MicroRNA Identification and Integrated Network Analyses for Age-Dependent Flavonoid Biosynthesis in Ginkgo biloba
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction and Quantification of Flavonol and Total Flavonoids
2.3. sRNA Library Construction and Sequencing
2.4. Identification of Known and Novel miRNAs
2.5. Differential miRNA Expression Analysis
2.6. miRNA Target Prediction and Functional Enrichment Analysis
2.7. Coexpression Analyses of miRNAs and Flavonoid Compounds
2.8. Statistical Analysis
3. Results
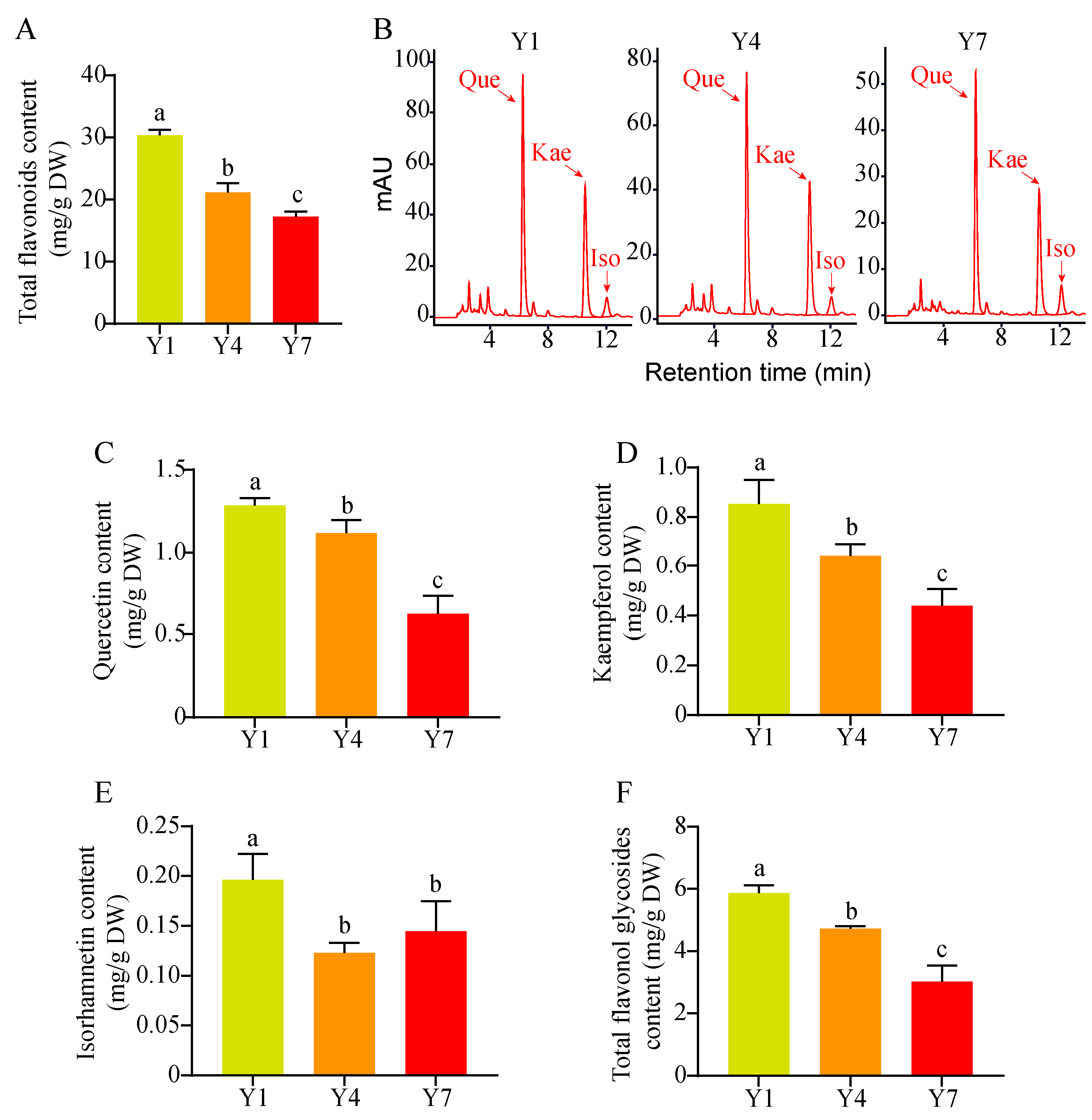
3.1. Flavonoid Analyses of Ginkgo Leaves of Different Ages
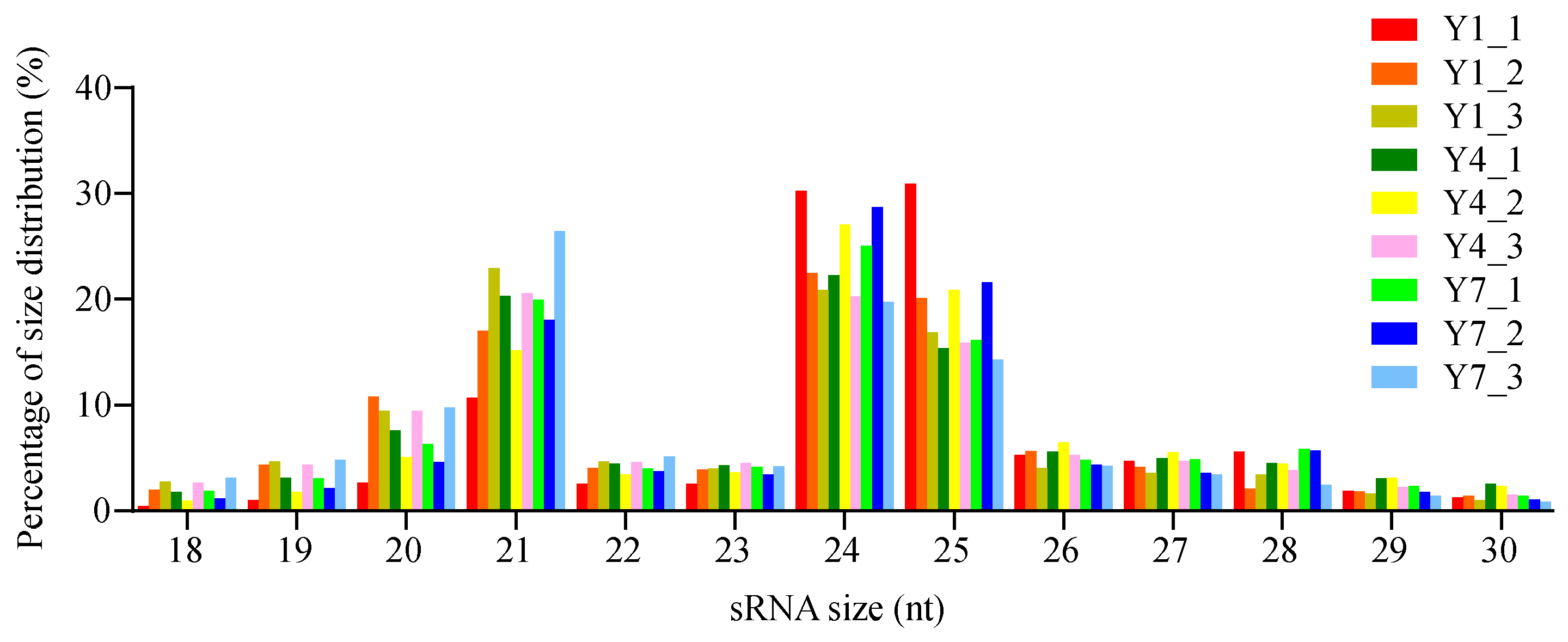
3.2. sRNA Sequencing and Identification Results for Conserved and Novel miRNAs
3.3. Differential Analysis of Candidate miRNAs According to Plant Age
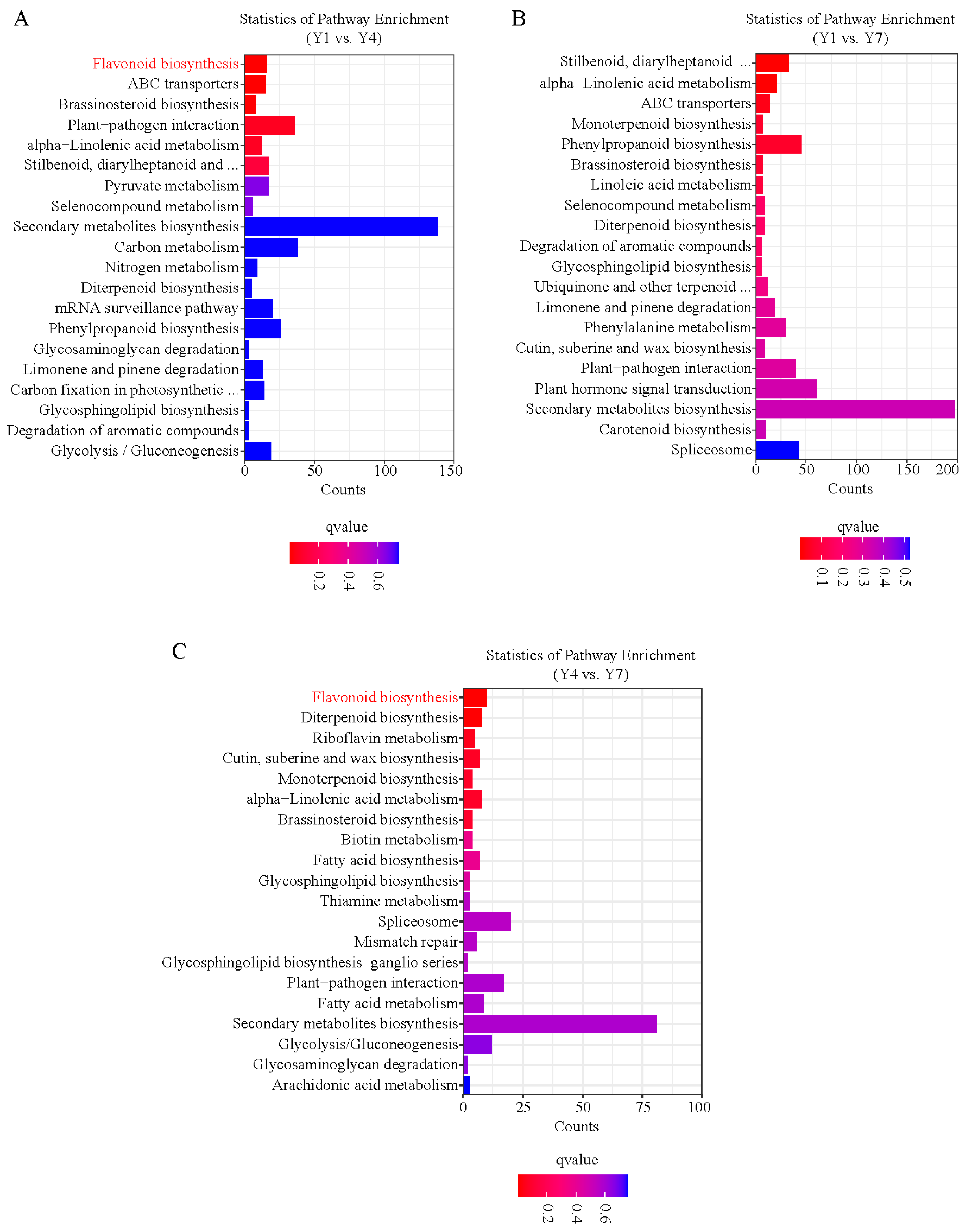
3.4. Identification and Analysis of miRNA Target Genes
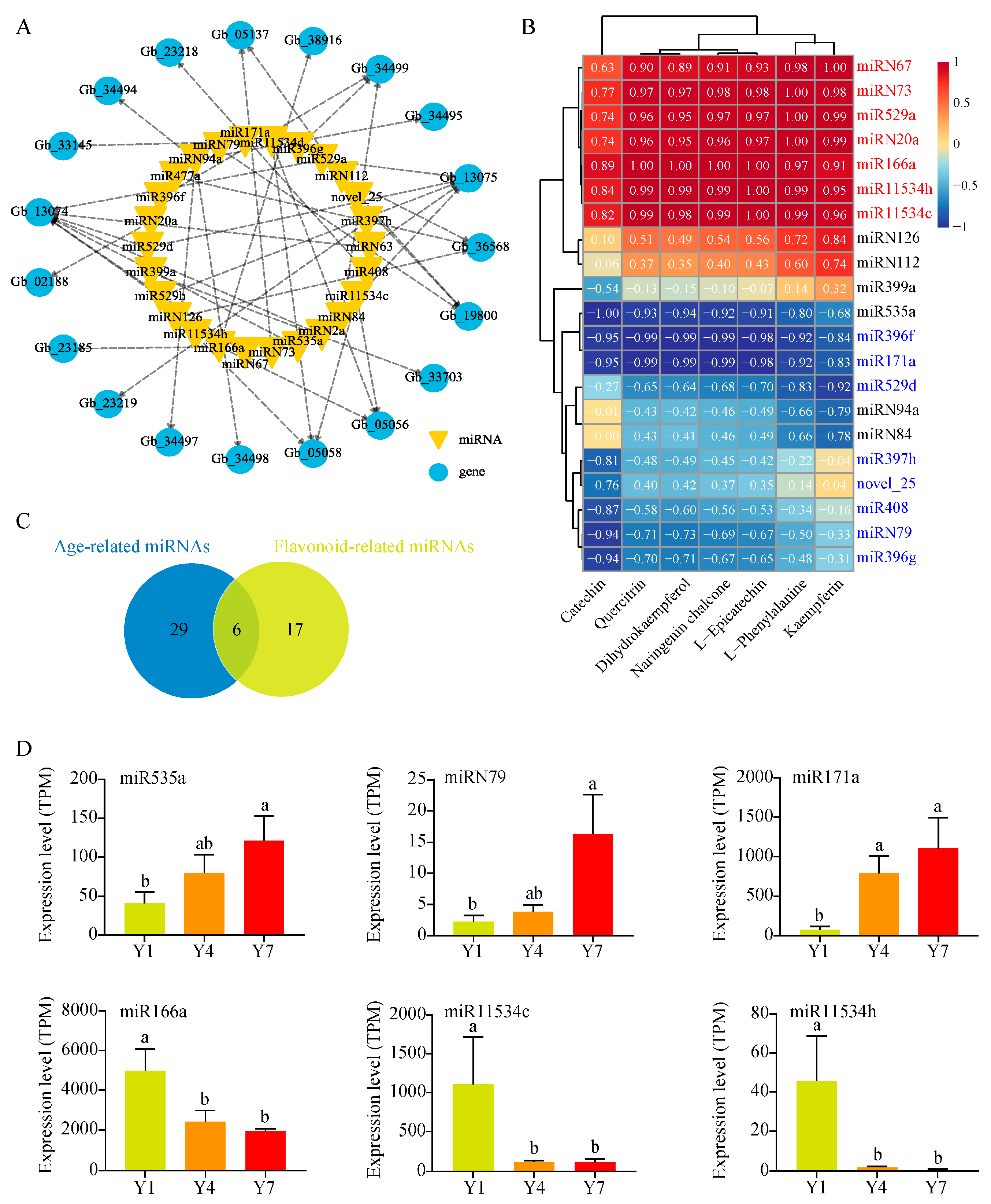
3.5. Prediction of miRNAs Related to the Flavonoid Synthesis
3.6. miRNAs Associated with Photosynthesis
3.7. miRNAs Associated with Plant Hormones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Ogo, Y.; Ozawa, K.; Ishimaru, T.; Murayama, T.; Takaiwa, F. Transgenic rice seed synthesizing diverse flavonoids at high levels: A new platform for flavonoid production with associated health benefits. Plant Biotechnol. J. 2013, 11, 734–746. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, K.; Lyu, S.; Ren, L.; Huang, C.; Pei, D.; Xing, Y.; Wang, Y.; Xu, Y.; et al. Comparison analysis of widely-targeted metabolomics revealed the variation of potential astringent ingredients and their dynamic accumulation in the seed coats of both Carya cathayensis and Carya illinoinensis. Food Chem. 2022, 374, 131688. [Google Scholar] [CrossRef]
- Yokotani, N.; Sato, Y.; Tanabe, S.; Chujo, T.; Shimizu, T.; Okada, K.; Yamane, H.; Shimono, M.; Sugano, S.; Takatsuji, H.; et al. WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J. Exp. Bot. 2013, 64, 5085–5097. [Google Scholar] [CrossRef]
- Mao, D.; Zhong, L.; Zhao, X.; Wang, L. Function, biosynthesis, and regulation mechanisms of flavonoids in Ginkgo biloba. Fruit Res. 2023, 3, 18. [Google Scholar] [CrossRef]
- Hassani, D.; Fu, X.; Shen, Q.; Khalid, M.; Rose, J.K.; Tang, K. Parallel transcriptional regulation of artemisinin and flavonoid biosynthesis. Trends Plant Sci. 2020, 25, 466–476. [Google Scholar] [CrossRef]
- Wang, N.; Xu, H.; Jiang, S.; Zhang, Z.; Lu, N.; Qiu, H.; Qu, C.; Wang, Y.; Wu, S.; Chen, X. MYB12 and MYB22 play essential roles in proanthocyanidin and flavonol synthesis in red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant J. 2017, 90, 276–292. [Google Scholar] [CrossRef]
- Gou, J.Y.; Felippes, F.F.; Liu, C.J.; Weigel, D.; Wang, J.W. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef]
- Valares Masa, C.; Sosa Díaz, T.; Alías Gallego, J.C.; Chaves Lobón, N. Quantitative variation of flavonoids and diterpenes in leaves and stems of Cistus ladanifer L. at different ages. Molecules 2016, 21, 275. [Google Scholar] [CrossRef]
- Valkama, E.; Salminen, J.P.; Koricheva, J.; Pihlaja, K. Changes in leaf trichomes and epicuticular flavonoids during leaf development in three birch taxa. Ann. Bot. 2004, 94, 233–242. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. MicroRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol. 2006, 57, 19–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Czech, B.; Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.; Weigel, D.; Poethig, R.S. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef]
- Wang, J.W. Regulation of flowering time by the miR156-mediated age pathway. J. Exp. Bot. 2014, 65, 4723–4730. [Google Scholar] [CrossRef]
- Dai, Z.; Tan, J.; Zhou, C.; Yang, X.; Yang, F.; Zhang, S.; Sun, S.; Miao, X.; Shi, Z. The OsmiR396–OsGRF8–OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa). Plant Biotechnol. J. 2019, 17, 1657–1669. [Google Scholar] [CrossRef]
- Lin, S.; Singh, R.K.; Moehninsi, M.; Navarre, D.A. R2R3-MYB transcription factors, StmiR858 and sucrose mediate potato flavonol biosynthesis. Hortic. Res. 2021, 8, 25. [Google Scholar] [CrossRef]
- Fan, Y.; Li, W.; Li, Z.; Dang, S.; Han, S.; Zhang, L.; Qi, L. Examination of the transcriptional response to LaMIR166a overexpression in Larix kaempferi (Lamb.) Carr. Biology 2021, 10, 576. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, P.; Singh, R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef]
- LeJeune, T.M.; Tsui, H.Y.; Parsons, L.B.; Miller, G.E.; Whitted, C.; Lynch, K.E.; Ramsauer, R.E.; Patel, J.U.; Wyatt, J.E.; Street, D.S.; et al. Mechanism of action of two flavone isomers targeting cancer cells with varying cell differentiation status. PLoS ONE 2015, 10, e0142928. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Meng, Z.; Cao, M.; Liu, S.; Kato-Noguchi, H.; Yu, W.; Jin, B.; Wang, L. Integration of morphological, physiological and multi-omics analysis reveals the optimal planting density improving leaf yield and active compound accumulation in Ginkgo biloba. Ind. Crops Prod. 2021, 172, 114055. [Google Scholar] [CrossRef]
- Liu, X.G.; Wu, S.Q.; Li, P.; Yang, H. Advancement in the chemical analysis and quality control of flavonoid in Ginkgo biloba. J. Pharm. Biomed. Anal. 2015, 113, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Dong, Y.; Xie, Y.; Wang, L.; Wang, J.; Liu, Y.; Zhao, L.; Cao, F. Effects of β-glucosidase and α-rhamnosidase on the Contents of Flavonoids, Ginkgolides, and Aroma Components in Ginkgo Tea Drink. Molecules 2019, 24, 2009. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, Y.; Mao, X.; Yu, W.; Lu, J.; Wang, L. Integration of morphological, physiological, cytological, metabolome and transcriptome analyses reveal age inhibited accumulation of flavonoid biosynthesis in Ginkgo biloba leaves. Ind. Crops Prod. 2022, 187, 115405. [Google Scholar] [CrossRef]
- Wen, M.; Shen, Y.; Shi, S.; Tang, T. miREvo: An Integrative microRNA Evolutionary Analysis Platform for Next-generation Sequencing Experiments. BMC Bioinf. 2010, 13, 140. [Google Scholar] [CrossRef]
- Friedlander, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2011, 40, 37–52. [Google Scholar] [CrossRef]
- Wu, H.J.; Ma, Y.K.; Chen, T.; Wang, M.; Wang, X.J. PsRobot: A web-based plant small RNA meta-analysis toolbox. Nucleic Acids Res. 2012, 40, W22–W28. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T.; Xin, Y.; Wang, G.; Xu, L.A. Overexpression of the GbF3′H1 gene enhanced the epigallocatechin, gallocatechin, and catechin contents in transgenic Populus. J. Agric. Food Chem. 2020, 68, 998–1006. [Google Scholar] [CrossRef]
- Yant, L.; Mathieu, J.; Dinh, T.T.; Ott, F.; Lanz, C.; Wollmann, H.; Chen, X.; Schmid, M. Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 2010, 22, 2156–2170. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Ahammed, G.J.; Li, Y.T.; Wei, J.P.; Yan, P.; Zhang, L.P.; Han, X.; Han, W.Y. Salicylic acid acts upstream of nitric oxide in elevated carbon dioxide-induced flavonoid biosynthesis in tea plant (Camellia sinensis L.). Environ. Exp. Bot. 2019, 161, 367–374. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Kovinich, N.; Durkin, P. Hormone deficient mutants have distinct flavonoid proportion fingerprints in response to abiotic stress. Plant Signal. Behav. 2018, 13, e1542241. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.B.; Liu, Y.Q.; Chen, D.Y.; Chen, F.Y.; Fang, X.; Hong, G.J.; Wang, L.J.; Wang, J.W.; Chen, X.Y. Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 2017, 8, 13925. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Galvão, V.C.; Zhang, Y.C.; Horrer, D.; Zhang, T.Q.; Hao, Y.H.; Feng, Y.Q.; Wang, S.; Schmid, M.; Wang, J.W. Gibberellin regulates the Arabidopsis floral transition through miR156-targeted SQUAMOSA PROMOTER BINDING–LIKE transcription factors. Plant Cell 2012, 24, 3320–3332. [Google Scholar] [CrossRef] [PubMed]
- Izawa, T. What is going on with the hormonal control of flowering in plants? Plant J. 2021, 105, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Gan, Q.; Xu, Y.; Lu, J.K.; Zhong, L.; Wang, M.; Liu, S.; Wang, L. Pruning improves seedling development and bioactive secondary metabolite accumulation in the leaves of Ginkgo biloba. Trees 2022, 36, 953–966. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, L.; Lu, J.; Shen, N.; Wang, L.; Liu, S.; Wang, Q.; Yu, W.; Kato-Noguchi, H.; Li, W. Rejuvenation increases leaf biomass and flavonoid accumulation in Ginkgo biloba. Hortic. Res. 2022, 9, uhab018. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Shang, E.; Zhou, G.; Tang, Y.; Guo, S.; Su, S.; Jin, C.; Qian, D.; Qin, Y.; Duan, J.A. Comparative characterization of total flavonol glycosides and terpene lactones at different ages, from different cultivation sources and genders of Ginkgo biloba leaves. Int. J. Mol. Sci. 2012, 13, 10305–10315. [Google Scholar] [CrossRef]
- Gao, H.; Chen, X.; Li, Y.; Gao, X.; Wang, J.; Qian, M.; Tong, X.; Wang, S.; Wang, Y.; Feng, J.; et al. Quality evaluation of ginkgo biloba leaves based on non-targeted metabolomics and representative ingredient quantification. J. Chromatogr. B 2023, 1214, 123549. [Google Scholar] [CrossRef]
- Bergonzi, S.; Albani, M.C.; Ver Loren van Themaat, E.; Nordström, K.J.; Wang, R.; Schneeberger, K.; Moerland, P.D.; Coupland, G. Mechanisms of age-dependent response to winter temperature in perennial flowering of Arabis alpina. Science 2013, 340, 1094–1097. [Google Scholar] [CrossRef]
- Chuck, G.; Cigan, A.M.; Saeteurn, K.; Hake, S. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat. Genet. 2007, 39, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Shen, J.; Hou, X.; Yao, J.; Li, X.; Xiao, J.; Xiong, L. Gradual increase of miR156 regulates temporal expression changes of numerous genes during leaf development in rice. Plant Physiol. 2012, 158, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Ozawa, S.; Sentoku, N.; Itoh, J.I.; Nagato, Y.; Yokoi, S. Change of shoot architecture during juvenile-to-adult phase transition in soybean. Planta 2013, 238, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.; Carlsbecker, A.; Sundås-Larsson, A.; Vahala, T. APETALA2 like genes from Picea abies show functional similarities to their Arabidopsis homologues. Planta 2007, 225, 589–602. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.H.; Amerson, H.; Chiang, V.L. MicroRNAs in loblolly pine (Pinus taeda L.) and their association with fusiform rust gall development. Plant J. 2007, 51, 1077–1098. [Google Scholar] [CrossRef]
- Wang, L.; Cui, J.; Jin, B.; Zhao, J.; Xu, H.; Lu, Z.; Li, W.; Li, X.; Li, L.; Liang, E.; et al. Multifeature analyses of vascular cambial cells reveal longevity mechanisms in old Ginkgo biloba trees. Proc. Natl. Acad. Sci. USA 2020, 117, 2201–2210. [Google Scholar] [CrossRef]
- Niu, S.H.; Liu, C.; Yuan, H.W.; Li, P.; Li, Y.; Li, W. Identification and expression profiles of sRNAs and their biogenesis and action-related genes in male and female cones of Pinus tabuliformis. BMC Genom. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Niu, S.; Yuan, H.; Sun, X.; Porth, I.; Li, Y.; El-Kassaby, Y.A.; Li, W. A transcriptomics investigation into pine reproductive organ development. New Phytol. 2016, 209, 1278–1289. [Google Scholar] [CrossRef]
- Li, Y.; Chen, T.; Khan, W.U.; An, X. Regulatory roles of miRNAs associated with the aging pathway in tree vegetative phase changes. For. Res. 2023, 3, 9. [Google Scholar] [CrossRef]
- Ma, J.J.; Chen, X.; Song, Y.T.; Zhang, G.F.; Zhou, X.Q.; Que, S.P.; Mao, F.; Pervaiz, T.; Lin, J.X.; Li, Y.; et al. MADS-box transcription factors MADS11 and DAL1 interact to mediate the vegetative-to-reproductive transition in pine. Plant Physiol. 2021, 187, 247–262. [Google Scholar] [CrossRef]
- Liu, G.; Xue, X.; Feng, J.; Cao, D.; Lin, J.; Xu, H. Age-dependent microRNAs in regulation of vascular cambium activity in Chinese fir (Cunninghamia lanceolata). Trees 2021, 35, 1451–1466. [Google Scholar] [CrossRef]
- Li, W.F.; Qi, L.W.; Yang, W.H. Age-Related miRNA-Mediated Regulatory Networks Orchestrating Chronological Development of Meristems in Larix kaempferi. J. Plant Growth Regul. 2022, 41, 2305–2318. [Google Scholar] [CrossRef]
- Sharma, D.; Tiwari, M.; Pandey, A.; Bhatia, C.; Sharma, A.; Trivedi, P.K. MicroRNA858 is a potential regulator of phenylpropanoid pathway and plant development. Plant Physiol. 2016, 171, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Meng, Z.; Zhang, H.; Chu, Y.; Qiu, Y.; Jin, B.; Wang, L. Identification and characterization of thirteen gene families involved in flavonoid biosynthesis in Ginkgo biloba. Ind. Crops Prod. 2022, 188, 115576. [Google Scholar] [CrossRef]
- Um, T.; Choi, J.; Park, T.; Chung, P.J.; Jung, S.E.; Shim, J.S.; Kim, Y.S.; Choi, I.Y.; Park, S.C.; Oh, S.J.; et al. Rice microRNA171f/SCL6 module enhances drought tolerance by regulation of flavonoid biosynthesis genes. Plant Direct 2022, 6, e374. [Google Scholar] [CrossRef]
- Gupta, O.P.; Dahuja, A.; Sachdev, A.; Kumari, S.; Jain, P.K.; Vinutha, T.; Praveen, S. Conserved miRNAs modulate the expression of potential transcription factors of isoflavonoid biosynthetic pathway in soybean seeds. Mol. Biol. Rep. 2019, 46, 3713–3730. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, X.; Tan, J.; Xu, F.; Cheng, S.; Chen, Z.; Zhang, W.; Liao, Y. Global identification of Ginkgo biloba microRNAs and insight into their role in metabolism regulatory network of terpene trilactones by high-throughput sequencing and degradome analysis. Ind. Crops Prod. 2020, 148, 112289. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, J.; Wang, T.; Cao, F.; Wang, G. Metabolomic and transcriptomic analyses of mutant yellow leaves provide insights into pigment synthesis and metabolism in Ginkgo biloba. BMC Genom. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Naik, J.; Misra, P.; Trivedi, P.K.; Pandey, A. Molecular components associated with the regulation of flavonoid biosynthesis. Plant Sci. 2022, 317, 111196. [Google Scholar] [CrossRef]
- Du, T.; Fan, Y.; Cao, H.; Song, Z.; Dong, B.; Liu, T.; Yang, W.; Wang, M.; Niu, L.; Yang, Q.; et al. Transcriptome analysis revealed key genes involved in flavonoid metabolism in response to jasmonic acid in pigeon pea (Cajanus cajan (L.) Millsp.). Plant Physiol. Biochem. 2021, 168, 410–422. [Google Scholar] [CrossRef]
- Sun, T.P. The molecular mechanism and evolution of the GA–GID1–DELLA signaling module in plants. Curr. Biol. 2011, 21, R338–R345. [Google Scholar] [CrossRef] [PubMed]
- Tirumalai, V.; Swetha, C.; Nair, A.; Pandit, A.; Shivaprasad, P.V. miR828 and miR858 regulate VvMYB114 to promote anthocyanin and flavonol accumulation in grapes. J. Exp. Bot. 2019, 70, 4775–4792. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Man, C.; Xie, Y.; Yan, J.; Chu, J.; Huang, J. A crucial role of GA-regulated flavonol biosynthesis in root growth of Arabidopsis. Mol. Plant 2019, 12, 521–537. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Mao, X.; Xu, Y.; Liu, S.; Wang, L. MicroRNA Identification and Integrated Network Analyses for Age-Dependent Flavonoid Biosynthesis in Ginkgo biloba. Forests 2023, 14, 1706. https://doi.org/10.3390/f14091706
Lu J, Mao X, Xu Y, Liu S, Wang L. MicroRNA Identification and Integrated Network Analyses for Age-Dependent Flavonoid Biosynthesis in Ginkgo biloba. Forests. 2023; 14(9):1706. https://doi.org/10.3390/f14091706
Chicago/Turabian StyleLu, Jinkai, Xinyu Mao, Yuan Xu, Sian Liu, and Li Wang. 2023. "MicroRNA Identification and Integrated Network Analyses for Age-Dependent Flavonoid Biosynthesis in Ginkgo biloba" Forests 14, no. 9: 1706. https://doi.org/10.3390/f14091706



