Correlation between Non-Structural Carbohydrates and C:N:P Stoichiometric Ratio of Haloxylon ammodendron under Different Water–Salt Gradients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Experimental Design
2.3. Sample Collection and Laboratory Measurement
2.3.1. Determination of Soil Moisture Content (SWC), Soil Salt Content (SSC), Soil pH, Soil Organic Carbon (SOC), Soil Total Nitrogen (TN), and Soil Total Phosphorus (TP) under the Canopy
2.3.2. Determination of Plant C, N, and P
2.3.3. Determination of Plant NSC Content
2.4. Data Analysis
3. Results
3.1. Characteristics of Soil Environmental Factors
3.2. Morphological Characteristics of H. ammodendron under Different Water−Salt Gradients
3.3. Changes in Water Potential of Assimilating Branches and Secondary Branches of H. ammodendron with Water–Salt Gradients
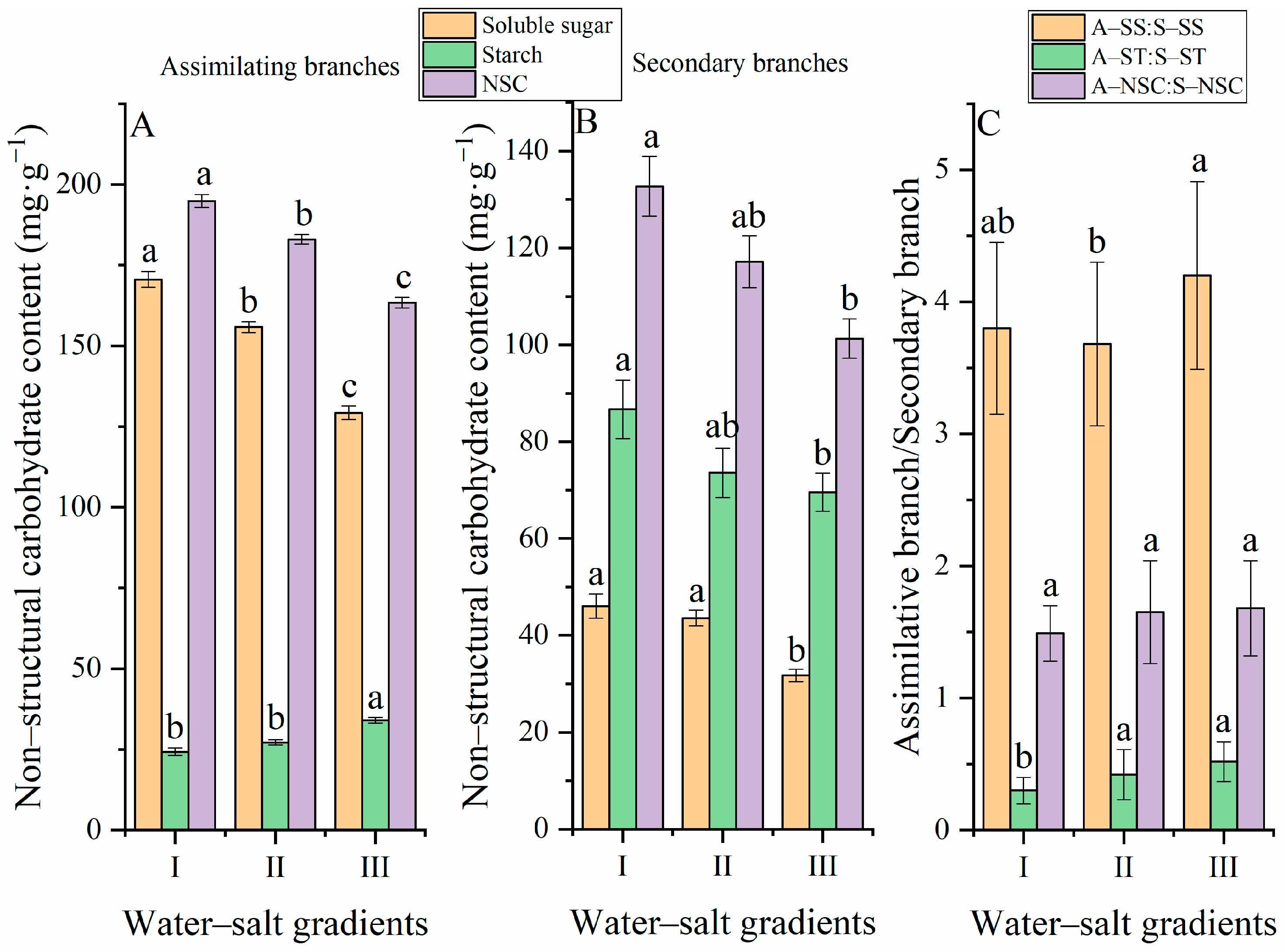
3.4. Variation Characteristics of NSC and the Composition of H. ammodendron under Different Water–Salt Gradients
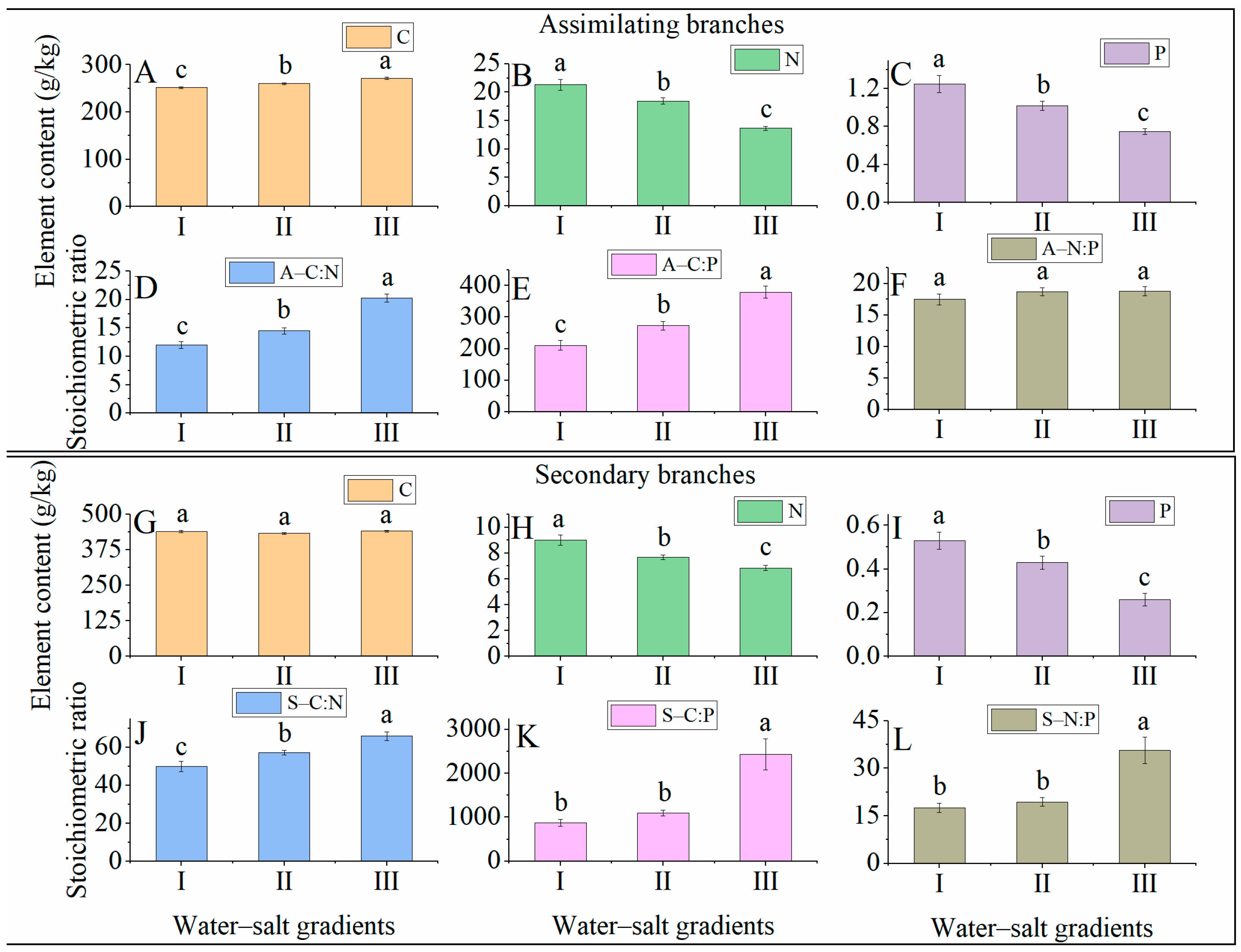
3.5. Variation Ecostoichiometric Characteristics of H. ammodendron under Different Water–Salt Gradients
3.6. The Relationship between the NSC of the Assimilation Branches and Secondary Branches of H. ammodendron and the Element Ratio Characteristics
4. Discussion
4.1. Effects of Different Water–Salt Gradients on NSC of H. ammodendron
4.2. Changes in the C:N:P Dosing Ratio Characteristics of H. ammodendron with Different Water–Salt Gradients
4.3. The Relationship between the Characteristics of H. ammodendron NSC and the C:N:P Ratio under Different Water–Salt Gradients
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooney, H.A. The Carbon Balance of Plants. Annu. Rev. Ecol. Syst. 1972, 3, 315–346. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural Carbon in Woody Plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.L.; Noctor, G. Photosynthetic nitrogen assimilation: Inter-pathway control and signaling. In Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism; Foyer, C.H., Noctor, G., Eds.; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Ramírez-Briones, E.; Rodríguez-Macías, R.; Salcedo-Pérez, E.; Martínez-Gallardo, N.; Tiessen, A.; Molina-Torres, J.; Délano-Frier, J.P.; Zañudo-Hernández, J. Seasonal variation in non-structural carbohydrates, sucrolytic activity and secondary metabolites in deciduous and perennial Diospyros species sampled in Western Mexico. PLoS ONE 2017, 12, e0187235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.T.; Yu, M.K.; Cheng, X.Y. Leaf non-structural carbohydrate allocation and C:N:P stoichiometry in response to light acclimation in seedlings of two subtropical shade-tolerant tree species. Plant Physiol. Biochem. 2018, 124, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ditmarová, L.; Kurjak, D.; Palmroth, S.; Kmeť, J.; StřElcovÁ, K. Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiol. 2009, 30, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Sun, Y.; Ou, Y.; Zheng, X.; Feng, Q.; Zhang, H.; Fei, Y.; Luo, J.; de Dios, V.R.; Yao, Y. Pretreating poplar cuttings with low nitrogen ameliorates salt stress responses by increasing stored carbohydrates and priming stress signaling pathways. Ecotox Environ. Safe 2021, 225, 112801. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.A.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.W. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Lebauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shen, C.; Sun, B.; Wang, X.N.; Wei, D.; Lyu, L.Y. Effects of drought stress on C, N and P stoichiometry of Ulmus pumila seedlings in Horqin sandy land. J. Appl. Ecol. 2018, 29, 2286–2294. [Google Scholar]
- Patel, A.D.; Jadeja, H.; Pandey, A.N. Effect of salinization of soil on growth, water status and nutrient accumulation in seedlings of Acacia auriculiformis (Fabaceae). J. Plant Nutr. 2010, 33, 914–932. [Google Scholar] [CrossRef]
- Rong, Q.; Liu, J.; Cai, Y.; Lu, Z.; Zhao, Z.; Yue, W.; Xia, J. Leaf carbon, nitrogen and phosphorus stoichiometry of Tamarix chinensis Lour. in the Laizhou Bay coastal wetland, China. Ecol. Eng. 2015, 76, 57–65. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Ogaya, R. Drought-induced changes in C and N stoichiometry in a Quercus ilex Mediterranean forest. Forest Sci. 2008, 54, 513–522. [Google Scholar]
- Van Dijk, G.; Smolders, A.J.P.; Loeb, R.; Bout, A.; Roelofs, J.G.M.; Lamers, L.P.M. Salinization of coastal freshwater wetlands; effects of constant versus fluctuating salinity on sediment biogeochemistry. Biogeochemistry 2015, 126, 71–84. [Google Scholar] [CrossRef]
- Sun, X.; Gao, Y.; Wang, D.; Chen, J.; Zhang, F.; Zhou, J.; Yan, X.; Li, Y. Stoichiometric variation of halophytes in response to changes in soil salinity. Plant Biol. 2017, 19, 360–367. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Nat. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [Green Version]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial Redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability-Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; p. 1132. [Google Scholar]
- Yan, S.; Weihong, Z.; Kequan, P.; Keping, M.A. Genetic variation within and among populations of a dominant desert tree Haloxylon ammodendron (Amaranthaceae) in China. Ann. Bot. 2005, 96, 245–252. [Google Scholar]
- Gong, X.W.; Lyu, G.H.; Ran, Q.Y.; Yang, X.D. Response of vegetative growth and biomass allocation of Lappula semiglabra seedlings to dew gradient. J. Appl. Ecol. 2016, 27, 2257–2263. [Google Scholar]
- Yang, X.D.; Lü, G.H.; Tian, Y.H.; Yang, J.; Zhang, X.M. Ecological groups of plants in Ebinur Lake Wetland Nature Reserve of Xinjiang. Chin. J. Ecol. 2009, 28, 2489–2494. [Google Scholar]
- Mcmahon, T.A.; Kronauer, R.E. Tree structures: Deducing the principle of mechanical design. J. Theor. Biol. 1976, 59, 443. [Google Scholar] [CrossRef]
- von Arx, G.; Arzac, A.; Fonti, P.; Frank, D.; Zweifel, R.; Rigling, A.; Galiano, L.; Gessler, A.; Olano, J.M. Responses of sapwood ray parenchyma and non-structural carbohydrates of Pinus sylvestris to drought and long-term irrigation. Funct. Ecol. 2017, 31, 1371–1382. [Google Scholar] [CrossRef] [Green Version]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Todea, I.M.; González-Orenga, S.; Boscaiu, M.; Plazas, M.; Sestras, A.F.; Prohens, J.; Vicente, O.; Sestras, R.E. Responses to water deficit and salt stress in Silver Fir (Abies alba Mill.) seedlings. Forests 2020, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Gleason, S.M.; Westoby, M.; Jansen, S.; Choat, B.; Hacke, U.G.; Pratt, R.B.; Bhaskar, R.; Brodribb, T.J.; Bucci, S.J.; Cao, K.F.; et al. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytol. 2016, 209, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Brodribb, T.J.; Cochard, H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Niu, D.; Zhang, C.; Ma, P.; Fu, H.; Elser, J.J. Responses of leaf C: N: P stoichiometry to water supply in the desert shrub Zygophyllum xanthoxylum. Plant Biol. 2019, 21, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.Z.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.N.; Bargerj, B.; Okin, G. Dryland Ecosystems Nutrient Cycling in Terrestrial Ecosystems; Marschner, P., Rengel, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 271–308. [Google Scholar]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; p. 357. [Google Scholar]
- Wang, J.Y.; Wang, J.N.; Wu, T.G. Stoichiometric homeostasis, physiology, and growth responses of three tree species to nitrogen and phosphorus addition. Trees 2018, 32, 1377–1386. [Google Scholar] [CrossRef]
- Yin, H.; Zheng, H.; Zhang, B.; Tariq, A.; Lv, G.; Zeng, F.; Graciano, C. Stoichiometry of C:N:P in the Roots of Alhagi sparsifolia Is More Sensitive to Soil Nutrients Than Aboveground Organs. Front. Plant Sci. 2021, 12, 698961. [Google Scholar] [CrossRef]
- Ding, D.; Arif, M.; Liu, M.; Li, J.; Hu, X.; Geng, Q.; Yin, F.; Li, C. Plant-soil interactions and C:N:P stoichiometric homeostasis of plant organs in riparian plantation. Front. Plant Sci. 2022, 13, 979023. [Google Scholar] [CrossRef]
- Vitousek, P. Nutrient cycling and nutrient use efficiency. Am. Nat. 1982, 119, 553–572. [Google Scholar] [CrossRef]
- Larsen, K.S.; Andresen, L.C.; Beier, C.; Jonasson, S.; Albert, K.; Ambus, P.; Arndal, M.F.; Carter, M.S.; Christensen, S.; Holmstrup, M.; et al. Reduced N cycling in response to elevated CO2, warming, and drought in a Danish heathland: Synthesizing results of the CLIMAITE project after two years of treatments. Global Change Biol. 2011, 17, 1884–1899. [Google Scholar] [CrossRef]
- Fan, H.B.; Wu, J.P.; Cai, Q.K. Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Y.M. Coupling of plant and soil C:N:P stoichiometry in black locust (Robinia pseudoacacia) plantations on the Loess Plateau, China. Trees 2017, 31, 1559–1570. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, Q.; Li, K.; Gong, Y.; Liu, Y.; Han, W. Patterns of nitrogen and phosphorus stoichiometry among leaf, stem and root of desert plants and responses to climate and soil factors in Xinjiang, China. Catena 2020, 199, 105100. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2018, 337, 280–2899. [Google Scholar] [CrossRef]
- Warren, C.R.; Adams, M.A.; Chen, Z.L. Is photosynthesis related to concentrations of nitrogen and Rubisco in leaves of Australian native plants? Funct. Plant Biol. 2000, 27, 407–416. [Google Scholar] [CrossRef]
- Qi, D.D.; Feng, F.J.; Fu, Y.M. C:N:P stoichiometry of different soil components after the transition of temperate primary coniferous and broad-leaved mixed forests to secondary forests. Soil Till Res. 2022, 216, 105260. [Google Scholar] [CrossRef]
- Barbaroux, C.; Breda, N. Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiol. 2002, 22, 1201–1210. [Google Scholar] [CrossRef] [PubMed]


| Clustering Results | Sample Number | SWC (%) | SSC (g·kg−1) |
|---|---|---|---|
| I | 1–8, 10, 22 | 18.39 ± 0.63 a | 9.02 ± 0.53 a |
| II | 9, 11–21, 23–34, 37, 39 | 9.84 ± 0.40 b | 5.22 ± 0.19 b |
| III | 35–36, 38, 40–62 | 3.46 ± 0.25 c | 2.48 ± 0.12 c |
| Water–Salt Gradient | pH | SOC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) |
|---|---|---|---|---|
| I | 8.55 ± 0.08 a | 7.66 ± 1.02 a | 1.26 ± 0.12 a | 0.62 ± 0.01 a |
| II | 8.20 ± 0.06 b | 1.90 ± 0.12 b | 0.41 ± 0.02 b | 0.48 ± 0.01 b |
| III | 7.71 ± 0.04 c | 1.06 ± 0.07 b | 0.20 ± 0.01 c | 0.37 ± 0.01 c |
| Water–Salt Gradient | Base Diameter (cm2) | Tree Height (m) | Crown Area (m2) |
|---|---|---|---|
| I | 22.73 ± 2.71 a | 4.10 ± 0.28 a | 33.86 ± 4.95 a |
| II | 15.69 ± 1.49 b | 3.04 ± 0.13 b | 15.72 ± 2.83 b |
| III | 10.21 ± 0.47 c | 2.53 ± 0.09 c | 8.73 ± 0.75 c |
| Water–Salt Gradient | Assimilating Branches | Secondary Branches | ||
|---|---|---|---|---|
| Predawn | Midday | Predawn | Midday | |
| I | −4.62 ± 0.29 ab | −5.92 ± 0.94 b | −4.94 ± 0.82 c | −5.91 ± 1.09 c |
| II | −4.24 ± 0.79 a | −4.77 ± 0.95 a | −3.55 ± 0.75 a | −3.78 ± 1.02 a |
| III | −4.93 ± 0.76 b | −5.29 ± 0.85 ab | −4.23 ± 0.85 b | −4.53 ± 1.12 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Lv, G.; Qie, Y. Correlation between Non-Structural Carbohydrates and C:N:P Stoichiometric Ratio of Haloxylon ammodendron under Different Water–Salt Gradients. Forests 2023, 14, 1185. https://doi.org/10.3390/f14061185
Yang F, Lv G, Qie Y. Correlation between Non-Structural Carbohydrates and C:N:P Stoichiometric Ratio of Haloxylon ammodendron under Different Water–Salt Gradients. Forests. 2023; 14(6):1185. https://doi.org/10.3390/f14061185
Chicago/Turabian StyleYang, Fang, Guanghui Lv, and Yadong Qie. 2023. "Correlation between Non-Structural Carbohydrates and C:N:P Stoichiometric Ratio of Haloxylon ammodendron under Different Water–Salt Gradients" Forests 14, no. 6: 1185. https://doi.org/10.3390/f14061185






