. Wood Chem. Technol. 2002, 20, 307–320. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Chao, Y.; Nawawi, D.S.; Akiyama, T.; Yokoyama, T.; Matsumoto, Y. Analysis of lignin aromatic structure in wood based on the IR spectrum. J. Wood Chem. Technol. 2012, 32, 294–303. [Google Scholar] [CrossRef]
- Gelbrich, J.; Mai, C.; Militz, H. Chemical changes in wood degraded by bacteria. Int. Biodeterior. Biodegrad. 2008, 61, 24–32. [Google Scholar] [CrossRef]
- Pena, M.M.G.; Curling, S.F.; Hale, M.D.C. On the effect of heat on the chemical composition and dimensions of thermally-modified wood. Polym. Degrad. Stab. 2009, 94, 2184–2193. [Google Scholar] [CrossRef]
- Riley, R.; Salamov, A.A.; Brown, D.W.; Nagy, L.G.; Floudas, D.; Held, B.W.; Levasseur, A.; Lombard, V.; Morin, E.; Otillar, R.; et al. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. USA 2014, 111, 9923–9928. [Google Scholar] [CrossRef]
- Schilling, J.S.; Kaffenberger, J.T.; Held, B.W.; Ortiz, R.; Blanchette, R.A. Using wood rot phenotypes to illuminate the “gray” among decomposer fungi. Front. Microbiol. 2020, 11, 1288. [Google Scholar] [CrossRef] [PubMed]
- Rouhier, M.M. Wood as a hostile habitat for ligninolytic fungi “Wood Degradation and Ligninolytic Fungi”. Adv. Bot. Res. 2021, 99, 115–149. [Google Scholar]
- Clerivet, A.; El Modafar, C. Vascular modifications in Platanus acerifolia seedlings inoculated with Ceratocystis fimbriata f. sp. platani. Eur. J. Forest Pathol. 1994, 24, 1–10. [Google Scholar] [CrossRef]
- Rioux, D.; Nicole, M.; Simard, M.; Ouellette, G.B. Immunocytochemical evidence that secretion of pectin occurs during gel (gum) and tylosis formation in trees. Phytopathology 1998, 88, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Clerivet, A.; Deon, V.; Alami, I.; Lopez, F.; Geiger, J.P.; Nicole, M. Tyloses and gels associated with cellulose accumulation in vessels are responses of plane tree seedlings (Platanus acerifolia) to the vascular fungus Ceratocystis fimbriata f. sp. platani. Trees 2000, 15, 25–31. [Google Scholar] [CrossRef]
- Malaysian Timber Industrial Board (MTIB). Medium Hardwood Eucalyptus. In 100 Malaysian Timbers, 2010th ed.; Malaysian Timber Industry Board: Kuala Lumpur, Malaysia, 2010; pp. 132–133. [Google Scholar]
- Eucalyptus Urophylla. Available online: https://en.wikipedia.org/wiki/Eucalyptus_urophylla (accessed on 20 December 2022).
- Eucalyptus grandis. Available online: https://en.wikipedia.org/wiki/Eucalyptus_grandis (accessed on 20 December 2022).
- Bayle, G.K. Ecological and social impacts of Eucalyptus tree plantation on the environment. Biodivers. Conserv. Bioresour. Manag. 2019, 5, 93–104. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Flavours and Fragrances of Plant Origin; Food and Agriculture Organization: Rome, Italy, 1995. [Google Scholar]
- Toloza, A.C.; Lucia, A.; Zerba, Z.; Masuh, H.; Picollo, M.I. Interspecific hybridization of Eucalyptus as a potential tool to improve the bioactivity of essential oils against permethrin-resistant head lice from Argentina. Bioresour. Technol. 2008, 99, 7341–7347. [Google Scholar] [CrossRef]
- Guenther, E. The Essential Oils; Krieger Publishing Company: Malabar, FL, USA, 1972. [Google Scholar]
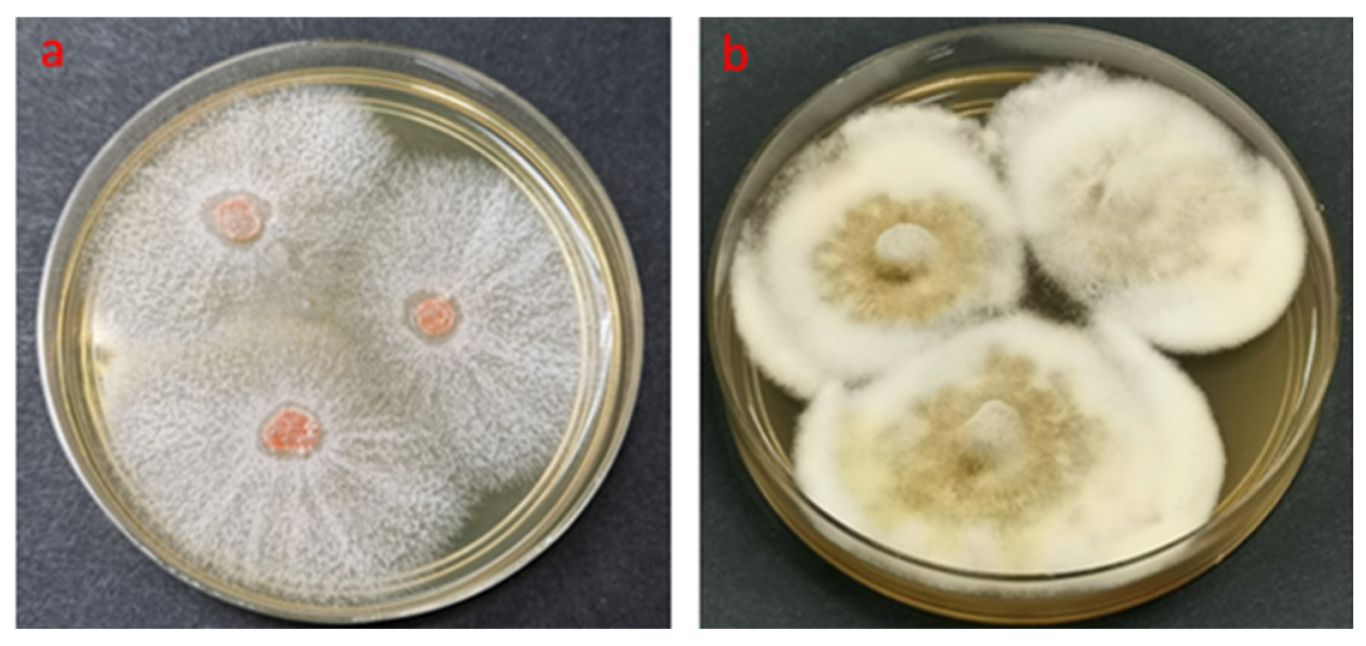



| Class | Category | Symptom |
|---|---|---|
| 1 | Healthy | Stem appears normal without any symptom of being infected |
| 2 | Moderate | Swollen bark (callus) |
| Cracking | ||
| Fruiting structure | ||
| Fresh kino pocket | ||
| Canker | ||
| 3 | Severe | Swollen bark (callus) |
| Cracking | ||
| Fruiting structure | ||
| Fresh kino pocket and fresh kino/gummosis | ||
| Canker | ||
| Sunken | ||
| Rotten | ||
| 4 | Very severe | Swollen bark (callus) |
| Cracking | ||
| Fruiting structure | ||
| Dried kino pocket & dried kino/gummosis | ||
| Canker | ||
| Sunken | ||
| Rotten | ||
| Shoot |
| Infection Classes | EMC (%) | Density (Kg/m3) | Volsh (%) |
|---|---|---|---|
| 1 | 10.5 | 670.8 | 15.1 |
| 2 | 10.1 | 618.9 | 14 |
| 3 | 10.2 | 706.8 | 14.5 |
| 4 | 9.7 | 542.3 | 12.9 |
| Sample Condition | Mean Weight Loss (%) | Resistance Class |
|---|---|---|
| Highly resistant | 0–10 | I |
| Resistant | 11–24 | II |
| Moderately resistant | 25–44 | III |
| Slightly resistance or non-resistant | ≥45 | IV |
| Sample Condition | Mean Weight Loss (%) | Resistance Class |
|---|---|---|
| Very resistant | <3.52 | I |
| Resistant | 3.52–7.50 | II |
| Moderate | >7.50–10.96 | III |
| Poor | >10.96–18.94 | IV |
| Very poor | >18.94 | V |
| Visual Rating Classification | Rating |
|---|---|
| Sound | 10 |
| Trace, surface nibbles permitted | 9.5 |
| Slight attack, up to 3% of cross-sectional area affected | 9 |
| Moderate attack, 3%–10% of cross-sectional area affected | 8 |
| Moderate/severe attack, penetration, 10%–30% of cross-sectional area affected | 7 |
| Severe attack, 30%–50% of cross-sectional area affected | 6 |
| Very severe attack, 50%–75% of cross-sectional area affected | 4 |
| Failure | 0 |
| Severity Classes | Chemical Composition (%) | |||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | Other Component | |
| 1 (Healthy) | 53.2 | 14.1 | 18.1 | 14.6 |
| 2 (Moderate) | 50.9 | 11.2 | 19.9 | 18.0 |
| 3 (Severe) | 49.8 | 11.6 | 19.0 | 19.6 |
| 4 (Very severe) | 45.4 | 13.9 | 20.5 | 20.2 |
| Wavenumber (cm−1) | Functional Group/Band Assignment |
|---|---|
| 3570–3200 (broad) | -OH stretching hydrogen bonds in cellulose |
| 2935–2915 | Asymmetric -CH stretching of methylene (CH2) in lignin |
| 1750–1700 | C=O stretching of non-conjugated carbonyl compound in xylan |
| 1615–1580 | C=O aromatic ring stretching |
| 1515–1500 | Aromatic ring (benzene) stretching vibrations |
| 1460 | C-H deformations in xylan |
| 1420 | Aromatic ring of lignin and C-H bending in cellulose |
| 1324–1322 | CN stretching in ether and oxy compound, and stretching P=O in simple hetero-oxy compound |
| 1225–950 (several) | Aromatic C-H in-plane bend |
| 1190–1130 | Secondary amine, CN stretch |
| 1047–1004 | C-O stretching in cellulose I and cellulose II |
| 896 | Asymmetric C-H out-of-plane bending deformation in cellulose and hemicellulose |
| Infection Classes | Value | WLdecay (%) | Resistance Classes | ||
|---|---|---|---|---|---|
| P. sanguineus | C. puteana | P. sanguineus | C. puteana | ||
| 1 (Healthy) | Mean | 14.4 a | 11.2 a | Resistant (II) | Resistant (II) |
| SD | 2.7 | 2.8 | |||
| 2 (Moderate) | Mean | 12.3 b | 8.0 b | Resistant (II) | Highly resistant (I) |
| SD | 3.0 | 2.3 | |||
| 3 (Severe) | Mean | 10.3 c | 7.8 c | Highly resistant (I) | Highly resistant (I) |
| SD | 2.5 | 3.1 | |||
| 4 (Very severe) | Mean | 9.5 c | 6.1 c | Highly resistant (I) | Highly resistant (I) |
| SD | 1.9 | 2.6 | |||
| p-value | 0.000 *** | 0.000 *** | |||
| Infection Classes | Value | WLtermite (%) | Visual Rating | Resistant Classes |
|---|---|---|---|---|
| 1 (Healthy) | Mean | 20.12 a | 7 | Very poor (V) |
| SD | 4.5 | |||
| 2 (Moderate) | Mean | 16.44 ab | 7 | Poor (IV) |
| SD | 8.8 | |||
| 3 (Severe) | Mean | 12.49 bc | 7 | Poor (IV) |
| SD | 5.5 | |||
| 4 (Very severe) | Mean | 9.86 c | 8 | Moderately resistant (III) |
| SD | 6.0 | |||
| p-value | 0.000 *** |
| Correlations | |||||
|---|---|---|---|---|---|
| Class | Decay | Termite | |||
| P. sanguineus | C. puteana | C. curvignathus | |||
| Class | Pearson correlation | 1 | −0.594 ** | −0.540 ** | −0.528 ** |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | ||
| N | 80 | 80 | 80 | 80 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahali, R.; Lee, S.H.; Md Tahir, P.; Salim, S.; Hishamuddin, M.S.; Che Ismail, A.; Khoo, P.S.; Krystofiak, T.; Antov, P. Influence of Chrysoporthe deuterocubensis Canker Disease on the Chemical Properties and Durability of Eucalyptus urograndis against Wood Rotting Fungi and Termite Infestation. Forests 2023, 14, 350. https://doi.org/10.3390/f14020350
Dahali R, Lee SH, Md Tahir P, Salim S, Hishamuddin MS, Che Ismail A, Khoo PS, Krystofiak T, Antov P. Influence of Chrysoporthe deuterocubensis Canker Disease on the Chemical Properties and Durability of Eucalyptus urograndis against Wood Rotting Fungi and Termite Infestation. Forests. 2023; 14(2):350. https://doi.org/10.3390/f14020350
Chicago/Turabian StyleDahali, Rasdianah, Seng Hua Lee, Paridah Md Tahir, Sabiha Salim, Muhammad Syahmi Hishamuddin, Atikah Che Ismail, Pui San Khoo, Tomasz Krystofiak, and Petar Antov. 2023. "Influence of Chrysoporthe deuterocubensis Canker Disease on the Chemical Properties and Durability of Eucalyptus urograndis against Wood Rotting Fungi and Termite Infestation" Forests 14, no. 2: 350. https://doi.org/10.3390/f14020350








