Quercus suber Bark as a Sustainable Source of Value-Added Compounds: Experimental Studies with Cork By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Physicochemical Characterization
Particle Size
Colour
Moisture Content
Hygroscopicity
pH
Heavy Metals Content
Volatile Compound Analysis
Preparation of P1 Ethanolic Extracts
DPPH Scavenging Activity
Folin-Ciocalteu Assay
NIR Spectral Acquisition
Chemometric Analysis
3. Results and Discussion
3.1. General Physicochemical Characterization
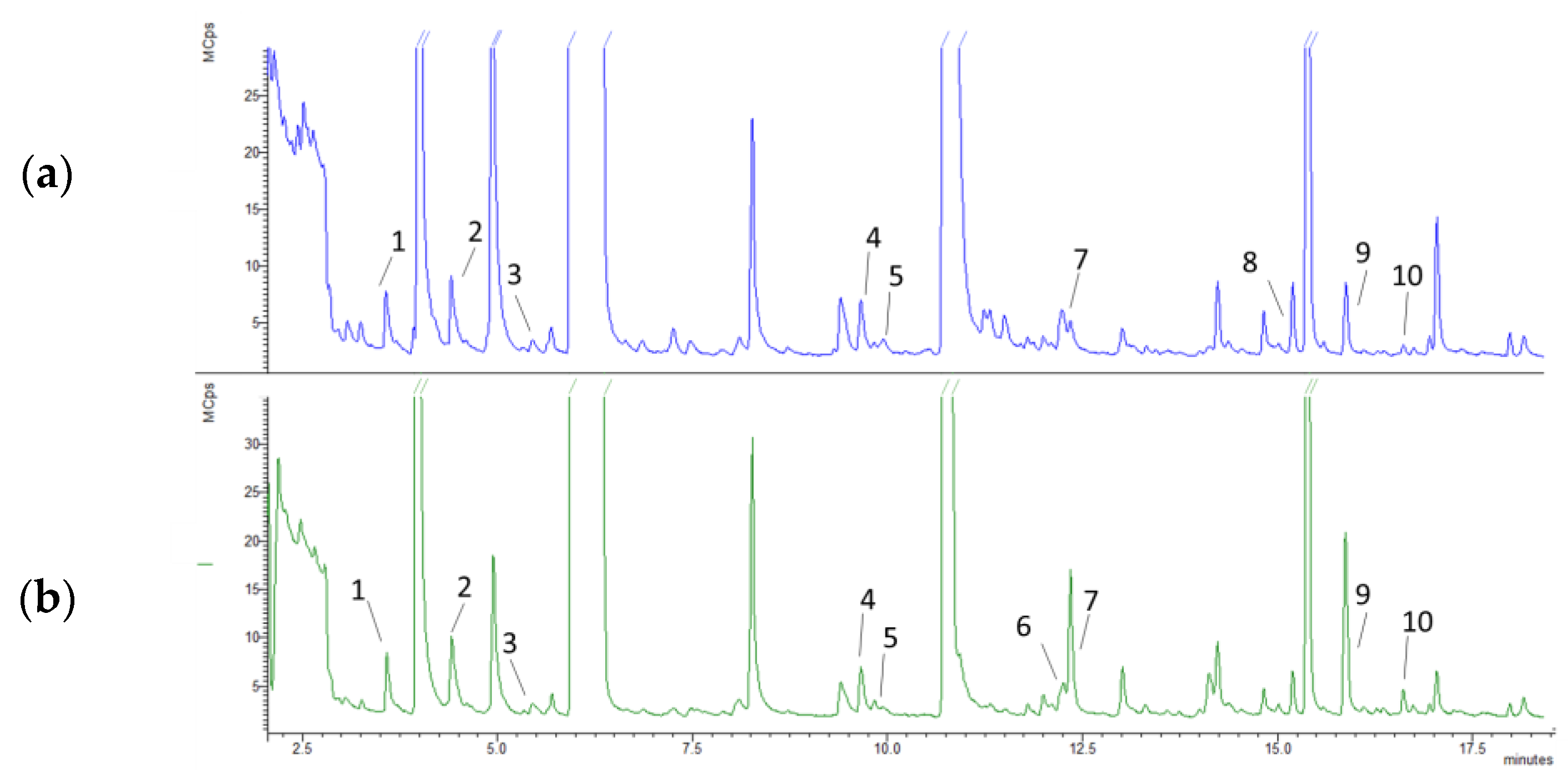
3.2. Volatile Compound Analysis
3.3. DPPH Scavenging Activity and Folin Ciocalteau Assay
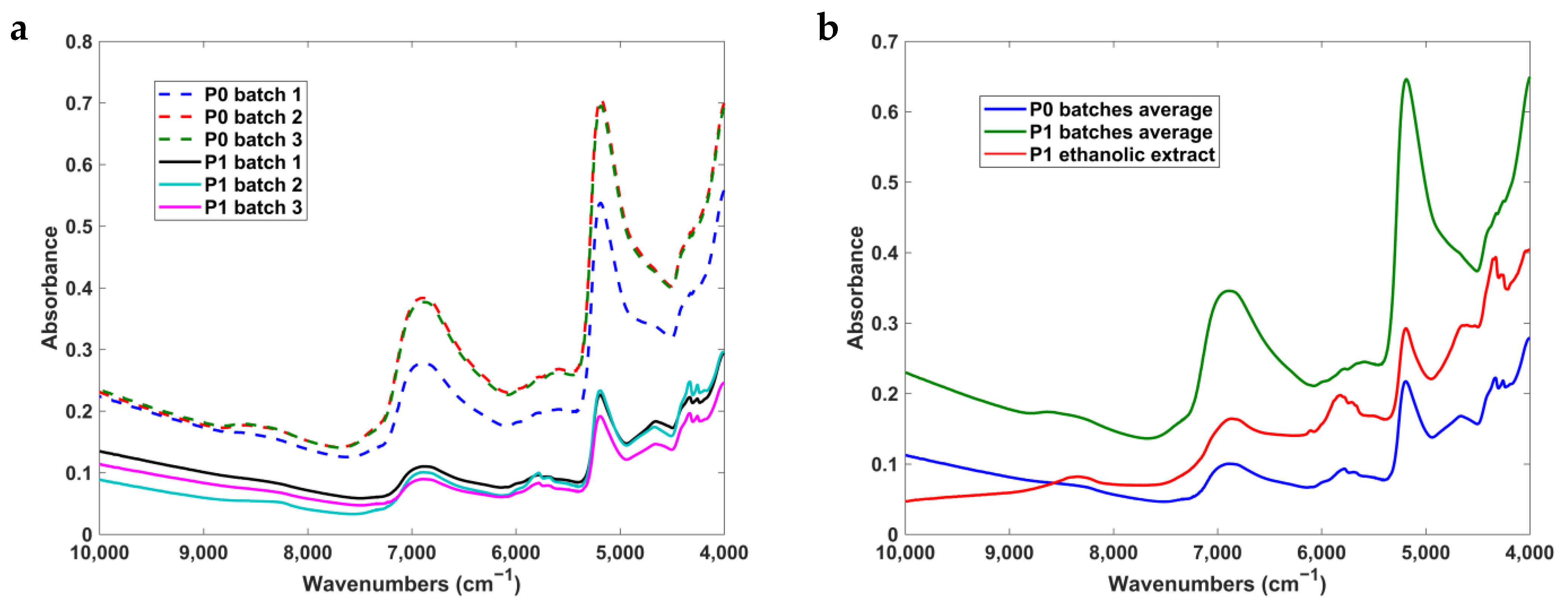
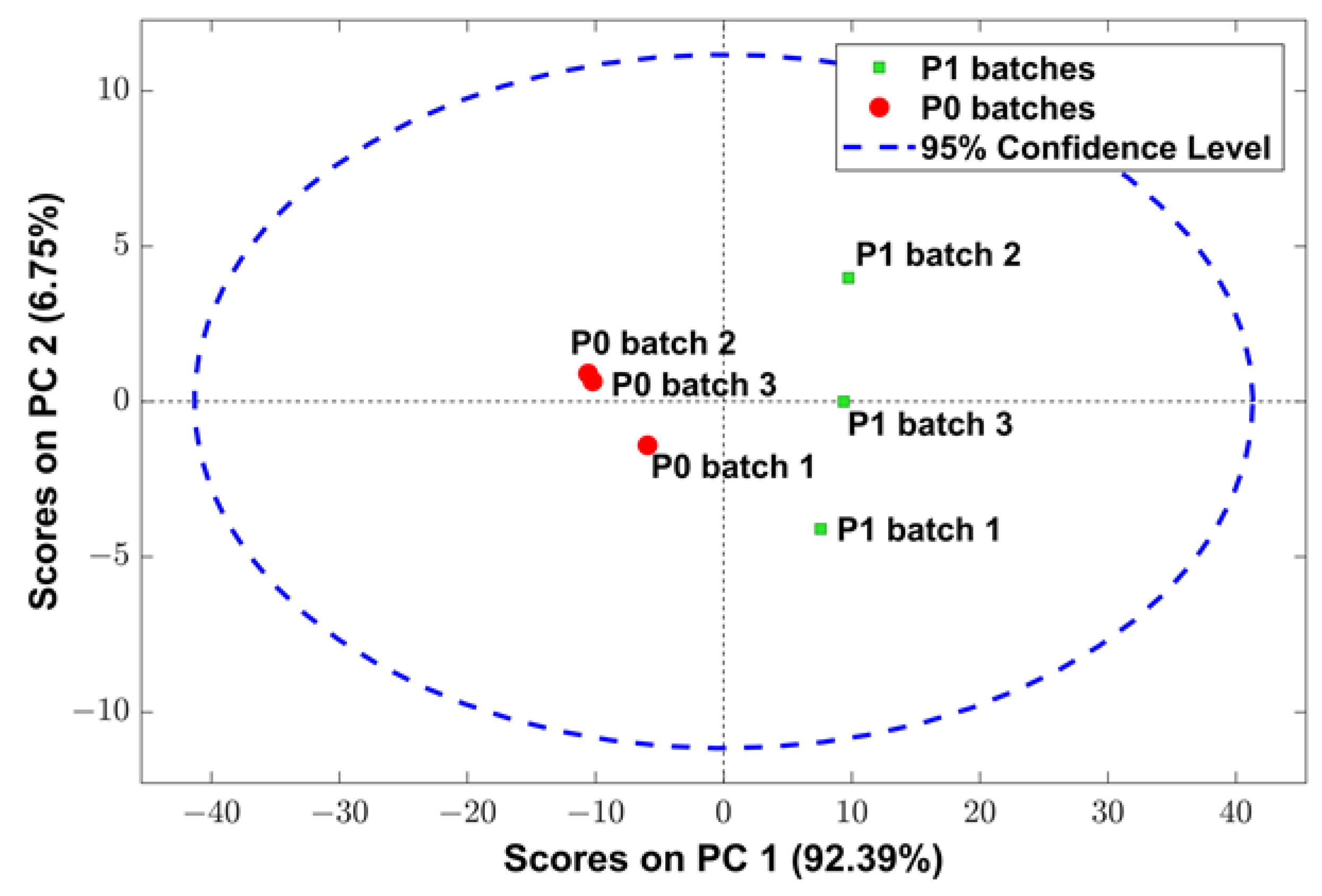
3.4. NIR Spectra Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruggerio, C.A. Sustainability and sustainable development: A review of principles and definitions. Sci. Total Environ. 2021, 786, 147481. [Google Scholar] [CrossRef] [PubMed]
- United Nations. The 17 Goals—History. Available online: https://sdgs.un.org/goals (accessed on 25 November 2022).
- Economy, E.P. Circular Economy: Definition, Importance and Benefits. Available online: https://www.europarl.europa.eu/news/en/headlines/economy/20151201STO05603/circular-economy-definition-importance-and-benefits (accessed on 28 November 2022).
- Mota, S.; Pinto, C.; Cravo, S.; Rocha e Silva, J.; Afonso, C.; Sousa Lobo, J.M.; Tiritan, M.E.; Cidade, H.; Almeida, I.F. Quercus suber: A Promising Sustainable Raw Material for Cosmetic Application. Appl. Sci. 2022, 12, 4604. [Google Scholar] [CrossRef]
- Morganti, P.; Gao, X.; Vukovic, N.; Gagliardini, A.; Lohani, A.; Morganti, G. Food Loss and Food Waste for Green Cosmetics and Medical Devices for a Cleaner Planet. Cosmetics 2022, 9, 19. [Google Scholar] [CrossRef]
- Teixeira, R.T. Cork Development: What Lies Within. Plants 2022, 11, 2671. [Google Scholar] [CrossRef]
- Quinto-Canas, R.; Cano-Ortiz, A.; Raposo, M.; Piñar Fuentes, J.C.; Cano, E.; Barbosa, N.; Pinto Gomes, C.J. Cork oak vegetation series of southwestern iberian peninsula: Diversity and ecosystem services. In New Metropolitan Perspectives. NMP 2020. Smart Innovation, Systems and Technologies; Bevilacqua, C., Calabrò, F., Della Spina, L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 178, pp. 1279–1290. [Google Scholar] [CrossRef]
- Cortiça, A.-A.P.D. Montado—Environmental Sustainability. Available online: https://www.apcor.pt/en/montado/sustainability/environmental-sustainability/ (accessed on 28 November 2022).
- Oliveira, G.; Costa, A. How resilient is Quercus suber L. to cork harvesting? A review and identification of knowledge gaps. For. Ecol. Manag. 2012, 270, 257–272. [Google Scholar] [CrossRef]
- Pérez-Girón, J.C.; Díaz-Varela, E.R.; Álvarez-Álvarez, P. Climate-driven variations in productivity reveal adaptive strategies in Iberian cork oak agroforestry systems. For. Ecosyst. 2022, 9, 100008. [Google Scholar] [CrossRef]
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Gil, L. Cork: Sustainability and New Applications. Front. Mater. 2015, 1, 38. [Google Scholar] [CrossRef]
- Associação Portuguesa da Cortiça. CORK_2020. Boletim Estatístico. Available online: https://www.apcor.pt/portfolio-posts/boletim-estatistico-2020/ (accessed on 13 December 2022).
- Gil, L. Cork powder waste: An overview. Biomass Bioenergy 1997, 13, 59–61. [Google Scholar] [CrossRef]
- Carriço, C.; Ribeiro, H.M.; Marto, J. Converting cork by-products to ecofriendly cork bioactive ingredients: Novel pharmaceutical and cosmetics applications. Ind. Crops Prod. 2018, 125, 72–84. [Google Scholar] [CrossRef]
- Sousa, V.B.; Leal, S.; Quilho, T.; Pereira, H. Characterization of Cork Oak (Quercus suber) Wood Anatomy. Iawa J. 2009, 30, 149–161. [Google Scholar] [CrossRef]
- Fernandes, A.; Sousa, A.; Mateus, N.; Cabral, M.; de Freitas, V. Analysis of phenolic compounds in cork from Quercus suber L. by HPLC-DAD/ESI-MS. Food Chem. 2011, 125, 1398–1405. [Google Scholar] [CrossRef]
- Mislata, A.M.; Puxeu, M.; Ferrer-Gallego, R. Aromatic potential and bioactivity of cork stoppers and cork by-products. Foods 2020, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Cadahía, E.; García-Vallejo, M.C.; González-Adrados, J.R. Chemical characterization of reproduction cork from spanish Quercus suber. J. Wood Chem. Technol. 1998, 18, 447–469. [Google Scholar] [CrossRef]
- Reis, S.F.; Lopes, P.; Roseira, I.; Cabral, M.; Mateus, N.; Freitas, V. Recovery of added value compounds from cork industry by-products. Ind. Crops Prod. 2019, 140, 111599. [Google Scholar] [CrossRef]
- Sousa, A.F.; Pinto, P.C.; Silvestre, A.J.; Pascoal Neto, C. Triterpenic and other lipophilic components from industrial cork byproducts. J. Agric. Food Chem. 2006, 54, 6888–6893. [Google Scholar] [CrossRef]
- Carriço, C.; Pinto, P.; Graça, A.; Gonçalves, L.M.; Ribeiro, H.M.; Marto, J. Design and characterization of a new Quercus suber-based pickering emulsion for topical application. Pharmaceutics 2019, 11, 131. [Google Scholar] [CrossRef]
- Associação Portuguesa da Cortiça. Cork Processing—Industrial Path—Natural Cork Stoppers. Available online: https://www.apcor.pt/en/cork/processing/industrial-path/natural-cork-stoppers/ (accessed on 15 December 2022).
- Faé, G.S.; Montes, F.; Bazilevskaya, E.; Añó, R.M.; Kemanian, A.R. Making Soil Particle Size Analysis by Laser Diffraction Compatible with Standard Soil Texture Determination Methods. Soil Sci. Soc. Am. J. 2019, 83, 1244–1252. [Google Scholar] [CrossRef]
- Konen, M.E.; Burras, C.L.; Sandor, J.A. Organic Carbon, Texture, and Quantitative Color Measurement Relationships for Cultivated Soils in North Central Iowa. Soil Sci. Soc. Am. J. 2003, 67, 1823–1830. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- United States Environmental Protection Agency (EPA). Test Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; EPA SW-846, Revision 3; EPA: Washington, DC, USA, 1996. [Google Scholar]
- Pinto, J.; Oliveira, A.S.; Lopes, P.; Roseira, I.; Cabral, M.; de Lourdes Bastos, M.; Guedes de Pinho, P. Characterization of chemical compounds susceptible to be extracted from cork by the wine using GC-MS and 1H NMR metabolomic approaches. Food Chem. 2019, 271, 639–649. [Google Scholar] [CrossRef]
- Cruz, I.; Puthongking, P.; Cravo, S.; Palmeira, A.; Cidade, H.; Pinto, M.; Sousa, E. Xanthone and Flavone Derivatives as Dual Agents with Acetylcholinesterase Inhibition and Antioxidant Activity as Potential Anti-Alzheimer Agents. J. Chem. 2017, 2017, 8587260. [Google Scholar] [CrossRef]
- Vale, A.P.; Cidade, H.; Pinto, M.; Oliveira, M.B. Effect of sprouting and light cycle on antioxidant activity of Brassica oleracea varieties. Food Chem. 2014, 165, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Alves, R.C.; Costa, A.S.G.; Jerez, M.; Casal, S.; Sineiro, J.; Núñez, M.J.; Oliveira, B. Antiradical Activity, Phenolics Profile, and Hydroxymethylfurfural in Espresso Coffee: Influence of Technological Factors. J. Agric. Food Chem. 2010, 58, 12221–12229. [Google Scholar] [CrossRef] [PubMed]
- Ireneusz, O.; Marcin, C.; Mateusz, S.; Karolina, L.; Marcin, O.; Mateusz, P. Studies on Moisture Effects on Powder Flow and Mechanochemical Improvement of Powder Flowability. Adv. Sci. Technol. Res. J. 2021, 15, 228–246. [Google Scholar] [CrossRef]
- Hunt, R.W.G.; Pointer, M.R. Measuring Color; John Wiley & Sons Inc.: New York, NY, USA, 2011. [Google Scholar]
- MacDougall, D. Colour in Food: Improving Quality; Elsevier Science: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Guideline Q3D (R2) on Elemental Impurities; European Medicines Agency: Amsterdam, The Netherlands, 2022. [Google Scholar]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Off. J. Eur. Union 2009, L 342, 59–209. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L 364, 5–24. [Google Scholar]
- European Commission. Commission Regulation (EU) No. 488/2014 amending Regulation (EC) No. 1881/2006 as regards maximum levels of cadmium in foodstuffs. Off. J. Eur. Union 2021, L 138, 75–79. [Google Scholar]
- European Commission. Commission Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2021, L 314M, 558–577. [Google Scholar]
- Moreira, N.; Lopes, P.; Cabral, M.; Guedes de Pinho, P. HS-SPME/GC-MS methodologies for the analysis of volatile compounds in cork material. Eur. Food Res. Technol. 2016, 242, 457–466. [Google Scholar] [CrossRef]
- Furtado, I.; Oliveira, A.S.; Amaro, F.; Lopes, P.; Cabral, M.; de Lourdes Bastos, M.; Guedes de Pinho, P.; Pinto, J. Volatile profile of cork as a tool for classification of natural cork stoppers. Talanta 2021, 223, 121698. [Google Scholar] [CrossRef] [PubMed]
- Furtado, I.; Lopes, P.; Oliveira, A.S.; Amaro, F.; de Lourdes Bastos, M.; Cabral, M.; Guedes de Pinho, P.; Pinto, J. The Impact of Different Closures on the Flavor Composition of Wines during Bottle Aging. Foods 2021, 10, 2070. [Google Scholar] [CrossRef] [PubMed]
- Viant, M.R.; Kurland, I.J.; Jones, M.R.; Dunn, W.B. How close are we to complete annotation of metabolomes? Curr. Opin. Chem. Biol. 2017, 36, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Soares-Castro, P.; Soares, F.; Santos, P.M. Current Advances in the Bacterial Toolbox for the Biotechnological Production of Monoterpene-Based Aroma Compounds. Molecules 2021, 26, 91. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Seol, G.H.; Kim, K.Y. Eucalyptol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor--a fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Song, R.; Wu, Q.; Zhao, L.; Yun, Z. Advances on antioxidants in research and applications. E3S Web Conf. 2019, 131, 01009. [Google Scholar] [CrossRef]
- Griffiths, K.; Aggarwal, B.B.; Singh, R.B.; Buttar, H.S.; Wilson, D.; De Meester, F. Food Antioxidants and Their Anti-Inflammatory Properties: A Potential Role in Cardiovascular Diseases and Cancer Prevention. Diseases 2016, 4, 28. [Google Scholar] [CrossRef]
- Brudzyńska, P.; Kurzawa, M.; Sionkowska, A.; Grisel, M. Antioxidant Activity of Plant-Derived Colorants for Potential Cosmetic Application. Cosmetics 2022, 9, 81. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Pinto, P.C.R.O.; Silvestre, A.J.D.; Neto, C.P. Chemical composition and antioxidant activity of phenolic extracts of cork from Quercus suber L. Ind. Crops Prod. 2010, 31, 521–526. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Conde, E.; Cadahia, E.; GarciaVallejo, M.C.; de Simon, B.F.; Adrados, J.R.G. Low molecular weight polyphenols in cork of Quercus suber. J. Agric. Food Chem. 1997, 45, 2695–2700. [Google Scholar] [CrossRef]
- Castola, V.; Marongiu, B.; Bighelli, A.; Floris, C.; Laï, A.; Casanova, J. Extractives of cork (Quercus suber L.): Chemical composition of dichloromethane and supercritical CO2 extracts. Ind. Crops Prod. 2005, 21, 65–69. [Google Scholar] [CrossRef]
- Fernandes, A.; Fernandes, I.; Cruz, L.s.; Mateus, N.; Cabral, M.; de Freitas, V. Antioxidant and Biological Properties of Bioactive Phenolic Compounds from Quercus suber L. J. Agric. Food Chem. 2009, 57, 11154–11160. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.; Fernandes, A.; Oliveira, J.; Brás, N.F.; Reis, S.; Lopes, P.; Roseira, I.; Cabral, M.; Mateus, N.; de Freitas, V. Reactivity of Cork Extracts with (+)-Catechin and Malvidin-3-O-glucoside in Wine Model Solutions: Identification of a New Family of Ellagitannin-Derived Compounds (Corklins). J. Agric. Food Chem. 2017, 65, 8714–8726. [Google Scholar] [CrossRef]
- Araújo, A.R.; Pereira, D.M.; Aroso, I.M.; Santos, T.; Batista, M.T.; Cerqueira, M.T.; Marques, A.P.; Reis, R.L.; Pires, R.A. Cork extracts reduce UV-mediated DNA fragmentation and cell death. RSC Adv. 2015, 5, 96151–96157. [Google Scholar] [CrossRef]
- Cunha, M.; Lourenço, A.; Barreiros, S.; Paiva, A.; Simões, P. Valorization of Cork Using Subcritical Water. Molecules 2020, 25, 4695. [Google Scholar] [CrossRef]
- Batista, M.; Rosete, M.; Ferreira, I.; Ferreira, J.; Duarte, C.; Matias, A.; Poejo, J.; Crespo, J.; Valério, R.; Fraga, M.; et al. Extracto Hidro-Glicólico de Cortiça, Processo para a sua Preparação, Formulações Compreendendo o Referido Extracto e sua Utilização. International Patent WO2015152746A1; filed 1 April 2015, issued 8 October, 2015. [Google Scholar]
- Aroso, I.M.; Araújo, A.R.; Fernandes, J.P.; Santos, T.; Batista, M.T.; Pires, R.A.; Mano, J.F.; Reis, R.L. Hydroalcoholic extracts from the bark of Quercus suber L. (Cork): Optimization of extraction conditions, chemical composition and antioxidant potential. Wood Sci. Technol. 2017, 51, 855–872. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Villaverde, J.J.; Sousa, A.F.; Coelho, J.F.J.; Neto, C.P.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of industrial cork by-products. Ind. Crops Prod. 2013, 47, 262–269. [Google Scholar] [CrossRef]
- Prades, C.; García-Olmo, J.; Romero-Prieto, T.; García de Ceca, J.L.; López-Luque, R. Methodology for cork plank characterization (Quercus suber L.) by near-infrared spectroscopy and image analysis. Meas. Sci. Technol. 2010, 21, 065602. [Google Scholar] [CrossRef]
- Prades, C.; Gómez-Sánchez, I.; Garcia-Olmo, J.; González-Hernández, F.; Gonzalez-Adrados, J.R. Application of VIS/NIR spectroscopy for estimating chemical, physical and mechanical properties of cork stoppers. Wood Sci. Technol. 2014, 48, 811–830. [Google Scholar] [CrossRef]
- Prades, C.; Cardillo-Amo, E.; Beira-Dávila, J.; Serrano-Crespín, A.; Nuñez-Sánchez, N. Evaluation of Parameters that Determine Cork Plank Quality (Quercus suber L.) by Near Infrared Spectroscopy. J. Wood Chem. Technol. 2017, 37, 369–382. [Google Scholar] [CrossRef]
- Pérez-Terrazas, D.; González-Adrados, J.R.; Sánchez-González, M. Qualitative and quantitative assessment of cork anomalies using near infrared spectroscopy (NIRS). Food Packag. Shelf Life 2020, 24, 100490. [Google Scholar] [CrossRef]
- Burns, D.A.; Ciurczak, E.W. Chapter 17—Calibration Transfer. In Handbook of Near-Infrared Analysis; CRC Press: Boca Raton, FL, USA, 2007; pp. 347–386. [Google Scholar]
- Joshi, R.; Sathasivam, R.; Jayapal, P.K.; Patel, A.K.; Nguyen, B.V.; Faqeerzada, M.A.; Park, S.U.; Lee, S.H.; Kim, M.S.; Baek, I.; et al. Comparative Determination of Phenolic Compounds in Arabidopsis thaliana Leaf Powder under Distinct Stress Conditions Using Fourier-Transform Infrared (FT-IR) and Near-Infrared (FT-NIR) Spectroscopy. Plants 2022, 11, 836. [Google Scholar] [CrossRef]
- Muthudoss, P.; Tewari, I.; Chi, R.L.R.; Young, K.J.; Ann, E.Y.C.; Hui, D.N.S.; Khai, O.Y.; Allada, R.; Rao, M.; Shahane, S.; et al. Machine Learning-Enabled NIR Spectroscopy in Assessing Powder Blend Uniformity: Clear-Up Disparities and Biases Induced by Physical Artefacts. AAPS PharmSciTech 2022, 23, 277. [Google Scholar] [CrossRef]
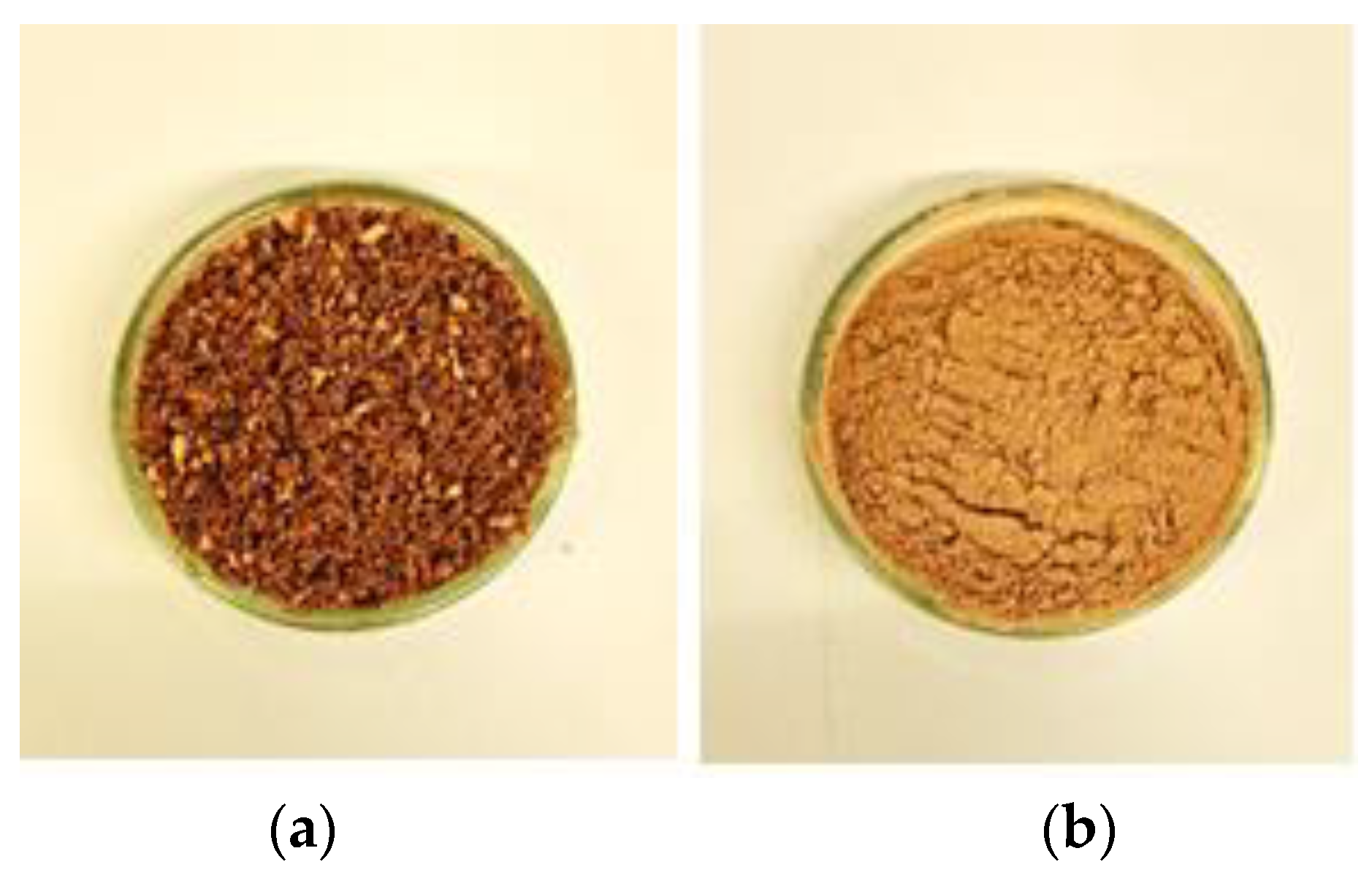
| Parameter | P0 | P1 |
|---|---|---|
| Particle size (µm) | ||
| Dx10 | 463 ± 286 | 6.7 ± 0.0 |
| Dx50 | 1200 ± 257 | 24.6 ± 0.1 |
| Dx90 | 2340 ± 198 | 72.5 ± 1.2 |
| Color | L* = 40.20 ± 0.40 | L* = 54.83 ± 0.16 |
| a* = 6.97 ± 0.30 | a* = 7.67 ± 0.15 | |
| b* = 11.20 ± 0.27 | b* = 16.62 ± 0.17 | |
| Moisture content (%) | 19.3 ± 7.2 | 7.6 ± 1.0 |
| Hygroscopicity (%) | --- | 0.88 ± 0.73 |
| pH | 6.70 ± 0.05 | 5.51 ± 0.01 |
| Heavy metals (µg/g) | As = 0.21 ± 0.02 | As = 0.40 ± 0.02 |
| Cd = 0.058 ± 0.003 | Cd = 0.106 ± 0.003 | |
| Hg = 0.008 ± 0.000 | Hg = 0.007 ± 0.000 | |
| Pb = 1.04 ± 0.14 | Pb = 2.40 ± 0.14 |
| Compounds | RT (min) | m/z | R Match | Area (AU) | Odor Descriptor | |
|---|---|---|---|---|---|---|
| P0 ± SD (10⁶) | P1 ± SD (10⁶) | |||||
| Ketones | ||||||
| Pent-2-one L1 | 3.60 | 58, 71, 86 | 895 | 21.60 ± 5.72 | 19.00± 6.25 | Fruity [44] |
| 4-methylpent-2-one L2 | 4.43 | 58, 85, 100 | 805 | 33.60 ± 7.51 | 23.90 ± 14.70 | |
| Hex-2-one L1 | 5.44 | 58, 85, 100 | 869 | 6.07 ± 0.98 | 4.81 ± 1.19 | Ether [44] |
| Aromatic aldehydes | ||||||
| Vanillin L1 | 9.66 | 193, 209 | 686 | 18.50 ± 1.48 | 15.40 ± 2.22 | Vanilla like [28] |
| Monoterpenes | ||||||
| Camphene L2 | 9.83 | 93, 121 | 925 | 1.19 ± 0.04 | 2.74 ± 0.71 | Camphor, herbal, woody [28] |
| Limonene L1 | 12.25 | 67, 68, 93 | 871 | --- | 2.32 ± 0.68 | Lemon, orange [28] |
| Eucalyptol L1 | 12.35 | 81, 93, 108 | 800 | 2.37 ± 1.45 | 46.40 ± 8.59 | Mint, herbal [28] |
| Camphor L1 | 15.87 | 95, 109 | 901 | 22.30 ± 0.81 | 62.00 ± 1.50 | Camphor [28] |
| Borneol L2 | 16.61 | 95, 100 | 809 | 2.02 ± 0.15 | 7.18 ± 0.60 | Camphor like [28] |
| Alcohols | ||||||
| Phenylethyl alcohol L1 | 15 | 91, 92 | 824 | 0.97 ± 0.09 | --- | Rose, honey, talc [28] |
| 40 °C | RT | |
|---|---|---|
| Total Phenolic Compounds (mg GAE/g of extract) | 468.5 ± 0.57 | 354.7 ± 0.21 |
| DPPH (IC50 = μg of extract/mL) | 3.67 ± 0.02 | 7.33 ± 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rego, L.; Mota, S.; Torres, A.; Pinto, C.; Cravo, S.; Silva, J.R.e.; Páscoa, R.N.M.J.; Almeida, A.; Amaro, F.; Pinho, P.G.; et al. Quercus suber Bark as a Sustainable Source of Value-Added Compounds: Experimental Studies with Cork By-Products. Forests 2023, 14, 543. https://doi.org/10.3390/f14030543
Rego L, Mota S, Torres A, Pinto C, Cravo S, Silva JRe, Páscoa RNMJ, Almeida A, Amaro F, Pinho PG, et al. Quercus suber Bark as a Sustainable Source of Value-Added Compounds: Experimental Studies with Cork By-Products. Forests. 2023; 14(3):543. https://doi.org/10.3390/f14030543
Chicago/Turabian StyleRego, Liliana, Sandra Mota, Ana Torres, Cláudia Pinto, Sara Cravo, Joana Rocha e Silva, Ricardo N. M. J. Páscoa, Agostinho Almeida, Filipa Amaro, Paula Guedes Pinho, and et al. 2023. "Quercus suber Bark as a Sustainable Source of Value-Added Compounds: Experimental Studies with Cork By-Products" Forests 14, no. 3: 543. https://doi.org/10.3390/f14030543
APA StyleRego, L., Mota, S., Torres, A., Pinto, C., Cravo, S., Silva, J. R. e., Páscoa, R. N. M. J., Almeida, A., Amaro, F., Pinho, P. G., Cidade, H., Silva, J. P., Sousa Lobo, J. M., & Almeida, I. F. (2023). Quercus suber Bark as a Sustainable Source of Value-Added Compounds: Experimental Studies with Cork By-Products. Forests, 14(3), 543. https://doi.org/10.3390/f14030543










