Abstract
Somatic mmbryogenesis receptor-like kinase (SERK) is a kind of protein kinase widely distributed in plants. It plays a vital role in regulating plant immunity and responses to abiotic stress. The SERK gene family has not been systematically studied in moso bamboo (Phyllostachys edulis). In this study, we identified six PeSERK genes and classified them into four groups in moso bamboo. PeSERKs of each group shared a highly similar distribution of conserved domains. Cis-element analysis indicated that many stress and hormone response elements are distributed on the promoters of PeSERKs. Moreover, we analyzed the chromosomal locations and synteny of PeSERKs. A collinear gene pair, PeSERK1 and PeSERK3, shared a high similarity, 93%, and the expression analysis showed similar expression patterns. Compared to PeSERK3, PeSERK1 had a higher expression in all tissues examined and all stages of shoot development. PeSERK3 was expressed mainly in leaf sheaths but with a low expression in other tissues. The expressions of PeSERKs were analyzed in seedlings under abiotic and hormone treatments using qRT-PCR. Except for PeSERK1 and PeSERK3, the expressions of most genes were downregulated or had no big difference at 0 h of drought treatment. Under drought treatment, PeSERK1 and PeSERK3 had a similar expression trend of increasing first and then decreasing. However, the expression level of PeSERK3 was higher than PeSERK1 after 3 h of drought treatment. PeSERK3 might play a more vital role in the drought stress response than PeSERK1. This study provides a theoretical basis for the further study of the SERK response to stress conditions in moso bamboo.
1. Introduction
Somatic embryogenesis receptor-like kinases (SERKs), belonging to the leucine-enriched repeat receptor-like kinase Ⅱ family, are highly conserved in plants [1,2]. SERK proteins have four conserved domains: a signal receptor domain, transmembrane domain, intracellular kinase activity domain, and SPP-motif extension [3]. In 1997, Schmidt et al. discovered a gene encoding somatic embryos from the embryonic cells of carrot hypocotyl. The gene was named somatic embryogenesis-related receptor-like protein kinase gene (SERK), identified as the first SERK in plants [4]. SERKs have been reported in Oryza sativa (rice) [5], Arabidopsis thaliana [6], Triticum aestivum (wheat) [7], Hordeum vulgare (barley) [8], Zea mays (maize) [9], Theobroma cacao (cocoa) [10], Dendrobium officinale [11], Camellia sinensis [12], Solanum tuberosum (potato) [13] and Glycine max (soybean) [14]. Aan den Toorn et al. compared the amino acid sequences of the SERK proteins of monocot, dicot and non-vascular plants, and found that SERKs can be divided into four clades. The authors also established that monocot SERKs are more closely related to a member of the dicot SERK1-2 cluster [15].
Previous research has shown that SERK genes are associated with in vitro cellular reprograming and differentiation events, such as the acquisition of embryogenic competence by somatic cells [4]. However, SERKs not only play an irreplaceable role in somatic embryogenesis and plant development [16], but also regulate the immune response [17], and the response to abiotic stress [14]. In soybean, GmSERK16 was upregulated under polyethylene glycol (PEG), abscisic acid (ABA), salicylic acid (SA) and jasmonic acid (JA) treatments [14]. In Ananas comosus (pineapple), AcSERK1 expression was upregulated by JA, SA, and low temperature treatments [18]. In apple, the expression of MdSERK2, MdSERK5 and MdSERK3 was upregulated under ABA and methyl JA (MeJA) treatments, whereas MdSERK4 and MdSERK10 were activated by salt treatment [19]. All HvSERKs were upregulated under salt stress [20]. Taking the above into account, SERKs may also be involved in improving the adaptability of plants to abiotic stress.
Bambusoideae, generally called bamboo, belongs to the grass family (Poaceae) [21]. It is one of the most important forest resources with significant economic value [22], ecological value [23], and cultural value [24]. There are more than 1400 bamboo species worldwide [25], mainly distributed in subtropical and tropical regions. They possess the advantages of rapid forest formation and good material properties. Among all bamboo species, moso bamboo has a fast growth rate, up to 1 m/day, and can reach a height of 20 m in 45–60 days [26,27]. It also has the highest economic value, with the broadest distribution and largest area in China [28]. Because of these beneficial features, bamboo is widely used as wood as well as in paper, artwork and food. However, environmental stress has affected the growth of moso bamboo, and research into the underlying mechanism has become a popular topic [29]. The whole genome sequence of moso bamboo was recently completed [30]. Several gene families involved in abiotic stress response that were sequentially identified and analyzed in the species include SOD [31], PP2C [32], Raf22 [33], APX [34] and WNK [35].
PeSERKs have been identified in diverse plants. However, SERK genes in moso bamboo have not undergone genome-wide identification. In this study, we analyzed SERKs in moso bamboo to characterize their sequence, evolutionary relationships and expression patterns under various abiotic stress treatments. These results will provide a foundation for further research of the function of PeSERKs under abiotic stress.
2. Materials and Methods
2.1. Plant Materials and Treatments
The seeds of moso bamboo were collected from Guilin, Guangxi Province, China, and cultivated in the intelligent greenhouse at Zhejiang A&F University. Seedlings were grown in a hydroponic culture (ddH2O supplemented with Hoagland nutrient solution) under a temperature of 25 °C and humidity of 70%. Two-month-old seedlings were exposed to 25% PEG 6000, 200 mM NaCl, 200 µM ABA or 1 mM SA for abiotic stress or phytohormone treatment. Seedlings with no treatment were the control. At 0, 3 and 24 h after treatments, five leaves of the seedlings were sampled, frozen in liquid nitrogen, and kept at −80 °C for later study.
2.2. Identification and Characterization of the PeSERK Family
Genomic data of moso bamboo were downloaded from the moso bamboo genome database (ftp://parrot.genomics.cn/gigadb/pub/10.5524/100001_101000/100498/(accessed on 10 May 2021)). The accession number of SERKs in rice and Arabidopsis were from references [36,37], and the SERK CDS and protein sequences were obtained from the NCBI database. We used the SERKs of rice and Arabidopsis as queries to blast against the moso bamboo protein V2 database with e-value cut-offs of 10−10. According to the conserved domains of SERK proteins, the putative PeSERKs were annotated using the NCBI Conserved Domain database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi (accessed on 10 May 2021)). The putative proteins with typical domains in moso bamboo were screened out.
2.3. Analysis of Physicochemical Properties and Prediction of Transmembrane Domains of PeSERKs
We used the online ProtParam (http://web.expasy.org/protparam/ (accessed on 18 July 2021)) to analyze the physical and chemical properties of PeSERKs [38]. The TMHMM Server V.2.0 (http://www.cbs.dtu.dk/services/TMHMM/ (accessed on 15 June 2021)) was used to predict and analyze the transmembrane domain of PeSERKs [39]. The subcellular localizations of PeSERKs were predicted using Euk-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/euk-multi-2/ (accessed on 18 July 2021)).
2.4. Phylogenetic Analysis and Collinearity Analysis
To better understand the structures of PeSERKs, we used MEME (http://meme-suite.org/tools/meme/ (accessed on 18 July 2021)) [40] to predict the conserved motifs of SERK proteins in moso bamboo. TBtools was used to draw the motif distribution and gene structure of PeSERKs. To study the evolutionary relationship of PeSERK genes with other species, a maximum likelihood tree was produced and assessed using bootstrap value with default parameters in MEGA11.0 [39] The amino acid sequences of PeSERK proteins and other species were compared and the evolutionary tree was built.
2.5. Cis-Acting Elements in PeSERK Promoter Regions
The 1500 bp regions upstream of the initiation codon were considered the proximal promoters of PeSERKs. Promoters were submitted to the PlantCARE database for cis-element analysis (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 18 August 2021)). Collinearity analysis of PeSERKs, Arabidopsis and rice involved using McScanX [41].
2.6. Real-Time PCR Analysis
After extracting total RNA from each sample using the Fast Pure Plant Total RNA Isolation kit (Nanjing Vazyme Biotech, Nanjing, China, RC401), the first strand cDNA was synthesized from total RNA (1 μg) using gDNA Purge according to the manufacturer’s instructions. The sequence was amplified with gene-specific primers using 2 × NovoStart SYBR qPCR, with an NTB gene as the internal control. The primer sequences used for qRT-PCR are in Table 1. The qRT-PCR cycling parameters were 95 °C for 30 s, followed by 39 cycles at 95 °C for 5 s and 60 °C for 30 s, with a melting curve analysis. All reactions were performed in triplicate to ensure the reproducibility of the results. Relative expression was calculated according to the 2−∆∆Ct method [42].

Table 1.
Primers for qRT-PCR.
2.7. Tissue-Specific Analysis of PeSERKs
To study the expression of PeSERKs in different tissues and at different growth stages of moso bamboo, we used the NCBI Short Read Archive database (SRA) (https://www.ncbi.nlm.nih.gov/sra/ (accessed on 18 August 2021)) to obtain the different organizations of the transcriptome data for moso bambooo (retrieved for SRX2408703). The transcriptome data were calculated and the heat map was created using TBtools software [43].
3. Results
3.1. Genome-Wide Identification of PeSERKs
To predict the SERK genes in the moso bamboo genome, we used the SERK proteins from rice and Arabidopsis [36,37] as queries to blast against the moso bamboo protein V2 database with an e-value cut-off of 10−10. Six PeSERKs were obtained and named PeSERK1 to PeSERK6 based on their position in chromosomes (Figure 1 and Table 2). The PeSERKs were about 590–629 amino acids long, with a molecular weight from 65,128.33 to 69,287.06 kDa (Table 2). The isoelectric point ranged from 5.23 to 6.34 (Table 2). Thus, the proteins encoded by the PeSERKs were acidic proteins, and the calculation of the instability index predicted that PeSERKs were stable proteins. According to the hydrophilicity–hydrophobicity value (GRAVY), the PeSERK proteins were all hydrophilic proteins (Table 2). All PeSERKs were predicted as cell-membrane-located proteins consistent with their functions as membrane-binding receptors sensing intracellular and/or extracellular signals [15,44].

Figure 1.
Comparison of SERKs in moso bamboo and rice. The signal receptor domain, transmembrane domain, intracellular kinase activity domain, SPP-motif and C-terminal domain are underlined.

Table 2.
Characteristics of PeSERKs in moso bamboo.
3.2. Phylogenetic Analysis of PeSERKs
To study the evolutionary relationship of PeSERKs, we constructed a phylogenetic tree using PeSERKs and SERKs from other species, including Arabidopsis, rice, Malus domestica (apple), maize, barley, and carrot (Figure 2, sequences listed in Supplementary Material). Class A, included PeSERK5, PeSERK6, OsSERK2, ZmSERK1 and HvSERK1. Class B included PeSERK1, PeSERK3, ZmSERK2 and ZmSERK3, which indicates that SERK genes in moso bamboo were more closely related to grass plants, such as rice, barley and maize. PeSERK4 and OsSERK1 were clustered in the same branch of Class C. PeSERK2 was separated in class F. According to the evolutionary relationship, PeSERKs were more homologous to SERKs from Poaceae.
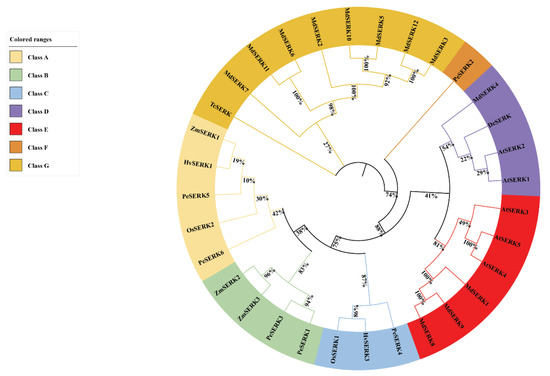
Figure 2.
Maximum likelihood phylogenetic tree of SERK proteins. The tree was constructed with protein sequences encoded by the longest transcript of each SERK in moso bamboo, and SERK protein sequences in Arabidopsis thaliana (At), Malus domestica (Md), Oryza sativa (Os), Hordeum vulgare (Hv), Zea mays (Zm), Daucus carota (Dc) and Theobroma cacao (Tc) with bootstrap values of replicates. Different groups of SERK proteins are distinguished by different colors.
3.3. Gene Structure and Motif Analysis of PeSERKs
We constructed a phylogenetic tree with only PeSERKs to compare the gene structure and conserved domain distribution of homologous PeSERKs (Figure 3). PeSERK proteins were divided into four subclasses: Ia, Ib, Ic and Id, with 2, 2, 1 and 1 members, respectively (Figure 3A). Motif analysis (Figure 3B) and gene structure analysis (Figure 3C) indicated that their function may be similar. PeSERKs contained 11 exons, which is consistent with other plants, such as Dendrobium officinale [11], rice [5], soybean [14] and Arabidopsis [6]. Motif analysis revealed that except for PeSERK5, PeSERKs have 10 motifs. Each exon and its coding protein functional domains are almost in a one-to-one correspondence [45].
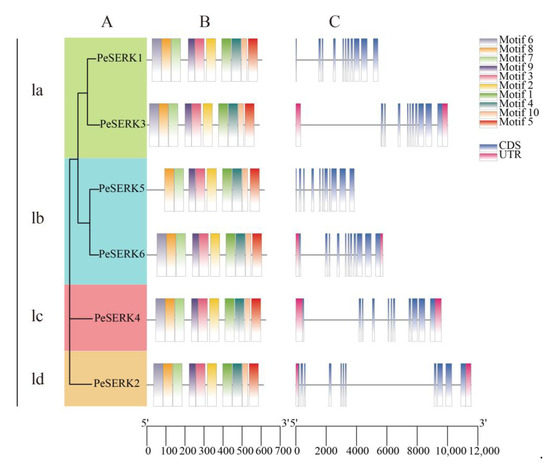
Figure 3.
Phylogenetic relationships, motif compositions, and gene structure of moso bamboo PeSERKs. (A) Phylogenetic tree and classification of PeSERK proteins. PeSERKs can be divided into four clades. (B) Schematic representation of the motifs among PeSERK proteins obtained by MEME analysis. Each color represents a specific motif. (C) Exon/intron organization of PeSERKs. Exons, introns and untranslated regions are represented by orange boxes, fold lines and purple boxes, respectively.
3.4. Collinearity Analysis of PeSERKs
PeSERKs were distributed on six different scaffolds (Figure 4). Gene replication events are common in all species, they generate new functional genes and drive family expansion [46]. MCScanX genomic collinearity analysis was used to explore the repetition of PeSERKs. Six pairs of collinearity genes were detected (Figure 4). Except for PeSERK2 and PeSERK4, other PeSERK genes exhibited linear relationships (Figure 4).
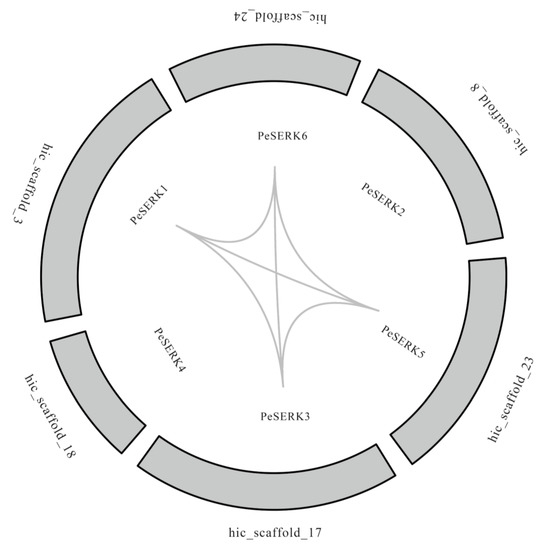
Figure 4.
Chromosome locations and syntenic relationships between PeSERKs in moso bamboo. Gray lines indicate the syntenic PeSERK gene pairs.
We also analyzed the synteny of SERKs between moso bamboo, rice and Arabidopsis (Figure 5). Collinearity analysis revealed six pairs of SERKs between rice and moso bamboo. Thus, the SERK genes of moso bamboo and rice were highly homologous, so they probably have similar functions. However, we found no linear relationship of SERKs in moso bamboo and Arabidopsis, so the SERKs of moso bamboo and Arabidopsis had undergone huge evolutionary changes.
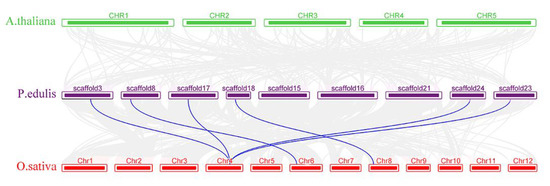
Figure 5.
Syntenic relationships between PeSERKs in moso bamboo and SERK genes in two representative plant species. Gray lines in the background indicate the collinear blocks within P. edulis and other plant genomes. Blue lines indicate the syntenic SERK gene pairs.
3.5. Cis-Acting Element Analysis in the Promoter Regions of PeSERKs
To understand the potential transcriptional regulatory mechanisms of PeSERKs, we analyzed the 1500 bp sequences of PeSERK promoter regions using PlantCARE [47]. We removed the unknown function elements and general transcription regulatory elements (Figure 6). The PeSERK promoter regions contain many light response elements, which may indicate that these proteins are involved in the plant response to light fluctuations. Moreover, the promoters of most PeSERKs also possess various cis-elements related to abiotic stress, such as ABA, MeJA, SA, drought, low temperature and salt stress responsive elements. PeSERK3, PeSERK5 and PeSERK6 have low temperature responsive elements, which may indicate that these proteins are involved in the plant response to light fluctuations. According to the evolutionary tree, PeSERK1 and PeSERK3 are highly homologous (Figure 3A,B). They contained similar cis-elements such as a drought responsive element, which suggests that they may be involved in resisting drought stress. The PeSERK gene family also has a variety of hormone-responsive elements, so PeSERKs may participate in the growth and development of moso bamboo via different hormonal regulatory pathways.
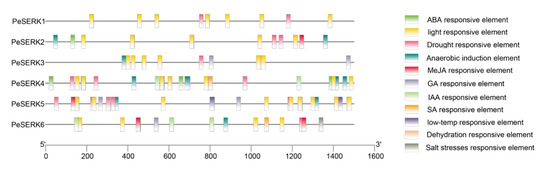
Figure 6.
Cis-acting element analysis of PeSERK promoters. The 1500 bp sequence upstream of the PeSERK start codon was analyzed using PlantCARE. Binding sites in the promoter region are represented by boxes of different colors, and the graph shows the number of binding sites.
3.6. Expression Pattern of PeSERKs in Different Tissues
To study the expression pattern of PeSERKs in different tissues and the development stages in moso bamboo, we analyzed the expression of PeSERKs in 26 different tissues and development stages using bamboo transcriptome data (retrieved for SRX2408703). PeSERK1 and PeSERK2 showed the same expression trend in all tissues, PeSERK1 was highly expressed in different tissues, and PeSERK2 was poorly expressed in all tissues (Figure 7A), so PeSERK1 may play an important role in the development of all tissues in moso bamboo. PeSERKs can be divided into two groups (Figure 7B). The first group includes PeSERK6. The expression was higher for PeSERK6 than other PeSERKs in the middle of a 3 m bamboo shoot, with low expression in other tissues, so this gene might play important roles in the development of the 3 m bamboo shoot. The second group included PeSERK3, PeSERK4 and PeSERK5, which were expressed in different tissues and in different growth periods. PeSERK4 showed high expression in leaves, 0.5 cm shoots and 6.7 m bamboo shoots; PeSERK3 had a high expression in 0.1 cm shoots and lower buds; and PeSERK5 had a high expression in middle buds.
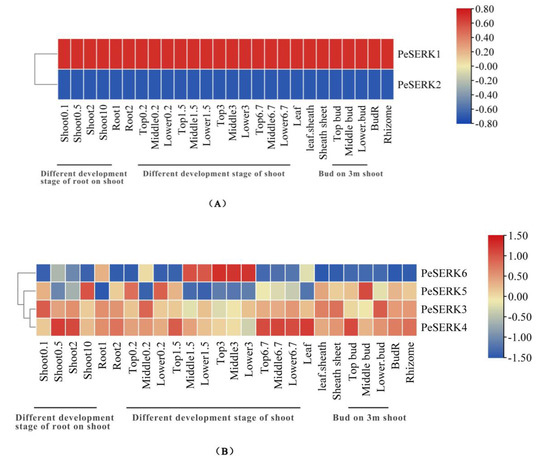
Figure 7.
Expression patterns of PeSERKs in different tissues of moso bamboo. (A) Differential expression patterns of PeSERK1 and PeSERK2 in development stages of root on shoot, development stages of shoots, root on rhizome, bud on 3 m shoot, BudR, rhizome, leaf and leaf sheath. (B) Differential expression patterns of PeSERK3, PeSERK4, PeSERK5 and PeSERK6 in development stages of root on shoot, development stages of shoots, root on rhizome, bud on 3 m shoot, BudR, rhizome, leaf and leaf sheath. The development stages of root on shoot (0.1, 0.5, 2 and 10 cm shoot), development stages of shoots (top 0.2 m, middle 0.2 m, lower 0.2 m, top 1.5 m, middle 1.5 m, lower 1.5 m top 1.5 m, top 3 m, middle 3 m, lower 3 m top 6.7 m, middle 6.7 m and lower 6.7 m), root on rhizome, bud on 3 m shoot (top bud, middle bud and lower bud), BudR, rhizome, leaf and leaf sheath.
3.7. Expression Patterns of PeSERKs under Abiotic and Hormone Treatment
To obtain further insights into the potential function of SERK genes in moso bamboo, we used qRT-PCR to detect the expression profiles of the six PeSERKs under different abiotic and hormone treatments (Figure 8). Except for PeSERK4, other PeSERKs were downregulated under NaCl, ABA, SA or PEG treatment after 24 h. With ABA treatment for 3 h, the exogenous hormone could strongly stimulate the upregulated expression of PeSERK4, so PeSERK4 might play a significant role in hormone signal transduction. PeSERK2, PeSERK5 and PeSERK6 were significantly downregulated under ABA treatment. The expression of PeSERK1 and PeSERK3 was not significantly changed under ABA treatment, so PeSERKs had different response modes to ABA treatment. Under PEG6000 treatment for 3 h, the expression of PeSERK1, PeSERK2 and PeSERK3 was increased, with PeSERK3 showing the largest change. PeSERK3 may have a major physiological function in moso bamboo under drought stress. Under salt stress after 3 h, except for PeSERK1 and PeSERK3, the expression of other PeSERKs showed a similar expression trend, one of low expression, so PeSERK1 and PeSERK3 may play a role in the response to salt stress.
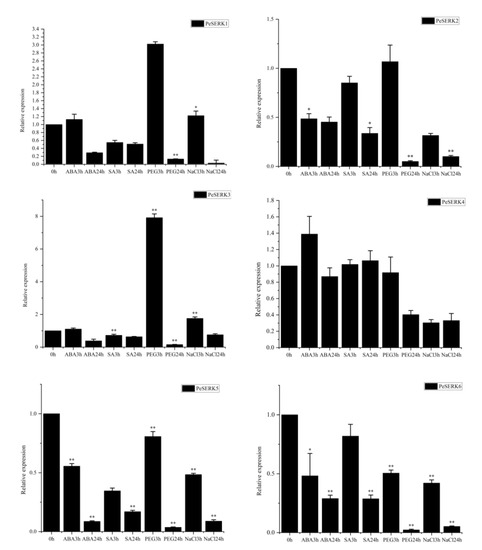
Figure 8.
Expression levels of PeSERKs in moso bamboo under abiotic and phytohormone treatment. The expression of NTB was the internal control to standardize the RNA samples for each reaction. Data are the mean ± SE from three samples, * p ≤ 0.05, ** p ≤ 0.01.
4. Discussion
In plants, SERK genes are widespread and have a variety of biological functions, such as participating in plant innate immunity [16], as well as regulating cell death [14], plant disease resistance and plant growth and development. SERKs have been studied in a wide range of species, such as rice [5], A. thaliana [6], wheat [7], barley [8] and maize [9], but not in moso bamboo. In this study, we identified six PeSERKs from the moso bamboo genome. We found that PeSERKs contain 11 exons, which is consistent with other plants, such as D. officinale [11], rice [5], soybean [14] and Arabidopsis [6]. SERK genes from dicotyledonous and monocotyledonous plants have a highly conserved structural organization. The genomic sequence contains 11 exons [48], and PeSERKs also have 11 exons.
In some plants, there are several SERK genes with embryonic development specificities, which include LaSERK1 [49], DiSERK1 [50], CpSERKa and CpSERKb [51], LhSERK1 [52] and SeSERK [53]. However, for the function of SERKs not limited to embryonic development, AtSERK1 and AtSERK2 were found essential for tapetum cell layer development in Arabidopsis [54,55], and SeSERK2 was found highly expressed in bud, leaf and petiole [53]. CiSERK1 was only expressed in embryonic cells, not in non-embryonic cells [56]. TcSERK was abundantly expressed in embryogenic guelder and proliferating embryos, and also had trace expression in leaves, but no expression in roots, petals and staminodes [10]. MdSERK2 and MdSERK6 were highly expressed in phloem and xylem. MdSERK8, MdSERK9 and MdSERK10 were highly expressed in leaves [19]. Here, we found that PeSERK4 (homologous to OsSERK1) was highly expressed in leaves (Figure 2 and Figure 7). PeSERK1 (a homologous gene of ZmSERK2) was highly expressed in all tissues (Figure 2 and Figure 7).
Some homologous genes originating from duplication events have similar expression patterns [46]. For instance, ZmSERK2 and ZmSERK3, homologous genes arising from duplication events, had similar expression patterns in all tissues of maize [9]. In this study, PeSERK1 and PeSERK3 had the same response pattern under different abiotic and hormone treatments, but showed a disparate pattern during normal development. They might be under diverse transcriptional regulation during tissue development. Gene replication plays an important role in the expansion and functional diversity of gene family members [57,58]. The gene pair PFGLO1 and PFDEF have different functions due to changes in B-class MADS box elements [59]. The amino acid change in PpMYB10.1 and PpMYB10.2 during gene duplication led to functional divergence [60]. In this study, we found that PeSERK3 lacks a signal peptide, which may cause it to function differently from other PeSERKs. Mutations in the N-terminal signal peptide of Cry1Ia led to differences in the thermal stability of Bacillus thuringiensis [61]. The lack of this domain may have led to the functional differentiation of PeSERK1 and PeSERK3. Their specific function needs to be verified through molecular biology experiments.
Cis-acting elements are functional elements in the gene promoter region and play important roles in regulating gene expression [62]. We found that most PeSERKs have various types of cis-elements related to hormone response, light response, and stress responses. PeSERKs showed different expression patterns under different abiotic stress treatments, so they may affect moso bamboo plant growth in response to abiotic stress. Moreover, SERKs play an essential role in regulating abiotic stress [14,20]. Previous studies showed that salt treatment could activate the expression of MdSERK4 and MdSERK10; SA treatment increased the expression of MdSERK2/5 and MdSERK12; and ABA treatment increased that of MdSERK2/5, MdSERK3 and MdSERK6/11 [20]. OsSERK1 was induced within 1 h and reached its highest expression within 3 h with 1 mmol/L SA or 0.3 mmol/L benzothiadiazole, which lasted for 12 h. OsSERK1 was induced after 6 h and remained at a high level for 12 h. When treated with 0.1 mmol/L ABA, OsSERK1 was slightly induced within 1 h and reached a fairly high expression after 12 h. When treated with 0.1 mmol/L benzothiadiazole, OsSERK1 was induced within 1 h and reached a high expression for 12 h. When treated with 0.1 mmol/L ABA, OsSERK1 was slightly induced within 1 h and reached high expression after 12 h [5]. The expression of GmSERKl, GmSERK3, GmSERK4 and GmSERK16 was upregulated under salt treatment [14]. However, little is known about the potential roles of SERKs in regulating stress responses in moso bamboo, or whether PeSERKs are associated with SA, ABA, drought and salt signals. Therefore, we monitored the expression pattern of PeSERKs under different stress conditions. Most PeSERKs were downregulated under salt treatment. PeSERKs might have different functions in moso bamboo under salt treatment. Furthermore, PeSERK4 (homologous to OsSERK1) was upregulated under ABA treatment (Figure 2 and Figure 8) [5]. PeSERK4 may have similar functions in moso bamboo under ABA treatment.
5. Conclusions
This is the first comprehensive and systematic genome-wide analysis of the SERK gene family in moso bamboo using bioinformatics analysis. Six PeSERK genes were identified and classified into four groups in moso bamboo. The bioinformatics analysis of the PeSERK family included physicochemical, motif, phylogenetic relationships and promoter cis-elements. PeSERKs of each group shared a highly similar distribution of conserved domains. Cis-element analysis revealed many stress and hormone response elements distributed on the promoters of PeSERKs. We found a collinear gene pair, PeSERK1 and PeSERK3, with the similar expression trend under different abiotic and hormone treatments; PeSERK3 was more sensitive to drought treatment than PeSERK1, but PeSERK3 was more sensitive to drought treatment than PeSERK1. PeSERK3 might play a more vital role in the stress response than PeSERK1. This study preliminarily discusses the role of the SERK gene family in moso bamboo, which provides a reference for further functional studies of SERK genes, and lays a theoretical basis for the further study of the SERK response to stress conditions in moso bamboo.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14030540/s1. Sequences list.
Author Contributions
Conceptualization, D.H. and X.L.; methodology, P.Z. and H.L.; software, H.W.; validation, H.Z. and Q.L.; formal analysis, P.Z., Z.H. and H.Z.; resources, D.H. and X.L.; data curation, P.Z.; writing—original draft preparation, P.Z., Z.H. and H.Z.; writing—review and editing, P.Z. and D.H.; visualization, J.Z.; supervision, D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Foreign Expert Project of China (G2022016012L), the Scientific Research and Development Fund Project of ZAFU University (2021LFR049), the Natural Science Foundation of Zhejiang Province (LZ20C160002), the National Natural Science Foundation of China (31971735) and the National Natural Science Foundation of China (32271970).
Data Availability Statement
The authors do not want to disclose it.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shiu, S.H.; Bleecker, A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. PNAS 2001, 98, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Kou, S.J.; Liu, Y.L.; Fang, Y.N.; Xu, Q.; Guo, W.W. Genomewide analysis of small RNAs in nonembryogenic and embryogenic tissues of citrus: microRNA- and siRNA-mediated transcript cleavage involved in somatic embryogenesis. Plant Biotechnol. J. 2015, 13, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Becraft, P.W. Receptor kinases in plant development. Trends Plant Sci. 1998, 3, 384–388. [Google Scholar] [CrossRef]
- Schmidt, E.D.; Guzzo, F.; Toonen, M.A.; de Vries, S.C. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 1997, 124, 2049–2062. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L.; Yang, Y. Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 2005, 222, 107–117. [Google Scholar] [CrossRef]
- Hecht, V.; Vielle-Calzada, J.P.; Hartog, M.V.; Schmidt, E.D.; Boutilier, K.; Grossniklaus, U.; de Vries, S.C. The Arabidopsis somatic embryogenesis receptor-like kinases1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001, 127, 803–816. [Google Scholar] [CrossRef]
- Singh, A.; Khurana, P. Ectopic expression of Triticum aestivum SERK genes (TaSERKs) control plant growth and development in Arabidopsis. Sci. Rep. 2017, 7, 12368. [Google Scholar] [CrossRef]
- Yang, S.S.; Li, Y.B.; Tan, H.X.; Guo, G.M. Cloning and bioinformatics analysis of SERK gene family in barley (Hordeum vulgare L.). Acta Agric. Shanghai 2016, 32, 5–13. [Google Scholar]
- Baudino, S.; Hansen, S.; Brettschneider, R.; Hecht, V.F.; Dresselhaus, T.; Lörz, H.; Dumas, C.; Rogowsky, P.M. Molecular characterisation of two novel maize LRR receptor-like kinases, which belong to the SERK gene family. Planta 2001, 213, 1–10. [Google Scholar] [CrossRef]
- Santos, M.; Romano, E. Characterisation of the cacao somatic embryogenesis receptor-like kinase (SERK) gene expressed during somatic embryogenesis. Plant Sci. 2005, 68, 723–729. [Google Scholar] [CrossRef]
- Liang, Y.; Li, L.J.; Ma, J.H.; Liu, C.Q.; Li, C.; Wang, W.J. Identification and expression analysis of SERK gene in Dendrobium officinale. J. Biol. 2019, 36, 11–16. [Google Scholar]
- Weng, H.; Lai, Z.X. Cloning and Bioinformatics Analysis of Cn-SERK Gene from Somatic Embryos in Camellia nitidissima. Chin. J. Trop. Crops 2013, 34, 699–1707. [Google Scholar]
- Zhang, J.L.; Wu, Y.J.; Xiao, C.B. Isolation of SERKs family genes in potato and their functions in plant immune signal. J. Gansu Agric. Univ. 2019, 54, 88–99. [Google Scholar]
- He, F.L.; Liu, X.; Zhang, B. Bioinformatics of soybean SERK gene family and expression analysis under salt stress. Acta Agric. Boreali-Occident. Sin. 2019, 28, 1708–1717. [Google Scholar]
- Aan den Toorn, M.; Albrecht, C.; de Vries, S. On the origin of SERKs: Bioinformatics analysis of the somatic embryogenesis receptor kinases. Mol. Plant 2015, 8, 762–782. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, R.; Lin, Q. Biological functions of plant somatic cell embryogenesis receptor-like protein kinases. Genetics 2012, 34, 46–54. [Google Scholar]
- Wang, Z.Y. Brassinosteroids modulate plant immunity at multiple levels. Proc. Natl. Acad. Sci. USA 2012, 109, 7–8. [Google Scholar] [CrossRef]
- Ma, Y.H.; He, Z.Y.; Hu, S.; Kanakala, W.T.; Xu, J.X.; Xia, C.H.; Guo, S.Q.; Lin, C.J.; Chen, C.H. Histological analysis of somatic embryogenesis in pineapple: AcSERK1 and its expression validation under stress conditions. J. Plant Biochem. Biotechnol. 2016, 25, 49–55. [Google Scholar] [CrossRef]
- Zheng, L.W.; Ma, J.J.; Mao, J.P.; Fan, S.; Zhang, D.; Zhao, C.; An, N.; Han, M.Y. Genome-wide identification of SERKs in apple and analyses of their role in stress responses and growth. BMC Genom. 2018, 19, 962. [Google Scholar] [CrossRef]
- Li, Y.B.; Liu, C.H.; Guo, G.M.; He, T.; Chen, Z.W.; Gao, R.H. Expression analysis of three SERK-like genes in barley under abiotic and biotic stresses. J. Plant Interact. 2017, 12, 279–285. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, X.J.; He, J.; Jin, S.H.; Guo, H.D.; Yu, X.F.; Zhou, Y.J.; Li, X. Genome-Wide Identification, Characterization, and Stress-Responsive Expression Profiling of Genes Encoding LEA (Late Embryogenesis Abundant) Proteins in Moso Bamboo (Phyllostachys edulis). PloS ONE 2016, 11, e0165953. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F. Analysis on economic and ecological benefit of bamboo planting. Mod. Hortic. 2014, 018, 25. [Google Scholar]
- Xu, Y.; Hu, Q.T.; Hou, D.; Lu, H.; Lin, X.C. Genome-wide Identification and Expression Analysis of NCED Gene Family in Phyllostachys edulis. J. Agric. Biotechnol. 2021, 29, 1061–1072. [Google Scholar]
- Wang, F.D.; Zhang, Y.F. Development of bamboo cultural tourism resources in China. J. Anhui Agric. Sci. 2007, 188–191+301. [Google Scholar] [CrossRef]
- Yang, Y.M. Bamboo resources and the development direction of bamboo industry economy in Yunnan. Ecol. Econ. 1992, 4, 28–32. [Google Scholar]
- Li, F.F.; Sun, H.J.; Jiao, Y.B. Viral infection-induced endoplasmic reticulum stress and a membrane-associated transcription factor NbNAC089 are involved in resistance to virus in Nicotiana benthamiana. Plant Pathol. 2018, 67, 233–243. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, C.; Zhang, Y.; Hu, T.; Mu, S.; Li, X.; Gao, J. Transcriptome sequencing and analysis of the fast growing shoots of moso bamboo (Phyllostachys edulis). PloS ONE 2013, 8, e78944. [Google Scholar] [CrossRef]
- Li, B.Y.; Hu, S.L.; Cao, Y.; Xu, G. Bioinformatics analysis of NAC gene family in moso bamboo. Genom. Appl. Biol. 2015, 34, 1769–1777. [Google Scholar]
- Tao, G.Y.; Fu, Y.; Zhou, M.B. Research progress on the mechanism of rapid growth of bamboo plants. J. Agric. Biotechnol. 2018, 26, 871–887. [Google Scholar]
- Zhao, H.; Gao, Z.; Wang, L.; Wang, J.; Wang, S.; Fei, B.; Chen, C.; Shi, C.; Liu, X.; Zhang, H.; et al. Chromosome-level reference genome and alternative splicing atlas of moso bamboo (Phyllostachys edulis). Giga Sci. 2018, 7, giy115. [Google Scholar] [CrossRef]
- Cai, K. Bioinformatics and Functional Analysis of SOD Gene Family in Moso Bamboo; Zhejiang A&F University: Hang Zhou, China, 2018. [Google Scholar]
- Hu, Q.T.; Hou, D.; Wei, H.T.; Lin, X.C. Identification and expression analysis of PP2C gene family in moso bamboo. J Agric. Biotechnol. 2020, 28, 1776–1787. [Google Scholar]
- Yang, Y.; Wang, T.T.; Wei, W. Cloning and expression analysis of PeRaf22 gene in moso bamboo. Mol. Plant Breed. 2021, 19, 815–819. [Google Scholar]
- Song, X.L.; Kong, B.; Gao, Z.M. Identification and expression analysis of APX family genes in moso bamboo. J. Trop. Subtrop. Bot. 2020, 28, 255–264. [Google Scholar]
- Liu, R.X.; Vasupalli, N.; Hou, D.; Stalin, A.; Wei, H.T.; Zhang, H.C.; Lin, X.C. Genome-wide identification and evolution of WNK kinases in Bambusoideae and transcriptional profiling during abiotic stress in Phyllostachys edulis. PeerJ 2022, 10, e12718. [Google Scholar] [CrossRef] [PubMed]
- Ghai, D.; Alok, A.; Himani; Upadhyay, S.K.; Sembi, J.K. Genome wide characterization of the SERK/SERL gene family in Phalaenopsis equestris, Dendrobium catenatum and Apostasia shenzhenica (Orchidaceae). Comput. Biol. Chen. 2020, 85, 107210. [Google Scholar] [CrossRef]
- Gu, Q.M.; Zhao, Y.Y.; Peng, A.Y.; Cheng, F.J.; Luo, D.F. Genome-wide identification and phylogenetic analysis of SERK gene family in Brassica napus. Chin. J. Oil Crop Sci. 2021, 43, 783–794. [Google Scholar]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202-8. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Gou, X.; Yin, H.; He, K.; Du, J.; Yi, J.; Xu, S. Genetic evidence for an indispensable role of somatic embryogenesis receptor kinases in brassinosteroid signaling. PLoS Genet. 2012, 8, e1002452. [Google Scholar] [CrossRef]
- Lin, Q.G.; Cui, B.M.; Peng, M. Advanced study on SERK genes family. Hereditas 2007, 29, 681–687. [Google Scholar] [CrossRef]
- Ganko, E.W.; Meyers, B.C.; Vision, T.J. Divergence in expression between duplicated genes in Arabidopsis. Mol. Biol. Evol. 2007, 24, 2298–2309. [Google Scholar] [CrossRef]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Koehler, A.D.; Irsigler, A.S.T.; Carneiro, V.T.C.; Cabral, G.B.; Rodrigues, J.C.M.; Gomes, A.C.M.M. SERK genes identification and expression analysis during somatic embryogenesis and sporogenesis of sexual and apomictic Brachiaria brizantha (Syn. Urochloa brizantha). Planta 2020, 252, 39. [Google Scholar] [CrossRef]
- Li, L. Expression Pattern and Antibody Preparation of LaSERK1 Gene Related to Somatic Embryogenesis in Larch; Chinese Academy of Forestry: Beijing, China, 2013. [Google Scholar]
- Cai, Y.Q. Cloning and Expression Analysis of Embryonic Related Genes such as SERK during Longan Somatic Embryogenesis; Fujian Agriculture and Forestry University: Fuzhou, China, 2011. [Google Scholar]
- You, C.R. Characterization of Somatic Embryogenesis, Development and Expression of SERK Gene during Somatic Embryogenesis in Cyclamen D; Ocean University of China: Qingdao, China, 2009. [Google Scholar]
- Wei, P.W. Cloning and Expression Analysis of Somatic Embryogenesis Marker Genes in Liriodendron Hybrid; Nanjing Forestry University: Nanjing, China, 2009. [Google Scholar]
- Thomas, C.; Meyer, D.; Himber, C.; Steinmetz, A. Spatial expression of a sunflower SERK gene during induction of somatic embryogenesis and shoot organogenesis. Plant Physiol. Bioch. 2004, 42, 35–42. [Google Scholar] [CrossRef]
- Mehan, M.R.; Almonte, M.; Slaten, E.; Freimer, N.B.; Rao, P.N.; Ophoff, R.A. Analysis of segmental duplications reveals a distinct pattern of continuation-of-synteny between human and mouse genomes. HUM Genet. 2007, 121, 93–100. [Google Scholar] [CrossRef]
- Mehan, M.R.; Freimer, N.B.; Ophoff, R.A. A genome-wide survey of segmental duplications that mediate common human genetic variation of chromosomal architecture. HUM Genet. 2004, 1, 335–344. [Google Scholar] [CrossRef]
- Shimada, T.; Hirabayashi, T.; Endo, T. Isolation and characterization of the somatic embryogenesis receptor-like kinase gene homologue (CitSERK1) from Citrus unshiu Marc. Sci. Hortic. 2005, 103, 233–238. [Google Scholar] [CrossRef]
- Albrecht, C.; Russinova, E.; Hecht, V.; Baaijens, E.; de Vries, S. The Arabidopsis thaliana somatic embryogenesis receptor-like kinases1 and 2 control male sporogenesis. Plant Cell 2005, 17, 3337–3349. [Google Scholar] [CrossRef] [PubMed]
- Colcombet, J.; Boisson-Dernier, A.; Ros-Palau, R.; Vera, C.E.; Schroeder, J.I. Arabidopsis somatic embryogenesis receptor-like kinases1 and 2 are essential for tapetum development and microspore maturation. Plant Cell 2005, 17, 3350–3361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, J.S.; Zhao, J.; He, C. Distinct subfunctionalization and neofunctionalization of the B-class MADS-box genes in Physalis floridana. Planta 2015, 241, 387–402. [Google Scholar] [CrossRef]
- Zhou, H.; Liao, L.; Xu, S.; Ren, F.; Zhao, J.; Ogutu, C.; Wang, L.; Jiang, Q.; Han, Y. Two amino acid changes in the R3 repeat cause functional divergence of two clustered MYB10 genes in peach. Plant Mol. Biol. 2018, 98, 169–183. [Google Scholar] [CrossRef]
- Guo, P.J.; Liu, J.P.; Han, Q.Z.; Gao, J.H. Effect of N-terminal signal peptide of Cry1Ia protein on its protein expression. J. Shanxi Agric. Sci. 2021, 49, 694–698. [Google Scholar]
- Kang, R.; Zhao, R.; Wang, L.; Liu, C.; Zhang, F.; Zhou, Q. Genome-Wide Identification and Characterization of Calmodulin and Calmodulin-like Genes Family in Tea Plant and Their Roles under Abiotic Stress. Forests 2022, 13, 1578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).