An Integrative Analysis of Metabolome and Transcriptome Reveals the Molecular Regulatory Mechanism of the Accumulation of Flavonoid Glycosides in Different Cyclocarya paliurus Ploidies
Abstract
:1. Introduction
2. Results
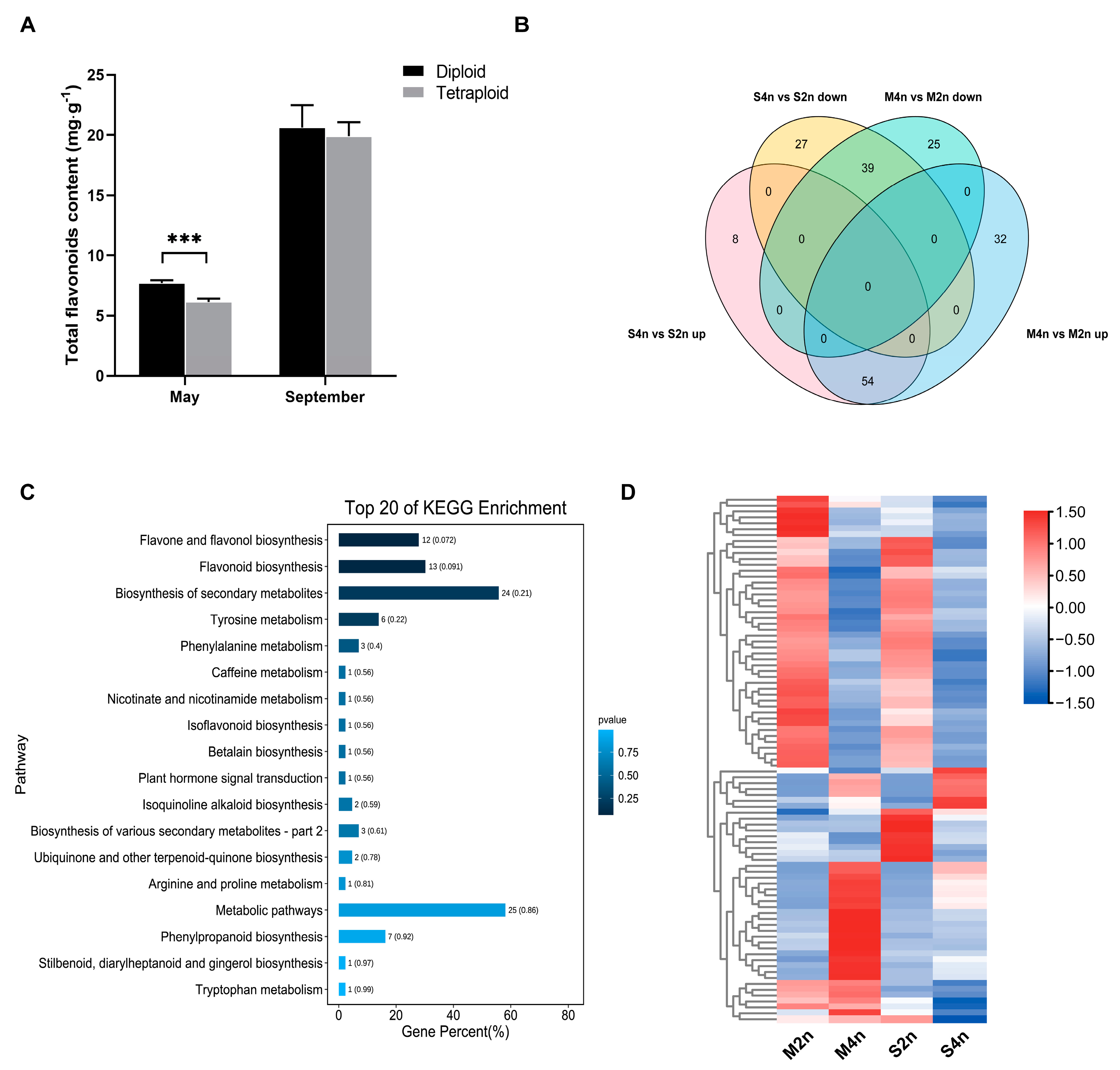
2.1. Accumulation Pattern of Flavonoids in Leaves Obtained from C. paliurus with Different Ploidies
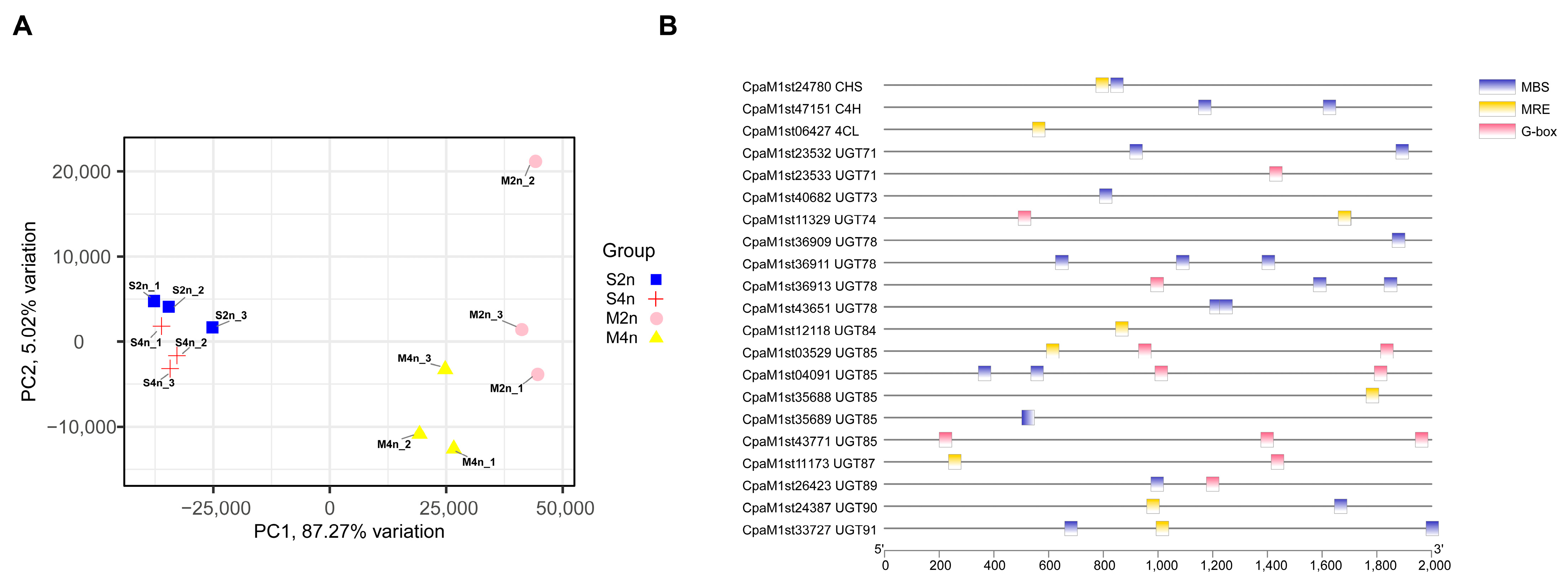
2.2. Differences in Flavonoid Glycosides in Two-Ploidy C. paliurus at the Transcriptome Level
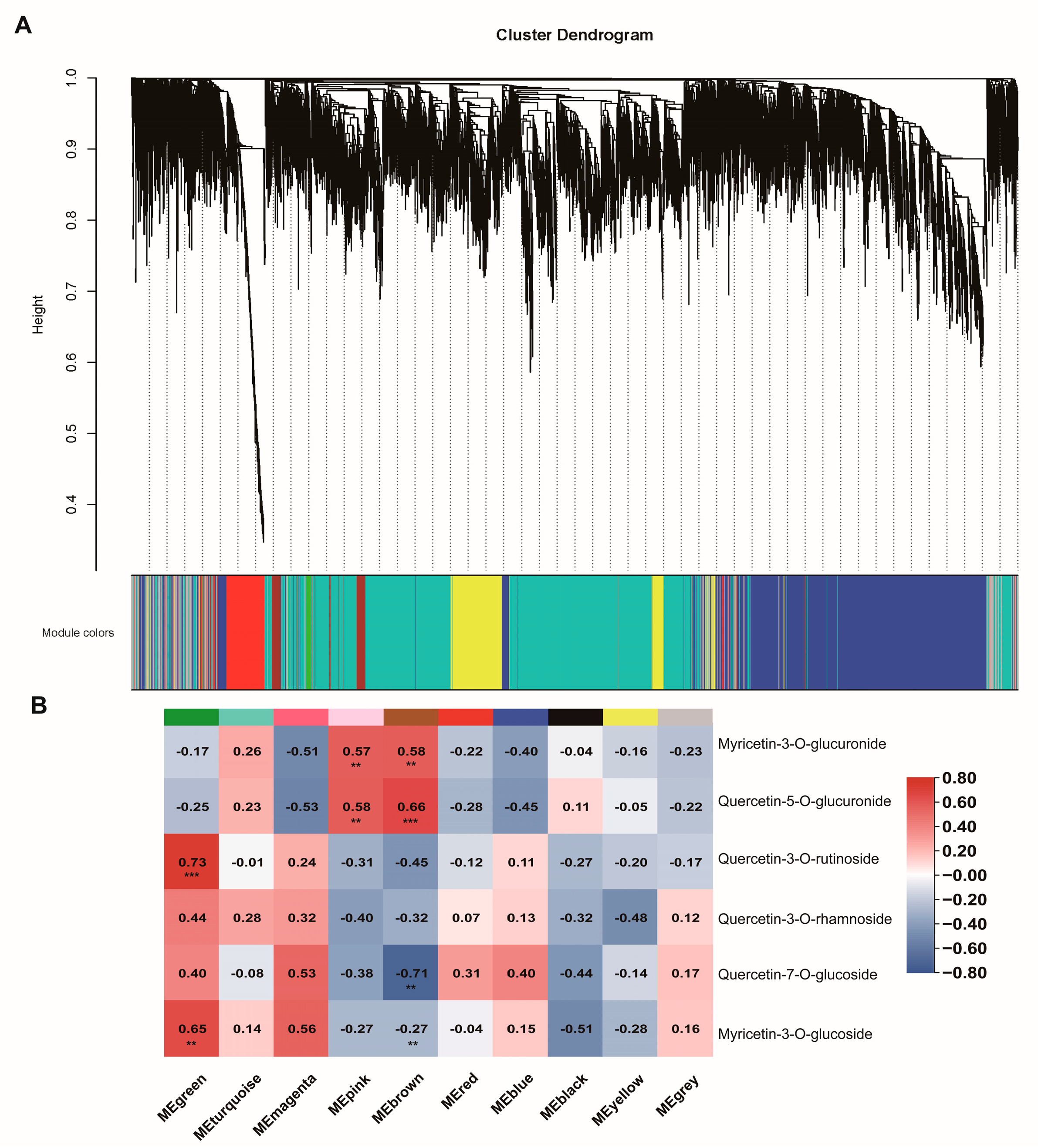
2.3. Co-Expression Network Analysis of Different C. paliurus Ploidies
3. Discussion
3.1. Differential Accumulation of Flavonoids between di- and Tetraploid C. paliurus
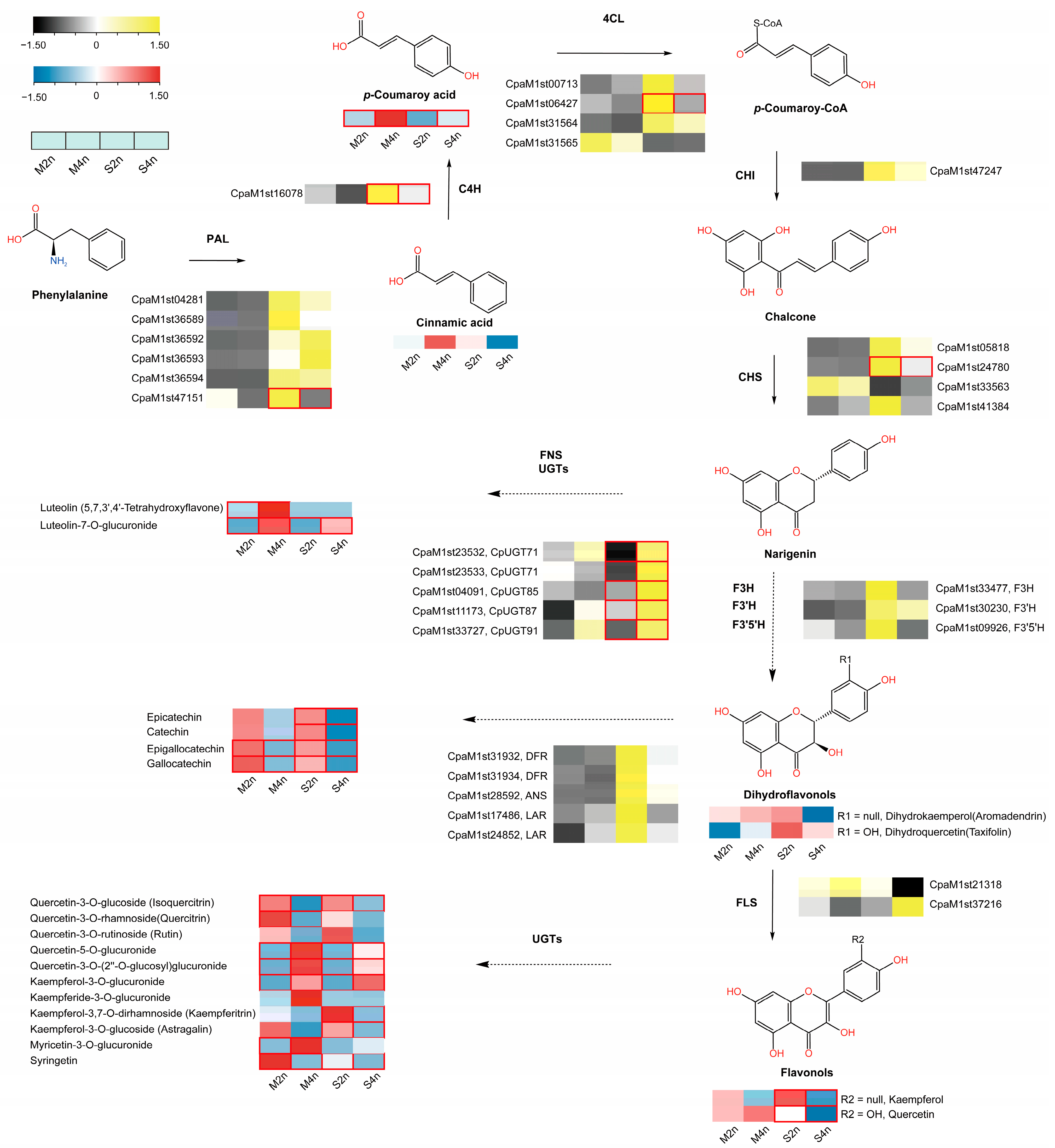
3.2. Differential Patterns of Gene Expressions between di- and Tetraploid C. paliurus
3.3. Ploidazation Can Affect Flavonoid Biosynthesis Occurring in C. paliurus
4. Materials and Methods
4.1. Materials and Samples
4.2. Flavonoid Content Measurement
4.3. Non-Targeted Metabolite Analysis Using the UPLC-MS/MS System
4.4. Transcriptomic Analysis and Co-Expression Network Construct
4.5. RT-qPCR Analysis
4.6. Phylogenic Tree Construct
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of Oxidative and Drought Tolerance in Arabidopsis by Overaccumulation of Antioxidant Flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of Phytohormone Signaling: A Primary Function of Flavonoids in Plant–Environment Interactions. Front. Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Miglio, C.; Morabito, G.; Ioannone, F.; Serafini, M. Flavonoids and Immune Function in Human: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 383–395. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Wang, X.; Wu, L.; Shen, T.; Ji, L.; Zhao, X.; Si, C.-L.; Jiang, Y.; Wang, G. Apigenin-7-O-β-d-Glucuronide Inhibits LPS-Induced Inflammation through the Inactivation of AP-1 and MAPK Signaling Pathways in RAW 264.7 Macrophages and Protects Mice against Endotoxin Shock. Available online: https://pubmed.ncbi.nlm.nih.gov/26750400/ (accessed on 20 January 2023).
- Cho, Y.-C.; Park, J.; Cho, S. Anti-Inflammatory and Anti-Oxidative Effects of Luteolin-7-O-Glucuronide in LPS-Stimulated Murine Macrophages through TAK1 Inhibition and Nrf2 Activation. Int. J. Mol. Sci. 2020, 21, 2007. [Google Scholar] [CrossRef] [Green Version]
- De Bruyn, F.; Maertens, J.; Beauprez, J.; Soetaert, W.; De Mey, M. Biotechnological Advances in UDP-Sugar Based Glycosylation of Small Molecules. Biotechnol. Adv. 2015, 33, 288–302. [Google Scholar] [CrossRef]
- Xiao, J. Dietary Flavonoid Aglycones and Their Glycosides: Which Show Better Biological Significance? Crit. Rev. Food Sci. Nutr. 2015, 57, 1874–1905. [Google Scholar] [CrossRef]
- Ross, J.; Li, Y.; Lim, E.-K.; Bowles, D.J. Higher Plant Glycosyltransferases. Genome Biol. 2001, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Structure, Mechanism and Engineering of Plant Natural Product Glycosyltransferases. FEBS Lett. 2009, 583, 3303–3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, A.; Horikawa, M.; Fukui, Y.; Fukuchi-Mizutani, M.; Iuchi-Okada, A.; Ishiguro, M.; Kiso, Y.; Nakayama, T.; Ono, E. Local Differentiation of Sugar Donor Specificity of Flavonoid Glycosyltransferase in Lamiales. Plant Cell 2009, 21, 1556–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, T.; Chen, R.; Chen, D.; Liu, J.; Xie, K.; Dai, J. Enzymatic Synthesis of Bioactive O-Glucuronides Using Plant Glucuronosyltransferases. J. Agric. Food Chem. 2019, 67, 6275–6284. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Yang, W.; Chu, X.; Shang, X.; She, C.; Fu, X. Provenance and Temporal Variations in Selected Flavonoids in Leaves of Cyclocarya paliurus. Food Chem. 2011, 124, 1382–1386. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, C.; Yao, N.; Li, Y.; Wang, Q.; Fang, S.; Shang, X.; Zhao, M.; Che, C.; Ni, Y.; et al. Antihyperlipidemic Effect of Cyclocarya paliurus (Batal.) Iljinskaja Extract and Inhibition of Apolipoprotein B48 Overproduction in Hyperlipidemic Mice. J. Ethnopharmacol. 2015, 166, 286–296. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Fang, S.; Wang, T.; Yin, Z.; Shang, X.; Yang, W.; Fu, X. Antidiabetic Effect of Cyclocarya paliurus Leaves Depends on the Contents of Antihyperglycemic Flavonoids and Antihyperlipidemic Triterpenoids. Molecules 2018, 23, 1042. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-M.; Yin, Z.-Q.; Zhao, M.-G.; Jiang, C.-H.; Zhang, J.; Pan, K. Pentacyclic Triterpenoids from Cyclocarya paliurus and Their Antioxidant Activities in FFA-Induced HepG2 Steatosis Cells. Phytochemistry 2018, 151, 119–127. [Google Scholar] [CrossRef]
- Zhou, M.; Lin, Y.; Fang, S.; Liu, Y.; Shang, X. Phytochemical Content and Antioxidant Activity in Aqueous Extracts of Cyclocarya paliurus Leaves Collected from Different Populations. PeerJ 2019, 7, e6492. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.; Shang, X.; Fang, S.; Li, Q.; Fu, X.; Su, J. Integrated Effects of Light Intensity and Fertilization on Growth and Flavonoid Accumulation in Cyclocarya paliurus. J. Agric. Food Chem. 2012, 60, 6286–6292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, S.; Yang, W.; Shang, X.; Fu, X. Light Quality Affects Flavonoid Production and Related Gene Expression in Cyclocarya paliurus. J. Photochem. Photobiol. B Biol. 2018, 179, 66–73. [Google Scholar] [CrossRef]
- Qu, Y.; Shang, X.; Zeng, Z.; Yu, Y.; Bian, G.; Wang, W.; Liu, L.; Tian, L.; Zhang, S.; Wang, Q.; et al. Whole-Genome Duplication Reshaped Adaptive Evolution in a Relict Plant Species, Cyclocarya paliurus. Genom. Proteom. Bioinform. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The Polyploidy and Its Key Role in Plant Breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef]
- De Jesus-Gonzalez, L.; Weathers, P.J. Tetraploid Artemisia Annua Hairy Roots Produce More Artemisinin than Diploids. Plant Cell Rep. 2003, 21, 809–813. [Google Scholar] [CrossRef]
- Xiang, Z.; Tang, X.; Liu, W.; Song, C. A Comparative Morphological and Transcriptomic Study on Autotetraploid Stevia rebaudiana (Bertoni) and Its Diploid. Plant Physiol. Biochem. 2019, 143, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.L.; Chen, B.J.; Zhu, D.N. In Vitro Production and Identification of Autotetraploids of Scutellaria baicalensis. Plant Cell Tissue Organ. Cult. 2002, 70, 289–293. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Fang, S.; Liu, Y.; Shang, X. Integrative Analysis of Metabolome and Transcriptome Reveals Molecular Regulatory Mechanism of Flavonoid Biosynthesis in Cyclocarya paliurus under Salt Stress. Ind. Crops Prod. 2021, 170, 113823. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New Perspectives on Genomes, Pathways, Diseases and Drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional Control of Flavonoid Biosynthesis by MYB–BHLH–WDR Complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zong, X.; Ren, P.; Qian, Y.; Fu, A. Basic Helix-Loop-Helix (BHLH) Transcription Factors Regulate a Wide Range of Functions in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 7152. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Osbourn, A.; Ma, P. MYB Transcription Factors as Regulators of Phenylpropanoid Metabolism in Plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comai, L. The Advantages and Disadvantages of Being Polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Abdoli, M.; Moieni, A.; Naghdi Badi, H. Morphological, Physiological, Cytological and Phytochemical Studies in Diploid and Colchicine-Induced Tetraploid Plants of Echinacea purpurea (L.). Acta Physiol. Plant 2013, 35, 2075–2083. [Google Scholar] [CrossRef]
- Tavan, M.; Sarikhani, H.; Mirjalili, M.H.; Rigano, M.M.; Azizi, A. Triterpenic and Phenolic Acids Production Changed in Salvia Officinalis via in Vitro and in Vivo Polyploidization: A Consequence of Altered Genes Expression. Phytochemistry 2021, 189, 112803. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Yoon, J.S.; Park, S.-Y.; Kim, J.K.; Park, S.U. Comparative Analysis of Secondary Metabolites and Metabolic Profiling between Diploid and Tetraploid Morus alba L. J. Agric. Food Chem. 2021, 69, 1300–1307. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional Roles of Flavonoids in Photoprotection: New Evidence, Lessons from the Past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lin, W.; Yu, B.; Zhu, J.; Li, J. Integrated Metabolomic and Transcriptomic Analysis of the Flavonoid Accumulation in the Leaves of Cyclocarya paliurus at Different Altitudes. Front. Plant Sci. 2022, 12, 794137. [Google Scholar] [CrossRef]
- Xia, J.; Ma, Y.J.; Wang, Y.; Wang, J.W. Deciphering Transcriptome Profiles of Tetraploid Artemisia Annua Plants with High Artemisinin Content. Plant Physiol. Biochem. 2018, 130, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The Flavonoid Biosynthetic Pathway in Arabidopsis: Structural and Genetic Diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, W.; Li, D.; Li, X.; Gao, Y.; Li, W.; Li, H.; Liu, J.; Liu, H.; Chen, W.; Luo, J.; et al. Metabolome-Based Genome-Wide Association Study of Maize Kernel Leads to Novel Biochemical Insights. Nat. Commun. 2014, 5, 3438. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Gao, Y.; Gong, L.; Lu, K.; Wang, W.; Li, Y.; Liu, X.; Zhang, H.; Dong, H.; Zhang, W.; et al. Genome-Wide Association Analyses Provide Genetic and Biochemical Insights into Natural Variation in Rice Metabolism. Nat. Genet. 2014, 46, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Wang, Y.; Huang, F.; Lu, S.; Zhao, L.; Ma, X.; Kai, G. SmMYB2 Promotes Salvianolic Acid Biosynthesis in the Medicinal Herb Salvia miltiorrhiza. J. Integr. Plant Biol. 2020, 62, 1688–1702. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, Y.; Lu, Q.; Lu, H.; Li, J. Combined Analysis of the Metabolome and Transcriptome Identified Candidate Genes Involved in Phenolic Acid Biosynthesis in the Leaves of Cyclocarya paliurus. Int. J. Mol. Sci. 2020, 21, 1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, M.; Su, H.-Y.; Li, M.-L.; Yang, D.-L.; Yao, R.-Y.; Li, M.-F.; Wei, J.-H. Effect of UV-B Radiation on Growth, Flavonoid and Podophyllotoxin Accumulation, and Related Gene Expression in Sinopodophyllum hexandrum. Plant Biol. J. 2021, 23, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zheng, P.; Zhang, K.; Liao, J.; Xiong, F.; Shen, Q.; Ma, Y.; Fang, W.; Zhu, X. Effects of GABA on the Polyphenol Accumulation and Antioxidant Activities in Tea Plants (Camellia sinensis L.) under Heat-Stress Conditions. Plant Physiol. Biochem. 2021, 159, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, L.; Cai, Z.; Chen, C.; Liu, Z.; Liu, S.; Zou, L.; Tan, M.; Chen, J.; Liu, X.; et al. Metabolite Profiling and Transcriptome Analysis Explains Difference in Accumulation of Bioactive Constituents in Licorice (Glycyrrhiza uralensis) Under Salt Stress. Front. Plant Sci. 2021, 12, 727882. [Google Scholar] [CrossRef]
- Pucker, B.; Selmar, D. Biochemistry and Molecular Basis of Intracellular Flavonoid Transport in Plants. Plants 2022, 11, 963. [Google Scholar] [CrossRef]
- Goodman, C.D.; Casati, P.; Walbot, V. A Multidrug Resistance–Associated Protein Involved in Anthocyanin Transport in Zea mays. Plant Cell 2004, 16, 1812–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Constabel, C.P. MYB Repressors as Regulators of Phenylpropanoid Metabolism in Plants. Trends Plant Sci. 2019, 24, 275–289. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB Transcription Factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Yu, C.; Huang, J.; Wu, Q.; Zhang, C.; Li, X.-L.; Xu, X.; Feng, S.; Zhan, X.; Chen, Z.; Wang, H.; et al. Role of Female-Predominant MYB39-BHLH13 Complex in Sexually Dimorphic Accumulation of Taxol in Taxus Media. Hortic Res. 2022, 9, uhac062. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Gu, L.; Liu, J.; Zhao, S.; Hong, X.; Schulenburg, K.; Schwab, W. Functional Characterization and Substrate Promiscuity of UGT71 Glycosyltransferases from Strawberry (Fragaria × ananassa). Plant Cell Physiol. 2015, 56, 2478–2493. [Google Scholar] [CrossRef] [Green Version]
- Adams, K.L.; Wendel, J.F. Polyploidy and Genome Evolution in Plants. Curr. Opin. Plant Biol. 2005, 8, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Yan, M.; Li, T.; Li, X.; Yin, Y.; Cui, M.; Fang, Y.; Liu, J.; Kong, Y.; Xu, P.; et al. Characterization of UDP-Glycosyltransferase Family Members Reveals How Major Flavonoid Glycoside Accumulates in the Roots of Scutellaria baicalensis. BMC Genom. 2022, 23, 169. [Google Scholar] [CrossRef]
- Ono, E. Functional Differentiation of the Glycosyltransferases That Contribute to the Chemical Diversity of Bioactive Flavonol Glycosides in Grapevines (Vitis vinifera). Available online: https://pubmed.ncbi.nlm.nih.gov/20693356/ (accessed on 9 November 2022).
- Cao, Y.; Fang, S.; Yin, Z.; Fu, X.; Shang, X.; Yang, W.; Yang, H. Chemical Fingerprint and Multicomponent Quantitative Analysis for the Quality Evaluation of Cyclocarya paliurus Leaves by HPLC–Q–TOF–MS. Molecules 2017, 22, 1927. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Langfelder, P.; Horvath, S. Fast R Functions for Robust Correlations and Hierarchical Clustering. J. Stat. Soft. 2012, 46, i11. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- MAFFT-DASH: Integrated Protein Sequence and Structural Alignment|Nucleic Acids Research|Oxford Academic. Available online: https://academic.oup.com/nar/article/47/W1/W5/5486273?login=true (accessed on 18 January 2023).
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing Large Minimum Evolution Trees with Profiles Instead of a Distance Matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Interactive Tree Of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation|Nucleic Acids Research|Oxford Academic. Available online: https://academic.oup.com/nar/article/49/W1/W293/6246398?login=true (accessed on 18 January 2023).
- Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Jin, J.; Gao, G. PlantRegMap: Charting Functional Regulatory Maps in Plants. Nucleic Acids Res. 2020, 48, D1104–D1113. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]



| Module | Gene ID | Gene Name | KME | Gene Significance (GS) | |
|---|---|---|---|---|---|
| Glucuronides | p | ||||
| Brown | CpaM1st09750 * | bHLH122-1 | 0.6338 | 0.6164 | 0.0328 |
| Brown | CpaM1st14736 | bHLH122-2 | 0.6114 | 0.5432 | 0.0680 |
| Brown | CpaM1st30271 | bHLH72 | 0.9094 | 0.5188 | 0.0839 |
| Brown | CpaM1st33475 | bHLH121 | 0.8237 | 0.5638 | 0.0562 |
| Brown | CpaM1st39770 | bHLH105 | 0.6208 | 0.5358 | 0.0726 |
| Brown | CpaM1st43854 * | bHLH107 | 0.6126 | 0.6875 | 0.0135 |
| Brown | CpaM1st44522 * | bHLH42 | 0.8650 | 0.6017 | 0.0385 |
| Brown | CpaM1st08920 * | MYB39-1 | 0.8779 | 0.6044 | 0.0374 |
| Brown | CpaM1st08932 * | MYB39-2 | 0.8025 | 0.7002 | 0.0112 |
| Brown | CpaM1st43861 * | MYB39-3 | 0.8756 | 0.6757 | 0.0159 |
| Brown | CpaM1st04091 * | UGT85 | 0.6957 | 0.7930 | 0.0021 |
| Brown | CpaM1st11173 | UGT87 | 0.7322 | 0.5472 | 0.0655 |
| Brown | CpaM1st33727 | UGT71-1 | 0.8409 | 0.4961 | 0.1009 |
| Pink | CpaM1st47487 * | bHLH51 | 0.8040 | 0.8040 | 0.0061 |
| Pink | CpaM1st22888 | bHLH15 | 0.7233 | 0.7233 | 0.1003 |
| Pink | CpaM1st23532 | UGT71-2 | 0.8487 | 0.8487 | 0.0525 |
| Pink | CpaM1st23533 * | UGT71-3 | 0.7877 | 0.7877 | 0.0397 |
| Module | Pair | Correlation Coefficient | p-Value | |
|---|---|---|---|---|
| Structure Genes | TFs | |||
| Brown | CpaM1st04091 | CpaM1st08920 | 0.72 | 0.01 |
| Brown | CpaM1st04091 | CpaM1st08932 | 0.83 | 0 |
| Brown | CpaM1st04091 | CpaM1st33475 | 0.57 | 0.05 |
| Brown | CpaM1st04091 | CpaM1st43854 | 0.69 | 0.01 |
| Brown | CpaM1st04091 | CpaM1st43861 | 0.71 | 0.01 |
| Brown | CpaM1st04091 | CpaM1st44522 | 0.69 | 0.01 |
| Brown | CpaM1st11173 | CpaM1st08920 | 0.7 | 0.01 |
| Brown | CpaM1st11173 | CpaM1st08932 | 0.62 | 0.03 |
| Brown | CpaM1st11173 | CpaM1st09750 | 0.62 | 0.03 |
| Brown | CpaM1st11173 | CpaM1st30271 | 0.62 | 0.03 |
| Brown | CpaM1st11173 | CpaM1st33475 | 0.62 | 0.03 |
| Brown | CpaM1st11173 | CpaM1st43854 | 0.85 | 0 |
| Brown | CpaM1st11173 | CpaM1st43861 | 0.61 | 0.04 |
| Brown | CpaM1st11173 | CpaM1st44522 | 0.73 | 0.01 |
| Brown | CpaM1st33727 | CpaM1st08920 | 0.77 | 0 |
| Brown | CpaM1st33727 | CpaM1st08932 | 0.76 | 0 |
| Brown | CpaM1st33727 | CpaM1st09750 | 0.97 | 0 |
| Brown | CpaM1st33727 | CpaM1st14736 | 0.71 | 0.01 |
| Brown | CpaM1st33727 | CpaM1st30271 | 0.83 | 0 |
| Brown | CpaM1st33727 | CpaM1st33475 | 0.77 | 0 |
| Brown | CpaM1st33727 | CpaM1st39770 | 0.61 | 0.04 |
| Brown | CpaM1st33727 | CpaM1st43861 | 0.75 | 0.01 |
| Brown | CpaM1st33727 | CpaM1st44522 | 0.62 | 0.03 |
| Pink | CpaM1st23532 | CpaM1st22888 | 0.61 | 0.03 |
| Pink | CpaM1st23532 | CpaM1st47487 | 0.70 | 0.01 |
| Pink | CpaM1st23533 | CpaM1st22888 | 0.67 | 0.02 |
| Pink | CpaM1st23533 | CpaM1st47487 | 0.66 | 0.02 |
| Gene ID | Gene Names | Primer Sequence (5′—3′) | |
|---|---|---|---|
| PAL1-F | CpaM1st04281 | PAL1 | TGCGTAAACACCGACCCTT |
| PAL1-R | PAL1 | CTCCGACAGCTCCACCTTC | |
| C4H-F | CpaM1st16078 | C4H | ACCTCGTGGTGGTGTCGTC |
| C4H-R | C4H | TTCCCAACCGAATCTGTGC | |
| 4CL2-F | CpaM1st06427 | 4CL2 | ACAGAGCAGCGGGGAGAAC |
| 4CL2-R | 4CL2 | ATGCCGAGCCTGTTGAGAC | |
| 4CL3-F | CpaM1st31564 | 4CL3 | AGTGATAACGACTGCTAACCCAT |
| 4CL3-R | 4CL3 | GAAATGCAACAAGCCCTCC | |
| CHS2-F | CpaM1st24780 | CHS2 | ATCTCCCAAACTGATTTTCCTG |
| CHS2-R | CHS2 | GCAACTCGACCACCGCTAT | |
| F3′H-F | CpaM1st30230 | F3′H | TCGCTGTCTTCCTCTTTTACC |
| F3′H-R | F3′H | CAAGAACTGGGAGGCTACG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Qu, Y.; Wang, S.; Wang, Q.; Shang, X.; Fu, X. An Integrative Analysis of Metabolome and Transcriptome Reveals the Molecular Regulatory Mechanism of the Accumulation of Flavonoid Glycosides in Different Cyclocarya paliurus Ploidies. Forests 2023, 14, 770. https://doi.org/10.3390/f14040770
Yu Y, Qu Y, Wang S, Wang Q, Shang X, Fu X. An Integrative Analysis of Metabolome and Transcriptome Reveals the Molecular Regulatory Mechanism of the Accumulation of Flavonoid Glycosides in Different Cyclocarya paliurus Ploidies. Forests. 2023; 14(4):770. https://doi.org/10.3390/f14040770
Chicago/Turabian StyleYu, Yanhao, Yinquan Qu, Shuyang Wang, Qian Wang, Xulan Shang, and Xiangxiang Fu. 2023. "An Integrative Analysis of Metabolome and Transcriptome Reveals the Molecular Regulatory Mechanism of the Accumulation of Flavonoid Glycosides in Different Cyclocarya paliurus Ploidies" Forests 14, no. 4: 770. https://doi.org/10.3390/f14040770





