Early Diagnosis of Pine Wood Nematode Disease Based on Chlorophyll Fluorescence Parameters and Organic Acids
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Material Preparation
2.2. PWN Inoculation and Disease Observation
2.3. Determination of Chlorophyll Fluorescence Parameters of Pinus hwangshanensis Needles
2.4. Determination of the Organic Acid Content of Pinus hwangshanensis Needles
2.5. Data Analysis
3. Results
3.1. Observation of the Condition of Pinus hwangshanensis
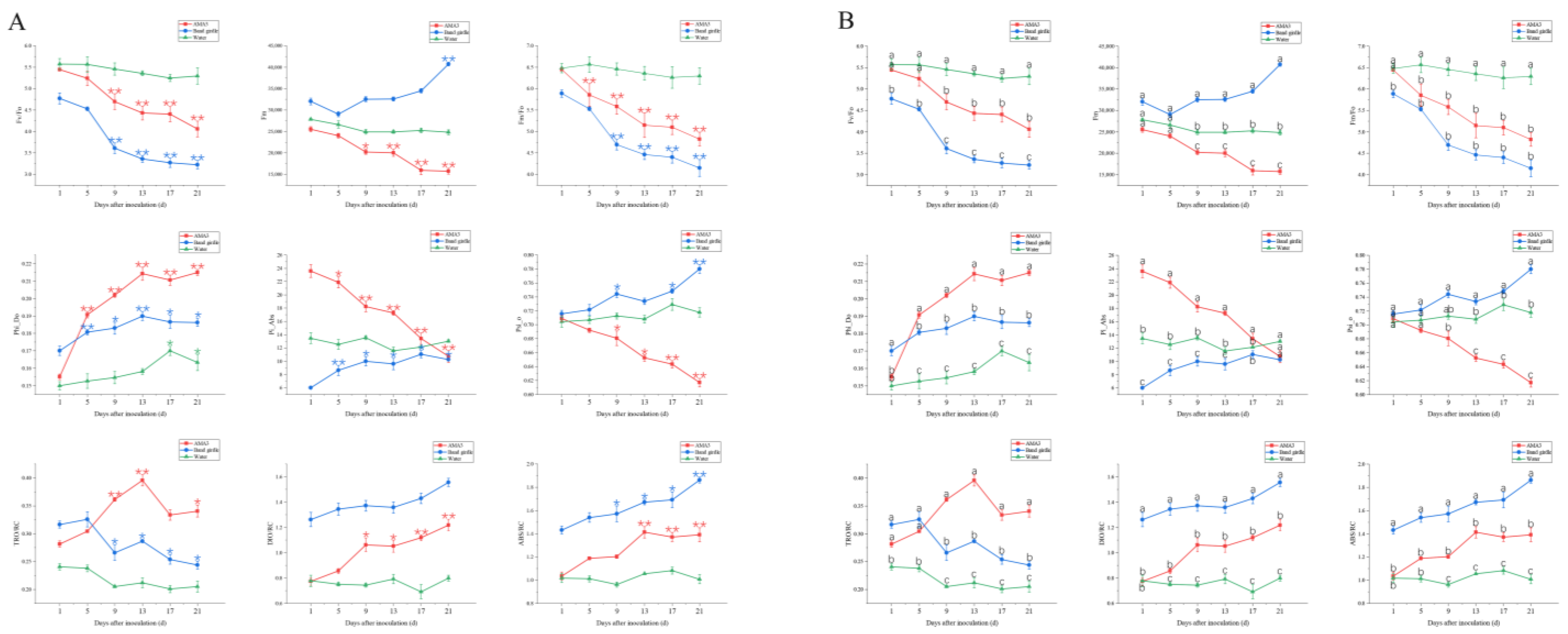
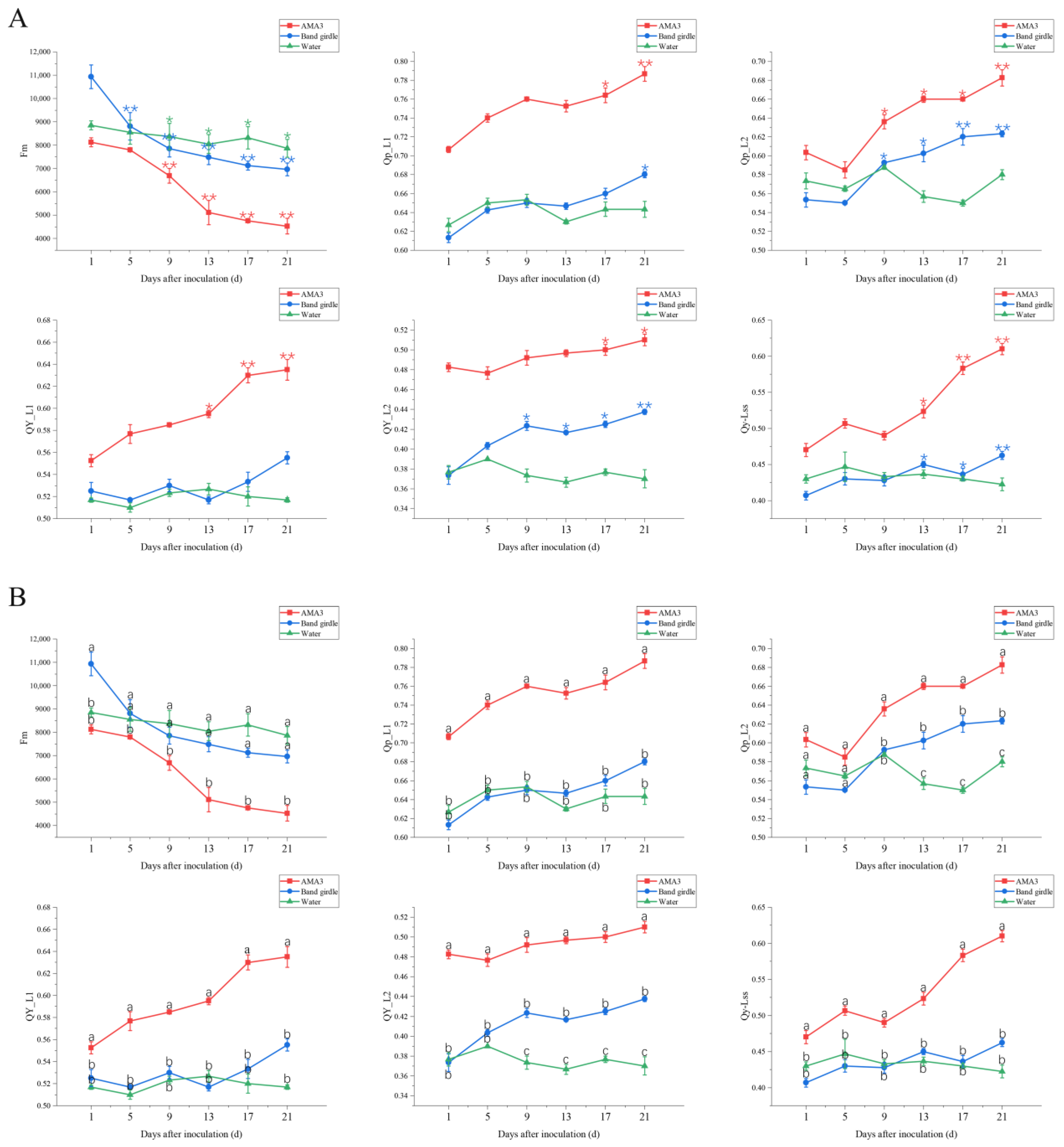
3.2. Effect of PWNs on Chlorophyll Fluorescence Parameters of Pinus hwangshanensis Needles
3.2.1. Variation in the OJIP Parameters of Pinus hwangshanensis Needles
3.2.2. Variation in the NPQ Parameters of Pinus hwangshanensis Needles
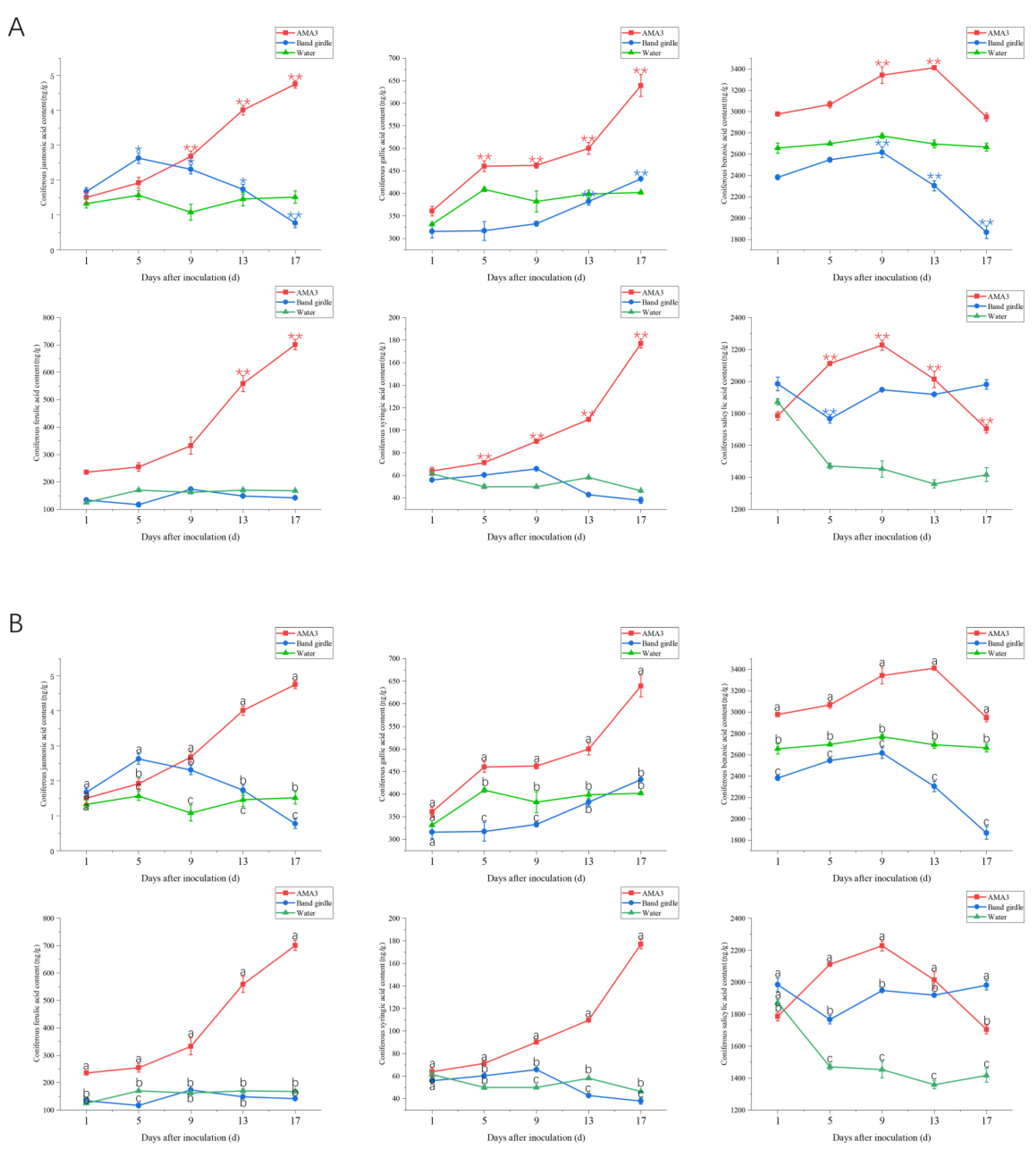
3.3. Effect of PWNs on the Organic Acid Content of the Needles of Pinus hwangshanensis
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kikuchi, T.; Cotton, J.A.; Dalzell, J.J.; Hasegawa, K.; Kanzaki, N.; McVeigh, P.; Takanashi, T.; Tsai, I.J.; Assefa, S.A.; Cock, P.J.; et al. Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog. 2011, 7, e1002219. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Wang, Y.; Li, R.H.; Liu, C.F.; Fan, Y.S. Damage, transmission route and control measures pf pine wood nematode disease. J. Jilin For. Sci. Technol. 2023, 52, 44–45. [Google Scholar]
- Ye, J.R.; Wu, X.Q. Research progress of pine wood nematode disease. For. Dis. Insects China 2022, 41, 1–10. [Google Scholar]
- Lim, E.T.; Do, M.S. Pine wilt disease detection based on deep learning using an unmanned aerial vehicle. KSCE J. Civ. Environ. Eng. Res. 2021, 41, 317–325. [Google Scholar]
- Lee, S.; Park, S.J.; Baek, G.; Kim, H.; Lee, C.W. Detection of Damaged Pine Tree by the Pine Wilt Disease Using UAV Image. Korean J. Remote Sens. 2019, 35, 359–373. [Google Scholar]
- Jiang, X.F.; Li, M.; Zhang, L.P.; Zhang, Y.; Wu, F.; Tong, Y.X.; Chen, F.M. Control strategies and measures of pine wood nematode disease. Sci. Biodisaster 2023, 46, 136–140. (In Chinese) [Google Scholar]
- Mota, M.; Vieira, P. (Eds.) Discovery of pine wood nematode in portugal and in Europe. In Proceedings of the International Workshop on Pine Wood Nematode, Bursaphelenchus xylophilus, Evora, Portugal, 20–22 August 2001; Volume 8, pp. 20–22. [Google Scholar]
- Zhang, X.R.; Guo, W.H.; Wang, R.Q.; Si, J.Y. Comparative study on chlorophyll fluorescence characteristics of three pine species. Shandong For. Sci. Technol. 2010, 40, 1–5. [Google Scholar]
- Li, X.; Feng, W.; Zeng, X.C. Advances in chlorophyll fluorescence analysis and its application. Acta Bot. Boreali-Occident. Sin. 2006, 26, 11. [Google Scholar]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant-pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef]
- Lemanczyk, G.; Kwasna, H. Effects of sharp eyespot (Rhizoctonia cerealis) on yield and grain quality of winter wheat. Eur. J. Plant Pathol. 2013, 135, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Fofana, B.; Banks, T.W.; McCallum, B.; Strelkov, S.E.; Cloutier, S. Temporal gene expression profiling of the wheat leaf rust pathosystem using cDNA microarray reveals differences in compatible and incompatible defence pathways. Int. J. Plant Genom. 2007, 2007, 17542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocal, N.; Sonnewald, U.; Sonnewald, S. Cell Wall-Bound Invertase Limits Sucrose Export and Is Involved in Symptom Development and Inhibition of Photosynthesis during Compatible Interaction between Tomato and Xanthomonas campestris pv vesicatoria. Plant Physiol. 2008, 148, 1523–1536. [Google Scholar] [CrossRef] [Green Version]
- Christen, D.; Schönmann, S.; Jermini, M.; Strasser, R.J.; Défago, G. Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ. Exp. Bot. 2007, 60, 504–514. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [Green Version]
- Ajigboye, O.O.; Bousquet, L.; Murchie, E.H.; Ray, R.V. Chlorophyll fluorescence parameters allow the rapid detection and differentiation of plant responses in three different wheat pathosystems. Funct. Plant Biol. 2016, 43, 356–369. [Google Scholar] [CrossRef]
- Linn, A.I.; Zeller, A.K.; Pfündel, E.E.; Gerhards, R. Features and applications of a field imaging chlorophyll fluorometer to measure stress in agricultural plants. Precis. Agric. 2021, 22, 947–963. [Google Scholar] [CrossRef]
- Recchia, I.; Sparla, F.; Pupillo, P. Photosynthetic properties of spring geophytes assessed by chlorophyll fluorescence analysis. Plant Physiol. Biochem. 2017, 118, 510–518. [Google Scholar] [CrossRef]
- Melakeberhan, H.; Toivonen, P.M.A.; Vidaver, W.E.; Webster, J.M.; Dube, S.L. Effect of Bursaphelenchus xylophilus on the water potential and water-splitting complex of photosystem II of Pinus sylvestris seedlings. Physiol. Mol. Plant Pathol. 1991, 38, 83–91. [Google Scholar] [CrossRef]
- Oku, H.S.T.; Kurozumi, S. Participation of toxin in wilting of Japanese pines caused by a nematode. Naturwissenschaften 1979, 66, 211. [Google Scholar] [CrossRef]
- Mamiya, Y. Inoculation of the first vear pine(Pinus densiflora) seedlings with Bursaphelenchns lignicolns and the histopathology of diseased seedlinns. J. Jpn. For. Soc. 1980, 62, 176–183. [Google Scholar]
- Li, Y.X.; Zhang, X.Y. Advances in Research on Pathogenic Mechanism of Pine Wood Nematode Disease. Chin. J. Environ. Entomol. 2018, 40, 11. [Google Scholar]
- Zhang, F.; Kajiwara, J.; Mori, Y.; Ohira, M.; Tsutsumi, Y.; Kondo, R. Metabolites from resistant and susceptible Pinus thunbergii after inoculation with pine wood nematode. Am. J. Plant Sci. 2013, 4, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Kawatsu; Kazuyoshi. Pathogenic Toxin of Pine Wood Disease. Kagaku Sebutsu 1998, 36, 120–124. [Google Scholar] [CrossRef]
- Hu, L.J.; Wu, X.Q. Research progress on the mechanism of resistance to pine wood nematode disease. Life Sci. 2018, 30, 659–666. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, M.; Hu, J.; Pan, M.; Shen, L.; Ye, J.; Tan, J. Early diagnosis of pine wilt disease in Pinus thunbergii based on chlorophyll fluorescence parameters. Forests 2023, 14, 154. [Google Scholar] [CrossRef]
- Viglierchio, D.R.; Schmitt, R.V. On the methodology of nematode extraction from field samples: Baermann funnel modifications. J. Nematol. 1983, 15, 438–444. [Google Scholar]
- Xie, L.Q.; Ju, Y.W.; Yang, Z.D.; Zhao, B.G. Changes of the number of bacteria and nematodes in the inoculated branches of Pinus thunbergii. J. Zhejiang AF Univ. 2005, 22, 310–314. [Google Scholar]
- Tan, J.J.; Wu, H.P. Effects of Pine Wood Nematode on Free Proline Content in Leaves of Pine. J. South China Agric. Univ. 2000, 21, 1. [Google Scholar]
- Ruiz-sola, M.A.; Petroutsos, D. A Toolkit for the characterization of the photoprotective capacity of green algae. Methods Mol. Biol. 2018, 1829, 315–323. [Google Scholar]
- Mou, S.; Zhang, X.; Ye, N.; Miao, J.; Cao, S.; Xu, D.; Fan, X.; An, M. Analysis of Delta pH and the xanthophyll cycle in NPQ of the Antarctic sea ice alga Chlamydomonas sp. ICE-L. Extremophiles 2013, 17, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Liu, Y.; Captoline, I.; Li, X.; Ye, D.; Wu, R. Citrus Huanglongbing detection based on polyphasic chlorophyll a fluorescence coupled with machine learning and model transfer in two citrus cultivars. Comput. Electron. Agric. 2021, 187, 106289. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Xi, Y.; Zhang, L.; Gao, Q.; Wang-Pruski, G. Effects of autotoxicity on seed germination, gas exchange attributes and chlorophyll fluorescence in melon seedlings. J. Plant Growth Regul. 2022, 41, 993–1003. [Google Scholar] [CrossRef]
- Nosek, M.; Kornaś, A.; Kuźniak, E.; Miszalski, Z. Plastoquinone redox state modifies plant response to pathogen. Plant Physiol. Biochem. 2015, 96, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Macioszek, V.K.; Gapińska, M.; Zmienko, A.; Sobczak, M.; Skoczowski, A.; Oliwa, J.; Kononowicz, A.K. Complexity of Brassica oleracea-Alternaria brassicicola susceptible interaction reveals downregulation of photosynthesis at ultrastructural, transcriptional, andphysiological levels. Cells 2020, 9, 2329. [Google Scholar] [CrossRef]
- Yang, Z.X.; Yang, Y.F.; Yu, S.Z.; Wang, R.G.; Wang, Y.; Chen, H.L. Photosynthetic, photochemical and osmotic regulation changes in tobacco resistant and susceptible to Alternaria alternata. Trop. Plant Pathol. 2018, 43, 413–421. [Google Scholar] [CrossRef]
- Rys, M.; Juhász, C.; Surówka, E.; Janeczko, A.; Saja, D.; Tóbiás, I.; Skoczowski, A.; Barna, B.; Gullner, G. Comparison of a compatible and an incompatible pepper-tobamovirus interaction by biochemical and non-invasive techniques: Chlorophyll a fluorescence, isothermal calorimetry and FT-Raman spectroscopy. Plant Physiol. Biochem. 2014, 83, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar]
- Liu, S.Y.; Minard, R.D.; Bollag, J.M. Oligomerization of Syringic Acid, a Lignin Derivative, by a Phenoloxidase. Soil Sci. Soc. Am. J. 1981, 45, 1100–1105. [Google Scholar] [CrossRef]
- Caño-Delgado, A.; Penfield, S.; Smith, C.; Catley, M.; Bevan, M. Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J. 2003, 34, 351–362. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, T.; Hu, J.; Han, R.; Zhu, Y.; Guan, Y.; Zhu, S. Relationship between endogenous salicylic acid and antioxidant enzyme activities in maize seedlings under cold stress. Exp. Agric. 2013, 49, 295–308. [Google Scholar] [CrossRef]
- Lee, I.H.; Kim, J.; Woo, K.S.; Jang, K.H.; Kim, Y.H.; Shim, D. De novo assembly and transcriptome analysis of the Pinus densiflora response to pine wilt disease in nature. Plant Biotechnol. Rep. 2018, 12, 229–236. [Google Scholar] [CrossRef]
- Truman, W.; Bennett, M.H.; Kubigsteltig, I.; Turnbull, C.; Grant, M. Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc. Natl. Acad. Sci. USA 2007, 104, 1075–1080. [Google Scholar] [CrossRef]
- Qian, W.Q.; Xiang, F.Q.; Wang, M.; Wang, S.L. Effects of Pine Wood Nematode Infection on Organic Acids and Enzymes in Masson Pine. Hunan For. Sci. Technol. 2009, 36, 3. [Google Scholar]
- Qin, S.J.; Qi, J.Y.; Liu, R.J.; Chen, F.M.; Hao, D.J.; Li, Z.X.; Sun, S.R. Physiological Response of Pinus tabulaeformis after Infection with Pine Wood Nematodes in Natural State. J. Shenyang Agric. Univ. 2021, 52, 625–632. [Google Scholar]
- Tan, J.J.; Ye, J.R.; Hao, D.J. Changes of organic acid contents of Pinus thunbergii after infection with pine wood nematode. Plant Pathol. Bull. 2008, 38, 3. [Google Scholar]
| Treatment | Disease Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 d | 5 d | 9 d | 13 d | 17 d | 21 d | 38 d | 55 d | 120 d | |
| AMA3 | 0 | 0 | 0 | 0 | 0 | 12.5 | 37.5 | 59.4 | 78.13 |
| Band girdle | 0 | 0 | 0 | 0 | 16.7 | 44.4 | 54.2 | 83.3 | 100 |
| Water | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Lin, X.; Liu, F.; Huang, Y.; Ye, J.; Tan, J. Early Diagnosis of Pine Wood Nematode Disease Based on Chlorophyll Fluorescence Parameters and Organic Acids. Forests 2023, 14, 1582. https://doi.org/10.3390/f14081582
Shen L, Lin X, Liu F, Huang Y, Ye J, Tan J. Early Diagnosis of Pine Wood Nematode Disease Based on Chlorophyll Fluorescence Parameters and Organic Acids. Forests. 2023; 14(8):1582. https://doi.org/10.3390/f14081582
Chicago/Turabian StyleShen, Luyang, Xiaoyu Lin, Fei Liu, Yingzhen Huang, Jianren Ye, and Jiajin Tan. 2023. "Early Diagnosis of Pine Wood Nematode Disease Based on Chlorophyll Fluorescence Parameters and Organic Acids" Forests 14, no. 8: 1582. https://doi.org/10.3390/f14081582





